Abstract
Background
Cannabis is the most widely used illicit substance and has been associated with cognitive impairment. It is unclear whether such impairment can occur in the absence of potential confounding influences of co-morbid axis-I disorders and use of other illicit substances.
Method
Young adult volunteers (18–29 years) were recruited from the general community on the basis of having no axis-I disorders or history of illicit substance use other than cannabis use. Subjects were then grouped according to presence or absence of cannabis use (>1 time/week over past 12 months). Cognition was compared between groups using selected paradigms from the CANTAB.
Results
Cannabis users (n=16) and controls (n=214) did not differ significantly on salient demographic characteristics. Compared to controls, cannabis users showed significant impairments on quality of decision-making (Cambridge Gamble task), and executive planning (One Touch Stockings of Cambridge task). Response inhibition, spatial working memory, and sustained attention were intact.
Conclusions
This study identified cognitive deficits in cannabis users even in the absence of axis-I disorders and a history of using other illicit drugs. Future work should use longitudinal designs to track whether these deficits predate cannabis use or are due to its consumption.
Keywords: Addiction, Cognition, Cannabis, Impulsivity
1. INTRODUCTION
Cannabis is the most widely used illicit substance (Substance Abuse and Mental Health Services Administration, 2010), and has been associated with poor academic achievement, unemployment, legal problems, and heightened risk of developing a psychotic disorder (Hall and Degenhardt, 2009). Despite considerable research, there are conflicting opinions regarding cannabis use and its association with cognitive dysfunction (Iversen, 2003; Hart et al., 2010).
There is broad consensus that cannabis intoxication often results in short-term dysfunction across a range of cognitive domains in healthy volunteers (Makela et al., 2006; Almeida et al., 2008; Hunault et al., 2009), though not all studies have been consistent in this regard (Heishman et al., 1997; Almeida et al., 2008; Hart et al., 2010). The extent to which chronic cannabis use is also associated with cognitive impairments remains less clear. Early cross-sectional studies reported associations between heavy chronic cannabis use and impaired verbal fluency and word recognition memory; some studies reported that these deficits persisted for just a few days following cessation of cannabis intake (Pope et al., 2001), while others suggested that these deficits persisted for a month or longer (Bolla et al., 2002; Pope et al., 2003). Research using various neuropsychological tests has confirmed that chronic cannabis use can be associated with dysfunction across a range of functions including aspects of memory, attention, inhibitory control, and executive planning (Almeida et al., 2008; Solowij and Pesa, 2010).
Limitations afflicting this extant literature, which may have contributed to the findings, include: use of different cognitive paradigms, some of which were not well-validated; recruitment of cannabis users with potentially confounding axis-I disorders; and the recruitment of cannabis users with a history of using other illicit drugs (Hart et al., 2010; Solowij and Pesa, 2010).
We recruited a cohort of young adults without potential confounders and investigated cognitive function in cannabis users using a range of well-validated translational paradigms (Owen et al., 1990; Coull et al., 1995; Rogers et al., 1999; Aron et al., 2003). Potential advantages of these paradigms include their validation in animal and human studies involving focal lesions and neuroimaging (Chamberlain et al., 2007; Robbins and Arnsten, 2009; Clark, 2010; Chamberlain et al., 2011). We hypothesized that cannabis users would exhibit deficits across a range of cognitive domains, consistent with the notion that cannabis use in young people is associated with deleterious effects on cortico-sub-cortical circuitry.
2. METHOD
2.1 Subjects
Participants comprised non-treatment-seeking adults aged 18–29 years who were recruited via media advertisements for a study examining impulsivity. Exclusion criteria included presence of axis-I disorders (besides cannabis dependence/abuse), history of any non-cannabis illicit drug use, and inability to understand/undertake the procedures and provide written consent.
The study procedures were carried out in accordance with the Declaration of Helsinki. The Institutional Review Board of the University of Minnesota approved the study and the consent. After all procedures were explained, subjects provided voluntary written informed consent.
2.2 Assessments
Raters assessed each subject using the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998) and a semi-structured instrument examining a range of behaviors (e.g., nicotine, alcohol and illicit substance use). Subjects were asked about frequency of cannabis use during the last 12 months (average frequency of use per typical week), and about any lifetime use of cannabis (yes/no).
