Abstract
Monoclonal antibody (mAb) therapy was first established upon the approval of a mouse antibody for treatment of human acute organ rejection. However, the high incidence of immune response against the mouse mAb restricted therapeutic utility. Development of chimeric, “humanized” and human mAbs broadened therapeutic application to immune-mediated diseases requiring long-term treatment. Indeed, mAb therapeutics targeting soluble cytokines are highly effective in numerous immune-mediated disorders. A recent example is ustekinumab, a first-in-class therapeutic human immunoglobulin (Ig) G1 kappa mAb that binds to the interleukins (IL)-12 and IL-23, cytokines that modulate lymphocyte function, including T-helper (Th) 1 and Th17 cell subsets. Ustekinumab was generated via recombinant human IL-12 immunization of human Ig (hu-Ig) transgenic mice. Ustekinumab binds to the p40 subunit common to IL-12 and IL-23 and prevents their interaction with the IL-12 receptor β1 subunit of the IL-12 and IL-23 receptor complexes. Ustekinumab is approved for treatment of moderate-to-severe plaque psoriasis and has demonstrated efficacy in Crohn disease and psoriatic arthritis. The clinical characterization of ustekinumab continues to refine our understanding of human immune pathologies and may offer a novel therapeutic option for certain immune-mediated diseases.
Key words: ustekinumab, psoriasis, monoclonal antibody, interleukin-12/23p40
Monoclonal Antibody Therapies for Immune-Mediated Disorders
The concept of antibodies as therapeutic agents was initially described by Paul Ehrlich, where he reasoned that if a compound could be designed to selectively target a disease-causing organism, then a toxin for that organism could be delivered along with the agent of selectivity.1 Functional and structural characterization of antibodies culminated in several precedent discoveries on the generation and maturation of the humoral immune response.2 The key scientific breakthrough that advanced the evaluation of antibodies as therapeutic modalities was the development of hybridoma technology, which afforded the ability to reliably produce sufficient quantities of “monospecific” or identical antibody moieties, i.e., monoclonal antibodies (mAbs).
The first successful clinical development of a mAb therapeutic agent was a fully mouse anti-CD3 immunoglobulin (Ig) G (muromononab-CD3) for treatment of acute organ rejection.3 However, frequent and significant immune-mediated toxicities were associated with administration of fully mouse mAbs, particularly upon repeated administration. Advancements in genetic engineering resulted in the development of chimeric, humanized and fully human therapeutic mAbs. The reduction or elimination of non-human amino acid sequences resulted in a significant decrease in immune-mediated associated toxicities, which in turn, broadened the potential therapeutic applications.4 Indeed, therapeutic mAbs have become an increasingly important component of pharmacotherapy. It is estimated that more than 300 mAbs are currently in development and, approximately 30 mAbs are approved by the United States Food and Drug Administration under Biologic License Applications.5 The majority of approved and experimental mAbs are for oncologic indications, but indications also include chronic immune-mediated, respiratory, metabolic and central nervous system (CNS) disorders.
Therapeutic mAbs targeting soluble cytokines or lymphocyte cell surface molecules have demonstrated efficacy in treating oncologic, as well as immune-mediated disorders. One mechanism of mAbs targeting cell surface receptors is depletion of a cell subtype or subtypes. Such an example is rituximab, a mouse/ human IgG1 chimeric mAb that binds to the cluster of differentiation (CD)20 antigen present on certain B lymphocytes.6 CD20 cell surface binding can lead to cell lysis via complement-dependent cytotoxicity (CDC) or antibody-dependent cellular cytotoxicity (ADCC). Rituximab is currently approved for both oncologic (i.e., non-Hodgkin's lymphoma and chronic lymphocytic leukemia) and immune-mediated disorders (i.e., rheumatoid arthritis (RA), and Wegener's granulomatosis). Alternately, mAbs or Fc-fusion proteins targeting cell surface receptors can function through blockade of ligand-mediated receptor signaling. For example, abatacept is an Fc-fusion protein of the extracellular domain of human cytotoxic T lymphocyte-associated antigen (CTLA)-4.7 Abatacept binds to the CD80/CD86 receptor on T cells and blocks the interaction of CD80/CD86 with CD28, a costimulatory signal required for full activation of T lymphocytes. The mechanistic properties of abatacept may include inhibition of tumor necrosis factor alpha (TNFα), and interferon gamma (IFNγ) production by activated T cells. Abatacept is currently approved for the treatment of adult RA and juvenile idiopathic arthritis.
Currently, one of the largest classes of therapeutic mAbs and Fc-fusion proteins are those that bind and neutralize TNFα, a pro-inflammatory cytokine primarily produced by macrophages. TNFα induces the expression of innate cytokines interleukin (IL)-1β, IL-6 and IL-8, resulting in the rapid recruitment of neutrophils upon exposure to infection.8 A putative mechanistic action of TNFα in immune-mediated disorders is inhibition of matrix metalloproteinase-producing neutrophils in the synovial fluid of affected joints. In addition, clinical response of TNF antagonism in RA is associated with the downregulation of peripheral blood genes associated with acute phase reactant proteins.9 Compounds in this group include a chimeric IgG1 mAb (infliximab), human IgG1 mAbs (golimumab and adalimumab), a pegylated Fab' fragment of humanized mAb (certolizumab), and a soluble dimeric Fc-fusion protein of the extracellular ligand-binding portion of the human 75 kD (p75) TNF receptor (etanercept).10 These TNF antagonists bind to TNFα and inhibit the interaction of soluble TNFα with its cell surface receptors, thus inhibiting biologic responses initiated or mediated by TNFα. TNFα can also exist as a cell-surface molecule. Therefore, some component of TNF antagonist mechanisms of action may include direct binding to cell surfaces. These TNF antagonists are approved for the treatment of a number of rheumatologic, gastroenterologic and dermatologic indications.
