Abstract
Sex- and age-typical responses to ethanol and novel stimuli tend to emerge postpubertally, suggesting a potential organizational or activational role for pubertal hormones in these behaviors. To test this possibility, male and female rats were gonadectomized (GX) or received sham gonadectomy (SH) either prepubertally on postnatal day (P) 23 (early) or in adulthood on P70 (late). Animals were tested as adults for response to novelty and, on the following day, challenged with either saline or ethanol (1g/kg) prior to social interaction testing with an unfamiliar partner in a familiar setting under low light conditions. Gonadectomy did not influence ethanol-induced social inhibition in either sex, but instead altered the microstructure of social behavior, with GX animals exhibiting proportionally less time in social investigation and proportionally more time in contact behavior than SH animals, regardless of age of gonadectomy. The early sham surgical manipulation process itself influenced social motivation, with early SH surgery eliminating ethanol-induced decreases in social preference in both sexes. Response to novelty was unaffected by gonadectomy, but was suppressed in early compared to late SH manipulated animals. These results suggest that adult-typical responses to ethanol and novelty-directed behaviors are little influenced by gonadal hormones during puberty or in adulthood. However, the experience of surgical manipulation itself during development exerts behavioral and pharmacological consequences that last into adulthood.
Keywords: Sex differences, social inhibition, response to novelty, ethanol sensitivity, gonadal hormones, puberty
1. Introduction
The actions of gonadal hormones on the nervous system can be considered either “activational” or “organizational”. Traditionally, organizational effects of gonadal hormones were thought to be restricted to the early perinatal period [1]. Evidence has emerged, however, to support the suggestion that gonadal hormones may exert organizational effects outside of this early developmental period [2]. Specifically, puberty has been suggested to be a second organizational period for steroid-dependent organization of brain structure and behavior during adolescence, with the increases in sex hormones associated with pubertal onset acting to further refine neural connections in brain regions previously organized during the perinatal period to give rise to sex-typical behaviors in adulthood [see 3 for review]. For example, gonadal hormone exposure during puberty has been shown to be necessary for the development of sexually dimorphic reproductive behaviors, along with other male sex-typical behaviors such as aggression- and anxiety-related behaviors, including aggression toward an intruder, territorial flank marking and unfamiliar environment-induced decreases in social behaviors [see 4, 5, 6, 7, 8,].
Sex differences have been reported in patterns of alcohol consumption, sensitivity to certain alcohol effects and susceptibility for abuse and dependence. Although women often report drinking fewer drinks per occasion than men [9], they have shorter intervals between the onset of drinking and the emergence of problem drinking than men [10]. Sensitivity to a number of acute and chronic consequences of ethanol (EtOH), such as ethanol-induced cognitive and behavioral impairments, as well as subjective intoxication, also differs between the sexes [e.g., 11, 12, 13, 14, 15]. For instance, when blood ethanol concentration (BEC) was held constant across sex, women were less sensitive than men to alcohol-induced impairment of inhibitory control [11], but more sensitive to alcohol-related cognitive disruptions in attention [12] and to impairments of short- and long-term memory [13].
Although animal studies of alcohol effects have traditionally focused on male rodents, some modest sex differences have been observed in studies using laboratory animals. For example, in studies of adult rats and mice, females have been shown to be less sensitive than males to ethanol-induced social inhibition [16, 17, 18], locomotor activity [19], and hangover related suppression of social behavior [20]. Sex differences in ethanol intake are also well-established in adult rodents, although in contrast to the typical sex difference seen in human consumption [9,10, 21], adult female rats have been found to drink more ethanol on a g/kg basis than adult males under a number of circumstances [e.g., 22, 23, 24, 25]. This increased ethanol intake among adult females does not appear to be due solely to fluctuations in gonadal hormones with phase of estrous. For example, some studies have found that total ethanol intake was unaffected by stage of estrous cycle when females were allowed to cycle freely [26, 27], although the microstructure of ethanol drinking was reported to vary across estrous phase [26].
