Abstract
Levels of numerous hormones vary across the day and night. Such fluctuations are not only attributable to changes in sleep/wakefulness and other behaviors but also to a biological timing system governed by the suprachiasmatic nucleus of the hypothalamus. Sleep has a strong effect on levels of some hormones such as growth hormone but little effect on others which are more strongly regulated by the biological timing system (e.g., melatonin). Whereas the exact mechanisms through which sleep affects circulating hormonal levels are poorly understood, more is known about how the biological timing system influences the secretion of hormones. The suprachiasmatic nucleus exerts its influence on hormones via neuronal and humoral signals but it is also now apparent that peripheral cells can rhythmically secrete hormones independent of signals from the suprachiasmatic nucleus. Under normal circumstances, behaviors and the biological timing system are synchronized and consequently hormonal systems are exquisitely regulated. However, many individuals (e.g., shift-workers) frequently undergo circadian misalignment by desynchronizing their sleep/wake cycle from the biological timing system. Recent experiments indicate that circadian misalignment has an adverse effect on metabolic and hormonal factors such as glucose and insulin. Further research is needed to determine the underlying mechanisms that cause the negative effects induced by circadian misalignment. Such research could aid the development of countermeasures for circadian misalignment.
Keywords: misalignment, night-work, shift-work, melatonin, cortisol
INTRODUCTION
In mammals, concentrations of many hormones fluctuate across the day and night. Numerous hormones are directly affected by sleep and behavioral activity. Thus, one may think that the daily rhythm in sleep and other behaviors fully explain why there exists a day/night rhythm in hormone levels. However, an endogenous timing system also affects the day/night fluctuations of hormones. The influence of sleep and the endogenous timing system actually interact in order to regulate some hormones. Under normal conditions, the behavioral cycle and endogenous timing system are synchronized and appropriately regulate levels of hormones. However, when the sleep/wake cycle and endogenous timing system are uncoupled or desynchronized (e.g., during night-work), normal day/night variations in numerous hormones are altered which may have adverse health consequences. In this review, we primarily discuss the influence of the circadian timing system, sleep and their de-synchronization on hormones and emerging consequences on metabolic function.
THE CIRCADIAN TIMING SYSTEM
A multitude of organisms, ranging from single cellular organisms, plants, and flies, to humans, have developed an endogenous timing system that optimally synchronizes physiology and behavior (e.g., rest/activity cycles) with the solar day. This circadian system (“circa”, around; “dies”, day) has two fundamental characteristics: (1) endogenous rhythmicity with a period of approximately 24 h that persists independently of oscillations in external factors such as the light/dark cycle; (2) the ability to have its timing shifted by external factors such as light and nutrient intake. Circadian clocks are located centrally and in most peripheral tissues such as the liver and heart (Yamazaki et al., 2000; Storch et al., 2002; Ko and Takahashi, 2006). Another important feature of the circadian system is that it creates a ‘biological night’ and ‘biological day’ that alternate in cyclic fashion, with their transitions being characterized by relatively abrupt changes in hormonal, electrophysiological, and behavioral variables such as the propensity to sleep (Wehr et al., 2001; Aeschbach et al., 2003b). The term biological night refers to the habitual dark episode, i.e., the time normally characterized by behavioral inactivity in diurnal (day-active) species and behavioral activity in nocturnal (night-active) species and the opposite holds true for the term biological day. The importance of the circadian system is further exemplified by the observation that circadian disruption leads to an array of disorders (e.g., sleep disorders, impaired glucose regulation and obesity) and decreased life expectancy (Hurd and Ralph, 1998; Turek et al., 2005). This indicates that optimal functioning of the circadian timing system is imperative for good health and thus warrants extensive research.
Suprachiasmatic nucleus: the master circadian clock
The suprachiasmatic nucleus (SCN) is a bilateral structure consisting of thousands of neurons (45,000–50,000 in humans and ~20,000 in rats) that is located in the anterior hypothalamus directly on top of the optic chiasm and next to the third ventricle (van den Pol, 1980; Güldner, 1983; Swaab et al., 1985; Hofman et al., 1988). Different lines of evidence indicate that the SCN regulates circadian rhythms: (1) lesions of the SCN obliterate circadian rhythms in physiology and behavior (Moore and Eichler, 1972; Stephan and Zucker, 1972; van den Pol and Powley, 1979); (2) transplantation of a donor-SCN can reinstate circadian rhythms in SCN-lesioned rodents (Lehman et al., 1987); (3) transplantation of a donor-SCN restore rhythms in a SCN-lesioned recipient with a period length characteristic of the donor (Ralph et al., 1990); and (4) the SCN displays a circadian rhythm in neural firing rate not only in vivo, but also in vitro (Green and Gillette, 1982; Groos and Hendriks, 1982; Meijer et al., 1997). Circadian clocks were once thought to be only present in the SCN, but several research groups have now demonstrated that peripheral cells express circadian clocks that can function independently of the SCN (Balsalobre et al., 1998; Yoo et al., 2004; Brown et al., 2005). This is not surprising considering the molecular machinery (transcription-translation feedback loops) that drive circadian rhythms in SCN and peripheral cells is similar (Yagita et al., 2001). Despite this, synchrony between peripheral clocks within organs is lost without input from the SCN (Guo et al., 2006). Thus, the SCN is considered to be the master circadian clock, i.e. the pacemaker that is necessary for optimal coordination of peripheral clocks.
Entrainment: setting the clock
In mammals—including humans—circadian clocks have a period of approximately, but not exactly, 24 h (Czeisler et al., 1999). Consequently, circadian clocks require external input (i.e., a time cue) in order to phase-shift and thus synchronize (or entrain) with the environment (e.g., the light/dark cycle). Circadian clocks are entrained by both photic and non-photic signals.
Light is the most potent Zeitgeber (German: “time giver”), or time cue, to the master circadian oscillator. A direct projection from the retina to the SCN in mammals was confirmed in the 1970s (Moore and Lenn, 1972; Moore, 1973). Since then, it has been established that light is detected by intrinsically photosensitive retinal ganglion cells (containing the photopigment melanopsin), rods and cones and this signal is passed on to the SCN via the retinohypothalamic tract (RHT; Moore et al., 1995; Gooley et al., 2001; Hattar et al., 2002). Moreover, the SCN also indirectly receives light information via another pathway; light information is conveyed to the intergeniculate leaflet from the optic tract, then to the SCN via the geniculohypothalamic tract (GHT; Pickard, 1985; Harrington et al., 1987). Dysfunction of the abovementioned pathways impairs light induced phase-shifts and entrainment to a light/dark cycle, with a stronger effect of RHT dysfunction. Lesioning the RHT prevents entrainment to a light/dark cycle whereas lesioning the GHT only slows the rate of entrainment to a light/dark cycle (Johnson et al., 1988; Johnson et al., 1989; Hattar et al., 2003; Panda et al., 2003). The effect of light upon the circadian system is dependent on the internal time of exposure (i.e., circadian time). Light exposure during the biological evening/early night (i.e., a time normally associated with the start of behavioral inactivity in diurnal animals and behavioral activity in nocturnal species) phase delays the circadian system (i.e., causes the circadian cycle to shift later relative to clock time). On the other hand, light exposure during the biological morning (i.e., typically the transition from behavioral inactivity to activity in diurnal species; and vice versa in nocturnal animals) results in a phase advance (i.e., causes the circadian cycle to shift earlier relative to clock time; Khalsa et al., 2003). The effectiveness of light is further determined by intensity, wavelength, duration, and the prior photic history (Van Cauter et al., 1994; Zeitzer et al., 2000b; Lockley et al., 2003; Revell et al., 2005; Chang et al., 2011a; Chang et al., 2011b). Other neural inputs to the SCN in addition to the abovementioned ones have been identified, but their precise physiological relevance is yet to be determined (Moga and Moore, 1997; Krout et al., 2002).
Research indicates that circadian entrainment can occur in the absence of light cues and thus suggests that non-photic signals can phase-shift and synchronize circadian clocks (Lockley et al., 2000; Sack et al., 2000). Such non-photic signals include exercise, melatonin, feeding and temperature (Reppert et al., 1988; McArthur et al., 1991; Lewy et al., 1998; Damiola et al., 2000; Lockley et al., 2000; Sack et al., 2000; Buxton et al., 2003; Barger et al., 2004; Escobar et al., 2009; Buhr et al., 2010; Burgess et al., 2010). Due to brevity of this review, we only discuss the influence of melatonin and temperature upon circadian entrainment as examples. Sack et al. (2000) and Lockley et al. (2000) demonstrated that daily melatonin administration can entrain free-running circadian rhythms of blind individuals. In vitro, melatonin can phase-shift the SCN electrical activity rhythm (McArthur et al., 1991). This indicates that the entrainment effect of melatonin may be caused by its direct action upon the SCN. This is perhaps not surprising considering the high level of expression of melatonin receptors in the SCN (Reppert et al., 1988). As with light, a temporal relationship exists in regard to the entrainment effect of exogenous melatonin; intake during the biological day/evening phases advances and consumption during the biological night/morning phase delays circadian rhythms (Lewy et al., 1998; Burgess et al., 2010).
Of interest, Buhr et al.(2010) recently demonstrated that subtle, and physiologically relevant, variations in temperature (36 to 38.5°C) can entrain peripheral oscillators but not the SCN in vitro. The SCN’s resistance to the entrainment effect of temperature may prevent interfering feedback of body temperature on SCN, since the circadian regulation of body temperature is a key role of the SCN in endothermic animals (i.e., mammals; Buhr et al., 2010). Thus, the abovementioned findings indicate that the robust circadian rhythm in mammalian core body temperature may serve to entrain peripheral clocks.
Output of the SCN
The SCN orchestrates circadian rhythms in behavior and physiology via endocrine and neural pathways. Hormonal systems that are under clear circadian control include melatonin, the hypothalamic-pituitary-adrenal (HPA) axis including cortisol, the hypothalamic-pituitary-thyroid axis, and epinephrine as demonstrated recently (Czeisler and Klerman, 1999; Scheer et al., 2010). Multi-synaptic projections from the SCN to different organ systems have been demonstrated in rodents utilizing the trans-synaptic retrograde tracer pseudorabies virus, including projections to the pineal gland, heart, kidneys, adrenal cortex, liver, pancreas, spleen, and white and brown adipose tissue (la Fleur et al., 2000; Bartness et al., 2001; Buijs et al., 2001; Scheer et al., 2001; Kreier et al., 2002; Buijs et al., 2003; Scheer et al., 2003; Shibata, 2004). In addition, hormones (e.g., glucocorticoids) under SCN control may serve to entrain circadian oscillations in peripheral tissues (Balsalobre et al., 2000).
The SCN’s influence on the autonomic system may explain why human cardiovascular markers (e.g., heart rate and blood pressure) exhibit circadian rhythmicity, both at rest and in their response to stressors such as exercise and head-up tilt (Kräuchi and Wirz-Justice, 1994; Burgess et al., 1997; Scheer et al., 2004a; Scheer et al., 2010; Hu et al., 2011; Shea et al., 2011). Indeed, plasma epinephrine and plasma norepinephrine, as measures of sympathetic activity, and heart rate variability markers, as measures of vagal (parasympathetic) cardiac modulation, show endogenous circadian rhythms in humans, i.e. independent of any daily changes in behavior or environment (Burgess et al., 1997; Scheer et al., 2010). Most notably, the largest increase of sympathetic markers and the largest decrease in parasympathetic markers in response to standardized exercise occurs at an endogenous circadian phase corresponding to ~9 AM (Scheer et al., 2010), i.e. the peak time for the occurrence heart attacks and stroke, as observed in epidemiologic studies (Muller et al., 1985; Elliott, 1998). These data, if replicated in vulnerable populations, would suggest that the circadian system can contribute to the morning peak in adverse cardiovascular events. Understanding the underlying circadian mechanisms may help develop novel therapeutic strategies (Scheer et al., 2004b; Cagnacci et al., 2005). Other projections from the SCN relevant in the regulation of sleep and hormones are discussed in sections below.