Role of Interleukin-12 and Interleukin-23 on Lymphocyte Development and Function
TNF antagonists established mAb-based cytokine targeting as an effective treatment approach for immune-mediated disease. Another cytokine thought to contribute to certain immune-mediated disorders is IL-12. IL-12 is primarily produced by phagocytic and dendritic cells in response to microbial stimulation, and drives cell-mediated immunity by inducing lymphokine-activated killer cells and activation of natural killer (NK) cells and T lymphocytes.11 CD4+ T cells can differentiate into T-helper (Th) effector lineages, which are typically classified by the environment leading to their development and the cytokine profiles they produce. The original Th lineages identified were designated Th1 and Th2.12 IL-12 is the key inducer of Th1 cells, which are characterized by utilization of T-bet transcription factor and robust IFNγ production. Th1 responses are thought to promote cell-mediated immunity to intracellular pathogens, delayed type hypersensitivity and macrophage activation.13 In contrast, the Th2 lineage is associated with the GATA-3 transcription factor and IL-4, IL-5 and IL-13 production. Th2 cells are thought to mediate humoral immunity, especially to extracellular pathogens. Concurrent with discovery of the Th1/Th2 lineages, animal model and clinical studies indicated abnormal Th1 responses were driving the pathology of immune-mediated disorders, whereas abnormal Th2 responses were proposed to mediate asthmatic and allergic disorders. Mouse Th1 and Th2 cells were shown to counter-regulate each other, thus establishing the Th1/Th2 paradigm.14 A number of key aspects of the Th1/Th2 paradigm are currently under debate, one example being irreversible lineage commitment of Th cells.
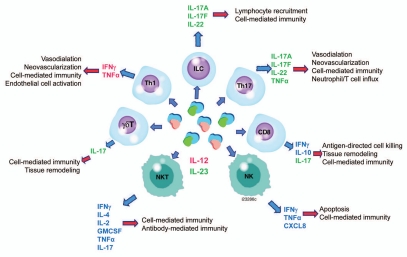
The Th1/Th2 paradigm was challenged by the identification of the Th17 lineage, characterized by cell surface CD161 and C-C chemokine receptor type (CCR)6 expression, as well as IL-17A and IL-17F production.15 Original reports in mouse systems suggested that a newly discovered cytokine, IL-23, was critical for Th17 differentiation.16 However, more recent studies conducted on human cells suggest a cocktail of cytokines, such as IL-23 and IL-1β, are required.15,17 Development and maintenance of the Th17 phenotype utilizes retinoid-related orphan receptor (ROR)γτ and RORα transcription factors and likely requires multiple cytokines, including IL-23. Differences exist between human and mouse Th17 cells in relation to cytokine requirements for lineage commitment and maintenance, as well as cytokine profiles.15,16 Human Th17 cells are thought to produce several pro-inflammatory cytokines, including IL-17A and F, TNFα, IL-22, IL-26 and IFNγ.18,19 Similar to IL-12, IL-23 can contribute to functional responses of several effector cell subtypes other than CD4+ T cells, including CD8+ T cells,20 NK, NKT,21,22 γδ T cells,23,24 and innate lymphoid cells (Fig. 1).25
Figure 1.
Function of interleukin (IL)-12 and IL-23 on effector cells. IL-12-specific specific effects are designated red; IL-23-specific effects are designated green. Properties attributed to both IL-12 and IL-23 are designated blue. CXCL, chemokine (C-X-C) motif; IFNγ, interferon gamma; GMCSF, granulocyte macrophage colony stimulating factor; NKT, natural killer cells; TNFα, tumor necrosis factor. Cited from references 12, 15, 18–21 and 23.
The discovery and characterization of Th17 cells and additional Th lineages such as T follicular helper, Th9, Th22 and T regulatory (Treg) cells are substantially altering our understanding of adaptive immune function and immune-mediated pathology.26–28 There is increasing evidence of plasticity amongst certain Th subtypes, depending upon the cytokine microenvironment.29 For instance, Foxp3 expression by Treg cells and IL-17 by Th17 cells can be altered by changing the cytokine milieu, suggesting these phenotypes are not stable. For example, activated mouse Treg cells have the capacity to differentiate into Th17 cells in vitro in the presence of exogenous IL-6.30 Alternately, Th17 cells can differentiate to Th1 cells in the presence of IL-12.29 Although contributions from IL-23 were not originally appreciated, Centocor chose to develop a mAb that attenuates Th cell function by modulation of the cytokine environment and thus, initiated discovery of an anti-IL-12 therapeutic mAb.
Interleukin-12 and Interleukin-23 Antibody Discovery and Generation
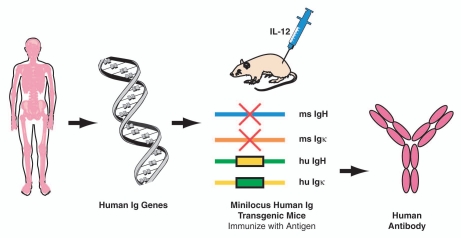
Ustekinumab is a human IgG1 kappa (κ) mAb generated by Centocor Research & Development, a division of Johnson & Johnson Pharmaceutical Research and Development, LLC, using human Ig (hu-Ig) transgenic mice obtained from GenPharm, which was subsequently acquired by Medarex and is currently part of Bristol-Meyers Squibb of Princeton, New Jersey. In these mice, four distinct genetic modifications replaced the mouse Ig loci with human antibody transgenes.31,32 The mouse antibody heavy chain joining (J) coding sequences were deleted, thereby preventing the DNA rearrangement process that is required to assemble a functional mouse antibody heavy chain gene. In addition, the mouse antibody κ light chain and constant region coding sequences were deleted, preventing expression of mouse κ light chains. The human heavy chain “minilocus” of DNA (∼80,000 bases in length), which contained coding sequences for four variable (V) regions, sixteen diversity segments, six J segments, IgM constant regions and IgG1 constant regions, were cloned and inserted into the mouse genome. In addition, a human κ light chain “minilocus” of DNA (∼450,000 bases in length), containing the coding sequences for at least ten V regions, five J segments and κ constant region, was inserted. These genetic modifications resulted in a mouse strain capable of producing human antibodies in response to immunizations to any antigen of interest (Fig. 2). The human Ig transgenic mouse technology enabled generation of diverse, high affinity, and highly specific mAbs with lower deleterious immunogenicity responses than previously developed rodent mAbs.
