Abstract
The photoreceptor/RPE complex must maintain a delicate balance between maximizing the absorption of photons for vision and retinal image quality while simultaneously minimizing the risk of photodamage when exposed to bright light. We review the recent discovery of two new effects of light exposure on the photoreceptor/RPE complex in the context of current thinking about the causes of retinal phototoxicity. These effects are autofluorescence photobleaching in which exposure to bright light reduces lipofuscin autofluorescence and, at higher light levels, RPE disruption in which the pattern of autofluorescence is permanently altered following light exposure. Both effects occur following exposure to visible light at irradiances that were previously thought to be safe. Photopigment, retinoids involved in the visual cycle, and bisretinoids in lipofuscin have been implicated as possible photosensitizers for photochemical damage. The mechanism of RPE disruption may follow either of these paths. On the other hand, autofluorescence photobleaching is likely an indicator of photooxidation of lipofuscin. The permanent changes inherent in RPE disruption might require modification of the light safety standards. AF photobleaching recovers after several hours although the mechanisms by which this occurs are not yet clear. Understanding the mechanisms of phototoxicity is all the more important given the potential for increased susceptibility in the presence of ocular diseases that affect either the visual cycle and/or lipofuscin accumulation. In addition, knowledge of photochemical mechanisms can improve our understanding of some disease processes that may be influenced by light exposure, such as some forms of Leber’s congenital amaurosis, and aid in the development of new therapies. Such treatment prior to intentional light exposures, as in ophthalmic examinations or surgeries, could provide an effective preventative strategy.
Keywords: Phototoxicity, Photochemical, Retina, Retinal pigment epithelium, Autofluorescence, Visual cycle, Lipofuscin, Bisretinoids
1. Introduction
Each day the retina of the average human absorbs approximately 1012 to 1015 photons and this can be greatly increased by workplace exposure (e.g. welders), activities in high light environments (such as sunshine during skiing) or medical imaging of the retina. With high level exposure to light, this hail of photons can cause irreparable damage to the retina. Brief exposure to extremely bright lights can produce an immediate thermal injury. On the other hand, exposure to light for an extended period of time may result in chemical changes in retinal cells that ultimately result in cell death. The latter is known as photochemical damage. Table 1 compares the properties of thermal and photochemical damage. Asunburn is a common example of photochemical damage to the skin. Better images of the retina bring with them increased risk of photochemical damage. For example, the importance for the retinal specialist of the brightest and clearest possible view of the retina must be weighed against the risk of phototoxicity. There is a new generation of high-resolution ophthalmoscopes under development for the retina that can provide microscopic views of the retina, but that require intra-ocular powers that increase with the square of the magnification they use (Porter et al. 2006).
Table 1.
Comparison of typical characteristics for thermal and photochemical damage
| Property | Thermal Damage | Photochemical Damage |
|---|---|---|
| Exposure duration | µs – 10 s | > 1 s |
| Wavelength | all λ | short visible (λ < 600 nm) |
| Time course | appear in < 24 hrs | appear in 24 – 48 hrs |
| Temperature change | > 20 °C temperature ↑ | < 10 °C temperature ↑ |
| Minimal lesion size | < beam diameter | = beam diameter |
| Reciprocity of time & power | no | yes |
| Scotoma | permanent | reparable |
| Damage threshold | ∝ (irradiated area diameter)−1 ∝ time−1/4 independent of λ (for 440 nm < λ < 700 nm) |
independent of irradiated area ∝ time−1 ∝ λ |
We have discovered two unexpected changes in images of retinal pigment epithelium (RPE) following long duration exposure to 568 nm light (Morgan et al. 2008). These effects raise interesting questions about how light interacts with the retina and which of these interactions are ultimately deleterious for the eye. The first is an immediate reduction in lipofuscin autofluorescence (AF) that recovers in several hours. This RPE AF photobleaching can be observed with exposures 2 orders of magnitude below current safety standards. At larger irradiances, we observed a second phenomenon characterized by a disruption in the RPE cell mosaic, which we call RPE disruption. What is especially striking is that both AF photobleaching and RPE disruption occur at light levels at or below the maximum permissible exposure (MPE) specified by currently published safety standards (American National Standards Institute. 2007).
Not all retinal cells are typically susceptible to damage from light. Inner retinal cells such as ganglion cells, Müller cells, amacrine cells, and bipolar cells, which are mostly transparent, are not known to be directly involved in phototoxicity. On the other hand, rods and cones, which require photopigments to absorb photons as the first step in seeing, are much more likely to be damaged by excess amounts of visible light. Similarly, the RPE cells contain light absorbers such as melanin, lipofuscin, and retinoids, which make them susceptible to photochemical damage. The study of phototoxicity is all the more important given that eyes are not equally susceptible to light damage. Vulnerability to photochemical damage can depend on many factors including age, diet, and pathology. For example, a dog model of retinitis pigmentosa exhibited enhanced sensitivity to the negative effects of light (Cideciyan et al. 2005). Furthermore, understanding photochemical mechanisms of damage may lead to greater understanding of the progression of some retinal diseases (Travis et al. 2007).
Here, we discuss the proposed mechanisms for photochemical damage in relation to our findings of RPE AF photobleaching and RPE photodamage. Each of these phenomena may involve a different mechanism. In addition, the potential for light damage will differ between normal and diseased ocular tissue, in which the particular molecules involved in phototoxicity may play an important role. Combined with the existing literature, these new results lead to specific recommendations about how to design and use ophthalmic instrumentation to minimize the risk of phototoxicity.
2. Mechanisms of photochemical retinal damage
Photochemical damage can occur when the energy in a photon of light induces changes in the irradiated molecules, such as changes in electron orbitals, or direct breakage of bonds. For instance, sequential transfer of energy from a photon to a photosensitive molecule, and then to oxygen causes changes in electron orbitals, creating reactive forms of oxygen, such as singlet oxygen (1O2). The subsequent reaction of singlet oxygen with surrounding molecules can break their molecular bonds, a process called photoxidation. If too many of these events occur, they can eventually result in cell damage or death. The mechanism of photochemical damage depends on the particular type of molecules that act as photosensitizers and the photon energy (related to wavelength) required to induce a chemical change.
Previous research has suggested two possible mechanisms for photochemical retinal damage. These two subtypes are often identified by the original experimenters in each category. Noell damage (Noell et al. 1966), also known as class I damage, has an action spectrum that suggests the involvement of photoreceptor photopigments. Ham damage (Ham et al. 1978), also known as class II or “blue-light” damage, has a damage threshold action spectrum that increases with wavelength and may be linked to chemical changes in lipofuscin. Current light safety standards only consider protection from photochemical hazards originating from Ham damage (American National Standards Institute. 2007). Kremers and van Norren (Kremers and van Norren. 1988) surveyed the existing photochemical data for a number of studies to reveal two threshold categories, consistent with the two prevailing theories. Although study of the two subtypes has traditionally involved differing experimental conditions and species (Table 2), it is the difference in the action spectra for these mechanisms of photochemical damage that most clearly distinguishes them (Mellerio. 1994). However, recent analyses of select datasets do not draw such clear distinctions between Noell and Ham type retinal damage mechanisms (van Norren and Gorgels. 2011).
Table 2.
Traditional experimental paradigms for studying the two subtypes of photochemical damage from visible light (Ham et al. 1979, Kremers and van Norren. 1988, Noell et al. 1966, Thumann et al. 1999, van Norren and Gorgels. 2011, Williams and Howell. 1983)
| Property | Noell Damage | Ham Damage |
|---|---|---|
| Class | I | II |
| Exposure duration | > 1.5 hours | < 5 hours |
| Source spectrum | green-filtered fluorescent & incandescent white | white & laser lines |
| Primary animal species | rats | primates |
| Exposure Size | large | small |
| Site of major impact | Photoreceptors, occasionally RPE | RPE |
| Action spectrum | resembles visual pigment absorption | peaks in UV |
Noell damage was first observed in response to long duration exposures (> 8 hours) to constant green (490 – 580 µm) light (Noell et al. 1966). Although primarily studied in rats, Noell type damage has been reported in mice (Reme. 2005), macaque (Harwerth and Sperling. 1975, Sperling and Harwerth. 1971), hamsters (Noell. 1968, Thumann et al. 1999), fish (Penn. 1985), chickens (Machida. 1994) and other species. However, none of the studies in species other than rats measured an action spectrum, thus the evidence for Noell damage is circumstantial. Threshold Noell damage occurs initially in the photoreceptors (1.5 – 48 hour exposures), with longer exposures leading to photoreceptor and RPE damage (8 – 50 day exposures) (Noell. 1980). However, in hamsters, the RPE cells exhibit the first signs of damage, with only minimal changes in the photoreceptors (Thumann et al. 1999). Because of the repair mechanisms that may come into play with multi-day exposures, there can be a breakdown of the reciprocity between exposure power and duration that is traditionally associated with photochemical damage (Table 1). In rats, known to have a rod-dominant retina, the action spectrum of this type of damage matched that of rhodopsin absorption (Noell et al. 1966, Williams and Howell. 1983). Hence, the pigment molecules themselves are the likely targets for the initial chemical change that brings about the subsequent cell damage. This may include retinoids, intermediate products of the visual cycle (Maeda et al. 2006). Although a single complete bleach resulting in a flood of retinoids does not cause light damage, sustained photopigment bleaching and hence over-accumulation of retinoids may be harmful. In addition, rats with a visual cycle mutation (rd) have a high susceptibility to Noell damage; for example, (Noell. 1980). The ability to target specific cone types using coloured lights provides further evidence in favor of this theory (Harwerth and Sperling. 1975, Machida. 1994, Sperling and Harwerth. 1971). The primary targets of Noell damage will be the photoreceptors and the RPE cells. Noell (Noell. 1980) also suggested that light may primarily act on molecules in the choroid, with secondary damage to RPE, but this idea has not been developed further as there are no known molecules within the choroid to serve as photosensitizer.
Ham damage has been studied in monkeys (Ham et al. 1976, Lund et al. 2006), rabbits (Hoppeler et al. 1988), rats (Busch et al. 1999, Gorgels and van Norren. 1995, van Norren and Schellekens. 1990) and squirrels (Collier et al. 1989). Ham damage has been postulated to occur with light exposures in which the visual pigments are almost instantly totally bleached (Kremers and van Norren. 1988). Thus, rhodopsin or the cone pigments themselves are not expected to be the site of insult that leads to this type of retinal damage. Across species, photoreceptors are the primary target of retinal damage caused by violet and ultraviolet light (van Norren and Gorgels. 2011). With Ham type exposures to visible light, the majority of damage is observed in the RPE with minor photoreceptor damage (Ham et al. 1978), indicating that changes in molecules located in the RPE are the primary suspects for damage initiation. Among others, these molecules could include melanin, lipofuscin or intermediate products of the visual cycle.
Melanin is not likely to play a primary role in photochemical damage for several reasons. Neither the Noell nor the Ham damage action spectra match those for melanin absorption or the uptake of oxygen (Mellerio. 1994). Melanin is thought to protect against singlet oxygen, rather than play a formative role (Wang et al. 2006a). In addition, photochemical damage occurs even in albino rodents lacking RPE melanin (Noell et al. 1966).
3. The role of the visual cycle in phototoxicity
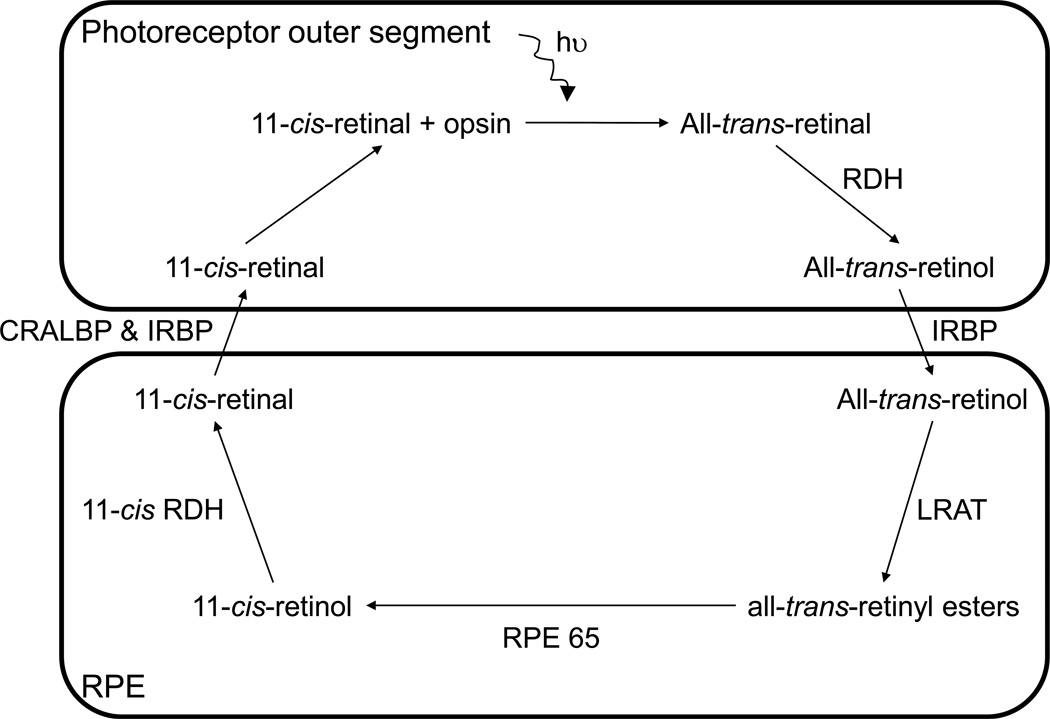
Absorption of a photon of light by rhodopsin or one of the cone opsins begins a cascade of events, known as the visual (or retinoid) cycle, whereby all-trans-retinal is converted back into 11-cis-retinal to regenerate photopigment (Figure 1). The steps of this process, carried out within the photoreceptors and RPE, generate retinoids that can act as photosensitizers. In cones, the 11-cis-retinal can be reformed by means of the above-mentioned pathway, or may be regenerated through a different mechanism (Jacobson et al. 2007), involving the Müller cells (Wang et al. 2009).
Figure 1.
The visual cycle. There are many thorough reviews of the visual cycle (e.g. (Lamb and Pugh. 2004, Sparrow et al. 2010, Travis et al. 2007)). The visual cycle is initiated as 11-cis-retinal, from the chromophore within an opsin molecule, is photoisomerized to all-trans-retinal and the process of converting it back into 11-cis-retinal begins. The all-trans-retinal molecule is moved from the photoreceptor disc membrane to the cytoplasmic space partly by ATP-binding cassette retina (ABCA4) and is then reduced by all-trans-retinol dehydrogenase (RDH) into all-trans-retinol. With an inter-photoreceptor retinol binding protein (IRBP) as a chaperone, all-trans-retinol is transported to the cytoplasm of the RPE where it is then chaperoned by cellular retinol binding protein (CRBP). The alcohol is esterified by the enzyme lecithin retinol acyl transferase (LRAT) to form all-trans-retinyl ester. Multiple all-trans-retinyl ester molecules can group together to form lipid bodies in which they are stored until needed (Imanishi et al. 2004). As the regulation of the visual cycle occurs by means of changing the rate of production of 11-cis-retinal within the RPE, a retinyl ester storage particle (REST; also known as a retinosome) can change in concentration and hence volume depending on the state and requirement of the visual system (Imanishi et al. 2004). To continue the visual cycle, all-trans-retinyl ester is isomerised to 11-cis-retinol by the RPE65 protein. The 11-cis-retinol is then oxidised into 11-cis-retinal by 11-cis retinol dehydrogenase (11-cis RDH) and transported back into the photoreceptors (chaperoned by cellular retinaldehyde binding protein (CRALBP) and IRBP). The 11-cis-retinal binds to an opsin in the outer segment membrane, becoming ready to absorb a photon of light and restart the visual cycle.