SLEEP
Sleep is characterized by an increased arousal threshold to sensory input, a reduction of motor output and the absence of consciousness. Humans spend approximately one third of their lives sleeping but the function of this behavior is not well understood. Nevertheless, sleep deprivation studies indicate that sleep is required for proper cognitive, motor and physiological function (Rechtschaffen et al., 1989; Pilcher and Huffcutt, 1996; Buxton et al., 2010).
Sleep is divided into non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep (Carskadon and Dement, 1996). NREM and REM sleep occur in cycles during a sleep period (Carskadon and Dement, 1996). Traditionally, NREM sleep has been subdivided into stages 1–4, with stages 3 and 4 often referred to as slow wave sleep (SWS). In 2007, the American Academy of Sleep Medicine published new criteria for characterizing sleep (Iber et al., 2007). The main changes from the traditional criteria of Rechtschaffen and Kales (1968) is that NREM sleep has three stages—N1, N2, and N3 (stages 3 and 4 from the previous criteria are combined into N3). SWS and EEG slow-wave activity (spectral power density in the 0.75–4.5 Hz range) typically dominate early in a sleep episode and then decrease, whereas stage 2 sleep, EEG spindle frequency activity (power density in the 12–15 Hz range) and REM sleep become more prominent as sleep progresses (Aeschbach and Borbély, 1993; Carskadon and Dement, 1996). Slow wave activity (SWA) is thought to be a measure of sleep intensity that dissipates in the course of a sleep episode (Borbély and Achermann, 2005). The sleep/wake dependent changes in the latter were the basis of Process S in the Two-Process model (see below).
A two-process model of sleep regulation
On the face of it, it is perplexing that humans do not typically feel increasingly sleepy in the course of a regular waking day, whereas sleepiness and sleep propensity rapidly increase if wakefulness is extended beyond a regular 16-h daytime episode. Research has shown that these observations have a biological basis in the interaction of two regulatory systems, conceptualized as Process S and Process C in the two-process model of sleep regulation by Borbély (1982). Process S corresponds to a homeostatic (i.e., need-based) drive for sleep that builds up with increasing time awake and declines during sleep. Process C corresponds to the circadian (i.e. sleep-wake independent) modulation in sleep propensity. The combined action of the homeostatic and circadian processes controls sleep propensity, sleep duration, and the quality of wakefulness including alertness and performance.
The basic tenets of the two-process model were later tested in a series of experimental protocols, in particular the forced desynchrony (FD) protocol which allowed for the mathematical separation and quantification of sleep-wake dependent (homeostatic) and circadian contributions to sleep propensity, sleep duration, sleep structure and waking quality. In the FD protocol, subjects are scheduled to live on a fixed sleep-wake cycle that is sufficiently different from 24 h and in dim light conditions such that the sleep-wake cycle is outside the range of entrainment of the master oscillator, causing the internal clock to ‘free run’ or drift according to its own internal period. The FD protocol, first developed by Kleitman (1963), allows the assessment of circadian and homeostatic influence by evenly spreading behavioral (e.g., sleep and wakefulness) and environmental factors across the circadian cycle. The circadian drive for sleep is greatest at the end of the biological night and lowest at the end of the biological day (Figure 1; Dijk and Czeisler, 1994). Thus, under entrained conditions, as the homeostatic drive for sleep (also referred to as homeostatic sleep pressure) dissipates during a sleep episode, the circadian sleep drive increases in a compensatory manner (Figure 2; Dijk and Czeisler, 1994). Conversely, under entrained conditions, as the homeostatic sleep drive increases during wakefulness, the circadian sleep drive decreases. In essence, under conditions of appropriate entrainment, the homeostatic and circadian systems interact to consolidate wakefulness during the biological day and to consolidate sleep during the biological night. Of note, the effect of Process S and C on sleep are not additive. This is best illustrated by comparing the magnitude of the circadian modulation of sleep propensity at the beginning of the sleep episode with that at the end: at the beginning it is very small, while it grows towards the end of the sleep opportunity (Figure 2). This concept has clear relevance for people that undertake shift-work for example. Falling asleep is typically not a problem for night-workers, but staying asleep is increasingly difficult when trying to sleep during the day, at an adverse circadian phase (e.g., trying to sleep between 11 AM–7 PM).
Figure 1.
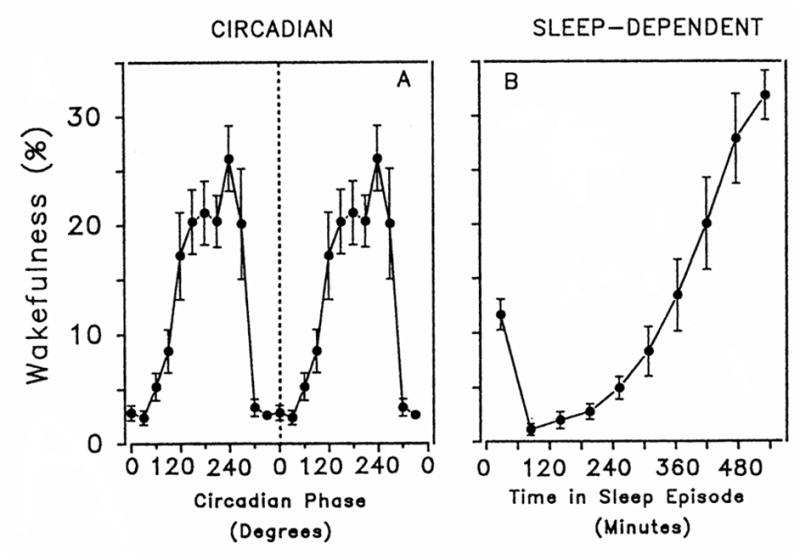
Independent influence of circadian phase (left panel) and time since start of sleep episode (right panel) on wakefulness in a scheduled sleep period. Reproduced with permission from Dijk and Czeisler (1994).
Figure 2.
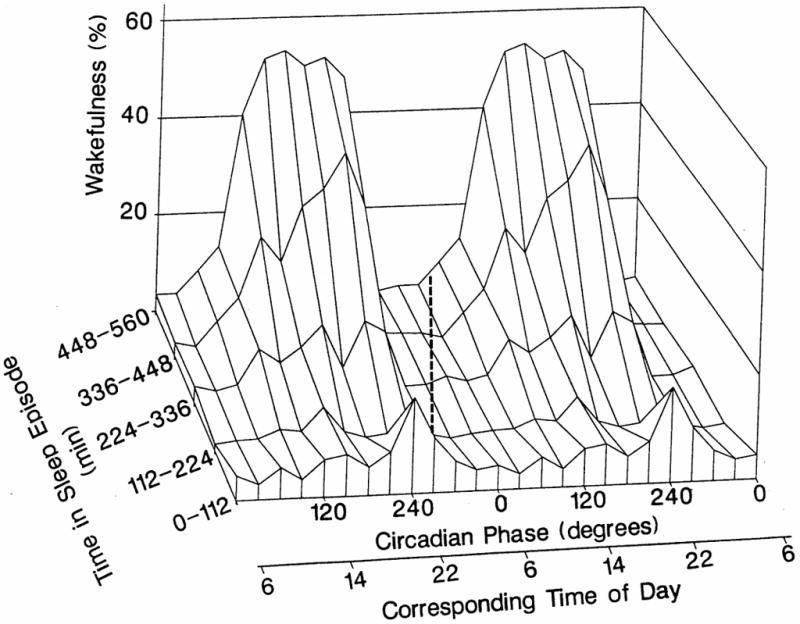
The interactive effect of the homeostatic and circadian systems on wakefulness during sleep. Percentage of wakefulness was assigned to the circadian phase (twelve 30-degree bins) and time into the sleep episode (five 112-min bins) that it occurred. Therefore, e.g., the ‘block’ at 0–112 min and 0 degrees represents wakefulness within the first 112 min of a sleep period that occurred between the circadian phases −15 to 15 degrees. Circadian phase is reported related to clock time under entrained conditions. The black dashed line represents the trajectory of circadian phase and time since the start of a scheduled sleep period, as would occur during nocturnal sleep in an entrained individual. Reproduced with permission from Dijk and Czeisler (1994).
Many aspects of sleep architecture are influenced by the homeostatic and circadian system, with the relative contribution varying among sleep parameters (Dijk et al., 1999). As proposed by the two-process model, the typical decline of SWS and EEG SWA during sleep is indeed due to a strong homeostatic influence, whereas the circadian influence is minimal (Figure 3; Dijk and Czeisler, 1995). In contrast, there is a strong circadian modulation of REM sleep and weaker homeostatic modulation (Figure 3). The maximum of the circadian rhythm in REM sleep occurs shortly after the minimum in the core body temperature rhythm. During entrained conditions, the combined sleep-dependent and circadian influences result in maximal REM sleep propensity close to habitual wake time, which has been thought to provide an optimal gate for the brain’s re-arousal (Lavie et al., 1979; Dijk and Czeisler, 1995). In addition, spindle frequency activity is influenced by both a sleep-dependent increase and a circadian modulation (Dijk and Czeisler, 1995; Aeschbach et al., 1997a).
Figure 3.
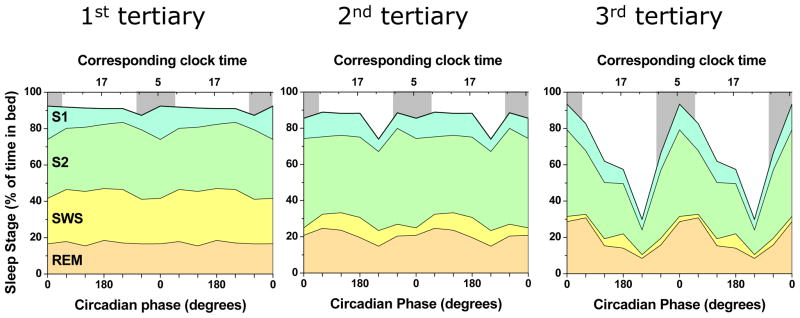
The separate and interacting effects of circadian and homeostatic systems on occurrence of sleep stages during sleep. Sleep was measured via polysomnography during a forced desyncrhony protocol. To assess the effect of the homeostatic system, sleep opportunities were split into 3 tertiaries (left, middle, and right panel). Slow wave sleep is primarily regulated by the homeostatic system (decreasing from first to third tertiary) whereas REM sleep is primarily regulated by the circadian system (with the peak at 0° and 60°, and the trough at 240°). Furthermore, the Figure highlights that the homeostatic and circadian influence are not additive: the circadian influence on (especially) REM sleep and Stage 2 sleep are small when homeostatic sleep pressure is high (left panel) and large when homeostatic sleep pressure is low (right panel). It also shows that the amount of slow wave sleep is not noticeably decreased when sleeping during the biological day (e.g., 180°), as occurs in night-workers, consistent with the fact that SWS is primarily homeostatically driven. S1, S2, SWS and REM indicate Stage 1, 2, slow wave sleep and rapid eye movement sleep respectively. Gray bars in the background indicate the circadian phases corresponding to the average habitual sleep episode in these subjects. Data are double plotted to improve visibility of rhythmicity. The data are unpublished, were provided by F.A. Scheer, T.J. Shea, M.F. Hilton and S.A. Shea, and based on Scheer et al. (2008).
Homeostatic and circadian processes are apparent not only during sleep. Quantitative markers of homeostatic sleep pressure and circadian sleep drive have also been derived from specific EEG frequency components during wakefulness (Aeschbach et al., 1997b; Cajochen et al., 2002).
ADENOSINE AS A MEDIATOR OF HOMEOSTATIC SLEEP PRESSURE
It is beyond the scope of this article to review fully the physiological basis of the changes in homeostatic sleep pressure that may underlie Process S. In short, Process S has been thought to be driven by a progressive build up of sleep-promoting chemicals during wakefulness. One chemical that has received a lot of scientific attention in recent decades is adenosine (for a full review see Landolt, 2008). Several findings are important in this context: (1) extracellular concentrations of adenosine in the basal forebrain—an area which promotes wakefulness—increase and decrease during elongated wakefulness and sleep, respectively (Porkka-Heiskanen et al., 1997; Porkka-Heiskanen et al., 2000); (2) intracerebroventricular injection of adenosine or an adenosine agonist promotes sleep (Virus et al., 1983; Benington et al., 1995; Schwierin et al., 1996); (3) adenosine antagonists such as caffeine promote wakefulness and alertness (Brezinova, 1974; Landolt et al., 1995a; Landolt et al., 1995b; Patat et al., 2000).
