Abstract
Although the roles of endothelial cells in cancer have been primarily considered to be related to tumor perfusion, the emerging appreciation of “angiocrine” regulation adds stromal regulatory capabilities to the expanding list of endothelial functions in tumors. We posit that understanding the state-dependent paracrine regulatory paradigms established in vascular disease and repair will be critical for a deep understanding of tumor biology, as endothelial cells regulate diverse processes in all vascularized tissues. We now outline the historical developments that led to the appreciation of the paracrine regulatory functions of endothelial cells, summarize classical views of blood vessels and stroma in cancer, and attempt to merge these ideas to include the stromal regulatory endothelial cell as a critical regulator of cancer. The notion of the endothelial cell as a biochemical regulator of cancer state in constant dynamic balance with its tumor could impact diagnosis, prognosis and treatment of cancer. Such concepts might well explain the mixed results from anti-angiogenic cancer therapeutics and how certain drugs that improve vascular health correlate with improved cancer prognosis.
Keywords: angiogenesis, endothelium, microenvironment, angiocrine, endothelial dysfunction
Introduction
“In the time available I have been able to show you a little of the current knowledge of the morphology of [endothelial] cells which fifteen years ago were thought to form little more than a sheet of nucleated cellophane.” Lord Florey, 1966 (1).
In the mid 19th century Virchow (2) included “abnormalities of the blood vessel wall” as one of the critical elements of his classic triad defining propensity for clotting– the others being blood coagulability and flow disruption. We interpret this abnormality to mean endothelial dysfunction, and seek to ascribe to Virchow the deepest insight as to the bioregulatory function of the endothelium, extending his triad of risk of venous thrombosis to all vascular pathologies. Current dogma holds that vascular health is synonymous with endothelial integrity and that disruption of endothelial health presages and contributes to vascular disease. Thus, we retrospectively attribute Virchow’s inclusion of mural abnormalities as early reference to the notion that the state of the endothelial cell determines if overlying blood will flow or clot, circulating leukocytes will adhere and transmigrate, underlying vessels will constrict or dilate and adjacent smooth muscle cells proliferate or regress.
As we continue to consider these issues in vascular biology, the analogy to tumor biology’s vascular dependence is obvious and intriguing. Factors released by endothelial cells can control many of cancer’s aggressive traits in a manner analogous to endothelial regulation of vascular repair (3). Reparative ECs – similar to those that inhibit vascular smooth muscle hyperplasia after vascular injury (4–5) – inhibit in vitro cancer proliferation and invasiveness, and in vivo tumor growth and metastasis (3). Controlled disruption of the endothelial phenotype – via silencing of the gene encoding perlecan, a heparan sulfate proteoglycan critical for endothelial inhibition of thrombosis after vascular repair (6) – eliminates the ability of ECs to inhibit cancer invasion and metastasis.
We now present our thoughts on the convergence of the biologies of vascular repair or injury with tumor control or spread, and especially how the use of matrix-embedded endothelial cells can help to reveal complex regulatory mechanisms in physiology and disease.
Vascular biology’s origins
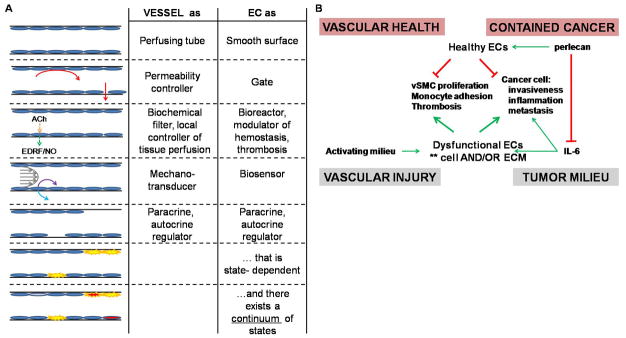
Functional studies of the vasculature originated with Ernest Starling in 1896, and later were expanded upon by Edmund Cowdry, Alfred Kohn, Ramon y Cajal, and others. Supported with a quantitative framework provided by John Pappenheimer they surmised that the endothelium served primarily as a selectively permeable vascular lining. Examination of endothelial cell control of vascular tone, thrombosis, hyperplasia, and inflammation (7–8) was complemented by investigation of endothelial sensitivity to biomechanical stimuli, including shear stress, hydrostatic pressure, and circumferential strain (Fig. 1A).