Figure 2.
Human antibody transgenic mice. Human heavy and light chain genes were used by GenPharm (later known as Medarex, now part of Bristol-Meyers Squibb) to prepare minilocus human immunoglobulin (hu-Ig) transgenic mice (HC4/KCo5). Mice were immunized with human interleukin-12 (IL-12) to produce human antibodies. H, heavy; κ, kappa; ms, mouse.
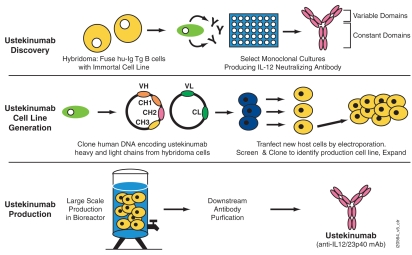
To elicit human anti-human IL-12 therapeutic mAbs, the transgenic mice were immunized with human IL-12 antigen. Mice that demonstrated positive serum titers for anti-IL-12 antibodies were selected for hybridoma fusion. Splenocytes, which contain antibody-producing B cells from IL-12 titer-positive mice, were fused with an immortal cell line, and the resulting hybridoma cells were cultured under selection conditions that allowed only hybridoma cells to grow. Growth-positive hybridomas secreting IL-12-specific antibodies were selected for limited dilution subcloning (Fig. 3). Binding and cell-based functional assays using human T cells were utilized to select antibodies that specifically bound IL-12 and inhibited IL-12-mediated responses. A monoclonal hybridoma clone that produced a human IgG1κ antibody capable of binding to and neutralizing human and non-human primate IL-12 was thus identified. The antibody, initially named 12B75, then CNTO1275, and later ustekinumab, was chosen for further development based on its superior IL-12 binding and neutralization activity.
Figure 3.
Ustekinumab discovery, cell line generation and antibody production. Ustekinumab is a human monoclonal antibody (mAb) discovered through the generation of hybridoma cultures from immunized human immunoglobulin (hu-Ig) transgenic (Tg) mice. Hybridomas secreting human mAbs that neutralized interleukin-12 (IL-12) were identified. Ustekinumab variable (V) and constant (C) domains were cloned from hybridoma cells and the heavy (H) and light (L) chains of the V and C domains were transfected into new host cells by electroporation. Ustekinumab is produced using perfusion fermentation culture and purified from the supernatant generated from the fermentation process.
As a first step towards preparing a stable cell line producing high quantities of ustekinumab, DNA encoding the entire heavy and light chain genes of the ustekinumab antibody was cloned from the hybridoma cells (Fig. 3). Sequencing of the cloned DNA encoding ustekinumab and their subsequent translation into amino acid sequences, followed by comparison to antibody databases, confirmed that ustekinumab was a human antibody with a human IgG1 heavy chain and a κ light chain. The heavy chain IgG1 constant region sequence is of the G1m (1,3) allotype. The cloned heavy and light chain genes were then introduced into a host cell line by electroporation. Transfected cell lines producing the highest titers of ustekinumab were selected for subcloning and expansion. A single cell line was chosen to support early development. Subsequently, further changes were made to support production using perfusion bioreactors in accordance with Good Manufacturing Practice guidelines, with the resultant recombinant antibody retaining the same amino acid sequence as found in the original hybridoma cell lines. Ustekinumab is purified from the supernatant generated from the bioreactor process.
Ustekinumab Mechanism of Action
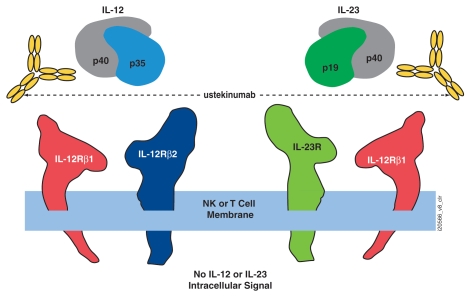
IL-12 is a heterodimeric cytokine containing two protein subunits named p40 and p35 according to their approximate molecular weight. Subunit binding analysis determined that ustekinumab binds to the IL-12p40 subunit. This was later confirmed by elucidation of the ustekinumab fragment antigen binding (Fab)/IL-12 co-crystal structure.33 IL-12 binds to a heterodimeric receptor complex consisting of IL-12 receptor (IL-12R) β1 and IL-12Rβ2 chains expressed on the surface of T cells or NK cells (Fig. 4).34 The IL-12Rβ1 chain binds to the p40 subunit, whereas IL-12p35 association with IL-12Rβ2 confers intracellular signaling. IL-12-mediated signaling includes intracellular phosphorylation of signal transduction activation of transcription (STAT)4 and STAT6 proteins, and functional responses including cell surface molecule expression, NK cell lytic activities and cytokine production, such as IFNγ.
Figure 4.