CIRCADIAN REGULATION OF SLEEP VIA PROJECTIONS IN THE BRAIN
Since reports of a circadian rhythm in sleep propensity and architecture, researchers have investigated how the SCN may regulate wakefulness and sleep. The influence of the SCN on the ventrolateral preoptic nucleus (VLPO) and orexin (also known as hypocretin) neurons found solely in the lateral hypothalamus has received special attention (Saper et al., 2005).
Lesioning the VLPO reduces sleep and thus suggests that these cells are involved in the promotion of sleep (Lu et al., 2000). Neurons of the VLPO presumably promote sleep by releasing inhibitory neurotransmitters (galanin and GABA) at various clusters of cells within the hypothalamus and brainstem that contribute to arousal, such as the locus coeruleus (Sherin et al., 1998; Saper et al., 2005). Orexin neurons project to the cerebral cortex along with cells in the hypothalamus and brainstem that partially mediate arousal and are predominately active during wakefulness (Peyron et al., 1998; Lee et al., 2005; Mileykovskiy et al., 2005). Mammals without orexin or its receptor exhibit narcoleptic behavior (e.g., excessive sleepiness during the period in which they are normally active) indicating that these neuropeptides promote wakefulness (Chemelli et al., 1999; Lin et al., 1999). The SCN has relatively few direct, but many possible indirect projections to the VLPO and orexin neurons (Chou et al., 2002; Yoshida et al., 2006). The indirect projections may occur via the following pathway: SCN to subparaventricular zone (SPZ), then dorsomedial nucleus of the hypothalamus (DMH) and finally VLPO and orexin neurons (Watts et al., 1987; Chou et al., 2002). Lesions of the ventral, but not dorsal SPZ impair circadian variation in sleep and wakefulness (Lu et al., 2001). This suggests that the SCN’s projection to the ventral SPZ is an important component of the circadian regulation of sleep and wakefulness.
CIRCADIAN SYSTEM, SLEEP AND ENDOCRINOLOGY
In this review, we only focus on hormones related to the circadian system and sleep that have been studied fairly extensively in humans. To determine the effect of the circadian system on variables, independent of changes in behavior (e.g., sleep and wakefulness) or environment, researchers studying humans have used a constant routine (CR) or FD (see above for a description) protocol. The CR protocol, first developed by Mills et al.(1978), enables the assessment of circadian variation in variables because the influences of behavioral and environmental factors are minimized or equally distributed across the circadian cycle by maintaining (1) constant wakefulness; (2) constant semi-recumbent posture; (3) limited physical movement; (4) dim light conditions; and (5) evenly distributing isocaloric snacks or continuous glucose infusion.
Melatonin
Circadian/sleep regulation of melatonin
Researchers have demonstrated that sleep per se (i.e., independent of the circadian phase at which it occurred) has little influence on circulating melatonin levels suggesting that the nocturnal onset of melatonin secretion is not a consequence of the transition from wakefulness to sleep (Figure 4; Dijk et al., 1999; Gooley et al., 2011). Moreover, scientists using either a CR or FD protocol have demonstrated robust circadian rhythmicity in melatonin, with levels being high during the biological night and low during the biological day (Figure 4; Dijk et al., 1999; Wehr et al., 2001; Cain et al., 2010; Gooley et al., 2011). The regulation of melatonin production is probably the best-known and most-studied physiological function regulated by the SCN via the autonomic nervous system. The SCN projects to the pineal gland via a multi-synaptic pathway that begins with a projection from the SCN to the paraventricular nucleus (PVN), then to the IML of the upper thoracic spinal cord (between the first and third thoracic vertebra), subsequently the superior cervical ganglion and finally the pineal gland (Moore, 1996; Teclemariam-Mesbah et al., 1999). Two lines of evidence indicate that the abovementioned projection is important for the circadian rhythm in production of melatonin. First, dysfunction of the pathway abolishes rhythmic production of melatonin in rodents (Klein et al., 1983; Hastings and Herbert, 1986). Second, humans with complete cervical spinal cord injury, which transects this pathway, exhibit no circadian rhythm in melatonin, while people with a spinal cord injury below the third thoracic vertebra, which leaves this pathway intact, display a normal circadian rhythm in melatonin (Zeitzer et al., 2000a).
Figure 4.
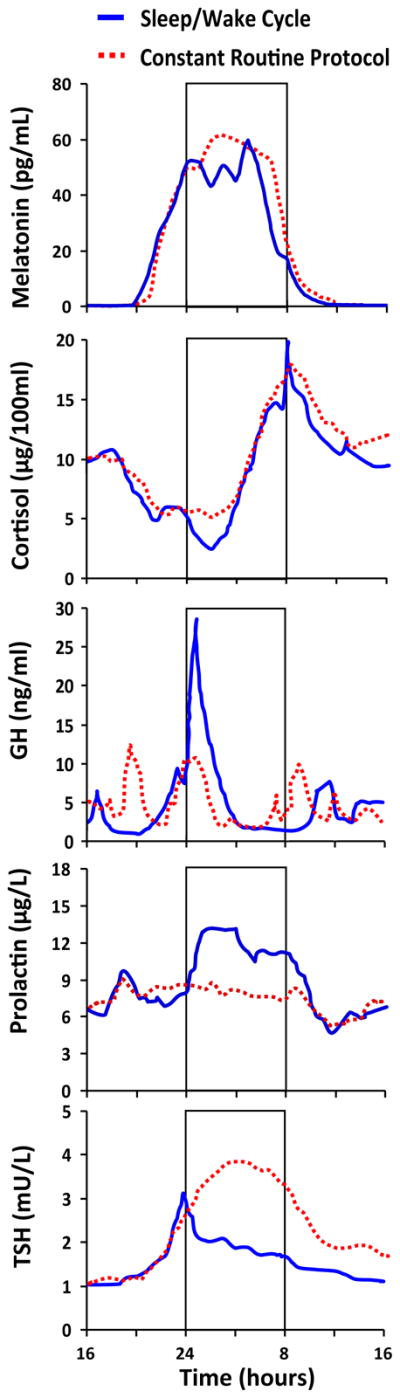
The effect of the sleep/wake cycle (solid line) and the endogenous circadian system (dotted line) on circulating levels of melatonin, cortisol, growth hormone (GH), prolactin and thyroid stimulating hormone (TSH). The solid line represents measurements taken whilst subjects maintained their habitual sleep/wake cycle including daytime activity and meals and nighttime rest and fasting. The dotted line represents data collected while subjects were under constant routine conditions including constant rest, semi-recumbent posture, wakefulness and hourly isocaloric snacks. For the sleep/wake cycle only, the vertical lines of the rectangle represent relative clock hour of habitual bed time (left line) and habitual wake time (right line) and thus the rectangle indicates scheduled nocturnal sleep. Note that the rectangle is not applicable to the constant routine conditions in which the subjects remained awake. The melatonin data were collected under dim light conditions (<3 lux during wakefulness and <1 lux during sleep periods) and are based on data from Gooley et al. (2011). Cortisol, GH and TSH data were collected under dim (<1 lux during sleep periods) and room light (~150 lux during wake periods) conditions and are based on data from Czeisler and Klerman (1999). The prolactin figure was based on data courteously provided by C.A. Czeisler. Copyright held by the Division of Sleep Medicine, Department of Medicine, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA. Reprinted with permission.
Melatonin effects on sleep
Several lines of evidence indicate that melatonin plays a role in the regulation of human sleep. First, exogenous melatonin administered outside of the phase of its endogenous production decreases sleep latency and—in particular in sustained-release and transdermal formulations—can increase total sleep time and sleep maintenance (Sack et al., 1997; Sharkey et al., 2001; Aeschbach et al., 2009). Second, exogenous melatonin induces specific changes in the spectral composition of the sleep EEG—including an increase in the spindle frequency range—that are similar to those attributed to the circadian pacemaker during the biological night (Dijk et al., 1995; Dijk et al., 1997; Knoblauch et al., 2003; Aeschbach et al., 2009). Third, pharmacological suppression of nocturnal melatonin with the beta-blocker Atenolol increases total wake time during a sleep opportunity, and administration of exogenous melatonin can restore normal sleep when given together with Atenolol (van den Heuvel et al., 1997). The adverse effects of beta-blockers on melatonin production and sleep raises the question whether melatonin supplementation may be beneficial in patient populations treated with beta-blockers, such as patients with hypertension, heart failure, arrhythmia, angina, or after a myocardial infarction (Scheer et al., 2004b; Scheer et al., 2007). Third, even chronic absence of endogenous melatonin production may be of clinical importance for sleep function. In a pilot study, patients with complete cervical spinal cord injury who produce no melatonin, had worse sleep efficiency than healthy people and individuals with thoracic spinal cord injury who had normal melatonin production (Scheer et al., 2006). It is worth noting that nocturnal species are active when endogenous melatonin levels are highest, when a soporific effect of melatonin would be counterproductive (Kalsbeek et al., 1996). Thus, interpreting data on the effects of melatonin on physiology in experimental rodents (typically nocturnal) and translating it to humans is not straight forward (Mendelson et al., 1980a).
Wyatt and co-workers (Wyatt et al., 2006), using a FD protocol, showed that exogenous melatonin (0.3 and 5 mg) in healthy people only improves sleep efficiency during the biological day when endogenous melatonin is absent and sleep quality is low, and failed to improve sleep during the biological night when endogenous melatonin levels and sleep quality are already high. The abovementioned researchers believe their results are consistent with the hypothesis that both exogenous and endogenous melatonin promotes sleep by opposing the wake-promoting signal from the master circadian clock. Interestingly, the intake of 0.3 and 5 mg of melatonin had a similar effect on sleep efficiency, perhaps indicating no additional benefit of exogenous melatonin concentrations above endogenous nighttime levels, consistent with previous observations (Zhdanova and Wurtman, 1997). Furthermore, these and other findings suggest that daytime melatonin intake may be useful for individuals (e.g., rotating shift-workers) who need to seek sleep during their biological day. Sustained release and transdermal melatonin delivery (Sack et al., 1997; Sharkey et al., 2001; Aeschbach et al., 2009), unlike immediate release melatonin, ensures that circulating melatonin levels are raised through much of a sleep period. Consequently, these formulations appear to be more effective than immediate release melatonin at improving daytime sleep.
In addition to its direct sleep-facilitating effect, melatonin can improve sleep through its chronobiotic effect by entraining the circadian system to a desired/imposed sleep/wake cycle (Lewy et al., 1992; Lockley et al., 2000; Sack et al., 2000; Burgess et al., 2010).
Cortisol
Circadian/sleep regulation of cortisol
Researchers utilizing either a CR or FD protocol have demonstrated a robust circadian rhythm in cortisol, with the trough during the early biological night, close to habitual bedtime, a rapid rise in the middle of the biological night when sleep would normally occur, and the peak in the biological morning, close to habitual waketime (Figure 4; El-Hajj Fuleihan et al., 1997; Wehr et al., 2001; Aeschbach et al., 2003b; Scheer et al., 2009). The SCN is thought to cause a circadian rhythm in corticosteroids (mainly cortisol in humans and corticosterone in rodents) cortisol levels via both hormonal and neuronal pathways. The hormonal pathway consists of projections from the SCN to the periventricular nucleus of the PVN and DMH which both then project to the medial parvocellular part of the PVN which stimulates the release of corticotropin-releasing hormone (Buijs et al., 1993; Buijs et al., 1999). This hormone then stimulates the secretion of adrenocorticotropin from the anterior pituitary gland that then acts upon the adrenal cortex to stimulate the release of corticosteroids (Buijs et al., 1993; Buijs et al., 1999). The neuronal pathway consists of a projection from the SCN to the PVN, then to the IML of the spinal cord and finally to the adrenal cortex (Buijs et al., 1999).
