Abstract
The ubihydroquinone: cytochrome c oxidoreductase, or cytochrome bc1, is a central component of photosynthetic and respiratory energy transduction pathways in many organisms. It contributes to the generation of membrane potential and proton gradient used for cellular energy production (ATP). The three-dimensional structures of cytochrome bc1 indicate that its two monomers are intertwined to form a symmetrical homodimer. This unusual architecture raises the issue of whether the monomers operate independently, or function cooperatively during the catalytic cycle of the enzyme. In this review, recent progress achieved in our understanding of the mechanism of function of dimeric cytochrome bc1 is presented. New genetic approaches producing heterodimeric enzymes, and emerging insights related to the inter monomer electron transfer between the heme b cofactors of cytochrome bc1 are described.
Keywords: cytochrome bc1, heterodimer, inter monomer electron transfer, Q cycle mechanism, Rhodobacter capsulatus
Introduction
The ubihydroquinone: cytochrome c oxidoreductase (cytochrome bc1 or complex III) is a multi-subunit membrane bound enzyme central to photosynthetic and respiratory electron transport chains of many organisms, including bacteria, archaea, eukaryotic mitochondria and chloroplasts [1–3]. This enzyme catalyzes oxidation of hydroquinone (QH2) to quinone (Q) and transfers the resulting electrons, usually via c-type cytochrome, to the reaction centers in photosynthetic and cytochrome c oxidases in aerobic respiratory growth conditions. It couples the free energy released by these reactions to the translocation of electrons and protons across membrane to contribute to the formation of proton motive force used for ATP synthesis [4].
The static three dimensional (3D) structures of cytochrome bc1 indicate that it is a symmetrical homodimer [5–9] with at least three catalytic subunits: the Rieske Fe/S protein with a high potential [2Fe2S] cluster, the cytochrome b with two b-type (one low potential (bL) and one high potential (bH)) hemes, and the cytochrome c1 with a c-type heme [10] (Fig. 1). Additional subunits in variable numbers are also present in some species [6–8,11]. The Q-cycle model is generally used to describe the mechanism of function of cytochrome bc1 [4,12,13]. Accordingly, two QH2 molecules are oxidized at a QH2 (Qo) site on the positive side, and one Q molecule is reduced at a Q reduction (Qi) site on the negative side, of the membrane. Each QH2 oxidation is initiated by the oxidized Fe/S protein, which accepts one electron and transfers it to cytochrome c1 via a large-scale movement of its cluster bearing extrinsic domain [14,15]. This electron is then transferred from cytochrome c1 to other downstream electron carriers and terminal acceptors. The second electron from QH2 oxidation is conveyed to the low potential hemes bL and bH, and then to a Q forming a stable semiquinone (SQ) at the (Qi) site (Fig. 1). Following a second turnover of a Qo site the SQ at the Qi site is converted to QH2 and released from the enzyme, completing the catalytic cycle [12]. This model accounts well for the electron transfer pathways between the cofactors of a monomeric cytochrome bc1. However, it does not describe whether the two consecutive QH2 oxidations, which are required to complete a full catalytic cycle, take place at the Qo site of a given monomer or at the two distinct Qo sites of the dimeric enzyme. The 3D structures of cytochrome bc1 revealed that its cofactors, except the mobile [2Fe2S] clusters, are distributed symmetrically within the dimeric enzyme. Moreover, the distances separating the hemes bL–bH in a given monomer, and the hemes bL–bL between the two monomers are quasi-identical (Fig. 1). These findings raised a number of intriguing questions (e.g., see [16,17]), including the independent or co-ordinated functions of cytochrome bc1 monomers. The architecture of cytochrome bc1 might play a functional role in addition to the structural stability of the enzyme if the two consecutive QH2 oxidations alternate between the two Qo sites of the dimeric enzyme, or if inter monomer electronic communication occurs between the heme cofactors. A number of hypothetical models, including “activated Q cycle” [18], “alternating Q cycle” [19] or “heterodimeric Q cycle” [20], invoking different structural and functional interactions between the Qo and Qi sites within one monomer, or between both monomers, of cytochrome bc1 have been described. Initially, peculiar transient kinetics data were interpreted as indication of a dimeric Q cycle mechanism for QH2 oxidation [18] despite other possible explanations. In recent years, more convincing experimental approaches [21–23] and theoretical calculations [24] were reported. In this review, we survey recent studies on inter monomer electronic communication within cytochrome bc1. We describe the different genetic systems used to produce asymmetric cytochrome bc1 heterodimers, and critically assess the outcomes of these efforts in understanding the mechanism of function of dimeric cytochrome bc1.
