Abstract
Isolated in vitro brainstem-spinal cord preparations are used extensively in respiratory neurobiology because the respiratory network in the pons and medulla is intact, monosynaptic descending inputs to spinal motoneurons can be activated, brainstem and spinal cord tissue can be bathed with different solutions, and the responses of cervical, thoracic, and lumbar spinal motoneurons to experimental perturbations can be compared. The caveats and limitations of in vitro brainstem-spinal cord preparations are well-documented. However, isolated brainstem-spinal cords are still valuable experimental preparations that can be used to study neuronal connectivity within the brainstem, development of motor networks with lethal genetic mutations, deleterious effects of pathological drugs and conditions, respiratory spinal motor plasticity, and interactions with other motor behaviors. Our goal is to show how isolated brainstem-spinal cord preparations still have a lot to offer scientifically and experimentally to address questions within and outside the field of respiratory neurobiology.
1. Strengths and limitations of in vitro brainstem-spinal cord preparations
The isolated in vitro brainstem-spinal cord preparation introduced by Suzue (1984) has been an instrumental part of studies that have made profound contributions to respiratory neurobiology in the past 25 years (>250 papers to date). This preparation, however, is highly criticized because of its experimental limitations. While acknowledging these limitations, the goal of this review is to recognize the importance of this preparation in the history of respiratory neurobiology and focus on its advantages and enduring versatility. We believe that this preparation is still useful, especially when combined with novel experimental techniques to address important scientific questions in respiratory motor control. Due to space limitations, the advantages of brainstem-spinal cord preparations will be discussed and supported by representative papers. A thorough review of all papers on a given topic using brainstem-spinal cord preparations is beyond the scope of this review.
Most isolated in vitro brainstem-spinal cord preparations are dissected from anesthetized pre- and postnatal rats or mice although brainstem-spinal cord preparations can also be isolated from ectothermic adult vertebrates (see Section 9). Brainstem-spinal cord preparations are typically placed in a recording chamber where hyperoxygenated artificial cerebrospinal fluid (aCSF) bathes the tissue at a fixed temperature (26-28°C). Spontaneous respiratory-related motor output is typically recorded from ventral spinal nerve roots on the cervical and thoracic spinal cord, or cranial nerve roots. One caveat is that brainstem-spinal cord preparations are superfused (instead of arterially perfused), which causes large PO2, K+, and H+ gradients to be established within the tissue (especially the brainstem) and in unstirred layers surrounding the tissue (Brockhaus et al., 1993; Okada et al., 1993). The hypoxic, hyperkalemic, acidic core in the brainstem is argued by some to transform the motor output of the respiratory network into gasping or a gasp-like rhythm (St. John, 1996; Wang et al., 1996). There is considerable controversy in the field with respect to whether rhythm produced by these preparations is related to normal breathing (Duffin, 2003; Ramirez and Lieske, 2003; Richter, 2003; St. John and Paton, 2003). Another limitation is that most brainstem-spinal cord preparations from older rodents either do not produce rhythmic respiratory-related motor output or only produce motor output for a short period of time. Postnatal changes in the developing respiratory control network are the main reasons for this age-dependent decrease in motor output, rather than age, mass, or the presence of an intact pons (Fong et al., 2008). Thus, neonatal rodent brainstem-spinal cord preparations are viable during a limited period of time during early development.
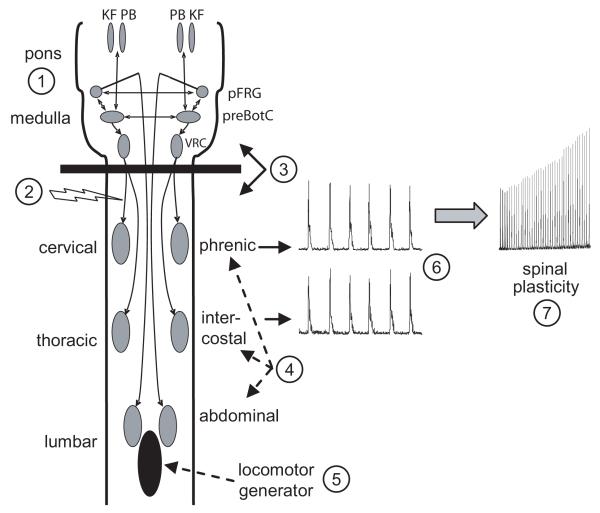
Despite these limitations, mammalian brainstem-spinal cord preparations still offer several experimental advantages. A key feature of these preparations (and other reduced in vitro preparations) is that spontaneous respiratory-related motor output can be recorded on nerves whose function is known. Respiratory motor output on cervical and thoracic spinal nerves pumps air in and out of the lungs, whereas respiratory motor output on cranial nerve roots (XII, X) control upper airway patency. Changes in cranial or spinal nerve respiratory motor output are physiologically interpretable and meaningful in terms of ventilation. Specific advantages of brainstem-spinal cord preparations are shown in Fig. 1 and discussed below in the following sections.
Fig. 1. Advantages of brainstem-spinal cord preparations.
A drawing of an isolated brainstem-spinal cord preparation illustrates several experimental advantages of this preparation. The circled numbers within the figure schematically highlight the advantages listed in the box below.
ADVANTAGES OF BRAINSTEM-SPINAL CORD PREPARATIONS
1. Central respiratory network in the pons and medulla is intact.
2. Monosynaptic descending bulbospinal connections can be electrically activated.
3. Brainstem and spinal cord can be bathed separately.
4. Phrenic, intercostal, and abdominal motoneuron physiology can be compared.
5. Interaction with other motor behaviors (locomotion) can be studied in vitro.
6. Fictive spinal respiratory motor output is correlated with ventilation.
7. Respiratory spinal plasticity can be studied in vitro.
2. Brainstem-spinal cord preparations reveal connectivity between respiratory-related neurons
One of the most important features of brainstem-spinal cord preparations is that a large portion of the central respiratory network is intact, which allows spatially separate pools of respiratory-related neurons to be revealed along with their connectivity. Interactions between pontine and medullary neurons can be studied in brainstem-spinal cord (and brainstem only) preparations in contrast to other less intact in vitro preparations, such as rhythmically active medullary slice (Smith et al., 1991), “tilted sagittal slab” (Mellen et al., 2003; Barnes et al., 2007), and medullary slice “island” preparations (Johnson et al., 2001).