Ustekinumab mechanism of action. Ustekinumab binds to the p40 subunit of interleukin (IL)-12 and IL-23 and prevents their interaction with the cell surface IL-12Rβ1 receptor, subsequently inhibiting IL-12- and IL-23-mediated cell signaling, activation and cytokine production (image not drawn to scale). NK, natural killer. Adapted from Benson et al.37
The p40 protein subunit of IL-12 was also found to associate with a p19 subunit to form IL-23.35 Both IL-12 and IL-23 exist only as secreted heterodimeric cytokines and neither IL-12p35 nor IL-23p19 subunits are secreted without intracellular covalent association with p40. IL-23 also utilizes the IL-12Rβ1 chain for binding to the cell surface of effector cells (Fig. 4). However, it is association of IL-23p19 to the second component of the IL-23 receptor complex (IL-23R) that confers IL-23-specific intracellular signaling, such as intracellular phosphorylation of STAT3 and lymphocyte activation and cytokine production, such as IL-17A (Fig. 4).36 Since IL-23 also contains the IL-12p40 protein subunit, ustekinumab was characterized for binding and neutralization activity against human IL-23. Interestingly, the description of IL-23 occurred subsequent to the discovery and preclinical development of ustekinumab. The opportunities and challenges of the unique dual specificity to the clinical development of ustekinumab have recently been described in detail.37
The p40 subunit of human IL-12 and IL-23 is comprised of three domains (D), i.e., D1–D3, two of which (D2 and D3) are involved in binding IL-12p35 and IL-23p19.38,39 Based on a crystal structure of ustekinumab Fab region complexed with human IL-12, the binding epitope for ustekinumab is located in the D1 domain of the p40 subunit, which is spatially distant from IL-12p35 and IL-23p19.33 Mutational analysis confirmed amino acid residues within D1 that were required for ustekinumab binding. Through isothermal titration calorimetry analysis, ustekinumab was shown to bind IL-12 and IL-23 equally, with the expected 2:1 antigen-to-antibody stoichiometry. Furthermore, ustekinumab did not bind to structurally related proteins or rodent IL-12/23. Overall, these studies determined the precise specificity and molecular interactions between ustekinumab and IL-12/23p40.
Ustekinumab prevents human IL-12 and IL-23 from binding to the IL-12Rβ1 receptor chain of IL-12 (IL-12Rβ1/β2) and IL-23 (IL-12Rβ1/23R) receptor complexes on the surface of NK and T cells (Fig. 4). This defines the molecular mechanism of action of ustekinumab. Ustekinumab cannot bind to endogenous IL-12 or IL-23 that is already bound to receptor complexes. Thus, ustekinumab is unlikely to mediate Fc effector functions, such as ADCC or CDC. In vitro, ustekinumab will neutralize IL-12-mediated responses, including intracellular phosphorylation of STAT4, cell surface marker expression and IFNγ cytokine production. IL-23-mediated responses are equally inhibited, including intracellular STAT3 phosphorylation and IL-17A, IL-17F and IL-22 cytokine protein production. Collectively, these data demonstrate that by preventing IL-12 and IL-23 from binding to the IL-12Rβ1 receptor, ustekinumab can effectively neutralize human IL-12- and IL-23-mediated cell signaling, activation and cytokine production. It is important to note that while ustekinumab will effectively neutralize IL-12- and IL-23-mediated functional responses, it will not affect immune responses stimulated through other cytokines or cellular activities.
Role of Interleukin-12 and Interleukin-23 in Immune-Mediated Diseases
Studies in animal models and with human disease samples have established a strong link between dysregulation of the Th1/Th17 pathways and dermatologic, rheumatic, gastrointestinal and neurologic disorders, namely psoriasis, RA, Crohn disease and multiple sclerosis (MS). Administration of IL-12 exacerbated disease in murine psoriasis,40 chronic colitis,41,42 collagen-induced arthritis (CIA) models,43 and experimental autoimmune encephalitis (EAE) models of MS,44 whereas administration of anti-IL-12/23p40 antibodies is either protective or attenuated disease severity. Subsequent studies in mouse models of EAE and CIA revealed that IL-12/23p40 or IL-23p19 inhibition through genetic ablation or antibody treatment is either protective or attenuates disease severity. In contrast, genetic ablation of IL-12p35 was not protective. Thus, in certain mouse systems, IL-23 mediates many disease pathologies previously attributed to IL-12.18,45–47
Human genetic and tissue analysis indicates both IL-12 and IL-23 pathways are involved in certain immune-mediated pathologies. However, given the overlap between human Th1 and Th17 pathways and the plasticity between human Th lineages in vivo, it is difficult to distinguish between IL-12 and IL-23 biologies. For example, overexpression of IL-12 was observed systemically or within diseased tissue from a number of human autoimmune/inflammatory disorders.48–51 In certain cases, such as MS, protein expression of the IL-12/23p40 in the serum or CNS correlated with disease severity.52,53 In addition, gene expression levels of IL-12, IFNγ and IL-23 are elevated in psoriasis skin lesions.54 Overexpression of both the p35 and p40 subunits of IL-12 are elevated in gastrointestinal tissue of Crohn disease patients and polymorphisms of genes that encode either IL-12/23p40 or the IL-23R are linked to psoriasis,55,56 and Crohn disease.57 In fact, the IL23R R381Q gene variant that protects against psoriasis, Crohn disease and ankylosing spondylitis was recently reported to exert its protective effects through selective attenuation of IL-23-induced Th17 cell effector function, without interfering with Th17 differentiation.58 Collectively, many published studies support dysregulation of either IL-12, IL-23, or both pathways in human immune-mediated diseases.