Photooxidation of all-trans-retinal has been suggested as a possible sensitizer for light damage (Delmelle. 1977), as has all-trans-retinol (Noell et al. 1966, Noell and Albrecht. 1971). Retinyl ester has also been suggested as a photosensitizer that forms anhydroretinol that is known to lead to cell apoptosis through oxidative stress (Lamb et al. 2001). An over-accumulation of all-trans-retinal may also lead to Noell type damage (Maeda et al. 2009). It is debatable whether or not all-trans-retinal and all-trans-retinol are stable and free for a long enough period of time to allow for either to build-up in photoreceptor outer segments and to act as a photosensitizer. Noell (Noell. 1980) suggested that retinol is unstable and, in rat, the concentration may be too low for it to be the photodynamic sensitizer. Moreover, the absorption spectra of these retinoids are almost negligible above 450 nm (Becker et al. 1971), making them unlikely photosensitizers for light damage with longer wavelengths. In addition, cultured RPE cells that do not contain lipofuscin or A2E do not exhibit damage or apoptosis when exposed to white or ‘blue’ (visible) light (Schutt et al. 2000, Sparrow et al. 2000). Other photosensitizers, such as cytochrome C oxidase (an enzyme involved in the electron transport chain) have been suggested. With in vitro RPE cells, Pautler and colleagues (Pautler et al. 1990) found that the action spectrum for cell death matched that of cytochrome C oxidase, rather than the absorption spectrum of all-trans-retinol. However, since all cells contain cytochrome C oxidase, the in vivo specificity of retinal damage to RPE and photoreceptors is inconsistent with this hypothesis.
In support of retinoids acting as photosensitizers, an increase in the rhodopsin content per rod outer segment length in aging rats was associated with an increase in light damage susceptibility (Rapp et al. 1990). Furthermore, the susceptibility to light damage was increased in knock-out mouse models (Rdh8−/−Abca4−/−) that prevented clearance of all-trans-retinal from photoreceptors (Maeda et al. 2008). Additionally, by using knock-out mouse models that prevent ocular accumulation of retinoids and lipofuscin (Lrat−/−Rdh8−/−Abca4−/− and Gnat1−/−Rdh8−/−Abca4−/−), Maeda and colleagues (Maeda et al. 2009) showed that light damage only occurred in the presence of 11-cis-retinal and all-trans-retinal. Dark-reared rats, that have increased levels of rhodopsin, are more susceptible to green light damage than normal cyclic light reared rats (Organisciak et al. 1998). Alternatively, Chen (Chen. 1993) observed similar retinal changes in dark-reared compared to cyclic light-reared rats exposed to blue light. This may be indicative of a strong wavelength dependence for the mechanism of primary retinal damage.
Fluctuations in the susceptibility to phototoxicity in rodents, concurrent with the circadian rhythm for phagocytosis, further suggests a role for the visual cycle in photochemical retinal damage (Duncan and O'Steen. 1985, Organisciak et al. 2000, Vaughan et al. 2002, White and Fisher. 1987, Wiechmann and O'Steen. 1992). Phagocytosis is the process by which the tips of the photoreceptor outer segments are internalized and digested by the RPE cells. In rodents reared with normal cyclic light, rod disc shedding occurs in the morning (Cahill and Besharse. 1995). Within the photoperiod, White and Fisher (White and Fisher. 1987) found enhanced photoreceptor damage for light exposures occurring during phagocytosis in the albino rat. This greater susceptibility to light damage when photopigment and retinoid concentrations in the RPE are elevated due to ingestion of the rod outer segments suggests their involvement in phototoxicity. Maximum susceptibility to light damage has been observed when light exposures occurred in the middle of the dark cycle (Organisciak et al. 2000, Vaughan et al. 2002), when whole eye rhodopsin concentration peaked by 5% to 10% (Organisciak et al. 2000, Vaughan et al. 2002). Furthermore, in rat, the superior hemisphere, which has the greatest rhodopsin concentration and rod outer segment length, is most sensitive to phototoxicity from green light (Vaughan et al. 2002). Given the broad spectrum of evidence, it is most likely that retinoids are photosensitizers for some photochemical retinal damage, but that, depending on wavelength and the state of the visual cycle, they are not always the cause of subsequent retinal damage.
4. The role of lipofuscin in phototoxicity
Lipofuscin, a conglomerate of modified lipids and bisretinoids (Bazan et al. 1990, Ng et al. 2008, Sparrow et al. 2010), accumulates with age in the lysosomes of the RPE as a by-product of the visual cycle and phagocytosis. Lipofuscin granules are autofluorescent, a property that has made it possible to image them in the living eye (Delori et al. 1995, Morgan et al. 2009). The peak of in vivo lipofuscin AF excitation, which includes absorption by the anterior ocular media, is near 510 nm (Delori et al. 1995). Bisretinoids account for the fluorescence of lipofuscin. They form as by-products of the visual cycle. All-trans-retinal and phosphatidylethanolamine (PE) can react to form N-retinylidene-PE. Rather than hydrolyzing back to all-trans-retinal and PE, some N-retinylidene-PE then reacts with a second all-trans-retinal (Sparrow et al. 2010). Subsequent reactions result in the formation of bisretinoids. These include, but are not limited to A2E, isomers of A2E, the all-trans-retinal dimer series (Fishkin et al. 2005, Parish et al. 1998) and A2-DHP-PE (Wu et al. 2009).
Lipofuscin is highly susceptible to photochemical changes that may lead to irreparable cellular damage and may be the photosensitizer for Ham damage. One of the most studied lipofuscin fluorophores is A2E. It has absorbance peaks at 338 nm and 447 nm (Ben-Shabat et al. 2002, Parish et al. 1998). A2E has seven known isomers that can be photo-induced, the most prevalent of which is 13′-cis-A2E (coined iso-A2E; λmax = 337 nm and 426 nm) which tends towards an equilibrium of 4:1 (A2E:iso-A2E) (Parish et al. 1998). In response to light exposure, the 438 nm absorbance of A2E is known to decrease in two stages. The first is associated with the photoisomerization of A2E leading to a blue shift in the absorbance peak (Parish et al. 1998). Photoisomerization is a non-toxic and reversible effect. In the second stage, there was a more gradual decline in the 438 nm absorbance related to the photooxidation of A2E and iso-A2E (Ben-Shabat et al. 2002). Lipofuscin photosensitization leads to formation of singlet oxygen by absorption of light by A2E and subsequent transfer of energy to ground state molecular oxygen (Ben-Shabat et al. 2002). Because A2E is also an excellent quencher of singlet oxygen, it is subsequently photooxidized by singlet oxygen at a carbon-carbon double bond (Ben-Shabat et al. 2002, Roberts et al. 2002, Sparrow et al. 2002). The oxygen containing moieties in photooxidized A2E include epoxides, furanoids and endoperoxides (Ben-Shabat et al. 2002, Hammer et al. 2006, Jang et al. 2005, Sparrow et al. 2002, Wang et al. 2006b). Also in response to visible light, aldehydes can form when the bisretinoids undergo photocleavage (photolysis) at sites in which singlet oxygen has added to carbon-carbon double bonds (Wu et al. 2010). In addition, photoexcitation of A2E can lead to the formation of other reactive oxygen intermediates (Gaillard et al. 2004), such as hydroperoxides (OOH) and the superoxide radical anion (O2•−) (Pawlak et al. 2003). When generated, any of these products could be harmful. However, the action spectrum for oxygen photoconsumption by A2E does not match that of lipofuscin, suggesting the involvement of additional bisretinoids in lipofuscin phototoxicity (Pawlak et al. 2002).
The second known class of lipofuscin fluorophores is the all-trans-retinal (atRAL) dimmer series (Fishkin et al. 2004, Fishkin et al. 2005, Kim et al. 2007) consisting of atRAL dimer, atRAL dimer-ethanolamine (E) and atRAL dimer-phosphatidylethanolamine (PE), with visible absorbance peaks at 432 nm, 510 nm and 511 nm, respectively (Kim et al. 2007). When isolated from the eyes of Abcr−/− mice, the atRAL dimer series is more abundant than A2E (Kim et al. 2007). This dimer series is expected to play a role in RPE phototoxicity, since it has been shown to photooxidize in response to blue light (Fishkin et al. 2005, Kim et al. 2007). When irradiated with 430 nm light, atRAL dimer-E is more efficient than A2E at generating and reacting with singlet oxygen (Kim et al. 2007). The photooxidation products of the atRAL dimer series are identified as furanoid oxides and cyclic peroxides (Kim et al. 2007).
5. The mechanism of RPE AF photobleaching
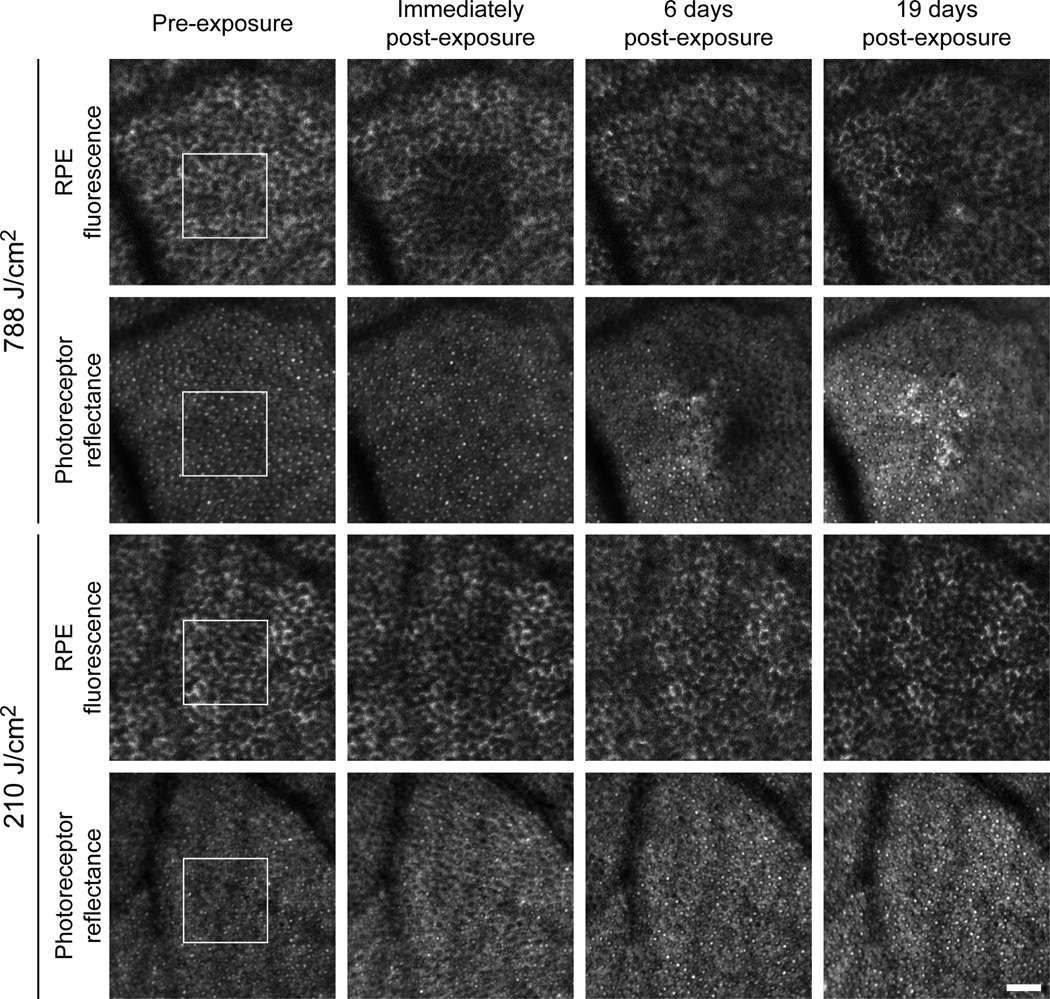
Using a unique capability to image in vivo individual cells of the RPE (Morgan et al. 2009), Morgan and colleagues (Morgan et al. 2008) have observed unexpected retinal changes following exposure to 568 nm visible light at irradiances below the ANSI photochemical MPE (562 J/cm2). Specifically, as seen in Figure 2, there is an immediate decrease in the magnitude of lipofuscin AF emission (retinal irradiances > 2 J/cm2) followed by either complete AF recovery in less than a week (retinal irradiances ≤ 210 J/cm2), or long-term disruption of the RPE mosaic (retinal irradiances ≥ 247 J/cm2). The magnitude of the AF photobleaching is correlated with the retinal radiant exposure.
Figure 2.
