Abstract
Ethanol withdrawal is a dysphoric condition that arises from termination of ethanol intake by dependent individuals. Common withdrawal symptoms include anxiety, increased reactivity to stimuli and increased seizure susceptibility as well as the risk of increased seizure severity. We use an animal model of dependence and withdrawal to study withdrawal behaviors and potential underlying neurobiological mechanisms. For a number of years, we have quantified pentylenetetrazol seizure thresholds as an assessment of ethanol withdrawal at both one day and three days of withdrawal. Typically, we see a significant decrease in seizure threshold (increased sensitivity to seizure induction) that persists through three days of withdrawal for male rats. Increasing evidence indicates that voluntary exercise affords protection against various challenges to physical and psychological health, including ethanol-related challenges. Therefore, the current study investigated the effect of voluntary wheel running on seizure susceptibility following chronic ethanol administration and withdrawal. We found that voluntary wheel running attenuated the increased sensitivity to pentylenetetrazol-induced seizures observed with ethanol withdrawal, at both the one-day and three-day time points. This result was especially interesting as animals with access to the running wheels consumed more of the ethanol-containing diet. These findings showed that chronic voluntary wheel running reduces the severity of ethanol withdrawal in our animal model and suggest that exercise-based interventions may have some utility in the clinical management of heavy drinking and alcohol withdrawal.
Keywords: Ethanol withdrawal, Activity wheel, GABAA receptors, Stress, Seizure susceptibility
1. Introduction
Alcohol (ethanol) withdrawal arises when an ethanol-dependent individual stops consumption. Ethanol withdrawal (EW) symptoms reflect a rebound central nervous system hyperexcitability resulting from removal of this central nervous system depressant, and include anxiety, agitation, insomnia, general dysphoria and tremors that may progress to seizures (Ballenger and Post, 1978; Goldstein and Pal, 1971). Preclinical studies with laboratory rats and mice have shown them to be useful models for studying ethanol dependence and withdrawal, as EW animals display a significant increase in seizure susceptibility and severity during early EW (Becker et al., 1997; Devaud et al., 1995a; Devaud and Morrow, 1999; Finn et al., 1995; Finn and Crabbe, 1999; Veatch et al., 2007). Seizure susceptibility measurements are used in these animal models as a quantifiable reflection of the rebound hyperexcitability that is unmasked during EW.
The CNS hyperexcitability of EW is believed to arise from neuroadaptations engendered by persistent ethanol intake. A number of brain adaptations in key neurotransmitter systems and cellular modulators occur (see Moonat et al., 2010; Olsen et al., 2007; Spanagal, 2009; Vengeliene et al., 2008 for review). Ethanol acts as a CNS depressant, largely by enhancing GABAergic transmission and inhibiting glutamatergic activity. Therefore, the homeostatic drive to limit the effects of persistent ethanol exposure results in a reduced responsiveness of GABAA receptors and increased responsiveness of glutamatergic systems, particularly NMDA receptors. These chronic ethanol-induced adaptations are believed to involve alterations in subunit composition of both of these receptor types (Alele and Devaud, 2005; Cagetti et al., 2003; Devaud et al., 1995b; 1998; Devaud and Morrow, 1999; Devaud and Alele, 2004; Mhatre and Ticku, 1994; Mehta and Ticku, 2005). While these adaptations are believed to contribute to several manifestations of withdrawal, such as the increased anxiety and seizure susceptibility, it is likely that the stress of withdrawal itself also exacerbates seizure sensitivity (Friedman et al., 2011).
Increasing evidence indicates that exercise exerts protective effects against a variety of challenges to physical and psychological health. In rats and mice, these effects can be effectively modeled by housing animals with free access to running wheels. Studies have shown that adaptation to running wheels promotes neuronal health by enhancing synaptic plasticity and neurogenesis, even in adult animals (Cotman and Berchold, 2002; Stranahan et al., 2007; van Praag et al., 1999a, 1999b). Voluntary wheel running improves the ability to manage stress exposure by reducing the HPA response and increasing production of brain growth factors, such as BDNF (Nyhius et al., 2010). These effects appear to account for the anxiolytic and antidepressant effects of running-wheel activity (Duman et al., 2008; Salam et al., 2009). Chronic voluntary wheel running has also been shown to reduce the intoxicating effects of acute ethanol administration in a mouse model (Mollenauer et al., 1991, 1992) while antagonizing both the antiproliferative (Crews et al., 2004) and neurotoxic effects of repeated binge-like ethanol administration in rats (Leasure and Nixon, 2010). Further, chronic intermittent ethanol exposure reduced running, especially during the active (night) phase (Logan et al., 2010), a finding that suggests ethanol dependence and withdrawal may reduce the beneficial effects of voluntary wheel running. Pentylenetetrazol (PTZ) is a chemoconvulsant and has been used in numerous investigations to assess seizure susceptibility in animal models. We have published a number of reports studying drug effects on PTZ seizure thresholds during EW and have now extended this approach to determine whether free access to running wheels modulates the increased sensitivity to pentylenetetrazole-induced seizures seen during EW.