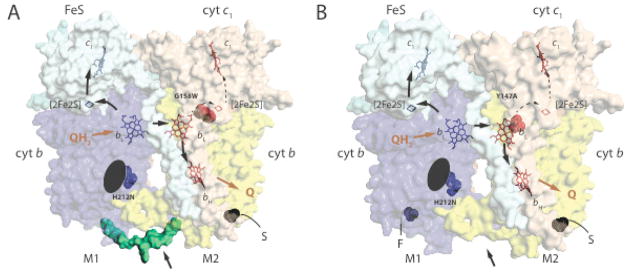
Fig. 1. Structure and function of heterodimeric cytochromes bc1.
A. A hypothetical structural model for a non-native heterodimeric cytochrome b-bc1. The 3D structure of cytochrome bc1 (PDB entry 1ZRT) was modified to include the Qi site H212N mutation (blue) on one monomer (M1) and the Qo site G158W mutation (red) on the other (M2) to depict a heterodimeric enzyme produced by the one-plasmid system [29]. The carboxyl end of one cytochrome b (blue) is linked to the amino end of the other (yellow) with a peptide (green) indicated by an arrow to create cytochrome b-b that is not naturally present in cytochrome bc1. S refers to the Strep–tag on the carboxyl terminal end of cytochrome b-b. The Fe/S and cytochrome c1 subunits are shown in light blue and light brown, with the cofactors ([2Fe2S] and hemes bH, bL and c1) in monomer M1 in blue and those in M2 in red. The black arrows indicate the electron transfer pathways across the monomers, Q and QH2 correspond to quinone and hydroquinone, respectively. The black ellipsoid indicates the absence of heme bH on monomer M1. B. A hypothetical structural model for a native-like heterodimeric cytochrome bc1. The 3D structure of cytochrome bc1 (PDB entry 1ZRT) was modified to include the Qi site H212N mutation (blue) on one monomer (M1) and the Qo site Y147A mutation (red) on the other (M2) to depict a heterodimeric enzyme produced by the two-plasmids system [32]. The labels are as in A, and note the absence (indicated by an arrow) of the peptide linking together the cytochromes b, and the presence of Flag- (F) and Strep (S)-tags on the carboxyl terminals of two cytochrome b on monomers M1 and M2, respectively.
Recent approaches to probe inter monomer electronic communication in cytochrome bc1
Half of the sites reactivity
Early on, Trumpower and colleagues described that one molecule of stigmatellin (a Qo site inhibitor) per dimer was sufficient to completely inhibit cytochrome bc1 [25], and that when yeast cytochrome bc1 is inhibited by antimycin A (a Qi site inhibitor) the amount of cytochrome c1 reduced corresponded to half of that present in cytochrome bc1 [21]. These studies assumed that equilibrium between the two hemes bH is attained via electron transfer between the hemes bL to support the “half of the sites reactivity” model for yeast [26] and bacterial [27] cytochrome bc1. More recently, a Paracoccus denitrificans strain that produces a quasi-wild type and a Qo site defective mutant variants of cytochrome bc1, tagged with His- and Strep-epitopes, respectively, (see below “two-plasmids system” for details) was described [28]. The quasi-wild type form of cytochrome bc1 used in this study lacked the amino terminal acidic part of cytochrome c1 and produced a dimeric enzyme, which otherwise forms a tetramer in its native state. From this strain, homodimeric (with two wild type Qo, or two mutant Qo sites) and heterodimeric (with a wild type Qo site in one monomer, and a mutant Qo site in the other monomer) cytochrome bc1 were purified by affinity chromatography with appropriate epitope-tags. Purified homodimeric wild type and heterodimeric mutant cytochromes bc1 exhibited comparable extents of cytochrome c1 and cytochrome bH reductions and enzyme activities, but antimycin-mediated stimulation was observed only with the homodimeric wild type enzyme. These findings suggested that only one of the two Qo sites of the wild type homodimer, or the mutant heterodimer is active, and that inter monomer electron transfer occurs in cytochrome bc1 [28]. These interesting findings are consistent with the alternating Q cycle model [19], although they do not prove directly the occurrence of either an inactive Qo site in wild type, or inter monomer electron transfer between the hemes bL in the heterodimeric mutant cytochrome bc1. In addition, this P. denitrificans mutant provides no information about the physiological ability of a heterodimeric cytochrome bc1 to support cellular growth.