Cognitive functions were assessed using well-validated computerized paradigms from the Cambridge Neuropsychological Test Automated Battery (CANTAB). We did not ask cannabis users to abstain from smoking or change their habits prior to participation, as we wished to evaluate cognition under normal circumstances in day-to-day life. Domains of interest were selected on the basis of their dissociable nature and the existing literature on effects of cannabis on cognition in healthy volunteers (tests comprised: Cambridge Gamble, One Touch Stockings of Cambridge, Stop-Signal, Spatial Working Memory, and Rapid Visual Information Processing tasks) (www.camcog.com provides citations to full task descriptions and previous validations).
2.3 Data Analysis
All subjects meeting inclusion criteria were grouped based on whether they used cannabis one or more times per week during the last 12 months (“cannabis users”) or had no use over the past 12 months (“controls”). The two groups were recruited from the same background population, using identical recruitment and screening, in order to avoid differential recruitment bias and to minimize confounds.
Group demographic and clinical characteristics, along with singular-type cognitive outcome variables where compared using t-tests and chi-squared tests. For the Cambridge Gamble and One-touch Stockings of Cambridge tasks, results were analyzed using analysis of variance (ANOVA) using task-appropriate within-subject factors and between-subject factor of group. This being an exploratory study using selected tests tapping orthogonal domains, significance was defined as p<0.05 uncorrected.
3. RESULTS
The group sizes were n=16 cannabis users and n=214 controls. None of the controls reported any lifetime cannabis use. Groups did not differ significantly on demographic characteristics (Table 1).
Table 1.
Comparison of demographic and clinical characteristics between cannabis users and controls
| Variables | Cannabis users (N=16) | Controls (N=214) | F / chi-s | p |
|---|---|---|---|---|
| Age (years), mean ± SD | 21.75 ± 2.91 | 21.19 ± 3.21 | 0.453 | 0.501 |
| Gender, male, N (%) | 10 (62.5%) | 153 (72.2%) | 0.583 | 0.445 |
| Annual income, USD, mean ± SD | 17206 ± 19623 | 15351 ± 16319 | 0.187 | 0.666 |
| Educational achievement, N (%) | ||||
| High School or below | 4 (25.0%) | 19 (9%) | 2.514 # | 0.285 |
| College | 11 (68.8%) | 170 (80.2%) | ||
| Beyond College | 1 (6.3%) | 23 (10.8%) | ||
| Nicotine users, N (%) | 5 (31.3%) | 36 (17%) | 1.245 # | 0.265 |
| Current Alcohol Use, N (%) | 14 (87.5%) | 141 (66.9%) | 2.257 # | 0.133 |
| Substance Use Disorder in First-Degree Family Member, N (%) | 5 (31.3%) | 50 (23.4%) | 0.168 # | 0.682 |
Yates' corrected
Subjects with current Axis I disorders, including substance use disorders (other than cannabis use) were excluded from the study
For those reporting cannabis use within the past 12 months, the mean frequency of use was 3.1 ± 2.2 times per week (range 1–7). Three users met criteria for cannabis dependence and two for cannabis abuse. None of the cannabis users and controls reported past substance use disorder otherwise.
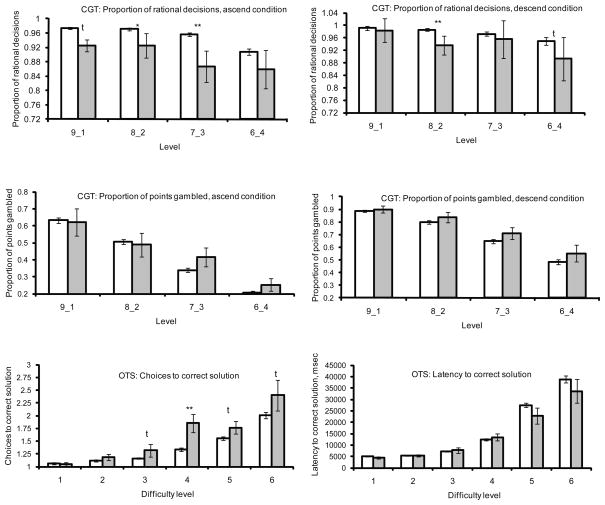
On the Cambridge Gamble Task (Figure 1), there was a main effect of group on the proportion of rational decisions made (F=6.139, p=0.014), due to cannabis users making significantly fewer rational decisions overall. There was no significant group by level interaction (F=0.409, p=0.747) or group by condition interaction (F=2.314, p=0.130). There was a significant main effect of level on the proportion of rational decisions (F=9.659, p<0.001), and of condition (ascend / descend, F=17.616, p<0.001), but there was no significant interaction (F=1.623, p=0.183). There was no significant effect of group on the proportion of points gambled (F=0.839, p=0.361), a significant group by level interaction (F=1.495, p=0.215), or group by condition interaction (F=0.142, p=0.706). There was a significant effect of level (F=145.45, p<0.001) and a significant effect of condition (F=105.78, p<0.001), but no significant interaction between the two (F=1.807, p=0.145).
