Abstract
Rationale
Despite recent advances in critical care and ventilator management, acute lung injury (ALI) and the Acute Respiratory Distress Syndrome (ARDS) continue to cause significant morbidity and mortality. Granulocyte-macrophage colony stimulating factor (GM-CSF) may be beneficial for patients with ARDS.
Objectives
To determine whether intravenous infusion of GM-CSF would improve clinical outcomes for patients with ALI/ARDS.
Design
A randomized, double-blind, placebo-controlled clinical trial of human recombinant GM-CSF vs. placebo. The primary outcome was days alive and breathing without mechanical ventilatory support within the first 28 days after randomization. Secondary outcomes included mortality and organ failure free days.
Setting
Medical and Surgical Intensive Care Units at three academic medical centers.
Patients
One hundred-thirty individuals with ALI of at least three days duration were enrolled, out of a planned cohort of 200 subjects.
Interventions
Patients were randomized to receive human recombinant GM-CSF (64 subjects, 250 μg/M2) or placebo (66 subjects) by intravenous infusion daily for 14 days. Patients received mechanical ventilation using a lung protective protocol.
Measurements and Main Results
There was no difference in ventilator-free days between groups (10.7 ± 10.3 days placebo vs. 10.8 ± 10.5 days GM-CSF, p=0.82). Differences in 28-day mortality (23% in placebo vs. 17% in patients receiving GM-CSF (p=0.31)) and organ failure free days (12.8 ± 11.3 days placebo vs. 15.7 ± 11.9 days GM-CSF, p=0.16) were not statistically significant. There were similar numbers of serious adverse events in each group.
Conclusions
In a randomized phase II trial, GM-CSF treatment did not increase the number of ventilator free days in patients with ALI/ARDS. A larger trial would be required to determine whether treatment with GM-CSF might alter important clinical outcomes such as mortality or multiorgan failure. (ClinicalTrials.gov number, NCT00201409 [ClinicalTrials.gov])
Keywords: Acute Respiratory Distress Syndrome, growth factors, sepsis, innate immunity
Introduction
Acute lung injury (ALI) and the acute respiratory distress syndrome (ARDS) represent a continuum of acute pulmonary disease that causes significant morbidity and mortality throughout the United States. ALI/ARDS is a common response to multiple systemic or lung-specific insults (1), although sepsis syndrome is the most common predisposing factor. The annual incidence of ALI/ARDS in the U.S. may be as high as 75 per 100,000 population (1-2). Since ARDS was first described in 1967 (3) there have been significant improvements in ancillary management strategies for care for these patients (4). In particular, a lung protective ventilatory strategy (5) and conservative approach to fluid management (6) have been shown to improve patient outcomes in large multicenter trials. Nonetheless, ALI/ARDS causes a significant burden of disease, with prolonged mechanical ventilation in the majority of patients and 30% mortality in recent series of selected patients (1, 4). ARDS leads to long-term limitations in health-related quality of life (HRQL) in survivors (4, 7-8). To date, the search for pharmacologic interventions targeted to the pathophysiology of ARDS has been disappointing (9-10). New therapeutic approaches that address the underlying pathophysiology and complications of this condition and improve outcomes are clearly needed.
Granulocyte-macrophage colony stimulating factor (GM-CSF) is critically important normal for pulmonary homeostasis. Local expression of GM-CSF in the lung is required for maturation of alveolar macrophages, and thus for normal surfactant clearance and pulmonary innate immunity (11). GM-CSF is also a growth and survival factor for alveolar epithelial cells, which are a primary site of injury in individuals with ARDS (12-13). In human patients with ALI/ARDS, increased expression of GM-CSF in BAL fluid is correlated with increased survival, supporting the hypothesis that GM-CSF plays an important protective role in the injured lung (14). Preclinical data from animal models suggest that GM-CSF might be of benefit in ALI/ARDS both by limiting early epithelial cell injury and by maintaining alveolar macrophage function (12, 15). Based on these considerations, we hypothesized that GM-CSF would act at multiple points to improve the outcome of patients with ALI/ARDS. Therefore, we carried out a prospective randomized placebo-controlled multicenter trial of recombinant human GM-CSF for patients with ALI/ARDS. The primary outcome was days free of mechanical ventilation in the first 28 days, while secondary outcomes included 28-day mortality and duration of organ failure.
Methods
Patient enrollment
All mechanically ventilated patients in the participating intensive care units at the University of Michigan Medical Center (Critical Care Medicine Unit, Surgical Intensive Care Unit, Cardiothoracic Surgery Intensive Care Unit), Emory University Hospitals (Medical Intensive Care Unit, Surgical Intensive Care Unit), and the University of Colorado Denver (Medical Intensive Care Unit) were screened by study coordinators on a daily basis. Those individuals meeting criteria for acute lung injury or ARDS by the American-European Consensus Conference definition were considered for enrollment (16). In addition to the exclusion criteria incorporated in this definition, patients were excluded if 1) if <18 years of age, 2) if >7 days had elapsed since onset of ALI/ARDS, 3) if there was evidence of pre-existing chronic respiratory failure, 4) if the patients was neutropenic (absolute neutrophil count <1000 cells/mm3, 5) if there was a history of hematological malignancy or bone marrow transplantation, 6) if the patient had entered other therapeutic trials, or 7) if there was a decision by the patient (or his/her legally authorized decision maker) or attending physician to forego aggressive care.