2. Methods
2.1. Animals
Male CR rats (Charles Rivers Lab) were approximately 42 days old at the start of experimental procedures.
2.2. Materials
Pentylenetetrazol (PTZ) from Sigma-Aldrich (St. Louis, MO) was dissolved in normal saline at a concentration of 5 mg/ml.
2.3. Activity wheel procedure
Animals were individually housed and randomly assigned to one of three running wheel conditions: (1) standard rat cages without wheels (No Wheel), (2) standard rat cages with wheels that are locked (Locked Wheel) or (3) standard rat cages with functioning running wheels (Free Wheel). The locked wheel condition was included to separate the possible effects of environmental complexity from exercise. The wheel condition was constant for 24 h each day throughout the course of the experiment. Running wheel activity was recorded by use of an external electronic LCD counter that was attached to the side of each free running wheel cage. Activity was recorded twice daily at 7:00 a.m. (start of rest phase) and at 5:00 p.m. (start of active phase). We chose these two times as lights on (7:00 a.m.) and approaching lights off (7:00 p.m.). Counters were manually reset after obtaining counts. All animals were housed under their respective conditions for 10 days prior to introduction of the liquid diets to allow for acclimation and adaptation to running wheels in Free Wheel animals.
2.4. Liquid diet procedure
Animals were made ethanol-dependent by administration of 6% ethanol, v/v, in a nutritionally complete liquid diet, which was slightly modified from the Frye liquid diet (Frye et al., 1983). Diet components were purchased individually with diet made at least twice per week and fresh diet was provided daily (MP Biomedical, Costa Mesa, CA) and administered for 14 days as previously described (Devaud and Morrow, 1994; Devaud et al., 1995a). Control animals were pair-fed the same liquid diet but with dextrose substituted isocalorically for the ethanol to ensure equivalent caloric intake and comparable nutritional status. The amount of liquid diet consumed was recorded daily.
After 14 days of liquid diet administration, the liquid diet was removed and regular lab chow provided ad libitum to all animals to maintain equivalent diet conditions. Seizure threshold testing was scheduled at 1 day or 3 days EW. All procedures were conducted in accordance with approved University of Maine Animal Welfare Protocols and NIH guidelines for the humane care and use of animals in an AAALAC-accredited facility.
2.5. PTZ Seizure threshold procedure
Constant tail vein infusion of the chemoconvulsant was used for the induction of seizures. A 25 g butterfly needle was inserted into a lateral tail vein while the animals were gently restrained and needle taped into place. The animal was then allowed to move freely while the observer gently held the tip of its tail. PTZ was infused at 1.6 ml/min and the time to the first myoclonic twitch of the face and/or neck indicated the endpoint of infusion (Alele and Devaud, 2007). Seizure thresholds were calculated from the time of infusion (minutes) times the dose (5 mg/ml×1.6 ml/min) of PTZ infused per body weight of the animal and are presented as mg PTZ per kilogram body weight.
2.6. Data analysis
Data were analyzed using one-way and two-way ANOVA, and post-hoc pair wise comparisons were performed using the least-significant difference (LSD) procedure to control type-1 error rate (SPSS, Chicago IL, USA).
3. Results
3.1. Body weights and ethanol consumption
Animals in all groups showed substantial weight gain over the course of the experiment from initial weights (day 1) until final weight determinations (day 25 or 27) (Table 1). Two-factor ANOVA conducted on bodyweights revealed a significant main effect of housing conditions (No Wheel, Locked Wheel, Free Wheel) [F(2,72)=16.64, P<0.001] but there was no effect of ethanol treatment group (1 day EW, 3 day EW, control) nor a housing by treatment interaction. Post-hoc pairwise comparisons indicated that Free Wheel animals weighed less than both No Wheel and Locked Wheel animals, which did not differ from each other.