Figure 1.
Performance on the Cambridge Gamble Task (CGT) and One-Touch Stockings of Cambridge Task (OTS). Shown in white bars, mean +/− SD controls, in grey mean +/−SD cannabis users. Group differences: t=trend (0.05<p<0.10), * p<0.05, ** p<0.01.
On the One-touch Stockings of Cambridge task (Figure 1), there was a main effect of group on choices to correct solution (F=6.711, p=0.010) and a significant group by difficulty interaction (F=3.332, p=0.005). This finding was attributable to cannabis users requiring more attempts to obtain correct solutions, especially at the harder levels of difficulty (3–6). There was a significant effect of difficulty overall (F=62.092, p<0.001). There was no main effect of group on latency to correct solution (F=0.782, p=0.378) or a significant group by difficulty interaction (F=0.915, p=0.470). There was a main effect of difficulty (F=78.355, p<0.001).
Groups did not differ significantly on Stop-Signal Reaction Time (SSRT) (171.25 ± 66.92, 170.39 ±43.45msec; t=0.074, p=0.941), on SSRT reaction times for go trials (407.16 ± 91.39, 439.10 ± 124.25msec; t=1.007, p=0.315), in terms of total errors (19.75 ± 18.95, 14.07 ± 13.32; t=1.591, p=0.113), or strategy scores on the Spatial Working Memory task (31.00 ± 6.49, 28.54 ± 6.09; t=1.550, p=0.123), or on target detection or false alarms on the Rapid Visual Information Processing task (0.743 ± 0.169, 0.743 ± 0.173; t=0.002, p=0.998; 0.011 ± 0.022, 0.007 ± 0.015; t=0.872, p=0.384, respectively).
4. DISCUSSION
This is the first study to explore associations between cannabis use and CANTAB cognitive performance in a sample of young people free from axis-I disorders (besides cannabis dependence/abuse) and free from a history of other illicit substance use. Though cannabis use has been associated with deficits across an array of domains in the literature, some of these findings may have been attributable to these confounds. The key finding was that cannabis use was associated with elevated risky decision-making on the Cambridge Gamble task and impaired executive planning on the Stockings of Cambridge task. These significant deficits occurred alongside relative sparing on measures of general motor performance, sustained attention, spatial working memory, and response inhibition. While this study highlights selective dysfunction in a relatively pure sample of cannabis users, it should be noted that many cannabis users have co-morbidities and use other substances, and that these users would be expected to manifest a broader range of executive dysfunction than found herein. Discrepancies between the current cognitive findings and those reported previously could also reflect other factors including differences in the nature of the samples (we studied young people and a relatively small proportion of cannabis users met criteria for dependence/abuse).
Cannabis users showed less rational decision-making on the Cambridge Gamble task than controls; they were more likely to make risky judgments and to select statistically unlikely, i.e., irrational, outcomes, despite resulting in punishment (negative feedback / loss of points). This impairment occurred across risk ratios, in the absence of more generalized problems with response speed or decisions regarding what proportion of points to gamble. We are unaware of any previous studies examining effects of cannabis or THC on this specific paradigm. Using the Iowa Gambling task, which bears parallels to the CGT, decision-making deficits have previously been reported in association with cannabis use (Whitlow et al., 2004; Hermann et al., 2009). These findings suggest that cannabis users are relatively insensitive to negative punishment. Interestingly, imaging research has demonstrated under-responsiveness of various neural regions (e.g., anterior cingulate, medial prefrontal, and superior parietal cortices) during decision-making in cannabis users (Wesley et al., 2011).
The ability to plan ahead is typically assessed in the laboratory using executive planning paradigms, such as the One-Touch Stockings of Cambridge task. Executive planning is dependent upon distributed neural circuitry including the dorsolateral prefrontal cortices (Owen et al., 1990; Williams-Gray et al., 2007). We found that cannabis users were less able to plan successfully, particularly at more challenging levels.