One-plasmid system
More recently, a different genetic system to probe inter monomer electron transfer in dimeric cytochrome bc1 was reported by Osyczka and collaborators [29]. They adapted a previously described Rhodobacter capsulatus genetic system [30] to carry an ingenuously modified petABC (structural genes of cytochrome bc1) operon. Two copies of petB (encoding cytochrome b) were connected to each other via a designed linker sequence to form a fused petB-B gene on a single plasmid, to produce a ‘cytochrome b-b’ subunit, which is tagged with Strep-epitope at its carboxyl end [29] (Fig. 2A). This “one-plasmid” system was considered to produce a non-native but fully active dimeric “cytochrome b-bc1” in which the two monomers are covalently linked to each other via a linker between two cytochromes b (Fig. 1A). The system was used to generate single and double mutations on either or both cytochrome b copies to yield homodimeric and heterodimeric cytochrome b-bc1 variants with active or inactive Qo and Qi sites. The cytochrome b G158W substitution that inhibits QH2 oxidation at the Qo site without affecting the other cofactors [30,31], and the H212N substitution that eliminates heme bH [16,22] were used to inactivate one or both monomers to produce heterodimers (Figs. 1A and 2A). Using appropriate cytochrome b-bc1 mutants and measuring their activities, the authors concluded that electrons move freely between the hemes bL and distribute without regulation among the hemes bH using an H-shaped electron transfer path (a “bus bar”) within a dimeric cytochrome b-bc1 [29].
Fig. 2. Genetic systems producing heterodimeric cytochromes bc1.
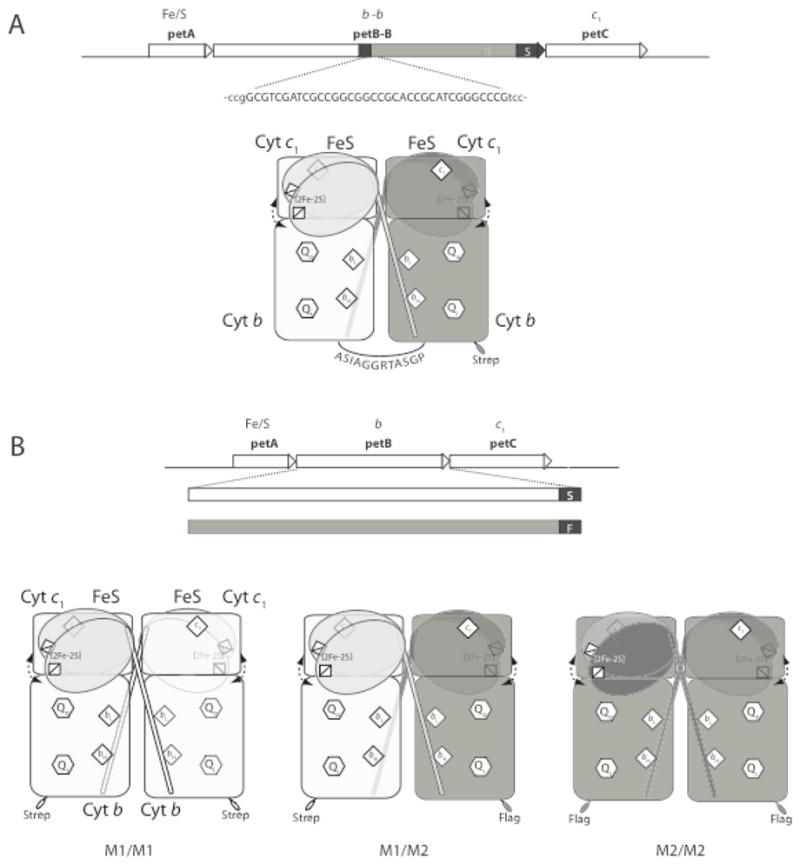
A. One-plasmid system is genetically less stable possibly due to high frequency intra-molecular rearrangements between petB genes (encoding cytochrome b) duplications carried by the plasmid, but produces only heterodimeric cytochromes bc1 [29]. petB-B encodes cytochrome b-b, formed by linking two cytochrome b copies by a dodecameric peptide. petA and petC refer to the structural genes of Fe/S protein and cytochrome c1, respectively. S indicates the Strep-tag epitope added to the carboxyl end of the second copy of cytochrome b. B. Two-plasmids system is genetically more stable, as only one copy of petABC (structural genes encoding cytochrome bc1) is carried by each plasmid. Various subunits of cytochrome bc1 are produced and re-assorted independently, leading the cells harboring this system to produce a combination of homodimeric (M1/M1 and M2/M2) and heterodimeric (M1/M2) cytochrome bc1 that are differentially tagged by the Flag (F) and Strep (S) epitopes, [32], as shown. Apparently no high frequency inter-molecular rearrangements are observed in this system, but the heterodimeric cytochromes bc1 thus produced need to be separated from homodimeric variants by affinity chromatography via appropriate epitope tags.