Sex differences in alcohol intake and sensitivity to the various effects of alcohol do not extend reliably to prepubertal adolescent animals [28, 20, 25] and tend to emerge with the onset of puberty in both humans and animals, suggesting a potential “organizational” and/or “activational” influence of rising gonadal hormones during puberty on these differences [29]. In recent research in our laboratory, we have sought to examine these possibilities by assessing the impact of removal of the gonads in both male and female rats prior to puberty or in adulthood on sex differences in ethanol intake typically seen in adulthood. In this study, gonadectomy in males, regardless of age of gonad removal, was found to elevate levels of ethanol consumption in adulthood that were significantly greater than those of sham-gonadectomized males and similar to those of adult females [30]. Similar findings of an increase in ethanol consumption in adult male rats following prepubertal gonadectomy was recently reported by another group [31]. A follow-up study conducted in our laboratory showed that elevations in ethanol consumption seen in GX males were reversed by testosterone replacement, supporting the suggestion that testosterone plays an “activational” (in contrast to an “organizational”) role in suppressing ethanol intake in male rats, thereby likely contributing to the sex differences typically seen in this behavior in adulthood [32]. In contrast, ovariectomy (at either age) had little impact on ethanol drinking of adult females [30].
Sensitivity to ethanol also differs as a function of age, with prepubertal adolescent animals being less sensitive to some effects of ethanol than their adult counterparts. For instance, prepubertal adolescent rats are less sensitive than older adolescent and adult males and females to ethanol-induced anxiolysis and social impairment [33, 34]. This increase in sensitivity to ethanol effects may emerge around the time of puberty, although no studies to our knowledge have examined the influence of pubertal hormones on developmental changes in ethanol responding typically observed across the adolescent-to-adult transition. To the extent that sex differences in ethanol intake levels are moderated by differences in sensitivity to social impairing and other aversive effects of ethanol [see 35], and that activational effects of gonadal hormones on ethanol drinking are restricted to male rats as shown in our prior work [30], it would be predicted that gonadectomy should attenuate sensitivity to ethanol-induced social impairment selectively in male rats, with this effect evident regardless of whether the gonads were removed prepubertally or in adulthood.
In addition to showing age-typical differences in ethanol sensitivity, adolescent male and female rats also display greater novelty-directed behavior than their adult counterparts [36, 37]. Although not correlated with physical or hormonal indices of pubertal development, this greater response to a novel object was found to peak around the time of puberty, then decline across the adolescent-to-adult transition [38]. While no sex differences in novelty-seeking were observed at any age in this [38] and similar studies examining response to novelty [36], other studies have reported adult male mice [39] and adolescent and adult male rats [40] to show greater preferences for novel objects than adult females. Indeed, increases in gonadal hormones associated with puberty have been suggested to underlie age- and sex-typical responses to novelty, a suggestion supported by data showing that long-term suppression of gonadal hormone production via prepubertal administration of Antide eliminated sex differences in novelty preference during late adolescence [41]. Yet, groups of similarly treated adults were not included in this study, and thus it is unclear whether the observed effects were related to activational or organizational effects of gonadal hormones.
The purpose of the present experiment was to examine whether increases in pubertal sex hormones play an organizational and/or activational role in postpubertally emerging sex-typical behaviors as indexed via novelty-seeking and sensitivity to ethanol-induced social inhibition. Both male and female rats were gonadectomized or received sham surgeries either prepubertally or in adulthood prior to testing each animal’s response to a novel stimulus and sensitivity to ethanol-induced social inhibition in adulthood. Because the surgical process itself in prepubertally sham manipulated males was found to influence ethanol drinking and preference in prior work [30], groups of non-manipulated control animals were also included for assessment of sham surgery effects.
2. Material and Methods
2.1 Subjects
A total of 192 male and female Sprague-Dawley rats bred in our facility at Binghamton University were used as experimental animals in this experiment. On postnatal day (P) 1, the day after birth, litters were culled to 8-10 pups, with 6 animals of one sex and 4 of the other kept whenever possible. Offspring were weaned and pair-housed with a same-sex littermate in a temperature-controlled vivarium on a 14:10 light/dark cycle (lights on at 0700) and given ad libitum access to food (Purina Rat Chow, Lowell, MA) and water until the onset of experimental procedures. At all times, animals were treated in accordance with guidelines for animal care established by the Institute of Laboratory Animal Research [42], using protocols approved by Binghamton University Institutional Animal Care and Use Committee. No more than one animal per litter was placed into any given experimental condition, with any extra animals from these litters used in other experiments in our laboratory.