These findings draw parallels with those of previous research conducted in adolescent cannabis users, which reported significant impairment on the Delis–Kaplan Executive Function System planning test (Medina et al., 2007). These findings may explain the association between cannabis use in adolescents and young adults and a range of problematic behaviors. Cannabis use has been associated with driving under the influence (Alvarez et al., 2007), high-risk sexual behavior (Hendershot et al., 2010), and poor school performance and school drop-out (Cox et al., 2007). It is likely that risky decision-making and impaired ability to plan, mediated by underlying dysregulation of fronto-striatal circuitry, may mediate such deleterious behaviors.
Several limitations of the current study should be noted. This was a cross-sectional study with the inherent limitations thereof; the cognitive findings may have been driven by unmeasured factors associated with cannabis use rather than by cannabis use itself. We did not ask cannabis users time since last intake and this clearly would have been useful in retrospect, as would more information about quantity of use. This study cannot clarify whether cognitive problems predate cannabis use, or are due to it, nor potential differential effects of intoxication, acute withdrawal, protracted withdrawal or residual effects as opposed to chronic use. Lifetime use estimates were also not recorded. In a post-hoc secondary analysis, however, we found no significant correlations between cognitive performance on deficient measures in the cannabis users and average weekly cannabis intake (all p>0.10, Spearman s r). The study was likely underpowered to detect such correlations. The selective nature of the cognitive deficits that were identified suggests that results are not simply attributable to more general problems e.g. relating to IQ. The group sizes were unmatched with a relatively small sample size in the cannabis group, and this may have reduced statistical power, though the study was sufficiently powered to identify core deficits versus controls. The sample size differences were an inevitable consequence of our strategy to recruit all people from the same underlying population using identical screening criteria, to avoid introducing differential recruitment bias. Other non-significant tasks on the CANTAB, however, may be less sensitive than the Cambridge Gamble task and Stockings of Cambridge task, and significant differences could possibly be obtained with larger sample sizes.
In summary, our results suggest that young adults who use cannabis demonstrate selective cognitive dysfunction in terms of decision-making and executive planning. The question remains whether such dysfunction predates the use of cannabis or rather may be due to it.
Acknowledgments
Role of Funding Source: This research is supported by a Center for Excellence in Gambling Research grant by the Institute for Responsible Gaming to Dr. Grant. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Acknowledgements: None
Footnotes
All work completed in Minneapolis, Minnesota, United States
Contributors: Dr. Grant, Dr. Chamberlain, Ms. Schreiber, and Mr. Odlaug contributed to the initial draft of the manuscript, data analysis, and revisions. Each author has studied the manuscript in its current form, agrees to the order of authorship, and approves it for submission.
Conflict of Interest: Dr. Grant has received research grants from National Institute on Drug Abuse (RC1-DA028279-01) and from Forest Pharmaceuticals. Dr. Chamberlain has consulted for Cambridge Cognition, P1Vital, and Shire Pharmaceuticals. Mr. Odlaug has received honoraria from Oxford University Press. Ms. Schreiber reports no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Almeida PP, Novaes MA, Bressan RA, Lacerda AL. Review: executive functioning and cannabis use. Rev Bras Psiquiatr. 2008;30:69–76. doi: 10.1590/s1516-44462008000100013. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Fierro I, Del Rio MC. Cannabis and driving: results from a general population survey. Forensic Sci Int. 2007;170:111–116. doi: 10.1016/j.forsciint.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Cervellione K, Cottone J, Ardekani BA, Sevy S, Kumra S. Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. J Psychiatr Res. 2009;43:189–204. doi: 10.1016/j.jpsychires.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Robbins TW, Winder-Rhodes S, Müller U, Sahakian BJ, Blackwell AD, Barnett JH. Translational approaches to frontostriatal dysfunction in attention-deficit/hyperactivity disorder using a computerized neuropsychological battery. Biol Psychiatry. 2011;69:1192–1203. doi: 10.1016/j.biopsych.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Clark L. Decision-making during gambling: an integration of cognitive and psychobiological approaches. Philos Trans R Soc Lond B Biol Sci. 2010;365:319–330. doi: 10.1098/rstb.2009.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Dombrovski AY, Siegle GJ, Butters MA, Shollenberger CL, Sahakian BJ, Szanto K. Impairment in risk-sensitive decision-making in older suicide attempters with depression. Psychol Aging. 2011;26:321–330. doi: 10.1037/a0021646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology (Berl) 1995;120:322–332. doi: 10.1007/BF02311180. [DOI] [PubMed] [Google Scholar]