The brainstem-spinal cord allows the timing of respiratory phases to be precisely determined, at least with respect to inspiration, because the inspiratory phase is defined by inspiratory-related cervical root discharge. Neuronal activity in the medulla can then be compared to cervical root discharge to classify neurons as inspiratory, expiratory, post-inspiratory, etc. For example, the pre-Bötzinger Complex (preBötC) and the para-facial respiratory group (pFRG)/retrotrapezoid nucleus (RTN) regions were first identified and characterized in newborn rodent brainstem-spinal cord preparations. The preBötC was discovered by sequentially sectioning the brainstem in a rostrocaudal direction using a newborn rat brainstem-spinal cord preparation (Smith et al., 1991). The preBötC is hypothesized to generate the inspiratory rhythm (Feldman and Del Negro, 2006) and an intact preBötC is necessary and sufficient for inspiratory rhythm generation (Gray et al., 1999, Wenninger et al., 2004; McKay et al., 2005).
Similarly, the pFRG was identified early on with its distinctive neurons that fire during the pre-inspiratory and post-inspiratory phases in relation to spinal nerve C4 inspiratory motor output in brainstem-spinal cord preparations (Onimaru and Homma, 1987). The pFRG wraps caudally and ventrally around the facial nucleus in the medulla and appears to overlap with RTN and the Bötzinger Complex, but its borders are relatively ill defined (Feldman and Del Negro, 2006). The pFRG is proposed to be the primary source of the respiratory rhythm (Onimaru et al., 1987; Onimaru 1995; Onimaru and Homma, 2006) because pFRG neurons fire prior to preBötC neurons as revealed in elegant imaging studies on brainstem-spinal cord preparations bathed in voltage-sensitive dyes (Onimaru and Homma, 2003). Using brainstem-spinal cord preparations was critical in identifying the importance of the pFRG/RTN and preBötC, but also in elucidating connectivity between these two regions (Mellen et al., 2003), which has led to a “dual oscillator” model of respiratory rhythm generation (Feldman and Del Negro, 2006; Janczewski and Feldman, 2006).
3. Spatial separation of accessible respiratory spinal motoneurons from brainstem rhythm-generating neurons is exploited experimentially
In brainstem-spinal cord preparations, spinal motoneurons that control pump muscles are spatially separated from rhythm-generating neurons in the brainstem, which confers several experimental advantages. A barrier placed at the spinomedullary border allows spinal respiratory neurons to be bathed with drugs or chemicals while the brainstem is bathed in normal solutions to maintain rhythmic motor activity. For example, neuromodulation of bulbospinal excitatory glutamatergic synaptic transmission can be studied with respect to pre- vs. postsynaptic sites of action. Bath application of an adenosine analog drug to the cervical spinal cord decreases inspiratory burst amplitude on cervical spinal roots without altering phrenic motoneuron input resistance or responsiveness to exogenous glutamate application (Dong et al., 1996). Instead, the adenosine analog drug decreases the frequency of miniature EPSPs, suggesting that adenosine acts presynaptically to reduce glutamate release (Dong et al., 1996). A similarly designed study shows that serotonin increases phrenic motoneuron excitability while reducing inspiratory synaptic currents most likely by a presynaptic mechanism (Lindsay and Feldman, 1993).
In addition, phrenic motoneurons in the cervical spinal cord are large and readily accessible, thereby permitting long-lasting whole-cell recordings that provide insights into the nature of monosynaptic glutamatergic excitatory synaptic transmission from brainstem premotor neurons (reviewed in Monteau and Hilaire, 1991). For example, exquisite low-noise whole-cell recordings of spontaneous excitatory postsynaptic potentials in phrenic motoneurons from neonatal rat brainstem-spinal cord preparations provided one of the first demonstrations of quantal synaptic transmission in the mammalian CNS (Liu and Feldman, 1992). Likewise, inspiratory currents recorded from phrenic motoneurons in neonatal rat brainstem-spinal cords were cleverly injected back into the phrenic motoneurons to evaluate the impact of high-frequency (>10 Hz) oscillations on action potential frequency (Parkis et al., 2003). The major finding was that high-frequency oscillatory inputs increase the efficiency with which motoneurons transduce synaptic inputs into action potentials.
Finally, respiratory spinal motor output provides a “window” into the rhythm-generating network located rostrally in the brainstem. For example, expiratory motor activity is produced by intercostal and abdominal spinal motoneurons in the caudal thoracic and lumbar spinal cord (reviewed in Iizuka, 2011). Since the pFRG is hypothesized to be a primary source of expiratory activity within the medullary dual oscillator (Pagliardini et al., 2011), studying spinal expiratory motor activity may provide insights into mechanisms that contribute to the expiratory phase. Neonatal rodent brainstem-spinal cord preparations are useful in this regard because spinal expiratory motor activity can be recorded on ventral thoracic spinal roots (Iizuka, 1999). For example, bath-applied bicuculline (GABAA receptor antagonist) causes expiratory-related spinal motor activity to overlap temporally with inspiratory motor activity, suggesting that tonic inhibition via GABAA receptors (and not glycine receptors) plays a role in regulating the timing of the expiratory phase (Iizuka, 2003). Thus, the separation of readily identified and accessible respiratory spinal motoneurons from rhythm-generating brainstem neurons is advantageous for a variety of experimental approaches addressing many different scientific questions.