Ustekinumab Clinical Development
As summarized previously, a strong body of pre-clinical and clinical data established an association between IL12/23p40 and a number of chronic immune-mediated disorders. Of these, psoriasis was chosen as the first-in-human population since it allowed the establishment of proof of concept early in clinical development and afforded the ability to collect and examine diseased tissue for pharmacodynamic effects via minimally invasive procedures. Psoriasis is a chronic immune-mediated skin disorder with significant co-morbidities such as psoriatic arthritis (PsA), depression, cardiovascular disease, hypertension, obesity, diabetes, metabolic syndrome and Crohn disease.59 Plaque psoriasis is the most common form of the disease and manifests in well-demarcated erythematous lesions topped with white silver scales.60 Plaques are pruritic, painful and often disfiguring, and a significant proportion of psoriatic patients have plaques on hands/nails, face, feet and genitalia. As such, psoriasis can impose physical and psychosocial burdens that extend beyond the physical dermatological symptoms and interfere with everyday activities. For example, psoriasis negatively impacts familial, spousal, social and work relationships,61,62 and is associated with a higher incidence of depression and increased suicidal tendencies.63
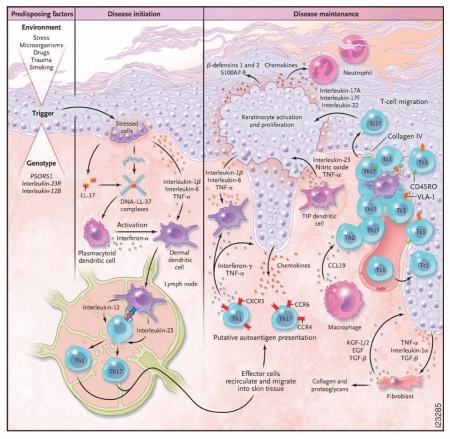
Histologic characterization of psoriasis lesions reveals a thickened epidermis resulting from aberrant keratinocyte proliferation and differentiation, as well as dermal infiltration and co-localization of CD3+ T lymphocytes and dendritic cells (Fig. 5).64 While the etiology of psoriasis is not well-defined, gene and protein analyses have shown that IL-12, IL-23 and their downstream molecules are overexpressed in psoriatic lesions,48,65 and some may correlate with psoriasis disease severity.66,67 Some therapies used in the treatment of psoriasis modulate IL-12 and IL-23 levels, which is speculated to contribute to their efficacy.68 As illustrated in Figure 5, Th1 and Th17 cells can produce effector cytokines that induce the production of vasodilators, chemoattractants and expression of adhesion molecules on endothelial cells, which, in turn, promote monocyte and neutrophil recruitment, T cell infiltration, neovascularization and keratinocyte activation and hyperplasia. Activated keratinocytes can produce chemoattractant factors that promote neutrophil, monocyte, T cell and DC trafficking, thus establishing a cycle of inflammation and keratinocyte hyperproliferation.
Figure 5.
Proposed model for psoriasis immunopathogenesis. Activated dendritic cells (DCs) induce differentiation of naive T cells into effector cells such as T-helper (Th) 1 and Th17 cells, which then release effector cytokines that induce the production of chemokines and adhesion receptors on endothelial cells. Effector cells infiltrate the skin and contribute to keratinocyte activation and proliferation. The overall result is a continuing cycle of T cell and DC activation resulting in the maintenance of psoriatic skin inflammation and plaque formation. Reproduced with permission from Nestle et al.82
Results of three Phase 3 clinical studies of ustekinumab in the treatment of moderate-to-severe plaque psoriasis have been published.69–71 Ustekinumab administered by subcutaneous injection at weeks 0 and 4 and then once every 12 weeks exhibited rapid and sustained clinical response, as assessed by the Psoriasis Area and Severity Index, a validated efficacy tool for psoriasis. A Phase 3 study comparing ustekinumab with etanercept, a TNF antagonist, demonstrated that the efficacy of ustekinumab was superior to that of etanercept over a 12-week period in patients with moderate-to-severe psoriasis.71 In two Phase 3 clinical studies, PHOENIX I and PHOENIX II, ustekinumab exhibited a half-life of approximately 3 weeks. Immune response rates against ustekinumab ranged from 3 to 5%.72 In addition, reported adverse events were relatively mild, with the majority of events including susceptibility to mild infections such as nasopharyngitis and upper respiratory tract infection. Rates of infection were not higher in ustekinumab-treated patients when compared with placebo-treated patients over 12 weeks of therapy; nor were they increased in association with higher, relative to lower, ustekinumab doses. Also, rates of serious infections, cardiovascular events, injection site reactions and malignancies were low.69,70 Taken together, the clinical observations of ustekinumab in psoriasis have supported its first-in-class status and confirmed the fundamental role of IL-12 or IL-23 in psoriasis pathogenesis.
Completed ustekinumab Phase 2 studies in Crohn disease and PSA indicate that blockade of IL-12/23p40 also results in clinical response in these diseases.73 Ustekinumab treatment resulted in significant attenuation of arthritis signs and symptoms of PsA in addition to diminishment of psoriatic plaques.74 The safety and efficacy of ustekinumab in PsA is currently being evaluated in a Phase 3 study.75 Ustekinumab was also recently shown to induce and maintain clinical response in patients with moderate-to-severe Crohn disease who had previously failed one or more TNF-antagonist mAbs.76 The efficacy and safety of ustekinumab in moderate-to-severe Crohn disease are currently being further evaluated in three Phase 3 studies.77 These clinical observations suggest that psoriasis, PsA and Crohn disease share common pathological immune pathways, which include IL-12 and IL-23 (Fig. 6). In contrast, a Phase 2 study of ustekinumab in patients with relapsing-remitting MS did not yield significant or clinically meaningful differences in the cumulative number of gadolinium-enhancing T-1 weighted lesions (a marker of CNS inflammation), or a reduction in the severity and duration of relapses.78 The discordance between animal model causality and human disease association of IL-12/23 and the ustekinumab clinical trial results in MS is not well understood.
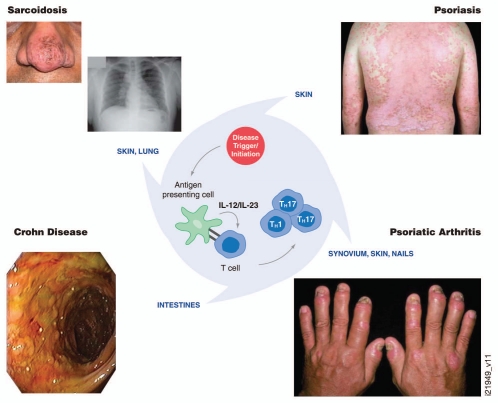
Figure 6.
