Abstract
NAA10 gene encodes the catalytic subunit of N(alpha)-acetyltransferase NatA that catalyzes the acetylation of the N-termini of many eukaryotic proteins. A homologous gene called NAA11 is also present in mammalian cells. hNaa10p and hNaa11p are reported to be co-expressed in human cell cultures. In mouse tissues, however, Naa11 transcripts can only be detected in gonadal tissues whereas Naa10 transcripts are present in various tissues. We re-examined the expression of NAA11 in human cell lines and expanded the test to normal as well as cancerous human tissues. Surprisingly, we did not detect the expression of NAA11 in human cell lines that previously were reported to express it. Similar to its mouse ortholog, NAA10 displayed widespread expression in human tissues. NAA11 transcripts, however, were only detected in testicular and placental tissues. The lack of NAA11 expression was also demonstrated in eight different types of human cancerous tissues. By methylation-specific polymerase chain reaction and bisulfite sequencing, we found that the absence of NAA11 expression correlated with hypermethylation of the CpG island located at the proximal promoter of NAA11 gene. We also found that the cloned NAA11 gene promoter fragment was active when introduced into non NAA11-expressing human cells and its promoter activity was lost upon in vitro DNA methylation. Taken together, our results indicate NAA11 expression is tissue-specific and is epigenetically regulated by DNA methylation.
Key words: arrest defective 1, N(α)-acetyltransferase, spermatogenesis, testis, placenta, methylation-specific polymerase chain reaction, CpG island methylation, promoter assay, retrogene
Introduction
In higher eukaryotes, protein acetylation occurs at either α-amino groups or ε-amino groups on polypeptide chains. While protein ε-acetylation at internal lysine residues is a reversible post-translational modification process,1 protein α-acetylation is irreversible and takes place co-translationally when the first 25 to 50 amino acid residues of a nascent polypeptide emerge from the ribosome.2 The transfer of an acetyl group from acetyl-CoA to N-termini of proteins is catalyzed by N(α)-acetyltransferases (NATs). At least three distinctive NAT complexes (namely NatA, NatB and NatC) exist in yeast and human cells.3 Among them, NatA is the most studied. NatA is composed of a catalytic subunit called Naa10p (also known as ARREST DEFECTIVE 1A; ARD1A) and an auxiliary subunit called Naa15p (also known as NMDA-Receptor Regulated 1; NARG1; NATH; NAT1) that is responsible for docking the NatA to ribosomes.4,5 Formation of ARD1A-NARG1 heterodimer is required for NAT activity.6 Recently, an alternative form of NatA, composed of hNaa10p and hNaa16p, was reported in HEK 293 cells.7
N(α)-acetylation is one of the most common protein modification processes occurring in eukaryotic proteins. Over 80% of mammalian proteins undergo this modification.2,8 The widespread occurrence of protein N(α)-acetylation suggests that the enzymatic activity is essential for normal cellular functions. Nevertheless, the biological significance of this protein modification process remains largely unknown. Recent studies in yeast suggest that N(α)-acetylation creates specific degradation signals for proteins,9 and prevents proteins from being targeted to the endoplasmic reticulum.10 NatA was shown to regulate neuronal dendritic development in rodents11 and it may also be involved in maintaining cellular proliferation.12 Attenuation of NatA activity by silencing the expression of hNaa10p or hNaa15p with small interfering RNA results in a reduced viability and an increased level of apoptosis in HeLa cells.13 Furthermore, hNaa10p was demonstrated to promote cancer cell proliferation.14–17 An elevated expression level of hNaa10p is observed in a variety of human cancers such as breast, colorectum and lung.16,18–21 Overexpression of hNaa10p is also associated with a poor prognosis in cancers of colon19 and lung.21 Nevertheless, the proposition that hNaa10p is an oncoprotein remains controversial as the opposite effect has also been observed.22,23 hNaa10p level is reduced in neoplastic thyroid tissue when compared to its non-neoplastic counterpart.24 NAA10 transcript level is significantly reduced in non-small cell lung cancer as contrasted to adjacent non-tumor lung tissue.23 Increased levels of NAA10 transcripts and hNaa10p proteins were respectively found to correlate with better clinical outcome in breast cancer patients23 and survival of lung cancer patients.25 Similar to the yeast Naa10p, mouse Naa10p alone does not display NAT activity.12,26 However, hNaa10p alone was demonstrated to catalyze the acetylation of internal lysine residues in β-Catenin (CTNNB1),15 Myosin Light Chain Kinase (MLCK),22 and hNaa10p itself.14 A shorter isoform of mNaa10p (mNaa10p_NP_001171436) was shown to stimulate the degradation of Hypoxia Inducible Factor 1α (Hif1α) by acetylating an internal lysine residue of the protein.27 Interestingly, hNaa10p was shown in lung cancer cells to modulate the activity of DNA Methyltransferase 1 (DNMT1),21 and to suppress metastasis25 independently of its acetyltransferase activity.
