Abstract
Cocaine has been shown to have initial positive (euphoric) and delayed negative (anxiogenic) effects in both humans and animals. Cocaine-paired cues are consequently imbued with mixed positive and negative associations. The current study examines the relative roles of these dual associations in the enhanced drug-seeking observed upon presentation of cocaine-paired cues. Rats ran a straight alley once/day for a single i.v. injection of cocaine (1.0 mg/kg/inj) in the presence of a distinctive olfactory cue (scented cotton swabs placed under the apparatus). An alternate scent was presented in a separate cage 2-hr prior to runway testing. After 15 trials/days, the scents and cocaine reinforcer were removed and a series of extinction trials (lasting for one or three weeks) was initiated. Immediately following extinction, runway responding was tested during a single trial in the presence of the cocaine-paired or non-paired cue. As previously reported, while subjects initiated responding faster over trials (reduced latencies to leave the start box), they exhibited a progressive increase in approach-avoidance conflict behavior (“retreats”) regarding goal-box entry, reflecting cocaine’s dual positive + negative effects. Once established, retreat behaviors persisted over the course of 6 and 20 days of extinction. However, both run times and retreats decreased in response to presentation of the cocaine-paired but not the non-paired scent. These data suggest that, after reinforcer removal, cue-induced cocaine-seeking stems in part from a reduction in approach-avoidance conflict; i.e., a greater weakening of the negative relative to the positive associations that animals form with cocaine-paired stimuli.
Keywords: cocaine, runway, drug self-administration, cue-induced reinstatement, opponent process theory, discriminative stimuli
1. Introduction
Human cocaine addicts report that once the initial drug-induced state of euphoria subsides, they experience profound levels of anxiety, agitation, depression, and fatigue (Resnick and Resnick, 1984; Smith, 1986; Washton and Gold, 1984; Williamson et al., 1987). These negative effects of cocaine occur even while plasma levels of the drug remain high, suggesting that the onset of such effects is not a result of drug withdrawal, but more directly related to the inherent mixed or opponent-process properties of cocaine (Van Dyke and Byck, 1982). The negative/aversive actions of cocaine have also been demonstrated in animal studies. Cocaine increases the reluctance of animals to explore an open field task (Simon et al., 1994; Yang et al., 1992) or enter the open arms of an elevated plus maze (Rogerio and Takahashi, 1992). Cocaine administration has been reported to potentiate an animal’s avoidance of an inherently negative environment (Costall et al., 1989) and decrease responding in conflict tests (Fontana and Commissaris, 1989). While animals develop conditioned preferences for distinct environments paired with the immediate effects of i.v. cocaine, they exhibit aversions for environments paired with the effects present 15-min post-injection (Ettenberg et al., 1999; Ettenberg and Bernardi, 2007; Jhou et al., personal communication; Knackstedt et al., 2002; Pliakas et al., 2001). Additionally, cocaine has long been known to produce increases in plasma and/or brain levels of stress hormones such as adrenocorticotropin and corticotropin-releasing factor (Goeders, 2002; Goldstein, 1991; Koob, 1999; Moldow and Fischman, 1987; River and Vale, 1987) and more recently its negative effects have been associated with actions within structures of the extended-amygdala (e.g. Wenzel et al., 2011) that have themselves been implicated in fear, stress and anxiogenic states (e.g., Davis et al., 2008; de la Mora et al., 2010; Tanimoto et al., 2003; Walker and Davis, 2008). Collectively, these studies provide clinical, behavioral, and neurobiological evidence that demonstrate the presence of profound aversive/anxiogenic properties of cocaine administration.
The demonstration that cocaine produces dual and opposing actions would seem to suggest that the motivation of organisms to seek the drug must involve both positive (approach) and negative (avoidance) features. To assess the nature of these dual actions of cocaine, our laboratory has developed and employed a runway model of drug self-administration in which animals traverse a straight alley once a day to enter a goal box where they receive an i.v. injection of the drug reinforcer. In this model the time it takes the subject to re-enter the goal area each day provides an index of that subject’s motivation to seek the drug. The runway has been successfully employed to assess the motivation of subjects to seek a variety of drug and natural reinforcers (e.g. for a review see Ettenberg, 2009). Early on, it was found that animals running for cocaine, exhibited a unique pattern of responding that had not and has not been observed with other drug reinforcers (e.g., Ettenberg and Geist, 1993; Su et al., 2011). While cocaine-reinforced animals initiate responding (leave the start box) more and more quickly as trials progress (demonstrating the positive motivational “pull” of the drug), they develop a progressive increase in “retreat behaviors” in which they approach the goal quickly, but then stop at the threshold, turn, and run back toward the start box (Ettenberg and Geist, 1991). These retreat behaviors have been shown to reflect an approach-avoidance conflict about goal-box entry that stems from the mixed positive (rewarding) and negative (anxiogenic) associations with the goal box that in turn stem from cocaine’s opponent-process properties (see review by Ettenberg, 2004). Thus, the runway self-administration model provides a unique means of investigating the dual positive and negative actions of cocaine administration within the same subject on the same trial.