Figure 1. Evolution of endothelial regulatory roles in vascular repair and homologies with roles in cancer.
(A) The endothelium, the cellular lining of the vasculature, is a remarkably plastic and responsive organ with far-reaching regulatory roles. (B) State-dependent regulatory paradigms identified in vascular disease and repair may be useful as guides for the examination of endothelial roles in cancer.
Insight into the structural biology of the endothelium was made possible by technical achievements linked to deep scientific insight – Karnovsky’s work on novel cytochemical investigations into intact vascular ultrastructure amongst the many important findings. Florey cites this work in his tome on endothelial physiology (1) – a work that was astonishingly prescient in its scientific implications and general perspectives regarding the synergistic progress of the science and enabling technological innovations of vascular biology. Drugs that regulate clotting, blood pressure, cholesterol metabolism and heart failure and endovascular implants could not have been conceived of, developed or refined without deep understanding of vascular biology, and the use of these drugs and devices provided new means of probing physiologic systems. Detailed examination of endothelial cell biology was propelled further by two pioneering descriptions of the (9–10) the stable culture, identification and study of isolated endothelial cells.
The endothelial cell, endothelium and vascular structure
Large vessels are endothelial-lined tubes and like epithelial-lined tubes have a trilaminate architecture. Three vascular mural tunics interface with the lumen from within and the viscera from without, with a muscular layer in between. The innermost intima contains endothelial cells and their underlying extracellular matrix (ECM) layer, the basement membrane, and in larger vessels vascular smooth muscle cells. Beneath the intima, separated by the internal elastic lamina, is the media, with phalanges of smooth muscle cells separated into packets by fascia and connective tissue sheets. The densest of these sheets bounds the media – on the intimal side as the internal elastic lamina and on the interface with the adventitia as the external elastic lamina. The adventitia contains nerves (“vasa nervosum”), fibroblasts, extracellular matrix, and capillaries (“vasa vasorum”). The capillaries, made of endothelial cells and sparse supporting pericytes, comprise a second vascular network parallel to the vessel. As the vessel wall thickens beyond a critical limit additional perfusing vessels and a network of vasa interna, connected to the externa via communicating capillaries, becomes necessary. This thickness limit is reached always in large arteries, in particular in atherosclerotic vessels. Atherosclerotic plaques (11) and catheter-induced intimal hyperplastic regions (12) are rich in and dependent upon vasa vasorum.
There are then two primary sources of endothelial cells in large vessels– those that reside at the lumen of the large vessel and those of the vasa vasorum that run parallel to and then course through the vessel wall. The large vessels’ endothelial cells regulate and sense flow, interact with blood-borne elements, and modulate permeability. The capillary endothelial cells of the vasa vasorum are far more abundant and their ubiquity provides that every cell in the vessel wall is adjacent to and under the potential regulatory control of an endothelial cell. Since all tissues contain microvasculature, every cell in every tissue is under similar potential control. It is then the endothelial cells of vessels and indeed of all tissues that convey vital systemic and regional environmental cues like inflammation and fluid dynamics inward, and endothelial cell secreted factors that impose tight regulatory control over inflammation and flow in turn.