4. Perinatal brainstem-spinal cord preparations reveal insights into early motor network development
The central respiratory control network must be functional at birth, but still undergoes substantial postnatal development in parallel with developing airway, lungs, and muscles (Chatonnet et al., 2002, Borday et al., 2003, Chatonnet et al., 2003). Brainstem-spinal cords isolated from prenatal rodents are ideal for studying early development of the respiratory control system because these preparations are: (1) large enough to be isolated from embryos with minimal mechanical damage; (2) smaller and contain less myelin compared to postnatal preparations, thereby minimizing the diffusion limitations of nutrients and gases to the center of the tissue; (3) viable throughout the prenatal period; and (4) spontaneously produce respiratory-related motor activity for hours.
For example, brainstem-spinal cord preparations show that phasic contractions of the diaphragm and abdomen (fetal breathing movements) begin at embryonic day 15 (E15) for the mouse (Thoby-Brisson et al., 2005) and E17 for the rat (Pagliardini et al., 2003, Huxtable et al., 2009). Respiratory neuron activity in the brainstem at these early stages is demonstrated by calcium imaging and electrophysiological recordings from brainstem-spinal cord and rhythmic medullary slice preparations (Di Pasquale et al., 1992, Greer et al., 1992, Abadie et al., 2000, Pagliardini et al., 2003, Thoby-Brisson et al., 2005). The primary source of the underlying rhythm appears to be correlated with the onset of activity in the preBötC (reviewed in Thoby-Brisson and Greer, 2008). These fetal breathing movements are postulated to facilitate lung maturation and growth, and influence the development of respiratory motoneurons and muscles. Thus, prenatal brainstem-spinal cord preparations provide novel information into how the respiratory motor network develops prior to birth.
5. Brainstem-spinal cord preparations can be isolated from animals with genetic mutations or prenatal exposures to toxins
Since brainstem-spinal cords can be isolated prenatally or immediately after birth, studies can be performed on mutant animals that otherwise wouldn’t survive following birth (e.g., lethal knockouts). Demonstrating that a brainstem-spinal cord preparation from a mutant animal produces a normal respiratory motor output argues against respiratory failure as the cause of death. For example, mutant mice lacking the NMDAR1 gene die within 24-48 h after birth (Forrest et al., 1994), but the respiratory rhythm in P0 brainstem-spinal cord and rhythmic slice preparations is identical to the respiratory rhythm in control wild-type mouse preparations, suggesting that the lethality of the NMDAR1 knockout is not due to disruption of respiratory rhythm generation (Funk et al., 1997).
In addition, respiratory motor output can be analyzed and compared in brainstem-spinal cord preparations from mutant versus wild type rodents to gain insights as to the potential functions of specific genes. For example, murine brainstem-spinal cord preparations from Phox2a mutants have poorly developed adrenergic A6 regions and increased respiratory cycle variability compared to control mice (Morin et al., 1997; Viemari et al., 2004), thereby linking the loss of adrenergic modulation with irregular breathing. Respiratory disturbances (e.g., apneas and irregular breathing) can be characterized in brainstem-spinal cord preparations from Prader-Willi mice (Ren et al., 2003; Pagliardini et al., 2005; Zanella et al., 2008), or mice deficient in serotonin (Hodges et al., 2009) or deficient in the expression of Lbx-1 (Pagliardini et al., 2008), tachykinin-1 receptors (Berner et al., 2007), and monoamine oxidase (Bou-Flores et al., 2000; Bou-Flores and Hilaire, 2000).
Finally, pregnant rodents can be exposed to toxins and viable brainstem-spinal cords can be isolated and tested for long-lasting changes or damage to the respiratory control system. Infants born to mothers that smoke while pregnant have abnormal breathing patterns (reduced tidal volume and increased respiratory rate), higher frequency of apnea, blunted responses to hypoxia, and decreased ability to autoresuscitate during severe hypoxia (reviewed in Hafström et al., 2005). Isolated brainstem spinal-cord preparations allow investigators to detect similar changes in respiratory function and explore underlying mechanisms in rodents. For example, prenatal nicotine exposure causes neonatal rat brainstem-spinal cord preparations to be less responsive to nicotine (Pilarski and Fregosi, 2009), and more responsive to GABAA and glycine agonist drugs (Luo et al., 2004, 2007) and AMPA (Pilarski and Fregosi, 2009). Similarly, prenatal nicotine exposure causes neonatal murine brainstem-spinal cord preparations to produce slower, more irregular respiratory-related motor bursts, and the bath acidification response switched from being muscarinic to a nicotinic-based mechanism (Eugenin et al., 2008; Coddou et al., 2009). Thus, brainstem-spinal cord preparations provide viable tissue from newborn animals that may not survive long following birth due to respiratory-related disturbances.
6. Brainstem-spinal cord preparations permit rapid screening of drugs and pathological conditions
The neonatal rodent brainstem-spinal cord preparation is relatively easily to isolate and use for experiments compared to other relatively intact in situ preparations, such as the working heart-brainstem preparation (Paton, 1996), and perfused rat and guinea pig semi-intact preparations (Piantadosi et al., 1985; Richerson and Getting, 1987; Hayashi et al., 1991; Morin-Surun et al., 1992). Thus, neonatal rodent brainstem-spinal cord preparations provide the opportunity to rapidly screen for the effects of drugs of abuse, anesthetics, or pathological conditions on a spontaneously active, vertebrate rhythmic motor network. Results from these studies may have clinical relevance and lead to new insights with respect to underlying mechanisms.