2.2 Experimental Design
The basic design of this experiment was a 2 sex × 2 surgery condition (gonadectomy; sham) × 2 surgery age (early; late) × 2 drug (saline; ethanol) factorial, with 8-10 animals in each of the 16 groups specified by this factorial design. Both male and female rats were gonadectomized (GX) or received sham gonadectomy (SH). Animals received surgery at one of two ages, either prepubertally on P23 (early) or postpubertally in adulthood on P70 (late), with all animals tested during adulthood for response to novelty (on P79) and then challenged with either saline or ethanol prior to social interaction testing (on P80). Four additional groups of animals were also tested. These groups were non-manipulated (NM) adult male and female rats from the same birth cohort as the surgically manipulated animals that were tested identically to the other groups on P79 and after challenge with either saline or ethanol on P80. These 4 NM groups (male NM challenged with saline; male NM challenged with ethanol; female NM challenged with saline; female NM challenged with ethanol) were included as controls for the SH manipulated groups to provide appropriate non-manipulated comparison groups to determine whether the surgical manipulation process itself at either age or sex influenced the dependent measures. Sample sizes for these additional 4 groups of NM animals were also 8-10 per group, as for the 16 experimentally manipulated groups in this study.
2.3 Surgery
Animals were rehoused with a littermate of the same age and sex and assigned to the same surgery condition two days before surgery. On the day of surgery, animals were anesthetized using isoflurane (3-3.5% initially, with anesthetization maintained during the surgery by nose cone supplementation as necessary). In males, castration included removal of each testis, suturing of each tunic and the inguinal ring and closure of the surgical site with Vetbond tissue adhesive (3M, St. Paul, MN). For ovariectomy of females, an incision was made on the side of the animal, caudal to the last rib and through the skin perpendicular to the midline. Using blunt dissection, an opening in the muscle wall was made on each side of the incision, and then the oviduct on each side was sutured proximal to the ovary, prior to excising the ovary. Sutures were then placed in the muscle wall and the surgical site closed with skin staples. For sham gonadectomies, the same procedures were followed, except that reproductive tissue was not manipulated, nor were the gonads removed. Animals received a subcutaneous injection of the anti-inflammatory agent Carprofen (5 mg/kg) immediately after surgery and twice the next day. Animals were returned to their home cages post surgery following recovery from the anesthetic where they were separated from their housing partner using a wire-mesh divider for a recovery period of approximately 72 h, after which the divider was removed.
2.4 Testing Procedure
The test apparatus consisted of a Plexiglas chamber (30 × 20 × 20 cm for adolescents and 45 × 30 × 30 cm for adults) containing clean wood shavings and divided along the long axis into 2 equally sized compartments by a clear Plexiglas partition containing an aperture (7 × 5 cm for adolescents and 9 × 7 cm for adults) allowing movement of the animals between compartments [43, 44]. A video camera mounted approximately 60 cm above each chamber was used to record all sessions. All testing was conducted under low light conditions (3 lux).
All animals were placed individually into the social interaction chambers one day prior to testing (P79) to acclimate them to the testing environment. Animals were allowed to explore the social interaction chamber for 25 min. Such pre-test acclimation has previously been shown to facilitate higher levels of social behavior and magnify differences in ethanol sensitivity relative to when the test environment is unfamiliar [33], with the latter viewed as an anxiogenic context that suppresses levels of social activity [45]. At the end of the 25 min acclimation period, each animal was given a 5-min novel object test using a novel object exposure procedure previously used in our laboratory [36]. While still in the social interaction chamber, a novel object (e.g., a new cotton ball approximately 2.5 cm in diameter) was placed on one side of the social interaction chamber for 5 minutes and the animal’s behavior was videotaped for later analysis of time spent in contact with the novel object as well as time spent sniffing the object. Pilot data conducted in our laboratory revealed no significant differences in quality or quantity of social interactions on test day in animals that received the novel object test at the end of the acclimation period relative to those who did not receive the novel object test during this acclimation period on the day prior to social interaction testing.