Proposed central role of interleukin (IL)-12/23 and T helper (Th)1/17 cells in psoriasis, psoriatic arthritis, Crohn disease and sarcoidosis pathologies. Observations to date from clinical studies with ustekinumab suggest common immune pathways between psoriasis, psoriatic arthritis and Crohn disease. The role of IL-12/23 in sarcoidosis is under evaluation.
New indications for ustekinumab are also being explored. One example is sarcoidosis, which is a chronic, heterogenic and multi-systemic granulomatous disease of unknown cause. Release of cytokines such as TNFα and IL-12 during the formation of sarcoid granulomas and upregulation of IL-12 in lung tissue are reported in patients with pulmonary involvement.79 However, the role of IL-12 or IL-23 in the development of cutaneous sarcoid lesions is not yet clearly elucidated. Genes linked with the Th1 pathway, as well as expression of IL-23 and IL-23R, are associated with cutaneous sarcoidosis.80 In fact, gene expression of IL-12/23p40 was comparable or higher, than levels observed in psoriatic skin lesions. The effect of ustekinumab on granuloma formation in sarcoidosis is currently being assessed in a Phase 2 proof of concept study.81 Collectively, observations to date from clinical studies with ustekinumab suggest common immune pathways between psoriasis, PSA and Crohn disease, with the role of IL-12/23 in sarcoidosis under evaluation (Fig. 6).
Summary
Ustekinumab is a “first-in-class” anti-IL-12/23p40 mAb approved for the treatment of moderate-to-severe plaque psoriasis and is one of the first approved therapeutic mAbs generated directly through hu-Ig mice technology with no further molecular engineering. The mAb binds to the p40 subunit of both IL-12 and IL-23, preventing the interaction of both cytokines with the IL-12Rβ1 subunit that is common to both IL-12 and IL-23 cell surface receptors. Ustekinumab prevents IL-12- and IL-23-mediated downstream signaling, gene activation and cytokine production. Ustekinumab exhibits a long biologic half-life and low immune response rate, which translates into 12-week dosing intervals for treatment of moderate-to-severe psoriasis. The positive clinical results of ustekinumab observed in psoriasis and other immune-mediated disorders, such as Crohn disease and PSA, indicate that Th1 or Th17 lineages play a critical role in the underlying pathologic processes of these immune disorders. Similar to the TNF antagonists, ustekinumab further demonstrates that mAb-directed cytokine targeting can effectively attenuate cytokine-mediated pathologic processes, presumably through altering the local cytokine environment within diseased tissues. The relative roles of IL-12 and IL-23 in immune pathologies are not clearly defined and would require further clinical evaluation with agents specifically targeting the individual cytokines.t
Acknowledgments
The authors would like to thank Kim Staquet for her contributions to ustekinumab hybridoma production and Tom Nesspor for his contributions to ustekinumab cell line development. In addition, we thank Michelle Perate, M.S. and Dr. Mary Whitman of Janssen Biotech, Inc., for assistance with the preparation of the manuscript, as well as Christine Delaurentis and Ray Heslip of Janssen Biotech, Inc., for assistance with graphical displays.
We are dedicating this manuscript to the memory of Michael Brigham-Burke as a tribute to his contribution to ustekinumab analysis. Michael was dedicated to bio-molecular interactions analysis, being one of the pioneers in the area of surface plasmon resonance and a specialist in analytical ultracentrifugation analysis. He made significant scientific contributions that advanced the development of pharmaceutical sciences and several biotherapeutics, including ustekinumab.
Abbreviations
- ADCC
antibody-dependent cellular cytotoxicity
- CCL
chemokine (C-C motif) ligand
- CCR
C-C chemokine receptor type
- CXCL
chemokine (C-X-C motif) ligand
- CD
cluster of differentiation
- CDC
complement-dependent cytotoxicity
- CIA
collagen-induced arthritis
- CNS
central nervous system
- CTLA
cytotoxic T lymphocyte-associated antigen
- D
domain
- EAE
experimental autoimmune encephalomyelitis
- Fab
fragment antigen binding
- GMCSF
granulocyte macrophage stimulating factor
- Hu-Ig
human immunoglobulin
- IFNγ
interferon gamma
- IL
interleukin
- IL-12R
interleukin-12 receptor complex
- IL-23R
IL-23 receptor complex
- J
joining
- κ
kappa
- mAb
monoclonal antibody
- NK
natural killer
- NKT
natural killer T cells
- MS
multiple sclerosis
- PsA
psoriatic arthritis
- RA
rheumatoid arthritis
- ROR
retinoid-related orphan receptor
- STAT
signal transduction and activation of transcription
- Th
T-helper
- TNFα
tumor necrosis factor alpha
- Treg
T regulatory
- V
variable
Notes
This work was supported by Centocor Research & Development. With the exception of D.P. and M.A.M., all authors are currently employees of Centocor Research & Development and hold and own shares of Johnson & Johnson, the parent company of Centocor Research & Development. Johnson & Johnson has a commercial interest in drugs mentioned in this article. M.A.M. is a paid consultant for Centocor Research & Development and Janssen Biotech, Inc., and owns shares of Johnson & Johnson stock.
References
- 1.Winau F, Westphal O, Winau R. Paul Ehrlich—in search of a magic bullet. Microbes Infect. 2004;6:786–789. doi: 10.1016/j.micinf.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Raju TN. The Nobel chronicles. 1972: Gerald M Edelman (b 1929) and Rodney R Porter (1917–85) Lancet. 1999;354:1040. doi: 10.1016/s0140-6736(05)76658-7. [DOI] [PubMed] [Google Scholar]
- 3.Orthoclone OKT®3 prescribing information. www.janssenbiotech.com/assets/OKT3_PI.pdf.