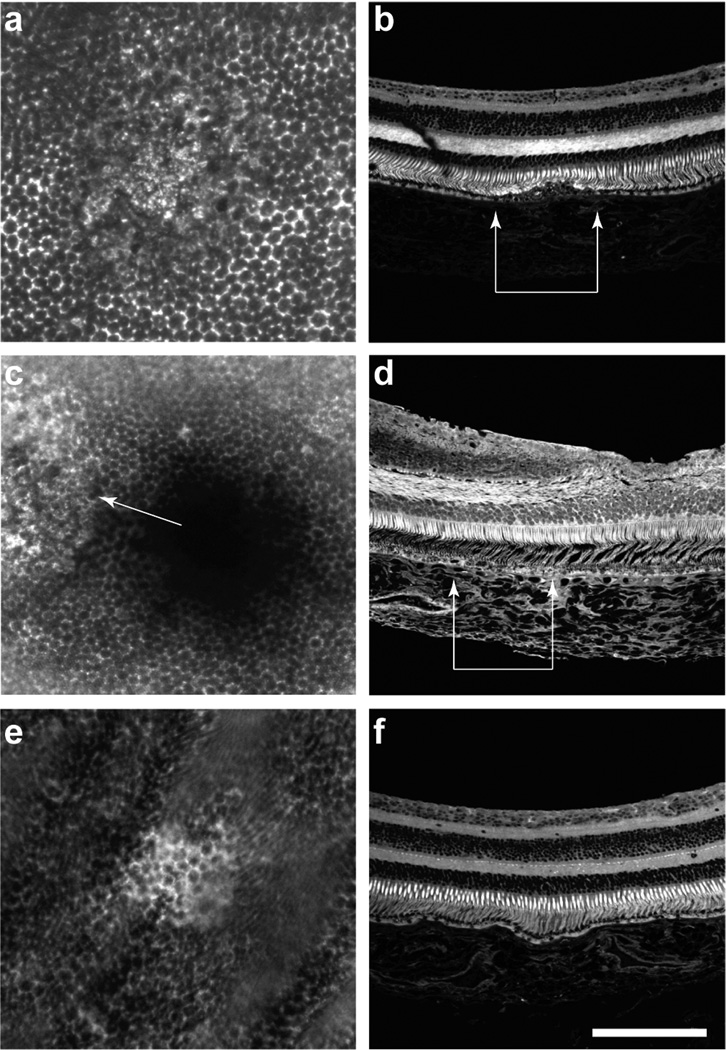
In vivo images of RPE AF photobleaching and disruption. A series of repeated images in the living macaque eye obtained using a fluorescence-enabled adaptive optics scanning light ophthalmoscope (AOSLO) shows the sequence of light-induced changes in RPE AF and photoreceptor reflectance that was observed following 568 nm light exposure. The honeycomb mosaic of discrete RPE cells can be seen because the cell nucleus does not contain lipofuscin and appears dark, whereas the cytoplasm surrounding the nucleus appears bright due to lipofuscin AF. The white outline in the pre-exposure image indicates the region of the retina exposed to either 788 J/cm2 or 210 J/cm2. The abrupt reduction in RPE AF intensity is visible in the immediately post-exposure images. There are no immediate changes in the appearance of the photoreceptor mosaic. Six days post-exposure, a long-term disruption in the RPE mosaic and an alteration in the photoreceptor reflectance (origin unknown) is seen with 788 J/cm2. Although less pronounced these changes persist over time, as seen 19 days post exposure. No long term changes were observed in the RPE or photoreceptors for 210 J/cm2 exposures.
AF photobleaching was only observed with exposure to visible, and not near-infrared (830 nm), light (Morgan et al. 2008). It was independent of the method of light delivery, whether scanning or uniform illumination (Morgan et al. 2008). The magnitude of the reduction in AF exhibits reciprocity of exposure irradiance and duration (Morgan et al. 2009). These properties are consistent with AF photobleaching being a photochemical phenomenon (Morgan et al. 2009). AF photobleaching is not caused by photopigment bleaching. That would predict a decrease in the absorption of fluorescence emission as it passes back through the photoreceptors (Theelen et al. 2008), thereby predicting AF brightening rather than reduction. Because AF photobleaching is observed by imaging the AF of lipofuscin, AF photobleaching almost certainly involves one or more of the fluorescent molecules contained within RPE lipofuscin. Without a clear understanding of the mechanism of this photochemical process, it is impossible to know if AF photobleaching is a marker of a benign or a potentially toxic event which should be avoided.
5.1 Multiple fluorophores are involved in AF photobleaching
Previously, AF photobleaching has only been investigated for 568 nm light exposures. The observed changes in RPE AF intensity could stem from one or several of the fluorescent molecules that comprise lipofuscin, potentially making it difficult to identify the particular molecules involved. Any of the bisretinoids are candidates for involvement in AF photobleaching and may be linked to the process that leads to permanent retinal damage. In A2E-laden ARPE-19 cells, irradiation with three times more green than blue light had a minimal impact on the number of non-viable cells (Sparrow et al. 2000), making it questionable whether A2E is involved in the effects that we are observing with 568 nm light, although it likely exhibits AF photobleaching when excited at lower wavelengths. The role of A2E in AF photobleaching was tested in an in vitro model (Section 5.2). Rozanowska and Sarna (Rozanowska and Sarna. 2005) found that the non-A2E, chloroform-soluble components of lipofuscin absorb considerably more 568 nm light than A2E. Given that atRAL dimer-E and atRAL dimer-PE have the longest peak absorbance wavelength of any of the identified components of lipofuscin (Kim et al. 2007), they are key candidates for involvement in the mechanism of AF photobleaching observed in response to 568 nm light. As a first step towards understanding the mechanism of AF photobleaching, it is important to determine whether only one or if more than one of the fluorescent molecules contained within the lipofuscin granules are susceptible to AF photobleaching.
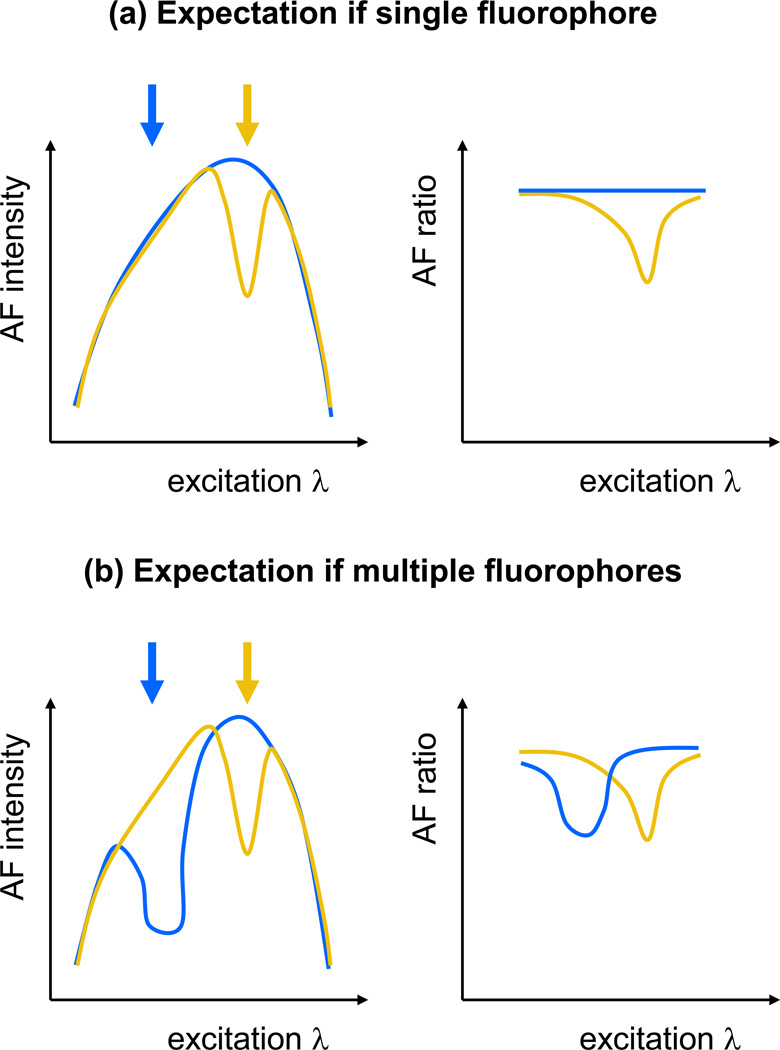
To determine this, the emitted AF intensity response, quantified by the AF ratio (Morgan et al. 2008, Morgan et al. 2009), for blue and yellow light exposures were measured for different AF excitation wavelengths. The AF ratio compares the AF within and surrounding an exposure region between pre- and post-exposure RPE images of a 2° field of view that were obtained under matched conditions (~1 J/cm2 at 568 nm or ~0.5 J/cm2 at 488 nm). The in vivo excitation spectrum of lipofuscin is a combination of the excitation spectra of the individual fluorophores and absorption of the anterior media. If one fluorophore is depleted, then the excitation spectrum will be altered in a manner similar to the excitation spectrum of the individual fluorophore (Figure 3a, yellow curve). If a light exposure does not deplete any fluorophores, then the excitation spectrum will not change from its original form and the AF ratio will be constant with excitation wavelength (Figure 3a, blue curve). If exposures to different wavelengths result in depletion of multiple fluorophores, then the AF ratio, as a function of excitation wavelength, will display different variations for each exposure wavelength and will represent the excitation spectra of the different fluorophores altered by each exposure wavelength (Figure 3b).
Figure 3.
Models of AF photobleaching. Schematic representation depicting AF ratio outcomes if a single fluorophore (a) or if multiple fluorophores (b) are involved in AF photobleaching. The arrows indicate the colour of the light exposure and are matched to the corresponding colour in the AF excitation spectra and AF ratio plots.
By performing exposures at 2 wavelengths (488 nm and 568 nm) and then calculating the AF ratio as measured with AF excitation at the same 2 wavelengths (Hunter et al. 2009), a plot of the AF ratio versus excitation wavelength shows different slopes of the separate curves for the 2 different exposure wavelengths (Figure 4). This suggests that multiple lipofuscin fluorophores are involved in the light-induced reduction of AF. Because the shape of the action spectrum for AF photobleaching changes depending on the wavelength of the light exposure, more than one lipofuscin fluorophore is involved. Of course, not all lipofuscin fluorophores are necessarily susceptible to AF photobleaching; this result only confirms that more than one is involved. Interestingly, there appears to be similar susceptibility to photobleaching by the AF molecules excited with 488 nm and 568 nm light, suggesting that the molecular species at each wavelength have similar photobleaching efficiency.
Figure 4.
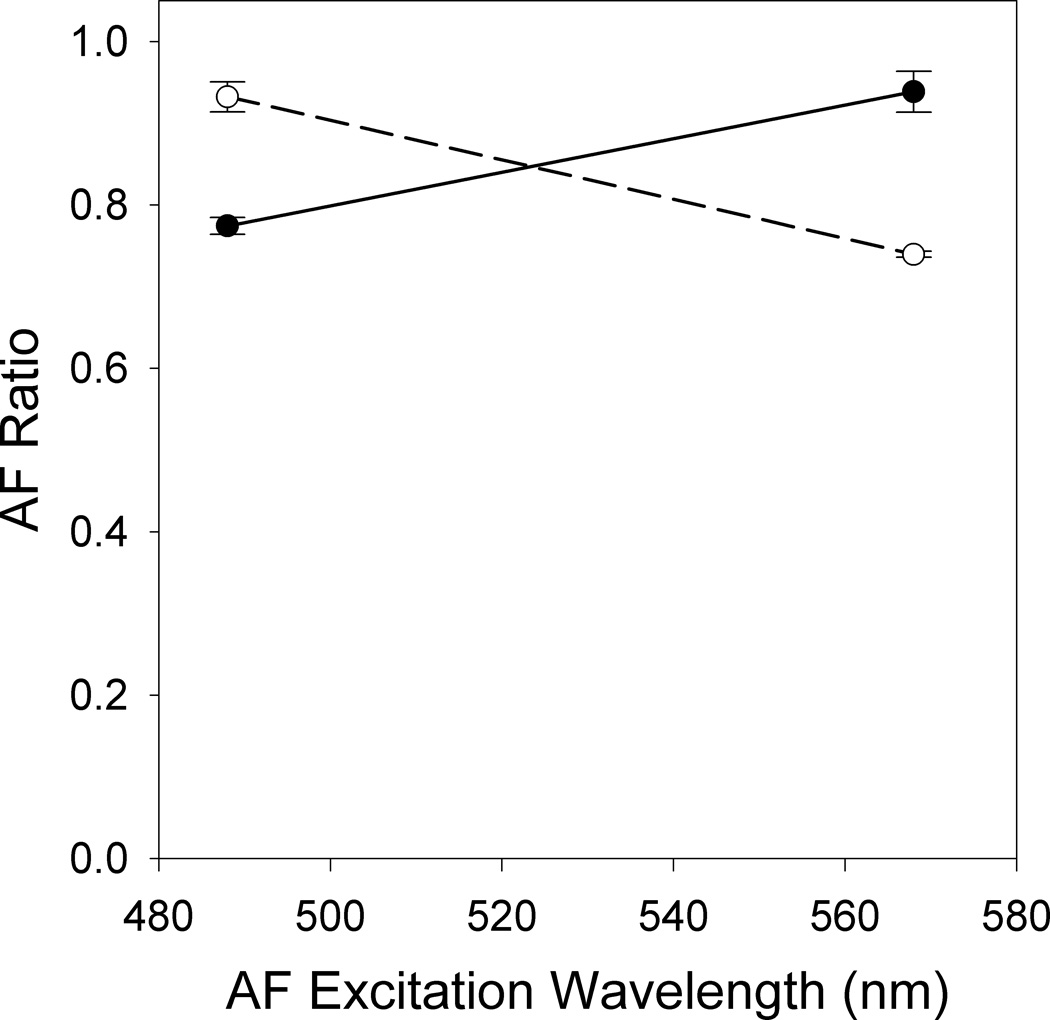
AF photobleaching involves multiple molecules. Average RPE AF ratio immediately post exposure to 88 J/cm2 of 488 nm (solid circles) or 130 J/cm2 of 568 nm (open circles) light plotted versus the wavelength used for AF excitation in the pre and post exposure images. Error bars represent the standard error of the mean.
5.2 In vitro models of AF photobleaching
To further elucidate properties of AF photobleaching, including the involvement of metabolic activity and whether A2E is a contributor to the AF photobleaching observed with 568 nm light exposures, we explored the AF response of 2 different ex vivo preparations for comparison to our in vivo results (Morgan et al. 2008). The response to 568 nm light was studied in fixed flat-mounted human RPE with the retina removed and fixed ARPE-19 cells laden with synthesized A2E. Using a model eye in our fluorescence-equipped adaptive optics scanning light ophthalmoscope (AOSLO), without employment of the adaptive optics, the AF in these cells was imaged before and after prescribed light exposures.