Our ongoing experiments indicate that the R. capsulatus one-plasmid system with cytochrome b-b duplications, constructed as reported in [29], exhibit extreme genetic instability (~ >10−2) and does not confer normal photosynthetic growth to R. capsulatus cytochrome bc1-minus mutant. Even under respiratory conditions, where growth can be maintained via a cytochrome bc1 independent branch (i.e., via an alternate hydroquinone oxidase), selective pressure against intra-molecular direct cytochrome b repeats appears to be strong. Plasmids seem to rearrange immediately to discard one of the cytochrome b copies to yield wild type-like homodimeric cytochrome bc1. We also observe that high frequency intra-molecular rearrangements similar to those described above (petAB-BC) (Fig. 2A) occur when the entire petABC operon is duplicated as either direct (petABC-ABC) or inverted (petABC-CBA) repeats on a single plasmid. These strains also exhibit poor photosynthetic growth and high frequency of intra-molecular rearrangements, to quickly generate uncontrollable mixtures of homo and heterodimeric cytochromes bc1 (manuscript in preparation). Until we understand and control the occurrence of frequent intra-molecular rearrangements, these undesirable features restrict severely the use of the one-plasmid system for studies of heterodimeric cytochromes bc1.
Two plasmids system
A genetic system, similar to that used in [28], for studying inter monomer electron transfer in dimeric cytochromes bc1 was initiated about a decade ago by our group, and reported recently [32]. This system also derives from earlier described R. capsulatus genetic system [30] and harbors two different plasmids (marked with different antibiotic resistance, KanR and TetR). Each of the plasmids has a single copy of petABC operon where each petB is carboxyl-terminally tagged with a different (Strep and Flag) epitope [32] (Fig. 2B). The cytochrome bc1 subunits being produced and assorted independently in cells, the “two-plasmids” system yields two sets of homodimeric cytochrome bc1 with the same epitope at their monomers (Strep-Strep or Flag-Flag) together with a set of heterodimeric cytochrome bc1 with a different tag at each monomer (Flag-Strep) (Fig. 1B). Unlike the one-plasmid system, the two-plasmids system carrying wild type petABC operons confers quasi-wild type like photosynthetic growth to R. capsulatus cytochrome bc1-minus mutants. Inter-molecular genetic rearrangements among the two plasmids appear to be less frequent (~ < 10−4) than intra-molecular events seen with the one-plasmid system and intra-molecular duplications. With two-plasmids system, use of different replicons of different plasmid copy numbers changes steady-state amounts of homodimeric and heterodimeric cytochrome bc1 variants in cells. In addition, the two-plasmid system is not restricted to heteromerization of cytochrome b only as it can yield heterodimers with different combinations of all cytochrome bc1 subunits. However, extensive purification is required to isolate the desired heterodimeric cytochrome bc1 variant among its multiple combinations produced.