Enrollment and Randomization
After informed consent was obtained from the patient or the patient's legal surrogate, demographic data and physiologic measurements were collected from the time of entry into the study. The window for randomization and initiation of study drug infusion was 3 to 7 days after meeting criteria for ALI/ARDS. Thereafter, time points are defined relative to the day of randomization. Patients were randomized (1:1) to receive either recombinant human GM-CSF or placebo. The study used a randomized block design at each site, generated by the biostatistics core and provided to the pharmacies in sequential, sealed, opaque envelopes. At each site, subjects were stratified based on age (≤ or >65 years) in consideration of the possibility that age might be an independent factor influencing outcome, especially in patients with sepsis (17). The investigators, study coordinators and clinicians involved in patient care all were blinded as to treatment and outcomes for the duration of the study.
Study Protocol
Following randomization, subjects received recombinant human GM-CSF (250 μg/M2) or placebo, administered by slow intravenous infusion over 4 hrs, once daily for 14 days. The study drug and identical appearing placebo were provided to the investigational pharmacies at the different sites by the manufacturer (initially Berlex Laboratories; subsequently Bayer A.G., and then Genzyme) and prepared by the pharmacy as 200 ml infusions. These manufacturers provided the study drug and placebo without charge, but provided no other support for the study. The protocol stipulated that study drug infusions were to be held on days on which the WBC was >40,000 cells/mm3 or the ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (P/F ratio) was <80. Standardized ventilator management was based upon the ARDS Network low tidal volume protocol, targeting a tidal volume of 6 ml/kg ideal body weight and plateau pressure less than 30 mmHg (5). Weaning from mechanical ventilation was standardized, based on the ARDS Network protocol. This protocol was implemented by respiratory therapists and used pressure-support ventilation.
Each subject was assessed daily by the study coordinators and study parameters were recorded, including survival, requirement for mechanical ventilation, P/F ratio, hemodynamics, and laboratory studies. The primary outcome of this study was ventilator free days (VFD), defined as the number of days within the first 28 days after enrollment on which the patient was alive and breathing without ventilatory assistance, so long as the period of unassisted breathing had lasted at least 48 hrs. Secondary outcomes, defined prospectively, included 28-day and 6-month all cause mortality and organ failure free days within the first 28 days. Failure of extrapulmonary organ systems was defined as in recent ARDS Network studies (5-6, 18). The oxygenation index (calculated as [(mean airway pressure × FiO2)/PaO2] × 100) was compared between groups on the day of first study drug administration and on days 4, 8, and 15 after randomization for subjects continuing to receive mechanical ventilation.
Data safety and monitoring
The study was approved by a data safety and monitoring board assembled by the National Heart Lung and Blood Institute, and by the institutional review board at each site. The data safety and monitoring board then reviewed the study for safety on a regular basis.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed using standard technique prior to randomization in a subset of patients. One hundred sixty ml of sterile isotonic saline was instilled in 20 ml aliquots, with gentle suctioning after each aliquot. Cells were collected by centrifugation, and the cell-free supernatant was frozen at -70°C for subsequent assays. Bronchoscopy was not performed for research purposes if the P/F ratio was <100, the patient was deemed to be too unstable by the attending clinician, or the surrogate declined participation in this portion of the study.
GM-CSF and biomarker measurements
GM-CSF protein was measured in BAL fluid and serum by ELISA (R & D Systems, Minneapolis, MN, sensitive to 3 pg/ml). Serum samples were obtained in the morning, prior to initiation of the day's infusion of study drug. Tumor necrosis factor alpha (TNFα), interleukin (IL-) 8 and IL-6 in serum were measured by ELISA (R & D Systems) according to the manufacturer's protocol. Samples were assayed in duplicate and individual data points presented as mean values.
Statistical analysis and sample size calculations
We planned to enroll 100 patients in each group. This number of subjects would provide 80% power to detect a difference of 3.9 VFD between GM-CSF and placebo groups using a 5% level of significance. This potential effect on VFD was estimated based on the potential mechanisms of GM-CSF effects from preclinical studies, and the change in VFD in response to change in tidal volume in the initial ARDS Network study (19). When comparing the two treatment groups, p-values are computed by Fisher's exact test, log-rank test or by the Mann-Whitney rank sum t-test. The Mann-Whitney nonparametric two-tailed two-sample test was used for all group comparisons involving ordinal data, since several of the variables had a very skewed distribution. All p-values are two-tailed. Summary statistics are presented as percents, mean ± standard deviation or median ± interquartile range. All analyses were performed using SAS® version 9.1.3.
