Abstract
Objective
We developed a novel pyrrole analogue of etomidate, (R)-ethyl 1-(1-phenylethyl)-1H-pyrrole-2-carboxylate (carboetomidate), which retains etomidate’s desirable anesthetic and hemodynamic properties but lacks its potent inhibitory affect on adrenocorticotropic hormone-stimulated steroid synthesis. The objective of this study was to test the hypothesis that in contrast to etomidate, carboetomidate neither suppresses the adrenocortical response to endotoxemia nor enhances the accompanying production of pro-inflammatory cytokines.
Design
Animal study
Setting
University research laboratory
Subjects
Male Sprague-Dawley rats
Interventions
For both single and multiple anesthetic dose studies, rats were injected with Escherichia coli lipopolysaccharide immediately followed by a hypnotic dose of etomidate, carboetomidate or vehicle alone (dimethyl sulfoxide) as a control. For single dose studies, no additional anesthetic (or vehicle) was administered. For multiple anesthetic dose studies, additional doses of anesthetic (or vehicle) were administered every 15 min for a total of eight anesthetic (or vehicle) doses.
Measurements and Main Results
Plasma adrenocorticotropic hormone, corticosterone, and cytokine concentrations were measured before lipopolysaccharide administration and intermittently throughout the 5 hour experiment. In single anesthetic dose studies, plasma adrenocorticotropic hormone and cytokine concentrations were not different at any time point among the etomidate, carboetomidate, and vehicle groups whereas plasma corticosterone concentrations were briefly (60–120 min) reduced in the etomidate group. In multiple anesthetic dose studies, plasma corticosterone concentrations were persistently lower and peak plasma IL-1β and IL-6 concentrations were higher in the etomidate group versus the carboetomidate and control groups. Peak plasma IL-10 concentrations were similarly elevated in the etomidate and carboetomidate groups versus the control group.
Conclusions
Compared to etomidate, carboetomidate produces less suppression of adrenocortical function and smaller increases in pro-inflammatory cytokine production in an endotoxemia model of sepsis. These findings suggest that carboetomidate could be a useful alternative to etomidate for maintaining anesthesia for a prolonged period of time in patients with sepsis.
Keywords: Lipopolysaccharides, Endotoxemia, Sepsis, Etomidate, Anesthetic, Corticosterone, Cytokines
Introduction
Patients with sepsis commonly need general anesthesia for important therapeutic interventions. Unfortunately, nearly all anesthetics produce cardiovascular depression (1–6), which can be life threatening to critically ill patients. Etomidate is an imidazole-based sedative-hypnotic that is distinguished from other anesthetics by its lesser effects on cardiovascular function (7–17). However because it binds with high affinity to the cytochrome P450 enzyme 11β-hydroxylase and inhibits the enzyme’s function, it potently suppresses the synthesis of adrenocortical steroids that help to restore homeostasis and enhance survival during sepsis (18–20). Such “pharmacological adrenalectomy” precludes the administration of multiple etomidate doses or continuous etomidate infusions to maintain anesthesia for a prolonged period of time and has raised concerns regarding the administration of even a single intravenous dose for anesthetic induction (21–29).
Carboetomidate (figure 1) is a novel pyrrole analogue of etomidate. It retains etomidate’s anesthetic activity, rapid recovery profile, hemodynamic stability, and high therapeutic index, but binds with significantly lower affinity to 11β-hydroxylase and does not suppress ACTH-stimulated steroid production (30). It’s design was based on the hypothesis that etomidate binds with high affinity to 11β-hydroxylase because the basic nitrogen in etomidate’s imidazole ring forms a coordination bond with the heme iron at the enzyme’s active site (31). In carboetomidate, etomidate’s basic nitrogen has been replaced with a -CH moiety to eliminate the ability to form this coordination bond and reduce affinity for 11β-hydroxylase.
Figure 1.
Chemical structures of etomidate and carboetomidate.
Because of carboetomidate favorable characteristics, we hypothesize that it might be a suitable replacement for etomidate that would allow the safe administration of multiple boluses or prolonged continuous infusions. Such drugs are predicted not to impact the normal adrenocortical response to sepsis that is thought to play an important role in suppressing the accompanying pro-inflammatory cytokine storm (32–35). As a test of this hypothesis, we defined the impact of single and multiple doses of carboetomidate and etomidate on plasma levels of ACTH, corticosterone, and cytokines in rats challenged with Escherichia coli lipopolysaccharide (LPS) to determine whether these two agents differentially affect the inflammatory response to endotoxemia.
Materials and Methods
Animals
This study was approved by the Subcommittee on Research Animal Care at the Massachusetts General Hospital, Boston, Massachusetts. The care and handling of the animals was in accord with National Institutes of Health guidelines for ethical animal research. Adult male Sprague-Dawley rats (320–370 gm) were purchased from Charles River Laboratories (Wilmington, MA) with pre-implanted femoral venous catheters. Rats were individually caged with controlled temperature (21± 2°C) and light-dark cycles (7 A.M. to 7 P.M.) in the Massachusetts General Hospital Center for Comparative Medicine animal care facility with food and water provided at libitum.