In the current study, we employed the self-administration runway to examine a topic of primary interest in the drug abuse field, namely the role of drug-paired environmental stimuli as a motivating factor for the return to drug-seeking behavior observed after a period of drug abstinence (Childress et al., 1987, 1988; O’Brien et al., 1992; Robbins and Ehrman, 1992). Previously we had reported that while heroin reinforcement supported more robust goal-directed performance in the runway (i.e, faster start and run times) compared to cocaine (in the same subjects), those animals were subsequently more responsive to the presentation of cocaine-paired cues than heroin-paired cues after the drug reinforcers had been removed (i.e., after a period of non-reinforced responding) (Su et al., 2011). More specifically, in animals with a history of both cocaine and heroin reinforcement, only the cocaine-paired cue (and not the heroin cue) induced a reduction in runway retreat behaviors during extinction. This decrease in retreats in the presence of the cocaine-cue suggests that the strength of the negative associations with that cue (i.e., the factor motivating avoidance of the goal box) weakens faster than the positive associations with the cue (the factor motivating approach behavior) – hence goal-directed behavior is potentiated. If correct, this would represent a novel explanation for cue-induced response reinstatement/relapse of drug-seeking behavior. Of course it remains unclear whether or not the results obtained by Su et al. (2011) were dependent upon the animals prior comparative experience with both heroin and cocaine. The current study was therefore devised to extend these findings by focusing solely on cocaine and comparing the runway performance of animals presented with either a cocaine-paired or non-paired cue after varying periods of non-cued/non-reinforced trials. If the strength and/or persistence of the positive associations with a cocaine-paired cue are indeed stronger than that of the negative associations with that cue, then the impact of non-cued/non-reinforced responding should be a shift in the relative valence of the cue toward the positive -- thereby resulting in a reduction in the frequency of approach-avoidance retreat behaviors when the cocaine-paired cue is again presented. The present experiment tested this prediction.
2.0 Material and Methods
2.1 Subjects
Male adult albino Sprague-Dawley rats (n=37), weighing 330–360g at the time of surgery, were obtained from Charles River Laboratories (Wilmington, Massachusetts, USA) and served as subjects. Animals were individually housed in plastic cages within a temperature-controlled (23°C) vivarium maintained under a reverse 12-hour light-dark cycle (lights off at 0800 h). Animals were provided ad libitum access to food (Purina Rat Chow) and water throughout the duration of the study. All animal handling and experimental procedures adhered to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by University of California at Santa Barbara’s Institutional Animal Care and Use Committee.
2.2 Surgery
Rats were acclimated to human handling and vivarium conditions for a minimum of one week prior to i.v. catheterization. Each animal was then deeply anesthetized via isoflurane inhalation (4% for induction and 1.5–2.5% for maintenance). During surgery, rats were administered atropine (0.02 mg/kg, i.m.) to prevent respiratory congestion, and a non-opiate analgesic, flunixin meglumine (FluMeglumine; Phoenix Pharmaceuticals, Belmont, California, USA) (2.0 mg/kg, s.c.) to reduce post-surgical pain. Each animal was fitted with a chronic indwelling catheter (13 mm of polyethylene tubing, 0.3 mm inner diameter, 0.64 outer diameter; Dow Corning Corporation, Midland, MI, USA) inserted into the right jugular vein and secured in place by silk sutures. The open end of the catheter was passed subcutaneously to a stainless steel guide cannula (Item 313G; Plastics One, Roanoke, VA, USA) that exited though a small 2 mm hole on the midline of the animal’s back. The cannula was secured to a 2 cm square surgical Mersilene mesh (Bard; Warwick, RI) with dental cement and laid flat against the subdermal tissue. Following surgery, animals received the antibiotic, ticarcillin disodium/clavulanate potassium (Timentin; 50 mg/kg i.v. in 0.25 ml sterile 0.9 % physiological saline) and 0.1 ml of anticoagulant heparin (66 IU/0.1 ml i.v. prepared in 0.9% physiological saline) to protect against microbial infection and to promote catheter patency.
All animals were allowed a minimum of one week to recover from surgery before the onset of operant runway training. During this time, the catheters were flushed once daily with Timentin antibiotic (20mg in 0.1 ml), followed by 0.1 ml of heparinized 0.9% physiological saline. In addition, animals received 3.0 ml of 0.9% physiological saline subcutaneously once each day for 3 consecutive days to prevent dehydration. Topical antibiotic (Neosporin, Pfizer, New York, NY, USA) was applied to all incisions to aid healing and prevent infection. Prior to the start of the experiment, catheter patency was confirmed in all animals by observing the behavioral impact of an i.v. injection of the fast-acting barbiturate, methohexital sodium (Brevital; 2.0 mg/kg in 0.1 ml filtered nanopure water). Animals that were unresponsive to the Brevital (did not exhibit loss of their righting reflex) were re-implanted with a new catheter using the left jugular vein and given additional days for recovery.