Results
Patients
Enrollment began in July 2004. The study was originally designed with an enrollment target of 200 subjects. However, enrollment proved slower than anticipated and the trial was closed in March 2009, after a total of 132 subjects had been enrolled. Early closure of enrollment was entirely attributable to feasibility concerns; neither the investigators nor the DSMB proposed closure due to safety. Sixty-five subjects were randomized to receive GM-CSF and 67 subjects to receive placebo. Two individuals, one from each group, did not receive infusions after randomization. One subject died following randomization, prior to drug infusion. In a second subject, treatment was withheld because new information obtained after randomization suggested that the patient should have failed entry criteria, based on a possible diagnosis of hematologic malignancy. These two patients are excluded from the analysis.
The demographics, clinical characteristics and initial physiologic characteristics of the GM-CSF and placebo groups were quite similar (Table 1). The mean age of the entire population was 48.4 ± 14.6 years. Fifty-seven percent of the subjects were male and 74.2% were white. Sepsis and pneumonia were most commonly identified as the precipitating event leading to ALI/ARDS in both groups. The severity of illness at the time of enrollment was indicated by Acute Physiology Scores (APS) from Acute Physiology and Chronic Health Evaluation (APACHE) III (range 0-299) and the SOFA score. The patients in both groups were critically ill, and the two groups did not differ with respect to severity of illness at enrollment, as indicated by mean APS at enrollment (57.3 ± 16.0 in the placebo group vs. 56.6 ± 16.1 in the GM-CSF group; p=0.61) or SOFA score (p=0.67). Respiratory failure was severe, with mean P/F ratio in both groups < 200, indicative of ARDS, rather than ALI. Mean arterial pressure, was slightly, yet statistically lower in the placebo group (63.9 ± 11.1 vs. 68.4 ± 10.9, p=0.04). Subjects were enrolled a median of 3 days after meeting criteria for ALI/ARDS (maximum 6 days in the GM-CSF group and 7 days in the placebo group) and the first infusion of study drug was begun within 24 hours of enrollment.
Table 1. Patient characteristics*.
| Placebo | GMCSF | p-value Fisher exact test | |
|---|---|---|---|
| Sample size | 66 | 64 | |
| Age | 48.5±15.6 | 48.3±13.8 | 0.82 |
| Sex (%) | 0.72 | ||
| Male | 59.1 | 54.7 | |
| Female | 40.9 | 45.3 | |
| Hispanic | 4.6 | 1.6 | 0.62 |
| Race(%) | 0.41 | ||
| White | 72.7 | 76.6 | |
| Black or African American | 27.3 | 20.3 | |
| Asian | 0 | 1.6 | |
| Other | 0 | 1.6 | |
| Etiology (%) | 0.67 | ||
| primary sepsis | 21.2 | 32.3 | |
| pneumonia | 28.8 | 32.3 | |
| aspiration | 18.2 | 17.7 | |
| trauma | 10.6 | 8.1 | |
| pancreatitis | 9.1 | 4.8 | |
| transfusion | 4.6 | 1.6 | |
| post-op | 6.1 | 1.6 | |
| other | 1.5 | 1.6 | |
| Any sepsis(%) | 66.7 | 72.6 | 0.56 |
| Location(%) | 0.10 | ||
| MICU | 57.6 | 71.9 | |
| SICU | 42.4 | 28.1 | |
| Placebo | GMCSF | p-value Mann-Whitney | |
| APS score | 57.3±16.0 | 56.6±16.1 | 0.61 |
| Sofa score | 8.1±4.0 | 7.8±3.8 | 0.67 |
| Qualifying P/F ratio | 128±54 | 123±57 | 0.48 |
| Day of randomization: | |||
| Mean arterial pressure | 63.9±11.1 | 68.4±10.9 | 0.039 |
| Hemoglobin (gm/dL) | 10.3±1.5 | 10.1±1.7 | 0.30 |
| WBC (×103 cells/mm3) | 14.9±7.7 | 16.3±8 | 0.29 |
| Platelet (×103 cells/mm3) | 185±128 | 205±160 | 0.65 |
| P/F ratio | 127±45 | 135±57 | 0.62 |
Plus-minus values are mean ±SD. Etiology denotes the final presumed etiology of ALI/ARDS.
APS score denotes the Acute Physiology Score from APACHE III. P/F ratio denotes (partial pressure of arterial oxygen/fraction of inspired oxygen). The Qualifying P/F ratio is from the day of enrollment, and thus may be different than the P/F ratio on the day of randomization.