Drugs and Chemicals
Carboetomidate was synthesized by Aberjona Laboratories (Beverly, MA) as previously described (30). Etomidate was from Bachem Americas (Torrance CA). LPS from Escherichia coli serotype 055:B5 and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Chemical Co (St. Louis, MO).
General Procedure
All animal studies were begun at 8:30 AM to minimize diurnal variations. Each rat received supplemental oxygen (100%, 2 L/min) through a port in the chamber throughout the experiment. After weighing, rats were allowed to acclimate for two hours to restriction within a 3-inch diameter, 9-inch long acrylic chamber with a tail exit port. A sub-lethal dose of LPS (1mg/kg dissolved in 1 mL of saline) was administered through the femoral venous catheter.
For single anesthetic dose studies (n = 5 rats/group), a single dose of etomidate (2 mg/kg in DMSO), carboetomidate (14 mg/kg in DMSO), or DMSO vehicle alone (as a control) was administered through the femoral catheter immediately after injection of LPS followed by a 1 mL saline flush. These anesthetic doses are twice the respective ED50s for loss of righting reflexes in rats and produce similar durations of hypnosis (30, 36). The concentrations of etomidate and carboetomidate were 2.85 mg/mL and 20 mg/mL respectively, which maintained the volume of DMSO vehicle injected into each rat at 0.7 mL/kg. Rats in the control group similarly received 0.7 ml/kg DMSO, but without anesthetic.
For multiple anesthetic dose studies (n = 5 rats/group), etomidate (2 mg/kg in DMSO), carboetomidate (14 mg/kg in DMSO), or DMSO vehicle alone was administered through the femoral catheter immediately after injection of LPS. Anesthetic in DMSO (or DMSO vehicle alone) was re-administered every 15 minutes for a total of 8 doses (i.e. 15, 30, 45, 60, 75, 90, and 105 minutes post-LPS injection). For these studies, the concentrations of etomidate and carboetomidate were 11.4 mg/mL and 80mg/mL in DMSO, respectively. This maintained the total volume of DMSO vehicle injected into each rat at 1.4 mL/kg. Rats in the control group similarly received 1.4 mL/kg DMSO, but without anesthetic. No rats died during our experiments. However in the multiple anesthetic dose study, a single rat in the carboetomidate group had a plasma IL-6 concentration before LPS injection that was an order of magnitude higher than the average of all other rats and the IL-6 concentration failed to increase after LPS injection. Because this suggested the presence of a pre-existing infection perhaps related to placement of the femoral venous line, all data from this rat was eliminated from analyses.
For both single and multiple anesthetic dose studies, blood samples (400 ul) were collected through the femoral catheter immediately before LPS administration and 2, 15, 30, 60, 120, 160 and 300 minutes post-LPS injection. This blood was replaced with 1 mL of saline. Upon collection, blood samples were mixed with EDTA (3 mg) and centrifuged at 1,530g for 15min. The resultant plasma was stored at −80°C until assayed.
Plasma ACTH and corticosterone concentrations in all blood draws were measured individually for each animal using an Enzyme-Linked ImmunoSorbent Assay (ELISA) with kits from MD Bioproducts (St. Paul, MN) and Immunodiagnostics Systems (Fountain Hills, AZ), respectively. Plasma cytokine concentrations were measured for each animal at time points 0 min, 30 min, 60 min, 120 min, and 300 min post-LPS injection using a MILLPLEX MAP Rat Cytokine/Chemokine Panel (Millipore, St. Charles, MO).
Statistical Analysis
All data are reported as mean +/− SE. Statistical analyses were done using Prism v5.0 for the Macintosh (GraphPad Software, Inc., LaJolla, CA). To compare differences in plasma corticosterone or cytokine concentrations among groups at each time point, a repeated measures two-way analysis of variance was performed where the factors were time after LPS administration and drug group, followed by a Bonferroni post–test. For all statistical analyses, P values of less than 0.05 were considered to indicate statistical significance.
