Abstract
The primary cilium is a non-motile singular cellular structure that extends from the surface of nearly every cell in the body. The cilium has been shown to play numerous roles in maintaining tissue homeostasis, through regulating signaling pathways and sensing both biophysical and biochemical changes in the extracellular environment. The structural performance of the cilium is paramount to its function as defective cilia have been linked to numerous pathologies. In particular, the cilium has demonstrated a mechanosensory role in tissues such as the kidney, liver, endothelium and bone, where cilium deflection under mechanical loading triggers a cellular response. Understanding how cilium structure and subsequent mechanical behavior contributes to the roles the cilium plays in regulating cellular behavior is a compelling question, yet is a relatively untouched research area. Recent advances in biophysical measurements have demonstrated the cilium to be a structurally intricate organelle containing an array of load bearing proteins. Furthermore advances in modeling of this organelle have revealed the importance of these proteins at regulating the cilium’s mechanosensitivity. Remarkably, the cilium is capable of adapting its mechanical state, altering its length and possibly it’s bending resistance, to regulate its mechanosensitivity demonstrating the importance of cilium mechanics in cellular responses. In this review, we introduce the cilium as a mechanosensor; discuss the advances in the mechanical modeling of cilia; explore the structural features of the cilium which contribute to its mechanics and finish with possible mechanisms in which alteration in structure may affect ciliary mechanics, consequently affecting ciliary based mechanosensing.
Keywords: Primary Cilium, Mechanics, Cellular mechanosensor, Structure, Model
1. Introduction
Primary cilia are non-motile, solitary extensions that protrude from the apical surface of nearly every cell in the human body. These organelles were originally discovered over a century ago and, until recently, their function was a mystery. Indeed, they were believed by some to be vestigial (Davenport and Yoder, 2005). In the past several decades a plethora of studies have revealed the primary cilium to be a multifunctional antenna, sensing both mechanical (fluid flow, pressure, touch, vibration) and chemical (light, odor, PDGF) changes in the extracellular environment (Singla and Reiter, 2006). More recently the primary cilium has also been implicated as a complex signaling center for the cell, regulating key signaling pathways during development such as Hedgehog and Wingless (Berbari et al., 2009). The structural integrity of this versatile extracellular sensor is paramount to its function as defects in the primary cilium have been linked to numerous pathologies (ciliopathies) such as arthritis, osteoporosis, polycystic kidney disease, heart failure, obesity and cancer (Badano et al., 2006; Adams et al., 2008; Veland et al., 2009; Hildebrandt et al., 2011).
The basic structure of the primary cilium is very similar to that of the better understood motile cilium and flagellum. They all consist of a membrane bound axoneme containing nine circumferentially arranged doublet microtubules which extend upwards from the mother centriole/basal body out into the extracellular space. Despite the similarities, there are distinct structural differences which dramatically affect the mechanics of each. Both motile cilia and flagella have two additional central microtubules which are attached to each of the surrounding doublets by connections called radial spokes. Therefore motile cilia are commonly referred to as 9+2 cilia in contrast to the primary cilium’s 9+0 arrangement. In addition, the doublets of the 9+2 cilium are connected via nexin links, which in combination with the radial spokes, reinforce the axoneme resulting in an order of magnitude increase in the resistance to bending (flexural rigidity) in comparison to the primary cilium (Rikmenspoel and Sleigh, 1970; Schwartz et al., 1997b). This increase in flexural rigidity demonstrates that even a small change in molecular arrangement at these scales can yield a dramatic difference in organelle level mechanical behavior.
Changes in the structural mechanics of primary cilia greatly affect the molecular mechanisms of mechanosensing. For example recent studies have demonstrated that upon mechanical perturbation the primary cilium decreases in length coinciding with a reduction in amiloride-sensitive sodium current (Resnick and Hopfer, 2007; Besschetnova et al., 2010; Gardner et al., 2011). Therefore alterations in primary cilium length, and possibly other mechanical properties and features, may be a central mechanism for regulating cellular mechanosensitivity. It may also alter responsiveness to biochemical stimuli and be a fundamental synergistic mechanism whereby cells sense, integrate, and coordinate responses to combined physical and chemical signals. In this review, we introduce the cilium as a cellular mechanosensor (section 2); discuss the recent advances in the mechanical modeling of the primary cilium (section 3); explore the structural features of the cilium which contribute to its mechanics (section 4), and finish with possible mechanisms in which alteration in cilia structure may affect ciliary mechanics and as a consequence, affect ciliary based cellular mechanosensing (section 5).
2. The Mechanosensing Primary Cilium
The primary cilium first emerged as a mechanosensor in the kidney after defects in cilia structure and function were found to correlate with cyst formation. Further study revealed that fluid flow within the kidney resulting in the deflection of the primary cilium causes an extracellular calcium dependent increase in intracellular calcium, a response that was lost with removal of the primary cilium (Praetorius and Spring, 2001, 2003). Nauli et al first demonstrated that this calcium response is mediated by a mechanosensory complex located at the base of the cilium. This complex consists of two membrane bound proteins, Polycystin 1 and a stretch activated cationic channel known as Polycystin 2 (Nauli et al., 2003). In addition to this complex regulating calcium influx, unloading of the cilium has been shown to result in an intra-membrane proteolysis of PC1, which acts as a transcription factor in association with STAT6 and P100 (Low et al., 2006). This mechanosensing mechanism has also been reported in liver cholangiocytes, where bending of the cilium not only results in an influx of calcium but also suppresses a forskolin stimulated increase in the second messenger cyclic adenosine monophosphate (cAMP) (Masyuk et al., 2006). The primary cilium has also emerged as a mechanosensor in bone where deflection of the primary cilium under fluid flow resulted in an increase in the osteogenic gene Cox-2. Furthermore, an inactivating mutation in the Pkd1 gene, which encodes the ciliary protein PC1, in a mouse model results in an osteopenic phenotype (Xiao and Quarles, 2010). Interestingly primary cilia in bone cells do not mediate flow-induced intracellular calcium assayed by Fura-2, indicating that the mechanotransduction pathway in bone differs relative to that in kidney and liver (Malone et al., 2007). Additional studies in bone have revealed that upon deflection of the primary cilium a rapid and transient decrease in cAMP occurs and that this decrease is dependent on adenylyl cyclase 6 (AC6) which is also localized to the cilium (Kwon et al., 2010).
In addition to sensing fluid flow, primary cilia have been shown to sense other mechanical signals such as pressure, touch and vibration. For example, the stretch activated ion channel TRPV4 localizes to the primary cilia of cholangiocytes and chondrocytes and has been shown to be involved in sensing osmotic pressure (Gradilone et al., 2007; Phan et al., 2009). In Drosophila, neurons extend a cilium that attaches into a cavity created by support cells known as scolopale (Ernstrom and Chalfie, 2002). Vibrations are then detected by the stretching of the cilium resulting in a rapid electrical response mediated by ion channels in the membrane. Furthermore in C. elegans, cilia are located along the dendrites of neurons which are known to be mechanoresponsive. Although the exact mechanism of mechanotransduction in these cells is not known, the primary cilia are surrounded by extracellular matrix (ECM) and disruption of this ECM results in impaired mechanosensation (Ernstrom and Chalfie, 2002). It has been postulated that the cilium acts to transfer loads from the ECM to the underlying cytoskeleton regulating the activity of sodium channels mec-4 and mec-10 (ENaC superfamily) and converts the stimuli into a rapid electrical response.