This two-plasmids genetic system was used to probe inter monomer electronic communication between the hemes bL of cytochrome bc1, by selective inactivation of one Qo site of one monomer and one Qi site of the other monomer in a heterodimeric construct (Fig. 1B and 2B). Based on trials and errors, the previously characterized cytochrome b Y147A and H212N substitutions for inactivating the Qo and Qi sites, respectively were chosen. The former mutation affects drastically the rate of electron transfer from QH2 oxidation to heme bL at the Qo site [33], whereas the latter causes loss of heme bH [16,22], eliminating SQ formation at the Qi site. Strains that produced only homodimeric mutant cytochromes bc1 (i. e, carrying cytochrome b Y147A or H212N mutations on both plasmids) were unable to grow in photosynthesis and produced inactive enzymes. In contrast, strains producing heterodimeric cytochrome bc1 (i.e., carrying cytochrome b Y147A and H212N mutations on two different plasmids) exhibited slow photosynthetic growth compared with a wild type strain and had cytochrome bc1 activity [32]. Thus, electron transfer between the hemes bL can interconnect across the enzyme the active Qo site on one monomer to the active Qi site on the other monomer of the heterodimeric cytochrome bc1 to generate enough enzyme activity to support photosynthetic growth. Detailed, molecular genetic, biochemical and biophysical studies of cells with two-plasmids revealed semi-quantitatively that inter molecular heme bL–bL–bH electron transfer occurs more slowly than intra-monomer heme bL–bH electron transfer, but is sufficient to sustain slow photosynthetic growth. Precise determination of inter molecular hemes bL–bL electron transfer rates is complicated due to electronic coupling between the photosynthetic reaction centers and cytochromes bc1 in membranes. Vigorous photosynthetic growth was observed when higher amounts of heterodimeric cytochromes bc1 were produced [32] (manuscript in preparation), documenting the physiological relevance of the two-plasmids system with respect to the efficiency and amount of a heterodimeric cytochrome bc1.
Summary and future studies
Genetic approaches that were initiated in recent years are now probing functional roles of the dimeric architecture of cytochrome bc1 [28,29,32]. Experiments are revealing, with varying degrees of rigor and reliability, the occurrence of slow but appreciable inter monomer electron transfer between the hemes bL, at least in mutant heterodimeric cytochrome bc1 enzymes that are forced to support photosynthetic growth [32]. Further studies of electronic communication between the hemes bL, especially in a wild type cytochrome bc1, is of paramount importance to our understanding of the mechanism of function of a dimeric enzyme, but this requires additional work. First, currently available heterodimeric cytochrome bc1 production systems need improvements. Choosing appropriate mutations and respiration-deficient backgrounds might assess the physiological capability of P. denitrificans heterodimeric mutant cytochrome bc1 enzyme reported in [28]. Inactivating RecA enzyme, which is known to reduce the frequency of homologous recombination in many organisms [34], might reduce molecular rearrangements to increase genetic stability of the one-plasmid system. Using different kinds of epitope tag or different Qo and Qi site mutations might improve the stability of purified active heterodimeric cytochromes bc1 produced by the two-plasmids R. capsulatus [32] (manuscript in preparation).
Second, inter monomer electronic communication per se deserves further studies. Direct determinations of inter monomer hemes b electron transfer rates might be achieved using photo-activatable ruthenium cytochrome c derivatives [35]. The role of aromatic residues, like F195 and Y199 of cytochrome b, located between the hemes bL at the interface of the monomers in the dimeric cytochrome bc1 with respect to inter monomer electron transfer [36,37] need reexamination. A two-plasmids system producing appropriate heterodimeric enzymes with an inactive Qo site in one monomer and an inactive Qi site in the other monomer, together with selected cytochrome b F195 or Y199 substitutions, might probe better the role of these residues on inter monomer electronic communication in dimeric cytochrome bc1. Moreover, availability of a reliable heterodimeric cytochrome bc1 with one fully active and one fully inactive monomer generated via the two-plasmids system might probe if intra-monomer hemes bL–bH electron transfer alone can support photosynthetic growth without any hemes bL-bL electronic coupling. Comparing the amounts of non-productive bypass reactions (i.e., ROS production) generated by an appropriate heterodimeric cytochrome bc1 having only one active monomer with those produced by a wild type homodimeric enzyme with two active monomers, might be eye opening in respect to physiological regulation of cytochrome bc1. Could steady state inter monomer electronic communications within a dimeric cytochrome bc1 minimize unproductive by-pass reactions? Could it play a protective role to minimize ROS mediated oxidative damages under specific physiological conditions, like high membrane potential or limited oxygen availability, rendering hemes bL partially reduced [24]?