2.3 Drug
Cocaine hydrochloride (cocaine, 1.0 mg/kg) was provided by the National Institute of Drug Abuse. The drug was dissolved in sterile 0.9% physiological saline and delivered in a volume of 0.1 ml over a period of 4.6 s via a 10-ml syringe seated in a motorized syringe pump (Razel Scientific Instruments, St Albans, Vermont, US). The drug dose for this project is the standard dose that has been employed in prior studies in our laboratory and has been shown to produce optimal responding in the runway (i.e., fastest start latencies and run times; Raven et al., 2000).
2.4 Runway apparatus
All trials for this experiment utilized two identical wooden straight-arm alleys (160 cm in length × 12 cm wide × 44 cm high). A start box and goal box (each 23 cm × 20 cm × 44 cm) were attached to opposite ends of each runway. The floor of each apparatus consisted of 3 cm steel rods, laid in parallel 1.2 cm apart perpendicular to the runway walls. Aligned along the length of the runway were 13 infrared photodetector-emitter pairs evenly spaced along the length of the apparatus. The first pair was located within the start box and the 13th pair inside the goal box. The output from these sensors was fed to a desktop computer via a custom Any-Maze Ami interface (Stoelting Co, Wood Dale, IL, USA) that permitted the precise location of the animal in the alley to be recorded in real time throughout each trial.
Suspended above each apparatus were two long magnetic rails (spaced 3 cm apart) that ran in parallel along the entire length of the runway. Positioned between the rails was a liquid swivel (375-22PS, Instech Laboratories Inc., Plymouth Meeting, PA, USA) that connected the guide cannula on the animal’s back to a 10-ml drug-filled syringe via polyethyne-20 tubing. Around the midsection of the swivel, a flat plastic disk was secured that prevented it from falling through between the magnetic rails. A pot magnet was attached to the underside of the plastic disk with the polarity aligned to repel that of the magnetic rails. The magnetic repulsion between the swivel assembly and the rails permitted the swivel to float slightly above the tracks and thereby provided a low-friction and low-resistance mechanism that permitted the rat to move freely throughout the alley, pulling the swivel assembly along behind and above the animal as it moved (for a more complete description of the runway apparatus, see Geist and Ettenberg, 1990).
2.5 Procedure
Animals were habituated to the apparatus during a single 10-min trial prior to the start of drug self-administration. During runway testing, each subject was removed from its home cage and placed in a plastic holding tub for 10-min during which it was exposed to one of two distinct olfactory cues (McCormick’s pure orange or almond food extract; Sparks, MD, USA). 2-hr after the non-paired cue exposure animals were connected to the drug delivery system, and placed in the start box. After 5-sec, the start door was opened to allow the animal access to the alley. Upon entry into the goal box (detected by interruption of the infrared photobeam within the goal box) the goal door was closed (to prevent re-tracing) and the syringe pump activated to deliver a single i.v. infusion of cocaine. Animals were left in the goal box for an additional 5-min and then returned to their home cages. Animals experienced 15 single-daily runway trials for cocaine reinforcement each of which was conducted in the presence of the alternate olfactory cue from that to which the animal had been exposed in the holding cage. In the runway, these cues were introduced by infusing cotton swabs with 3 ml of the scented extract and placed in plastic trays under the start box, halfway down the runway, and under the goal box. A fan was used to continuously aerate the runways with the odor. Thus, upon completion of the 15-day self-administration phase of the experiment, each animal had been exposed to both scents, only one of which was explicitly paired with the runway and the drug reinforcer. The order of presentation and assignment of the paired and non-paired cue were counterbalanced between and within groups.
Following the completion of this self-administration phase, animals underwent one or three weeks of one trial/day non-reinforced non-cued runway trials followed by a single test trial in which runway responding was assessed upon the presentation of either the cocaine- or nondrug-paired cue. This produced four groups of animals: two groups that experienced one week of extinction and were tested in either in the presence of the cocaine-paired cue (n=10) or the non-paired cue (n=9), and groups that experienced three weeks of extinction prior to presentation of either the cocaine-paired cue (n=9) or the non-paired cue (n=7).
2.6 Dependent measures
Three dependent measures were collected on every runway trial throughout the experiment: start latency (the time to leave the start box once the start door was opened), run time (the time required to traverse the runway and enter the goal box once the rat had left the start box), and retreat frequency (the number of times the animal ceased its forward locomotion toward the goal box, stopped, and reversed its direction back toward the start box).