On the social interaction test day (P80), animals were injected i.p. with either 1.0 g/kg ethanol (12.6% v/v) or an equivalent volume of the saline (0.9% w/v) vehicle solution, and placed individually into a pre-test holding cage for 25 min. Immediately following the 25-min social isolation period, pairs of test animals were placed simultaneously together into the chamber for a 5 min modified social interaction test. Each test pair consisted of unfamiliar non-littermates of the same age, sex, surgery condition, and surgery age, with one given saline and the other challenged with ethanol prior to testing. This test procedure varies from that routinely used by our group [e.g., 33] where each experimental animal was paired with a non-manipulated, non-injected partner of the same age and sex. Due to the labor intensive surgeries required for the present study, a pilot study was conducted in our laboratory to determine whether two experimental animals (one assigned to each acute drug condition) could be tested together, thereby halving the necessary sample sizes needed. The results of this study revealed no significant differences in social interactions between adult animals placed with an injected social partner and those placed with a non-manipulated partner. All other experimental conditions, such as lighting level, dose of ethanol, time between injection and testing, and length of social interaction test were similar to those used in previous social interaction studies [e.g., 33].
The frequencies of social behaviors recorded during the testing sessions were later scored by experimenters blind to the surgery condition, ethanol dose, and age of surgery using procedures in routine use by our group [e.g., see 33, 18]; inter-rater reliability scores were >95%. Social behaviors scored included: frequency of social investigation (sniffing of partner), contact behavior (sum of crawling over/under partner and social grooming), and play fighting (sum of pouncing or nape attack, following and chasing, and pinning). To explore the relative proportion of each of these behaviors contributing to overall social behavior, each component behavior for each animal was also converted to a percent of the total amount of social behavior exhibited by that animal; for example, investigation as a percentage of total social behavior was calculated as follows: (counts of investigation/(counts of investigation + contact + play fighting)) × 100. Number of crossovers to and from a side containing the social partner was also used as an index of social motivation, via calculating a social preference/avoidance coefficient [(# of crossovers toward the partner - # of crossovers away from the partner)/(total # of crossovers) × 100].
Immediately following the social interaction session, animals were sacrificed and whole trunk blood collected for later analysis of BEC, testosterone in males and estradiol and progesterone in females. For BECs, blood was collected in heparinized tubes and briefly placed on ice prior to storing the samples at −80°C until the time of analysis. For hormone assays, after collection, the blood samples were centrifuged at 2°C for 20 min at 3,000 rpm, and the plasma removed and subsequently stored at −80°C until the time of assay.
2.5 Blood Analyses
For analysis of BECs, blood samples were thawed and BECs determined by means of head-space gas chromatography, using a Hewlett Packard (HP) 5890 series II Gas Chromatograph, a HP 7694E Headspace Sampler, and HP Chemstation software. This software allows for comparison of the peak area under the curve in each sample to standard curves derived from reference standard solutions. Prior to the BEC assay, whole blood samples were placed in airtight vials, and then heated for 7 min in a water bath at 55 °C. Using an airtight syringe, a 1.0 ml sample from the gas head space was then extracted and injected onto the column [46].
Plasma testosterone in males and estradiol and progesterone levels in females were used to confirm removal of gonadal tissue in the gonadectomized groups, with hormone levels in the sham and non-manipulated groups used to confirm assay effectiveness. Testosterone, estradiol and progesterone levels were assessed from thawed blood samples via radioimmunoassay using a 125I RIA double antibody kit from MP Biomedicals (Solon, OH), with specificities of 100% for each hormone assayed. Testosterone assay sensitivity was 0.03 ng/ml, with inter- and intra-assay coefficients of variation of 10.1% and 6.6%, respectively. Sensitivity of the estradiol assay was 7.2 pg/ml, with inter-assay coefficients of 9% and intra-assay coefficients of 7.3%. Progesterone assay sensitivity was 0.11 ng/ml, with inter-assay coefficients of 7.9% and intra-assay coefficients of 5.9%. Samples and standards for each gonadal hormone assay were run in duplicate, using a Packard Cobra II Autogamma Counter, with disintegrations per min averaged against a standard curve.