A homolog of Naa10p, called Naa11p (also known as ARREST DEFECTIVE 1B; ARD1B; ARD2), was identified in the mouse26 and human.28 The genes encoding Naa11 and NAA11 are believed to be the functional autosomal copies of their respective X-linked progenitors (Naa10 and NAA10) through retroposition.26,28 Except for the divergent C-termini, the polypeptide sequences of Naa10p and Naa11p are highly conserved between the mouse and human. Both mNaa11p and hNaa11p can reconstitute a functional NAT in the presence of mNaa15p and hNaa15p, respectively. In the mouse, Naa11 is expressed predominantly in the testis; its expression level is upregulated during meiosis when Naa10 expression is downregulated.26 In contrast, Naa10 is expressed in somatic tissues that do not show Naa11 expression. It is therefore believed that mNaa11p is expressed to compensate for the loss of mNaa10p during spermatogenesis.26 On the other hand, NAA11 was found to co-express with NAA10 in several human cell lines.17,25,28 The induction of differentiation of promyelocytic leukemia NB4 cells leads to a downregulation of hNaa10p and hNaa15p expression. However, the level of hNaa11p remains unchanged, which implies a role for hNaa11p in the cellular differentiation process.28 The loss of heterozygosity in NAA11 was shown to correlate with a poor prognosis in hepatocellular carcinoma patients.29 Other than these, the biological functions of mNaa11p and hNaa11p are not known. The presence of two similar NatA complexes sharing the same ribosome docking subunit, but different catalytic subunits in the same human cells,28 may imply a complementary role in regulating similar biological processes. Alternatively, the two NatA complexes may display different protein substrate specificity and thus, biological functions. Intrigued by this hypothesis, we examined whether co-expression of NAA10 and NAA11 is a common phenomenon in human tissues. Contrary to our expectation, we could not reproduce the co-expression of NAA10 and NAA11 in human cell cultures. NAA11 expression was detected only in the testis and placenta obtained from normal human subjects. Except for a few cases, NAA11 expression was also absent in a variety of human cancer tissues. We examined the methylation status of the CpG island in NAA11 gene promoter and tested the promoter activity in the presence or absence of DNA methylation. Our findings indicate the expression of NAA11 gene is epigenetically regulated by DNA methylation of its proximal promoter, which explains the tissue-specific expression pattern of the gene.
Results
Expression analysis of NAA10 and NAA11 in human tissues and cell lines.
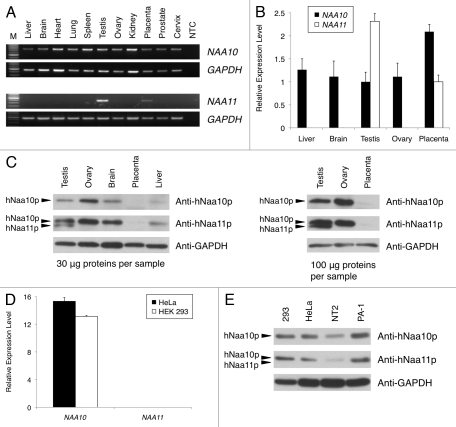
To examine the tissue expression pattern of NAA10 and NAA11 in different adult human tissues, we performed RT-PCR experiments with PCR primers spanning the exon-intron junctions of both genes. NAA11 transcripts were detected only in the testis and placenta (Fig. 1A). In contrast, NAA10 was expressed in all tissues. These findings were confirmed in a separate Q-PCR analysis, from which the expression level of NAA11 was more than 2-fold higher in human testis than placenta (Fig. 1B).
Figure 1.
Expression analysis of NAA10 (NM_003491) and NAA11 (NM_032693) gene products in human tissues and cell lines. (A) Expression pattern of NAA10 and NAA11 transcripts in normal human tissues by RT-PCR. Expression of GAPDH was analyzed as an endogenous control. M: DNA molecular weight ladder. NTC: no-template control reaction. Sizes of amplicons: NAA10 (708 bp), NAA11 (382 bp) and GAPDH (452 bp). (B) Q-PCR analysis of NAA10 and NAA11 expression in selected human tissues. Relative expression levels of NAA10 (black bars) and NAA11 (white bars) after normalizing to that of 18S rRNA were presented using the 2(-Delta Delta C(T)) method. (C) Expression analysis of hNaa10p and hNaa11p in human tissues by western blotting. The detection of GAPDH protein was performed as an endogenous control. The amount of tissue lysates used is listed. The immuno-reactive band corresponding to hNaa11p appears at a slightly smaller molecular weight than that of hNaa10p; and the anti-hNaa11p antibody was cross-reactive to hNaa10p. (D) Q-PCR analysis of NAA10 and NAA11 expression in HeLa and HEK 293 cells. Relative expression levels of NAA10 and NAA11 after normalizing to that of 18S rRNA were presented using the delta C(T) method. NAA10 expression was detected in HeLa cells (black bar) and HEK 293 cells (white bar), whereas no NAA11 expression was detected. Data shown were averaged from triplicate measurements of duplicate preparations of total RNA samples from each cell line. (E) Expression analysis of hNaa10p and hNaa11p in human cell lines by western blotting. Thirty micrograms of total protein lysates were loaded per sample. No immuno-reactivity corresponding to hNaa11p was observed in any of the cell lysates. The detection of GAPDH protein was performed as an endogenous control. In figures (B and D), error bars represent the standard deviations of relative gene expression levels.
We further examined the expression of hNaa10p and hNaa11p in human tissues by western blotting. Despite the fact that the anti-hNaa11p antibody displayed cross-reactivity to hNaa10p, an immunoreactive band of hNaa11p, which appeared at a slightly smaller molecular weight than that of hNaa10p, was detected only in human testis (Fig. 1C). We did not observe similar immunoreactivity of hNaa11p in human placental tissues even when higher amounts of tissue lysates were used in the western blotting experiment (Fig. 1C). Consistent with the RT-PCR result, hNaa10p was expressed in different human tissues.
Based on the differential expression pattern of NAA11 in human tissues we observed, we re-examined the expression pattern of NAA10 and NAA11 in HeLa and HEK 293 cells. NAA10 transcripts were readily detected in these cells, but no NAA11 expression could be detected (Fig. 1D). Similar findings were obtained when we examined the expression of hNaa11p in these and other human cell lines (Fig. 1E).
Expression analysis of NAA10 and NAA11 in cancer tissues by Q-PCR.