3. Results
3.1 Runway self-administration
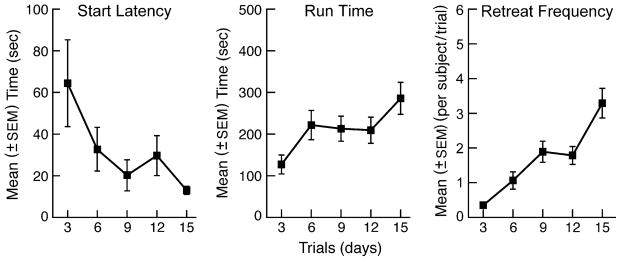
Figure 1 depicts the runway behavior averaged across all subjects during the 15-day self-administration phase of the experiment. The data are expressed as 3-day mean (±S.E.M.) scores to smooth the variability inherent when data are plotted from single daily measures. Individual one-way analyses of variance (ANOVA) were computed for each of the three dependent measures depicted in the figure. The results confirmed that start latencies significantly decreased over the course of testing (F(4, 36)=3.639, p < .01) suggesting that the goal box experience was motivating subjects to initiate responding with greater speed as trials progressed. In contrast to the decrease in start latencies that was observed over trials, subjects took longer to actually enter the goal box as testing progressed (F(4,36)=6.497, p < .001), an effect likely attributable to the significant increase in approach-avoidance retreat frequency that also increased over trials (F(4,36)=28.003, p < .001).
Fig. 1.
Mean (± SEM) start latency, run time, and retreats for animals running an alley for a single daily injection of 1.0 mg/kg/inj i.v. cocaine. Data are expressed as three-day averages to smooth variability across trials. Note that while animals initiate trials with progressively shorter start latencies as testing continued, there are concurrent increases in run times and approach-avoidance retreat responses.
3.2 Cue-Induced Responding
To ensure that there were no inherent group differences in performance prior to test day, runway data from the final three non-cued, non-reinforced trials were compared for the groups that would be presented with either the drug-paired or non-paired cue on the next trial (test day). Separate comparisons were made for groups in the one-week and three-week conditions and for each of the three dependent measures: start latency, run time, and retreat frequency. None of these comparisons yielded significant effects (two-tailed independent group Bonferroni-corrected t-tests, p = n.s.). Thus, any observed differences between the animals exposed to the drug-paired versus non-paired cue on test day were not likely a consequence of inherent differences already present during the non-cued, non-reinforced trials.
Start Latency
While start times improved dramatically over the course of acquisition (Fig 1), the removal of the cocaine reinforcer unexpectedly had little impact – rats continued to leave the start box quickly throughout the experiment. Comparisons of the last three days of acquisition to the last three days of extinction in both the one-week and three-week extinction groups produced no statistically reliable effects (Bonferroni-corrected repeated-measures t-tests, p = n.s.). There were also no differences in the start latency when the period of extinction was extended from one to three weeks. Comparisons of the last three days of extinction for the one-week group to the three-week group again yielded no significant effects (Bonferroni-corrected independent-group t-tests, p = n.s.). On test day, the application of the drug-paired cue produced a modest but nonsignificant potentiation in responding relative to the non-paired cue. In animals having experienced one-week of extinction, the mean (±S.E.M.) start latency in response to the cocaine-paired cue was 5.3 sec (±1.4) compared to 6.2 sec (±0.7) in subjects exposed to the non-paired. Similarly, in animals having experienced three weeks of single daily extinction trials, the cocaine-paired cue produced a mean start latency of 3.8 sec (±0.6) compared to 8.52 sec (±2.2) for the non-paired cue group. Thus animals appeared to be more responsive to the cocaine-paired cue than the non-paired cue. However, a two-factor (Cue × Withdrawal) independent-group ANOVA computed on the data from these four groups yielded no statistically significant effects.
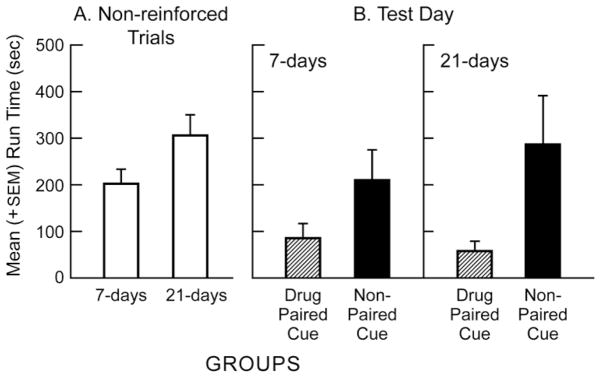
Run Times
As was the case for Start Latency, there were no significant differences between the mean run times obtained during the last three days of acquisition and the last three days of extinction in either the one-week or three-week extinction groups (Bonferroni-corrected repeated-measures t-tests, p = n.s.). Similarly, while run times predictably slowed as extinction progressed (from one week to three weeks; See Fig 2-A), this difference was not statistically reliable (Bonferroni-corrected independent group t-tests, p = n.s.). In contrast to the start latency data, groups behaved differently to the drug-paired versus non-paired cue on test day. Mean (+SEM) run times for the four groups of subjects on test day are depicted on the right side of Figure 2 (panel B). A two-factor (Cue × Withdrawal) independent-group ANOVA computed on the data from the four groups depicted in Fig 2-B revealed a strong and significant main effect for Cue (F(1,31) = 9.36, p < .006) thereby confirming that animals entered the goal box more quickly when tested in the presence of the drug-paired cue compared to the non-paired cue. There was no main effect for “withdrawal” period indicating that, when averaged across both cue conditions, animals behaved comparably after one or three weeks of extinction (p>.05). Additionally, while the presentation of the drug-paired cue appeared to produce faster running after three-weeks compared to one-week of extinction (compare the two dashed bars in Fig 2-B), and while the response to the non-paired cue seemed to weaken with prolonged extinction (compare the two dark bars of Fig 2-B), these effects did reach statistical significance as there was no Cue × Withdrawal interaction (p > .05).