2.6 Data Analyses
All data were analyzed using Statistica version 9 (StatSoft, Tulsa, OK). Data analyses focused on two issues: (a) assessment of the effects of early vs. late gonadectomy; and (b) determination of whether the surgical process itself, at either or both ages, altered expression of the targeted behaviors. The design of the first set of ANOVAs (i.e., those focusing on gonadectomy effects) was a 2 sex × 2 surgery age (early; late) × 2 surgery condition (GX; SH) factorial for each measure, with the additional variable of dose (saline vs. ethanol) included for the assessments involving ethanol challenge. The second set of analyses (focusing on consequences of the surgical process per se) compared early and late SH animals with NM animals using a 2 sex × 3 surgical manipulation (early SH; late SH; non-manipulated) factorial, again with the addition of a dose factor (saline vs. ethanol) for the ethanol challenge data. Fisher’s LSD tests were used to determine the locus of significant main effects and interactions in all ANOVAs.
Prior to the ANOVAs, Levene’s tests were used to test for homogeneity of variance (HV) in each data set. Measures violating this assumption included contact behavior, play fighting, and progesterone; HV in these data were improved using log(10) (n+1) transformation and were so transformed prior to the analyses described above. There was also an HV violation for the testosterone data that was not improved via standard transformations due to floor effects in testosterone levels among GX males; these data were analyzed using nonparametric Kruskal-Wallis and Mann-Whitney U tests. For ease of interpretation, non-transformed data with parametric descriptors (means +/− SEMs) are shown in all figures and tables.
3. Results
Results of the ANOVAs for assessment of gonadectomy effects are summarized in Table 1, whereas a summary of the significant findings in the ANOVAs assessing surgical manipulation effects are shown in Table 2.
Table 1.
Significant ANOVA results for the analyses focused on effects of gonadectomy
| Dependent Variable | Sex (S) | Surgery Age (A) |
Surgery Condition (C) |
Drug (D) | Significant Interactions |
|---|---|---|---|---|---|
| Novelty | |||||
| Sniffing | ---- | 4.19 p< .05 |
---- | ---- | |
| Contact | ---- | 4.43 p< .05 |
---- | ---- | |
| Social Behavior | |||||
| Investigation | 20.22 p< .01 |
---- | ---- | 138.96 p< .01 |
---- |
| Contact | ---- | ---- | 8.58 p< .01 |
68.41 p< .01 |
---- |
| Play | ---- | ---- | ---- | 23.54 p< .01 |
---- |
| Preference Coefficient | ---- | ---- | ---- | ---- | A × C × D 4.49 p< .05 |
| % Investigation | ---- | ---- | 7.50 p< .01 |
19.66 p< .01 |
---- |
| % Contact | ---- | ---- | 13.30 p< .01 |
20.18 p< .01 |
---- |
| % Play | ---- | ---- | ---- | ---- | ---- |
| BEC | ---- | ---- | ---- | ---- | ---- |
| Hormone | |||||
| Testosterone | ---- | 62.87 p< .01 |
---- | ---- | |
| Progesterone | ---- | 16.34 p< .01 |
---- | ---- |
Table 2.