Clinical Endpoints
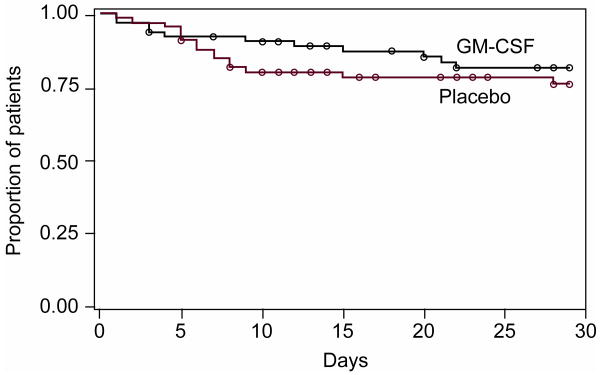
There was no difference between subjects who received placebo and those who received GM-CSF with respect to VFD, the primary endpoint of the study (Table 2). There was also no difference in days on mechanical ventilation or duration of hospitalization. Mortality at 28 days in the placebo-control group was 23% compared to 17% in the GM-CSF group (Figure 1), a difference which was not statistically significant (p=0.31 by log-rank test). At 6 months three additional patients had died in each group. Similarly, the number of organ failure free days was greater in the GM-CSF group compared to the placebo group (Table 2), although this difference did not achieve statistical significance.
Table 2. Mechanical ventilation and organ failure*.
| Placebo | GMCSF | p-value | |
|---|---|---|---|
| Ventilator-free days (VFD) | 10.7±10.3 8.5(0,21) |
10.8±10.5 10(0,21.5) |
0.82 |
| Days on mechanical ventilation | 13.7±9.5 10(6,23) |
15.0±10.1 13(5,24.5) |
0.59 |
| Days on study drug | 10.2±4.2 12(7,14) |
8.9±4.2 9(6,13) |
0.06 |
| Days drug with held | 1.3±1.9 1(0,2) |
2.8±3.1 2(1,4) |
0.0011 |
| Days without organ failure | 12.8±11.3 11(0,24) |
15.7±11.9 20.5(0.5,26.5) |
0.16 |
| Days with organ failure | 11.6±9.7 8(3,20) |
10.2±10.1 5(2,16.5) |
0.24 |
| Days free of circulatory failure | 17.8±10.9 22(4,27) |
20.1±10.1 24.5(15,28) |
0.25 |
| Days free of coagulation failure | 22.6±10.4 --(18,--) |
23.7±9.4 --(23.5,--) |
0.95 |
| Days free of renal failure | 17.8±12.9 28(2,--) |
20.4±12.4 --(4,--) |
0.17 |
| Days free of hepatic failure | 20.8±11.1 28(9,--) |
23±9.4 28(21.5,--) |
0.43 |
VFD is the number of days within the first 28 days following enrollment on which the subject was alive and breathing without mechanical ventilatory support.
Days without organ failure is number of days within the first 28 days following enrollment on which the subject was alive and without organ failure.
Data are expressed as mean ± SD and median(25th,75th %-ile); Mann-Whitney p-values are shown.
-- Greater than or equal to 29, the upper bound of days that were included in the study.
Figure 1. Patient survival through day 29 after randomization.
p=0.31 using log-rank test.
Physiologic responses to treatment
It had been anticipated that GM-CSF infusion might influence peripheral leukocyte counts. At baseline the mean WBC count was slightly, but not significantly, greater in the GM-CSF group compared to the placebo group (Table 3). From baseline to study day 4, WBC increased significantly in patients who received GM-CSF (p=0.0028) but was unchanged in those who received placebo (p=0.29) (Table 3).
Table 3. Effect of treatment on WBC and gas exchange*.
| Placebo | Placebo N = | GM-CSF | GM-CSF N= | p-value | |
|---|---|---|---|---|---|
| WBC at randomization | 14.9±7.7 | 16.3±8.0 | 0.29 | ||
| WBC day 4 after randomization | 19.1±13.0 | 25.0±10.7 | 0.0001 | ||
| Change in WBC, day 4 – randomization | 4.1±11.0 | 8.2±11.0 | 0.0054 | ||
| p-value (vs. randomization) | p=0.29 | p=0.0028 | |||
| Oxygenation index at First Drug (mean±SD) | 13.6±8.0 | 55 | 12.7±8.1 | 57 | 0.5795 |
| Oxygenation index at Day 4 | 10.9±7.7 | 44 | 12.7±8.0 | 39 | 0.2779 |
| Difference (Day 4 - First Drug) | -3.4±7.7 | 39 | -1.6±6.5 | 36 | 0.2730 |
| p-value vs. first drug | 0.0096 | 0.1570 | |||
| Oxygenation index at Day 8 | 8.8±7.6 | 26 | 12.1±6.3 | 31 | 0.0894 |
| Difference (Day 8 - First Drug) | -5.3±8.2 | 23 | -4.3±9.6 | 29 | 0.6961 |
| p-value vs. first drug | 0.0054 | 0.0217 | |||
| Oxygenation index at Day 15 | 6.7±2.0 | 10 | 12.1±10.3 | 17 | 0.0522 |
| Difference (Day 15 - First Drug) | -4.6±4.5 | 8 | -3.5±10.4 | 14 | 0.7438 |
| p-value vs. first drug | 0.0244 | 0.2302 |
WBC counts are × 103 cells/mm3 ; Oxygenation index = [(mean airway pressure × FiO2)/PaO2] × 100
Data are expressed as mean ± SD; Mann-Whitney p-values are shown.