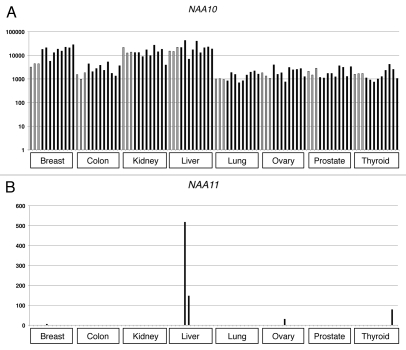
To determine if NAA10 and NAA11 were co-expressed in the malignant state, we performed Q-PCR to examine the gene expression patterns in normal and cancerous tissues derived from breast, colon, kidney, liver, lung, ovary, prostate and thyroid of individual donors. Despite different levels of expression, NAA10 transcripts could be detected in all cancerous tissues as well as their normal counterparts (Fig. 2A). In contrast, NAA11 transcripts could only be detected in 5 of the 72 cancer tissues examined (one from breast cancer, two from liver cancer, one from ovarian cancer and one from thyroid cancer), and their relative abundance was much lower than that of NAA10 (Fig. 2B). None of the tissue samples from normal donors showed NAA11 expression.
Figure 2.
Expression analysis of NAA10 and NAA11 in cancerous tissues by Q-PCR. Y-axis: relative expression level of respective gene after normalizing to that of ACTB. Grey bars represent the gene expression levels obtained from normal tissues, whereas black bars refer to the expression levels detected in cancerous tissues. Br: breast; Co: colon; Ki: kidney; Lv: liver; Ln: lung; Ov: ovary; Pr: prostate; Ty: thyroid; N: normal tissues; T: cancer tissues. Numbers refer to the individual donors.
Analysis of the methylation status of NAA10 and NAA11 promoters.
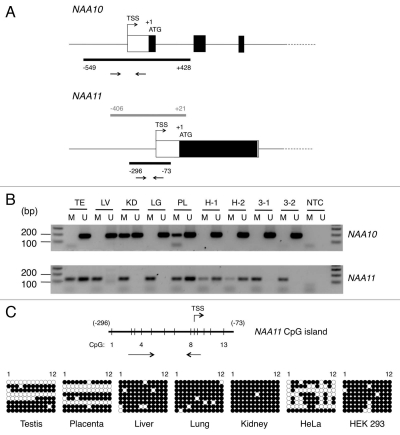
The expression pattern of NAA11 suggests its expression is regulated in a tissue-specific manner. In a search of regulatory sequence elements present in the upstream genomic regions of NAA10 and NAA11 genes, a CpG island was identified at the proximal promoter region of both genes (Fig. 3A). In both cases, the CpG island spans the transcription start site (TSS) of the gene. The presence of CpG island at TSS suggests that the transcription of NAA11 may be susceptible to regulation by DNA methylation. To examine this possibility, we performed MS-PCR to analyze the methylation status of the CpG islands in both NAA10 and NAA11 genes in human tissues (including testis, liver, kidney, lung and placenta) and cell lines (HeLa and HEK 293). PCR primers were designed to detect the corresponding genomic loci in methylated (M) and non-methylated (U) states after bisulfite treatment. For NAA10 gene, U primers generated PCR products of the expected size in all tissues and cell lines examined. On the other hand, reactions with M primers generated PCR products in kidney and placenta (Fig. 3B). The PCR products from the latter may be derived from non NAA10-expressing cell types present in these tissues. For NAA11 gene, PCR products generated with U primers were limited to testis, placenta and surprisingly, HeLa cells. PCR products produced with M primers were detected readily in all tissues and cell lines examined.
Figure 3.
Identification of CpG islands in proximal promoter regions of NAA10 and NAA11 genes and analysis of their methylation status. (A) Genomic organization of NAA10 and NAA11 genes at their 5′ ends. Black bars represent the locations of CpG islands in both genes. The arrow pairs denote the positions of primers used for MS-PCR. Grey bar refers to the genomic fragment cloned for NAA11 promoter assay. TSS, transcription start site. The genomic positions of CpG islands and NAA11 promoter fragment are annotated with respect to the start codon (ATG) of the respective gene, with A denoted as +1. (B) Analysis of methylation status of NAA10 and NAA11 gene proximal promoters in human tissues and cell lines by MS-PCR. Sizes of amplicons: for NAA10 gene: M primers (178 bp), U primers (183 bp); for NAA11 gene: M primers (129 bp), U primers (126 bp). Duplicate sets of genomic DNA samples from HeLa (H-1 and H-2) and HEK 293 (3-1 and 3-2) cells, which were harvested from the same preparations of cells for NAA10 and NAA11 expression analysis in Figure 1D, were examined. TE, testis; LV, liver; KD, kidney; LG, lung; PL, placenta; NTC, no-template control reaction. Forty cycles of amplification were performed. (C) Bisulfite sequencing analysis of the CpG island in NAA11 gene. Vertical lines on horizontal bar refer to the position of CpG dinucleotides (1–13) in the CpG island. Annotations are defined as in (A). Owing to the constraints in primer design, the 13th CpG dinucleotide was not analyzed in this experiment. For HeLa and HEK 293 cells, only one genomic DNA sample from each cell line was analyzed. Black circle, methylated CpG dinucleotide; white circle, non-methylated CpG dinucleotide.