For example, ethanol application to neonatal rat brainstem-spinal cords has no effect on phrenic motoneuron activity but significantly reduces hypoglossal activity, suggesting that pathways to upper airway motoneurons are selectively disrupted (Di Pasquale et al., 1995). In contrast, barbiturates, such as sodium pentobarbital, decrease respiratory burst frequency, suggesting that rhythm-generating neurons are altered (Tarasiuk et al., 1991; Fregosi et al., 2004). Brainstem-spinal cord preparations are also used to examine the effects of different anesthetic drugs on brainstem respiratory neuron function, such as propofol (Kashiwagi et al., 2004), enflurane, halothane, and isoflurane (Otsuka 1998), and sevoflurane (Takita and Morimoto, 2010). The results with sevoflurane are particularly interesting because sevoflurane (at a specific concentration) inhibits pre-I neurons within the pFRG with minimal effects on other brainstem respiratory neurons, which may permit testing whether pre-I neurons are necessary for rhythm generation (Takita and Morimoto, 2010). Each drug appears to alter specific components of the brainstem respiratory control network and does not simply cause a global depression within the entire network. Finally, experiments using brainstem-spinal cord preparations and rhythmically active medullary slice preparations show that ampakine drugs reverse opioid-induced respiratory depression without altering antinociception (Ren et al., 2006).
Pathological conditions, such as central hypothermia, can be studied under in vitro conditions using brainstem-spinal cord preparations because the temperature of the tissue can be controlled without confounding compensatory responses to counteract hypothermia. For example, the mechanisms underlying the abrupt decrease in respiratory motor output with hypothermia and subsequent autoresuscitation upon rewarming are poorly understood. Brainstem-spinal cord preparations from neonatal rats show that hypothermia-induced respiratory arrest is due to reversible failure in the network and not to irreversible neuronal damage in respiratory neurons (Mellen et al., 2002; Zimmer and Milsom, 2004). The ability to resuscitate upon rewarming is riluzole-dependent in hamster, but not rat, brainstem-spinal cord preparations (Fong et al, 2009), which reveals important species differences and the value of a comparative experimental approach with respect to hypothermia. Thus, the low cost and ease of setting up rodent brainstem-spinal cord preparations makes these preparations attractive for rapidly testing the effects of drugs, toxins, or different conditions on respiratory motor control.
7. Brainstem-spinal cord preparations permit in vitro studies on respiratory motor plasticity
Respiratory plasticity is defined as an alteration in future system performance based on experience (Mitchell and Johnson 2003). Brainstem-spinal cord preparations are used to study various forms of respiratory spinal plasticity, such as activity-dependent plasticity, pattern-sensitive serotonin-dependent plasticity, and changes in the brainstem during recovery from acute spinal cord injury. In these studies, brainstem-spinal cord preparations take advantage of the spatial separation of the brainstem from the respiratory spinal motoneurons, the ability to stimulate primarily monosynaptic bulbospinal synaptic inputs to respiratory spinal motoneurons, the ability to record from different pools of respiratory spinal motoneurons that serve different functions, and the ability to control the timing of drug application to the spinal cord.
For example, activity-dependent spinal plasticity is the alteration in synaptic efficacy due to previous activity at that synapse. Short-term (0-15 min) activity-dependent changes occur in bulbospinal synaptic inputs to respiratory spinal motoneurons (reviewed in Johnson and Mitchell, 2002), but long-term (>1 h) changes are also observed in adult turtle brainstem-spinal cord preparations (Johnson and Mitchell, 2000). In the turtle brainstem-spinal cord preparation, it is possible to record from separate expiratory and inspiratory nerves and there is sufficient spinal cord length to allow reversible inactivation of spontaneous respiratory-related bulbospinal synaptic inputs (Johnson and Mitchell, 2000). These experiments show that descending synaptic inputs to spinal motoneurons following high or low frequency stimulation are biased towards potentiation in inspiratory motoneurons, but depression in expiratory motoneurons, suggesting the importance of preserving inspiration for survival (Johnson and Mitchell 2000, 2002).
With respect to serotonin-dependent spinal plasticity, long-lasting augmentation of cervical and thoracic spinal motor output is induced by three spinal serotonin applications (Lovett-Barr et al., 2006). This demonstrated that episodic spinal serotonin receptor activation in vitro was sufficient to increase spinal respiratory motor output in a manner similar to intermittent hypoxia-induced phrenic long-term facilitation (Fuller et al., 2000; Mitchell et al., 2001). Surprisingly, sustained spinal serotonin application for 9 min induced a long-lasting increase in thoracic, but not cervical, spinal respiratory motor output (Lovett-Barr et al., 2006), demonstrating that serotonin-dependent plasticity in the cervical spinal cord is pattern-dependent whereas thoracic plasticity is pattern-independent.
Finally, after two days of recovery from a cervical C2 spinal cord lesion, brainstem-spinal cord preparations from neonatal rats respond differently to changes in bath pH compared to preparations from sham-operated and control neonatal rats (Zimmer et al., 2007). When bath pH is increased, cervical spinal respiratory burst duration and area increases in sham control preparations but decreases in preparations from spinally injured rats. Furthermore, there are significant changes in critical proteins related to respiratory motor control (e.g., glutamate receptor subunits, adenosine receptors, and neurokinin-1 receptors) in the medulla rostral to the spinal cord injury (Zimmer et al., 2007). Thus, neonatal rat brainstem-spinal cord preparations provide a novel experimental approach for studying changes in respiratory function following spinal cord injury in neonatal animals.
8. Interactions between respiration and other rhythmic motor behaviors
Respiratory motor activity is coordinated with other rhythmic motor activities, such as locomotion or swallowing. Recent comparisons between respiratory and locomotor networks are intriguing because critical interneurons within their central pattern generators appear to share similar genetic phenotypes and possibly similar functions (reviewed in Grossman et al., 2010; see also Gray 2008, Gray et al., 2010; Champagnat et al., 2011). In addition, insights can be gained into neuronal mechanisms that coordinate rhythmic motor behaviors because isolated neonatal rodent brainstem-spinal cord preparations contain other motor central pattern generators. Mammalian locomotor-respiratory coupling is hypothesized to prevent a lack of oxygen during exercise but the mechanisms underlying the coupling are poorly understood (Daffertshofer et al., 2004). Brainstem-spinal cord preparations from neonatal rodents show that respiratory activity can be reset and entrained by stimulating ascending lumbar or cervical propriospinal pathways that originate from the hindlimb or forelimb muscles (Morin and Viala, 2002).