Endothelial cells are ubiquitous, plastic, paracrine regulators
The power of endothelial-derived regulation resides not only in its interfacial position but also in its plasticity. The same cells can respond differentially based on subtle alterations in microenvironmental cues and in their own state (13). For example, the density-dependence of endothelial regulatory phenotype modulates control of vascular smooth muscle hyperplasia (4, 14) and attraction and trafficking of leukocytes. Healthy endothelium responds to increased blood flow by releasing vasodilatory factors like NO, while injured endothelial layers respond to the identical stimulus with the release of a vasoconstricting factors. This latter response of macrovascular endothelium to increased fluid flow was termed paradoxical and formed the original definition of “endothelial dysfunction” (15). The state-dependent endothelial regulatory phenotype has been extended to explain perivascular angiogenesis (16), hematopoiesis and thrombopoiesis (17), and most aspects of inflammation (18). Within a vast potential spectrum of control over diverse physiologic and pathophysiologic processes we posit that there are at least three fundamental states: dormant or quiescent, physiologically activated and reparative, and dysfunctionally activated and therefore disease-stimulatory. It is clear that atherosclerosis (19), and therefore coronary and peripheral arterial disease, kidney disease and uremia (20), diabetes mellitus and metabolic syndromes (21), rheumatoid arthritis, hypertension (22), pre-eclampsia, and many other pathologic states strongly correlate with endothelial dysfunction, which can function as a surrogate for disease severity. That endothelial state could now explain diverse elements of tumor biology is intriguing (3, 23–24).
Endothelial cell state and substratum interactions
Endothelial state is both manifested in and regulated by the composition of the underlying extracellular matrix (ECM). The basement membrane on which endothelial cells reside provides critical adhesion molecule ligand-binding sites (25) and serves as a depot for signaling molecules and growth factors that regulate endothelial and neighboring cells (26). Turnover or degradation of basement membrane by matrix-digesting enzymes can cause profound changes in the local environment (27). It is not surprising then that particular ECM molecules can promote endothelial reparative capabilities – e.g. the heparan sulfate proteoglycan perlecan (6) – and modification or degradation of these molecules or increasing the activity of opposing molecules can promote dysfunction. Destructive stimuli causing endothelial dysfunction may therefore target both the cell and matrix components.
Embedding endothelial cells within porous polymeric scaffolds stabilizes endothelial phenotype by controlling cell-substratum interactions. Regulatory units of precise number and with controlled biosecretion patterns can be created for facile implantation in a variety of culture and animal models. Our laboratory has used such cellular devices as reparative endothelium and demonstrated the power of this engineered organ in models of hyperplastic disease (28–29). Unlike injections of isolated cells that require time for homing, engraftment, accommodation, maturation, etc., a matrix-embedded endothelial cell construct is immediately effective. Regulatory effects and potency are sustained long after constructs erode and without generating a significant inflammatory or immune response, even when allogeneic or xenogeneic cells are used (30). Matrix-embedded endothelial cells placed perivascularly provide long-term inhibition of intimal hyperplasia following vascular injury and inhibit thrombosis in a manner directly dependent on embedded cell expression of perlecan (6). Such cell implants also promote healing of other structures, including the trachea (29) and now solid tumors (3). Matrix-embedded endothelial cell implants in disease models can provide unique insights not easily obtainable by delivery of isolated factors.
Classical views of endothelial cells in cancer: role in tumor angiogenesis
“The presence of a tumor-angiogenesis factor suggests a transfer of information from tumor cells to capillary endothelial cells. The relationship between tumor cells and endothelial cells may be interdependent.” Folkman et al, 1971 (31).
Vascularization is essential for the development of physiologic and pathologic tissues. The calor, tumor, and rubor of inflammation arise from vascularization, and modern schemata of cancer biology must include vessels for continued growth and eventual metastasis. The paradigm first described by Folkman (32) posited that since growing tumors need a blood supply for perfusion, interruption of the blood supply should interrupt tumor growth. Normally equilibrium between pro-angiogenic and anti-angiogenic factors maintains vessel homeostasis, and balances vascular network expansion and pruning. Tumor vessels’ unchecked expansion is likely driven by the incorporation of endothelial cells derived from existing local vessels and perhaps circulating cells as well (33). Without a vasculature tumors are unable to grow to more than ~1 mm3 in volume, remaining small and dormant. Once a tumor flips the “angiogenic switch”, new vessels are recruited that first increase tumor microvascular density and later increase tumor growth and invasiveness.