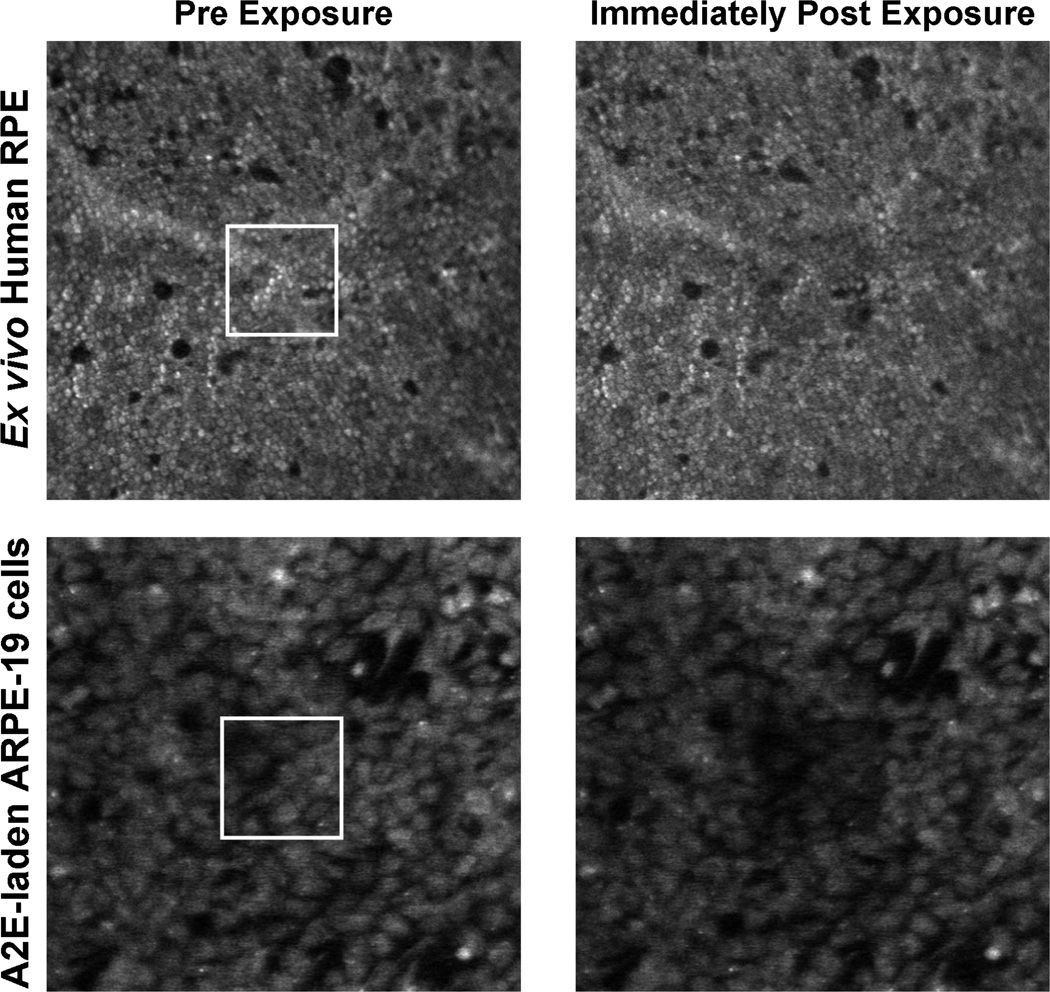
The response of the ex vivo preparations to light were similar to those observed in the living macaque (Morgan et al. 2008, Morgan et al. 2009). Exposure to near-IR light did not alter the AF intensity in either preparation. On the other hand, both ex vivo preparations showed similar and significant (t-tests; p ≤ 0.03) decreases in AF intensity immediately following illumination with the prescribed 568 nm light exposures. The region of the exposure is clearly visible as a darkened square in the post exposure AF images as compared to the pre exposure images (Figure 5). This indicates that A2E contributes to AF photobleaching from 568 nm light, even though this would be unexpected given its absorption spectrum and previous observations of decreased cell viability following blue light compared to green exposures (Sparrow et al. 2000). With increasing radiant exposure to 568 nm light, the AF immediately post exposure decreased significantly for the fixed ex vivo human RPE cells (ANOVA, p < 0.0005) and the ARPE-19 cells (ANOVA, p = 0.001). On the time scales of these exposures, the AF ratio showed reciprocity of duration and power. Thus, exposures of equivalent radiant exposure will have similar effects. This is characteristic of a photochemical process and thus the AF decrease reflects an intrinsic property of the fluorophores involved, including A2E. Within hours, partial recovery of the AF intensity was observed in the fixed tissue (Figure 6), implying that the recovery of RPE AF following light exposure is not entirely a metabolic process. The living eye is 10 times less sensitive to AF photobleaching than these ex vivo preparations and shows complete recovery (Morgan et al. 2008), hence there may be in vivo mechanisms that actively protect against light-induced changes in RPE. An in vitro model of live ARPE-19 cells laden with A2E also exhibited AF photobleaching and complete recovery after exposure to 480 nm light (Zhou et al. 2011). Our ex vivo results provide evidence that in vitro studies are a useful model of AF photobleaching in which the production of photooxidation products can be monitored. To understand the safety and mechanism of AF photobleaching, further investigations into the molecular origins of the observed phenomena are necessary.
Figure 5.
Ex vivo AF photobleaching. Pre (left) and immediately post 568 nm exposure (right) images of the ex vivo human RPE cells exposed to 30.6 J/cm2 (top) and the A2E-laden ARPE19 cells exposed to 106.4 J/cm2 (bottom). The exposure locations are outlined in white in the pre exposure images and are clearly visible in the post exposure images.
Figure 6.
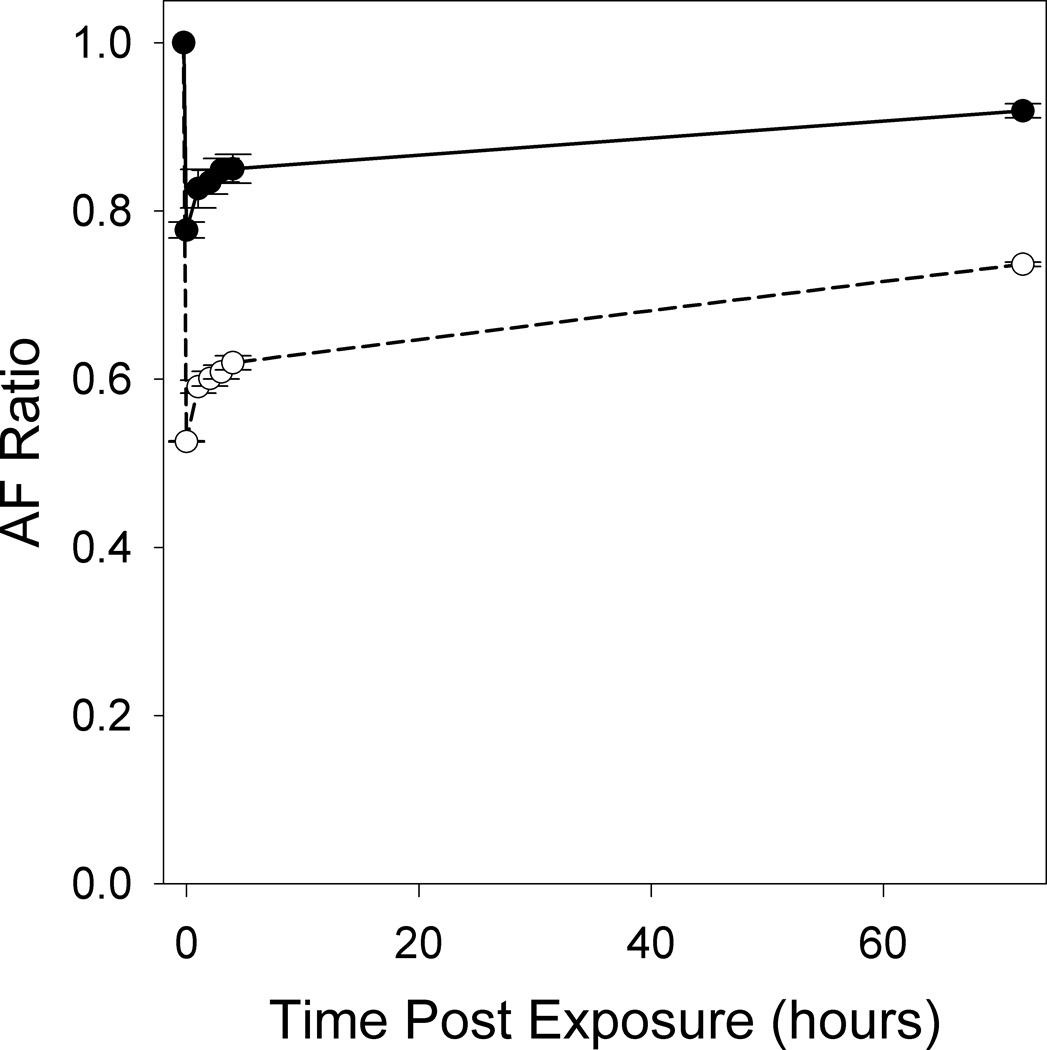
Quantifying ex vivo AF photobleaching and partial recovery. The reduction and partial recovery over 3 days of RPE AF is quantified by the AF ratio in A2E-laden ARPE-19 cells exposed to 14.2 J/cm2 (solid circles) or 106.4 J/cm2 (open circles) of 568 nm light. Pre and post exposure images were taken in a 2° field using 568 nm light. Each location received only one exposure and none of the regions overlapped. Error bars represent the standard error of the mean of 2 measurements.
5.3 Is AF photobleaching safe?
The decrease in RPE AF, observed after exposure to 568 nm light, may be a consequence of either the photoisomerization or photooxidation of lipofuscin fluorophores such as A2E. Photoisomerization is a non-toxic, reversible effect and if it is the cause of the observed phenomenon, then light exposures that lead to AF photobleaching should be considered safe. On the other hand, the molecular changes causing the photobleaching could be hazardous to the retina and may require a more conservative re-evaluation of current light safety standards which currently only protect against acute damage. By monitoring with ultraperformance liquid chromatography (UPLC), mass spectrometry, absorbance and fluorescence, the bisretinoids in A2E-laden ARPE-19 cells exposed to 0.3 – 0.5 J/cm2 of 480 or 430 nm light, suggests that AF photobleaching is associated with decreased A2E absorbance and increased levels (measured as absorbance) of some photooxidized forms of A2E. These results indicate that photooxidation of A2E is associated with AF photobleaching (Zhou et al. 2011). Therefore, AF photobleaching may be an indicator of in vivo lipofuscin photooxidation, which may be harmful to the cell. In this case, the observed recovery of AF photobleaching would not represent a reversal of photooxidation, but possibly a reorganization of A2E within the lipofuscin granules, such as migration of unbleached, previously unexcited, fluorophores between domains of the lysosomal organelle; a hypothesis that requires further research. As such, AF photobleaching could be a forerunner of photodamage. As an additional hazard, AF photobleaching may reflect the chronic accumulation of toxic products in RPE over a lifetime. The cumulative nature of photochemical reactions to light exposures has been demonstrated (Griess and Blankenstein. 1981, Ham et al. 1979). There have been reports of a linkage between age-related macular degeneration (AMD) and habitual light exposure (Taylor et al. 1992, Tomany et al. 2004), however other studies have not found such a relationship (Darzins et al. 1997).
6. The mechanism of RPE disruption
Although AF photobleaching in response to visible light (> 2 J/cm2 of 568 nm light) is a transient effect, at high exposures (≥ 247 J/cm2 of 568 nm light) a subsequent disruption in the RPE mosaic was observed (Figure 2) approximately a week after exposure in the living non-human primate eye with a fluorescence-equipped AOSLO with single cell resolution (Morgan et al. 2008). In these experiments, the retinal irradiances were confined to 0.5° square areas of the retina and exposures lasted 15 minutes (900 second). Images of the RPE in a 2° square region encompassing the exposure area and surrounding cells were obtained using ~1 J/cm2 of 568 nm light. That the RPE disrupting light exposures can produce a maximum retinal temperature increase of 1.5°C implies that it is photochemical and not thermal in origin (Morgan et al. 2008). RPE disruption occurs at light levels at or slightly below the MPE, which is alarming because the MPE is typically about 10 times below the damage threshold for small lesions and 2–3 times below for large lesions (American National Standards Institute. 2007). With the strongest exposures studied (788 J/cm2), in addition to RPE disruption, there was an altered appearance of the photoreceptor mosaic, as seen with AOSLO reflectance imaging (Figure 2). RPE disruption lasts as long as we have followed individual animals, ~3 years, although some remodeling appears to occur. These changes were also visible as discolorations in fundus images and as window-defects with fluorescein angiography (Morgan et al. 2008). They appear as dark regions in visible and infrared fundus AF images (Heidelberg SLO) indicating loss of lipofuscin and melanin, respectively. In addition, we have observed RPE disruption with 900 second exposures to 488 nm light with radiant exposures ≥ 79 J/cm2 (Hunter et al. 2009). Although the threshold for damage may be lower, this is already a factor of 2 below the published threshold for photochemical damage from a 1000 second exposure (Ham et al. 1976). However, this is comparable to the published thresholds of 79 J/cm2 (Ham et al. 1976) and 71 J/cm2 (Lund et al. 2006) reported for 100 second exposures to 488 nm and 476 nm light, respectively. For 488 nm exposures, no RPE disruption was observed at or below the ANSI photochemical MPE of 15 J/cm2 (American National Standards Institute. 2007). The discrepancy with previously published damage thresholds may stem from our ability to accurately and precisely monitor the retinal location at a cellular scale during the exposure and to dynamically correct for eye motion. Measurements of damage thresholds for additional wavelengths are necessary to clarify the action spectrum.
The retinal changes observed in vivo, were confirmed with histology in one macaque that was exposed to 568 nm light 6 months and 12 days prior to sacrifice. After the macaque was perfused with saline and 4% paraformaldehyde, the retinas were removed and wholemounted. Using a fluorescence confocal microscope (Zeiss Meta 510 Laser Scanning Microscope), we found persistent morphological retinal changes in the whole-mounted retinas for exposures ≥ 247 J/cm2 (Figure 7), confirming the in vivo appearance of disrupted lipofuscin fluorescence in the RPE at the sites of the exposures. The retinas were then embedded in paraffin and cut into 6 µm thick sections. For the 788 J/cm2 exposures, disruption of the RPE layer and cone outer segments was visible, although less so in the exposures that were 6 months old. Although the exposure site was clearly visible in the wholemount, there were no obvious changes in the 6 µm section of retina exposed to 247 J/cm2.
Figure 7.
Histology of RPE disruption. Fluorescence images of 3 light exposed locations in macaque retina seen in both wholemounted retina (a, c, e) and 6 µm paraffin sectioned (b, d, f) macaque retina, 12 days (a, b, e, f) and 6 months (c, d) post-exposure to 568 nm light with retinal radiant exposures of 788 J/cm2 (a–d) and 247 J/cm2 (e, f). In c, the central black region is the fovea and the bright region on the left (white arrow) is the location of the exposure showing RPE disruption. In the images of the paraffin sections, only the 788 J/cm2 exposures (edges denoted by white arrows in b and d) produced detectable changes in the photoreceptors and RPE. Scale bar is 130 µm.
Even though RPE disruption produces observable permanent changes in the AF pattern in the RPE, it is not clear whether and to what extent this results in loss of photoreceptor function and/or visual sensitivity. Using an AOSLO at 514 nm (0.12 µJ/cm2), recovery of photopigment density following a full bleach in 4 retinal locations was measured 2 to 6 months following 568 nm exposure. With these preliminary functional measurements, there was no detectable difference in the concentration of photopigment or its rate of recovery following a full bleach between regions of healthy RPE and areas of RPE disruption (Masella et al. 2011). In addition, one month after a 568 nm light exposure expected to cause RPE disruption, contrast thresholds for orientation discrimination (Gabor, σ = 0.15°, 2.5 cycles/degree) were not significantly different between exposed and surrounding retinal locations at the same eccentricity (Masella et al. 2011).