In the brainstem, respiration and swallowing are highly coordinated because disruption of the interaction between respiratory and swallowing central pattern generators can cause lethal aspiration pneumonia. In neonatal rodent brainstem-spinal cord preparations, electrical stimulation of the superior laryngeal nerve activates the swallowing central pattern generator and increases the expiration phase duration (Yamanishi et al, 2010). The swallowing-induced inhibition of the respiratory rhythm generator is blocked by an alpha-2 adrenergic agonist and enhanced by and alpha-2 adrenergic antagonist, thereby revealing a key role of alpha-2 adrenergic receptors in the interaction between swallowing and respiratory motor networks (Yamanishi et al., 2010). Thus, brainstem-spinal cord preparations are valuable tools to begin unraveling complex neuronal interactions between different rhythmic motor networks.
9. Brainstem-spinal cords from other non-mammalian vertebrates – a comparative perspective
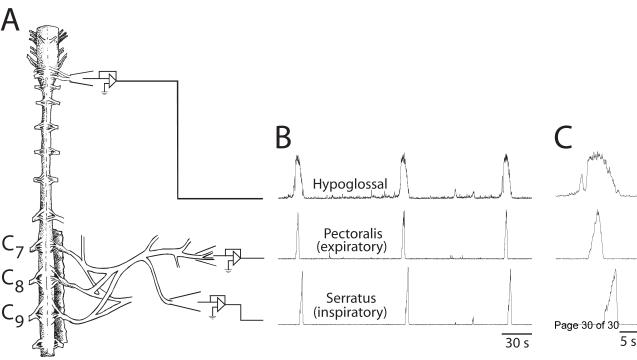
Brainstem-spinal cords from reptiles, such as the red-eared slider turtle, can be used to address similar questions in respiratory motor control. However, turtle brainstem-spinal cord preparations have unique advantages over neonatal rodent preparations. First, turtles are extremely hypoxia-resistant, which means that isolated brainstems (and presumably brainstem-spinal cords) from fully mature adult turtles can produce respiratory motor output under in vitro conditions for days (Wilkerson et al., 2003). Second, adult turtle brainstem-spinal cords can produce active expiratory and inspiratory activity on their appropriate spinal nerves that is similar to intact turtles (Johnson and Mitchell, 1998) at a physiological temperature (i.e., room temperature) and normal bath [K+] (Fig. 2; Johnson et al., 1998). Third, turtle brainstems appear to produce all three phases of breathing: inspiratory, expiratory, and post-inspiratory (post-inspiratory activity = unpublished observations in S.M. Johnson laboratory). To our knowledge, no other isolated adult vertebrate brainstem-spinal cord preparation has these advantages. Finally, studying mechanisms underlying respiratory motor control in turtles may provide insights into which mechanisms are evolutionarily conserved compared to amphibians and mammals.
Fig. 2. Expiratory and inspiratory spinal motor activity produced by an adult vertebrate brainstem-spinal cord preparation.
(A) A drawing of an isolated brainstem-spinal cord preparation from an adult turtle shows that respiratory-related motor activity is produced on pectoralis (expiratory) and serratus (inspiratory) nerves. (B) Rhythmic expiratory and inspiratory motor activity on nerves is correlated with respiratory activity on hypoglossal nerve roots in the brainstem. (C) Expiratory activity on pectoralis is typically bell-shaped while inspiratory activity on serratus is slowly-incrementing and rapidly decrementing. The resistance of turtle brain and spinal cord to hypoxia allows this preparation to produce spinal respiratory activity similar to intact adult turtles (discussed in Johnson et al., 1998). In contrast, the nature of respiratory-related motor output produced by neonatal rodent brainstem-spinal cord preparations is controversial. Abbreviations: KF = Kölliker-Fuse nucleus; PB = parabrachial nucleus; pFRG = para-facial group; preBötC = pre-Bötzinger Complex; VRC = ventral respiratory column.
10. Summary and conclusions
It is said that “no preparation is perfect”, which means that every experimental preparation has its limitations and shortcomings. The choice of which experimental preparation to use depends, of course, on the question to be addressed. The in vitro isolated vertebrate brainstem-spinal cord preparation has had its share of legitimate criticism over the years. Nevertheless, this preparation holds a prominent place in the history of respiratory neurobiology research, and still has a lot to offer scientifically and experimentally to address questions within and outside the field of respiratory neurobiology.
Acknowledgements
This work was supported by the following NIH grants: HL07654 (S.M.F. Turner), HL69064 (A.G. Huxtable, F. Ben-Mabrouk), and HL80209 (A.G. Huxtable).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
These preparations have several experimental advantages despite their limitations.
This review describes these advantages and highlights examples in the literature.