- 4.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJT. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. 2010;9:325–338. doi: 10.1038/nrd3003. [DOI] [PubMed] [Google Scholar]
- 5.Reichert JM. Antibodies to watch in 2010. mAbs. 2010;2:84–100. doi: 10.4161/mabs.2.1.10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rituximab prescribing information. 2001. Apr, www.gene.com/gene/products/information/pdf/rituxan-prescribing.pdf.
- 7.Abatacept prescribing information. packageinserts.bms.com/pi/pi_orencia.pdf.
- 8.Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNFalpha) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 1993;94:354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koczan D, Drynda S, Hecker M, Drunda A, Guthke R, Kekow J, et al. Molecular discrimination of responders and nonresponders to anti-TNFalpha therapy in rheumatoid arthritis by etanercept. Arthritis Res Ther. 2008;10:R50. doi: 10.1186/ar2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 13.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 17.Duvallet E, Semerano L, Assier E, Falgarone G, Bossier MC. Interleukin-23: A key cytokine in inflammatory diseases. Ann Med. 2011:1–9. doi: 10.3109/07853890.2011.577093. [DOI] [PubMed] [Google Scholar]
- 18.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 20.Eijnden SV, Goriely S, De Wit D, Willems F, Goldman M. IL-23 upregulates IL-10 and induces IL-17 synthesis by polyclonally activated naïve T cells in human. Eur J Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 21.Van de Wetering D, de Paus RA, van Dissel JT, van de Vosse E. IL-23 modulates CD56+/CD3− NK cell and CD56+/CD3+ NK-like T cell function differentially from IL-12. Internat Immunol. 2008;21:145–153. doi: 10.1093/intimm/dxn132. [DOI] [PubMed] [Google Scholar]
- 22.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγτ and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ness-Schwickerath KJ, Jin C, Morita CT. Cytokine requirements for the differentiation and expansion of IL-17A—and IL-22-producing human V γ2Vδ2 T cells. J Immunol. 2010;184:7268–7280. doi: 10.4049/jimmunol.1000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien RL, Roark CL, Born WK. IL-17-producing γδ T cells. Eur J Immunol. 2009;39:634–675. doi: 10.1002/eji.200839120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geremia A, Aranchibia-Cárcamo CV, Fleming MPP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208:1127–1133. doi: 10.1084/jem.20101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 27.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–872. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 28.Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N, et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGFβ. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 31.Fishwild DM, O'Donnell SL, Bengoechea T, Hudson DV, Harding F, Bernhard SL, et al. High-avidity human IgG(kappa) monoclonal antibodies from a novel strain of minilocus transgenic mice. Nat Biotechnol. 1996;14:845–851. doi: 10.1038/nbt0796-845. [DOI] [PubMed] [Google Scholar]
- 32.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–1125. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 33.Luo J, Wu SJ, Lacy ER, Orlovsky Y, Baker A, Teplyakov A, et al. Structural basis for dual recognition of IL-12 and IL-23 by ustekinumab. J Mol Biol. 2010;402:797–812. doi: 10.1016/j.jmb.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 34.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, et al. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/s1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 36.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 37.Renson JM, Sachs CW, Treacy G, Zhou H, Pendley CE, Brodmerkel C, et al. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nature Biotech. 2011;29:615–624. doi: 10.1038/nbt.1903. [DOI] [PubMed] [Google Scholar]
- 38.Yoon C, Johnston SC, Tang J, Stahl M, Tobin JF, Somers WS. Charged residues dominate a unique interlocking topography in the heterodimeric cytokine interleukin-12. EMBO J. 2000;19:3530–3541. doi: 10.1093/emboj/19.14.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupardus PJ, Garcia KC. The structure of interleukin-23 reveals the molecular basis of p40 subunit sharing with interleukin-12. J Mol Biol. 2008;382:931–941. doi: 10.1016/j.jmb.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong K, Chu A, Lúdivíksson BR, Berg EL, Ehrardt RO. IL-12, independently of IFNγ plays a crucial role in the pathogenesis of a murine psoriasis-like skin disorder. J Immunol. 1999;162:7480–7491. [PubMed] [Google Scholar]
- 41.Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFNγ, plays a major role in sustaining the chronic phase of cholitis in IL-10 deficient mice. J Immunol. 1998;161:3143–3149. [PubMed] [Google Scholar]
- 42.Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malfait AM, Butler DM, Presky DH, Maini RN, Brennan FM, Feldmann M. Blockade of IL-12 during the induction of collagen-induced arthritis (CIA) markedly attenuates the severity of the arthritis. Clin Exp Immunol. 1998;111:377–383. doi: 10.1046/j.1365-2249.1998.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leonard JP, Waldburger KE, Goldman SJ. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin 12. J Exp Med. 1995;181:381–386. doi: 10.1084/jem.181.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, et al. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-deficient mice are susceptible to experimental autoimmune encephalomyelitis: evidence for redundancy in the IL-12 system in the induction of central nervous system autoimmune demyelination. J Immunol. 2002;169:7104–7110. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 47.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 48.Yawalker N, Karlen S, Hunger R, Brand CU, Braathen LR. Expression of IL-12 is increased in psoratic skin. J Invest Dermatol. 1998;111:1053–1057. doi: 10.1046/j.1523-1747.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 49.Monteleone G, Biancone L, Marasco R, Morrone G, Marasco O, Luzza F, et al. Interleukin 12 is expressed and actively released by Crohn's disease intestinal lamina propria mononuclear cells. Gastroenterology. 1997;112:1169–1178. doi: 10.1016/s0016-5085(97)70128-8. [DOI] [PubMed] [Google Scholar]