The mechanism of the observed RPE disruption is as yet unidentified, but is expected to be either Noell or Ham damage. RPE disruption occurs at irradiances that result in AF photobleaching and fully bleached photopigment, consistent with Ham damage. Thus, RPE disruption may be indicative of levels of lipofuscin photooxidation that overwhelm the cell. That threshold damage appears to be confined to the RPE, where the lipofuscin resides, is suggestive of a Ham damage mechanism, but is also consistent with observations of Noell damage in hamsters (Thumann et al. 1999). RPE disruption could be linked to photoreceptor photopigment and the retinoids of the visual cycle. It is unknown whether the RPE is the primary site of visible light absorption leading to retinal damage or if rods or cones are the initial targets for light absorption where toxic molecules are generated and then absorbed by the RPE. Until recently (Dubra A et al. 2011), we have been unable to image rod photoreceptors in the living primate eye to assess their viability in the presence of RPE disruption. It is significant that we observe RPE photodamage at longer wavelengths than expected for Ham damage, yet we observe changes in the RPE, even when photoreceptors appear normal (Morgan et al. 2008). There is a strong overlap between the 568 nm light exposures studied and the peaks of the scotopic (~510 nm) and photopic (~555 nm) visual sensitivity curves, indicators of photopigment absorption for rods and cones, respectively, suggesting Noell damage.
7. Factors affecting susceptibility to phototoxicity
The potential for light damage is influenced by a number of factors including age, diurnal fluctuations, and pathology. In turn, the role of these factors in susceptibility to damage is linked to the mechanism of damage.
7.1 Susceptibility and age
As the eye ages, the optical density increases and the cutoff wavelength of the anterior optics shifts towards 450 nm (as reviewed in van de Kraats J et al. 2007). This is primarily a result of yellowing of the crystalline lens with age. As such, the potential for blue light to reach the retina decreases with age. However, lipofuscin accumulates with age, potentially making older adults more susceptible to light damage from a mechanism that involves lipofuscin. If lipofuscin is the target molecule for a phototoxicity pathway, then infants, that naturally lack lipofuscin, will be less susceptible to Ham damage than older adults, even though their anterior optics transmit some near-UV light. To protect pseudophakic eyes from the potentially damaging effects of blue light and to potentially slow the progression of AMD (Section 7.3), blue-blocking intraocular lenses (IOLs) have been developed, but their value has been highly debated (Henderson and Grimes. 2010, Mainster and Turner. 2010). Although blue-blocking IOLs appear to have no significant effect on most measures of visual performance, they may impact scotopic vision and interrupt circadian rhythms (Henderson and Grimes. 2010).
Macular pigment, located in Henle fibers and inner plexiform layer of the central 3° of the macula, blocks short wavelength light from reaching the photoreceptors and RPE (Snodderly et al. 1984a, Snodderly et al. 1984b). Macular pigment absorbance is less than 5% for light longer than 520 nm (Snodderly et al. 1984b). Therefore, although macular pigment may play a role in foveal protection from blue light, it is unlikely to protect against Noell type damage mechanisms.
If the mechanism of damage is linked to retinoids of the visual cycle, then older adults with slowed dark adaptation kinetics (Jackson et al. 1999, Steinmetz et al. 1993), likely related to a decreased concentration of 11-cis-retinal in the RPE (Lamb and Pugh. 2004), may be less susceptible to retinal damage than young children. It is highly probable that both lipofuscin and the visual cycle play a role in phototoxicity, resulting in a complex trade-off among susceptibility, mechanism and age.
7.2 Susceptibility in diseases that affect the visual cycle
Phototoxicity becomes a serious consideration in the presence of retinal disease because of the potential for changes in the visual cycle and lipofuscin accumulation. Because of the potential difference in susceptibility, light damage in diseased eyes may have a different spectral dependence than in normal healthy eyes. In recessive Stargardt’s macular dystrophy, a mutation in the ABCR (ABCA4) gene leads to a progressive loss of central vision with delayed dark adaptation. By slowing the removal of all-trans-retinal from the photoreceptor disc membrane there is an accelerated accumulation of lipofuscin in the RPE. As a consequence, the RPE degenerates, followed by the photoreceptors (Travis et al. 2007). Whether the mechanism of photochemical light damage is related to changes in lipofuscin or molecules within the visual cycle, such as all-trans-retinal, individuals with recessive Stargardt’s disease will be highly susceptible to injury. People with Best disease, who have an excess accumulation of lipofuscin (Frangieh et al. 1982, Weingeist et al. 1982), are also potentially more susceptible to light damage, if the photochemical mechanism is linked to lipofuscin.
If the mechanism is directly related to retinoids involved in the visual cycle, then diseases that affect the regeneration of photopigments will have altered susceptibility to photochemical light damage as compared to healthy individuals. Fundus albipunctatus is a mildly progressive form of stationary night-blindness with delayed dark adaptation (Dryja. 2000). It is caused by an RDH5 mutation. This affects the conversion of 11-cis-retinol to 11-cis-retinal within the RPE, possibly resulting in local accumulations of retinyl esters, seen in a fundus exam as white dots scattered across the retina (Travis et al. 2007). These accumulations may have increased susceptibility to the effects of light. In a mouse model of Oguchi disease, another form of stationary night blindness caused by mutations that lead to nonfunctional arrestin and rhodopsin kinase, animals were highly susceptible to light damage, even showing photoreceptor loss in response to normal cyclic light rearing conditions (Chen et al. 1999). Mouse models of retinitis pigmentosa (a group of progressively blinding retinal diseases caused by genetic mutations that affect rhodopsin and the visual cycle), with a three-point mutation in rhodopsin (Wang et al. 1997) or transgenic T17M rhodopsin (White et al. 2007), showed increased susceptibility of the retina to light damage. Similarly, a dog model of retinitis pigmentosa also shows increased susceptibility to retinal damage (Cideciyan et al. 2005). Unfortunately, reducing light exposure in 2 people with retinitis pigmentosa did not slow the progression of the disease over a 5 year period as compared to their contralateral eye (Berson. 1980).
Leber’s congenital amaurosis results from a mutation in RPE65, RDH12 or LRAT genes. Patients exhibit abnormal development or premature degeneration of photoreceptors resulting in a severe loss of vision within a few months after birth (Travis et al. 2007). People with RPE65 mediated LCA are unable to form 11-cis-retinal and thus, the visual cycle is greatly reduced from the birth. Since the visual cycle in total is reduced, these LCA patients would have less susceptibility to Noell light damage. In addition, because the visual cycle does not progress as normal, these patients do not accumulate lipofuscin and do not show AF. Thus, they would also be less susceptible to Ham light damage. In contrast, Maeda and colleagues (Maeda et al. 2006), postulate that the photoreceptor dysfunction in Leber’s congenital amaurosis from an RDH12 mutation does not result from a lack of chromophore, but from an increased susceptibility to the toxic effects of light on an increased generation of retinoids. In any case, increased care should be taken to prevent light damage in the presence of diseases that affect the visual cycle.
7.3 Susceptibility and age-related macular degeneration
Several epidemiological studies have investigated the possibility of a link between light exposure and AMD with conflicting conclusions. Such retrospective epidemiological studies are difficult because their gross estimates of lifetime sunlight exposure require good memory recall in an elderly population. Often sunlight exposure is a crude estimate of the average amount of time spent outdoors, ignoring variations in spectral properties with location, time of day, or season, even though our knowledge of photochemical processes suggests that the eye will be most susceptible to visible and UV radiation (Sliney. 2005). Because UV exposure does not reach the retina in primates, it cannot contribute to the development of AMD (Arnarsson et al. 2006, Clemons et al. 2005, Taylor et al. 1992). A few studies, such as those taking place in Croatia (Plestina-Borjan and Klinger-Lasic. 2007, Vojnikovic et al. 2007, Vojnikovic et al. 2009), and Beaver Dam (Tomany et al. 2004), found a strong correlation between sunlight exposure, particularly visible light, and development of AMD. The European Eye Study (Fletcher et al. 2008) only found a significant association between blue light exposure and AMD in people with a low intake of anti-oxidants. They also suggested that blue light may be more damaging with increasing age (Fletcher et al. 2008). A study in Australia found a non-significant trend for greater mean annual ocular sun exposure in people with AMD as compared to those without (McCarty et al. 2001). On the other hand, no associations were found between AMD and estimated lifetime light exposure for studies taking place in Coastal Southern France (Delcourt et al. 2001), Reykjavik Iceland (Arnarsson et al. 2006), England (Khan et al. 2006), United States (Hyman et al. 1983), Australia (Darzins et al. 1997) and Finland (Hirvela et al. 1996). However, the Pathologies Oculaires Liées à l'Age (POLA) study did find a significant negative relationship between the use of sunglasses and formation of soft drusen (Delcourt et al. 2001). The Australian study noted an increased risk for AMD in people who were particularly glare sensitive in young adulthood (Darzins et al. 1997). This may relate to the fact that some individuals have larger pupils than others in the same outdoor daylight environment (Sliney, unpublished data). Given the possibility that AMD may be related to visible light exposure, until further research clarifies any relationship, unnecessary illumination should be avoided, especially in people with early signs of AMD as these eyes may be more prone to visible light damage.
It is surprising that there is so little epidemiological evidence for a role of light in development of AMD (Arnarsson et al. 2006, Clemons et al. 2005, Plestina-Borjan and Klinger-Lasic. 2007, Tomany et al. 2004, Vojnikovic et al. 2007, Vojnikovic et al. 2009) and other degenerative retinal diseases. Total lifetime light exposures among different people with different lifestyles must be orders of magnitude apart, but often with apparently little or no consequences based on the epidemiological literature (Arnarsson et al. 2006, Clemons et al. 2005, Darzins et al. 1997, Delcourt et al. 2001, Fletcher et al. 2008, Hirvela et al. 1996, Hyman et al. 1983, Khan et al. 2006, McCarty et al. 2001). We speculate that there are no clear effects of environmental light dose on phototoxicity and AMD incidence because the light exposure changes occur gradually and the retina has a chance to protect itself against these potential insults, similar to tanning protecting skin from sunburn. There is some evidence in rats that pre-treatment with bright light can reduce the extent of retinal damage (Li et al. 2003, Liu et al. 1998), although the mechanism of action is unclear. It could be that it is not the total lifetime light exposure that contributes to AMD, but that rapid increases in light exposure (i.e. a large first derivative of the light exposure) may escape preventative mechanisms, resulting in retinal damage.
Potentially consistent with this hypothesis, is the varying susceptibility to light-induced retinal damage with diurnal fluctuation and the level of dark-adaptation prior to light exposure (Duncan and O'Steen. 1985, Organisciak et al. 2000, Vaughan et al. 2002, White and Fisher. 1987, Wiechmann and O'Steen. 1992). Light exposure at night or in the morning is potentially more harmful than in the afternoon and evening. Such exposures may be more harmful because of the larger change in irradiance between the environment and the damaging exposure. However, as discussed previously, this could also stem from fluctuations in the ocular concentrations of photopigment and retinoids. Regardless of the mechanism, since phagocytosis follows a circadian cycle in the mammalian retina (Kevany and Palczewski. 2010), clinicians may wish to consider such findings when scheduling surgery and retinal exams in highly susceptible individuals.
7.4 Susceptibility and general pathology
Even some diseases that are not directly related to defects in the visual cycle or lipofuscin accumulation can result in increased susceptibility to retinal light damage. For example, Smith-Lemli-Opitz syndrome is a genetic defect that affects the production of cholesterol, resulting in an accumulation of cholesterol precursors that are highly prone to oxidation. Symptoms of Smith-Lemli-Opitz syndrome can include visual loss and photosensitivity. A rat model of Smith-Lemli-Opitz syndrome was more sensitive and exhibited more severe light-induced retinal damage than normal and albino rats (Vaughan et al. 2006).
7.5 Susceptibility, nutrition and pharmaceuticals
Diet, prescription medications and the use of herbal supplements can influence susceptibility to light damage. An anti-oxidant rich diet can help to prevent light-induced retinal damage and increase damage thresholds (Vaughan et al. 2002). Conversely, susceptibility to light damage may increase with the use of antidepressants (E.g. Thorazine and Prondol, but not Prozac) or antibiotics (E.g. tetracycline) with a tricyclic, heterocyclic or porphyrin ring system (Wang et al. 1992). A known potential side effect of the herbal supplement, St John’s wort (Hypericum perforatum L.), is photosensitivity and when combined with antibiotics or birth control pills, the risk of sun sensitivity may increase (MedlinePlus. 2010). Damage thresholds may be lower in people using such drugs or supplements. Therefore, a thorough medical history should be considered in order to prevent retinal damage as a result of retinal examination or ocular surgery.
7.6 Susceptibility in the Optometric and Ophthalmologic practice
The devices used for monitoring disease, such as a direct ophthalmoscope or a fundus camera, require that the retina be illuminated. Even a prolonged slit-lamp examination has been shown to result in retinal damage with fluorescein (Hochheimer et al. 1979). The altered phototoxic susceptibility in diseased eyes creates a further challenge for treatment since illumination may result in accelerated retinal damage. This was observed with a dog model of retinitis pigmentosa, in which the animals were exposed to retinal radiant exposures equivalent to those of a standard fundus camera (0.06 J/cm2). These exposures would not be expected to cause RPE disruption or AF photobleaching. Over the subsequent 24 hours, the site of the exposure became clearly visible as regions of altered reflectance in images obtained using an infrared scanning laser ophthalmoscope (Cideciyan et al. 2005). Patients with diabetic retinopathy have been linked to the occurrence of phototoxic maculopathy, even with short cataract surgeries lasting less than 30 minutes (Kleinmann et al. 2002). This would indicate that as little visible light as possible should be used in treating patients with retinitis pigmentosa and other diseases which may have increased phototoxic susceptibility.
In the operating room, surgeons require illumination to perform the procedures necessary to restore vision. There are countless reports of light damage resulting from cataract surgery, during which the light illuminating the crystalline lens also exposes the retina; for example, (Boldrey et al. 1984, Harada et al. 1988, Knox Cartwright et al. 2007, McDonald and Irvine. 1983, Menezo et al. 2002). Using an operating microscope at its highest illumination setting, Irvine and co-workers (Irvine et al. 1984) produced visible retinal lesions in pseudophakic rhesus macaque retinas with exposures that lasted slightly less than 8 minutes. In a retrospective analysis, phototoxic maculopathy resulted after IOL implantation in 8.5% of the surgeries performed (Kweon et al. 2009)}. This may occur even with surgeries lasting less than 30 minutes (Kleinmann et al. 2002). Light-induced damage has also been reported following corneal keratoplasty (Brod et al. 1986). In retinal surgery, an endoilluminator may be inserted into the vitreous and directed onto the area on which the surgeon is operating. The safety of these endoilluminators has been called into question (van den Biesen et al. 2000). Cell death in response to exposures from endoilluminators has been reported (Yanagi et al. 2006, Yanagi et al. 2007). There are several reports of phototoxic damage to the RPE following vitrectomy (Banker et al. 1997, McDonald et al. 1986, Michels et al. 1992, Poliner and Tornambe. 1992, Postel et al. 1998). With improved technology, the light output of endoilluminators is increasing, as is the illuminated area. However, with increased education about light safety and maximum exposure duration as well as adjustments to the spectral tuning of the light sources, the safety of surgical illumination may be improved. Alternatively, operating microscopes that detect infrared light in combination with infrared illumination are a safer option (Komaromy et al. 2008).