Jain and colleagues extended the concepts surrounding perfusion-mediated effects of tumor vessels by realizing that these vessels, comprised mainly of endothelial cells, possess abnormal architecture due to an imbalance of pro- and anti-angiogenic factors (34). This architectural dysregulation includes heightened permeability, which contributes to intratumoral hypoxia/acidosis and elevated interstitial pressure, facilitating the outward spread of cancers and impeding soluble molecule flux into the tumor. They suggest that “normalization” of the tumor vasculature by doses of anti-angiogenesis agents insufficient to destroy the vasculature instead restores the balance of pro- and anti-angiogenic factors and partially explains the efficacy of such therapies. Other tumor endothelial phenotypic abnormalities include an “activated” integrin expression pattern (35), dysregulated leukocyte adhesion (36), abnormal responses to oxidative stress (37), and abnormal mechanosensing (38). Similar derangements have been characterized in dysfunctional endothelial cell phenotypes in vascular disease (7, 39).
Most of the explosive research in tumor angiogenesis concentrated on how vessels are recruited (40) and structurally distorted (34) to promote tumor growth. Some have proposed a more direct role for the endothelial cells themselves in cancer regulation, including contact-dependent and -independent regulation (41–42). Yet the homology to vascular repair has not been fully recognized and interest in such has partially receded. Moreover, the endothelial cell has been rarely (43) and inconsistently mentioned as part of the population of stromal cells such as fibroblasts (44) and myeloid cells (45) that are increasingly recognized as essential elements of tumor biology. As “angiocrine” paradigms for stromal regulatory endothelial cells have begun to appear (23) it is worth considering how endothelial cell-derived paracrine regulatory models in the biology of vascular homeostasis and repair (7) can contribute to cancer sciences (3).
The genetically normal stromal cells of the cancer microenvironment are potentially attractive therapeutic targets, offering the potential for lower toxicity and intervention at multiple and shared events in tumor evolution (46). Furthermore, there may be means to utilize endogenous stroma to inhibit, rather than support, cancer aggression (47–48). Strategies toward this goal could include both pharmacologic and biophysical cues to normalize the tumor microenvironment or the placement of healthy regulatory cells within or adjacent to the deranged tumor milieu (3).
The impact of anti-angiogenic therapy on cancer is revealing. Drugs designed to limit tumor vascularization have mixed effects on patient survival (49). Some have even proposed that specific modes of anti-angiogenic therapy that target VEGF might accelerate tumor invasion and metastasis while shrinking primary tumors (50–51), although the implications of these preclinical studies have not yet been fully evaluated in human specimens (52). Thus, there remain elusive details about the crosstalk between cancer cells and endothelial cells, and the effects of endothelial cell state – e.g. quiescent, reparative or dysfunctional – on such processes must be taken into consideration. Along this line, it is intriguing that the cholesterol-lowering drugs that inhibit HMG-CoA reductase (“statin” drugs) and non-steroidal anti-inflammatory drugs (NSAIDs) both improve vascular integrity and endothelial health (53–54) and are associated with improved cancer prognoses (55).
The emerging “angiocrine” paradigm: dysfunctional tumor-associated endothelial cells
Butler et al recently proposed a model (23) which combines the cancer-stroma interaction and angiogenesis paradigms. They proposed that endothelial cells are recruited to tumors to provide “angiocrine” support for tumor growth and spread. This model has been used to identify, for example, that the endothelial cell EphA2 receptor negatively regulates the secretion of a cancer-stimulatory angiocrine factor Slit2 (24). This notion is consistent with the increasingly appreciated roles of inflammation and stromal regulatory elements present in the tumor milieu (56), which could in combination cause endothelial cell dysfunction.