In the past two decades the primary cilium has been demonstrated to play an important mechanosensory role in numerous tissues across many species and organisms. The cilium’s ability to do so depends on its mechanical properties. For example, an interesting feature of the primary cilium is its ability to dynamically modulate its length, fine tuning its sensitivity to the extracellular environment. Besschetnova et al (Besschetnova et al., 2010) recently demonstrated that by blocking calcium ion, Ca++ entry (using gadolinium) and increasing intracellular cAMP (using forskolin) the length of the primary cilium in mammalian epithelial and mesenchymal cells increased 2-fold in 3 hours (Besschetnova et al., 2010). After the application of fluid shear, which is known to increase intracellular Ca++ and decrease intracellular cAMP the average cilia length decreased by 20%-35%. Furthermore it has been demonstrated that overloading of chondrocytes results in a decrease in cilia length and conversely stress deprivation in tendon cells results in an immediate and significant increase in length (McGlashan et al., 2010; Gardner et al., 2011). Therefore understanding the mechanics of the primary cilium and how ciliary mechanics may change in a diseased state may yield insight into the cause of the numerous debilitating ciliopathies.
3. Modeling Primary Cilia Mechanics
The primary cilium is emerging as a central nexus for sensing a number of biochemical and biophysical extracellular signals. As a mechanosensor, its mechanical properties dictate how external stimuli such as fluid flow translate into cilia deformation and subsequently a cellular response. For example, it is often hypothesized that bending of the cilium contributes to the opening of ion channels such as PC2. In this case, a stiff cilium will experience lower membrane strains than a more flexible cilium when exposed to the same bending force. Thus, a stiff cilium would be expected to be a less sensitive mechanotransducer. However, the mechanical properties of primary cilia are virtually unknown. To date, only a few studies have investigated the mechanical behavior of the primary cilium, all of which focused on the bending response of the primary cilium in kidney cells (Figure 2).
Figure 2.
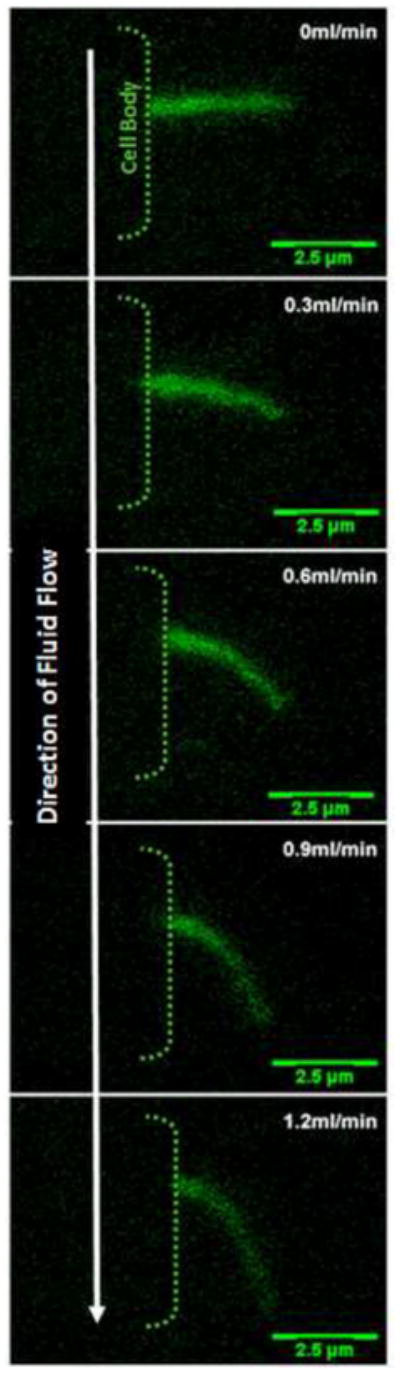
XZ bending profiles of a kidney cell primary cilium under increasing flow rates. There is a one-to-one functional relationship between flow rate and degree of bending. Cilia are visualized by transfection with a SSTR3-GFP fusion protein which specifically localizes to the cilium.
The bending behavior of the primary cilium was first modeled mathematically by Schwartz et al (Schwartz et al., 1997b). Using the assumption that a primary cilium under fluid shear acts as a uniform cylindrical cantilevered beam subjected to a unidirectional load perpendicular to its long axis, the bending behavior of the primary cilium can be described using the large-rotation Euler-Bernoulli formulation (Schwartz et al., 1997b). This equation has never yielded a closed form solution and so is solved by various numerical approximation methods. The fluid drag was estimated assuming the closed- form solution for 2D laminar flow around a cylinder. Using this mathematical approach Schwartz et al accurately predicted the bending profile of kidney epithelial cilia observed experimentally under numerous physiological flow profiles demonstrating a one-to-one functional correspondence between bending profile and applied fluid shear. Furthermore, with this model they were able to measure the flexural rigidity, (EI), of the primary cilium and determined it to be 3.1±0.8×10−23 Nm2, which is approximately one order of magnitude less stiff than the motile cilium. This difference in stiffness can most likely be ascribed to structural features such as radial spokes and nexin links that act to reinforce the axoneme of motile cilia. Given the close correspondence between the experimental bending profile and the profile predicted using a cantilevered beam model (i.e. assumes the cilium is fixed at the base) it was concluded that the cilium is firmly anchored to the cell. This anchorage is primarily achieved through the attaching microtubules and possibly by striated rootlets which extend out from the base of the basal body. Such elements are discussed in greater detail below (see section 4). Both the difference in EI between motile and primary cilia and the anchorage of the cilium within the cell highlight the importance of structural elements within the ciliary complex contributing to its mechanical behavior.
Liu et al developed a more precise model of the fluid flow around an array of primary cilia by numerically solving the Stokes equations (Liu et al., 2003). Modeling an array of cilia, it was predicted that hydrodynamic interactions between cilia diminished the expected increase in the fluid velocity at the tip, total drag force along the length, and also the torque generated at the base of the cilium as the cilium increases in length. However, even with this interaction, the drag force and the torque experienced by an 8μm cilium were 6-fold (10-fold without interaction) and 22-fold (33-fold without interaction) greater than a 2.5μm cilium respectively. This relationship between cilium length and force transmission supports the hypothesis that cilia alter their length to adjust their mechanosensitivity (Besschetnova et al., 2010). Interestingly, the authors calculated that the shear stress experienced by the apical cell membrane under their flow conditions was not sufficient to elicit a calcium response. Therefore the authors concluded that the calcium response elicited by flow must be mediated by the primary cilium such that deformation of the ciliary shaft resulting in the opening of ion channels or the torque generated at the anchoring microtubules at the base of the cilium is the activating mechanical stimulus.