Fig 2.
Mean (+SEM) run times of animals having experienced either one-week or three-weeks of non-cued, non-reinforced runway responding (left panel A). The data reflect an average of the last three extinction trials. Panel B (right side) depicts the mean (+SEM) run times of animals on a single test day following either one or three weeks of extinction. Half the animals in each condition were presented with either a cocaine-paired olfactory cue predictive of cocaine availability in the goal box, or a “neutral” non-paired cue. Across both extinction conditions, animals entered the goal box more quickly in the presence of the drug-paired cue compared to the non-paired cue.
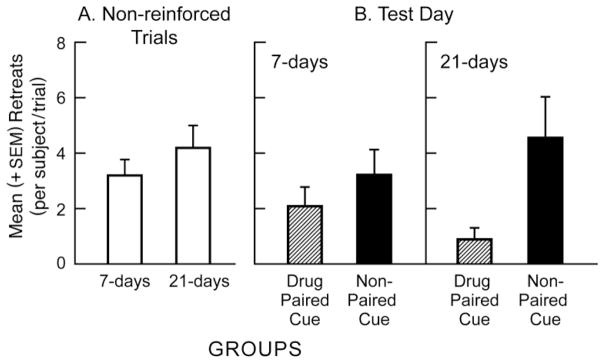
Retreats
The statistical analysis of retreat data exactly paralleled the results described above for Run Time. Thus, once again, there were no significant differences in the mean retreat frequency during the last three days of acquisition and the last three days of extinction in either of the two extinction groups (Bonferroni-corrected repeated-measures t-tests, p = n.s.), nor was the small increase in retreat frequency observed as extinction progressed (a comparison of the two clear bars in Fig 3-A) statistically reliable (Bonferroni-corrected independent group t-tests, p = n.s.). However, a two-factor ANOVA computed on the retreat data from test day (the four bars in Fig 3-B) identified a significant reduction in retreats among subjects presented with the drug-paired cue compared to the group provided the non-paired cue on test day (a main effect of Cue; F(1,31)=7.65, p <.01). Once again, there was no overall main effect of Withdrawal period (p>.05). Additionally, after three weeks of extinction, while the response to the drug-paired cue seemed to be stronger and the response to the non-paired cue weaker than the comparison groups in the one-week extinction condition, these effects did not reach statistical significance (i.e., there was no Cue × Withdrawal interaction; p>.05).
Fig 3.
Mean (+SEM) frequency of approach-avoidance retreat behaviors/rat/trial in subjects having experienced either one-week or three-weeks of non-cued, non-reinforced runway responding (left panel A). The data reflect an average of the last three extinction trials. Panel B (right side) depicts the mean (+SEM) number of retreats/animal on a single test day following either one or three weeks of extinction. Half the animals in each condition were presented with either a cocaine-paired olfactory cue predictive of cocaine availability in the goal box, or a “neutral” non-paired cue. Across both extinction conditions, animals emitted fewer approach-avoidance retreats in the presence of the drug-paired cue compared to the non-paired cue.
4. Discussion
The classic Opponent Process Theory of motivation (Solomon and Corbit, 1974) postulates that all affective emotional stimuli produce diametrically opposite and temporally dissociated actions. Thus, a rewarding drug that produces an initial positive hedonic state would be expected to also produce a negative affective state whose onset is delayed, whose duration is longer, and whose function is to return the organism to homeostasis. Consistent with this theory, we have previously reported that animals running a straight alley for i.v. cocaine develop over trials an ambivalence about goal-box entry that is thought to stem from cocaine’s mixed positive and negative properties (see reviews by Ettenberg 2004, 2009). The current results confirm these observations -- while animals initiated responding faster and faster over trials (thereby exhibiting an increased motivation to seek cocaine) they concurrently developed a progressive increase in retreat behaviors reflecting a growing ambivalence about goal box entry (see Fig 1). Note that this result is similar to that of animals working for natural reinforcers that are presented concurrently with aversive foot-shock. Martin and Ross (1964), for example, trained thirsty animals to run a straight alley for water whose delivery was paired with brief pulses of foot-shock. They reported that animals lapped the water more slowly, but exhibited faster start times with repeated testing. Similarly Miller and Hunt (1944) demonstrated that animals running an alley for food+shock continued to exhibit progressive increases in start speeds even while developing a hesitancy to enter a goal box. In this latter example, as in the current study, the decrease in start latencies is explained by the conditioned positive incentive properties of the goal box, while the ambivalence about goal box entry is thought to reflect the conditioned negative incentive properties of that same goal box.