State-dependence might deepen this perspective (Fig. 1B). The notion that endothelial cells promote physiologic repair when healthy and disease processes when dysfunctional more likely represents points on a regulatory spectrum of the remarkably plastic endothelium (57). Precisely as in atherosclerotic vascular disease, where reparative endothelial cells inhibit disease processes like inflammation, hyperplasia, or thrombosis, and dysfunctional endothelial cells stimulate the same, endothelial cells may regulate cancer pathophysiology in a state-dependent manner. Reparative endothelial cells should suppress cancer cell malignant properties like proliferation and invasion, and dysfunctional endothelial cells stimulate the same. Regulatory factors identified in vascular disease and repair may also contribute to cancer biology. Although advanced cancers must eventually recruit and corrupt the cells in their microenvironment, it may be possible to pharmacologically reverse the phenotype of endogenous tumor endothelial cells - from dysfunctional to quiescent and even reparative - and regain control over the tumor milieu.
As in vascular disease, the ECM should contribute to tumor biology by regulating endothelial state and thereby affect adjacent cancer cells within a tumor. Control over cell-matrix interactions may help to ensure that endothelial cells placed within or adjacent to the tumor milieu retain their regulatory phenotype to overcome or resist the disruptive stimuli and promote homeostasis. We recently showed that secretions from reparative endothelial cells suppress cancer proliferation, invasiveness, and inflammatory signaling in vitro, and tumor growth and experimental metastasis in vivo (3). These experiments utilized the intact endothelial secretome, as opposed to constituent isolated factors, since combinations of individual factors can elicit even qualitatively different responses from target cells depending on dose and presence of cofactors. For example, prior work from our laboratory identified context-dependent roles for endothelial heparan sulfate proteoglycans in inhibiting vascular smooth muscle cell proliferation and showed how other factors emitted by endothelial cells either augment or reverse such inhibition (5). Our use of matrix-embedded endothelial cell implants to control the behavior of solid tumors in animals was another example in which prior work in vascular repair presaged experiments in cancer. Reparative matrix-embedded endothelial cell implants reduced tumor growth and normalized tumor structure (3), just as such implants help to guide repair after vascular (28) and tracheal (29) injury.
Although we have found that it is the synergistic action of endothelial cell-secreted factors that most efficiently guides repair (5), manipulation of specific endothelial cell-secreted products can help to elucidate partial mechanisms of endothelial-derived regulation. We previously showed that silencing endothelial expression of perlecan, the predominant endothelial-secreted heparan sulfate proteoglycan, abrogated the ability to inhibit occlusive vascular thrombosis, but not intimal hyperplasia, after vascular injury (6). We concluded that perlecan expression is critical for maintenance of the disease-inhibitory endothelial cell phenotype and hypothesized that endothelial perlecan expression may bolster endothelial anti-cancer effects. Indeed, knockdown of perlecan caused transcriptional upregulation of IL-6 and eliminated the ability of endothelial cells to inhibit cancer cell invasion and metastasis (3), supporting our vascular-cancer paradigm homology. Thus the phenotype of tumor-associated endothelium may be modified either by direct action of molecular mediators on the endothelial cells themselves or by modification of the subendothelial ECM.
The transfer of regulatory paradigms from vascular repair to cancer may be useful in identifying previously unrecognized processes in endothelial-cancer crosstalk. High-throughput gene expression studies have offered abundant data (58), but few tumor endothelial genes have been identified as paracrine regulators. Intriguingly, of the genes that have been identified many encode ECM or matrix-remodeling molecules (58), which could support the notion that particular modifications of the ECM contribute to tumor progression by modification of the endothelial phenotype.
Summary: Paracrine context-dependent regulatory roles of endothelial cells in cancer
“Perhaps you will be kind enough to look on what I have said today as one more interim report on endothelium. Our knowledge is still far from being definitive, and I should expect to see the next ten years yield a rich harvest of new knowledge about the cells which stand between the blood and lymph streams and the cells of the tissue. I would expect to see exemplified the dicta that the introduction of a new technique is certain to be followed by new discoveries and that the pushing of a known technique to greater heights of technical achievement will produce new accretions of knowledge.” Lord Florey 1966 (1).