Different experimental designs indicate that sleep per se suppress cortisol levels although its effect is relatively weak compared to that of the circadian system (Figure 4). Weitzman and co-workers (1983) reported that cortisol levels are decreased during the first few hours of both day and night time sleep. Also when Weitzman et al. (1974) imposed healthy subjects to a 3-h sleep/wake cycle (i.e. 2 h of wakefulness/1 h of scheduled sleep) for 10 days, cortisol levels were generally suppressed during sleep, regardless of the time of day it occurred. It is not known what aspect of sleep influences cortisol levels. Because sleep suppresses cortisol levels during the early part of a sleep period when SWS is most present, it has been hypothesized that SWS has an inhibitory effect on cortisol secretion. Follenius et al. (1992) demonstrated that SWS and REM sleep are often temporally related to decreased cortisol secretion but their study could not determine if either sleep stage has an inhibitory effect on cortisol secretion or vice versa.
HPA-axis influences on sleep
Both continuous and pulsatile intravenous infusion of cortisol increases SWS and decreases REM sleep in humans (Born et al., 1991; Friess et al., 1994; Bohlhalter et al., 1997; Friess et al., 2004). Considering SWS decreases and REM sleep increases as a sleep episode progresses, it is unlikely that the circadian increase in cortisol during the nocturnal sleep in entrained individuals serves to regulate sleep.
Growth hormone
Circadian/sleep regulation of growth hormone
Uchiyama and co-workers using a CR protocol observed spikes in growth hormone levels during the biological evening and night when one would typically sleep (Uchiyama et al., 1998). However, the influence of sleep on growth hormone secretion is stronger than the circadian system’s influence (Figure 4). Indeed, growth hormone levels are increased during sleep, with the major increase occurring soon after sleep onset, irrespective of the time of day that sleep takes place (Honda et al., 1969; Born et al., 1988; Pietrowsky et al., 1994; Weibel et al., 1997). When an individual’s nocturnal sleep is interrupted, a growth hormone surge occurs fairly soon after recommencement of sleep (Beck et al., 1975). Growth hormone surges can occur multiple times during a sleep period (Takahashi et al., 1968; Van Cauter et al., 1992). For example, Van Cauter and co-workers (1992) reported a growth hormone pulse approximately every 2 hours during sleep. These intermittent pulses of growth hormone are thought to be associated with the cyclic occurrence of SWS that occurs during a sleep period. Indeed, SWS and SWA are reported to be positively correlated with growth hormone level by some but not all researchers (Sassin et al., 1969b; Jarrett et al., 1990; Gronfier et al., 1996). Moreover, Holl et al. (1991) who measured growth hormone every 30 seconds during sleep noted that levels of the hormone were significantly higher during SWS compared to stage 1, 2 and REM sleep. It has been reported that growth hormone levels are lower in some individuals that are deprived of SWS, whereas stimulation of SWS with gamma-hydroxybutyrat (GHB) was found to be associated with an increase in growth hormone secretion (Sassin et al., 1969a; Van Cauter et al., 1997). Moreover, depriving individuals of REM sleep has no effect on growth hormone secretion during a nocturnal sleep period (Honda et al., 1969).
Growth hormone influences on sleep
Inconsistent results regarding the influence of growth hormone on human sleep have been reported. Some researchers have reported that intravenous infusion of growth hormone decreases SWS and increases REM sleep whereas others have observed no effect on sleep architecture (Mendelson et al., 1980b; Kern et al., 1993).
Prolactin
Circadian/sleep regulation of prolactin
Waldstreicher and co-workers (1996) used a CR protocol to determine if a circadian rhythm in prolactin levels was present in women and men. Men displayed a circadian rhythm in prolactin levels, with a nadir in the late biological morning/early afternoon and a plateau during the late biological afternoon, evening and night. Circadian variation in prolactin level was also present in women, but the characteristic of this rhythm was dependent on menstrual phase. In women studied during their follicular phase, prolactin levels rose in the late biological afternoon and remained high across the biological night until the start of the biological morning after which concentrations decreased to a nadir in the late biological morning/early afternoon. In women studied in their luteal phase, prolactin concentrations peaked in the late biological afternoon/early evening, then declined in the few hours before habitual bedtime (late biological evening), then reached a second peak during the late biological nighttime and then reached a nadir in the late biological morning/early afternoon.
Prolactin levels increase soon after the beginning of sleep and then remain high, regardless of the time of day at which sleep takes place (Parker et al., 1973; Sassin et al., 1973; Logue et al., 1992; Spiegel et al., 1996). The independent influence of sleep on prolactin levels appears to be stronger than the circadian system’s influence. Thus, prolactin concentrations appear to be primarily regulated by the sleep/wake cycle as opposed to the circadian system (Figure 4).
Conflicting findings regarding the relationship between specific sleep stages and prolactin secretion have been reported. For example, researchers have reported that prolactin levels are positively related to REM sleep and negatively or unrelated to SWS, and that prolactin secretory rate is low at the time of REM sleep onset (Vaughan et al., 1978; Spiegel et al., 1994). Moreover, Spiegel et al. (1995) reported that prolactin concentrations are positively correlated with EEG SWA. Clearly, more research is needed to clarify the relationship between sleep structure and prolactin levels.
Prolactin influences on sleep
In rats, systemic or intrahypothalamic infusion of prolactin increases and decreases REM sleep when administered during the day and night, respectively (Roky et al., 1993; Roky et al., 1994). Little research regarding the effect of prolactin on human sleep has been undertaken. However, it has been reported that patients with hyperprolactinemia have more SWS but similar REM sleep compared with healthy individuals (Frieboes et al., 1998). The influence of prolactin on human sleep warrants further exploration using better research designs.
Hypothalamic-pituitary-thyroid (HPT) axis
Circadian/sleep regulation of HPT-axis
For thyroid stimulating hormone (TSH), CR protocols have revealed a maximum in the middle of the biological night and nadir during the biological afternoon (Figure 4; Wehr et al., 1993; Allan and Czeisler, 1994). No circadian rhythm was present in total triiodothyronine (T3) or thyroxine (T4) concentrations (Allan and Czeisler, 1994), whereas a circadian rhythm in free T3 has been reported by others (Aeschbach et al., 2003a). Nighttime TSH levels are higher in the absence than in the presence of sleep indicating that sleep has an inhibitory influence on TSH secretion and opposes the circadian influence on this hormone (Figure 4; Parker et al., 1976; Brabant et al., 1990).
TSH values are negatively correlated with both SWS and SWA (Goichot et al., 1992; Gronfier et al., 1995). Moreover, recovery sleep following sleep deprivation suppresses TSH levels to larger extent than normal sleep does (Brabant et al., 1990), further suggesting a relationship between NREM sleep intensity and the magnitude of TSH suppression.
Glucose and insulin
Circadian/sleep regulation of glucose and insulin
Researchers using a CR protocol have determined circadian rhythmicity in glucose and insulin levels (Morgan et al., 1998; Shea et al., 2005). Peaks occurred during the late biological night/early biological morning. Scheer and colleagues (2009), using a FD protocol, reported a circadian rhythm in glucose (peak during the biological night) but not in insulin levels. The relatively small circadian insulin rhythm may have been masked by the strong stimulatory effect of the larger meals in the FD protocol compared to the CR protocol. Moreover, researchers have demonstrated that SCN intact-rats that have a fixed 6-meals-per-day feeding schedule (to uniformly distribute food intake across the circadian cycle) display a 24-h rhythm in glucose levels; whereas this rhythm is abolished in SCN-lesioned rats (la Fleur et al., 1999). Projections from the SCN to the liver and pancreas have been identified (la Fleur et al., 2000; Buijs et al., 2001). These projections provide neuroanatomical pathways by which the master circadian pacemaker—along with peripheral clocks—could influence levels of both glucose and insulin.
Glucose levels and insulin secretion rate increase during both nocturnal and daytime sleep under conditions of constant glucose infusion (Frank et al., 1995). It is thought that glucose levels increase during sleep—when glucose infusion is kept constant—due, in part, to a decrease in brain glucose metabolism possibly related to the lower brain activity that generally occurs during sleep compared to wakefulness (Boyle et al., 1994). Also, the rise in glucose during sleep under the above-described conditions may be partially due to a lack of glucose uptake by inactive muscles and due to growth hormone secretion pulses which can have an anti insulin-like effect (Moller et al., 1990; Van Cauter, 2005). In the latter part of a sleep period—when glucose infusion is kept constant—glucose levels and insulin secretion decrease and this may be due to an increase in glucose utilization during wakefulness and REM sleep—both of which occur more frequently at the end rather than beginning of a sleep bout (Boyle et al., 1994; Van Cauter, 2005).
Ghrelin and leptin
Circadian/sleep regulation of ghrelin and leptin
Animal and human research indicates that ghrelin and leptin promote and suppress food intake, respectively (Coleman, 1978; Friedman and Leibel, 1992; Clement et al., 1998; Friedman and Halaas, 1998; Wren et al., 2001; Esler et al., 2007). To the authors’ knowledge, no researcher has published data regarding the influence of the human circadian system on ghrelin levels. However, LeSauter et al. (2009) demonstrated that expression of ghrelin by stomach cells was higher at circadian time (CT) 6—with CT12 being circadian onset of activity in nocturnal rodents—than at CT18 in mice kept in constant darkness and fed ad libitum or that were food-deprived. Natalucci and co-workers (Natalucci et al., 2005) reported that ghrelin levels rise before and decrease after habitual meal times in individuals that are fasting and similar findings have been reported in rats (Drazen et al., 2006). Future studies are required to test if the circadian system regulates ghrelin levels in humans.
Dzaja and co-workers (2004) reported that ghrelin levels are increased during the early part of sleep and that this response is blunted when sleep does not occur. These findings provide some evidence for sleep per se having an effect on ghrelin levels. However, no significant relationship between ghrelin and any sleep stage has been observed under normal conditions (Schuessler et al., 2005).
Shea and co-workers (2005), who used a CR protocol, reported a circadian rhythm in leptin concentrations, with levels increasing during the biological night and peaking in the biological morning whereas Scheer and colleagues (2009), using a FD protocol, observed no circadian rhythm in leptin levels. The circadian leptin rhythm may have been masked by a stimulatory effect of the larger meals in the FD protocol. A projection from the SCN to adipose tissue—the primary site for leptin secretion—has been indentified in rodents that could be involved in circadian rhythms in leptin levels (Kalsbeek et al., 2001; Kreier et al., 2006). Simon and colleagues (1998) reported that, under conditions of continuous enteral nutrition, leptin levels increase with the onset of sleep, regardless of the time of day that sleep takes place.
Ghrelin and leptin influences on sleep
Repetitive intravenous infusion of ghrelin increases growth hormone levels and SWS, and decreases REM sleep (Weikel et al., 2003). Considering that ghrelin increases growth hormone levels, it is possible that the effect of ghrelin on SWS is mediated via stimulating the secretion of growth hormone-releasing homone which is known to promote SWS when infused into humans (Steiger et al., 1992).
Regarding the potential effect of leptin on sleep, in rats, systemic administration of leptin increases SWS and decreases REM sleep (Sinton et al., 1999).
CIRCADIAN MISALIGNMENT AND CARDIOMETABOLIC HEALTH
Given that the sleep and hormonal systems are tightly integrated, it is conceivable that disruptions of their synchrony may have adverse health consequences. Research in this area is just at the beginning, but some results—particularly in the area of cardiometabolic health and circadian misalignment—are already emerging. Circadian misalignment occurs when the behavioral cycle (including the sleep/wake and fasting/feeding cycle) is not appropriately in alignment with the circadian timing system (e.g., during shift-work, during let lag, and in certain circadian rhythm sleep disorders).
Society’s demand for 24-h services (e.g., healthcare, industry and transport) has resulted in a high prevalence of shift-work. In the US, at least 5.7% of the work force regularly undertake night-work, either on permanent night shifts or rotating shifts (United States Department of Labor, 2005). This high prevalence of night-work is of considerable significance for public health considering that shift-work (relative to day-work) has been shown to be a risk factor for health problems such as cardiovascular disease, impaired glucose metabolism and obesity (Tenkanen et al., 1997; Morikawa et al., 2005; Suwazono et al., 2008; Suwazono et al., 2009). It is not fully understood why shift-work is a risk factor. However, recent experimental work indicates that circadian misalignment (i.e. an altered relationship between the sleep/wake and fasting/feeding cycle on one hand, and the endogenous circadian cycle on the other hand) can have profound adverse effects on metabolism.