It is noteworthy that the genomic DNA samples for HeLa and HEK 293 cells were extracted from the same preparations of cells used for NAA10 and NAA11 expression analysis (Fig. 1D). To obtain a better resolution of the methylation status of the CpG islands at single nucleotide level, we performed bisulfite sequencing on the same set of human tissues and cells. Owing to the large size (978 bp) of the CpG island in NAA10 gene, we were not able to design primers that could amplify the corresponding genomic locus for sequencing analysis after bisulfite conversion. We focused on delineating the methylation status of the CpG island in NAA11 gene. As shown in Figure 3C, the CpG island was hyper-methylated in liver, lung, kidney and HEK 293 cells which did not show NAA11 expression. In both testis and placenta, we observed two distinctive CpG methylation profiles that were indicative of the presence of hyper-methylated and non-methylated CpG islands. This finding is consistent with the presence of non NAA11-expressing and NAA11-expressing cell types, respectively, in these tissues. On the contrary, the CpG methylation profile in HeLa cells was less distinctive, with the CpG island displaying various degree of methylation among the different clones of sequences. The presence of non-methylated CpG dinucleotides allowed a productive MS-PCR with the U primers, which explained the detection of both methylated and non-methylated version of NAA11 CpG island in HeLa cells. Nevertheless, our findings suggest the CpG island in NAA10 gene is generally non-methylated in human tissues and cell lines. With the exception of HeLa cells, the CpG island in NAA11 gene is extensively methylated in human tissues and cell lines that do not show NAA11 expression; the genomic locus is non-methylated in tissues that show NAA11 expression.
Suppression of NAA11 promoter activity by CpG methylation.
The MS-PCR and bisulfite sequencing data indicate the involvement of DNA methylation in the suppression of NAA11 transcription. We therefore examined if DNA methylation would directly suppress NAA11 promoter activity. A 427-bp contiguous genomic DNA fragment covering the proximal promoter and the first 7 codons of NAA11 gene was cloned into reporter vector pGL3-Basic to generate the promoter construct pGL3-Basic/NAA11-pro (Fig. 3A). Upon transfection of HeLa cells, the promoter construct produced a significantly higher level of luciferase activity than cells transfected with the empty vector (data not shown). The same effect was also observed in HEK 293 cells, suggesting the NAA11 promoter fragment was active in both cell lines even though these cells do not express endogenous NAA11.
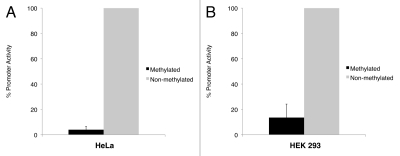
The same experiment was performed with cells transfected with in vitro methylated empty vector and promoter construct. In both cell lines, the luciferase activity produced by the methylated promoter construct was significantly lower than its non-methylated counterpart. To eliminate the interference that may be contributed by the vector sequence (i.e., to study the effect of DNA methylation on the NAA11 promoter fragment only), we compared the luciferase activities between the methylated and non-methylated promoter construct after normalizing to those obtained from the methylated and non-methylated empty vector, respectively. From this, we observed a 25.4-fold reduction of NAA11 promoter activity in HeLa cells after DNA methylation (Fig. 4A). In HEK 293 cells, a 7.4-fold reduction of NAA11 promoter activity was obtained (Fig. 4B). Our data clearly demonstrated that the activity of NAA11 promoter was susceptible to suppression by DNA methylation.
Figure 4.
NAA11 promoter activity is regulated by DNA methylation. Promoter assays were performed in HeLa and HEK 293 cells that were transiently transfected with pGL3-Basic and pGL3-Basic/NAA11-pro vectors with or without in vitro methylation treatment. Black bars and grey bars correspond to the promoter activities displayed by methylated and non-methylated NAA11 promoter fragment, respectively. Data shown are averaged results from three independent experiments. Promoter activity displayed by the non-methylated NAA11 promoter fragment was set to be 100%. Error bars represent the standard deviations of percentage promoter activities.
Discussion
Acetylation has long been a subject of interest in the study of protein co- and post-translational modification processes. The discovery of alternative substrate specificity of hNaa10p suggests the homologous hNaa11p may also acetylate amino groups other than α-amino groups on polypeptides. The human cell lines shown to co-express hNaa10p and hNaa11p17,25,28 therefore represent a convenient platform to investigate the biological activity of the two catalytic subunits, as well as their associated enzymatic complexes. We sought to evaluate this possibility. In this study, we first compared the expression of NAA10 and NAA11 gene products in different normal human tissues and established human cell lines. Similar to the mouse Naa11 gene, we found that NAA11 was predominantly expressed in the human testis; a lower expression level of the gene was detected in human placenta. On the protein level, however, we observed the presence of hNaa11p only in human testis. We speculate the NAA11 transcripts may be translationally repressed in placenta in a way similar to the Naa11 transcripts in the mouse testis.26 Alternatively, the expression of hNaa11p may be confined to a small population of cells within the placenta. As a result, the abundance of the protein may be lower than the detection limit of western blotting assay. The identification of the cellular expression pattern of NAA11 gene products in placenta tissues will help explain the discrepancy.
Unexpectedly, we did not detect NAA11 expression in the cell lines that were originally reported to express the gene. As these cells are either transformed or cancerous in nature, we also surveyed the expression of NAA11 in specimens that were extracted from multiple types of human cancer. The cancer specimens examined (e.g., breast, liver, lung and thyroid) covered the tissue origins of some of the cell lines that were previously analyzed for NAA11 and hNaa11p expression.17,25,28 NAA11 expression was not detected in the cancerous tissues, with the exception in several cases of relatively weak expression of the gene. On the other hand, NAA10 demonstrated a housekeeping expression pattern in all cases. All together, our data are consistent with our previous study on the mouse orthologs26 that shows that the expression of NAA10 is constitutive, but tissue-specific for NAA11. This observation implies that the regulatory mechanisms controlling the expression of the two genes are different. The fact that the NAA11 promoter fragment is active in non NAA11-expressing cells suggests the tissue-restricted expression of NAA11 is not entirely mediated by the action of cell- or tissue-specific factors. Instead, our data indicate the CpG island in the proximal promoter of NAA11 gene is a target of transcriptional regulation.