Resnick and Hopfer investigated the mechanosensing capacity of primary cilia under orbital shaking induced fluid flow. It was demonstrated that the transepithelial ENaC current decreased by 60% and cilium length decreased by 30% after 24hrs of shaking at a frequency of 2Hz (Resnick and Hopfer, 2007). In order to understand how mechanical stimulation can affect a cellular response they calculated the forces exerted on a cilium under such a loading regime. Unfortunately, since the flow field under such conditions has not been mathematically characterized, the analysis was restricted to limiting estimates. The cilium was assumed to be a rigid cylinder, again allowing for the close-formed calculation of drag force. Furthermore, a hemispherical ciliary cap was introduced allowing the drag force across the tip of the cilium to be determined. The drag force on the primary cilia in response to shaking induced fluid flow was estimated to be maximally 4 times that of thermal noise, yet was sufficient to elicit a cellular response demonstrating that the cilium is a highly sensitive mechanosensory organelle. Interestingly, according to their model over half of the total drag force experienced by the cilium is exerted on the ciliary cap. It must be noted that their calculation assumed a cilium in the upright position and so represents the maximal drag force. Upon fluid flow induced bending of the cilium the drag force across the cap may be dramatically reduced. Nevertheless, this model highlights the ciliary cap as an important feature and therefore should be incorporated into models of fluid flow mediated deflection of the cilium. Interestingly, various authors have visualized bulges at the tip of the cilium (Menco and Farbman, 1985; Roth et al., 1988), the presence of which could significantly alter the flow field around the cilium tip and resulting drag force.
Rydholm et al. recently investigated the bending behavior and subsequent calcium response of primary cilia in kidney epithelial cells under continuous and pulsatile flow at different frequencies and flow rates. Upon application of flow (19 mPa shear) the cilium was found to deflect rapidly (~0.5s) to a maximum angle. Analysis of the calcium response revealed a time delay of ~20s from application of flow. Furthermore, high frequency (2Hz) pulsatile flows elicited a reduced calcium response compared to continuous flow. In order to gain insight into these findings the authors developed a three- dimensional, structural mechanical model of a primary cilium using a finite element method (FEM) (Rydholm et al., 2010). The ciliary axoneme was modeled as a cylindrical elastic beam with a hemispherical cap (Young’s Modulus, E = 178kPa (Schwartz et al., 1997a)) and fixed at its base. The ciliary membrane was also modeled and was continuous with a viscoelastic plasma membrane. Utilizing this model, Rydholm et al. found that with deflection of the primary cilium, the stress in the ciliary membrane builds at a much slower rate than that of the axoneme itself. Furthermore the stress in the membrane was found to be maximal towards the base of the cilium. Based on these findings, the authors hypothesized that due to the viscoelastic nature of the membrane, the buildup of stress upon deflection to a level sufficient to open an ion channel located at the base of the cilium would take several seconds of continuous flow. This would therefore explain the 20s delay between onset of flow and a calcium response in kidney epithelial cells. This finding also explains the lack of response from high frequency loadings as the stress in the membrane cannot reach the required level to activate the channels within the short loading period. Rydholm et al. concluded that the primary cilium acts a low pass filter sensing slow steady flow while fast fluctuations in flow (< 5s) are ignored. Furthermore, by comparing the bending profile of their model, which utilizes a constant flexural rigidity along its length, to that of a bending profile of a cilium in vitro, they determined that the cilium is stiffer towards its base and that this change in flexural rigidity is more pronounced in longer cilia. Experimentally it has been shown that the circumferential arrangement of the doublets becomes disorganized towards the tip of the cilium (Odor and Blandau, 1985b; Yamamoto and Kataoka, 1986a) which may be the molecular mechanism of change in the mechanical behavior along the length of the cilium. Although the cause of this disrupted organization is unclear it is most likely due to the lack of interconnecting attachments such as nexin links between doublets in primary cilia. Although this model yields interesting explanations for experimental observations, the output of the model will be strongly influenced by the viscoelastic properties attributed to the membrane. Therefore, biophysical measurements of ciliary membrane properties are required to fully validate this model.
The current models of the primary cilia provide vital insights into the mechanics of this sensory organelle. However, to date, these models are relatively unsophisticated, treating the cilium as a cantilevered beam. In order to fully understand the mechanical behavior of the cilium, more comprehensive modeling approaches such as multiscale modeling and molecular dynamics should be implemented incorporating the numerous structural elements of the primary cilium. These models coupled with more advanced biophysical measurements will provide vital insights into how different structural defects can affect the mechanosensory function of the cilium.
4. Primary Cilia Structure
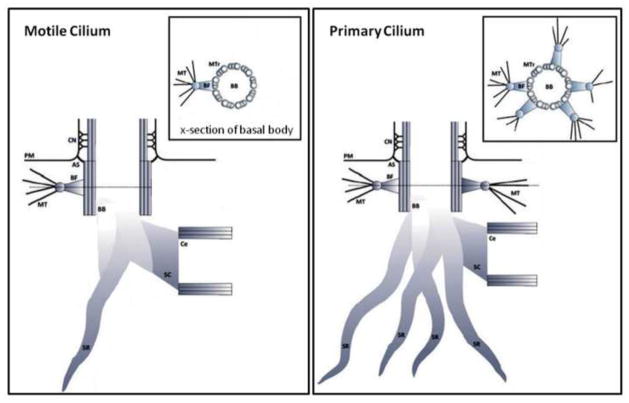
Recent morphological investigations have revealed the primary cilium to be an intricate arrangement of structural elements which, when combined, contributes to the structural integrity of this organelle. The various components are predominantly unique to the cilium can be divided into two different substructures, referred to as the ciliary and sub-ciliary compartments respectively. The ciliary compartment which extends out from the surface of the cell consists of the nine doublet microtubules and associated structural elements. The sub-ciliary consists of the basal body and associated anchoring elements within the interior of the cell body. All primary cilium related structural features discussed below are illustrated in figure 3.
Figure 3.