- Cox RG, Zhang L, Johnson WD, Bender DR. Academic performance and substance use: findings from a state survey of public high school students. J Sch Health. 2007;77:109–115. doi: 10.1111/j.1746-1561.2007.00179.x. [DOI] [PubMed] [Google Scholar]
- Hall W, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, Foltin RW. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacol Biochem Behav. 2010;96:333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacol Biochem Behav. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Magnan RE, Bryan AD. Associations of marijuana use and sex-related marijuana expectancies with HIV/STD risk behavior in high-risk adolescents. Psychol Addict Behav. 2010;24:404–414. doi: 10.1037/a0019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Lemenager T, Gelbke J, Welzel H, Skopp G, Mann K. Decision making of heavy cannabis users on the Iowa Gambling Task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. Eur Addict Res. 2009;15:94–98. doi: 10.1159/000189788. [DOI] [PubMed] [Google Scholar]
- Hunault CC, Mensinga TT, Bocker KB, Schipper CM, Kruidenier M, Leenders ME, de Vries I, Meulenbelt J. Cognitive and psychomotor effects in males after smoking a combination of tobacco and cannabis containing up to 69 mg delta-9-tetrahydrocannabinol (THC) Psychopharmacology (Berl) 2009;204:85–94. doi: 10.1007/s00213-008-1440-0. [DOI] [PubMed] [Google Scholar]
- Indlekofer F, Piechatzek M, Daamen M, Glasmacher C, Lieb R, Pfister H, Tucha O, Lange KW, Wittchen HU, Schutz CG. Reduced memory and attention performance in a population-based sample of young adults with a moderate lifetime use of cannabis, ecstasy and alcohol. J Psychopharmacol. 2009;23:495–509. doi: 10.1177/0269881108091076. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of delta-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology. 2006;31:462–470. doi: 10.1038/sj.npp.1300871. [DOI] [PubMed] [Google Scholar]
- Mata I, Rodriguez-Sanchez JM, Pelayo-Teran JM, Perez-Iglesias R, Gonzalez-Blanch C, Ramirez-Bonilla M, Martinez-Garcia O, Vazquez-Barquero JL, Crespo-Facorro B. Cannabis abuse is associated with decision-making impairment among first-episode patients with schizophrenia-spectrum psychosis. Psychol Med. 2008;38:1257–1266. doi: 10.1017/S0033291707002218. [DOI] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. J Int Neuropsychol Soc. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Downes JJ, Sahakian BJ, Polkey CE, Robbins TW. Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia. 1990;28:1021–1034. doi: 10.1016/0028-3932(90)90137-d. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Zakzanis KK, George TP. The effects of cannabis use on neurocognition in schizophrenia: a meta-analysis. Schizophr Res. 2011;128:111–116. doi: 10.1016/j.schres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Arnsten AF. The neuropsychopharmacology of fronto-executive function: monoaminergic modulation. Annu Rev Neurosci. 2009;32:267–87. doi: 10.1146/annurev.neuro.051508.135535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of National Findings. I. Office of Applied Studies; Rockville, MD: 2010. Results from the 2009National Survey on Drug Use and Health. NSDUH Series H-38A, HHS Publication No. SMA 10–4586. [Google Scholar]
- Schwartz RH, Gruenewald PJ, Klitzner M, Fedio P. Short-term memory impairment in cannabis-dependent adolescents. Am J Dis Child. 1989;143:1214–1219. doi: 10.1001/archpedi.1989.02150220110030. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. quiz 34–57. [PubMed] [Google Scholar]
- Solowij N, Pesa N. Cognitive abnormalities and cannabis use. Rev Bras Psiquiatr. 2010;32(Suppl 1):S31–S40. [PubMed] [Google Scholar]
- Takagi M, Lubman DI, Cotton S, Fornito A, Baliz Y, Tucker A, Yucel M. Executive control among adolescent inhalant and cannabis users. Drug Alcohol Rev. 2010 doi: 10.1111/j.1465–3362.2010.00256.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Hanlon CA, Porrino LJ. Poor decision-making by chronic marijuana users is associated with decreased functional responsiveness to negative consequences. Psychiatry Res. 2011;191:51–59. doi: 10.1016/j.pscychresns.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J. Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis. 2000;19:1–22. doi: 10.1300/J069v19n01_01. [DOI] [PubMed] [Google Scholar]