Results
Anesthetic Modulation of Plasma ACTH Concentrations following LPS Injection: Single and Multiple Anesthetic Dose Studies
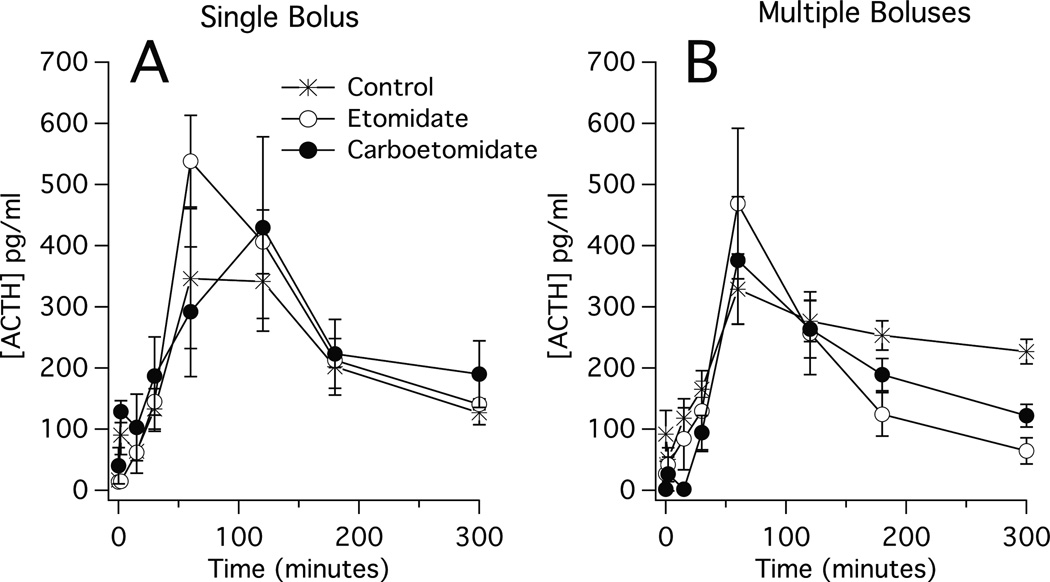
In single and multiple anesthetic dose studies, injection of LPS produced a rapid increase in plasma ACTH concentrations in all three groups (i.e. control, etomidate, and carboetomidate groups). Respective peak plasma ACTH concentrations in the single and multiple dose studies were 350 ± 114 pg/mL and 330 ± 58 pg/mL in the control groups, 540 ± 75 pg/mL and 470 ± 123 pg/mL in the etomidate groups, and 430 ± 148 pg/mL and 430 ± 116 pg/mL in the carboetomidate groups 30–120 min post-LPS injection (figure 2). After peaking, plasma ACTH concentrations in all groups remained elevated throughout the remainder of the 5-hour experiment. In each study, we detected no significant difference at any time point in plasma ACTH concentrations among the three groups.
Figure 2.
Time-dependent changes in plasma adrenocorticotropic hormone concentrations following administration of lipopolysaccharide (1 mg/kg at 0 min) and either (A) a single dose of anesthetic or vehicle (also at time 0) or; (B) eight doses of anesthetic or vehicle (one dose every 15 min beginning at 0 min and ending at time 105 min). Each etomidate and carboetomidate dose was 2 mg/kg and 14 mg/kg, respectively, which is twice the ED50 for loss of righting reflexes in rats (30, 36). Vehicle was dimethyl sulfoxide. Each data point is the mean ± SEM of 4 or 5 animals.
Anesthetic Modulation of Plasma Corticosterone Concentrations following LPS Injection
Single Anesthetic Dose Studies
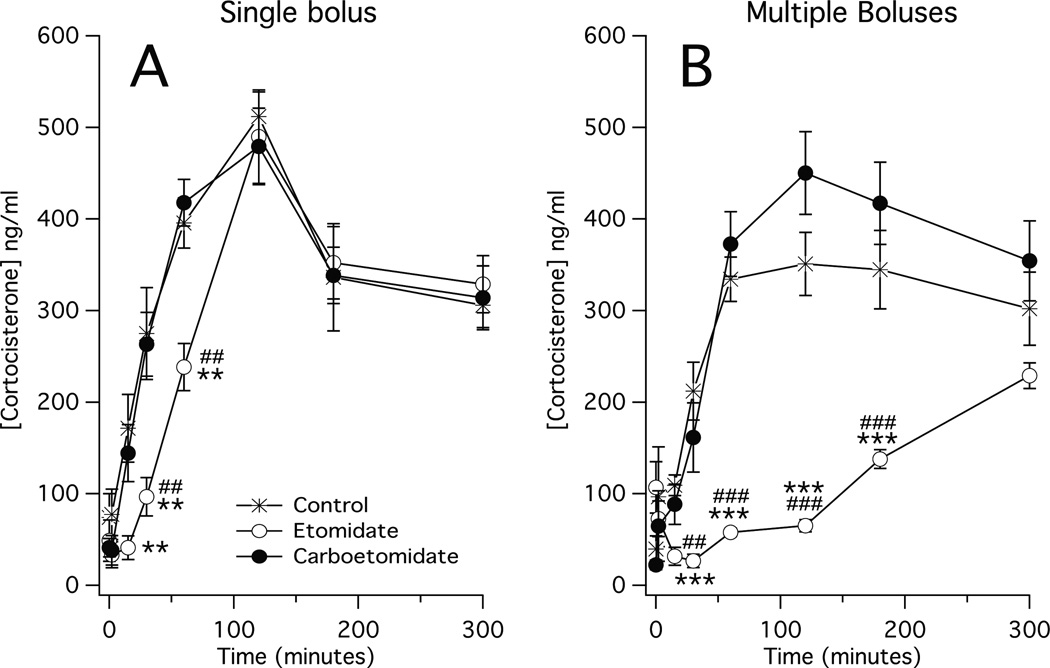
In all three groups, plasma corticosterone concentrations increased following LPS injection and peaked 120 min post-LPS injection (control group: 510 ± 27 ng/mL, etomidate group: 490 ± 51 ng/mL, and carboetomidate group: 480 ± 42 ng/mL; figure 3A). At no time point was the plasma corticosterone concentration in the carboetomidate group significantly different from the corresponding one in the control group. However at two time points prior to reaching a peak (30 min, and 60 min post-LPS injection), plasma corticosterone concentrations in the etomidate group were significantly lower than the corresponding ones in the carboetomidate group.
Figure 3.