References
- Abadie V, Champagnat J, Fortin G. Branchiomotor activities in mouse embryo. Neuroreport. 2000;11:141–145. doi: 10.1097/00001756-200001170-00028. [DOI] [PubMed] [Google Scholar]
- Barnes BJ, Tuong CM, Mellen NM. Functional imaging reveals respiratory network activity during hypoxic and opioid challenge in the neonate rat tilted sagittal slab preparation. J Neurophysiol. 2007;97:2283–2292. doi: 10.1152/jn.01056.2006. [DOI] [PubMed] [Google Scholar]
- Berner J, Shvarev Y, Lagercrantz H, Bilkei-Gorzo A, Hökfelt T, Wickström R. Altered respiratory pattern and hypoxic response in transgenic newborn mice lacking the tachykinin-1 gene. J Appl Physiol. 2007;103:552–559. doi: 10.1152/japplphysiol.01389.2006. [DOI] [PubMed] [Google Scholar]
- Borday C, Abadie V, Chatonnet F, Thoby-Brisson M, Champagnat J, Fortin G. Developmental molecular switches regulating breathing patterns in CNS. Respir Physiol Neurobiol. 2003;135:121–132. doi: 10.1016/s1569-9048(03)00031-4. [DOI] [PubMed] [Google Scholar]
- Bou-Flores C, Lajard AM, Monteau R, De Maeyer E, Seif I, Lanoir J, Hilaire G. Abnormal phrenic motoneuron activity and morphology in neonatal monoamine oxidase A-deficient transgenic mice: possible role of a serotonin excess. J Neurosci. 2000;12:4646–4656. doi: 10.1523/JNEUROSCI.20-12-04646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou-Flores C, Hilaire G. 5-Hydroxytryptamine(2A) and 5-hydroxytryptamine(1B) receptors are differently affected by the monoamine oxidase A-deficiency in the Tg8 transgenic mouse. Neurosci Lett. 2000;296:141–144. doi: 10.1016/s0304-3940(00)01653-0. [DOI] [PubMed] [Google Scholar]
- Brockhaus J, Ballanyi K, Smith JC, Richter DW. Microenvironment of respiratory neurons in the in vitro brainstem-spinal cord of neonatal rats. J. Physiol. 1993;462:421–445. doi: 10.1113/jphysiol.1993.sp019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Morin-Surun MP, Bouvier J, Thoby-Brisson M, Fortin G. Prenatal development of central rhythm generation. Respir. Physiol. Neurobiol. 2011;178:146–155. doi: 10.1016/j.resp.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Domínguez del Toro E, Thoby-Brisson M, Champagnat J, Fortin G, Rijli FM, Thaëron-Antôno C. From hindbrain segmentation to breathing after birth: developmental patterning in rhombomeres 3 and 4. Mol Neurobiol. 2003;28:277–294. doi: 10.1385/mn:28:3:277. [DOI] [PubMed] [Google Scholar]
- Chatonnet F, Thoby-Brisson M, Abadie V, Domínguez del Toro E, Champagnat J, Fortin G. Early development of respiratory rhythm generation in mouse and chick. Respir. Physiol. Neurobiol. 2002;131:5–13. doi: 10.1016/s1569-9048(02)00033-2. [DOI] [PubMed] [Google Scholar]
- Coddou C, Bravo E, Eugenín J. Alterations in cholinergic sensitivity of respiratory neurons induced by pre-natal nicotine: a mechanism for respiratory dysfunction in neonatal mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:2527–2535. doi: 10.1098/rstb.2009.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffertshofer A, Huys R, Beek PJ. Dynamical coupling between locomotion and respiration. Biol. Cybern. 2004;90:157–164. doi: 10.1007/s00422-004-0462-x. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G, Iscoe S. Effects of ethanol on respiratory activity in the neonatal rat brainstem-spinal cord preparation. Brain Res. 1995;695:271–274. doi: 10.1016/0006-8993(95)00903-4. [DOI] [PubMed] [Google Scholar]
- Dong XW, Morin D, Feldman JL. Multiple actions of 1S,3R-ACPD in modulating endogenous synaptic transmission to spinal respiratory motoneurons. J. Neurosci. 1996;16:4971–4982. doi: 10.1523/JNEUROSCI.16-16-04971.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J. A commentary on eupnoea and gasping. Respir. Physiol. Neurobiol. 2003;139:105–111. doi: 10.1016/s1569-9048(03)00194-0. [DOI] [PubMed] [Google Scholar]
- Eugenín J, Otárola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R. Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J. Neurosci. 2008;28:13907–13917. doi: 10.1523/JNEUROSCI.4441-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat. Rev. Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Marshall LH, Milsom WK. Riluzole disrupts autoresuscitation from hypothermic respiratory arrest in neonatal hamsters but not rats. Respir Physiol Neurobiol. 2009;166:175–183. doi: 10.1016/j.resp.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Fong AY, Corcoran AE, Zimmer MB, Andrade DV, Milsom WK. Respiratory rhythm of brainstem-spinal cord preparations: Effects of maturation, age, mass and oxygenation. Respir. Physiol. Neurobiol. 2008;164:429–440. doi: 10.1016/j.resp.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Forrest D, Yuzaki M, Soares HD, Ng L, Luk D, Sheng M, Stewart CL, Morgon JI, Connor JA, Curran T. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Luo Z, Iizuka M. GABAA receptors mediate postnatal depression of respiratory frequency by barbiturates. Respir. Physiol. Neurobiol. 2004;140:219–230. doi: 10.1016/j.resp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir. Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Funk GD, Johnson SM, Smith JC, Dong XW, Lai J, Feldman JL. Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J. Neurophysiol. 1997;78:1414–1420. doi: 10.1152/jn.1997.78.3.1414. [DOI] [PubMed] [Google Scholar]
- Gray PA. Transcription factors and the genetic organization of brain stem respiratory neurons. J. Appl. Physiol. 2008;104:1513–1521. doi: 10.1152/japplphysiol.01383.2007. [DOI] [PubMed] [Google Scholar]
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenín J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J. Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Respiratory and locomotor patterns generated in the fetal rat brain stem-spinal cord in vitro. J Neurophysiol. 1992;67:996–999. doi: 10.1152/jn.1992.67.4.996. [DOI] [PubMed] [Google Scholar]