Cardiometabolic consequences of circadian misalignment
Hampton and co-workers (1996) reported that a 9-h phase advance significantly increased postprandial responses of glucose and insulin to a standardized meal that was preceded by a high-fat meal, while integrated levels of triglycerides were unaffected. In a follow-up study, Ribeiro and colleagues (1998), using a very similar protocol, reported that a 9-h phase advance had no effect on postprandial response of glucose or insulin to a test meal when preceded by a low-fat meal. Triglyceride levels increased to a greater extent from baseline in the phase-shift condition. Differences between these two studies may have been due to difference in macronutrient content of meals preceding the test meals, which warrants further study.
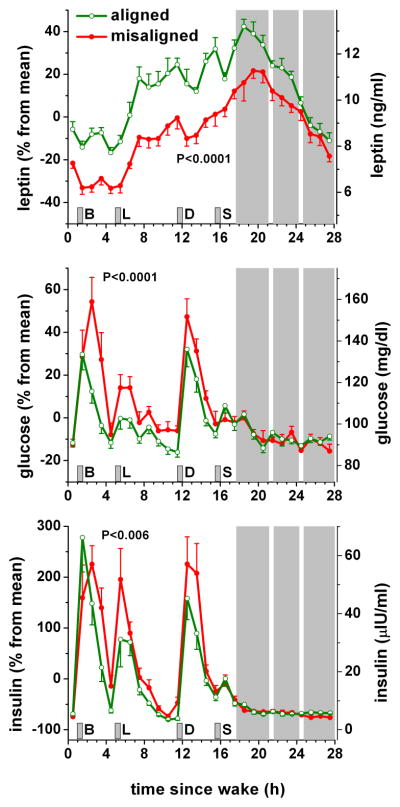
The above studies provide insightful data regarding the effect of simulated shift-work on postprandial metabolism. However, such studies are unable to determine the independent effect of circadian misalignment on postprandial metabolism because the time that elapsed from wake time and the consumption of the test meal differed between experimental conditions. Recently, Scheer and co-workers (2009) used a FD protocol to ensure that the time elapsed from wake time and the consumption of isocaloric meals etc. was the same across experimental conditions. The study demonstrated that circadian misalignment per se significantly reduces leptin levels, increases wake time blood pressure, and increases postprandial concentrations of glucose and insulin (Figure 5; Scheer et al., 2009). It is worth noting that circadian misalignment caused increases in postprandial glucose and insulin both during the subjects’ breakfast (i.e., the meal shortly after awakening) and dinner (close to bedtime), while they were almost 12 h apart. This shows that the effects in this study are not solely due to the meal being given at a different circadian time, and suggest an influence of circadian misalignment. Further studies are required to determine the precise molecular and physiological mechanisms involved.
Figure 5.
The effect of circadian misalignment on circulating levels of leptin, glucose and insulin. Gray area indicates scheduled sleep episode and short vertical grey bars represent meal times. The white strips within the scheduled sleep period indicate when participants were briefly awoken to perform pulmonary function tests. B, L, D and S indicate breakfast, lunch, dinner and snack respectively. Reproduced with permission from Scheer and colleagues (2009).
The study by Scheer et al. (2009) was not able to determine the long-term effect of circadian misalignment. Nevertheless, Arble et al. (2009), using an animal model, investigated the longer-term effect of circadian misalignment on body mass. The researchers fed mice for 6 weeks either in the 12 h dark phase (biological night; the normal feeding time for mice) or the 12 h light phase (biological day). Mice fed during the light phase gained significantly more body mass and tended to have a greater body fat percentage than mice fed during the dark phase. The light-fed mice tended to consume more energy and undertake less locomotor activity than the dark fed mice, although these group differences did not reach statistical significance. Thus, it is possible that the additive effects of increased energy consumption and decreased locomotor activity caused the increased body mass and fat content in light-fed mice (Arble et al., 2009). Moreover, it is possible that diet-induced thermogenesis was lower in the mice fed at the ‘wrong time’ (i.e., light-fed mice) than ‘correct time’ (i.e., dark-fed mice) as observed in humans (Romon et al., 1993).
Although not the focus of this review, it is worth mentioning that other forms of circadian disruption also induce an array of disorders. For example, Turek et al. (2005) demonstrated that Clock (a primary circadian clock gene) mutant mice are obese, and show hyperlipidemia and hyperglycaemia (for a full review of this area see the work of Bass and Takahashi 2010).
Are adverse effects of circadian misalignment mediated through sleep loss?
Shift-workers more frequently report difficulty initiating and in particular maintaining sleep than day-workers (Åkerstedt, 1998; Ohayon et al., 2002). These findings are at least in part due to shift-workers attempting to sleep during an adverse circadian phase, when the circadian drive for wakefulness is high (Figure 2; Dijk and Czeisler, 1994). It is known that insufficient sleep causes adverse physiological effects such as impaired glucose tolerance (Spiegel et al., 1999; Buxton et al., 2010). Furthermore, SWS suppression per se (thus independent of changes in sleep amount) can lead to impaired glucose tolerance (Tasali et al., 2008). Therefore, it is conceivable that adverse health problems associated with shift-work are mediated by poor sleep. However, the recently observed effects of circadian misalignment in humans on cardiometabolic biomarkers appear to be beyond any effect of a decrease in sleep efficiency (Scheer et al., 2009). Furthermore, there was no decrease in the amount of SWS during circadian misalignment (i.e., when sleeping during the day) in that study (Figure 3). This is not surprising because SWS is primarily regulated by homeostatic sleep pressure. These results indicate that the effects of circadian misalignment cannot easily be explained by changes in sleep alone, although other aspects of sleep regulation may be involved as well.
Emerging countermeasures for circadian misalignment: beyond re-entrainment strategies
The abovementioned studies indicate that strategies are needed to prevent or attenuate the adverse effects of circadian misalignment. A strategy (e.g., appropriate timing of light exposure and/or melatonin intake) that quickly promotes full or even partial entrainment to a shift-worker’s schedule would be ideal. Such practice has been demonstrated to be possible under laboratory and field conditions (Czeisler et al., 1990; Boivin and James, 2002; Crowley et al., 2003; Smith et al., 2009). However, some shift-workers may find it difficult to appropriately time their light exposure and/or melatonin intake. Thus, in addition to strategies to entrain individuals to changing sleep/wake cycles, other strategies should also be researched. One such strategy is exercise. An acute bout of moderate intensity exercise in the evening significantly reduces blood pressure during subsequent simulated night-work (Fullick et al., 2009). Furthermore, daytime exercise is known to suppress concentrations of the ‘hunger hormone’ acylated ghrelin (Broom et al., 2007). Consequently, Morris and co-workers (2010) investigated whether early evening exercise could reduce perceived hunger and circulating concentrations of acylated ghrelin and leptin during a simulated night-shift. Surprisingly, evening exercise increased concentrations of both acylated ghrelin and leptin but had no effect on average ratings of perceived hunger. It is unknown if exercise affects food consumption during night-work and future studies should assess this in addition to measuring hormones related to appetite. A promising countermeasure to prevent adverse metabolic consequences of circadian misalignment may be food timing. In rats undertaking ‘night-work’ for several weeks by being placed in a slow-rotating drum during their habitual rest phase, the development of abdominal obesity and metabolic changes could be prevented by scheduling the animals food intake to only occur during the animal’s habitual active phase (Salgado-Delgado et al., 2010). Moreover, the body mass gained in mice exposed to dim light rather than darkness at night is prevented by restricting food consumption to the animal’s active phase (Fonken et al., 2010).
Open research questions
Additional research is needed to further determine adverse affects of circadian misalignment on many more physiological factors relating to metabolism (e.g., ghrelin), cardiovascular measures, inflammatory markers, gastrointestinal functioning, and cancer risk. Currently, little is not known regarding if ‘real’ shift-workers respond differently to circadian misalignment than non-shift workers (the population typically included in experimental studies). Real shift-workers may have adapted to the physiological strain of circadian misalignment and thus may respond differently to circadian misalignment than day-workers. Moreover, it is not known what aspect (e.g., sleep/wakefulness and meals) of circadian misalignment is most important. For example, does eating at an atypical time have more adverse consequences than being awake and/or asleep at an unusual time? In addition, more research regarding interventions (e.g., timing of meals and exercise) that reduce the adverse effects of circadian misalignment is desperately needed.
SUMMARY
Concentrations of numerous hormones fluctuate across the day and night. Such fluctuations do not occur randomly. Sleep per se influences levels of many hormones and this partially explains why there is a day/night rhythm in hormone concentrations. The sleep/wake cycle is also associated with rhythms in other behaviors, such as behavioral activity, food intake and postural changes, and environmental exposures, which contribute to day/night rhythms in hormones. In addition, the internal circadian timing system—independent of sleep and wakefulness—has a profound effect on an array of hormones and thus contributes to the day/night rhythm in hormone levels. Under entrained conditions, the behavioral cycle and circadian system are synchronized and consequently levels of hormones are appropriately controlled. However, dissociating the behavioral cycle and the circadian system (e.g., as occurs during shift-work) has a profound adverse affect on many physiological processes (e.g., endocrine function) which may cause health problems. Further research is needed to clarify how circadian misalignment affects various physiological systems. Such research will enable the development of appropriate strategies to prevent or attenuate the adverse consequences of circadian misalignment.