Time-dependent changes in plasma cortocosterone concentrations following administration of lipopolysaccharide (1 mg/kg at 0 min) and either (A) a single dose of anesthetic or vehicle (also at time 0) or; (B) eight doses of anesthetic or vehicle (one dose every 15 min beginning at 0 min and ending at time 105 min). Each etomidate and carboetomidate dose was 2 mg/kg and 14 mg/kg, respectively, which is twice the ED50 for loss of righting reflexes in rats (30, 36). Vehicle was dimethyl sulfoxide. Each data point is the mean ± SEM of 4 or 5 animals. *, P < 0.05 versus vehicle. **, P < 0.01 versus vehicle alone. ***, P < 0.001 versus vehicle alone. ##, P < 0.01 versus carboetomidate. ###, P < 0.001 versus carboetomidate.
Multiple Anesthetic Dose Studies
In the control and carboetomidate groups, plasma corticosterone concentrations increased following LPS injection, peaking 120 min post-LPS injection (350 ± 34 ng/mL and 410 ± 52 ng/mL, respectively; figure 3B) and at no time point were plasma corticosterone concentrations significantly different between the two groups. Conversely in the etomidate group, plasma corticosterone concentrations initially decreased significantly (from 110 ± 28 ng/mL prior to LPS injection to 32 ± 10 ng/mL 15 min post-LPS injection and 27 ± 7 ng/mL 30 min post-LPS injection) and then failed to increase significantly above the pre-LPS injection value until the final time point of our study. As such, the plasma corticosterone concentrations in the etomidate group were significantly lower than the corresponding ones in the control and carboetomidate groups at all time points between 30 min and 180 min post-LPS injection.
Anesthetic Modulation of Plasma Cytokine Concentrations following LPS Injection
Single Anesthetic Dose Studies
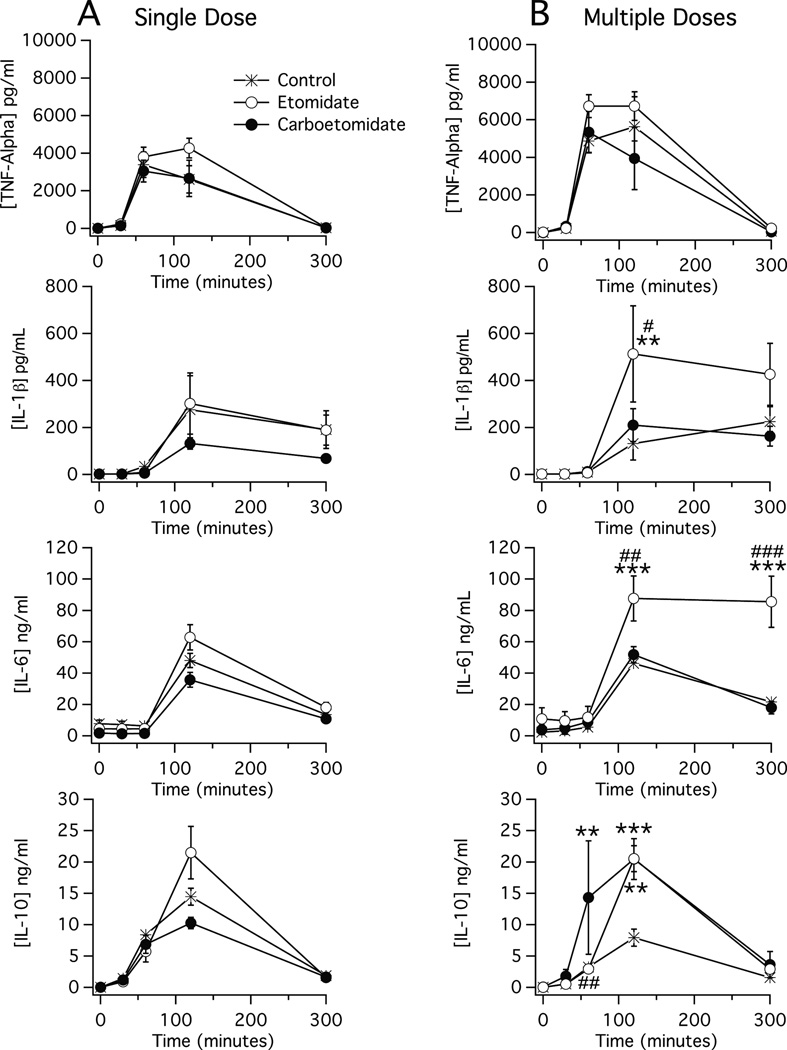
In all three groups, plasma TNF-α, IL-1β, IL-6 and IL-10 concentrations increased by at least 10-fold following LPS injection and peaked by 120 min post-LPS injection (figure 4A). There was no statistically significant difference in the plasma concentration of any cytokine in either anesthetic group versus that in the control group at any time point.
Figure 4.
Time-dependent changes in plasma cytokine concentrations following administration of lipopolysaccharide (1 mg/kg at 0 min) and either (A) a single dose of anesthetic or vehicle (also at time 0) or; (B) eight doses of anesthetic or vehicle (one dose every 15 min beginning at 0 min and ending at time 105 min). Each etomidate and carboetomidate dose was 2 mg/kg and 14 mg/kg, respectively, which is twice the ED50 for loss of righting reflexes in rats (30, 36). Each data point is the mean ± SEM of 4 or 5 animals. **, P < 0.01 versus vehicle alone. ***, P < 0.001 versus vehicle alone. #, P < 0.05 versus carboetomidate. ##, P < 0.01 versus carboetomidate. ###, P < 0.001 versus carboetomidate.