Third, time is ripe to start using heterodimeric cytochromes bc1 to probe intra and inter monomer Qo–Qi sites interactions in particular for initiation and regulation of the first versus the second QH2 oxidation during cytochrome bc1 catalytic cycle. Recent structural rationalization [38] of the heterodimeric Q cycle model [20] begs experimental testing. Inspection of cytochrome b structure indicates that its “rotating” E helix (providing all necessary ligand interactions at the Qi site) and its ef loop extension on the Qo site might be tethered to the more “static” D helix upon SQ formation at the Qi site. Faster electron transfer rates between hemes bL–bH as compared to those between hemes bL–bL–bH [32] might favor intra, instead of inter, monomer SQ formation at the cognate Qi sites of cytochrome bc1 monomers. Consequently, following the first QH2 oxidation conformation of the ef loop could change upon SQ production on the other side of the membrane, and inhibit access of oxidized Fe-S protein to the cognate Qo site on the same monomer [20,38]. After the second QH2 oxidation, only dismutation of the SQ molecules via inter monomer electronic communication would then complete the catalytic cycle to produce Q and QH2, and relax the SQ-tethered Qi sites, to render the cognate Qo sites active for the next turnover of the enzyme. Production of appropriate heterodimeric cytochrome bc1 variants should be invaluable to assess the validity, if any, of various aspects of speculative models [20].
In summary, the establishment of a dimeric architecture for cytochrome bc1 raised in recent years interesting questions aimed at complementing the Q-cycle scheme generally used to describe the mechanism of function of a monomeric enzyme. In particular, do the monomers operate independently or function cooperatively? Do the two consecutive QH2 oxidations required to complete the catalytic cycle occur in the same or different monomers? Does electronic communication occur between the hemes b cofactors of cytochrome bc1? If so, is there any selective or beneficial advantage? These and other related outstanding questions awaiting answers enticed the development of new genetic approaches to produce novel heterodimeric cytochromes bc1. Ongoing studies of these variant enzymes are progressing on our understanding of the structure-function relationship of dimeric cytochrome bc1. Hopefully, future studies will better define whether or not the mechanism of function of a dimeric cytochrome bc1 invokes intra and inter monomer cooperation and electronic communications.
Acknowledgments
This work was supported by grants from NIH (GM 38237) and DOE (91ER 20052) to F. D.
Abbreviations
- Q
quinone
- SQ
semiquinone
- QH2
hydroquinone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Darrouzet E, Moser CC, Dutton PL, Daldal F. Large scale domain movement in cytochrome bc1: a new device for electron transfer in proteins. Trends Biochem Sci. 2001;26:445–51. doi: 10.1016/s0968-0004(01)01897-7. [DOI] [PubMed] [Google Scholar]
- 2.Osyczka A, Moser CC, Dutton PL. Fixing the Q cycle. Trends Biochem Sci. 2005;30:176–82. doi: 10.1016/j.tibs.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Cape JL, Bowman MK, Kramer DM. Understanding the cytochrome bc complexes by what they don’t do. The Q-cycle at 30. Trends Plant Sci. 2006;11:46–55. doi: 10.1016/j.tplants.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976;62:327–67. doi: 10.1016/0022-5193(76)90124-7. [DOI] [PubMed] [Google Scholar]