Acknowledgments
C.J.M. was supported by the National Space Biomedical Research Institute through NASA NCC 9-58. D.A. was supported by National Institute of Health Grants R01-HL077399 and P01-AG009975. F.A.J.L.S. was supported by National Institute of Health Grants P30-HL101299 and R01 HL094806. We are very grateful for Miss Joanna I. Garcia’s assistance in preparing Figure 4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Borbély AA. All-night dynamics of the human sleep EEG. Journal of Sleep Research. 1993;2:70–81. doi: 10.1111/j.1365-2869.1993.tb00065.x. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Dijk DJ, Borbély AA. Dynamics of EEG spindle frequency activity during extended sleep in humans: Relationship to slow-wave activity and time of day. Brain Research. 1997a;748:131–136. doi: 10.1016/s0006-8993(96)01275-9. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Lockyer BJ, Dijk DJ, Lockley SW, Nuwayser ES, Nichols LD, Czeisler CA. Use of transdermal melatonin delivery to improve sleep maintenance during daytime. Clinical Pharmacology & Therapeutics. 2009;86:378–382. doi: 10.1038/clpt.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Dynamics of the human EEG during prolonged wakefulness: Evidence for frequency-specific circadian and homeostatic influences. Neuroscience Letters. 1997b;239:121–124. doi: 10.1016/s0304-3940(97)00904-x. [DOI] [PubMed] [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. SLEEP. Chicago, Illinois: 2003a. Dissimilar Circadian Regulation of Thyrotropin and Free Thyroid Hormone Between Short Sleepers and Long Sleepers; p. A0271. [Google Scholar]
- Aeschbach D, Sher L, Postolache TT, Matthews JR, Jackson MA, Wehr TA. A longer biological night in long sleepers than in short sleepers. Journal of Clinical Endocrinology and Metabolism. 2003b;88:26–30. doi: 10.1210/jc.2002-020827. [DOI] [PubMed] [Google Scholar]
- Åkerstedt T. Shift work and disturbed sleep/wakefulness. Sleep Medicine Reviews. 1998;2:117–128. doi: 10.1016/s1087-0792(98)90004-1. [DOI] [PubMed] [Google Scholar]
- Allan JS, Czeisler CA. Persistence of the circadian thyrotropin rhythm under constant conditions and after light-induced shifts of circadian phase. Journal of Clinical Endocrinology and Metabolism. 1994;79:508–512. doi: 10.1210/jcem.79.2.8045970. [DOI] [PubMed] [Google Scholar]
- Arble D, Bass J, Laposky A, Vitaterna M, Turek F. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2348. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Barger LK, Wright KP, Jr, Hughes RJ, Czeisler CA. Daily exercise facilitates phase delays of circadian melatonin rhythm in very dim light. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2004;286:R1077–R1084. doi: 10.1152/ajpregu.00397.2003. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK, Demas GE. SCN efferents to peripheral tissues: implications for biological rhythms. Journal of Biological Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian Integration of Metabolism and Energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck U, Brezinova V, Hunter WM, Oswald I. Plasma growth hormone and slow wave sleep increase after interruption of sleep. Journal of Clinical Endocrinology and Metabolism. 1975;40:812–815. doi: 10.1210/jcem-40-5-812. [DOI] [PubMed] [Google Scholar]
- Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Research. 1995;692:79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- Bohlhalter S, Murck H, Holsboer F, Steiger A. Cortisol enhances non-REM sleep and growth hormone secretion in elderly subjects. Neurobiology of Aging. 1997;18:423–429. doi: 10.1016/s0197-4580(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Boivin DB, James FO. Circadian adaptation to night-shift work by judicious light and darkness exposure. Journal of Biological Rhythms. 2002;17:556–567. doi: 10.1177/0748730402238238. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A two process model of sleep regulation. Human Neurobiology. 1982;1:195–204. [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep Homeostasis and models of sleep regulation. In: Kryger MH, et al., editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; Philadelphia, PA: 2005. pp. 405–417. [Google Scholar]
- Born J, DeKloet ER, Wenz H, Kern W, Fehm HL. Gluco- and antimineralocorticoid effects on human sleep: A role of central corticosteriod receptors. American Journal of Physiology. 1991;260:E183–E188. doi: 10.1152/ajpendo.1991.260.2.E183. [DOI] [PubMed] [Google Scholar]
- Born J, Muth S, Fehm HL. The significance of sleep onset and slow wave sleep for nocturnal release of growth hormone (GH) and cortisol. Psychoneuroendocrinology. 1988;13:233–243. doi: 10.1016/0306-4530(88)90021-2. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. The Journal of Clinical Investigation. 1994;93:529–535. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabant G, Prank K, Ranft U, Schuermeyer T, Wagner TOF, Hauser H, Kummer B, Feistner H, Hesch RD, von sur Mühlen A. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. Journal of Clinical Endocrinology and Metabolism. 1990;70:403–409. doi: 10.1210/jcem-70-2-403. [DOI] [PubMed] [Google Scholar]
- Brezinova V. EFFECT OF CAFFEINE ON SLEEP - EEG STUDY IN LATE MIDDLE-AGE PEOPLE. British Journal of Clinical Pharmacology. 1974;1:203–208. doi: 10.1111/j.1365-2125.1974.tb00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. Journal of Applied Physiology. 2007;102:2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, Chicheportiche R, Dayer JM, Albrecht U, Schibler U. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biology. 2005;3:e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo S-H, Takahashi JS. Temperature as a Universal Resetting Cue for Mammalian Circadian Oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. Journal of Comparative Neurology. 2001;431:405–423. doi: 10.1002/1096-9861(20010319)431:4<405::aid-cne1079>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, Kalsbeek A, Nagai K, Niijima A. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. The Journal of Comparative Neurology. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou Y-X, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: A light and electron microscopic study. The Journal of Comparative Neurology. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, van Heerikhuize JJ, Feenstra MGP, Horst GJT, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. European Journal of Neuroscience. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI. Human Phase Response Curves to Three Days of Daily Melatonin: 0.5 mg Versus 3.0 mg. Journal of Clinical Endocrinology and Metabolism. 2010;95:3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Trinder J, Kim Y, Luke D. Sleep and Circadian Influences on Cardiac Autonomic Nervous System Activity. Am J Physiol Heart Circ Physiol. 1997;273:H1761–H1768. doi: 10.1152/ajpheart.1997.273.4.H1761. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Lee CW, L’Hermite-Bal‚riaux M, Turek FW, Van Cauter E. Exercise elicits phase shifts and acute alterations of melatonin that vary with circadian phase. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 2003;284:R714–R724. doi: 10.1152/ajpregu.00355.2002. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep Restriction for 1 Week Reduces Insulin Sensitivity in Healthy Men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnacci A, Cannoletta M, Renzi A, Baldassari F, Arangino S, Volpe A. Prolonged melatonin administration decreases nocturnal blood pressure in women. American Journal of Hypertension. 2005;18:1614–1618. doi: 10.1016/j.amjhyper.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SS, Santhi N, Schoen M, Czeisler CA, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. Journal of Biological Rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of circadian and wake duration-dependent modulation of EEG activation during wakefulness. Neuroscience. 2002;114:1047–1060. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Dement WC. Normal human sleep: An overview. In: Kryger MH, et al., editors. Principles and Practice of Sleep Medicine. Saunders; Philadelphia, PA: 1996. pp. 16–25. [Google Scholar]
- Chang A-M, Scheer FAJL, Czeisler CA. The human circadian system adapts to prior photic history. The Journal of Physiology. 2011a;589:1095–1102. doi: 10.1113/jphysiol.2010.201194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Santhi N, Bradstreet DS, Lockley SW, Duffy JF, Kronauer RE, Czeisler CA. DURATION RESPONSE CURVE TO BRIGHT LIGHT IN HUMANS. SLEEP. 2011b:A162. doi: 10.1113/jphysiol.2011.226555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. Journal of Neuroscience. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Coleman D. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–8. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI. Combinations of bright light, scheduled dark, sunglasses, and melatonin to facilitate circadian entrainment to night shift work. Journal of Biological Rhythms. 2003;18:513–523. doi: 10.1177/0748730403258422. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Johnson MP, Duffy JF, Brown EN, Ronda JM, Kronauer RE. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253–1259. doi: 10.1056/NEJM199005033221801. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Progress in Hormone Research. 1999;54:97–132. [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes and Development. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neuroscience Letters. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. Journal of Neuroscience. 1995;15:3526–3538. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. Journal of Physiology (London) 1999;516.2:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijk DJ, Roth C, Landolt HP, Werth E, Aeppli M, Achermann P, Borbély AA. Melatonin effect on daytime sleep in men: suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neuroscience Letters. 1995;201:13–16. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. Journal of Physiology (London) 1997;505.3:851–858. doi: 10.1111/j.1469-7793.1997.851ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a Fixed Meal Pattern on Ghrelin Secretion: Evidence for a Learned Response Independent of Nutrient Status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. American Journal of Physiology - Endocrinology And Metabolism. 2004;286:E963–E967. doi: 10.1152/ajpendo.00527.2003. [DOI] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G, Klerman EB, Brown EN, Choe Y, Brown EM, Czeisler CA. The parathyroid hormone circadian rhythm is truly endogenous-a general clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1997;82:281–286. doi: 10.1210/jcem.82.1.3683. [DOI] [PubMed] [Google Scholar]
- Elliott WJ. Circadian variation in the timing of stroke onset: a meta-analysis. Stroke. 1998;29:992–996. doi: 10.1161/01.str.29.5.992. [DOI] [PubMed] [Google Scholar]
- Escobar C, Cailotto C, Angeles-Castellanos M, Delgado RS, Buijs RM. Peripheral oscillators: the driving force for food-anticipatory activity. European Journal of Neuroscience. 2009;30:1665–1675. doi: 10.1111/j.1460-9568.2009.06972.x. [DOI] [PubMed] [Google Scholar]
- Esler WP, Rudolph J, Claus TH, Tang W, Barucci N, Brown S-E, Bullock W, Daly M, DeCarr L, Li Y, Milardo L, Molstad D, Zhu J, Gardell SJ, Livingston JN, Sweet LJ. Small-Molecule Ghrelin Receptor Antagonists Improve Glucose Tolerance, Suppress Appetite, and Promote Weight Loss. Endocrinology. 2007;148:5175–5185. doi: 10.1210/en.2007-0239. [DOI] [PubMed] [Google Scholar]
- Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J. Nocturnal cortisol release in relation to sleep structure. Sleep. 1992;15:21–27. doi: 10.1093/sleep/15.1.21. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank SA, Roland DC, Sturis J, Byrne MM, Refetoff S, Polonsky KS, Van Cauter E. Effects of aging on glucose regulation during wakefulness and sleep. American Journal of Physiology. 1995;269:E1006–E1016. doi: 10.1152/ajpendo.1995.269.6.E1006. [DOI] [PubMed] [Google Scholar]
- Frieboes RM, Murck H, Stalla GK, Antonijevic IA, Steiger A. Enhanced slow wave sleep in patients with prolactinoma. Journal of Clinical Endocrinology and Metabolism. 1998;83:2706–2710. doi: 10.1210/jcem.83.8.5016. [DOI] [PubMed] [Google Scholar]
- Friedman J, Halaas J. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Leibel RL. TACKLING A WEIGHTY PROBLEM. Cell. 1992;69:217–220. doi: 10.1016/0092-8674(92)90402-x. [DOI] [PubMed] [Google Scholar]
- Friess E, Tagaya H, Grethe C, Trachsel L, Holsboer F. Acute cortisol administration promotes sleep intensity in man. Neuropsychopharmacology. 2004;29:598–604. doi: 10.1038/sj.npp.1300362. [DOI] [PubMed] [Google Scholar]
- Friess E, Van Bardeleben U, Wiedemann K, Lauer CJ, Holsboer F. Effects of pulsatile cortisol infusion on sleep-EEG and nocturnal growth hormone release in healthy men. Journal of Sleep Research. 1994;3:73–79. doi: 10.1111/j.1365-2869.1994.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Fullick S, Morris C, Jones H, Atkinson G. Prior Exercise Lowers Blood Pressure During Simulated Night-Work With Different Meal Schedules. American Journal of Hypertension. 2009;22:835–841. doi: 10.1038/ajh.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goichot B, Brandenberger G, Saini J, Wittersheim G, Follenius M. Nocturnal plasma thyrotropin variations are related to slow-wave sleep. Journal of Sleep Research. 1992;1:186–190. doi: 10.1111/j.1365-2869.1992.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SBS, Rajaratnam SMW, Van Reen E, Zeitzer JM, Czeisler CA, Lockley SW. Exposure to Room Light before Bedtime Suppresses Melatonin Onset and Shortens Melatonin Duration in Humans. Journal of Clinical Endocrinology & Metabolism. 2011;96:E463–E472. doi: 10.1210/jc.2010-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nature Neuroscience. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Research. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. Temporal link between plasma thyrotropin levels and electroencephalographic activity in man. Neuroscience Letters. 1995;200:97–100. doi: 10.1016/0304-3940(95)12082-f. [DOI] [PubMed] [Google Scholar]
- Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. A quantitative evaluation of the relationships between growth hormone secretion and delta wave electroencephalographic activity during normal sleep and after enrichment in delta waves. Sleep. 1996;19:817–824. doi: 10.1093/sleep/19.10.817. [DOI] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neuroscience Letters. 1982;34:283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Güldner FH. Numbers of neurons and astroglial cells in the suprachiasmatic nucleus of male and female rats. Experimental Brain Research. 1983;50:373–376. doi: 10.1007/BF00239203. [DOI] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Lehman MN, Bittman EL. Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. Journal of Neuroscience. 2006;26:6406–6412. doi: 10.1523/JNEUROSCI.4676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton SM, Morgan LM, Lawrence N, Anastasiadou T, Norris F, Deacon S, Ribeiro D, Arendt J. Postprandial hormone and metabolic responses in simulated shift work. Journal of Endocrinology. 1996;151:259–267. doi: 10.1677/joe.0.1510259. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Nance DM, Rusak B. Double-labeling of neuropeptide Y-immunoreactive neurons which project from the geniculate to the suprachiasmatic nuclei. Brain Research. 1987;410:275–282. doi: 10.1016/0006-8993(87)90325-8. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Herbert J. Neurotoxic lesions of the paraventriculo-spinal projection block the nocturnal rise in pineal melatonin synthesis in the Syrian hamster. Neuroscience Letters. 1986;69:1–6. doi: 10.1016/0304-3940(86)90404-0. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman MA, Fliers E, Goudsmit E, Swaab DF. Morphometric analysis of the suprachiasmatic and paraventricular nuclei in the human brain: Sex differences and age-dependent changes. Journal of Anatomy. 1988;160:127–143. [PMC free article] [PubMed] [Google Scholar]
- Holl RW, Hartman ML, Veldhuis JD, Taylor WM, Thorner MO. Thirty-second sampling of plasma growth hormone in man: correlation with sleep stages. Journal of Clinical Endocrinology and Metabolism. 1991;72:854–861. doi: 10.1210/jcem-72-4-854. [DOI] [PubMed] [Google Scholar]
- Honda Y, Takahashi K, Takahashi S, Azumi K, Irie M, Sakuma M, Tsushima T, Shizume K. Growth hormone secretion during nocturnal sleep in normal subjects. Journal of Clinical Endocrinology. 1969;29:20–29. doi: 10.1210/jcem-29-1-20. [DOI] [PubMed] [Google Scholar]
- Hu K, Scheer FAJL, Laker M, Smales C, Shea SA. Endogenous Circadian Rhythm in Vasovagal Response to Head-Up Tilt. Circulation. 2011;123:961–970. doi: 10.1161/CIRCULATIONAHA.110.943019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MW, Ralph MR. The Significance of Circadian Organization for Longevity in the Golden Hamster. Journal of Biological Rhythms. 1998;13:430–436. doi: 10.1177/074873098129000255. [DOI] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Americal Academy of Sleep Medicine; Westchester, Illinois: 2007. [Google Scholar]
- Jarrett DD, Greenhouse JB, Miewald JM, Fedorka IBB, Kupfer DJ. A reexamination of the relationship between growth hormone secretion and slow wave sleep using delta wave analysis. Biological Psychiatry. 1990;27:497–509. doi: 10.1016/0006-3223(90)90441-4. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Research. 1988;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Lateral geniculate lesions alter circadian activity rhythms in the hamster. Brain Research Bulletin. 1989;22:411–422. doi: 10.1016/0361-9230(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Drijfhout WJ, Westerink BH, van Heerikhuize JJ, van der Woude TP, van dV, Buijs RM. GABA receptors in the region of the dorsomedial hypothalamus of rats are implicated in the control of melatonin and corticosterone release. Neuroendocrinology. 1996;63:69–78. doi: 10.1159/000126937. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Romijn JA, la Fleur SE, Wortel J, Bakker O, Endert E, Buijs RM. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology. 2001;142:2677–2685. doi: 10.1210/endo.142.6.8197. [DOI] [PubMed] [Google Scholar]
- Kern W, Halder R, al-Reda S, Spath-Schwalbe E, Fehm H, Born J. Systemic growth hormone does not affect human sleep. Journal of Clinical Endocrinology and Metabolism. 1993;76:1428–1432. doi: 10.1210/jcem.76.6.8501147. [DOI] [PubMed] [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. Journal of Physiology (London) 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Smoot R, Weller JL, Higa S, Markey SP, Creed GJ, Jacobowitz DM. Lesions of the paraventricular nucleus area of the hypothalamus disrupt the suprachiasmatic - spinal cord circuit in the melatonin rhythm generating system. Brain Research Bulletin. 1983;10(5):647–652. doi: 10.1016/0361-9230(83)90033-3. [DOI] [PubMed] [Google Scholar]
- Kleitman N. Sleep and Wakefulness. University of Chicago Press; Chicago: 1963. [Google Scholar]
- Knoblauch V, Martens W, Wirz-Justice A, Krauchi K, Cajochen C. Regional differences in the circadian modulation of human sleep spindle characteristics. European Journal of Neuroscience. 2003;18:155–163. doi: 10.1046/j.1460-9568.2003.02729.x. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Human Molecular Genetics. 2006;15:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. American Journal of Physiology. 1994;267:R819–R829. doi: 10.1152/ajpregu.1994.267.3.R819. [DOI] [PubMed] [Google Scholar]
- Kreier F, Fliers E, Voshol PJ, Van Eden CG, Havekes LM, Kalsbeek A, Van Heijningen CL, Sluiter AA, Mettenleiter TC, Romijn JA, Sauerwein HP, Buijs RM. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat--functional implications. Journal of Clinical Investigation. 2002;110:1243–1250. doi: 10.1172/JCI15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from Fat Tissue, Liver, and Pancreas: A Neuroanatomical Framework for the Role of the Brain in Type 2 Diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- Krout KE, Kawano J, Mettenleiter TC, Loewy AD. CNS inputs to the suprachiasmatic nucleus of the rat. Neuroscience. 2002;110:73–92. doi: 10.1016/s0306-4522(01)00551-6. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. A suprachiasmatic nucleus generated rhythm in basal glucose concentrations. Journal of Neuroendocrinology. 1999;11:643–652. doi: 10.1046/j.1365-2826.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Research. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- Landolt HP. Sleep homeostasis: a role for adenosine in humans? Biochemical Pharmacology. 2008;75:2070–2079. doi: 10.1016/j.bcp.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Dijk DJ, Gaus SE, Borbély AA. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacology. 1995a;12:229–238. doi: 10.1016/0893-133X(94)00079-F. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Werth E, Borbély AA, Dijk DJ. Caffeine intake (200 mg) in the morning affects human sleep and EEG power spectra at night. Brain Research. 1995b;675:67–74. doi: 10.1016/0006-8993(95)00040-w. [DOI] [PubMed] [Google Scholar]
- Lavie P, Oksenberg A, Zomer J. It’s time, you must wake up now. Perceptual and Motor Skills. 1979;49:447–450. doi: 10.2466/pms.1979.49.2.447. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. The Journal of Neuroscience. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. Journal of Neuroscience. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proceedings of the National Academy of Sciences. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiology International. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Bauer VK, Ahmed S, Thomas KH, Cutler NL, Singer CL, Moffit MT, Sack RL. The human phase response curve (PRC) to melatonin is about 12 hours out of phase with the PRC to light. Chronobiology International. 1998;15:71–83. doi: 10.3109/07420529808998671. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. Journal of Clinical Endocrinology and Metabolism. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, James K, Thapan K, Wright J, Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. Journal of Endocrinology. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- Logue FC, Fraser WD, O’Reilly DSJ, Christie J, Cameron DA, Wallace DC, Beastall GH. Sleep shift dissociates the nocturnal peaks of parathyroid hormone, nephrogenous cyclic adenosine monophosphate, and prolactin in normal men. Journal of Clinical Endocrinology and Metabolism. 1992;75(1):25–29. doi: 10.1210/jcem.75.1.1320050. [DOI] [PubMed] [Google Scholar]
- Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. Journal of Neuroscience. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. Journal of Neuroscience. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur AJ, Gillette MU, Prosser RA. Melatonin directly resets the rat suprachiasmatic circadian clock in vitro. Brain Research. 1991;565:158–161. doi: 10.1016/0006-8993(91)91748-p. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Schaap J, Watanabe K, Albus H. Multiunit activity recordings in the suprachiasmatic nuclei: in vivo versus in vitro models. Brain Research. 1997;753:322–327. doi: 10.1016/s0006-8993(97)00150-9. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Gillin JC, Dawson SD, Lewy AJ, Wyatt RJ. Effects of melatonin and propranolol on sleep of the rat. Brain Research. 1980a;201:240–244. doi: 10.1016/0006-8993(80)90793-3. [DOI] [PubMed] [Google Scholar]
- Mendelson WB, Slater S, Gold P, Gillin JC. The effect of growth hormone administration on human sleep: A dose-response study. Biological Psychiatry. 1980b;15:613–618. [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral Correlates of Activity in Identified Hypocretin/Orexin Neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JN, Minors DS, Waterhouse JM. Adaptation to abrupt time shifts of the oscillator[s] controlling human circadian rhythms. Journal of Physiology (London) 1978;285:455–470. doi: 10.1113/jphysiol.1978.sp012582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. The Journal of Comparative Neurology. 1997;389:508–534. doi: 10.1002/(sici)1096-9861(19971222)389:3<508::aid-cne11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. American Journal of Physiology - Endocrinology And Metabolism. 1990;258:E86–E91. doi: 10.1152/ajpendo.1990.258.1.E86. [DOI] [PubMed] [Google Scholar]
- Moore RY. Retinohypothalamic projection in mammals: a comparative study. Brain Research. 1973;49:403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- Moore RY. Neural control of the pineal gland. Behavioural Brain Research. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. Journal of Comparative Neurology. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Card JP. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. Journal of Comparative Neurology. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- Morgan L, Arendt J, Owens D, Folkard S, Hampton S, Deacon S, English J, Ribeiro D, Taylor K. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. Journal of Endocrinology. 1998;157:443–451. doi: 10.1677/joe.0.1570443. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Naruse Y, Suwazono Y, Nogawa K. Shift work and the risk of diabetes mellitus among Japanese male factory workers. Scandinavian Journal of Work, Environment and Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Morris CJ, Fullick S, Gregson W, Clarke N, Doran D, MacLaren D, Atkinson G. PARADOXICAL POST-EXERCISE RESPONSES OF ACYLATED GHRELIN AND LEPTIN DURING A SIMULATED NIGHT SHIFT. Chronobiology International. 2010;27:590–605. doi: 10.3109/07420521003663819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Hartwell TD, Scheiner E, Gold HK, Jaffe AS, Raabe DS, Rude RE, Passamani E, Roberts R, Robertson T, Sobel BE, Willerson JT, Braunwald E, The MSG. Circadian variation in the frequency of onset of acute myocardial infarction. New England Journal of Medicine. 1985;313:1315–1322. doi: 10.1056/NEJM198511213132103. [DOI] [PubMed] [Google Scholar]
- Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. European Journal of Endocrinology. 2005;152:845–850. doi: 10.1530/eje.1.01919. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M. Prevalence and consequences of sleep disorders in a shift worker population. Journal of Psychosomatic Research. 2002;53:577–583. doi: 10.1016/s0022-3999(02)00438-5. [DOI] [PubMed] [Google Scholar]
- Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. Melanopsin is required for non-image-forming photic responses in blind mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- Parker DC, Pekary AE, Hershman JM. Effect of normal and reversed sleep-wake cycles upon nyctohemeral rhythmicity of plasma thyrotropin: Evidence suggestive of an inhibitory influence in sleep. Journal of Clinical Endocrinology and Metabolism. 1976;43:318–329. doi: 10.1210/jcem-43-2-318. [DOI] [PubMed] [Google Scholar]
- Parker DC, Rossman LG, Vanderlaan EF. Sleep-related, nyctohemeral and briefly episodic variation in human plasma prolactin concentrations. Journal of Clinical Endocrinology and Metabolism. 1973;36:1119–1124. doi: 10.1210/jcem-36-6-1119. [DOI] [PubMed] [Google Scholar]
- Patat A, Rosenzweig P, Enslen M, Trocherie S, Miget N, Bozon M-C, Allain H, Gandon J-M. Effects of a new slow release formulation of caffeine on EEG, psychomotor and cognitive functions in sleep-deprived subjects. Human Psychopharmacology: Clinical and Experimental. 2000;15:153–170. doi: 10.1002/(SICI)1099-1077(200004)15:3<153::AID-HUP154>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neuroscience Letters. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pietrowsky R, Meyrer R, Kern W, Born J, Fehm HL. Effects of diurnal sleep on secretion of cortisol, luteinizing hormone, and growth hormone in man. Journal of Clinical Endocrinology and Metabolism. 1994;78:683–687. doi: 10.1210/jcem.78.3.8126142. [DOI] [PubMed] [Google Scholar]