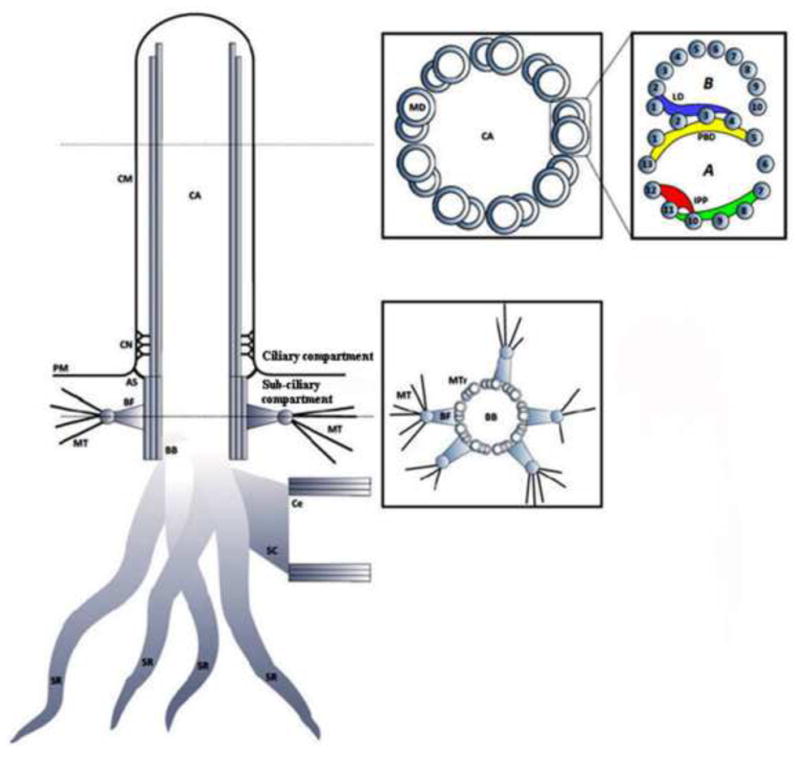
Schematic illustrating the ciliary and sub-ciliary structural components of the primary cilium. CA – Ciliary Axoneme; CM – Ciliary Membrane; CN – Ciliary Necklace; PM – Plasma Membrane; AS – Alar Sheet; BF – Basal Foot; BB – Basal Body; MT – Microtubule; Ce – Centriole; SC – Striated Connector; SR – Striated Rootlet; MD – Microtubule Doublet; MTr – Microtubule Triplet; LD – Linker Density; PBD – Partition Bridge Density; IPP – Interprotofilament Protein.
4.1 Ciliary compartment
The core structure of the ciliary compartment is the axoneme. The axoneme is a cylindrical array of nine doublet microtubules (MT) which extend from the triplet microtubule arrangement of the mother centriole or basal body. The doublets consist of two tubules; a complete microtubule containing 13 protofilaments (PF), refered to as tubule A, and an incomplete tubule containing 10 protofilaments, refered to as tubule B (Downing and Sui, 2007). The high rigidity of this doublet MT arises from its hollow tubular organization where the mass of the MT is distributed in its wall, thereby increasing the second moment of area. This structural feature greatly increases the doublets resistance to bending which is essential for a cilium which undergoes repeated loading due to fluid induced shear (Praetorius and Spring, 2005). Interestingly unlike singlet microtubules found within the cytoplasm, tubule A of the ciliary doublet is not spherical in nature and is known to form an elliptical shape with an elongation of 8% in the axonemes radial direction (Sui and Downing, 2006).
To our knowledge, no studies investigating the molecular architecture of the primary cilium microtubule doublet have been undertaken to date. However recent cryo-electron tomography studies in sperm flagella may provide some insight into protein structures which contribute to the linkages between protofilaments within tubules, connections between tubules, and possibly also act as docking sites for other structural proteins. Sui and Downing recently identified a range of non-tubulin proteins located within the microtubule doublet of sperm flagella (Sui and Downing, 2006). The connection between tubule A and B (PF A2–4 to B1–2, figure 3) was found to be relatively loose and consisted of a collection of proteins spanning numerous protofilaments at 16nm periodicities in the longitudinal direction. This collection of proteins is referred to as the linker density (Sui and Downing, 2006). Interestingly, it has been shown that these proteins have numerous domains that are predicted to bind Ca++ and produce a conformational change. The consequence of this is, as yet, unknown but previous studies have demonstrated that Ca++ can increases the stiffness of stereocilia (Pae and Saunders, 1994) and also increase the rate of depolymerization of microtubules (OBrien et al., 1997). The overlapping section between tubule A and B (PF A1–4, figure 3) is another area of high density highlighted from cryo-electron tomography. This area corresponds to a filamentous ribbon like structure which runs longitudinally along the protofilament A3 and has additional projections which reach out radially to adjacent protofilaments (Linck, 1976). These projections form a bridge like structure which Sui and Downing have referred to as the partition bridge density (Sui and Downing, 2006). This non tubulin ribbon consists of Tektin A, B and C which share a similar structure with intermediate filament proteins (Linck et al., 1987). The Linker density and the Tektin filaments are ideally located to stabilize the partition between the tubules A and B and have been hypothesized to contribute to the oval shape of the A tubule, hence increase the bending resistance of the doublet.
One of the major differences between the microtubules within the cytoplasm and the primary cilium is that the ciliary microtubules are known to undergo an array of reversible post-translational modifications including acetylation, polyglutamylation, tyrosination/detyrosination, and phorylation (Westermann and Weber, 2003). The majority of these modifications tend to affect the carboxy-terminal domain of tubulin (Nogales et al., 1999) which is located on the outside of the microtubule where it is ideally positioned to influence interactions with other proteins such as microtubule associated proteins (MAPs). Common MAPs include MAP1, MAP2, MAP4 and Tau, each of which has different binding affinities to tubulin (Takemura et al., 1992; Felgner et al., 1997; Chang et al., 2001). MAP1 in particular has been shown to localize to the axoneme of the cilium (Wiche et al., 1986; Sellner et al., 1991). The most studied form of the post-translational modifications is acetylation. Although it is unknown whether acetylation changes the mechanics of microtubules and hence the cilium directly, the occurrence of microtubule associated proteins (MAPs) is known to coincide with acetylation and the binding of MAPs has been shown to increase the flexural rigidity of microtubules as much as 8-fold (Takemura et al., 1992; Felgner et al., 1996). It was recently demonstrated that physiological levels of cyclic stretch in vivo and in vitro result in a significant increase in acetylation of microtubules in a magnitude and duration dependant manner. This appears to be due to a decrease in the activity of the deacetylation enzyme HDAC6 (Geiger R, 2009). In addition, mutant C. elegans which do not possess the acetyltransferase, αTAT1, lack the ability to acetylate microtubules and also have defective touch mechanosensation capabilities (Shida et al., 2010). Given that the microtubules of the primary cilium are known to be heavily acetylated, these findings suggest a possible mechanism by which the primary cilium could alter its flexural rigidity in response to loading, tuning its sensitivity to the extracellular environment.
The ciliary membrane is a continuation of the plasma membrane (Rohatgi and Snell, 2010) but there are indications of physiological differences between the two. For example, the ciliary membrane has been shown to have different ion-binding and osmotic behavior from the plasma membrane (Satir and Gilula, 1970). Furthermore the ciliary membrane shares certain diffraction characteristics with nerve myelin which is a dielectric sheath known to dramatically increase electrical resistance across the cell membrane (Silvester, 1964). Gilula and Satir, using a freeze-etch technique, found very few particles along the length of the ciliary membrane. However, they did discover a unique differentiation of the ciliary membrane which they called the ciliary necklace (Gilula and Satir, 1972). The ciliary necklace is an intra-membrane structure consisting of a complex of membrane associated particles located at the neck of the cilium, just above the transition zone between the axoneme and sub-axonemal compartments. Interestingly, the necklace consists of three strands of particles, arranged in scalloped rows. Each scallop is connected to a corresponding microtubule doublet via 50nm long champagne-glass-like structures. Immunostaining studies have repeatedly found localization of ion channels and receptors at the base of the cilium which may correspond to this ciliary necklace region (Zhang et al., 2004; Quarmby, 2009). Give that certain ion channels are stretch activated, the champagne-glass structures may act to stretch the membrane in this region, sensitizing the channels response to deformation of the ciliary axoneme.