DNA methylation at CpG dinucleotides is an epigenetic mechanism commonly associated with gene silencing. The process is involved in the control of developmental or cell-type specific gene expression patterns in normal cells.30 Meanwhile, dysregulated gene expression owing to aberrant DNA methylation is frequently observed in cancer cells.31 DNA methylation inhibits gene transcription by interfering directly with the binding of transcription factors to their cognate DNA sequences, or by recruiting methyl-CpG-binding domain proteins and their associated transcriptional co-repressors to induce a repressive chromatin state.32,33 The effect of CpG island methylation on suppression of NAA11 transcription was demonstrated in our promoter assay in which the activity of NAA11 promoter was dramatically reduced after in vitro methylation. The involvement of DNA methylation in controlling NAA11 expression was further corroborated by the MS-PCR and bisulfite sequencing analyses. We found that the CpG island in NAA11 gene was hyper-methylated in non NAA11-expressing cells and tissues. NAA11 gene products were also absent in HeLa cells despite the existence of a less extensively methylated CpG island. In contrast, the CpG island in NAA10 gene was not methylated, which correlates with its housekeeping expression pattern across different cell types and tissues. Consistently, NAA11 expression was present in normal testis and placenta in which a non-methylated CpG island was detected. Similar to the observations from male germ cell-specific genes such as Pdha234 and LDH-C35 and cancer testis antigens (e.g., MAGE-A1 and LAGE-1),36 our findings suggest that DNA methylation is the regulatory mechanism specifically utilized to suppress NAA11 transcription in cells and tissues that do not require its activity. The low level of NAA11 expression detected in the few cancer specimens may have resulted from a loss of DNA methylation that would lead to the re-activation of gene transcription. In this regard, the re-expression of NAA11 could be mimicked by treating non NAA11-expressing cells with inhibitor of DNA methyltransferase (Pang et al. manuscript in preparation).
To conclude, we demonstrated that the expression of NAA11 is tissue-specific and is governed by DNA methylation. Although NAA10 and NAA11 were found to be co-expressed in several human cell lines, our current observations point to the opposite: co-expression of NAA10 and NAA11 in the same cells is not a common phenomenon. In agreement with our previous findings,26 the NAT constituted by mNaa10p/hNaa10p and mNaa15p/hNaa15p is the major form of NatA in mammalian cells. The NatA composed of mNaa11p/hNaa11p and mNaa15p/hNaa15p is present only in specialized cell types such as male germ cells. We do not know the exact reasons for the discrepancy in NAA11 and hNaa10p expression pattern observed between our data and previous reports. The specificities of the different reagents and differences in experimental methodology may have contributed to the disparities. Other than that, the expression of NAA11 may become “leaky” in response to environmental factors such as variations in cell culturing conditions; this awaits further investigation. No matter what the potential cause may be, a more extensive analysis of NAA11 expression in various human cell lines is necessary before they can be used adequately as an in vitro model to study the biological roles of NAA11 gene products.
Materials and Methods
Cell lines, genomic DNA and total RNA isolation.
NT2/D1 (CRL-1973), PA-1 (CRL-1572), HeLa (CCL-2) and HEK 293 (CRL-1573) cell lines were obtained from ATCC and cultured according to ATCC recommendation. Genomic DNA samples from human tissues (testis: D1234260; liver: D1234149; kidney: D1234142; lung: D1234152; placenta: D1234200) were obtained from Biochain; those from human cell lines were extracted with Puregene Cell kit (Gentra D-5010A). Total RNA samples from human tissues were obtained from Ambion (AM6000); those from human cell lines were extracted using TRIzol reagent (Invitrogen 15596-018).
Reverse-transcription polymerase chain reaction (RT-PCR).
One microgram of total RNA was reverse-transcribed in the presence of random hexamers (Invitrogen 48190-011) and SuperScript II reverse transcriptase (Invitrogen 18064-014). The first-strand cDNA sample was diluted 5 times in nuclease free water (Ambion AM9937). One microliter of the diluted cDNA was used in 20-µL PCR with 2.5 mM of MgCl2, 0.5 mM of dNTPs, 0.5 µM of primers and 1 unit of Platinum Taq DNA polymerase (Invitrogen 10966-083). PCR products were resolved on 2% TAE agarose gel (EmbiTec GE-3642). The sequences of primers used for RT-PCR of NAA10, NAA11 and GAPDH were listed in Table 1.
Table 1.
List of primers used in this study
| For RT-PCR |
| NAA10-F: ATG AAC ATC CGC AAT GCG AGG |
| NAA10-R: CTA GGA GGC TGA GTC GGA GG |
| NAA11-F: CAG CAC ACT TTC TGA TTC TGA AG |
| NAA11-R: GTA ATG GCA GGT CTC AAA GTT C |
| GAPD-5: ACC ACA GTC CAT GCC ATC AC |
| GAPD-3: TCC ACC ACC CTG TTG CTG TA |
| For cloning of NAA11 promoter* |
| NAA11-pro-F: TAA GGC ACG CGT TGT TAT ATG GGT TTC TCC AGT TTG TG |
| NAA11-pro-R: TAA GGC AAG CTT CTG AGC GTT GCG GAT GTT C |
| For DNA sequencing |
| M13-R: GGA AAC AGC TAT GAC CAT |
| M13-20: GTA AAA CGA CGG CCA GTG |
| For MS-PCR |
| M-NAA10-1F: GTT TCG CGT ATT GGT TAA GC |
| M-NAA10-1R: GTA AAA ACG CAA TCA ACT ACC G |
| U-NAA10-1F: GTT TGT TTT GTG TAT TGG TTA AGT G |
| U-NAA10-1R: CAT AAA AAC ACA ATC AAC TAC CAC C |
| M-NAA11-F: TGC GTA CGT ATA CGT ATA ATG AAG C |
| M-NAA11-R: ATA AAA AAC GAA TAA CGA AAA AAC G |
| U-NAA11-F: GTG TAT GTA TAT GTA TAA TGA AGT GA |
| U-NAA11-R: AAA AAA CAA ATA ACA AAA AAA CAA A |
| For bisulfite sequencing |
| BSS-NAA11-F: ATT TAA ATT AAA AAG TTA AAA TTG AAA TAA |
| BSS-NAA11-R: AAA AAA CAC TAT TTA CCT CAA AAA TC |
| BSS-NAA11-NR: AAT CCA AAA AAC TAA CAC CAC C |
All primers are listed in 5′ to 3′ orientation.