The short term effect of study drug infusion on gas exchange was determined by comparing the change in oxygenation index between the day of first study drug administration and days 4, 8, and 15 after randomization, for subjects who continued to receive mechanical ventilation (Table 3). The oxygenation index at baseline was not different between the groups (p=0.58), and fell over the course of the study in both groups, indicative of improving gas exchange in survivors. Treatment with GM-CSF had little impact on the change in gas exchange over time in subjects who continued to require mechanical ventilation.
GM-CSF expression
A subset of subjects had levels of GM-CSF measured in serum or in BAL prior to randomization, early after initiation of treatment (days 1-5) and at later time points during treatments (Table 4). As anticipated, serum GM-CSF levels were quite low and were not different between the two groups at baseline. Serum levels did not change in either group over the course of the trial. Levels of GM-CSF in BAL fluid at baseline were also similar in the two groups. Changes in GM-CSF concentration in BAL fluid over time were not statistically significant.
Table 4. Levels of GM-CSF in plasma and BAL fluid*.
| Plasma GM-CSF (pg/ml) | pretreatment | days 1-5 | days 6-11 |
|---|---|---|---|
| Placebo | 5.39±11.43 | 5.98±11.53 | 5.12±10.13 |
| GM-CSF | 8.86±13.11 | 7.69±15.83 | 6.39±13.39 |
| BAL GM-CSF (pg/ml) | |||
| Placebo | 23.64±32.11 | 6.65±11.21 | 7.29±5.96 |
| n | 24 | 21 | 12 |
| GM-CSF | 21.51±37.68 | 22.07±51.4 | 15.84±26.39 |
| n | 20 | 25 | 18 |
GM-CSF levels were measured at baseline (prior to randomization and treatment) and subsequently after initiation of study drug in the time frame indicated.
Data are expressed as mean ± SD.
Inflammatory Biomarkers
At baseline, prior to treatment, there were no differences between the two groups in serum levels of IL-6 or IL-8. Similarly, serum IL-6 and IL-8 after the initiation of GM-CSF therapy were not different between the groups at days 1-5 or days 6-11 post treatment (Table 5). Because it was considered possible that treatment with GM-CSF might induce increased expression of TNFα, we also compared baseline and post-treatment TNFα levels between the groups. There was no difference in serum TNFα between the groups at baseline; nor were there differences in levels measured days 1-5 or days 6-11.
Table 5. Serum inflammatory cytokine levels at baseline and over time*.
| Cytokine (pg/ml) | placebo (n=40) | GM-CSF (n=39) | p-value |
|---|---|---|---|
| TNFα | |||
| baseline | 11.5±29.7 | 21.2±94.1 | 0.35 |
| Days 1-5 | 36.0±161.3 | 6.3±8.2 | 0.29 |
| Days 6-11 | 20.3±74.3 | 8.4±13.4 | 0.38 |
| IL-8 | |||
| baseline | 71.8±109.6 | 66.4±108.2 | 0.59 |
| Days 1-5 | 67.0±87.1 | 55.5±87.7 | 0.37 |
| Days 6-11 | 53.6±77.3 | 33.8±35.8 | 0.53 |
| IL-6 | |||
| baseline | 718.8±1305.8 | 251.2±372.6 | 0.27 |
| Days 1-5 | 138.8±264.9 | 105.4±178.4 | 0.12 |
| Days 6-11 | 101.0±228.2 | 73.8±81.8 | 0.98 |
Inflammatory cytokines were measured in serum by ELISA.
Subjects shown had serum obtained at baseline (prior to randomization and treatment) and subsequently after initiation of study drug in the time frame indicated.
Data are expressed as mean ± SD. Mann-Whitney p-values are as indicated.
Protocol compliance
There was good compliance with the treatment protocol overall. However, the study infusion was withheld more frequently in subjects receiving GM-CSF compared to those receiving placebo (20.6% of treatment days vs. 10.4% of treatment days, p<0.0001). Multiple factors contributed to the decisions to withhold drug infusions in these critically ill patients, and more than one factor was often present in the same subject. Leukocytosis was a common reason for withholding infusion in the GM-CSF group, as defined in the study protocol. In 7 subjects the study drug infusion was withheld the majority of days when it might have been given. Five of these individuals were in the GM-CSF group and two were in the placebo group. Elevated WBC above the predetermined threshold (40,000 cells/mm3) was a contributing factor in 4 of these 7 subjects. A P/F ratio below the predetermined safety threshold of 80 was a contributing factor in 3 subjects.
Ventilator management was by ARDS Network guidelines, targeting tidal volume ≤ 6 ml/kg ideal body weight, and plateau pressure ≤30 cm H2O. Both pressure limited and volume cycle ventilation were allowed in the protocol. Using a cutoff of 6.5 ml/kg, measured tidal volume was within range 62.3% of days measured, although on most days out of compliance the tidal volume remained < 8 ml/kg (85% of days measured). There was no difference between the two groups in days with tidal volume >6.5 ml/kg (39.7% GM-CSF vs. 35.1% placebo) or >8 ml/kg (16.0% GM-CSF vs. 14.5% placebo). There was no difference between the two groups in the number of days on which plateau pressure exceeded 30 cm H2O (15.8% GM-CSF vs. 11.5% placebo).