Table 1.
Starting and ending body weights for rats across diet and wheel conditions
| Initial body weight (g) | Final body weight (g) | % Increase in body weight | |
|---|---|---|---|
| Control | |||
| No Wheel | 169.2 ± 8.0 | 377.4 ±13.4 | 123% |
| Locked Wheel | 176.7 ± 7.0 | 381.0 ±13.6 | 116% |
| Free Wheel | 163.7 ±11.6 | 307.6 ±17.5* | 88% |
| Ethanol fed | |||
| No Wheel | 165.6 ± 7.9 | 379.6 ±12.6 | 129% |
| Locked Wheel | 171.4 ±9.1 | 366.1 ±12.3 | 114% |
| Free Wheel | 164.4 ±6.1 | 335.8 ± 20.2* | 104% |
P<0.001 compared to both No Wheel and Locked Wheel conditions.
Despite the fact that Free Wheel animals gained less weight relative to the other two housing conditions, Table 2 shows that Free Wheel animals actually consumed nearly 10% more of the ethanol-containing liquid diet than either No Wheel or Locked Wheel animals. One-way ANOVA showed a significant effect of housing conditions on ethanol diet consumption [F(2,48)=12.133, P<0.001], while post-hoc comparisons indicated that Free Wheel animals consumed significantly more than either No Wheel or Locked Wheel animals, but that No Wheel and Locked Wheel animals did not differ. Consumption for all wheel conditions was at levels of intake required to engender dependence (Devaud et al. 1995a; 1996). Blood ethanol concentrations were not assessed in these experiments due to the disruptive nature of collection and that emphasis was on assessing behavioral outcomes. Previous investigations by our group and others found that blood ethanol concentrations were highly variable throughout the course of ethanol administration by liquid diet, as the animals tend to drink in unpredictable bouts.
Table 2.
Ethanol consumption (g/kg/day) across wheel conditions for the final 7 days of ethanol diet administration
| Running wheel condition | g/kg/day |
|---|---|
| No Wheel | 9.3 ± 0.15 |
| Locked Wheel | 9.3 ± 0.12 |
| Free Wheel | 10.2 ±0.16* |
P<0.001 compared to both No Wheel and Locked Wheel conditions.
3.2. Running wheel activity
Activity levels increased gradually over the 10-day habituation period (Fig. 1). Following introduction of liquid diet, activity levels increased somewhat in animals on the control (non-ethanol) liquid diet, but significantly declined in ethanol-fed animals. Mean levels of 24-hour wheel-running activity were determined for days 6–10 of the habituation period and for all days of liquid diet exposure. Two-factor mixed ANOVA was used to compare activity levels in ethanol-fed and control diet animals under baseline and liquid-diet conditions (Fig. 2). This analysis revealed significant main effects of liquid diet [F(1,27)= 5.332, P=0.029] and ethanol feeding [F(1,27)=11.667, P<0.001], as well as a liquid-diet by ethanol-feeding interaction [F(1,27)=101.117, P<0.001]. This interaction was explored using post-hoc pairwise comparisons, which showed that ethanol-fed and control animals differed during liquid diet administration but not under baseline conditions. Further, ethanol-fed animals had reduced overall running wheel activity under liquid diet conditions. In contrast, control diet-fed animals showed increased activity with the continued access to free running wheels (Fig. 2).
Fig. 1.
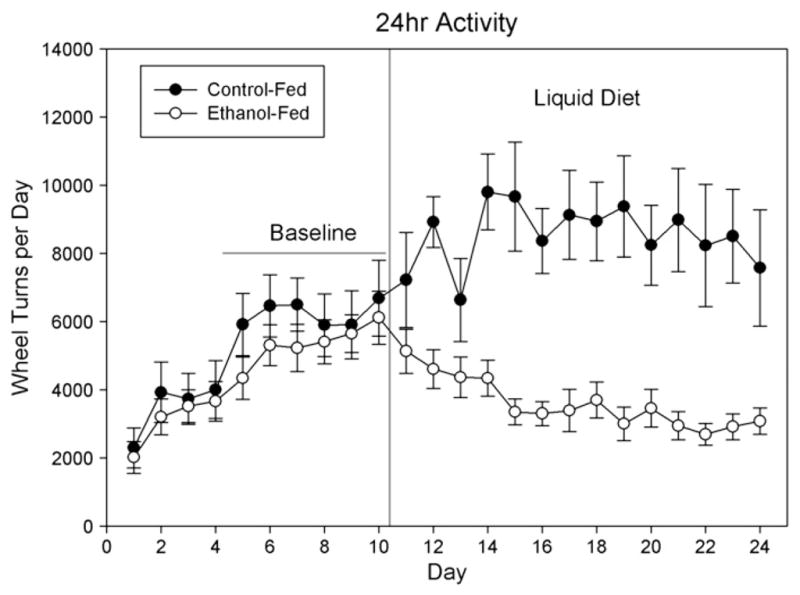
Day-by-day comparison of wheel turns during baseline (when all animals were on chow) and when switched to liquid diet. N=12–17 animals per diet condition.