Multiple Anesthetic Dose Studies
In all three groups, plasma TNF-α, IL-1β, IL-6 and IL-10 concentrations increased by at least 10-fold following LPS injection and peaked by 120 min post-LPS injection (figure 4B). There was no statistically significant difference at any time point in plasma TNF-α concentrations among the three groups. However, the peak plasma concentration of IL-1β was significantly higher in the etomidate group than in the control or carboetomidate groups (500 ± 200 pg/mL vs. 130 ± 20 pg/mL and 210 ± 70 pg/mL, respectively). Similarly, the peak plasma concentration of IL-6 was significantly higher in the etomidate group than in the control or carboetomidate groups (90 ± 14 ng/mL vs. 46 ± 6 ng/mL and 52 ± 5 ng/mL) and remained higher 300 min post-LPS injection. In contrast, the peak plasma concentration of IL-10 was similarly elevated in the etomidate and carboetomidate groups versus the control group (21 ± 2 ng/mL and 21 ± 3 ng/mL vs. 8 ± 1 ng/mL).
Discussion
This is the first study of the pyrrole etomidate analogue carboetomidate in a model of sepsis. It defined the effects of carboetomidate on plasma ACTH, corticosterone, and cytokine concentrations and compared them to the effects of etomidate and vehicle alone following an LPS challenge in rats. Our results reveal that carboetomidate and etomidate differentially affect the inflammatory response that results from LPS administration. This difference was most pronounced in studies using multiple anesthetic doses administered over a prolonged period of time as etomidate significantly reduced plasma corticosterone concentrations and increased peak plasma concentrations of the pro-inflammatory cytokines IL-1β, IL-6 and the anti-inflammatory cytokine IL-10 whereas carboetomidate only increased plasma concentrations of IL-10.
Several animal models of sepsis have been used to replicate either the signs and symptoms or the laboratory findings observed in human sepsis (37). These include bacterial infusion models, polymicrobial peritonitis models, and endotoxin models. Each model replicates certain aspects of clinical sepsis but also has its own limitations. For example, bacterial infusion models are not exactly analogous to clinical situations where there is commonly a focus of infection providing continuous dissemination of bacteria that colonize and replicate (38, 39). Bacterial peritonitis models closely resemble the clinical scenario of sepsis following bowel perforation, but the model has wide variability in terms of the host inflammatory and physiological responses, and the level of bacteremia and mortality rates (40, 41). We chose the endotoxin model in the present study because the model is highly reproducible and mimics the hyperinflammatory response that often occurs in patients in septic shock, while acknowledging that it differs from clinical sepsis because it lacks an infectious focus and produces larger and more transient cytokine responses.
Numerous cytokines have been identified that play important roles in the pathophysiology of sepsis (42). The pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 have been shown to both modulate and to be modulated by the hypothalamic-pituitary-adrenal axis (32, 43–46). Such crosstalk suggests that drugs known to inhibit adrenocortical function (e.g. etomidate, but not carboetomidate) might alter the production of these cytokines. In contrast, IL-10 possesses strong anti-inflammatory properties that may counteract some of the actions of these pro-inflammatory cytokines and improve survival (45).
Our results demonstrate that etomidate inhibits LPS-stimulated corticosterone production in vivo. Following LPS injection, a single intravenous etomidate dose significantly reduced plasma corticosterone concentrations (relative to vehicle controls) for 60–120 min. This duration of adrenocortical suppression is shorter than that observed in humans (20, 47, 48), which may reflect the presence of an aliesterase found in rat blood that rapidly metabolizes etomidate (49). With multiple etomidate doses, plasma corticosterone concentrations initially decreased after LPS administration. This likely reflects the combined effects of metabolism of existing plasma corticosterone along with inhibition of new corticosterone synthesis during prolonged etomidate administration. Etomidate reduced plasma corticosterone concentrations without impacting the ACTH response to LPS injection, consistent with etomidate’s known direct inhibitory action on 11β-hydroxylase activity (18, 50).
Carboetomidate’s affect on the adrenocortical response to an LPS challenge was significantly less than that of etomidate and not different from that of vehicle alone even when multiple doses were administered over a nearly two-hour time period. This implys that carboetomidate does not significantly inhibit 11β-hydroxylase activity and is consistent with our previous observation that carboetomidate does not suppress the adrenocortical response to exogenously administered ACTH (30).
In addition to differences between etomidate and carboetomidate with respect to the adrenocortical response to LPS injection, we also observed differences in their impact on cytokine production as peak plasma IL-1β and IL-6 concentrations were significantly higher following administration of etomidate versus carboetomidate (or vehicle control). These differences between etomidate and carboetomidate may relate to their differential effects on adrenocortical function as glucocorticoids reduce pro-inflammatory cytokine synthesis. For example, dexamethasone administration reduces the serum IL-1 concentration of mice challenged with LPS (51) and hydrocortisone infusion reduces plasma levels of IL-6 in patients in septic shock (34). Similarly, reducing glucocorticoid synthesis by either surgical adrenalectomy (32) or pharmacological adrenalectomy with mifepristone (44) increases plasma concentrations of IL-6 after LPS administration.