An unexpected finding in the current study was the persistence of runway behavior even after the cocaine-reinforcer was removed from the goal box. Although 21 single-daily extinction trials produced a modest slowing of start latency and run time, and a small decrease in retreat frequency, these effects were not statistically significant. Thus the unique behavioral profile of rats running an alley for cocaine would appear to hold true for both acquisition and extinction. While the underlying explanation for this result is unknown, evidence from the animal learning literature provides some relevant insights. Studies of approach-avoidance conflict (i.e., where animals run or lever-press for food or water delivered concurrently with foot-shock) suggest that mixed positive + negative events dramatically increase the subjects resistance to extinction compared to that of animals working for “pure” positive stimuli (e.g., Brown and Wagner, 1964; Farber, 1948; Fallon, 1968, 1969). In this context, cocaine, which produces the same runway behavioral profile as food+shock (Geist and Ettenberg, 1997), would be expected to sustain responding during extinction longer than a purely positive reinforcer. Additionally, since animals exhibit greater resistance to extinction when reward magnitude is small compared to when it is large (e.g., Campbell and Kruger, 1979; Ratcliff and Ratcliff, 1971), goal box events that are inherently mixed (positive+ negative) would be expected to have greater resistance to extinction due to the smaller “net” reward that subjects obtain relative to a pure positive stimulus. Together these findings might account for why cocaine-reinforced runway responding in the current experiment did not significantly weaken over the course of extinction compared to what we have previously observed with food, water or heroin reinforcers (e.g., Ettenberg and Camp, 1986a, 1986b; Ettenberg and Horvitz, 1990; McFarland and Ettenberg, 1997).
Of particular interest in the current study was the impact of a drug-paired cue on the runway behavior of cocaine-reinforced rats after one or three weeks of non-reinforced extinction trials. Note that since the animals did not exhibit a reliable weakening of runway performance during the 7 or 21 days of once-daily extinction trials, the current experiment should not be conceived of as a test of “response reinstatement” or a model of relapse. Rather, the results demonstrate: a) that cocaine-paired cues can potentiate or enhance the motivation for drug-seeking behavior; b) that such effects can occur even without a prior and lengthy period of drug abstinence, and most importantly, c) that the cue-induced potentiation in drug-seeking behavior appears to stem from a reduction in the approach-avoidance conflict that otherwise exists during reinforced responding.
The demonstration that animals emit fewer retreat behaviors upon presentation of the cocaine-paired cue after a period of drug removal may, of course, stem from either an increase in the strength of the positive or a reduction in the strength of the negative associations (or both) that animals had previously formed between the drug and the goal box during acquisition. We note, however, that start latencies remained fast for all groups during both non-reinforced and test trials suggesting that the motivation to seek (approach) the goal, once established during acquisition, remained strong throughout the course of the experiment, while the motivation to retreat away from the goal box (avoidance) was weaker on test day when a drug-paired cue was presented. Therefore, we hypothesize that during extinction trials the strength of the dual positive and negative associations between cocaine and the goal box that were formed during reinforced responding, weaken at differential rates with the negative associations exhibiting less persistence over time. This hypothesis would therefore predict that cue-induced increases in drug-seeking might be attributable in part to the relative increase in the impact of the positive incentive properties of cocaine-paired cues that result from a weakening of the response-inhibiting associations with such cues.
An alternative explanation for the cue-induced reduction in retreat behaviors observed on test day, is that during acquisition rats preferentially associate the olfactory cue with the positive and not the negative effects of cocaine. We note, however, that retreat behaviors have been repeatedly observed in rats running the alley for cocaine in the absence of any olfactory cues (Ettenberg, 2009) thereby demonstrating that the inherent cues present in the start box and runway are by themselves sufficient to produce retreats behavior. Hence in the absence of olfactory cues, animals readily learn to associate the sights, sounds and smells of the apparatus with the dual and opposing actions of cocaine – and consequently come to develop retreat behaviors over trials. It therefore seems reasonable to assume that if one provided an additional highly salient stimulus to the apparatus (such as an olfactory cue) it too would be readily associated with the dual positive and negative consequences of the cocaine. And indeed we have shown that rats can readily associate external stimuli (including olfactory cues) with both the positive and negative properties of cocaine as measured in the conditioned place preference test (see review, Ettenberg 2004).