- Grossmann KS, Giraudin A, Britz O, Zhang J, Goulding M. Genetic dissection of rhythmic motor networks in mice. Prog Brain Res. 2010;187:19–37. doi: 10.1016/B978-0-444-53613-6.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafström O, Milerad J, Sandberg KL, Sundell HW. Cardiorespiratory effects of nicotine exposure during development. Respir. Physiol. Neurobiol. 2005;149:325–341. doi: 10.1016/j.resp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Jiang C, Lipski J. Intracellular recording from respiratory neurones in the perfused ‘in situ’ rat brain. J. Neurosci. Methods. 1991;36:63–70. doi: 10.1016/0165-0270(91)90138-p. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J. Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, Funk GD. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci. 2009;29:14713–14725. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. Intercostal expiratory activity in an in vitro brainstem-spinal cord-rib preparation from the neonatal rat. J Physiol. 1999;520:293–302. doi: 10.1111/j.1469-7793.1999.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. GABAA and glycine receptors in regulation of intercostal and abdominal expiratory activity in vitro in neonatal rat. Physiol. 2003;551:617–633. doi: 10.1113/jphysiol.2003.042689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka M. Respiration-related control of abdominal motoneurons. Respir. Physiol. Neurobiol. 2011 doi: 10.1016/j.resp.2011.01.003. in press. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Feldman JL. Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol. 2006;570:407–420. doi: 10.1113/jphysiol.2005.098848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. N-methyl-D-aspartate-mediated bulbospinal respiratory drive is pH/P(CO2)-insensitive in turtle brainstem-spinal cord. Respir. Physiol. 1998;113:201–212. doi: 10.1016/s0034-5687(98)00079-6. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity of descending synaptic inputs to spinal motoneurons in an in vitro turtle brainstem-spinal cord preparation. J. Neurosci. 2000;20:3487–3495. doi: 10.1523/JNEUROSCI.20-09-03487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Bötzinger complex “island”. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Mitchell GS. Activity-dependent plasticity in descending synaptic inputs to respiratory spinal motoneurons. Respir. Physiol. Neurobiol. 2002;131:79–90. doi: 10.1016/s1569-9048(02)00039-3. [DOI] [PubMed] [Google Scholar]
- Kashiwagi M, Okada Y, Kuwana S, Sakuraba S, Ochiai R, Takeda J. A neuronal mechanism of propofol-induced central respiratory depression in newborn rats. Anesth. Analg. 2004;99:49–55. doi: 10.1213/01.ANE.0000117226.45704.65. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J. Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Feldman JL. Quantal synaptic transmission in phrenic motor nucleus. J. Neurophysiol. 1992;68:1468–1471. doi: 10.1152/jn.1992.68.4.1468. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr MR, Mitchell GS, Satriotomo I, Johnson SM. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142:885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABA(A) receptor-mediated inhibition of respiratory rhythm in neonatal rats. J. Physiol. 2004;561:387–393. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, McMullen NT, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure alters glycinergic and GABAergic control of respiratory frequency in the neonatal rat brainstem-spinal cord preparation. Respir. Physiol. Neurobiol. 2007;157:226–234. doi: 10.1016/j.resp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Janczewski WA, Bocchiaro CM, Feldman JL. Opioid-induced quantal slowing reveals dual networks for respiratory rhythm generation. Neuron. 2003;37:821–826. doi: 10.1016/s0896-6273(03)00092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellen NM, Milsom WK, Feldman JL. Hypothermia and recovery from respiratory arrest in a neonatal rat in vitro brain stem preparation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R484–R491. doi: 10.1152/ajpregu.00049.2001. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr. Invited review: Intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Monteau R, Hilaire G. Spinal respiratory motoneurons. Prog. Neurobiol. 1991;37:83–144. doi: 10.1016/0301-0082(91)90024-u. [DOI] [PubMed] [Google Scholar]
- Morin D, Viala D. Coordinations of locomotor and respiratory rhythms in vitro are critically dependent on hindlimb sensory inputs. J Neurosci. 2002;22:4756–4765. doi: 10.1523/JNEUROSCI.22-11-04756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X, Cremer H, Hirsch MR, Kapur RP, Goridis C, Brunet JF. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron. 1997;18:411–423. doi: 10.1016/s0896-6273(00)81242-8. [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Boudinot E, Sarraseca H, Fortin G, Denavit-Saubié M. Respiratory network remains functional in a mature guinea pig brainstem isolated in vitro. Exp. Brain Res. 1992;90:375–383. doi: 10.1007/BF00227251. [DOI] [PubMed] [Google Scholar]
- Okada Y, Mückenhoff K, Holtermann G, Acker H, Scheid P. Depth profiles of pH and PO2 in the isolated brain stem-spinal cord of the neonatal rat. Respir. Physiol. 1993;93:315–326. doi: 10.1016/0034-5687(93)90077-n. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Respiratory rhythm generator neurons in medulla of brainstem-spinal cord preparation from newborn rat. Brain Res. 1987;403:380–384. doi: 10.1016/0006-8993(87)90080-1. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Localization of respiratory rhythm-generating neurons in the medulla of brainstem-spinal cord preparations from newborn rats. Neurosci Lett. 1987;78:151–155. doi: 10.1016/0304-3940(87)90624-0. [DOI] [PubMed] [Google Scholar]
- Onimaru H. Studies of the respiratory center using isolated brainstem-spinal cord preparations. Neurosci. Res. 1995;21:183–190. doi: 10.1016/0168-0102(94)00863-b. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Homma I. Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Point: the PFRG is the primary site of respiratory rhythm generation in the mammal. J. Appl. Physiol. 2006;100:2094–2095. doi: 10.1152/japplphysiol.00119.2006. [DOI] [PubMed] [Google Scholar]