4.2 Sub-ciliary compartment
In comparison to the axoneme, little attention has been paid to the structure of the sub-axonemal compartment of the cilium. It includes a vast subsurface array of structural elements, the function of which are not entirely understood. Although primarily believed to anchor and stabilize the cilium it has also been postulated that they act as a transport network facilitating the transfer of proteins to and from the cilium.
At the base of the cilium is the basal body, a protein-based structure consisting of nine triplet microtubules arranged circumferentially and is believed to be the template for ciliogenesis. The basal body is commonly known as the mother centriole. Although primarily known for its role in cell division, it has been suggested that the mother centrioles primary role may be for ciliogenesis given that cells can still divide without a centriole (Mahoney et al., 2006). The basal body maintains the transition zone between the axoneme and cytoplasmic compartments of the cell and is attached to the cell membrane via distal appendages called transitional fibers or alar sheets (Hagiwara et al., 1997a; Rohatgi and Snell, 2010). Alar sheets are fibrous triangular structures which emanate from the distal side of each triplet microtubule within the basal body and extend out to the plasma membrane partitioning the cell membrane from the ciliary membrane. The function of these sheets is largely unknown but it is believed to act as a sieve regulating the transport of proteins into and out of the primary cilium.
In the region proximal to the alar sheets is a collar of conical structures known as basal feet (Albrecht-Buehler and Bushnell, 1980). Basal feet extend laterally forming attachment points for cytoskeletal microtubules. Interestingly, the number of basal feet is known to differ depending on whether the cilium is a motile beating cilium or a non motile primary cilium. For example in motile cilia, only one basal foot is present and is always orientated in the direction of cilia beating or deflection (Boisvieux-Ulrich and Sandoz, 1991). This orientation strongly indicates the basal foot as an integral structural component stabilizing the cilium in the plane of beating. In the primary cilium, numerous (1–5) basal feet are present, orientated laterally in all directions. This addition of basal feet increases the cilia’s structural integration with the cytoskeleton which would significantly increase its stability and load transfer to the attaching microtubules (see figure 4). The basal body is one of the two centrioles which make up the microtubule organizing center (MTOC) but it is the parent centriole/basal body from which the vast majority of MTs assemble. This phenomenon significantly contributes to the structural stability of the mother centriole over the daughter which is noteworthy given the mother centriole’s role in anchoring the primary cilium. Efforts to count the number of MTs emanating from the centrosome range from 10–100 MTs depending on cell type (Alieva, 1992). Gudima et al (Gudima et al., 1983b, 1983a) discovered that the mean number and length changed depending on cell attachment, spreading and migration where long MTs were more prominent in spread out, fully adhered cells. In a fully adhered, polarized cell, the cilium would extend perpendicular into the extracellular environment. When loaded, such a cilium would experience large moments at its base, and therefore, the extension of long microtubules may be a cellular mechanism to stabilize the cilium. In addition to stabilizing the cilium this connection provides a mechanism of load transfer from the extracellular environment through the cilium to the cytoskeleton. For example Hierck et al disrupted the microtubule network in epithelial cells while maintaining the primary cilium and found a decrease in the induction of the shear response marker KLF2 by an applied fluid flow (Hierck et al., 2008).
Figure 4.
Schematic illustrating the structural differences in sub ciliary components in motile versus primary cilia. For motile cilia, the plane of beating would be in the direction of the single basal foot. CM – Ciliary Membrane; CN – Ciliary Necklace; PM – Plasma Membrane; AS – Alar Sheet; BF – Basal Foot; BB – Basal Body; MT – Microtubule; Ce – Centriole; SC – Striated Connector; SR – Striated Rootlet; MTr – Microtubule Triplet;
Striated rootlets are a collection of filamentous structures with periodic striations that radiate from the proximal end of the basal body into the cytoplasm of the cell. Although the exact function of these structures is unknown, numerous hypotheses have been put forward. Wheatley suggested that these rootlets act as ‘nanomachines’ (similar to muscle) for contraction, pulling on cytokeratins within the cytoplasm (Wheatley, 2008). This may explain the ability of some primary cilia to be pulled rapidly into the cell. Fariss et al put forward the hypothesis that rootlets may act as highways for the transport of protein molecules from the Golgi apparatus to the plasma membrane (Fariss et al., 1997). In a knockout animal of the rootlet protein rootletin, photoreceptor cells exhibit disrupted membranous saccule transport (Yang et al., 2005). However given the location, orientation, and structure of these filaments it is also likely that they act to anchor the basal body/primary cilium complex in the cell most likely by interacting with the cell’s cytoskeleton (Kobayashi and Hirokawa, 1988). The occurrence and orientation of striated rootlets differs depending on the function of the cilium. For example, motile cilia which function as a propeller in some cell types, have only one striated rootlet which runs straight from the basal body to the nucleus (Hagiwara et al., 1997b). In contrast, immotile primary cilia have numerous striated rootlets which are found to be orientated in all directions appearing as arched, looped, and branched around the cytoplasm (Hagiwara et al., 2002) (see figure 4).
5. Mechanisms of alteration in primary cilia mechanics
Despite the extensive morphological analysis which has been performed on the primary cilium, little is known about what role the structures identified thus far play in regulating the mechanics of the cilium. It is well known that dysfunctional cilia have been associated with numerous pathologies. Therefore it is interesting to consider alterations in these structures and speculate as to what effect they may have on the mechanics of this organelle. Therefore this section explores how changes in structure may affect ciliary mechanics altering the mechanosensing capability of the primary cilium.
As the core element of the axoneme, the doublet microtubules bear the greater share of the loads transmitted from the extracellular environment. Within each doublet, there are numerous mechanisms which could affect its flexural rigidity. The proteins of the linker density undergo conformational changes as a result of Ca2+ binding. Ca2+ has been shown to increase the stiffness of stereocilia (Pae and Saunders, 1994) and also increase the rate of depolymerization of microtubules (OBrien et al., 1997). Therefore in response to cilia deflection and subsequent Ca2+ entry via cation channels, the primary cilium could increase in stiffness via contraction of the linker density and/or decrease in length via microtubule depolymerization, the latter having already been demonstrated experimentally (Besschetnova et al., 2010).