In human drug users, drug-paired cues have been reported to trigger cravings that are thought to motivate increased drug consumption or produce a relapse of drug self-administration even after a prolonged period of abstinence (Childress et al., 1987, 1988; O’Brien et al., 1992; Robbins and Ehrman, 1992). This phenomenon has also been demonstrated in the animal laboratory where the presentation of drug-paired cues has been shown to reinstate drug-reinforced responding that had been weakened through a period of non-reinforced extinction responding (Crombag et al., 2008; Shaham et al., 2003). Such findings have typically been conceptualized in terms of “conditioned reinforcement” where an originally neutral environmental cue is imbued with reinforcing properties due to its repeated pairing with the drug reinforcer. We note, however, that in the “real” world, the drug user does not first make a response and then receive a drug-paired cue (the conditioned reinforcer) in place of the drug, nor does the user typically work for drug-paired cues alone. Rather exposure to the drug-paired environmental stimulus occurs first and it in turn motivates drug-seeking i.e., the cue acts as a discriminative stimulus or S+ that predicts drug availability and hence motivates drug seeking behavior (Alleweireldt et al., 2001; Ettenberg and McFarland, 2003; McFarland and Ettenberg, 1997; Weiss et al., 2000). In the current experiment, the olfactory cue is present in the start box and runway prior to the animal’s experience of the drug and hence serves as an S+ that predicts the availability of cocaine in the goal box. Note that the non-paired cue here is not an S- since it does not predict the non-availability of cocaine in the runway, but rather remains a neutral cue that is not explicitly paired with any specific outcome. During the non-cued, non-reinforced trials, the removal of both the cocaine and the S+ cue served to strengthen the significance and salience of that cue so that when presented during withdrawal, the cue produces a strong potentiation in runway responding (see Figs 2-B and 3-B). The current results therefore suggest that, as we have previously reported for heroin reinforcement (Ettenberg and McFarland, 2003; McFarland and Ettenberg, 1997) and for rats with a mixed history of cocaine and heroin reinforcement (Su et al., 2011), subjects learn to identify a discriminative stimulus predictive of cocaine availability and hence exhibit an increase in cocaine-seeking behavior upon its presentation, even in the absence of extinguished responding.
Highlights.
Cocaine administration has mixed and opposing actions
Rats exhibited approach-avoidance conflict about entering a goal-box associated with cocaine
After 7–21 extinction trials, a cocaine-paired cue potentiated runway responding
The drug-paired cue elicited approach responding but weakened avoidance behavior
Cue-induced increases in drug-seeking may stem from a greater weakening of the negative relative to the positive associations formed with the drug-paired cue.
Acknowledgments
The authors acknowledge, with gratitude, Stephanie Waldroup and Rebeccah Baird for their assistance in various aspects of the project. This research was funded by NIDA grant DA05041 awarded to A.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alleweireldt AT, Weber SM, Neisewander JL. Passive exposure to a contextual discriminative stimulus reinstates cocaine-seeking behavior in rats. Pharmacol Biochem Behav. 2001;69:555–60. doi: 10.1016/s0091-3057(01)00573-1. [DOI] [PubMed] [Google Scholar]
- Brown RT, Wagner AR. Resistance to punishment and extinction following training with shock or nonreinforcement. J Exp Psychol. 1964;68:503–7. doi: 10.1037/h0042696. [DOI] [PubMed] [Google Scholar]
- Campbell PE, Kruger BM. Magnitude of water reward in the runway: a parametric investigation. Bull Psychonom Soc. 1979;14:165–8. [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Extinction of conditioned responses in abstinent cocaine or opioid users. NIDA Res Monogr. 1987;76:189–95. [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role for relapse. NIDA Res Monogr. 1988;84:25–40. [PubMed] [Google Scholar]
- Costall B, Kelly ME, Naylor RJ, Onaivi ES. The actions of nicotine and cocaine in a mouse model of anxiety. Pharmacol Biochem Behav. 1989;33:197–203. doi: 10.1016/0091-3057(89)90450-4. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. Opponent processes properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–8. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–7. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of busiprone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–8. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. Haloperidol induces a partial reinforcement extinction effect in rats: implications for a dopamine involvement in food reward. Pharmacol Biochem Behav. 1986a;25:813–21. doi: 10.1016/0091-3057(86)90392-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Camp CH. A partial reinforcement extinction effect in water-reinforced rats intermittently treated with haloperidol. Pharmacol Biochem Behav. 1986b;25:123l–1235. doi: 10.1016/0091-3057(86)90117-6. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. An animal model for investigating the anxiogenic effects of self-administered cocaine. Psychopharmacology. 1991;103:455–61. doi: 10.1007/BF02244244. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Geist TD. Qualitative and quantitative differences in the operant runway behavior of rats working for cocaine and heroin reinforcement. Pharmacol Biochem Behav. 1993;44:191–6. doi: 10.1016/0091-3057(93)90298-8. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Horvitz JC. Pimozide prevents the response-reinstating effects of water reinforcement in rats. Pharmacol Biochem Behav. 1990;37:465–9. doi: 10.1016/0091-3057(90)90014-9. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, McFarland K. Effects of haloperidol on cue-induced autonomic and behavioral indices of heroin reward and motivation. Psychopharmacology (Berl) 2003;168:139–45. doi: 10.1007/s00213-002-1266-0. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence for opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Fallon D. Resistance to extinction following learning with punishment of reinforced and nnoreinforced licking. J Exp Psychol. 1968;76:550–7. doi: 10.1037/h0025712. [DOI] [PubMed] [Google Scholar]