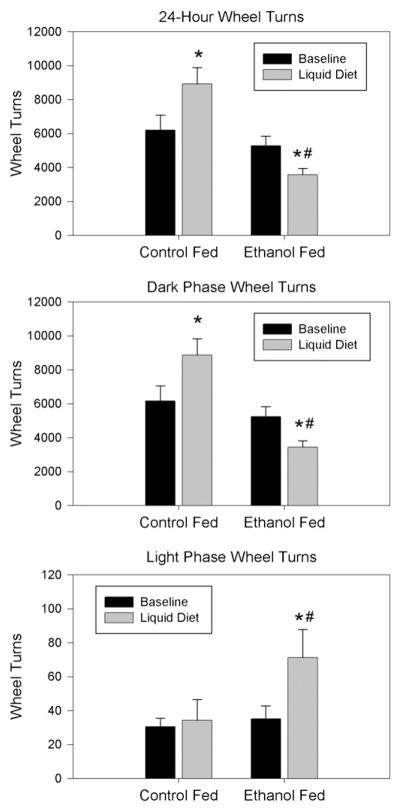
Fig. 2.
Average number of wheel turns. The top panel shows the average number of wheel turns per 24 h period, with the middle panel showing wheel turns during the active (night) phase and the bottom panel showing turns during the day (rest) phase. N=8–10 animals per treatment condition. #: P<0.01 compared to baseline activity; *: P<0.05 compared to control group liquid diet activity.
Similar analyses were also conducted on dark-phase and light-phase activity levels. Not surprisingly, analysis of dark-phase activity produced results that were essentially identical to those seen for total 24-hour activity. Values were 6170±136 wheel turns for animals assigned to control diet and 5240±233 wheel turns for animals assigned to ethanol diet. Specifically, ethanol-fed animals showed significantly reduced activity (reduced by 34% to an average of 3434±182 wheel turns per phase) during liquid-diet conditions relative to their own baseline. In contrast, however, ethanol-fed animals showed significant increases in light-phase activity relative to both baseline and control-diet conditions (from 35.4±6.3 to 117.8±19.8 wheel turns per phase; a 230% increase). Thus, as seen in Fig. 2, ethanol feeding reduced overall activity levels but also attenuated normal day–night differences in activity.
3.3. Seizure threshold
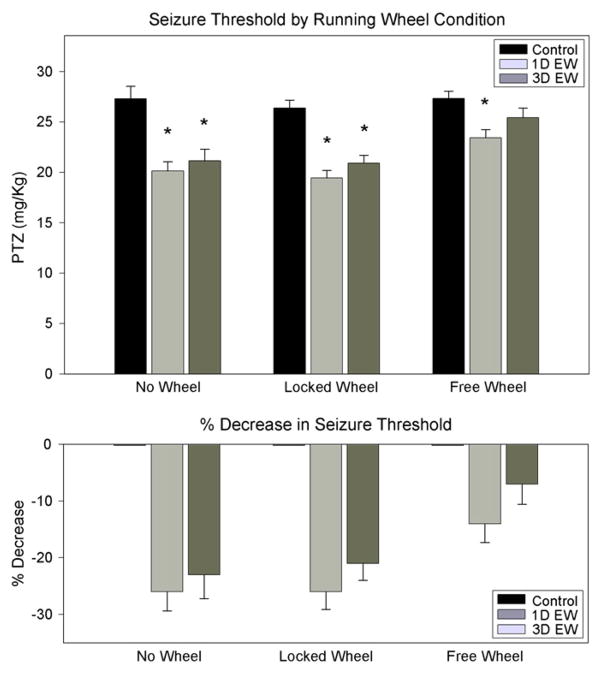
As shown in Fig. 3, basal seizure thresholds (mg/kg PTZ) did not differ across the three running wheel conditions [F(2,22)=0.267]. No Wheel thresholds were 27.3±1.2 mg/kg PTZ, Locked Wheel thresholds were 26.4±0.8 mg/kg PTZ and Free Wheel thresholds were 27.3±0.7 mg/kg PTZ. These data showed that the running wheel condition in and of itself did not alter basal seizure susceptibility. Two-factor ANOVA revealed significant main effects of both housing conditions [F(2,65)=4.245, P=0.019] and ethanol treatment group [F(2,65)=24.145, P<0.001] on seizure thresholds, but failed to detect a significant housing by treatment interaction. Nevertheless, since post-hoc pairwise comparisons showed that seizure thresholds in Free Wheel animals differed significantly from both No Wheel and Locked Wheel animals, while No Wheel and Locked Wheel groups did not differ, we conducted an additional ANOVA in which the No Wheel and Locked Wheel groups were combined and analyzed as a single condition. This analysis showed significant main effects of housing conditions [F(1,65)=18.015, P<0.001] and ethanol treatment group [F(2,65)=23.119, P<0.001], as well as a significant housing by ethanol treatment interaction [F(2,65) =3.648, P=0.032]. Further, post-hoc pairwise comparisons indicated that both 1 day EW and 3 day EW groups differed from their respective controls in both No Wheel and Locked Wheel animals, while only 1 day EW, not 3 day EW, animals differed from controls in Free Wheel animals.
Fig. 3.
Comparison of PTZ seizure thresholds during ethanol withdrawal across wheel condition. N=7–11 animals per treatment condition. *: P<0.01 compared to control diet seizure thresholds by wheel condition.
4. Discussion