Serious adverse events
As might be anticipated, serious adverse events occurred frequently in these critically ill patients. Over one half of all subjects had at least one SAE. These events were equally frequent in the two treatment groups (Table 6) and none were felt by the safety monitor to be clearly related to the study drug. Periodic safety reviews were carried out by the DSMB. Closure of the study was not due to safety concerns.
Table 6. Serious Adverse Events *.
| Placebo | GM-CSF | p-value | |
|---|---|---|---|
| Individuals with any SAE | 34 | 31 | p=0.86 |
| Total SAEs | 46 | 40 | |
| Pulmonary SAEs | 16 | 11 | |
| Subjects with SAE=Sepsis or multiorgan system failure | 13 | 11 | p=0.82 |
| Subjects with infection SAE | 9 | 7 | p=0.79 |
SAE are serious adverse events
Fisher's exact test p-values are shown
Discussion
We conducted a randomized, double blind, placebo-controlled trial of IV infusion of GM-CSF in patients with established ALI/ARDS at three academic centers. There was no significant difference in VFD between patients treated with GM-CSF and those treated with placebo. Thus, the two groups were not different with respect to the primary endpoint. Similarly, there was no difference in the secondary endpoints, mortality and organ failure free days, between the groups. GM-CSF infusion induced a leukocytosis in some individuals, but was well tolerated, with no increase in serious adverse events compared to placebo and with no serious adverse events clearly associated with the study drug.
ALI/ARDS remains a devastating condition causing very significant morbidity and mortality. Although important gains have been made as a result of improved strategies for mechanical ventilation (5) and fluid management (6), pharmacologic interventions have been generally disappointing (1). Recombinant GM-CSF is particularly appealing as a therapy for patients with ALI/ARDS because preclinical data suggest it may exert beneficial effects through several different mechanisms, including direct protective effects for alveolar epithelial cells (12), effects limiting progression from lung injury to fibrosis (20), and effects to maintain alveolar macrophage function for pulmonary innate immunity (15). These mechanisms might all act concurrently in a single patient, or may be manifest separately in different subgroups.
There are several potential reasons this study did not identify an effect of treatment with GM-CSF on VFD in patients with ALI/ARDS. It is possible that preclinical data concerning the effects of GM-CSF on alveolar epithelial injury and repair in animal models do not reflect human biology. Alternatively, there are several potential reasons this trial may have resulted in the negative outcome for the primary endpoint, whether or not our central hypothesis is correct. These include the number of subjects, the timing, dose and route of study drug administration and the choice of primary endpoint.
Although the enrollment target was 200 subjects, enrollment closed after 132 subjects had been randomized (of whom 130 individuals proceeded to treatment) due to the slow pace of subject accrual within a fixed period of support for the study. The planned sample size of 200 was based on 80% power to find an expected difference of 3.9 days in VFD with an SD of 9.2 days. Enrollment of 130 subjects provided 60% power to show this difference, or 80% power to show a difference in VFD of 4.6 days. A potential explanation for the negative result of this study for its primary endpoint is that the study was underpowered to detect a more modest effect on VFD. Although there was only a minimal difference in VFD between GM-CSF and placebo groups when the study closed, one can not extrapolate to the outcome had the additional 70 subjects been enrolled. The number of subjects actually treated was further reduced because prospectively determined safety rules resulted in withholding study drug infusion on a number of days. In seven patients, drug was withheld on at least half of the potential days of drug infusion, thus decreasing the opportunity for beneficial drug effects. Retrospective analysis excluding these subjects did not identify significant differences in VFD or other clinical endpoints.
It is possible that the decisions concerning the timing, dose or route of administration contributed to this outcome. In this trial, the window for initiation of treatment was between days 3 and 7 after onset ALI/ARDS. By waiting until day 3 of ALI to begin treatment, individuals who might recover from(21) or succumb to ALI quickly, prior to the point at which GM-CSF might be expected to change outcome, were not subjected to experimental treatment. Similarly, this delay in treatment avoided concerns that GM-CSF might increase levels of early response cytokines, such as TNFα, during the initial phase of illness. This delay in treatment decreased the opportunity for benefit during this early phase, a factor that might be particularly important if protection against alveolar epithelial cell injury and destruction is a key component of the effect of GM-CSF. Day 7 was chosen as the end of the enrollment period because patients beyond day 7 are increasingly likely to be in a more chronic phase of illness. Nonetheless, the inclusion of patients enrolled days 3-7 may have resulted in a relatively heterogeneous population and a less clear signal of therapeutic response.