Although there have been few clinical studies defining the effects of etomidate on cytokine production, they suggest a link between etomidate-induced adrenocortical suppression and increased pro-inflammatory cytokine production. Jameson et al. (52) showed that compared to thiopentone, etomidate reduced plasma cortisol concentrations and increased plasma IL-6 concentrations in women undergoing hysterectomies. In pediatric patients with meningococcal sepsis, Den Brinker et al (53) showed that plasma IL-6 concentrations were inversely related to the cortisol:ACTH ratios and increased in patients who had received etomidate. Yeager et al. (54) found that perioperative hydrocortisone replacement therapy administered to patients who received etomidate for cardiac surgery dose-dependently reduced postoperative plasma IL-6 concentrations.
In summary, our studies demonstrate that carboetomidate has lesser effects than etomidate on the adrenocortical and pro-inflammatory cytokine responses to LPS administration. This suggests that unlike etomidate, carboetomidate may be useful as an anesthetic maintenance agent in patients with sepsis.
Acknowledgement
The authors thank Hui Zheng of the Massachusetts General Hospital Biostatistics Center for advice on statistical analysis.
Supported by grants R01-GM087316, R21-DA029253, and K08-GM083216 from the National Institutes of Health, Bethesda, MD and the Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The Massachusetts General Hospital has submitted patent applications for carboetomidate and related analogues. Two authors (Raines and Cotten), and their respective laboratories, departments, and institutions could receive compensation related to the development or sale of these drugs. Dr. Raines Dr. Raines is the co-founder of Annovation Biopharma and has an equity interest in Annovation BioPharma, Inc., which has an option to license this technology for development. Dr. Raines is also the lead inventor of Carboetomidate and received royalties as an inventor. Dr. Cotten received patents and royalties from the National Institutes of Health. The remaining authors have not disclosed any potential conflicts of interest.
References
- 1.Coates DP, Monk CR, Prys-Roberts C, et al. Hemodynamic effects of infusions of the emulsion formulation of propofol during nitrous oxide anesthesia in humans. Anesth Analg. 1987;66(1):64–70. [PubMed] [Google Scholar]
- 2.Illievich UM, Petricek W, Schramm W, et al. Electroencephalographic burst suppression by propofol infusion in humans: hemodynamic consequences. Anesth Analg. 1993;77(1):155–160. [PubMed] [Google Scholar]
- 3.McKinney MS, Fee JP. Cardiovascular effects of 50% nitrous oxide in older adult patients anaesthetized with isoflurane or halothane. Br J Anaesth. 1998;80(2):169–173. doi: 10.1093/bja/80.2.169. [DOI] [PubMed] [Google Scholar]
- 4.Kikura M, Ikeda K. Comparison of effects of sevoflurane/nitrous oxide and enflurane/nitrous oxide on myocardial contractility in humans. Load-independent and noninvasive assessment with transesophageal echocardiography. Anesthesiology. 1993;79(2):235–243. doi: 10.1097/00000542-199308000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Benson M, Junger A, Fuchs C, et al. Use of an anesthesia information management system (AIMS) to evaluate the physiologic effects of hypnotic agents used to induce anesthesia. J Clin Monit Comput. 2000;16(3):183–190. doi: 10.1023/a:1009937510028. [DOI] [PubMed] [Google Scholar]
- 6.Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101(3):622–628. doi: 10.1213/01.ANE.0000175214.38450.91. table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Janssen PA, Niemegeers CJ, Schellekens KH, et al. Etomidate, R-(+)-ethyl-1-(− methyl-benzyl)imidazole-5-carboxylate (R 16659), a potent, short-acting and relatively atoxic intravenous hypnotic agent in rats. Arzneimittelforschung. 1971;21(8):1234–1243. [PubMed] [Google Scholar]
- 8.Gooding JM, Weng JT, Smith RA, et al. Cardiovascular and pulmonary responses following etomidate induction of anesthesia in patients with demonstrated cardiac disease. Anesth Analg. 1979;58(1):40–41. doi: 10.1213/00000539-197901000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Gillies GW, Lees NW. The effects of speed of injection on induction with propofol. A comparison with etomidate. Anaesthesia. 1989;44(5):386–388. doi: 10.1111/j.1365-2044.1989.tb11333.x. [DOI] [PubMed] [Google Scholar]
- 10.Boisson-Bertrand D, Taron F, Laxenaire MC. Etomidate vs. propofol to carry out suspension laryngoscopies. Eur J Anaesthesiol. 1991;8(2):141–144. [PubMed] [Google Scholar]