The circumferential arrangement of the doublets is an evolutionary design to maximize bending resistance while minimizing mass. However unlike the motile cilia which have interdoublet connections called nexin links, the doublets of the primary cilium do not appear attached to their neighbor. This results in the loss of the circumferential arrangement towards the tip of the cilium (Odor and Blandau, 1985a; Yamamoto and Kataoka, 1986b) resulting in a change in flexural rigidity along its length (Rydholm et al., 2010). Despite the lack of nexin links in primary cilia, the attachment of neighboring doublets via microtubule associated proteins was visualized by Poole et al (Poole et al., 2001) in the axoneme of the sterna chondrocytes of a chick embryo. Once again this alteration could be responsible for alterations in primary cilia mechanics in response to loading. Loading is also known to result in an increase in acetylation which has been linked to an increase in MAP binding. Acetylation occurs at the lysine 40 site located on the inside of the microtubule. However, the recently discovered acetyltransferase, αTAT1, responsible for acetylation is too large to enter the microtubule via diffusion (Shida et al., 2010). What has been hypothesized, and partly verified via cryo-electron studies, is a phenomenon called microtubule ‘breathing’ where large openings in the microtubule wall appear and close rapidly allowing entrance of αTAT1. Therefore it is interesting to speculate that increased loading and deflection of ciliary microtubules increases the rate of microtubule ‘breathing’, increasing acetylation, increasing the association of MAPs and subsequently altering ciliary mechanics. Preliminary observations in our lab have found a decrease in the degree of bending of primary cilia after a rest period was inserted during loading (figure 4) indicating that cilia may indeed alter flexural rigidity in response to loading.
Modulations of the sub-ciliary compartments could significantly affect cilia orientation and possibly protein transport to and from the cilium. An intriguing change in sub-ciliary mechanics occurs in post-menopausal woman. The striated rootlets of human oviduct epithelial cells appear shortened and lie perpendicular to the longitudinal axis of the cell, a 90° change in orientation. This alteration could greatly affect the function of the cilium by altering its orientation and vacuole trafficking to and from the membrane (Holley, 1984). If this post-menopausal alteration in cilia mechanics is organism-wide it may have far reaching effects in other mechano-regulated tissues such as bone which undergoes dramatically altered physiology in post-menopausal woman (Kelly et al., 1989). Further evidence of the structural role of striated rootlets originates from the finding that disruption of the microtubule network in PtK2 cells dramatically influences the thickness, number and orientation of the striated rootlets. It appears that when the structural support of the microtubules within the cytoskeleton is lost, the cell compensates by elongating and thickening the striated rootlets emanating from the basal body. Also surprisingly, striated rootlets begin to form throughout the cytoplasm independent of the centrosome (Hagiwara and Takata, 2002).
Another significant mechanism may involve alterations in the number and orientation of the basal feet and associated microtubules. The number of basal feet can vary between 2 and 5 in primary cilia which would indicate that this is not a fixed organization. Therefore it may be possible for a cell to re-orientate and possibly add or remove basal feet in response to stimuli. Alternating basal feet would dramatically affect not only the stability of the cilium but also the ability of the cilium to transmit external forces to the cytoskeleton. This may be critical to mechanotransduction because bending and buckling of the cytoskeletal microtubules has been shown to be a mechanosensing mechanism (Mofrad and Kamm, 2010).
6. Conclusions
The primary cilium is now an established cellular mechanosensor regulating key stages in development along with maintaining homeostasis of numerous adult tissues. The cilium is a mechanosensor capable of adapting its length and possibly its stiffness, altering its sensitivity to extracellular mechanical stimuli. Given the sophistication of the cilium’s function it is not surprising the cilium is one of the most structurally complex organelles in the cell. Understanding how the mechanical properties of the primary cilium may affect cellular mechanosensitivity is a compelling question and surprisingly is a relatively untouched research area. Incorporating what is known of the intricate structure of the primary cilium along with more detailed biophysical measurements of the cells response to cilium stimulation into more sophisticated multi-scale mathematical models will provide a deeper understanding of ciliary mechanics, further highlighting the remarkable influence of this complex organelle.
Figure 1.
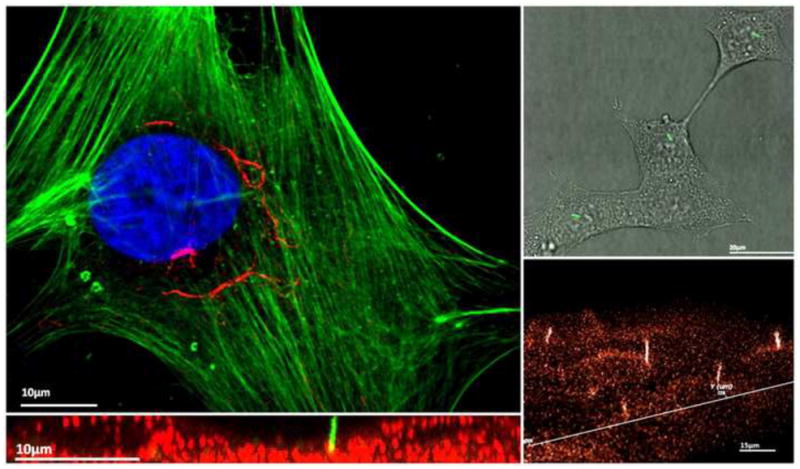
(Top Left) Immunoflouresence image of a human mesencyhmal stem cell illustrating a singular primary cilium enriched with acetylated alpha tubulin extending peri-nuclearly. Blue – Nucleus; Green – Actin; Red – Acetylated alpha Tublin. (Top Right) Kidney epithelial cells (IMCD) expressing a SSTR3-GFP fusion protein which is specific to the primary cilium. Primary cilia can be seen as green rod like structures. (Bottom Left) Immunoflouresence image of IMCD cells in the XZ plane. Green – SSTR3-GFP fusion protein (primary cilium); Red – Plasma membrane stain. (Bottom Right) 3D reconstruction of IMCD SSTR3-GFP cells illustrating an array of primary cilia projecting outwards from the cell plasma membrane.
Figure 5.
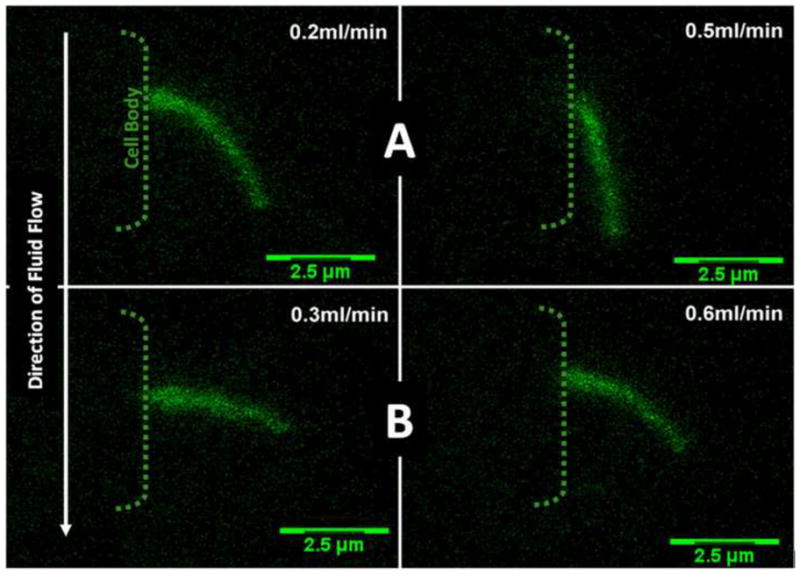
XZ bending profiles of a kidney cell primary cilium as seen in Figure 2. After an initial period of primary cilia bending in response to fluid flow (Images A), flow was stopped and the primary cilium was allowed to return to a no flow state. After a 15 minute rest period flow was reapplied (Images B). As can seen the degree of bending decreased in images B despite a higher flow rate indicating that the mechanics of the primary cilium has changed with time possible in response to the initial period of loading (Preliminary Observations).