- Fallon D. Resistance to extinction following partial punishment of reinforced and/or noreinforced responses during learning. J Exp Psychol. 1969;79:183–5. doi: 10.1037/h0025712. [DOI] [PubMed] [Google Scholar]
- Farber IE. Response fixation under anxiety and nonanxiety conditions. J Exp Psychol. 1948;38:111–31. doi: 10.1037/h0055806. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Commissaris RJ. Effects of cocaine on conflict behavior in the rat. Life Sci. 1989;45:819–27. doi: 10.1016/0024-3205(89)90175-6. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. A simple method for studying intravenous drug reinforcement in the runway. Pharmacol Biochem Behav. 1990;36:703–6. doi: 10.1016/0091-3057(90)90278-p. [DOI] [PubMed] [Google Scholar]
- Geist TD, Ettenberg A. Concurrent positive and negative goal box events produce runway behaviors comparable to those of cocaine-reinforced rats. Pharmacol Biochem Behav. 1997;57:145–50. doi: 10.1016/s0091-3057(96)00300-0. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goldstein A. Heroin addiction: neurobiology, pharmacology, and policy. J Psychoactive Drugs. 1991;23:123–33. doi: 10.1080/02791072.1991.10472231. [DOI] [PubMed] [Google Scholar]
- Jhou TC, Good CH, Hoffman AH, Rowley C, Mejias-Aponte CA, Xu S-P, Lupica CR, Ikemoto S. Unpublished manuscript. Lateral habenula-tegmental in cocaine aversion and opponent motivational processes. [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol Biochem Behav. 2002;72:931–6. doi: 10.1016/s0091-3057(02)00764-5. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Martin B, Ross LE. Effects of consummatory response punishment of consummatory and runway behavior. J Comp Physiol Psychol. 1964;58:243–7. doi: 10.1037/h0041368. [DOI] [PubMed] [Google Scholar]
- McFarland K, Ettenberg A. Reinstatement of drug-seeking behavior produced by heroin-predictive environmental stimuli. Psychopharmacology (Berl) 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- Moldow RL, Fischman AJ. Cocaine induced secretion of ACTH, betaendorphin and corticosterone. Peptides. 1987;8:819–22. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- Miller NE, Hunt JMcV. Experimental studies of conflict. In: Hunt JMcV., editor. Personality and them behavior disorders. Oxford, UK: Ronald Press; 1944. pp. 431–65. [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann NY Acad Sci. 1992;654:401–14. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- Pliakas Am, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with camp response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;24:7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp Clin Psychopharmacol. 2000;8:117–24. doi: 10.1037/1064-1297.8.1.117. [DOI] [PubMed] [Google Scholar]
- Ratliff EG, Ratliff AR. Runway acquisition and extinction as a joint function of magnoitude of reward and pefrcentage of rewarded acquisition trials. Learning Motivation. 1971;2:289–95. [Google Scholar]
- Rescorla RA, Cunningham CL. Recovery of the US representation over time during extinction. Learn Motiv. 1978;9:373–91. [Google Scholar]
- Resnick RB, Resnick EB. Cocaine abuse and its treatment. Psychiatric Clinics North Am. 1984;7:713–28. [PubMed] [Google Scholar]
- River C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422:403–6. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Designing studies of drug conditioning in humans. Psychopharmacology. 1992;58:78–87. doi: 10.1007/BF02801965. [DOI] [PubMed] [Google Scholar]
- Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus maze. Pharmacol Biochem Behav. 1992;43:631–3. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Constentin J. Thigmotaxis as an index of anxiety in mice: influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–45. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Smith DE. Cocaine-alcohol abuse: epidemiological, diagnostic and treatment considerations. J Psychoactive Drugs. 1986;18:117–29. doi: 10.1080/02791072.1986.10471392. [DOI] [PubMed] [Google Scholar]
- Su Z-I, Wenzel J, Baird R, Ettenberg A. Comparison of self-administration behavior and responsiveness to drug-paired cues in rats running an alley for intravenous heroin and cocaine. Psychopharmacology (Berl) 2011;214:769–78. doi: 10.1007/s00213-010-2088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto S, Nakagawa T, Yamauchi Y, Minami M, Satoh M. Differential contributions of the basolateral and central nuclei of the amygdala in the negative affective component of chemical somatic and visceral pains in rats. Eur J Neurosci. 2003;18:2343–50. doi: 10.1046/j.1460-9568.2003.02952.x. [DOI] [PubMed] [Google Scholar]
- Van Dyke C, Byck R. Cocaine. Sci Am. 1982;246:128–41. doi: 10.1038/scientificamerican0382-128. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer Brain Struct Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Washton AM, Gold MS. Chronic cocaine abuse: evidence for adverse effects on health and functioning. Psychiatr Ann. 1984;14:733–9. [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA. 2000;97:4321–26. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JM, Waldroup SA, Haber ZM, Su Z-I, Ben-Shahar O, Ettenberg A. Effects of lidocaine-induced inactivation of the bed nucleus of the stria terminalis, the central or the basolateral nucleus of the amygdala on the opponent-process actions of self-administered cocaine in rats. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2267-7. in press, Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strong J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1987;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Yang X-M, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–50. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]