It is possible that the dose of GM-CSF was inadequate, although this seems unlikely. The particular dose of recombinant GM-CSF chosen in this study was based on that used clinically for support of peripheral WBC counts and mobilization of hematopoetic stem cells. In our patients, changes in WBC counts in the active treatment group suggest that there were significant systemic drug effects. Because large portions of GM-CSF are bound to circulating antibodies and receptors on macrophages, protein measured by standard ELISA may not accurately reflect the available GM-CSF within the alveolar space (22). An argument might be made for provision of recombinant GM-CSF via the airway. However, the most injured portion of the lung in patients with ARDS often is not ventilated. Therefore, a systemic route of administration was preferred for this study. Systemic administration of the same dose of GM-CSF to animals in lung injury models resulted in very significant effects on alveolar macrophages and alveolar epithelial cells.
Although VFD is a clinically important and accepted end point for studies in patients with ARDS, it is possible that GM-CSF might act to influence other important endpoints, such as progression of organ failure or mortality. Potential mechanisms by which GM-CSF might limit the risk or duration of multiorgan failure include reversal of sepsis-induced immunoparalysis and leukocyte deactivation (23), stimulation of pulmonary innate immune function to limit ventilator-associated pneumonia (15, 24), or mobilization of tissue progenitor cells, including endothelial progenitor cells (25-26) and bone marrow derived mesenchymal stem cells (27-28). The 28-day mortality rate in the placebo group (23%) was comparable to that found in recent ARDS Network studies (29), while the mortality rate in patients receiving GM-CSF was 17%, a difference that was not significant. Similarly, the number of organ failure free days in was 12.8 ±11.3 in patients receiving placebo, and 15.7 ± 11.9 in those receiving GM-CSF. This study was not sufficiently large to detect differences of this magnitude for these endpoints. In the original ARDS Network study of the impact of low tidal volume, over 800 subjects were required to detect a comparable change in mortality (19).
Several recent trials have specifically investigated the potential benefit of GM-CSF treatment in patients with sepsis who were not neutropenic. These trials have varied significantly in the clinical characteristics of the patients enrolled, the dose and duration of GM-CSF treatment, and the endpoints measured. Separately, they have demonstrated reduced evidence of leukocyte deactivation and more rapid resolution of sepsis (30), more rapid clinical improvement in abdominal sepsis (31), and improved leukocyte function and pulmonary gas exchange in patients with severe sepsis and respiratory dysfunction (32-33). Although the doses, site of administration, and duration of GM-CSF administration have varied, these studies together indicate that treatment with GM-CSF is well tolerated in patients with severe sepsis and suggest trends toward improvements in secondary endpoints.
In our study, treatment with GM-CSF appears to have a good safety profile, even in this population of critically ill patients. It did not adversely impact mortality or days free of organ failure compared to placebo. The rate of serious adverse events did not differ between the groups. It was considered possible that treatment with GM-CSF might result in increased cytokine levels, fueling ongoing systemic inflammation. However, serum IL-6, IL-8 and TNFα were not increased in the GM-CSF group compared to those receiving placebo. The study was designed with study drug infusions to be withheld if the WBC count exceeded 40,000 cells/mm3. Similarly, in order to maximize patient safety, drug infusion was also withheld if the subject's P/F ratio was below 80. Whether the thresholds chosen were optimal has not yet been determined. Based on the experience in this trial, we believe these restrictions on drug administration could be loosened or removed in a future trial.
The approach to ventilator management has important consequences for outcomes in studies of patients with ALI/ARDS. Our protocol was designed to provide patients with lung protective ventilation (5). A small but significant number of subjects received tidal volumes greater than target, often in the setting of a pressure limited ventilatory mode. A much smaller number of individuals received ventilation with plateau pressures exceeding the ARDS Network target. Because these instances were equally frequent in the two groups, it is unlikely that issues related to ventilator management influenced the outcomes of this study.
In summary, in a multicenter trial of 130 patients with ALI/ARDS we found that infusion of GM-CSF (250 μg/M2/day) beginning between 3 and 7 days after onset of lung injury did not change the number of VFD in our patients. Effects on mortality and days free of organ failure were not statistically significant. GM-CSF treatment appears to be safe, with no difference in serious adverse events between subjects who received GM-CSF and those who received placebo. Thus, our results indicate that GM-CSF did not negatively impact patient outcomes in this critically ill populations. We believe the cumulative data from mechanistic preclinical studies, other investigations of GM-CSF in patients with sepsis, and the present study together suggest that GM-CSF be considered for further study of potential impact on mortality in patients with ARDS.
Acknowledgments
The authors wish to thank the research coordinators and critical care physicians, nurses and respiratory therapists at the University of Michigan Medical Center, Grady Memorial Hospital, the University of Colorado Hospital, and St. Anthony Central Hospital, without whom this work would not have been possible. We also wish to thank the members of the NHLBI-convened Data Safety and Monitoring Board for their careful attention to this study.
RP, RD, GM, AR, and EB received funding from the National Institutes of Health (NIH). All author claim that the manufacturers (Berlex, Bayer, Genzyme) provided study drug without charge, but no other support.