Acknowledgments
Funding provided by the an IRCSET-Marie Curie International Mobility Fellowship in Science, Engineering and Technology, New York State Stem Cell grant (N089-210) and NIH grants (AR45989 & AR54156). The authors would also to acknowledge Ms. Lisa Fitzgerald for her assistance with illustrations.
Footnotes
Conflict of interest statement
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams M, Smith UM, Logan CV, Johnson CA. Recent advances in the molecular pathology, cell biology and genetics of ciliopathies. J Med Genet. 2008;45:257–267. doi: 10.1136/jmg.2007.054999. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G, Bushnell A. The ultrastructure of primary cilia in quiescent 3T3 cells. Exp Cell Res. 1980;126:427–437. doi: 10.1016/0014-4827(80)90282-7. [DOI] [PubMed] [Google Scholar]
- Alieva IB, Nadezhdina ES, Vaisberg EA, Vorobjev IA. Microtubule and intermediate filament patterns around the centrosome in interphase cells. The Centrosome. 1992;15:103–129. [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Berbari NF, O’Connor AK, Haycraft CJ, Yoder BK. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Current Biology. 2010;20:182–187. doi: 10.1016/j.cub.2009.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell. 1991;72:3–14. doi: 10.1016/0248-4900(91)90072-u. [DOI] [PubMed] [Google Scholar]
- Chang W, Gruber D, Chari S, Kitazawa H, Hamazumi Y, Hisanaga S, Bulinski JC. Phosphorylation of MAP4 affects microtubule properties and cell cycle progression. J Cell Sci. 2001;114:2879–2887. doi: 10.1242/jcs.114.15.2879. [DOI] [PubMed] [Google Scholar]
- Davenport JR, Yoder BK. An incredible decade for the primary cilium: a look at a once-forgotten organelle. Am J Physiol Renal Physiol. 2005;289:F1159–1169. doi: 10.1152/ajprenal.00118.2005. [DOI] [PubMed] [Google Scholar]
- Downing KH, Sui H. Structural insights into microtubule doublet interactions in axonemes. Curr Opin Struct Biol. 2007;17:253–259. doi: 10.1016/j.sbi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Ernstrom GG, Chalfie M. Genetics of sensory mechanotransduction. Annu Rev Genet. 2002;36:411–453. doi: 10.1146/annurev.genet.36.061802.101708. [DOI] [PubMed] [Google Scholar]
- Fariss RN, Molday RS, Fisher SK, Matsumoto B. Evidence from normal and degenerating photoreceptors that two outer segment integral membrane proteins have separate transport pathways. J Comp Neurol. 1997;387:148–156. doi: 10.1002/(sici)1096-9861(19971013)387:1<148::aid-cne12>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M. Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol. 1997;138:1067–1075. doi: 10.1083/jcb.138.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner H, Frank R, Schliwa M. Flexural rigidity of microtubules measured with the use of optical tweezers. J Cell Sci. 1996;109 (Pt 2):509–516. doi: 10.1242/jcs.109.2.509. [DOI] [PubMed] [Google Scholar]
- Gardner K, Arnoczky SP, Lavagnino M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011;29:582–587. doi: 10.1002/jor.21271. [DOI] [PubMed] [Google Scholar]
- Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol. 2009;40:76–82. doi: 10.1165/rcmb.2007-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilula NB, Satir P. The ciliary necklace. A ciliary membrane specialization. J Cell Biol. 1972;53:494–509. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradilone SA, Masyuk AI, Splinter PL, Banales JM, Huang BQ, Tietz PS, Masyuk TV, Larusso NF. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc Natl Acad Sci U S A. 2007;104:19138–19143. doi: 10.1073/pnas.0705964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudima GO, Vorob’ev IA, Chentsov Iu S. Behavior of the cell center in the distribution of fibroblasts. Nauchnye Doki Vyss Shkoly Biol Nauki. 1983a:45–50. [PubMed] [Google Scholar]
- Gudima GO, Vorob’ev IA, Chentsov Iu S. Structural change in the cell center during fibroblast polarization and movement. Tsitologiia. 1983b;25:883–888. [PubMed] [Google Scholar]
- Hagiwara H, Aoki T, Fujimoto T. Ultrastructural observation on ‘transitional tubules’ in human oviductal ciliogenic cells. J Anat. 1997a;191 (Pt 2):285–290. doi: 10.1046/j.1469-7580.1997.19120285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Aoki T, Ohwada N, Fujimoto T. Development of striated rootlets during ciliogenesis in the human oviduct epithelium. Cell and Tissue Research. 1997b;290:39–42. doi: 10.1007/s004410050905. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Harada S, Maeda S, Aoki T, Ohwada N, Takata K. Ultrastructural and immunohistochemical study of the basal apparatus of solitary cilia in the human oviduct epithelium. J Anat. 2002;200:89–96. doi: 10.1046/j.0021-8782.2001.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara H, Takata K. Depolymerization of microtubules by colcemid induces the formation of elongated and centriole-nonassociated striated rootlets in PtK(2) cells. Cell Tissue Res. 2002;309:287–292. doi: 10.1007/s00441-002-0560-9. [DOI] [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley MC. The ciliary basal apparatus is adapted to the structure and mechanics of the epithelium. Tissue Cell. 1984;16:287–310. doi: 10.1016/0040-8166(84)90050-8. [DOI] [PubMed] [Google Scholar]
- Kelly PJ, Pocock NA, Sambrook PN, Eisman JA. Age and menopause-related changes in indices of bone turnover. J Clin Endocrinol Metab. 1989;69:1160–1165. doi: 10.1210/jcem-69-6-1160. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Hirokawa N. Cytoskeletal architecture and immunocytochemical localization of fodrin in the terminal web of the ciliated epithelial cell. Cell Motil Cytoskeleton. 1988;11:167–177. doi: 10.1002/cm.970110304. [DOI] [PubMed] [Google Scholar]
- Kwon RY, Temiyasathit S, Tummala P, Quah CC, Jacobs CR. Primary cilium-dependent mechanosensing is mediated by adenylyl cyclase 6 and cyclic AMP in bone cells. FASEB J. 2010 doi: 10.1096/fj.09-148007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck RW. Flagellar Doublet Microtubules - Fractionation of Minor Components and Alpha-Tubulin from Specific Regions of a-Tubule. J Cell Sci. 1976;20:405–439. doi: 10.1242/jcs.20.2.405. [DOI] [PubMed] [Google Scholar]
- Linck RW, Goggin MJ, Norrander JM, Steffen W. Characterization of antibodies as probes for structural and biochemical studies of tektins from ciliary and flagellar microtubules. J Cell Sci. 1987;88 (Pt 4):453–466. doi: 10.1242/jcs.88.4.453. [DOI] [PubMed] [Google Scholar]
- Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol. 2003;285:F998–F1012. doi: 10.1152/ajprenal.00067.2003. [DOI] [PubMed] [Google Scholar]
- Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Mahoney NM, Goshima G, Douglass AD, Vale RD. Making microtubules and mitotic spindles in cells without functional centrosomes. Current Biology. 2006;16:564–569. doi: 10.1016/j.cub.2006.01.053. [DOI] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–920. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan SR, Knight MM, Chowdhury TT, Joshi P, Jensen CG, Kennedy S, Poole CA. Mechanical loading modulates chondrocyte primary cilia incidence and length. Cell Biol Int. 2010;34:441–446. doi: 10.1042/CBI20090094. [DOI] [PubMed] [Google Scholar]
- Menco BP, Farbman AI. Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development. II. Olfactory epithelium, a morphometric analysis. J Cell Sci. 1985;78:311–336. doi: 10.1242/jcs.78.1.311. [DOI] [PubMed] [Google Scholar]
- Mofrad MRK, Kamm RD. Cellular mechanotransduction: diverse perspectives from molecules to tissues. Cambridge; New York: Cambridge University Press; 2010. [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- OBrien ET, Salmon ED, Erickson HP. How calcium causes microtubule depolymerization. Cell Motility and the Cytoskeleton. 1997;36:125–135. doi: 10.1002/(SICI)1097-0169(1997)36:2<125::AID-CM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Odor DL, Blandau RJ. Observations on the solitary cilium of rabbit oviductal epithelium: its motility and ultrastructure. Am J Anat. 1985a;174:437–453. doi: 10.1002/aja.1001740407. [DOI] [PubMed] [Google Scholar]
- Odor DL, Blandau RJ. Observations on the solitary cilium of rabbit oviductal epithelium: its motility and ultrastructure. American Journal of Anatomy. 1985b;174:437–453. doi: 10.1002/aja.1001740407. [DOI] [PubMed] [Google Scholar]
- Pae SS, Saunders JC. Intra- and extracellular calcium modulates stereocilia stiffness on chick cochlear hair cells. Proc Natl Acad Sci U S A. 1994;91:1153–1157. doi: 10.1073/pnas.91.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, Lee SH, Liedtke W, Guilak F. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Zhang ZJ, Ross JM. The differential distribution of acetylated and detyrosinated alpha-tubulin in the microtubular cytoskeleton and primary cilia of hyaline cartilage chondrocytes. J Anat. 2001;199:393–405. doi: 10.1046/j.1469-7580.2001.19940393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- Quarmby L. Ciliary ion channels: location, location, location. Curr Biol. 2009;19:R158–160. doi: 10.1016/j.cub.2008.12.038. [DOI] [PubMed] [Google Scholar]
- Resnick A, Hopfer U. Force-response considerations in ciliary mechanosensation. Biophys J. 2007;93:1380–1390. doi: 10.1529/biophysj.107.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikmenspoel R, Sleigh MA. Bending moments and elastic constants in cilia. J Theor Biol. 1970;28:81–100. doi: 10.1016/0022-5193(70)90065-2. [DOI] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KE, Rieder CL, Bowser SS. Flexible-substratum technique for viewing cells from the side: some in vivo properties of primary (9+0) cilia in cultured kidney epithelia. J Cell Sci. 1988;89 (Pt 4):457–466. doi: 10.1242/jcs.89.4.457. [DOI] [PubMed] [Google Scholar]
- Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical Properties of Primary Cilia Regulate the Response to Fluid Flow. Am J Physiol Renal Physiol. 2010 doi: 10.1152/ajprenal.00657.2009. [DOI] [PubMed] [Google Scholar]
- Satir P, Gilula NB. THE CELL JUNCTION IN A LAMELLIBRANCH GILL CILIATED EPITHELIUM: Localization of Pyroantimonate Precipitate. J Cell Biol. 1970;47:468–487. doi: 10.1083/jcb.47.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol Renal Physiol. 1997a;272:F132–F138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Schwartz EA, Leonard ML, Bizios R, Bowser SS. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997b;272:F132–138. doi: 10.1152/ajprenal.1997.272.1.F132. [DOI] [PubMed] [Google Scholar]
- Sellner W, Oberkanins C, Stockinger L, Wiche G. Ultrastructural localization of microtubule-associated proteins (MAP) 1 in cilia of the respiratory tract. Eur J Cell Biol. 1991;55:248–254. [PubMed] [Google Scholar]
- Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–21522. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvester NR. The Cilia of Tetrahymena Pyriformis: X-Ray Diffraction by the Ciliary Membrane. J Mol Biol. 1964;8:11–19. doi: 10.1016/s0022-2836(64)80143-1. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- Sui H, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature. 2006;442:475–478. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
- Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Increased microtubule stability and alpha tubulin acetylation in cells transfected with microtubule-associated proteins MAP1B, MAP2 or tau. J Cell Sci. 1992;103 (Pt 4):953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- Veland IR, Awan A, Pedersen LB, Yoder BK, Christensen ST. Primary Cilia and Signaling Pathways in Mammalian Development, Health and Disease. Nephron Physiol. 2009;111:39–53. doi: 10.1159/000208212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Bio. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- Wheatley DN. Nanobiology of the primary cilium--paradigm of a multifunctional nanomachine complex. Methods Cell Biol. 2008;90:139–156. doi: 10.1016/S0091-679X(08)00807-8. [DOI] [PubMed] [Google Scholar]
- Wiche G, Herrmann H, Dalton JM, Foisner R, Leichtfried FE, Lassmann H, Koszka C, Briones E. Molecular aspects of MAP-1 and MAP-2: microheterogeneity, in vitro localization and distribution in neuronal and nonneuronal cells. Ann N Y Acad Sci. 1986;466:180–198. doi: 10.1111/j.1749-6632.1986.tb38394.x. [DOI] [PubMed] [Google Scholar]
- Xiao ZS, Quarles LD. Role of the polycytin-primary cilia complex in bone development and mechanosensing. Ann N Y Acad Sci. 2010;1192:410–421. doi: 10.1111/j.1749-6632.2009.05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Kataoka K. Electron microscopic observation of the primary cilium in the pancreatic islets. Archivum Histologicum Japonicum. 1986a;49:449–457. doi: 10.1679/aohc.49.449. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Kataoka K. Electron microscopic observation of the primary cilium in the pancreatic islets. Arch Histol Jpn. 1986b;49:449–457. doi: 10.1679/aohc.49.449. [DOI] [PubMed] [Google Scholar]
- Yang J, Gao J, Adamian M, Wen XH, Pawlyk B, Zhang L, Sanderson MJ, Zuo J, Makino CL, Li T. The ciliary rootlet maintains long-term stability of sensory cilia. Mol Cell Biol. 2005;25:4129–4137. doi: 10.1128/MCB.25.10.4129-4137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci U S A. 2004;101:2311–2316. doi: 10.1073/pnas.0400073101. [DOI] [PMC free article] [PubMed] [Google Scholar]