Underlined sequence represents a random hexameric sequence included to protect the downstream MluI (ACGCGT) and HindIII (AAGCTT) restriction sites.
Gene expression in cancer tissues by quantitative real-time polymerase chain reaction (Q-PCR).
The expression level of NAA10, NAA11 and 18S rRNA in normal tissues and cell lines were analyzed with cDNA samples generated as described above. The expression of NAA10, NAA11 and ACTB in cancer tissues was analyzed on the TissueScan Cancer Survey Panel 96-I (OriGene Technologies CSRT501). Q-PCR was performed with Taqman gene expression assays for NAA10 (Hs00185854_m1), NAA11 (Hs00261953_m1) and 18S rRNA (TaqMan Ribosomal RNA Control Reagents 4308329) in the presence of Taqman Universal PCR master mix (4364338), all from Applied Biosystems. For measurement of ACTB transcript level, SYBR Green PCR master mix (Applied Biosystems 4364344) was used in the presence of ACTB-specific primer mix provided with the TissueScan Cancer Survey Panel 96-I. Expression levels of NAA10 and NAA11 were normalized to that of 18S rRNA or ACTB and calculated by 2(-Delta Delta C(T)) or delta C(T) method as specified.
Western blotting.
Total protein lysates from NT2/D1, PA-1, HeLa and HEK 293 cells were extracted in RIPA buffer (25 mM Tris-HCl, pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS) supplemented with Halt protease inhibitor cocktail (Pierce 87786). Total protein lysates from human tissues were obtained from Biochain (testis: P1234260; ovary: P1234183; brain: P1234035; liver: P1234149; placenta: P1234200). Proteins were transferred to PVDF membranes (BioRad 162-0177) in Protean transfer cell (BioRad 170-3930) according to standard procedure. Primary antibodies were diluted in blocking solution (1x PBS, 0.05% Tween-20 and 5% non-fat dry milk) as specified below: goat-anti-hNaa10p (Santa Cruz Biotechnology sc-33256) at 1:2,000; rabbit-anti-hNaa11p (ProteinTech Group 15670-1-AP) at 1:1,000; chicken-anti-GAPDH (Genway 15-288-22763) at 1:20,000. Different horse-radish peroxidase (HRP)-conjugated secondary antibodies (all diluted 10,000-fold in blocking solution) were used according to the origins of primary antibodies, including goat-anti-rabbit IgG-HRP (BioRad 170-6515), goat-anti-chicken IgY-HRP (abcam ab6877) and donkey-anti-goat IgG-HRP (Santa Cruz Biotechnology sc-2020). Immunoreactivities were visualized by film exposure after developing in SuperSignal West Pico chemiluminescent substrate (Pierce 34080). Membranes were re-probed after stripping in Restore western blot stripping buffer (Pierce 21059).
Cloning of NAA11 promoter fragment.
The −406 to +21 region of NAA11 gene (GenBank accession number: NM_032693, with the adenosine of start codon assigned as +1) was amplified by PCR in the presence of 1x HF buffer, 0.5 µM of each of NAA11-pro-F and NAA11-pro-R primers (Table 1), 0.5 mM of dNTPs, 20 ng of human testicular genomic DNA and 0.4 unit of Phusion Hot Start High-Fidelity DNA Polymerase (Finnzymes F-540L). The PCR product, with MluI and HindIII restriction sites incorporated at the respective 5′ end, was cloned into ZeroBlunt pCR4-TOPO vector (Invitrogen K2875-20) for DNA sequencing with M13 primers (Table 1). The promoter fragment was harvested by restriction digestion with the specified enzymes from New England Biolabs (MluI: R0198S; HindIII: R0104S) and ligated into firefly luciferase reporter vector pGL3-Basic (Promega E1751) to generate the pGL3-Basic/NAA11-pro vector. CpG islands on NAA10 and NAA11 genes were predicted using EMBOSS Cpgplot (www.ebi.ac.uk/emboss/cpgplot).
In vitro methylation.
The pGL3-Basic and pGL3-Basic/NAA11-pro vectors were separately methylated in vitro by M.SssI CpG methyltransferase (New England Biolabs M0226S). Each microgram of plasmid DNA was incubated at 37°C for 3 hours with 4 units of M.SssI CpG methyltransferase and 0.32 mM of S-adenosylmethionine. Completion of CpG methylation was confirmed by digestion of methylated plasmid with methylation-sensitive restriction enzyme HhaI (New England Biolabs R0139S). Methylated plasmid was purified using the Wizard SV Gel and PCR Clean-Up System (Promega A9282) and re-quantified by absorbance measurement using the NanoVue spectrophotometer (GE Healthcare 28924402).
Reporter assay.