- Otsuka H. Effects of volatile anesthetics on respiratory activity and chemosensitivity in the isolated brainstem-spinal cord of the newborn rat. Hokkaido Igaku Zasshi. 1998;73:117–136. [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Gray PA, Vandunk C, Gross M, Goulding M, Greer JJ. Central respiratory rhythmogenesis is abnormal in lbx1-deficient mice. J Neurosci. 2008;28:11030–11041. doi: 10.1523/JNEUROSCI.1648-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Wevrick R, Greer JJ. Developmental abnormalities of neuronal structure and function in prenatal mice lacking the prader-willi syndrome gene necdin. Am J Pathol. 2005;167:175–191. doi: 10.1016/S0002-9440(10)62964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini S, Ren J, Greer JJ. Ontogeny of the pre-Bötzinger complex in perinatal rats. J Neurosci. 2003;23:9575–95784. doi: 10.1523/JNEUROSCI.23-29-09575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkis MA, Feldman JL, Robinson DM, Funk GD. Oscillations in endogenous inputs to neurons affect excitability and signal processing. J. Neurosci. 2003;23:8152–8158. doi: 10.1523/JNEUROSCI.23-22-08152.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Sylvia AL, Saltzman HA, Jöbsis-Vandervliet FF. Carbon monoxide-cytochrome interactions in the brain of the fluorocarbon-perfused rat. J. Appl. Physiol. 1985;58:665–672. doi: 10.1152/jappl.1985.58.2.665. [DOI] [PubMed] [Google Scholar]
- Pilarski JQ, Fregosi RF. Prenatal nicotine exposure alters medullary nicotinic and AMPA-mediated control of respiratory frequency in vitro. Respir. Physiol. Neurobiol. 2009;169:1–10. doi: 10.1016/j.resp.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Lieske SP. Commentary on the definition of eupnea and gasping. Respir Physiol Neurobiol. 2003;139:113–119. doi: 10.1016/s1569-9048(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am. J. Respir. Crit. Care Med. 2006;174:1384–1391. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- Ren J, Lee S, Pagliardini S, Gérard M, Stewart CL, Greer JJ, Wevrick R. Absence of Ndn, encoding the Prader-Willi syndrome-deleted gene necdin, results in congenital deficiency of central respiratory drive in neonatal mice. J Neurosci. 2003;23:1569–1573. doi: 10.1523/JNEUROSCI.23-05-01569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Getting PA. Maintenance of complex neural function during perfusion of the mammalian brain. Brain Res. 1987;409:128–132. doi: 10.1016/0006-8993(87)90747-5. [DOI] [PubMed] [Google Scholar]
- Richter DW. Commentary on eupneic breathing patterns and gasping. Respir. Physiol. Neurobiol. 2003;139:121–130. doi: 10.1016/s1569-9048(03)00196-4. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? J. Appl. Physiol. 1996;81:1865–1877. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- St. John WM, Paton JF. Defining eupnea. Respir. Physiol. Neurobiol. 2003;139:97–103. doi: 10.1016/s1569-9048(03)00193-9. [DOI] [PubMed] [Google Scholar]
- Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J. Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takita K, Morimoto Y. Effects of sevoflurane on respiratory rhythm oscillators in the medulla oblongata. Respir. Physiol. Neurobiol. 2010;173:86–94. doi: 10.1016/j.resp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Tarasiuk A, Grossman Y, Kendig JJ. Barbiturate alteration of respiratory rhythm and drive in isolated brainstem-spinal cord of newborn rat: studies at normal and hyperbaric pressure. Br. J. Anaesth. 1991;66:88–96. doi: 10.1093/bja/66.1.88. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Greer JJ. Anatomical and functional development of the pre-Bötzinger complex in prenatal rodents. J Appl Physiol. 2008;104:1213–1219. doi: 10.1152/japplphysiol.01061.2007. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Trinh JB, Champagnat J, Fortin G. Emergence of the pre-Bötzinger respiratory rhythm generator in the mouse embryo. J Neurosci. 2005;25:4307–4318. doi: 10.1523/JNEUROSCI.0551-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Bevengut M, Burnet H, Coulon P, Pequignot JM, Tiveron MC, Hilaire G. Phox2a gene, A6 neurons, and noradrenaline are essential for development of normal respiratory rhythm in mice. J. Neurosci. 2004;24:928–937. doi: 10.1523/JNEUROSCI.3065-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Fung ML, Darnall RA, St John WM. Characterizations and comparisons of eupnoea and gasping in neonatal rats. J. Physiol. 1996;490:277–292. doi: 10.1113/jphysiol.1996.sp021143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol. 2004;97:1629–1636. doi: 10.1152/japplphysiol.00953.2003. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Wenninger MR, Mitchell GS, Johnson SM. Time-dependent changes in spontaneous respiratory activity in turtle brainstems in vitro. Respir Physiol Neurobiol. 2003;138:253–263. doi: 10.1016/j.resp.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Takao K, Koizumi H, Ishihama K, Nohara K, Komaki M, Enomoto A, Yokota Y, Kogo M. Alpha2-adrenoceptors coordinate swallowing and respiration. J Dent Res. 2010;89:258–263. doi: 10.1177/0022034509360312. [DOI] [PubMed] [Google Scholar]
- Zanella S, Watrin F, Mebarek S, Marly F, Roussel M, Gire C, Diene G, Tauber M, Muscatelli F, Hilaire G. Necdin plays a role in the serotonergic modulation of the mouse respiratory network: implication for Prader-Willi syndrome. J. Neurosci. 2008;28:1745–1755. doi: 10.1523/JNEUROSCI.4334-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spinal cord injury in neonates alters respiratory motor output via supraspinal mechanisms. Exp. Neurol. 2007;206:137–145. doi: 10.1016/j.expneurol.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Milsom WK. Effect of hypothermia on respiratory rhythm generation in hamster brainstem-spinal cord preparations. Respir Physiol Neurobiol. 2004;142:237–249. doi: 10.1016/j.resp.2004.06.005. [DOI] [PubMed] [Google Scholar]