- 5.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Crystal structure of the cytochrome bc1 complex from bovine heart mitochondria. Science. 1997;277:60–6. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata S, et al. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, et al. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–84. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 8.Hunte C, Koepke J, Lange C, Rossmanith T, Michel H. Structure at 2.3 A resolution of the cytochrome bc1 complex from the yeast Saccharomyces cerevisiae co-crystallized with an antibody Fv fragment. Structure. 2000;8:669–84. doi: 10.1016/s0969-2126(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 9.Berry EA, Huang LS, Saechao LK, Pon NG, Valkova-Valchanova M, Daldal F. X-Ray Structure of Rhodobacter capsulatus Cytochrome bc1: Comparison with its Mitochondrial and Chloroplast Counterparts. Photosynth Res. 2004;81:251–75. doi: 10.1023/B:PRES.0000036888.18223.0e. [DOI] [PubMed] [Google Scholar]
- 10.Berry EALD-W, Huang L-S, Daldal F. The Purple Phototrophic Bacteria. Springer; Berlin: 2009. Stuctural and mutational studies of the cytochrome bc1 complex. [Google Scholar]
- 11.Esser L, Elberry M, Zhou F, Yu CA, Yu L, Xia D. Inhibitor-complexed structures of the cytochrome bc1 from the photosynthetic bacterium Rhodobacter sphaeroides. J Biol Chem. 2008;283:2846–57. doi: 10.1074/jbc.M708608200. [DOI] [PubMed] [Google Scholar]
- 12.Crofts AR, Meinhardt SW, Jones KR, Snozzi M. The role of the quinone pool in the cyclic electron-transfer chain of Rhodopseudomonas sphaeroides: a modified Q-cycle mechanism. Biochim Biophys Acta. 1983;723:202–218. doi: 10.1016/0005-2728(83)90120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–12. [PubMed] [Google Scholar]
- 14.Darrouzet E, Valkova-Valchanova M, Moser CC, Dutton PL, Daldal F. Uncovering the [2Fe2S] domain movement in cytochrome bc1 and its implications for energy conversion. Proc Natl Acad Sci U S A. 2000;97:4567–72. doi: 10.1073/pnas.97.9.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darrouzet E, Daldal F. Movement of the iron-sulfur subunit beyond the ef loop of cytochrome b is required for multiple turnovers of the bc1 complex but not for single turnover Qo site catalysis. J Biol Chem. 2002;277:3471–6. doi: 10.1074/jbc.M107974200. [DOI] [PubMed] [Google Scholar]
- 16.Osyczka A, Moser CC, Daldal F, Dutton PL. Reversible redox energy coupling in electron transfer chains. Nature. 2004;427:607–12. doi: 10.1038/nature02242. [DOI] [PubMed] [Google Scholar]
- 17.Cramer WA, Hasan SS, Yamashita E. The Q cycle of cytochrome bc complexes: a structure perspective. Biochim Biophys Acta. 2011;1807:788–802. doi: 10.1016/j.bbabio.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gopta OA, Feniouk BA, Junge W, Mulkidjanian AY. The cytochrome bc1 complex of Rhodobacter capsulatus: ubiquinol oxidation in a dimeric Q-cycle? FEBS Lett. 1998;431:291–6. doi: 10.1016/s0014-5793(98)00768-6. [DOI] [PubMed] [Google Scholar]
- 19.Trumpower BL. A concerted, alternating sites mechanism of ubiquinol oxidation by the dimeric cytochrome bc1 complex. Biochim Biophys Acta. 2002;1555:166–73. doi: 10.1016/s0005-2728(02)00273-6. [DOI] [PubMed] [Google Scholar]
- 20.Cooley JW, Lee DW, Daldal F. Across membrane communication between the Qo and Qi active sites of cytochrome bc1. Biochemistry. 2009;48:1888–99. doi: 10.1021/bi802216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covian R, Gutierrez-Cirlos EB, Trumpower BL. Anti-cooperative oxidation of ubiquinol by the yeast cytochrome bc1 complex. J Biol Chem. 2004;279:15040–9. doi: 10.1074/jbc.M400193200. [DOI] [PubMed] [Google Scholar]
- 22.Cooley JW, Ohnishi T, Daldal F. Binding dynamics at the quinone reduction (Qi) site influence the equilibrium interactions of the iron sulfur protein and hydroquinone oxidation (Qo) site of the cytochrome bc1 complex. Biochemistry. 2005;44:10520–32. doi: 10.1021/bi050571+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covian R, Trumpower BL. Rapid electron transfer between monomers when the cytochrome bc1 complex dimer is reduced through center N. J Biol Chem. 2005;280:22732–40. doi: 10.1074/jbc.M413592200. [DOI] [PubMed] [Google Scholar]