- Pilcher JJ, Huffcutt AI. Effects of sleep deprivation on performance: A meta-analysis. Sleep. 1996;19:318–326. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Bergmann BM, Everson CA, Kushida CA, Gilliland MA. Sleep deprivation in the rat: X. integration and discussion of the findings. Sleep. 1989;12:68–87. [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human Subjects. U.S. Government Printing Office; Washington, D.C: 1968. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- Revell VL, Arendt J, Terman M, Skene DJ. Short-wavelength sensitivity of the human circadian system to phase-advancing light. Journal of Biological Rhythms. 2005;20:270–272. doi: 10.1177/0748730405275655. [DOI] [PubMed] [Google Scholar]
- Ribeiro D, Hampton SM, Morgan L, Deacon S, Arendt J. Altered postprandial hormone and metabolic responses in a simulated shift work environment. Journal of Endocrinology. 1998;158:305–310. doi: 10.1677/joe.0.1580305. [DOI] [PubMed] [Google Scholar]
- Roky R, Valatx J-L, Jouvet M. Effect of prolactin on the sleep-wake cycle in the rat. Neuroscience Letters. 1993;156:117–120. doi: 10.1016/0304-3940(93)90453-r. [DOI] [PubMed] [Google Scholar]
- Roky R, Valatx JL, Paut-Pagano L, Jouvet M. Hypothalamic injection of prolactin or its antibody alters the rat sleep-wake cycle. Physiology and Behavior. 1994;55:1015–1019. doi: 10.1016/0031-9384(94)90382-4. [DOI] [PubMed] [Google Scholar]
- Romon M, Edme J, Boulenguez C, Lescroart J, Frimat P. Circadian variation of diet-induced thermogenesis. The American Journal of Clinical Nutrition. 1993;57:476–480. doi: 10.1093/ajcn/57.4.476. [DOI] [PubMed] [Google Scholar]
- Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. New England Journal of Medicine. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- Sack RL, Hughes RJ, Edgar DM, Lewy AJ. Sleep-promoting effects of melatonin: At what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food Intake during the Normal Activity Phase Prevents Obesity and Circadian Desynchrony in a Rat Model of Night Work. Endocrinology. 2010;151:1019–1029. doi: 10.1210/en.2009-0864. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sassin JF, Frantz AG, Kapen S, Weitzman ED. The nocturnal rise of human prolactin is dependent on sleep. Journal of Clinical Endocrinology and Metabolism. 1973;37:436–440. doi: 10.1210/jcem-37-3-436. [DOI] [PubMed] [Google Scholar]
- Sassin JF, Parker DC, Johnson LC, Rossman LG, Mace JW, Gotlin RW. Effects of slow wave sleep deprivation on human growth hormone release in sleep: Preliminary study. Life Sciences. 1969a;8:1299–1307. doi: 10.1016/0024-3205(69)90034-4. [DOI] [PubMed] [Google Scholar]
- Sassin JF, Parker DC, Mace JW, Gotlin RW, Johnson LC, Rossman LG. Human growth hormone release: relation to slow-wave sleep and sleep-waking cycles. Science. 1969b;165:513–515. doi: 10.1126/science.165.3892.513. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proceedings of the National Academy of Sciences. 2010;107:20541–20546. doi: 10.1073/pnas.1006749107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Kalsbeek A, Buijs RM. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanisms in human and rat. Biological Chemistry. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Shea TJ, Hilton MF, Shea SA. An Endogenous Circadian Rhythm in Sleep Inertia Results in Greatest Cognitive Impairment upon Awakening during the Biological Night. Journal of Biological Rhythms. 2008;23:353–361. doi: 10.1177/0748730408318081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FA, Stone PH, Shea SA. Decreased sleep in heart failure: are medications to blame? Archives of Internal Medicine. 2007;167:1098–1099. doi: 10.1001/archinte.167.10.1098-b. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Ter Horst GJ, van der Vliet J, Buijs RM. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. American Journal of Physiology - Heart and Circulatory Physiology. 2001;280:H1391–H1399. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Van Doornen LJ, Buijs RM. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton Neurosci. 2004a;110:44–48. doi: 10.1016/j.autneu.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Van Montfrans GA, Van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004b;43:192–197. doi: 10.1161/01.HYP.0000113293.15186.3b. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2006;44:78–81. doi: 10.1038/sj.sc.3101784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler P, Uhr M, Ising M, Schmid D, Weikel J, Steiger A. Nocturnal ghrelin levels—relationship to sleep EEG, the levels of growth hormone, ACTH and cortisol—and gender differences. Journal of Sleep Research. 2005;14:329–336. doi: 10.1111/j.1365-2869.2005.00486.x. [DOI] [PubMed] [Google Scholar]
- Schwierin B, Borbély AA, Tobler I. Effects of N 6 -cyclopentyladenosine and caffeine on sleep regulation in the rat. European Journal of Pharmacology. 1996;300:163–171. doi: 10.1016/0014-2999(96)00021-0. [DOI] [PubMed] [Google Scholar]
- Sharkey KM, Fogg LF, Eastman CI. Effects of melatonin administration on daytime sleep after simulated night shift work. Journal of Sleep Research. 2001;10:181–192. doi: 10.1046/j.1365-2869.2001.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an Endogenous Circadian Blood Pressure Rhythm in Humans That Peaks in the Evening. Circulation Research. 2011;108:980–984. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. Journal of Clinical Endocrinology and Metabolism. 2005;90:2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. Journal of Neuroscience. 1998;18:4705–4721. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S. Neural regulation of the hepatic circadian rhythm. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2004;280:901–909. doi: 10.1002/ar.a.20095. [DOI] [PubMed] [Google Scholar]
- Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. Journal of Clinical Endocrinology and Metabolism. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Fitch TE, Gershenfeld HK. The effects of leptin on REM sleep and slow wave delta in rats are reversed by food deprivation. Journal of Sleep Research. 1999;8:197–203. doi: 10.1046/j.1365-2869.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: study 4. Journal of Biological Rhythms. 2009;24:161–172. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Follenius M, Simon C, Saini J, Ehrhart J, Brandenberger G. Prolactin secretion and sleep. Sleep. 1994;17(1):20–27. doi: 10.1093/sleep/17.1.20. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. Temporal relationship between prolactin secretion and slow-wave electroencephalic activity during sleep. Sleep. 1995;18:543–548. [PubMed] [Google Scholar]
- Spiegel K, Weibel L, Gronfier C, Brandenberger G, Follenius M. Twenty-four-hour prolactin profiles in night workers. Chronobiology International. 1996;13:283–293. doi: 10.3109/07420529609020908. [DOI] [PubMed] [Google Scholar]
- Steiger A, Guldner J, Hemmeter U, Rothe B, Wiedemann K, Holsboer F. Effects of growth hormone-releasing hormone and somatostatin on sleep EEG and nocturnal hormone secretion in male controls. Neuroendocrinology. 1992;56:566–573. doi: 10.1159/000126275. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Science. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. ShiftWork and Impaired Glucose Metabolism: A 14-Year Cohort Study on 7104 Male Workers. Chronobiology International. 2009;26:926–941. doi: 10.1080/07420520903044422. [DOI] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A Longitudinal Study on the Effect of Shift Work on Weight Gain in Male Japanese Workers. Obesity. 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Research. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kipnis DM, Daughaday WH. Growth hormone secretion during sleep. Journal of Clinical Investigation. 1968;47:2079–2090. doi: 10.1172/JCI105893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proceedings of the National Academy of Science. 2008;105:1044–1049. doi: 10.1073/pnas.0706446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Ter Horst GJ, Postema F, Wortel J, Buijs RM. Anatomical demonstration of the suprachiasmatic nucleus-pineal pathway. Journal of Comparative Neurology. 1999;406:171–182. [PubMed] [Google Scholar]
- Tenkanen L, Sjoblom T, Kalimo R, Alikoski T, Harma M. Shift work, occupation and coronary heart disease over 6 years of follow-up in the Helsinki Heart Study. Scandinavian Journal of Work, Environment and Health. 1997;23:257–265. doi: 10.5271/sjweh.218. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama M, Ishibashi K, Enomoto T, Nakajima T, Shibui K, Hirokawa GO, Okawa M. Twenty-four hour profiles of four hormones under constant routine. Psychiatry and Clinical Neurosciences. 1998;52:241–243. doi: 10.1111/j.1440-1819.1998.tb01053.x. [DOI] [PubMed] [Google Scholar]
- United States Department of Labor. Workers on flexible and shift schedules in May 2004. Bureau of Labor Statistics; 2005. [Google Scholar]
- Van Cauter E. Endocrine Physiology. In: Kryger, et al., editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; Philadelphia: 2005. pp. 266–282. [Google Scholar]
- Van Cauter E, Kerkhofs M, Caufriez A, Van Onderbergen A, Thorner MO, Copinschi G. A quantitative estimation of growth hormone secretion in normal man: reproducibility and relation to sleep and time of day. Journal of Clinical Endocrinology and Metabolism. 1992;74:1441–1450. doi: 10.1210/jcem.74.6.1592892. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Plat L, Scharf MB, Leproult R, Cespedes S, L’Hermite-Bal‚riaux M, Copinschi G. Simultaneous stimulation of slow-wave sleep and growth hormone secretion by gamma-hydroxybutyrate in normal young men. Journal of Clinical Investigation. 1997;100:745–753. doi: 10.1172/JCI119587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Bal‚riaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. American Journal of Physiology. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- van den Heuvel CJ, Reid KJ, Dawson D. Effect of atenolol on nocturnal sleep and temperature in young men: reversal by pharmacological doses of melatonin. Physiology and Behavior. 1997;61:795–802. doi: 10.1016/s0031-9384(96)00534-3. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: Intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Powley T. A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Research. 1979;160:307–326. doi: 10.1016/0006-8993(79)90427-x. [DOI] [PubMed] [Google Scholar]
- Vaughan GM, Allen JP, Tullis W, Siler-Khodr TM, dela Pena A, Sackman JW. Overnight plasma profiles of melatonin and certain adenohypophyseal hormones in men. Journal of Clinical Endocrinology and Metabolism. 1978;47:566–571. doi: 10.1210/jcem-47-3-566. [DOI] [PubMed] [Google Scholar]
- Virus RM, Djuricic-Nedelson M, Radulovacki M, Green RD. The effects of adenosine and 2′-deoxycoformycin on sleep and wakefulness in rats. Neuropharmacology. 1983;22:1401–1404. doi: 10.1016/0028-3908(83)90231-9. [DOI] [PubMed] [Google Scholar]
- Waldstreicher J, Duffy JF, Brown EN, Rogacz S, Allan JS, Czeisler CA. Gender differences in the temporal organization of prolactin (PRL) secretion: Evidence for a sleep-independent circadian rhythm of circulating PRL levels - a clinical research center study. Journal of Clinical Endocrinology and Metabolism. 1996;81:1483–1487. doi: 10.1210/jcem.81.4.8636355. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. The Journal of Comparative Neurology. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Wehr TA, Aeschbach D, Duncan WCJ. Evidence for a biological dawn and dusk in the human circadian timing system. Journal of Physiology. 2001;535:937–951. doi: 10.1111/j.1469-7793.2001.t01-1-00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, Bender C. Conservation of photoperiod-responsive mechanisms in humans. American Journal of Physiology. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- Weibel L, Follenius M, Spiegel K, Gronfier C, Brandenberger G. Growth hormone secretion in night workers. Chronobiology International. 1997;14:49–60. doi: 10.3109/07420529709040541. [DOI] [PubMed] [Google Scholar]
- Weikel JC, Wichniak A, Ising M, Brunner H, Friess E, Held K, Mathias S, Schmid DA, Uhr M, Steiger A. Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology: Endocrinology and Metabolism. 2003;284:E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Nogeire C, Perlow M, Fukushima D, Sassin J, McGregor P, Gallagher TF, Hellman L. Effects of a prolonged 3-hour sleep-wake cycle on sleep stages, plasma cortisol, growth hormone, and body temperature in man. Journal of Clinical Endocrinology and Metabolism. 1974;38:1018–1030. doi: 10.1210/jcem-38-6-1018. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Zimmerman JC, Czeisler CA, Ronda J. Cortisol secretion is inhibited during sleep in normal man. Journal of Clinical Endocrinology and Metabolism. 1983;56:352–358. doi: 10.1210/jcem-56-2-352. [DOI] [PubMed] [Google Scholar]
- Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin Enhances Appetite and Increases Food Intake in Humans. Journal of Clinical Endocrinology and Metabolism. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Dijk DJ, Ritz-De Cecco A, Ronda JM, Czeisler CA. Sleep facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29:609–618. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Science. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. The Journal of Comparative Neurology. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. Journal of Clinical Endocrinology and Metabolism. 2000a;85:2189–2196. doi: 10.1210/jcem.85.6.6647. [DOI] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. Journal of Physiology (London) 2000b;526.3:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdanova IV, Wurtman RJ. Efficacy of melatonin as a sleep-promoting agent. Journal of Biological Rhythms. 1997;12:644–650. doi: 10.1177/074873049701200620. [DOI] [PubMed] [Google Scholar]