7.7 Prevention of photochemical retinal damage
Ophthalmic and Optometric clinics can improve their light safety for all patients with the use of devices that employ sources with near infrared or longer wavelengths, in place of a standard fundus camera and direct ophthalmoscope which use broadband visible light. This will minimize the probability that the light entering the eye will be of sufficient energy to lead to photochemical damage. Such instruments include scanning laser ophthalmoscopes which image the retina using near-infrared light (e.g. Heidelberg HRA). Optical coherence tomography can provide a cross-sectional view of the retina as well as an en face view, showing features similar to a fundus photo. These devices, such as the Zeiss Cirrus OCT, use a broad spectrum of infrared light. The development of infrared fundus cameras could also improve safety and provide a more traditional view of the retina. Minimizing the illumination of the fixation targets in any of these devices, could improve their safety even further.
The use of fluorescence imaging and fluorescein angiography should be limited since the visible light used to excite the fluorophores could be damaging to highly susceptible retinas. From a light safety standpoint, angiography with indo-cyanine green (peak excitation 790 – 810 nm; (Landsman et al. 1976)) is a preferable alternative to the use of fluorescein (peak excitation 490 nm; (Novotny and Alvis. 1961)) because neither visual stimulation nor lipofuscin excitation occurs at these longer wavelengths. Near-IR AF imaging can safely visualize the melanin and melanolipofuscin distribution in the RPE and choroid in normal eyes (Keilhauer and Delori. 2006) and may provide useful diagnostic and progression information in some diseased eyes (Kellner et al. 2009a, Kellner et al. 2009b, Schmitz-Valckenberg et al. 2010). Fluorescence imaging of the retina with two-photon excitation, although not yet demonstrated in humans, is a viable replacement for traditional fluorescence imaging. With the use of pulsed infrared light, two-photon fluorescence imaging is photochemically safer than visible light fluorescence excitation. This technique has recently been demonstrated in vivo in non-human primate retina (Hunter et al. 2011). However, it is presently not efficient enough for safe use in humans because of thermal phototoxicity.
Understanding the mechanisms of photochemical light damage may provide the key to developing preventative therapies for use prior to intentional light exposure. In rats, pretreatment with antioxidants has been shown to reduce the severity of photochemical retinal damage (Vaughan et al. 2002). A vitamin A deficiency (Noell. 1979) or drugs, such as isotretinoin or retinylamine (Ret-NH2) (Travis et al. 2007), which inhibit reactions in the visual cycle, have been shown to increase the threshold for light damage in mice. Similar drugs are proposed for the treatment of ocular diseases, such as Stargardt’s disease (Travis et al. 2007). Halothane anesthesia prevents the regeneration of rhodopsin and cone photopigments and has been shown to prevent light-induced retina damage in mice and rats (Keller et al. 2001). Alternatively, drugs, such as 9-cis-retinyl acetate (9-cis-R-Ac), that enhance the production of 11-cis-retinal and accelerate the visual cycle, improving ERG performance and dark adaptation (Maeda et al. 2009) may increase susceptibility to light damage, particularly Noell damage.
8. Impact on safety standards
Light safety standards, such as the American National Standard Institute’s (ANSI) standard for the safe use of lasers (American National Standards Institute. 2007), provide maximum permissible exposures (MPE) that protect the eye from light-induced damage resulting from either intentional or accidental exposures. These standards are designed to protect the eye and skin from accidental light exposures. In addition, many commercial ophthalmic imaging and lighting devices (e.g. fundus cameras and endoilluminators), as well as advanced research instruments for high resolution imaging, adhere to these standards with additional constraints imposed for intentional ophthalmic exposures (section 8.3, (American National Standards Institute. 2007). The International Commission on Non-Ionizing Radiation Protection also has provided guidance for reducing ocular exposures from ophthalmic instruments (Sliney et al. 2005).
From a safety standard perspective, damage refers to an alteration in the retina that is manifested by a visible change in the retina and/or a deterioration of visual function, either of which may be temporary or permanent. To a particular cell type, these changes may be a direct consequence of photon absorption within that cell (primary) or may result from a chain of events originating from photon absorption in a different cell class (secondary). Traditionally, the presence of retinal damage is diagnosed in vivo by identifying a visible alteration in a fundus photo (Roach et al. 2006, Sliney et al. 2002). By repeatedly performing exposures of varying radiant exposure in the retina of an animal model, the probability of producing a visible lesion can be plotted versus the input power and fit with a probit curve. From this, the damage threshold is given by the retinal radiant exposure with a 50% probability of causing retinal damage (Roach et al. 2006, Sliney et al. 2002). Measures of visual function may be used to identify the presence and extent of retinal damage. Such methods may be objective, such as electroretinograms (ERG) on chickens (Machida. 1994) and rats (Noell et al. 1966, Sugawara et al. 2000), or subjective (e.g. blinded reading of fundus photos). Psychophysical tests have included a measure of critical flicker-fusion frequency in the light damaged albino rat (Williams et al. 1985), and acuity measures in monkey (Merigan et al. 1981). Damaged retina can also be identified by means of histological section; for example, (Ham et al. 1978). This provides cross-sectional insight into the consequences of light on a cellular level, but does not allow for longitudinal monitoring of retinal changes. In most cases, the ocular MPE is an order of magnitude below published thresholds for retinal damage.
High resolution imaging using adaptive optics provides an in vivo view of the cells that are altered by light providing a unique opportunity to longitudinally study phototoxicity in vivo. Our observations of RPE disruption and AF photobleaching at light levels below the ANSI photochemical MPE (560 J/cm2) are alarming. There are several plausible explanations for the discrepancy between our results and those upon which the light safety standards are based. Cellular-scale retinal imaging allows for precise monitoring and correction of eye drift to stabilize the light exposure throughout its duration (Morgan et al. 2008), whereas earlier experiments did not (Ham et al. 1979). Even when monkeys are anesthetized, movement of the eye is still observed and could spread the light exposure over a larger area than intended, thereby reducing the actual retinal radiant exposure. Furthermore, the ANSI photochemical MPE was only designed to protect against Ham damage. Given the long duration of the exposures that have been used to study Noell damage it is unlikely to occur in a practical setting. Some cases of solar retinopathy have been reported following sunbathing for more than 2 hours on unusually bright days, however, the mechanism of damage is not clear (Yannuzzi LA et al.). Because safety standards were designed to protect against accidental light exposures, protection from such long duration exposures to visible light was not deemed necessary.
Future light safety standards will reflect the findings of RPE disruption and AF photobleaching. In order to protect against RPE disruption by an order of magnitude, then the current ANSI photochemical MPE at 568 nm will need to drop by a factor of 20. Additional experiments are necessary to establish the spectral profile of these changes. Even though it is transient, AF photobleaching meets the safety standard damage criterion of an observable retinal change. Without understanding the mechanism of AF photobleaching, it is unclear whether or not it is necessary to avoid this effect. So far, our in vitro results suggest that AF photobleaching is an indication of lipofuscin photooxidation, the products of which can be harmful. Thus, AF photobleaching should be avoided. However, should further research provide evidence that AF photobleaching is benign, then safety standards should not impose such restrictive limits as to avoid its occurrence. Photopigment bleaching, which increases retinal reflectance, is an example of a harmless transient change that does not impact light safety standards. Therefore, it is necessary for future research to not only establish the thresholds for light damage, but to understand their mechanism.
9. Conclusions and future directions
Although the retina has evolved to convert light into a visual perception of the world around us, it is also highly susceptible to photochemical damage. Excess amounts of visible light result in the eventual deterioration of RPE and photoreceptors. Although debated (van Norren and Gorgels. 2011), currently, two distinct classes of photochemical damage have been established. These different mechanisms lead to damage that may manifest in similar ways, but with different spectral characteristics. The complexity of the retina and photochemical reactions suggests that these damage mechanisms are not distinct and that even more are possible.
In vitro assays are necessary to gain insight into the mechanisms of light damage by providing information about the molecular changes involved in RPE disruption and AF photobleaching. This may provide a tool for the development of preventative strategies for use in normal and diseased eyes. Susceptibility to light damage may be increased in abnormal retinas. Future drug development for disease treatment may be aided by an understanding of the mechanism of retinal light damage or vice versa.
In vivo retinal imaging is a highly useful tool in establishing light damage thresholds and may provide increased sensitivity for detection of such retinal changes as reduced AF. Cellular-scale fluorescence and reflectance imaging can be used to measure the action spectrum for threshold RPE disruption and changes in the photoreceptor layer. Not only will these results be used by ANSI to establish the appropriate modifications to the existing light safety standards, but they will provide insight into the mechanism of photochemical damage. The action spectra for light damage can be used to help establish the mechanisms involved in RPE disruption: photopigments, retinoids, or bisretinoids in lipofuscin.
Currently, there is very little information about whether RPE disruption and AF photobleaching actually result in loss of photoreceptor function and/or visual sensitivity. With the further application of photopigment densitometry and primate psychophysical measures, the impact of AF photobleaching and RPE disruption on rod and cone function can be established. In the future, two-photon imaging of light-induced concentration changes of retinoids can also be used to monitor the visual cycle in regions of AF photobleaching and retinal damage. Development of such techniques will also be useful in assessing visual function in diseased eyes.
Acknowledgements
The authors thank Tracy Bubel, François Delori, Alfredo Dubra, William Fischer, Benjamin Masella, Jennifer Norris, Lee Anne Schery, Jennifer Strazzeri, Richard Wang, Robert Wolfe, and Lu Yin. This research was supported by the National Institute for Health, Bethesda, Maryland (NIH EY014375, NIH EY01319, NIH EY07125, NIH EY12951), and Research to Prevent Blindness. This work has been supported in part by the National Science Foundation Science and Technology Center for Adaptive Optics (Santa Cruz, California), managed by the University of California at Santa Cruz (cooperative agreement no.: AST-9876783). Jessica I. W. Morgan is currently at the University of Pennsylvania.
Abbreviations
- ABCA4
ATP-binding cassette retina
- AF
autofluorescence
- AMD
age-related macular degeneration
- ANSI
American National Standards Institute
- AOSLO
adaptive optics scanning light ophthalmoscope
- CRALBP
cellular retinaldehyde binding protein
- CRBP
cellular retinol binding protein
- IOL
intraocular lens
- IRBP
inter-photoreceptor retinol binding protein
- LRAT
lecithin retinol acyl transferase
- MPE
maximum permissible exposure
- RDH
all-trans-retinol dehydrogenase
- REST
retinyl ester storage particle
- ROS
reactive oxygen species
- RPE
retinal pigment epithelium
- λ
wavelength
- 11-cis RDH
11-cis retinol dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American National Standards Institute. Anonymous American National Standard for safe use of lasers. Orlando, FL: American National Standards Institute; Laser Institute of America; 2007. American National Standard for Safe use of Lasers. [Google Scholar]
- Arnarsson A, Sverrisson T, Stefansson E, Sigurdsson H, Sasaki H, Sasaki K, Jonasson F. Risk factors for five-year incident age-related macular degeneration: the Reykjavik Eye Study. Am. J. Ophthalmol. 2006;142:419–428. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Banker A, Freeman W, Kim J, Munguia D, Azen S, Lai M, Abrams G, Dosick W, Feldman S, Ochabski R, Fine S, Bailey I, Aaberg T, Berger B, Blankenship G, Brucker A, deBustros S, Yoshida A, Gilbert H, Han D, Kokame G, McCuen B, Frambach D, Sipperley J, Teeters V, Wood W. Vision-threatening complications of surgery for full-thickness macular holes. Ophthalmology. 1997;104:1442–1452. doi: 10.1016/s0161-6420(97)30118-3. [DOI] [PubMed] [Google Scholar]
- Bazan HE, Bazan NG, Feeney-Burns L, Berman ER. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Invest. Ophthalmol. Vis. Sci. 1990;31:1433–1443. [PubMed] [Google Scholar]
- Becker RS, Inuzuka K, Balke DE. Comprehensive investigation of the spectroscopy and photochemistry of retinals. I. Theoretical and experimental considerations of absorption spectra. J. Am. Chem. Soc. 1971;93:38–42. doi: 10.1021/ja00730a006. [DOI] [PubMed] [Google Scholar]
- Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew. Chem. Int. Ed Engl. 2002;41:814–817. doi: 10.1002/1521-3773(20020301)41:5<814::aid-anie814>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Berson EL. Light deprivation and retinitis pigmentosa. Vision Res. 1980;20:1179–1184. doi: 10.1016/0042-6989(80)90057-7. [DOI] [PubMed] [Google Scholar]
- Boldrey EE, Ho BT, Griffith RD. Retinal burns occurring at cataract extraction. Ophthalmology. 1984;91:1297–1302. doi: 10.1016/s0161-6420(84)34150-1. [DOI] [PubMed] [Google Scholar]
- Brod RD, Barron BA, Suelflow JA, Franklin RM, Packer AJ. Phototoxic retinal damage during refractive surgery. Am. J. Ophthalmol. 1986;102:121–123. doi: 10.1016/0002-9394(86)90224-2. [DOI] [PubMed] [Google Scholar]