- 50.Berrebi D, Besnard M, Fromont-Hankard G, Paris R, Mougenot JF, De Lagausie P, et al. Interleukin-12 expression is focally enhanced in the gastric mucosa of pediatric patients with Crohn's disease. Am J Pathol. 1998;152:667–672. [PMC free article] [PubMed] [Google Scholar]
- 51.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, et al. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicoletti F, Patti F, Cocuzza C, Zaccone P, Nicoletti A, Di Marco R, et al. Elevated serum levels of interleukin-12 in chronic progressive multiple sclerosis. J Neuroimmunol. 1996;70:87–90. doi: 10.1016/s0165-5728(96)00101-4. [DOI] [PubMed] [Google Scholar]
- 53.Fassbender K, Ragoschke A, Rossol S, Schwartz A, Mielke O, Paulig A, et al. Increased release of interleukin-12p40 in MS: association with intracerebral inflammation. Neurology. 1998;51:753–758. doi: 10.1212/wnl.51.3.753. [DOI] [PubMed] [Google Scholar]
- 54.Torti DC, Feldman SR. Interleukin-12, interleukin-23, and psoriasis: current prospects. J Am Acad Dermatol. 2007;57:1059–1068. doi: 10.1016/j.jaad.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, Baumber L, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 57.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Meglio P, Di Cesare A, Laggner U, Chu CC, Napolitano L, Villanova F, et al. The IL23R R381Q gene variant protects against immune-mediated diseases by impairing IL-23-induced Th17 effector response in humans. PLoS ONE. 2011;6:17160. doi: 10.1371/journal.pone.0017160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lebwohl M. Psoriasis. Lancet. 2003;361:1197–1204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 60.Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26:314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 61.Eghlileb AM, Davies EEG, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. Br J Dermatol. 2007;156:1245–1250. doi: 10.1111/j.1365-2133.2007.07881.x. [DOI] [PubMed] [Google Scholar]
- 62.Basra MKA, Finlay AY. The family impact of skin diseases: the Greater Patient concept. Br J Dermatol. 2007;156:929–937. doi: 10.1111/j.1365-2133.2007.07794.x. [DOI] [PubMed] [Google Scholar]
- 63.Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420–426. doi: 10.1016/j.jaad.2005.11.1103. [DOI] [PubMed] [Google Scholar]
- 64.Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 65.Arican O, Aral M, Sasmaz S, Ciragil P. Serum levels of TNFα, IFNγ, IL-6, IL-8, IL 12, IL-17 and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;5:273–279. doi: 10.1155/MI.2005.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 67.Jacob SE, Nassiri M, Kerdel FA, Vincek V. Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediators Inflamm. 2003;12:309–313. doi: 10.1080/09629350310001619753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guttman-Yassky E, Krueger GG. Psoriasis: evolution of pathogenic concepts and new therapies through phases of translational research. Br J Dermatol. 2007;157:1103–1115. doi: 10.1111/j.1365-2133.2007.08135.x. [DOI] [PubMed] [Google Scholar]
- 69.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–1674. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]
- 70.Papp KA, Langley RG, Lebwohl M, Krueger GG, Szapary P, Yeilding N, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2) Lancet. 2008;371:1675–1684. doi: 10.1016/S0140-6736(08)60726-6. [DOI] [PubMed] [Google Scholar]
- 71.Griffiths CE, Strober BE, van de Kerkhof P, Ho V, Fidelus-Gort R, Yeilding N, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. doi: 10.1056/NEJMoa0810652. [DOI] [PubMed] [Google Scholar]
- 72.Ustekinumab prescribing information. 2010. Oct, www.Stelarainfo.com/pdf/PrescribingInformation.pdf.
- 73.Yeilding N, Szapary P, Brodmerkel C, Benson J, Plotnick M, Goyal K, et al. Development of IL-12/23 antagonist ustekinumab: past, present and future perspectives. Ann NY Acad Sci. 2011;1222:30–39. doi: 10.1111/j.1749-6632.2011.05963.x. [DOI] [PubMed] [Google Scholar]
- 74.Gottlieb A, Menter A, Mendelsohn A, Shen YK, Li S, Guzzo C, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet. 2009;373:633–640. doi: 10.1016/S0140-6736(09)60140-9. [DOI] [PubMed] [Google Scholar]
- 75.US National Institutes of Health. 2011. Nov, http://clinicaltrials.gov/ct2/show/NCT01077362?term=ustekinumab+psoriatic+arthritis&rank=3.
- 76.Sandborn WJ, Gasink C, Gao LL, Blank M, Johanns J, et al. A multicenter, randomized, doubleblind, placebo-controlled phase 2b study of ustekinumab, a human monoclonal antibody to IL-12/23p40, in patients with moderately to severely active Crohn's disease: results through week 22 from the Certifi Trial. Gastroenterology. 2011;140:109. [Google Scholar]
- 77.US National Institues of Health. 2011. Nov, http://clinicaltrials.gov/ct2/results?term=ustekinumab+Crohn%27s+disease.
- 78.Segal BM, Constantinescu CS, Raychaudhuri A, Fidelus-Gort R, Kasper LH. On behalf of the ustekinumab MS investigators. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. doi: 10.1016/S1474-4422(08)70173-X. [DOI] [PubMed] [Google Scholar]
- 79.Shigehara K, Shijubo N, Ohmichi M, Takahashi R, Kon S, Okamura H, et al. IL-12 and IL-18 are increased and stimulate IFNgamma production in sarcoid lungs. J Immunol. 2001;166:642–649. doi: 10.4049/jimmunol.166.1.642. [DOI] [PubMed] [Google Scholar]
- 80.Judson MA, Marchell RM, Mascelli MA, Piantone A, Barnathan ES, Petty KJ, et al. Molecular profiling and gene expression analysis in cutaneous sarcoidosis: the role of IL-12, IL-23, and the Th17 pathways. J Am Acad Dermatol. 2011 doi: 10.1016/j.jaad.2011.06.017. In press. [DOI] [PubMed] [Google Scholar]
- 81.US National Institutes of Health. 2011. Nov, http://clinicaltrials.gov/ct2/show/NCT00955279?term=ustekinumab+sarcoid&rank = 1.
- 82.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Eng J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]