The intent of the present study was to determine if voluntary wheel running afforded benefit against ethanol withdrawal severity. We, and others, have shown that one indicator of the CNS hyperexcitability of EW is increased seizure susceptibility. We consistently find an average 25% decrease in the amount of chemoconvulsant required to initiate first signs of a seizure in EW animals compared to ethanol naïve animals (Devaud et al., 1995a, 1995b, 1996; Devaud and Chadda, 2001). While most human alcoholics do not present with seizures during withdrawal, the ability to quantify changes in seizure susceptibility is a validated measure, which is indicative of the increased neuronal hyperexcitability that occurs with EW. Increased sensitivity to seizure induction during EW was found in this study, consistent with our previous reports, and supports that animals were made dependent by the liquid diet paradigm. No differences in basal seizure thresholds were observed across wheel conditions. This suggests that it was the voluntary exercise, not the cage configuration, which influenced EW seizure thresholds.
The major finding of this paper is that voluntary wheel running was protective against the increased seizure susceptibility of EW. This occurred in spite of the significant reduction in voluntary running caused by intake of the ethanol-containing liquid diet. Ethanol-fed animals ran 61% less during the active/night phase compared to control-fed animals. During the day (rest phase), animals significantly reduced running compared to the active/night phase. However, ethanol liquid diet administration disrupted the normal temporal pattern of activity by eliciting a 3-fold increase in running during the rest phase compared to activity for control diet-fed animals. The observed alteration in wheel running following ethanol exposure was consistent with a previous report showing that chronic intermittent ethanol exposure reduced wheel running in C57/BL6 mice, and that this reduction persisted for several days after termination of the ethanol exposure (Logan et al., 2010).
Ethanol withdrawal is known to be stressful and common symptoms are general dysphoria, increased anxiety, seizure risk and stress reactivity. The expression of EW is believed to result from a series of neuroadaptations that occur in response to the development of ethanol dependence. Major adaptations include reductions in the responsiveness of GABAergic systems, increased responsiveness of glutamatergic systems as well as alterations in cell signaling, all which likely contribute to the hyperexcitable state of EW. There are several possible mechanisms for the observed attenuation of the increased seizure risk of EW by voluntary wheel running. The most parsimonious explanation could involve pharmacokinetic factors. The ethanol diet animals may be less intoxicated, and, therefore, less dependent if there is enhanced clearance of ethanol in the voluntary running groups. Ardies et al. (1989) reported that exercising female rats showed increased clearance of ethanol following a single, low-dose intraperitoneal injection. However, a recent report by Leasure and Nixon (2010) reported that 14 days of voluntary wheel running did not change ethanol pharmacokinetics in their binge exposure model. Blood ethanol concentrations were not determined in the present set of studies due to the disruptive nature of this procedure and the desire to focus on identifying associations between exercise and EW seizure thresholds. A separate study that will monitor the time course for ethanol clearance after administration of a bolus ethanol injection at the completion of the liquid diet paradigm is needed to directly address this question.