The uniquely privileged and ubiquitous anatomic position of microvascular ECs might enable a global paradigm for disease control dictated by endothelial phenotype. The list of diseases impacted by endothelial anti- and pro-inflammatory regulation is immense, and perhaps now should prominently include cancer (3, 23–24). As with the foundational work of Virchow, Florey and all of those who came before us, our views will be refined with continued investigation. We have attempted to merge traditionally distinct fields of study into an updated report of the roles of the endothelium in health and disease. We hope that studies using matrix-embedded endothelial cells as convenient and controllable cellular implants will help define the extent of cancer-endothelial crosstalk. Such work may enable the design of pharmacologic therapies to reverse tumor endothelial phenotype from dysfunctional to reparative and guide the design of cellular implants that are able to resist the pressures present in the tumor milieu to effectively and permanently “heal” tumors.
Acknowledgments
The authors thank Dr. Morris Karnovsky for help editing the manuscript and Drs. Sangeeta Bhatia, Angelo Cardoso, David Housman and David Scadden for early discussions and insight into these issues.
Financial support was provided by NIH R01 GM49039 to E.R.E., NIH MSTP funding to J.W.F.
Footnotes
Disclosures: J.W.F. and E.R.E. are co-inventors on a patent owned by Massachusetts Institute of Technology that describes the use of cell implants to modulate cancer behavior. E.R.E. is a founder of Pervasis Therapeutics, which has licensed the patent application. No other authors have disclosures to declare.
References
- 1.Florey L. The endothelial cell. Br Med J. 1966;2:487–90. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virchow R. On occlusion of the pulmonary arteries. Collected papers in Medical Science. 1856 [Google Scholar]
- 3.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3:66ra5. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–60. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 5.Ettenson DS, Koo EW, Januzzi JL, Edelman ER. Endothelial heparan sulfate is necessary but not sufficient for control of vascular smooth muscle cell growth. J Cell Physiol. 2000;184:93–100. doi: 10.1002/(SICI)1097-4652(200007)184:1<93::AID-JCP10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci U S A. 2000;97:6722–7. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–74. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 8.Rogers C, Parikh S, Seifert P, Edelman ER. Endogenous cell seeding. Remnant endothelium after stenting enhances vascular repair. Circulation. 1996;94:2909–14. doi: 10.1161/01.cir.94.11.2909. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe EA, Hoyer LW, Nachman RL. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973;52:2757–64. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimbrone MA, Jr, Cotran RS, Folkman J. Endothelial regeneration: studies with human endothelial cells in culture. Ser Haematol. 1973;6:453–5. [PubMed] [Google Scholar]
- 11.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–7. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 12.Edelman ER, Nugent MA, Smith LT, Karnovsky MJ. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992;89:465–73. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parikh SA, Edelman ER. Endothelial cell delivery for cardiovascular therapy. Adv Drug Deliv Rev. 2000;42:139–61. doi: 10.1016/s0169-409x(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 14.Dodge AB, Lu X, D'Amore PA. Density-dependent endothelial cell production of an inhibitor of smooth muscle cell growth. J Cell Biochem. 1993;53:21–31. doi: 10.1002/jcb.240530104. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Drexler H, Wollschlager H, Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991;83:391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- 16.Nugent HM, Sjin RT, White D, Milton LG, Manson RJ, Lawson JH, et al. Adventitial endothelial implants reduce matrix metalloproteinase-2 expression and increase luminal diameter in porcine arteriovenous grafts. J Vasc Surg. 2007;46:548–56. doi: 10.1016/j.jvs.2007.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopp HG, Rafii S. Thrombopoietic cells and the bone marrow vascular niche. Ann N Y Acad Sci. 2007;1106:175–9. doi: 10.1196/annals.1392.004. [DOI] [PubMed] [Google Scholar]
- 18.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–15. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 19.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease--what have we learned in 10 years? Semin Dial. 2010;23:498–509. doi: 10.1111/j.1525-139X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 21.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pate M, Damarla V, Chi DS, Negi S, Krishnaswamy G. Endothelial cell biology: role in the inflammatory response. Adv Clin Chem. 2010;52:109–30. [PubMed] [Google Scholar]