- 11.Sokolove PE, Price DD, Okada P. The safety of etomidate for emergency rapid sequence intubation of pediatric patients. Pediatr Emerg Care. 2000;16(1):18–21. doi: 10.1097/00006565-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Vinson DR, Bradbury DR. Etomidate for procedural sedation in emergency medicine. Ann Emerg Med. 2002;39(6):592–598. doi: 10.1067/mem.2002.123695. [DOI] [PubMed] [Google Scholar]
- 13.Guldner G, Schultz J, Sexton P, et al. Etomidate for rapid-sequence intubation in young children: hemodynamic effects and adverse events. Acad Emerg Med. 2003;10(2):134–139. doi: 10.1111/j.1553-2712.2003.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi YF, Wong TW, Lau CC. Midazolam is more likely to cause hypotension than etomidate in emergency department rapid sequence intubation. Emerg Med J. 2004;21(6):700–702. doi: 10.1136/emj.2002.004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zed PJ, Abu-Laban RB, Harrison DW. Intubating conditions and hemodynamic effects of etomidate for rapid sequence intubation in the emergency department: an observational cohort study. Acad Emerg Med. 2006;13(4):378–383. doi: 10.1197/j.aem.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 16.Dhawan N, Chauhan S, Kothari SS, et al. Hemodynamic responses to etomidate in pediatric patients with congenital cardiac shunt lesions. J Cardiothorac Vasc Anesth. 2010;24(5):802–807. doi: 10.1053/j.jvca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Siedy J, Knapik P, Saucha W, et al. Comparison of propofol and etomidate anaesthesia for elective electrical cardioversion. Kardiol Pol. 2010;68(11):1249–1255. [PubMed] [Google Scholar]
- 18.de Jong FH, Mallios C, Jansen C, et al. Etomidate suppresses adrenocortical function by inhibition of 11 beta-hydroxylation. J Clin Endocrinol Metab. 1984;59(6):1143–1147. doi: 10.1210/jcem-59-6-1143. [DOI] [PubMed] [Google Scholar]
- 19.Wagner RL, White PF, Kan PB, et al. Inhibition of adrenal steroidogenesis by the anesthetic etomidate. N Engl J Med. 1984;310(22):1415–1421. doi: 10.1056/NEJM198405313102202. [DOI] [PubMed] [Google Scholar]
- 20.Duthie DJ, Fraser R, Nimmo WS. Effect of induction of anaesthesia with etomidate on corticosteroid synthesis in man. Br J Anaesth. 1985;57(2):156–159. doi: 10.1093/bja/57.2.156. [DOI] [PubMed] [Google Scholar]
- 21.Bloomfield R, Noble DW. Etomidate, pharmacological adrenalectomy and the critically ill: a matter of vital importance. Crit Care. 2006;10(4):161. doi: 10.1186/cc5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson WL., Jr Should we use etomidate as an induction agent for endotracheal intubation in patients with septic shock?: a critical appraisal. Chest. 2005;127(3):1031–1038. doi: 10.1378/chest.127.3.1031. [DOI] [PubMed] [Google Scholar]
- 23.Lipiner-Friedman D, Sprung CL, Laterre PF, et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007;35(4):1012–1018. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 24.Cotton BA, Guillamondegui OD, Fleming SB, et al. Increased risk of adrenal insufficiency following etomidate exposure in critically injured patients. Arch Surg. 2008;143(1):62–67. doi: 10.1001/archsurg.143.1.62. discussion 67. [DOI] [PubMed] [Google Scholar]
- 25.Fengler BT. Should etomidate be used for rapid-sequence intubation induction in critically ill septic patients? Am J Emerg Med. 2008;26(2):229–232. doi: 10.1016/j.ajem.2007.03.032. [DOI] [PubMed] [Google Scholar]
- 26.Hildreth AN, Mejia VA, Maxwell RA, et al. Adrenal suppression following a single dose of etomidate for rapid sequence induction: a prospective randomized study. J Trauma. 2008;65(3):573–579. doi: 10.1097/TA.0b013e31818255e8. [DOI] [PubMed] [Google Scholar]
- 27.Cuthbertson BH, Sprung CL, Annane D, et al. The effects of etomidate on adrenal responsiveness and mortality in patients with septic shock. Intensive Care Med. 2009 doi: 10.1007/s00134-009-1603-4. [DOI] [PubMed] [Google Scholar]
- 28.Edwin SB, Walker PL. Controversies surrounding the use of etomidate for rapid sequence intubation in patients with suspected sepsis. Ann Pharmacother. 2010;44(7–8):1307–1313. doi: 10.1345/aph.1M664. [DOI] [PubMed] [Google Scholar]
- 29.Kulstad EB, Kalimullah EA, Tekwani KL, et al. Etomidate as an induction agent in septic patients: red flags or false alarms? West J Emerg Med. 2010;11(2):161–172. [PMC free article] [PubMed] [Google Scholar]
- 30.Cotten JF, Forman SA, Laha JK, et al. Carboetomidate: a pyrrole analog of etomidate designed not to suppress adrenocortical function. Anesthesiology. 2010;112(3):637–644. doi: 10.1097/ALN.0b013e3181cf40ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roumen L, Sanders MP, Pieterse K, et al. Construction of 3D models of the CYP11B family as a tool to predict ligand binding characteristics. Journal of computer-aided molecular design. 2007;21(8):455–471. doi: 10.1007/s10822-007-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goujon E, Parnet P, Laye S, et al. Adrenalectomy enhances pro-inflammatory cytokines gene expression, in the spleen, pituitary and brain of mice in response to lipopolysaccharide. Brain Res Mol Brain Res. 1996;36(1):53–62. doi: 10.1016/0169-328x(95)00242-k. [DOI] [PubMed] [Google Scholar]