It is also possible that neuronal adaptations engendered by persistent exercise also contribute to its beneficial effects during ethanol diet consumption and withdrawal. EW is stressful and it has been shown that stress exacerbates risk for seizures in human epilepsy as well as in animal models (Friedman et al., 2011). Therefore, activation of the HPA axis and release of stress mediators may contribute to the increased seizure susceptibility of EW. Regular exercise has been shown to reduce the risk of stress-associated disorders by attenuating the HPA response to stressors (Nyhius et al., 2010). For example, voluntary wheel running was found to reduce the HPA axis response to audiogenic stress (Sasse et al., 2008). Cellular mechanisms contributing to these effects may include exercise-induced increases in brain-derived neurotrophic factor levels, synaptic spine density and hippocampal neurogenesis (Cotman and Berchold, 2002; Sartori et al., 2011; Stranahan et al., 2007; van Praag et al., 1999a, 1999b). Exercise was found to protect against hippocampal damage caused by stress (Snyder et al., 2009) as well as the hippocampal neurotoxicity associated with binge ethanol exposure (Leisure and Nixon, 2010). To provide further support of a direct protective effect of exercise on seizure susceptibility, a recent report showed that three weeks of voluntary wheel running reduced the severity of kainic acid-induced seizures (Reiss et al., 2009). Taken together, these studies suggest that exercise enhances the ability of the CNS to respond to stressful challenges and may contribute to protection against EW severity.
In summary, the present findings support the suggestion that regular physical activity affords protection against negative CNS responses to excessive ethanol consumption. The present observations of attenuation in seizure susceptibility during EW by chronic voluntary exercise could result from improved clearance of consumed ethanol and/or the enhanced neuronal plasticity and ability to adapt to challenges. Continued exploration of this question may help inform clinicians in their management of alcoholic patients.
Acknowledgments
The expert technical assistance of Zach Macklin and Jacob Wilson was greatly appreciated. This research was supported by NIH AA011877.
References
- Alele P, Devaud LL. Alterations in GABAergic and glutamatergic activity during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res. 2005;29:1027–34. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. J Pharmacol Exp Ther. 2007;320:427–36. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- Ardies CM, Morris GS, Erickson CK, Farrar RP. Both acute and chronic exercise enhance in vivo ethanol clearance in rats. J Appl Physiol. 1989;66:555–60. doi: 10.1152/jappl.1989.66.2.555. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndrome. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC, Diaz-Granados JL, Weathersby RT. Repeated ethanol withdrawal experience increases the severity of and duration of subsequent withdrawal seizures in mice. Alcohol. 1997;14:319–26. doi: 10.1016/s0741-8329(97)87949-9. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function and decreases behavioral responses to positive allosteric modulators of GABA-A receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchold Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurol. 2002:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele PE. Differential effects of chronic ethanol administration and withdrawal on γ-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rats brain. Alcohol Clin Exp Res. 2004;28:957–65. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res. 2001;25:1689–96. [PubMed] [Google Scholar]
- Devaud LL, Morrow AL. Effects of chronic ethanol administration on [3H]zolpidem binding in rat brain. Eur J Pharmacol. 1994;267:243–7. doi: 10.1016/0922-4106(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Morrow AL. Gender-selective effects of ethanol dependence on NMDA receptor subunit expression in cerebral cortex, hippocampus and hypothalamus. Eur J Pharmacol. 1999;369:331–4. doi: 10.1016/s0014-2999(99)00103-x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Morrow AL. The neurosteroid, 3α-hydroxy-5α-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995a;19:350–5. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Smith FD, Grayson DR, Morrow AL. Chronic ethanol consumption differentially alters the expression of GABAA receptor subunit mRNAs in rat cerebral cortex: competitive, quantitative RT/PCR analysis. Mol Pharmacol. 1995b;48:861–8. [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Finn DA, Morrow AL. Sensitization of γ-aminobutyric acidA receptors to neuroactive steroids in rats during ethanol withdrawal. J Pharmacol Exp Ther. 1996;278:510–7. [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Morrow AL. Influence of gender on GABAA receptor alterations elicited by ethanol dependence in rats. Brain Res. 1998;796:222–30. doi: 10.1016/s0006-8993(98)00357-6. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Russell DS, Duman RS. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–58. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Chronic ethanol differentially alters susceptibility to chemically induced convulsions in withdrawal seizureprone and -resistant mice. J Pharmacol Exp Ther. 1999;288:782–90. [PubMed] [Google Scholar]