- 23.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–46. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brantley-Sieders DM, Dunaway CM, Rao M, Short S, Hwang Y, Gao Y, et al. Angiocrine Factors Modulate Tumor Proliferation and Motility through EphA2 Repression of Slit2 Tumor Suppressor Function in Endothelium. Cancer Res. 2011;71:976–87. doi: 10.1158/0008-5472.CAN-10-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–8. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 26.Morss AS, Edelman ER. Glucose modulates basement membrane fibroblast growth factor-2 via alterations in endothelial cell permeability. J Biol Chem. 2007;282:14635–44. doi: 10.1074/jbc.M608565200. [DOI] [PubMed] [Google Scholar]
- 27.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–59. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nathan A, Nugent MA, Edelman ER. Tissue engineered perivascular endothelial cell implants regulate vascular injury. Proc Natl Acad Sci U S A. 1995;92:8130–4. doi: 10.1073/pnas.92.18.8130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zani BG, Kojima K, Vacanti CA, Edelman ER. Tissue-engineered endothelial and epithelial implants differentially and synergistically regulate airway repair. Proc Natl Acad Sci U S A. 2008;105:7046–51. doi: 10.1073/pnas.0802463105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methe H, Edelman ER. Tissue engineering of endothelial cells and the immune response. Transplant Proc. 2006;38:3293–9. doi: 10.1016/j.transproceed.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275–88. doi: 10.1084/jem.133.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 33.Yoder MC, Ingram DA. Endothelial progenitor cell: ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16:269–73. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 34.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 35.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 36.Castermans K, Griffioen AW. Tumor blood vessels, a difficult hurdle for infiltrating leukocytes. Biochim Biophys Acta. 2007;1776:160–74. doi: 10.1016/j.bbcan.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Houle F, Huot J. Dysregulation of the endothelial cellular response to oxidative stress in cancer. Mol Carcinog. 2006;45:362–7. doi: 10.1002/mc.20218. [DOI] [PubMed] [Google Scholar]
- 38.Ghosh K, Thodeti CK, Dudley AC, Mammoto A, Klagsbrun M, Ingber DE. Tumor-derived endothelial cells exhibit aberrant Rho-mediated mechanosensing and abnormal angiogenesis in vitro. Proc Natl Acad Sci U S A. 2008;105:11305–10. doi: 10.1073/pnas.0800835105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva R, D'Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–13. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 40.Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 41.Bandyopadhyay S, Zhan R, Chaudhuri A, Watabe M, Pai SK, Hirota S, et al. Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med. 2006;12:933–8. doi: 10.1038/nm1444. [DOI] [PubMed] [Google Scholar]
- 42.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the 'angiogenesis progression' hypothesis. Eur J Cancer. 1996;32A:2438–50. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 44.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 45.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet. 2009;25:30–8. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Ingber DE. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356–64. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17:320–9. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–16. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 50.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PK, Fazlollahi L, et al. Glioblastoma recurrence after cediranib therapy in patients: lack of "rebound" revascularization as mode of escape. Cancer Res. 2011;71:19–28. doi: 10.1158/0008-5472.CAN-10-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Gonzalez J, Badimon L. Mechanisms underlying the cardiovascular effects of COX-inhibition: benefits and risks. Curr Pharm Des. 2007;13:2215–27. doi: 10.2174/138161207781368774. [DOI] [PubMed] [Google Scholar]
- 54.Parmar KM, Nambudiri V, Dai G, Larman HB, Gimbrone MA, Jr, Garcia-Cardena G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J Biol Chem. 2005;280:26714–9. doi: 10.1074/jbc.C500144200. [DOI] [PubMed] [Google Scholar]
- 55.Xiao H, Yang CS. Combination regimen with statins and NSAIDs: a promising strategy for cancer chemoprevention. Int J Cancer. 2008;123:983–90. doi: 10.1002/ijc.23718. [DOI] [PubMed] [Google Scholar]
- 56.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 57.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 58.Aird WC. Molecular heterogeneity of tumor endothelium. Cell Tissue Res. 2009;335:271–81. doi: 10.1007/s00441-008-0672-y. [DOI] [PubMed] [Google Scholar]