- 33.Keh D, Boehnke T, Weber-Cartens S, et al. Immunologic and hemodynamic effects of "low-dose" hydrocortisone in septic shock: a double-blind, randomized, placebo-controlled, crossover study. Am J Respir Crit Care Med. 2003;167(4):512–520. doi: 10.1164/rccm.200205-446OC. [DOI] [PubMed] [Google Scholar]
- 34.Oppert M, Schindler R, Husung C, et al. Low-dose hydrocortisone improves shock reversal and reduces cytokine levels in early hyperdynamic septic shock. Crit Care Med. 2005;33(11):2457–2464. doi: 10.1097/01.ccm.0000186370.78639.23. [DOI] [PubMed] [Google Scholar]
- 35.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol. 2004;181(2):207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- 36.Cotten JF, Husain SS, Forman SA, et al. Methoxycarbonyl-etomidate: a novel rapidly metabolized and ultra-short-acting etomidate analogue that does not produce prolonged adrenocortical suppression. Anesthesiology. 2009;111(2):240–249. doi: 10.1097/ALN.0b013e3181ae63d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 38.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9(1):1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit Care Med. 2009;37(1 Suppl):S30–S37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]
- 40.Schultz MJ, van der Poll T. Animal and human models for sepsis. Ann Med. 2002;34(7–8):573–581. doi: 10.1080/078538902321117797. [DOI] [PubMed] [Google Scholar]
- 41.Rittirsch D, Huber-Lang MS, Flierl MA, et al. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2009;4(1):31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420(6917):885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 43.Uehara A, Gottschall PE, Dahl RR, et al. Stimulation of ACTH release by human interleukin-1 beta, but not by interleukin-1 alpha, in conscious, freely-moving rats. Biochem Biophys Res Commun. 1987;146(3):1286–1290. doi: 10.1016/0006-291x(87)90788-1. [DOI] [PubMed] [Google Scholar]
- 44.Morrow LE, McClellan JL, Conn CA, et al. Glucocorticoids alter fever and IL-6 responses to psychological stress and to lipopolysaccharide. Am J Physiol. 1993;264(5 Pt 2):R1010–R1016. doi: 10.1152/ajpregu.1993.264.5.R1010. [DOI] [PubMed] [Google Scholar]
- 45.Cavaillon JM. Pathophysiological Role of Pro- and Anti-Inflammatory Cytokines in Sepsis. Sepsis. 1998;2(2):127–140. [Google Scholar]
- 46.Haddad JJ, Saade NE, Safieh-Garabedian B. Cytokines and neuro-immune-endocrine interactions: a role for the hypothalamic-pituitary-adrenal revolving axis. J Neuroimmunol. 2002;133(1–2):1–19. doi: 10.1016/s0165-5728(02)00357-0. [DOI] [PubMed] [Google Scholar]
- 47.Allolio B, Stuttmann R, Leonhard U, et al. Adrenocortical suppression by a single induction dose of etomidate. Klin Wochenschr. 1984;62(21):1014–1017. doi: 10.1007/BF01711723. [DOI] [PubMed] [Google Scholar]
- 48.Fragen RJ, Shanks CA, Molteni A, et al. Effects of etomidate on hormonal responses to surgical stress. Anesthesiology. 1984;61(6):652–656. doi: 10.1097/00000542-198412000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Calvo R, Carlos R, Erill S. Etomidate and plasma esterase activity in man and experimental animals. Pharmacology. 1979;18(6):294–298. doi: 10.1159/000137268. [DOI] [PubMed] [Google Scholar]
- 50.Fry DE, Griffiths H. The inhibition by etomidate of the 11 beta-hydroxylation of cortisol. Clin Endocrinol (Oxf) 1984;20(5):625–629. doi: 10.1111/j.1365-2265.1984.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 51.Staruch MJ, Wood DD. Reduction of serum Interleukin-1-like activity after treatment with dexamethasone. J Leukoc Biol. 1985;37(2):193–207. doi: 10.1002/jlb.37.2.193. [DOI] [PubMed] [Google Scholar]
- 52.Jameson P, Desborough JP, Bryant AE, et al. The effect of cortisol suppression on interleukin-6 and white blood cell responses to surgery. Acta Anaesthesiol Scand. 1997;41(2):304–308. doi: 10.1111/j.1399-6576.1997.tb04683.x. [DOI] [PubMed] [Google Scholar]
- 53.den Brinker M, Joosten KF, Liem O, et al. Adrenal insufficiency in meningococcal sepsis: bioavailable cortisol levels and impact of interleukin-6 levels and intubation with etomidate on adrenal function and mortality. J Clin Endocrinol Metab. 2005;90(9):5110–5117. doi: 10.1210/jc.2005-1107. [DOI] [PubMed] [Google Scholar]
- 54.Yeager MP, Rassias AJ, Fillinger MP, et al. Cortisol antiinflammatory effects are maximal at postoperative plasma concentrations. Crit Care Med. 2005;33(7):1507–1512. doi: 10.1097/01.ccm.0000164565.65986.98. [DOI] [PubMed] [Google Scholar]