Footnotes
This work was supported by a Specialized Center for Clinical Research award (SCCOR) from the National Heart Lung and Blood Institute (P50-HL074024); Study drug was provided by Berlex Laboratories, Bayer A.G. and Genzyme.
References
- 1.Ware LB, Matthay MA. The Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and Outcomes of Acute Lung Injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg KP, Hudson LD. Acute lung injury and acute respiratory distress syndrome. The clinical syndrome. Clinics in Chest Medicine. 2000;21(3):401–417. vii. doi: 10.1016/s0272-5231(05)70156-8. [DOI] [PubMed] [Google Scholar]
- 5.The Acute Respiratory Distress Syndrome Network. Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. Epub 2006 May 2521. [DOI] [PubMed] [Google Scholar]
- 7.Davidson TA, Caldwell ES, Curtis JR, et al. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. Jama. 1999;281(4):354–360. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 8.Schelling G, Stoll C, Vogelmeier C, et al. Pulmonary function and health-related quality of life in a sample of long-term survivors of the acute respiratory distress syndrome. Intensive Care Medicine. 2000;26(9):1304–1311. doi: 10.1007/s001340051342. [DOI] [PubMed] [Google Scholar]
- 9.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. Jama. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 10.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30(1):1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Trapnell BC, Whitsett JA. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 12.Paine R, 3rd, Wilcoxen SE, Morris SB, et al. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol. 2003;163(6):2397–2406. doi: 10.1016/S0002-9440(10)63594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffman Reed JA, Rice WR, Zsengeller ZK, et al. GM-CSF enhances lung growth and causes alveolar type II epithelial cell hyperplasia in transgenic mice. American Journal of Physiology. 1997;273(4 Pt 1):L715–725. doi: 10.1152/ajplung.1997.273.4.L715. [DOI] [PubMed] [Google Scholar]
- 14.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Critical Care Medicine. 2000;28(1):1–7. doi: 10.1097/00003246-200001000-00001. see comments. [DOI] [PubMed] [Google Scholar]
- 15.Baleeiro CE, Christensen PJ, Morris SB, et al. GM-CSF and the impaired pulmonary innate immune response following hyperoxic stress. Am J Physiol Lung Cell Mol Physiol. 2006;291(6):L1246–1255. doi: 10.1152/ajplung.00016.2006. Epub 2006 Aug 1244. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 17.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 18.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 19.Ventilation with Lower Tidal Volumes as Compared with Traditional Tidal Volumes for Acute Lung Injury and the Acute Respiratory Distress Syndrome. New England Journal of Medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 20.Moore BB, Coffey MJ, Christensen P, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165(7):4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 21.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. Epub 2003 Oct 2016. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K, Nakata K, Suzuki T, et al. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood. 2009;113(11):2547–2556. doi: 10.1182/blood-2009-05-155689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz C, Carlet J, Fitting C, et al. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88(5):1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baleeiro CEO, Wilcoxen SE, Morris SB, et al. Sublethal Hyperoxia Impairs Pulmonary Innate Immunity. J Immunol. 2003;171(2):955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 25.Wang QR, Wang F, Zhu WB, et al. GM-CSF accelerates proliferation of endothelial progenitor cells from murine bone marrow mononuclear cells in vitro. Cytokine. 2009;45(3):174–178. doi: 10.1016/j.cyto.2008.12.002. Epub 2009 Jan 2014. [DOI] [PubMed] [Google Scholar]
- 26.Cho HJ, Kim HS, Lee MM, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation. 2003;108(23):2918–2925. doi: 10.1161/01.CIR.0000097001.79750.78. Epub 2003 Oct 2920. [DOI] [PubMed] [Google Scholar]
- 27.Rojas M, Xu J, Woods CR, et al. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33(2):145–152. doi: 10.1165/rcmb.2004-0330OC. Epub 2005 May 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. doi: 10.1038/nm.1905. Epub 2008 Nov 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The National Heart LaBIACTN. Higher versus Lower Positive End-Expiratory Pressures in Patients with the Acute Respiratory Distress Syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 30.Nierhaus A, Montag B, Timmler N, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29(4):646–651. doi: 10.1007/s00134-003-1666-6. Epub 2003 Feb 2021. [DOI] [PubMed] [Google Scholar]
- 31.Orozco H, Arch J, Medina-Franco H, et al. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg. 2006;141(2):150–153. doi: 10.1001/archsurg.141.2.150. discussion 154. [DOI] [PubMed] [Google Scholar]
- 32.Presneill JJ, Harris T, Stewart AG, et al. A randomized phase II trial of granulocyte-macrophage colony-stimulating factor therapy in severe sepsis with respiratory dysfunction. Am J Respir Crit Care Med. 2002;166(2):138–143. doi: 10.1164/rccm.2009005. [DOI] [PubMed] [Google Scholar]
- 33.Meisel C, Schefold JC, Pschowski R, et al. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180(7):640–648. doi: 10.1164/rccm.200903-0363OC. Epub 2009 Jul 2009. [DOI] [PubMed] [Google Scholar]