Eighty nanograms of pGL3-Basic vector or pGL3-Basic/NAA11-pro vector, and 1 ng of Renilla luciferase normalization vector pGL4.73[hRluc/SV40] (Promega E6911) were co-transfected into HeLa and HEK 293 cells seeded at a density of 20,000 cells/well in 24-well culture plates. In parallel, the same transfection experiment was performed with pGL3-Basic and pGL3-Basic/NAA11-pro vectors, both in vitro methylated. Two days after transfection, the transfected cells were harvested for reporter assay using the Dual Luciferase Reagent (Promega E1910). The ratios of luciferase activities from the reporter vector (pGL3-Basic series) and the normalization vector (pGL4.73[hRluc/SV40]) from all experiments (pGL3-Basic with or without methylation and pGL3-Basic/NAA11-pro with or without methylation) were obtained after background subtraction. To identify the effect of DNA methylation on reporter activity regulated by the NAA11 promoter fragment, the luciferase activity ratio from non-methylated pGL3-Basic/NAA11-pro was divided by the ratio from non-methylated pGL3-Basic and compared to that obtained from methylated pGL3-Basic/NAA11-pro and pGL3-Basic. The promoter activity of methylated NAA11 promoter fragment was expressed as a fraction of that of non-methylated NAA11 promoter fragment.
Bisulfite treatment of genomic DNA, methylation specific-polymerase chain reaction (MS-PCR) and bisulfite sequencing.
Genomic DNA samples were bisulfite-converted using the EZ DNA Methylation-Gold kit (Zymo Research D5005) and re-quantified with the NanoVue spectrophotometer. For MS-PCR, 10 ng of bisulfite-converted DNA was used per reaction in the presence of primers that would distinguish between methylated (M) and non-methylated (U) promoters of NAA10 and NAA11 genes after bisulfite conversion. For bisulfite sequencing, the modified CpG island in NAA11 gene from individual samples was amplified by regular PCR in the presence of 20 ng of bisulfite-converted DNA and primers BSS-NAA11-F and BSS-NAA11-R; followed by a hemi-nested PCR with 2 µL of the ten-fold diluted PCR product and primers BSS-NAA11-F and BSS-NAA11-NR. The resulting 272-bp PCR products were separately cloned into pCR4-TOPO vector and multiple clones were selected for sequencing analysis. All MS-PCR and bisulfite sequencing primers (Table 1) were designed using the MethPrimer tool.37
Acknowledgments
This research was supported by the Intramural Research Program of Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Abbreviations
- bp
base-pair
- MS-PCR
methylation specific-polymerase chain reaction
- NAA10
N(α)-acetyltransferase 10, NatA catalytic subunit
- NAA11
N(α)-acetyltransferase 11, NatA catalytic subunit
- NAA15
N(α)-acetyltransferase 15, NatA auxiliary subunit
- NAT
N(α)-acetyltransferase
- Q-PCR
quantitative-polymerase chain reaction
- RT-PCR
reverse transcription-polymerase chain reaction
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Yang XJ. Lysine acetylation and the bromodomain: a new partnership for signaling. Bioessays. 2004;26:1076–1087. doi: 10.1002/bies.20104. [DOI] [PubMed] [Google Scholar]
- 2.Driessen HP, de Jong WW, Tesser GI, Bloemendal H. The mechanism of N-terminal acetylation of proteins. CRC Crit Rev Biochem. 1985;18:281–325. doi: 10.3109/10409238509086784. [DOI] [PubMed] [Google Scholar]
- 3.Starheim KK, Gromyko D, Velde R, Varhaug JE, Arnesen T. Composition and biological significance of the human Nalpha-terminal acetyltransferases. BMC Proc. 2009;3:3. doi: 10.1186/1753-6561-3-S6-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautschi M, Just S, Mun A, Ross S, Rucknagel P, Dubaquie Y, et al. The yeast N(alpha)-acetyltransferase NatA is quantitatively anchored to the ribosome and interacts with nascent polypeptides. Mol Cell Biol. 2003;23:7403–7414. doi: 10.1128/MCB.23.20.7403-7414.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polevoda B, Brown S, Cardillo TS, Rigby S, Sherman F. Yeast N(alpha)-terminal acetyltransferases are associated with ribosomes. J Cell Biochem. 2008;103:492–508. doi: 10.1002/jcb.21418. [DOI] [PubMed] [Google Scholar]
- 6.Park EC, Szostak JW. ARD1 and NAT1 proteins form a complex that has N-terminal acetyltransferase activity. EMBO J. 1992;11:2087–2093. doi: 10.1002/j.1460-2075.1992.tb05267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnesen T, Gromyko D, Kagabo D, Betts MJ, Starheim KK, Varhaug JE, et al. A novel human NatA Nalpha-terminal acetyltransferase complex: hNaa16p-hNaa10p (hNat2-hArd1) BMC Biochem. 2009;10:15. doi: 10.1186/1471-2091-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, et al. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci USA. 2009;106:8157–8162. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 327:973–977. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forte GMA, Pool, Martin R, Stirling, Colin J. N-Terminal Acetylation Inhibits Protein Targeting to the Endoplasmic Reticulum. PLoS Biol. 2011;9:1001073. doi: 10.1371/journal.pbio.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohkawa N, Sugisaki S, Tokunaga E, Fujitani K, Hayasaka T, Setou M, et al. N-acetyltransferase ARD1-NAT1 regulates neuronal dendritic development. Genes Cells. 2008;13:1171–1183. doi: 10.1111/j.1365-2443.2008.01235.x. [DOI] [PubMed] [Google Scholar]