- Finn DA, Roberts AJ, Crabbe JC. Neuroactive steroid sensitivity in withdrawal seizureprone and -resistant mice. Alcohol Clin Exp Res. 1995;19:410–5. doi: 10.1111/j.1530-0277.1995.tb01523.x. [DOI] [PubMed] [Google Scholar]
- Friedman AR, Cacheaux LP, Ivens S, Kaufer D. Elucidating the complex interactions between stress and epileptogenic pathways. Cardiovasc Psychiatry Neurol. 2011:1–8. doi: 10.1155/2011/461263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye GD, McCown TJ, Breese GR. Characterization of susceptibility to audiogenic seizures in ethanol-dependent rats after microinjection of gamma-aminobutyric acid (GABA) agonists in to the inferior colliculus, substantia nigra or medial septum. J Pharmacol Exp Ther. 1983;227:663–70. [PMC free article] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–90. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–14. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Seggio JA, Robinson SL, Richard GR, Rosenwasser AM. Circadian wheel-running activity during ethanol withdrawal from chronic intermittent ethanol exposure in mice. Alcohol. 2010;44:239–44. doi: 10.1016/j.alcohol.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Effect of chronic administration of ethanol on GABA-A receptor assemblies derived from alpha2-, alpha3-, beta2- and gamma2-subunits in the rat cerebral cortex. Brain Res. 2005;1031:134–7. doi: 10.1016/j.brainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Mhatre MC, Ticku MK. Chronic ethanol treatment upregulates the GABA receptor beta subunit expression. Mol Brain Res. 1994;23:246–52. doi: 10.1016/0169-328x(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Mollenauer S, Bryson R, Speck C, Chamberlin JR. Voluntary wheel running reduced the effects of acute ethanol on activity and avoidance in C57BL/6J mice. Pharmacol Biochem Behav. 1991;39:821–4. doi: 10.1016/0091-3057(91)90173-y. [DOI] [PubMed] [Google Scholar]
- Mollenauer S, Bryson R, Speck C, Chamberlin JR. Effects of exercise on ethanol-induced hypothermia and loss of righting response in C57BL/6J mice. Pharmacol Biochem Behav. 1992;43:285–90. doi: 10.1016/0091-3057(92)90669-7. [DOI] [PubMed] [Google Scholar]
- Moonat S, Starkman BG, Sakharkar A, Pandey SC. Neuroscience of alcoholism: molecular and cellular mechanisms. Cell Mol Life Sci. 2010;67:73–88. doi: 10.1007/s00018-009-0135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyhius TJ, Masini CV, Sasse SK, Day HEW, Campeau S. Physical activity, but not environmental complexity, facilitates HPA axis response habituation to repeated audiogenic stress despite neurotrophin mRNA regulation in both conditions. Brain Res. 2010;1363:68–77. doi: 10.1016/j.brainres.2010.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–9. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss JI, Dishman RK, Boyd HE, Robinson JK, Holmes PV. Chronic activity wheel running reduces the severity of kainic acid-induced seizures in the rat: possible role of galanin. Brain Res. 2009;1266:54–63. doi: 10.1016/j.brainres.2009.02.030. [DOI] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF and proB DNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Sasse SK, Greenwood BN, Masini CV, Nyhius TJ, Fleshner M, Day HEW, et al. Chronic voluntary wheel running facilitates corticosterone response habituation to repeated audiogenic stress exposure in male rats. Stress. 2008;11:425–37. doi: 10.1080/10253890801887453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagal R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogeneis in the adult mouse dentate gyrus. Nat Neurosci. 1999a;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999b;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veatch LM, Wright TM, Randall CL. Only male mice show sensitization of handling-induced convulsions across repeated ethanol withdrawal cycles. Alcohol Clin Exp Res. 2007;31:477–85. doi: 10.1111/j.1530-0277.2006.00328.x. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Blibao, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]