- 24.Shinkarev VP, Wraight CA. Intermonomer electron transfer in the bc1 complex dimer is controlled by the energized state and by impaired electron transfer between low and high potential hemes. FEBS Lett. 2007;581:1535–41. doi: 10.1016/j.febslet.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez-Cirlos EB, Trumpower BL. Inhibitory analogs of ubiquinol act anti-cooperatively on the Yeast cytochrome bc1 complex. Evidence for an alternating, half-of-the-sites mechanism of ubiquinol oxidation. J Biol Chem. 2002;277:1195–202. doi: 10.1074/jbc.M109097200. [DOI] [PubMed] [Google Scholar]
- 26.Covian R, Trumpower BL. Regulatory interactions between ubiquinol oxidation and ubiquinone reduction sites in the dimeric cytochrome bc1 complex. J Biol Chem. 2006;281:30925–32. doi: 10.1074/jbc.M604694200. [DOI] [PubMed] [Google Scholar]
- 27.Covian R, Kleinschroth T, Ludwig B, Trumpower BL. Asymmetric binding of stigmatellin to the dimeric Paracoccus denitrificans bc1 complex: evidence for anti-cooperative ubiquinol oxidation and communication between center P ubiquinol oxidation sites. J Biol Chem. 2007;282:22289–97. doi: 10.1074/jbc.M702132200. [DOI] [PubMed] [Google Scholar]
- 28.Castellani M, Covian R, Kleinschroth T, Anderka O, Ludwig B, Trumpower BL. Direct demonstration of half-of-the-sites reactivity in the dimeric cytochrome bc1 complex: enzyme with one inactive monomer is fully active but unable to activate the second ubiquinol oxidation site in response to ligand binding at the ubiquinone reduction site. J Biol Chem. 2010;285:502–10. doi: 10.1074/jbc.M109.072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swierczek M, Cieluch E, Sarewicz M, Borek A, Moser CC, Dutton PL, Osyczka A. An electronic bus bar lies in the core of cytochrome bc1. Science. 2010;329:451–4. doi: 10.1126/science.1190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atta-Asafo-Adjei E, Daldal F. Size of the amino acid side chain at position 158 of cytochrome b is critical for an active cytochrome bc1 complex and for photosynthetic growth of Rhodobacter capsulatus. Proc Natl Acad Sci U S A. 1991;88:492–6. doi: 10.1073/pnas.88.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding H, Moser CC, Robertson DE, Tokito MK, Daldal F, Dutton PL. Ubiquinone pair in the Qo site central to the primary energy conversion reactions of cytochrome bc1 complex. Biochemistry. 1995;34:15979–96. doi: 10.1021/bi00049a012. [DOI] [PubMed] [Google Scholar]
- 32.Lanciano P, Lee DW, Yang H, Darrouzet E, Daldal F. Intermonomer electron transfer between the low-potential b hemes of cytochrome bc1. Biochemistry. 2011;50:1651–63. doi: 10.1021/bi101736v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saribas AS, Ding H, Dutton PL, Daldal F. Tyrosine 147 of cytochrome b is required for efficient electron transfer at the ubihydroquinone oxidase site (Qo) of the cytochrome bc1 complex. Biochemistry. 1995;34:16004–12. doi: 10.1021/bi00049a014. [DOI] [PubMed] [Google Scholar]
- 34.Cox MM. The bacterial RecA protein as a motor protein. Annu Rev Microbiol. 2003;57:551–77. doi: 10.1146/annurev.micro.57.030502.090953. [DOI] [PubMed] [Google Scholar]
- 35.Millett F, Durham B. Use of ruthenium photooxidation techniques to study electron transfer in the cytochrome bc1 complex. Methods Enzymol. 2009;456:95–109. doi: 10.1016/S0076-6879(08)04405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong X, Yu L, Xia D, Yu CA. Evidence for electron equilibrium between the two hemes bL in the dimeric cytochrome bc1 complex. J Biol Chem. 2005;280:9251–7. doi: 10.1074/jbc.M409994200. [DOI] [PubMed] [Google Scholar]
- 37.Crofts AR, et al. The Q-cycle reviewed: How well does a monomeric mechanism of the bc1 complex account for the function of a dimeric complex? Biochim Biophys Acta. 2008;1777:1001–19. doi: 10.1016/j.bbabio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooley JW. A structural model for across membrane coupling between the Qo and Qi active sites of cytochrome bc1. Biochim Biophys Acta. 2010;1797:1842–8. doi: 10.1016/j.bbabio.2010.05.013. [DOI] [PubMed] [Google Scholar]