- 12.Sugiura N, Adams SM, Corriveau RA. An evolutionarily conserved N-terminal acetyltransferase complex associated with neuronal development. J Biol Chem. 2003;278:40113–40120. doi: 10.1074/jbc.M301218200. [DOI] [PubMed] [Google Scholar]
- 13.Arnesen T, Gromyko D, Pendino F, Ryningen A, Varhaug JE, Lillehaug JR. Induction of apoptosis in human cells by RNAi-mediated knockdown of hARD1 and NATH, components of the protein N-alpha-acetyltransferase complex. Oncogene. 2006;25:4350–4360. doi: 10.1038/sj.onc.1209469. [DOI] [PubMed] [Google Scholar]
- 14.Seo JH, Cha JH, Park JH, Jeong CH, Park ZY, Lee HS, et al. Arrest defective 1 autoacetylation is a critical step in its ability to stimulate cancer cell proliferation. Cancer Res. 2010;70:4422–4432. doi: 10.1158/0008-5472.CAN-09-3258. [DOI] [PubMed] [Google Scholar]
- 15.Lim JH, Park JW, Chun YS. Human arrest defective 1 acetylates and activates beta-catenin, promoting lung cancer cell proliferation. Cancer Res. 2006;66:10677–10682. doi: 10.1158/0008-5472.CAN-06-3171. [DOI] [PubMed] [Google Scholar]
- 16.Yu M, Ma M, Huang C, Yang H, Lai J, Yan S, et al. Correlation of expression of human arrest-defective-1 (hARD1) protein with breast cancer. Cancer Invest. 2009;27:978–983. doi: 10.3109/07357900902769723. [DOI] [PubMed] [Google Scholar]
- 17.Fisher TS, Etages SD, Hayes L, Crimin K, Li B. Analysis of ARD1 function in hypoxia response using retroviral RNA interference. J Biol Chem. 2005;280:17749–17757. doi: 10.1074/jbc.M412055200. [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Gong J, Ma M, Yang H, Lai J, Wu H, et al. Immunohistochemical analysis of human arrest-defective-1 expressed in cancers in vivo. Oncol Rep. 2009;21:909–915. doi: 10.3892/or_00000303. [DOI] [PubMed] [Google Scholar]
- 19.Jiang B, Ren T, Dong B, Qu L, Jin G, Li J, et al. Peptide mimic isolated by autoantibody reveals human arrest defective 1 overexpression is associated with poor prognosis for colon cancer patients. Am J Pathol. 2010;177:1095–1103. doi: 10.2353/ajpath.2010.091178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren T, Jiang B, Jin G, Li J, Dong B, Zhang J, et al. Generation of novel monoclonal antibodies and their application for detecting ARD1 expression in colorectal cancer. Cancer Lett. 2008;264:83–92. doi: 10.1016/j.canlet.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Lee CF, Ou DS, Lee SB, Chang LH, Lin RK, Li YS, et al. hNaa10p contributes to tumorigenesis by facilitating DNMT1-mediated tumor suppressor gene silencing. J Clin Invest. 2010;120:2920–2930. doi: 10.1172/JCI42275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin DH, Chun YS, Lee KH, Shin HW, Park JW. Arrest defective-1 controls tumor cell behavior by acetylating myosin light chain kinase. PLoS One. 2009;4:7451. doi: 10.1371/journal.pone.0007451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuo HP, Lee DF, Chen CT, Liu M, Chou CK, Lee HJ, et al. ARD1 stabilization of TSC2 suppresses tumorigenesis through the mTOR signaling pathway. Sci Signal. 2010;3:9. doi: 10.1126/scisignal.2000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnesen T, Gromyko D, Horvli O, Fluge O, Lillehaug J, Varhaug JE. Expression of N-acetyl transferase human and human Arrest defective 1 proteins in thyroid neoplasms. Thyroid. 2005;15:1131–1136. doi: 10.1089/thy.2005.15.1131. [DOI] [PubMed] [Google Scholar]
- 25.Hua KT, Tan CT, Johansson G, Lee JM, Yang PW, Lu HY, et al. N-alpha-acetyltransferase 10 protein suppresses cancer cell metastasis by binding PIX proteins and inhibiting Cdc42/Rac1 activity. Cancer Cell. 2011;19:218–231. doi: 10.1016/j.ccr.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Pang AL, Peacock S, Johnson W, Bear DH, Rennert OM, Chan WY. Cloning, characterization and expression analysis of the novel acetyltransferase retrogene Ard1b in the mouse. Biol Reprod. 2009;81:302–309. doi: 10.1095/biolreprod.108.073221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 28.Arnesen T, Betts MJ, Pendino F, Liberles DA, Anderson D, Caro J, et al. Characterization of hARD2, a processed hARD1 gene duplicate, encoding a human protein N-alpha-acetyltransferase. BMC Biochem. 2006;7:13. doi: 10.1186/1471-2091-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang GL, Li BK, Zhang MY, Zhang HZ, Wei RR, Yuan YF, et al. LOH analysis of genes around D4S2964 identifies ARD1B as a prognostic predictor of hepatocellular carcinoma. World J Gastroenterol. 2010;16:2046–2054. doi: 10.3748/wjg.v16.i16.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illingworth RS, Bird AP. CpG islands-‘a rough guide’. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Berdasco M, Esteller M. Aberrant epigenetic landscape in cancer: how cellular identity goes awry. Dev Cell. 2010;19:698–711. doi: 10.1016/j.devcel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 34.Iannello RC, Young J, Sumarsono S, Tymms MJ, Dahl HH, Gould J, et al. Regulation of Pdha-2 expression is mediated by proximal promoter sequences and CpG methylation. Mol Cell Biol. 1997;17:612–619. doi: 10.1128/mcb.17.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang H, Goldberg E. Homo sapiens lactate dehydrogenase c (Ldhc) gene expression in cancer cells is regulated by transcription factor Sp1, CREB and CpG island methylation. J Androl. 2009;30:157–167. doi: 10.2164/jandrol.108.005785. [DOI] [PubMed] [Google Scholar]
- 36.De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–7335. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]