- Busch EM, Gorgels TG, van Norren D. Temporal sequence of changes in rat retina after UV-A and blue light exposure. Vision Res. 1999;39:1233–1247. doi: 10.1016/s0042-6989(98)00233-8. [DOI] [PubMed] [Google Scholar]
- Cahill G, Besharse JC. Circadian rhythmicity in vertebrate retina: Regulation by a photoreceptor oscillator. Prog. Retin. Eye Res. 1995;14 267–291-267–291. [Google Scholar]
- Chen E. Inhibition of cytochrome oxidase and blue-light damage in rat retina. Graefes Arch. Clin. Exp. Ophthalmol. 1993;231:416–423. doi: 10.1007/BF00919652. [DOI] [PubMed] [Google Scholar]
- Chen J, Simon MI, Matthes MT, Yasumura D, LaVail MM. Increased susceptibility to light damage in an arrestin knockout mouse model of Oguchi disease (stationary night blindness) Invest. Ophthalmol. Vis. Sci. 1999;40:2978–2982. [PubMed] [Google Scholar]
- Cideciyan AV, Jacobson SG, Aleman TS, Gu D, Pearce-Kelling SE, Sumaroka A, Acland GM, Aguirre GD. In vivo dynamics of retinal injury and repair in the rhodopsin mutant dog model of human retinitis pigmentosa. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5233–5238. doi: 10.1073/pnas.0408892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL, 3rd Age-Related Eye Disease Study Research Group. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier RJ, Waldron WR, Zigman S. Temporal sequence of changes to the gray squirrel retina after near-UV exposure. Invest. Ophthalmol. Vis. Sci. 1989;30:631–637. [PubMed] [Google Scholar]
- Darzins P, Mitchell P, Heller RF. Sun exposure and age-related macular degeneration. An Australian case-control study. Ophthalmology. 1997;104:770–776. doi: 10.1016/s0161-6420(97)30235-8. [DOI] [PubMed] [Google Scholar]
- Delcourt C, Carriere I, Ponton-Sanchez A, Fourrey S, Lacroux A, Papoz L POLA Study Group. Light exposure and the risk of age-related macular degeneration: the Pathologies Oculaires Liees a l'Age (POLA) study. Arch. Ophthalmol. 2001;119:1463–1468. doi: 10.1001/archopht.119.10.1463. [DOI] [PubMed] [Google Scholar]
- Delmelle M. Retinal damage by light: possible implication of singlet oxygen. Biophys. Struct. Mech. 1977;3:195–198. doi: 10.1007/BF00535819. [DOI] [PubMed] [Google Scholar]
- Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest. Ophthalmol. Vis. Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- Dryja TP. Molecular genetics of Oguchi disease, fundus albipunctatus, and other forms of stationary night blindness: LVII Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2000;130:547–563. doi: 10.1016/s0002-9394(00)00737-6. [DOI] [PubMed] [Google Scholar]
- Dubra A, Sulai Y, Norris JL, Cooper RF, Dubis AM, Williams DR, Carroll J. Noninvasive imaging of the human rod photoreceptor mosaic using a confocal adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011;2(7):1864–1876. doi: 10.1364/BOE.2.001864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, O'Steen WK. The diurnal susceptibility of rat retinal photoreceptors to light-induced damage. Exp. Eye Res. 1985;41:497–507. doi: 10.1016/s0014-4835(85)80007-5. [DOI] [PubMed] [Google Scholar]
- Fishkin N, Pescitelli G, Sparrow JR, Nakanishi K, Berova N. Absolute configurational determination of an all-trans-retinal dimer isolated from photoreceptor outer segments. Chirality. 2004;16:637–641. doi: 10.1002/chir.20084. [DOI] [PubMed] [Google Scholar]
- Fishkin NE, Sparrow JR, Allikmets R, Nakanishi K. Isolation and characterization of a retinal pigment epithelial cell fluorophore: an all-trans-retinal dimer conjugate. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7091–7096. doi: 10.1073/pnas.0501266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher AE, Bentham GC, Agnew M, Young IS, Augood C, Chakravarthy U, de Jong PT, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Vioque J. Sunlight exposure, antioxidants, and age-related macular degeneration. Arch. Ophthalmol. 2008;126:1396–1403. doi: 10.1001/archopht.126.10.1396. [DOI] [PubMed] [Google Scholar]
- Frangieh GT, Green WR, Fine SL. A histopathologic study of Best's macular dystrophy. Arch. Ophthalmol. 1982;100:1115–1121. doi: 10.1001/archopht.1982.01030040093017. [DOI] [PubMed] [Google Scholar]
- Gaillard ER, Avalle LB, Keller LM, Wang Z, Reszka KJ, Dillon JP. A mechanistic study of the photooxidation of A2E, a component of human retinal lipofuscin. Exp. Eye Res. 2004;79:313–319. doi: 10.1016/j.exer.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Gorgels TG, van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest. Ophthalmol. Vis. Sci. 1995;36:851–863. [PubMed] [Google Scholar]
- Griess GA, Blankenstein MF. Additivity and repair of actinic retinal lesions. Invest. Ophthalmol. Vis. Sci. 1981;20:803–807. [PubMed] [Google Scholar]
- Ham WT, Jr, Mueller HA, Ruffolo JJ, Jr, Clarke AM. Sensitivity of the retina to radiation damage as a function of wavelength. Photochem. Photobiol. 1979;29:735–743. doi: 10.1111/j.1751-1097.1979.tb07759.x. [DOI] [PubMed] [Google Scholar]
- Ham WT, Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976;260:153–155. doi: 10.1038/260153a0. [DOI] [PubMed] [Google Scholar]
- Ham WT, Jr, Ruffolo JJ, Jr, Mueller HA, Clarke AM, Moon ME. Histologic analysis of photochemical lesions produced in rhesus retina by short-wave-length light. Invest. Ophthalmol. Vis. Sci. 1978;17:1029–1035. [PubMed] [Google Scholar]
- Hammer M, Richter S, Guehrs KH, Schweitzer D. Retinal pigment epithelium cell damage by A2-E and its photo-derivatives. Mol. Vis. 2006;12:1348–1354. [PubMed] [Google Scholar]
- Harada T, Koizumi E, Saito A, Sugita K, Hisada H, Awaya S. Three cases with light-induced retinopathy. Doc. Ophthalmol. 1988;69:11–18. doi: 10.1007/BF00154414. [DOI] [PubMed] [Google Scholar]
- Harwerth RS, Sperling HG. Effects of intense visible radiation on the increment-threshold spectral sensitivity of the rhesus monkey eye. Vision Res. 1975;15:1193–1204. doi: 10.1016/0042-6989(75)90162-5. [DOI] [PubMed] [Google Scholar]
- Henderson BA, Grimes KJ. Blue-blocking IOLs: a complete review of the literature Surv. Ophthalmol. 2010;55:284–289. doi: 10.1016/j.survophthal.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Hirvela H, Luukinen H, Laara E, Sc L, Laatikainen L. Risk factors of age-related maculopathy in a population 70 years of age or older. Ophthalmology. 1996;103:871–877. doi: 10.1016/s0161-6420(96)30593-9. [DOI] [PubMed] [Google Scholar]
- Hochheimer BF, D'Anna SA, Calkins JL. Retinal damage from light. Am. J. Ophthalmol. 1979;88:1039–1044. doi: 10.1016/0002-9394(79)90413-6. [DOI] [PubMed] [Google Scholar]
- Hoppeler T, Hendrickson P, Dietrich C, Reme C. Morphology and time-course of defined photochemical lesions in the rabbit retina. Curr. Eye Res. 1988;7:849–860. doi: 10.3109/02713688808997242. [DOI] [PubMed] [Google Scholar]
- Hunter JJ, Masella B, Dubra A, Sharma R, Yin L, Merigan WH, Palczewska G, Palczewski K, Williams DR. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed. Opt. Express. 2011;2:139–148. doi: 10.1364/BOE.2.000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JJ, Morgan JIW, Merigan WH, Williams DR. Retinal phototoxicity observed using high resolution autofluorescence imaging; International Laser Safety Conference, Paper 202; 2009. [Google Scholar]
- Hyman LG, Lilienfeld AM, Ferris FL, 3rd, Fine SL. Senile macular degeneration: a case-control study. Am. J. Epidemiol. 1983;118:213–227. doi: 10.1093/oxfordjournals.aje.a113629. [DOI] [PubMed] [Google Scholar]
- Imanishi Y, Batten ML, Piston DW, Baehr W, Palczewski K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell Biol. 2004;164:373–383. doi: 10.1083/jcb.200311079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine AR, Wood I, Morris BW. Retinal damage from the illumination of the operating microscope. An experimental study in pseudophakic monkeys. Arch. Ophthalmol. 1984;102:1358–1365. doi: 10.1001/archopht.1984.01040031100034. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Owsley C, McGwin G., Jr Aging and dark adaptation. Vision Res. 1999;39:3975–3982. doi: 10.1016/s0042-6989(99)00092-9. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Aleman TS, Cideciyan AV, Heon E, Golczak M, Beltran WA, Sumaroka A, Schwartz SB, Roman AJ, Windsor EA, Wilson JM, Aguirre GD, Stone EM, Palczewski K. Human cone photoreceptor dependence on RPE65 isomerase. Proc. Natl. Acad. Sci. U. S. A. 2007;104:15123–15128. doi: 10.1073/pnas.0706367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YP, Matsuda H, Itagaki Y, Nakanishi K, Sparrow JR. Characterization of peroxy-A2E and furan-A2E photooxidation products and detection in human and mouse retinal pigment epithelial cell lipofuscin. J. Biol. Chem. 2005;280:39732–39739. doi: 10.1074/jbc.M504933200. [DOI] [PubMed] [Google Scholar]
- Keilhauer CN, Delori FC. Near-infrared autofluorescence imaging of the fundus: visualization of ocular melanin. Invest. Ophthalmol. Vis. Sci. 2006;47:3556–3564. doi: 10.1167/iovs.06-0122. [DOI] [PubMed] [Google Scholar]
- Keller C, Grimm C, Wenzel A, Hafezi F, Reme C. Protective effect of halothane anesthesia on retinal light damage: inhibition of metabolic rhodopsin regeneration. Invest. Ophthalmol. Vis. Sci. 2001;42:476–480. [PubMed] [Google Scholar]
- Kellner S, Kellner U, Weber BH, Fiebig B, Weinitz S, Ruether K. Lipofuscin- and melanin-related fundus autofluorescence in patients with ABCA4-associated retinal dystrophies. Am. J. Ophthalmol. 2009a;147:895–902. doi: 10.1016/j.ajo.2008.12.023. 902.e1. [DOI] [PubMed] [Google Scholar]
- Kellner U, Kellner S, Weber BH, Fiebig B, Weinitz S, Ruether K. Lipofuscin- and melanin-related fundus autofluorescence visualize different retinal pigment epithelial alterations in patients with retinitis pigmentosa. Eye (Lond) 2009b;23:1349–1359. doi: 10.1038/eye.2008.280. [DOI] [PubMed] [Google Scholar]
- Kevany BM, Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan JC, Shahid H, Thurlby DA, Bradley M, Clayton DG, Moore AT, Bird AC, Yates JR Genetic Factors in AMD Study. Age related macular degeneration and sun exposure, iris colour, and skin sensitivity to sunlight. Br. J. Ophthalmol. 2006;90:29–32. doi: 10.1136/bjo.2005.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinmann G, Hoffman P, Schechtman E, Pollack A. Microscope-induced retinal phototoxicity in cataract surgery of short duration. Ophthalmology. 2002;109:334–338. doi: 10.1016/s0161-6420(01)00924-1. [DOI] [PubMed] [Google Scholar]
- Knox Cartwright NE, Tole DM, Haynes RJ, Males JJ, Dick AD, Mayer EJ. Recovery from macular phototoxicity after corneal triple procedure. Cornea. 2007;26:102–104. doi: 10.1097/01.ico.0000240102.51852.5a. [DOI] [PubMed] [Google Scholar]
- Komaromy AM, Acland GM, Aguirre GD. Operating in the dark: a night-vision system for surgery in retinas susceptible to light damage. Arch. Ophthalmol. 2008;126:714–717. doi: 10.1001/archopht.126.5.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremers JJ, van Norren D. Two classes of photochemical damage of the retina. Laser and Light in Ophthalmology. 1988;2 41-52-41-52. [Google Scholar]
- Kweon EY, Ahn M, Lee DW, You IC, Kim MJ, Cho NC. Operating microscope light-induced phototoxic maculopathy after transscleral sutured posterior chamber intraocular lens implantation. Retina. 2009;29:1491–1495. doi: 10.1097/IAE.0b013e3181aa103b. [DOI] [PubMed] [Google Scholar]
- Lamb LE, Zareba M, Plakoudas SN, Sarna T, Simon JD. Retinyl palmitate and the blue-light-induced phototoxicity of human ocular lipofuscin. Arch. Biochem. Biophys. 2001;393:316–320. doi: 10.1006/abbi.2001.2492. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog. Retin. Eye Res. 2004;23:307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Landsman ML, Kwant G, Mook GA, Zijlstra WG. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976;40:575–583. doi: 10.1152/jappl.1976.40.4.575. [DOI] [PubMed] [Google Scholar]
- Li F, Cao W, Anderson RE. Alleviation of constant-light-induced photoreceptor degeneration by adaptation of adult albino rat to bright cyclic light. Invest. Ophthalmol. Vis. Sci. 2003;44:4968–4975. doi: 10.1167/iovs.03-0140. [DOI] [PubMed] [Google Scholar]
- Liu C, Peng M, Laties AM, Wen R. Preconditioning with bright light evokes a protective response against light damage in the rat retina. J. Neurosci. 1998;18:1337–1344. doi: 10.1523/JNEUROSCI.18-04-01337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund DJ, Stuck BE, Edsall P. Retinal injury thresholds for blue wavelength lasers. Health Phys. 2006;90:477–484. doi: 10.1097/01.HP.0000190115.83416.cb. [DOI] [PubMed] [Google Scholar]
- Machida S. Evaluation of retinal light damage in aphakic chicken eyes using monochromatic ERGs] Nippon Ganka Gakkai Zasshi. 1994;98:55–62. [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Chou S, Desai A, Hoppel CL, Matsuyama S, Palczewski K. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J. Biol. Chem. 2009;284:15173–15183. doi: 10.1074/jbc.M900322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J. Biol. Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda A, Maeda T, Imanishi Y, Sun W, Jastrzebska B, Hatala DA, Winkens HJ, Hofmann KP, Janssen JJ, Baehr W, Driessen CA, Palczewski K. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J. Biol. Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainster MA, Turner PL. Blue-blocking IOLs decrease photoreception without providing significant photoprotection Surv. Ophthalmol. 2010;55:272–289. doi: 10.1016/j.survophthal.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Masella B, Hunter JJ, Yin L, Strazzeri J, Dubra A, Merigan WH, Williams DR. No loss of photopigment kinetics or contrast sensitivity seen after photochemical insult to the retinal pigment epithelium. IOVS. 2011;52:3199–3199. [Google Scholar]
- McCarty CA, Mukesh BN, Fu CL, Mitchell P, Wang JJ, Taylor HR. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch. Ophthalmol. 2001;119:1455–1462. doi: 10.1001/archopht.119.10.1455. [DOI] [PubMed] [Google Scholar]
- McDonald HR, Irvine AR. Light-induced maculopathy from the operating microscope in extracapsular cataract extraction and intraocular lens implantation. Ophthalmology. 1983;90:945–951. doi: 10.1016/s0161-6420(83)80022-0. [DOI] [PubMed] [Google Scholar]
- McDonald HR, Verre WP, Aaberg TM. Surgical-Management of Idiopathic Epiretinal Membranes. Ophthalmology. 1986;93:978–983. doi: 10.1016/s0161-6420(86)33635-2. [DOI] [PubMed] [Google Scholar]
- MedlinePlus. St. John's wort.; 2010. p. 2011. [Google Scholar]
- Mellerio J. Light Effects on the Retina. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology. Philadelphia: W B Saunders; 1994. p. Ch 116. [Google Scholar]
- Menezo JL, Peris-Martinez C, Taboada Esteve J. Macular phototrauma after cataract extraction and multifocal lens implantation: case report. Eur. J. Ophthalmol. 2002;12:247–249. doi: 10.1177/112067210201200315. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Pasternak T, Zehl D. Spatial and temporal vision of macaques after central retinal lesions. Invest. Ophthalmol. Vis. Sci. 1981;21:17–26. [PubMed] [Google Scholar]
- Michels M, Lewis H, Abrams GW, Han DP, Mieler WF, Neitz J. Macular phototoxicity caused by fiberoptic endoillumination during pars plana vitrectomy. Am. J. Ophthalmol. 1992;114:287–296. doi: 10.1016/s0002-9394(14)71792-1. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Dubra A, Wolfe R, Merigan WH, Williams DR. In vivo autofluorescence imaging of the human and macaque retinal pigment epithelial cell mosaic. Invest. Ophthalmol. Vis. Sci. 2009;50:1350–1359. doi: 10.1167/iovs.08-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Hunter JJ, Masella B, Wolfe R, Gray DC, Merigan WH, Delori FC, Williams DR. Light-induced retinal changes observed with high-resolution autofluorescence imaging of the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 2008;49:3715–3729. doi: 10.1167/iovs.07-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JI, Hunter JJ, Merigan WH, Williams DR. The reduction of retinal autofluorescence caused by light exposure. Invest. Ophthalmol. Vis. Sci. 2009;50:6015–6022. doi: 10.1167/iovs.09-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Gugiu B, Renganathan K, Davies MW, Gu X, Crabb JS, Kim SR, Rozanowska MB, Bonilha VL, Rayborn ME, Salomon RG, Sparrow JR, Boulton ME, Hollyfield JG, Crabb JW. Retinal pigment epithelium lipofuscin proteomics. Mol. Cell. Proteomics. 2008;7:1397–1405. doi: 10.1074/mcp.M700525-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell WK. ERG in experimental retinal toxicology. 1968. 290-299-290-299. [Google Scholar]
- Noell WK. Effects of environmental lighting and dietary vitamin A on the vulnerability of the retina to light damage. Photochem. Photobiol. 1979;29:717–723. doi: 10.1111/j.1751-1097.1979.tb07756.x. [DOI] [PubMed] [Google Scholar]
- Noell WK. Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res. 1980;20:1163–1171. doi: 10.1016/0042-6989(80)90055-3. [DOI] [PubMed] [Google Scholar]
- Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971;172:76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest. Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- Novotny HR, Alvis DL. A method of photographing fluorescence in circulating blood in the human retina. Circulation. 1961;24:82–86. doi: 10.1161/01.cir.24.1.82. [DOI] [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Darrow RA, Kutty RK, Kutty G, Wiggert B. Light history and age-related changes in retinal light damage. Invest. Ophthalmol. Vis. Sci. 1998;39:1107–1116. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest. Ophthalmol. Vis. Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14609–14613. doi: 10.1073/pnas.95.25.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautler EL, Morita M, Beezley D. Hemoprotein(s) mediate blue light damage in the retinal pigment epithelium. Photochem. Photobiol. 1990;51:599–605. doi: 10.1111/j.1751-1097.1990.tb01972.x. [DOI] [PubMed] [Google Scholar]
- Pawlak A, Rozanowska M, Zareba M, Lamb LE, Simon JD, Sarna T. Action spectra for the photoconsumption of oxygen by human ocular lipofuscin and lipofuscin extracts. Arch. Biochem. Biophys. 2002;403:59–62. doi: 10.1016/S0003-9861(02)00260-6. [DOI] [PubMed] [Google Scholar]
- Pawlak A, Wrona M, Rozanowska M, Zareba M, Lamb LE, Roberts JE, Simon JD, Sarna T. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem. Photobiol. 2003;77:253–258. doi: 10.1562/0031-8655(2003)077<0253:cotapo>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Penn JS. Effects of continuous light on the retina of a fish, Notemigonus crysoleucas. J. Comp. Neurol. 1985;238:121–127. doi: 10.1002/cne.902380111. [DOI] [PubMed] [Google Scholar]
- Plestina-Borjan I, Klinger-Lasic M. Long-term exposure to solar ultraviolet radiation as a risk factor for age-related macular degeneration. Coll. Antropol. 2007;31 Suppl 1:33–38. [PubMed] [Google Scholar]
- Poliner L, Tornambe P. Retinal-Pigment Epitheliopathy After Macular Hole Surgery. Ophthalmology. 1992;99:1671–1677. doi: 10.1016/s0161-6420(92)31746-4. [DOI] [PubMed] [Google Scholar]
- Porter J, Queener HM, Lin JE, Thorn K, Awwal A, editors. Adaptive Optics for Vision Science: Principles, Practices, Design and applications. Hoboken, NJ: John Wiley & Sons, Inc.; 2006. [Google Scholar]
- Postel E, Pulido J, Byrnes G, Heier J, Waterhouse W, Han D, Mieler W, Guse C, Wipplinger W. Long-term follow-up of iatrogenic phototoxicity. Arch. Ophthalmol. 1998;116:753–757. doi: 10.1001/archopht.116.6.753. [DOI] [PubMed] [Google Scholar]
- Rapp LM, Tolman BL, Koutz CA, Thum LA. Predisposing factors to light-induced photoreceptor cell damage: retinal changes in maturing rats. Exp. Eye Res. 1990;51:177–184. doi: 10.1016/0014-4835(90)90070-b. [DOI] [PubMed] [Google Scholar]
- Reme CE. The dark side of light: rhodopsin and the silent death of vision the proctor lecture. Invest. Ophthalmol. Vis. Sci. 2005;46:2671–2682. doi: 10.1167/iovs.04-1095. [DOI] [PubMed] [Google Scholar]
- Roach W, Thomas R, Buffington G, Polhamus G, Notabartolo J, DiCarlo C, Stockton K, Stolarski D, Schuster K, Carothers V, Rockwell B, Cain C. Simultaneous Exposure Using 532 and 860 nm lasers for visible lesion thresholds in the rhesus retina. Health Phys. 2006;90:241–249. doi: 10.1097/01.HP.0000180773.07672.af. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Kukielczak BM, Hu DN, Miller DS, Bilski P, Sik RH, Motten AG, Chignell CF. The role of A2E in prevention or enhancement of light damage in human retinal pigment epithelial cells. Photochem. Photobiol. 2002;75:184–190. doi: 10.1562/0031-8655(2002)075<0184:troaip>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Rozanowska M, Sarna T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem. Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- Schmitz-Valckenberg S, Lara D, Nizari S, Normando EM, Guo L, Wegener AR, Tufail A, Fitzke FW, Holz FG, Cordeiro MF. Localisation and significance of in vivo near-infrared autofluorescent signal in retinal imaging. Br. J. Ophthalmol. 2010 doi: 10.1136/bjo.2010.189498. [DOI] [PubMed] [Google Scholar]
- Schutt F, Davies S, Kopitz J, Holz FG, Boulton ME. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest. Ophthalmol. Vis. Sci. 2000;41:2303–2308. [PubMed] [Google Scholar]
- Sliney D, Aron-Rosa D, DeLori F, Fankhauser F, Landry R, Mainster M, Marshall J, Rassow B, Stuck B, Trokel S, West TM, Wolffe M International Commission on Non-Ionizing Radiation Protection. Adjustment of guidelines for exposure of the eye to optical radiation from ocular instruments: statement from a task group of the International Commission on Non-Ionizing Radiation Protection (ICNIRP) Appl. Opt. 2005;44:2162–2176. doi: 10.1364/ao.44.002162. [DOI] [PubMed] [Google Scholar]
- Sliney DH. Exposure geometry and spectral environment determine photobiological effects on the human eye. Photochem. Photobiol. 2005;81:483–489. doi: 10.1562/2005-02-14-RA-439. [DOI] [PubMed] [Google Scholar]
- Sliney DH, Mellerio J, Gabel VP, Schulmeister K. What is the meaning of threshold in laser injury experiments? Implications for human exposure limits. Health Phys. 2002;82:335–347. doi: 10.1097/00004032-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Snodderly DM, Auran JD, Delori FC. The macular pigment. II. Spatial distribution in primate retinas. Invest. Ophthalmol. Vis. Sci. 1984a;25:674–685. [PubMed] [Google Scholar]
- Snodderly DM, Brown PK, Delori FC, Auran JD. The macular pigment. I. Absorbance spectra, localization, and discrimination from other yellow pigments in primate retinas. Invest. Ophthalmol. Vis. Sci. 1984b;25:660–673. [PubMed] [Google Scholar]
- Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest. Ophthalmol. Vis. Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- Sparrow JR, Wu Y, Kim CY, Zhou J. Phospholipid meets all-trans-retinal: the making of RPE bisretinoids. J. Lipid Res. 2010;51:247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest. Ophthalmol. Vis. Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- Sperling HG, Harwerth RS. Red-green cone interactions in the increment-threshold spectral sensitivity of primates. Science. 1971;172:180–184. doi: 10.1126/science.172.3979.180. [DOI] [PubMed] [Google Scholar]
- Steinmetz RL, Haimovici R, Jubb C, Fitzke FW, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br. J. Ophthalmol. 1993;77:549–554. doi: 10.1136/bjo.77.9.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T, Sieving PA, Bush RA. Quantitative relationship of the scotopic and photopic ERG to photoreceptor cell loss in light damaged rats. Exp. Eye Res. 2000;70:693–705. doi: 10.1006/exer.2000.0842. [DOI] [PubMed] [Google Scholar]
- Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch. Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- Theelen T, Berendschot TT, Boon CJ, Hoyng CB, Klevering BJ. Analysis of visual pigment by fundus autofluorescence. Exp. Eye Res. 2008;86:296–304. doi: 10.1016/j.exer.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Thumann G, Bartz-Schmidt KU, Kociok N, Kayatz P, Heimann K, Schraermeyer U. Retinal damage by light in the golden hamster: an ultrastructural study in the retinal pigment epithelium and Bruch's membrane. J. Photochem. Photobiol. B. 1999;49:104–111. doi: 10.1016/s1011-1344(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Tomany SC, Cruickshanks KJ, Klein R, Klein BE, Knudtson MD. Sunlight and the 10-year incidence of age-related maculopathy: the Beaver Dam Eye Study. Arch. Ophthalmol. 2004;122:750–757. doi: 10.1001/archopht.122.5.750. [DOI] [PubMed] [Google Scholar]
- Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu. Rev. Pharmacol. Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Kraats J, van Norren D. Optical density of the aging human ocular media in the visible and the UV. J Opt Soc Am A Opt Image Sci Vis. 2007;24(7):1842–1857. doi: 10.1364/josaa.24.001842. [DOI] [PubMed] [Google Scholar]
- van den Biesen PR, Berenschot T, Verdaasdonk RM, van Weelden H, van Norren D. Endoillumination during vitrectomy and phototoxicity thresholds. Br. J. Ophthalmol. 2000;84:1372–1375. doi: 10.1136/bjo.84.12.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Norren D, Gorgels TG. The action spectrum of photochemical damage to the retina: a review of monochromatic threshold data. Photochem. Photobiol. 2011;87:747–753. doi: 10.1111/j.1751-1097.2011.00921.x. [DOI] [PubMed] [Google Scholar]
- van Norren D, Schellekens P. Blue light hazard in rat. Vision Res. 1990;30:1517–1520. doi: 10.1016/0042-6989(90)90032-g. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Nemke JL, Fliesler SJ, Darrow RM, Organisciak DT. Evidence for a circadian rhythm of susceptibility to retinal light damage. Photochem. Photobiol. 2002;75:547–553. doi: 10.1562/0031-8655(2002)075<0547:efacro>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp. Eye Res. 2006;82:496–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vojnikovic B, Micovic V, Coklo M, Vojnikovic D. Sun exposure and visual field damage among children on the Adriatic Island Rab--possible initial risk factor in development of age-related macular degeneration. Coll. Antropol. 2009;33:747–749. [PubMed] [Google Scholar]
- Vojnikovic B, Njiric S, Coklo M, Spanjol J. Ultraviolet sun radiation and incidence of age-related macular degeneration on Croatian Island Rab. Coll. Antropol. 2007;31 Suppl 1:43–44. [PubMed] [Google Scholar]
- Wang JS, Estevez ME, Cornwall MC, Kefalov VJ. Intra-retinal visual cycle required for rapid and complete cone dark adaptation. Nat. Neurosci. 2009;12:295–302. doi: 10.1038/nn.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Lam TT, Tso MO, Naash MI. Expression of a mutant opsin gene increases the susceptibility of the retina to light damage. Vis. Neurosci. 1997;14:55–62. doi: 10.1017/s0952523800008750. [DOI] [PubMed] [Google Scholar]
- Wang RH, Dillon J, Reme C, Whitt R, Roberts JE. The potential ocular phototoxicity of antidepressant drugs. Lens Eye Toxic. Res. 1992;9:483–491. [PubMed] [Google Scholar]
- Wang Z, Dillon J, Gaillard ER. Antioxidant properties of melanin in retinal pigment epithelial cells. Photochem. Photobiol. 2006a;82:474–479. doi: 10.1562/2005-10-21-RA-725. [DOI] [PubMed] [Google Scholar]
- Wang Z, Keller LM, Dillon J, Gaillard ER. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochem. Photobiol. 2006b;82:1251–1257. doi: 10.1562/2006-04-01-RA-864. [DOI] [PubMed] [Google Scholar]
- Weingeist TA, Kobrin JL, Watzke RC. Histopathology of Best's macular dystrophy. Arch. Ophthalmol. 1982;100:1108–1114. doi: 10.1001/archopht.1982.01030040086016. [DOI] [PubMed] [Google Scholar]
- White DA, Fritz JJ, Hauswirth WW, Kaushal S, Lewin AS. Increased sensitivity to light-induced damage in a mouse model of autosomal dominant retinal disease. Invest. Ophthalmol. Vis. Sci. 2007;48:1942–1951. doi: 10.1167/iovs.06-1131. [DOI] [PubMed] [Google Scholar]
- White MP, Fisher LJ. Degree of light damage to the retina varies with time of day of bright light exposure. Physiol. Behav. 1987;39:607–613. doi: 10.1016/0031-9384(87)90160-0. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, O'Steen WK. Melatonin increases photoreceptor susceptibility to light-induced damage. Invest. Ophthalmol. Vis. Sci. 1992;33:1894–1902. [PubMed] [Google Scholar]
- Williams RA, Pollitz CH, Smith JC, Williams TP. Flicker detection in the albino rat following light-induced retinal damage. Physiol. Behav. 1985;34:259–266. doi: 10.1016/0031-9384(85)90114-3. [DOI] [PubMed] [Google Scholar]
- Williams TP, Howell WL. Action spectrum of retinal light-damage in albino rats. Invest. Ophthalmol. Vis. Sci. 1983;24:285–287. [PubMed] [Google Scholar]
- Wu Y, Fishkin NE, Pande A, Pande J, Sparrow JR. Novel lipofuscin bisretinoids prominent in human retina and in a model of recessive Stargardt disease. J. Biol. Chem. 2009;284:20155–20166. doi: 10.1074/jbc.M109.021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Yanase E, Feng X, Siegel MM, Sparrow JR. Structural characterization of bisretinoid A2E photocleavage products and implications for age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7275–7280. doi: 10.1073/pnas.0913112107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Inoue Y, Jang WD, Kadonosono K. A2e mediated phototoxic effects of endoilluminators. Br. J. Ophthalmol. 2006;90:229–232. doi: 10.1136/bjo.2005.076711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y, Iriyama A, Jang WD, Kadonosono K. Evaluation of the safety of xenon/bandpass light in vitrectomy using the A2E-laden RPE model. Graefes Arch. Clin. Exp. Ophthalmol. 2007;245:677–681. doi: 10.1007/s00417-006-0428-x. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Fisher YL, Krueger A, Slakter J. Solar retinopathy: a photobiological and geophysical analysis. Trans Am Ophthalmol Soc. 1987;85:120–158. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Hunter JJ, Masella B, Williams DR, Sparrow JR. Bleaching and recovery of RPE autofluorescence. IOVS. 2011;52:3201. [Google Scholar]