Significant ANOVA results for the analyses focused on effects of the surgical process
| Dependent Variable | Sex (S) | Surgical Manipulation (M) |
Drug (D) | Significant Interactions |
|---|---|---|---|---|
| Novelty | ||||
| Sniffing | ---- | 3.31 p< .05 |
---- | |
| Contact | ---- | 3.80 p< .05 |
---- | |
| Social Behavior | ||||
| Investigation | ---- | ---- | 103.54 p< .01 |
---- |
| Contact | ---- | ---- | 85.31 p< .01 |
---- |
| Play | ---- | ---- | 5.88 p< .05 |
---- |
| Preference Coefficient | ---- | ---- | ---- | M × D 5.34 p< .01 |
| % Investigation | ---- | ---- | 32.1 p< .01 |
---- |
| % Contact | ---- | ---- | 62.18 p< .01 |
---- |
| % Play | ---- | ---- | ---- | ---- |
| BEC | ---- | ---- | ---- | ---- |
| Hormone | ||||
| Testosterone | ---- | ---- | ---- | |
| Progesterone | ---- | ---- | ---- |
3.1. Response to novelty
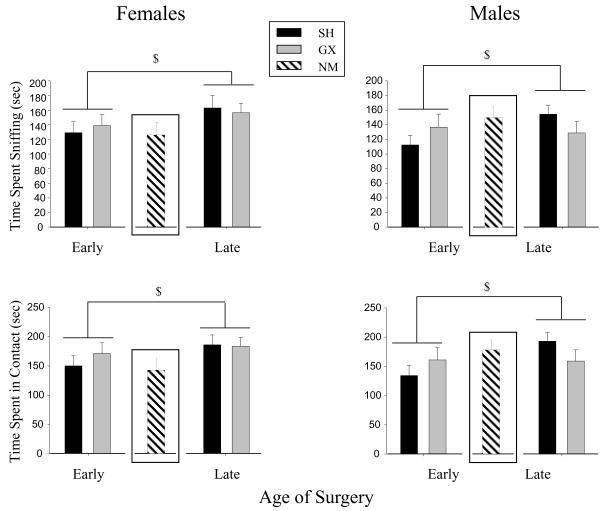
Only main effects of surgery age emerged in the ANOVAs focused on assessment of gonadectomy effects on novelty-associated behaviors. Animals of both sexes receiving early surgery (regardless of GX or SH assignment) showed less of each of these novelty-directed behaviors than late surgery animals. As seen in Fig. 1, this surgery age effect appeared to be driven largely by the SH animals, although surgery age did not interact significantly with surgery condition for either measure. Indeed, in the second set of ANOVAs focused on comparing early SH, late SH and NM animals, significant main effects of surgical manipulation were seen for both behaviors, with early SH animals exhibiting significantly less of these novelty-directed behaviors than late SH animals, and the behavior of NM animals not differing significantly from either of these SH groups.
Fig. 1.
Novel object directed behaviors of gonadectomized (GX) and sham (SH) males and females are shown by surgery age. The error bars indicate standard error of the mean in this and all subsequent figures. Although the non-manipulated (NM) groups were analyzed in a separate analysis with the early and late SH groups, for the sake of comparison, the means and standard errors of the NM data for each sex are also shown. The $ symbol indicates significant differences between the early and late surgery groups.
No significant effects of sex emerged in either set of analyses of behavior directed towards the novel object.
3.2 Ethanol-induced social inhibition
In ANOVAs focused on gonadectomy effects, the ethanol challenge was found to induce significant decreases in the frequency of social investigation (sniffing of the partner), contact (crawling over/under partner and social grooming), and play (pouncing, nape attack, following, chasing, and pinning)(see Table 3). Only one of these measures, contact behavior, was significantly influenced by gonadectomy, with contact behavior greater in GX than SH animals regardless of age at the time of surgery. As can be seen in Table 3, this gonadectomy effect appears to be driven more by the females, although sex did not interact significantly with surgery condition in the analysis of these data. Indeed, the only significant sex difference emerging in these analyses was in the analysis of social investigation, with females exhibiting significantly more social investigation than males.
Table 3.
Mean ( SEM) frequency of social investigation, contact and play behavior after challenge with 1 g/kg ethanol or saline
| Measure | Investigation | Contact | Play | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | |||||||
| Saline |
EtOH* |
Saline |
EtOH * |
Saline |
EtOH* |
Saline |
EtOH* |
Saline |
EtOH* |
Saline |
EtOH* |
|
| Early Surgery | ||||||||||||
|
| ||||||||||||
| SH | 39.4 (3.0) | 27.9 (4.1) # | 35.6 (2.2) | 16.8 (2.9) | 3.6 (0.8) | 0.6 (0.3) | 5.0 (1.8) | 1.3 (0.9) | 2.9 (0.6) | 1.4 (0.4) | 2.1 (0.7) | 1.0 (0.4) |
| GX | 39.0 (3.5) | 19.4 (3.0) | 35.7 (2.8) | 17.1 (3.4) | 6.9 (1.7) | 4.1 (1.8) | 6.7 (1.5) | 1.8 (0.8) † | 3.1 (1.3) | 0.8 (0.3) | 2.6 (0.9) | 1.1 (0.4) |
|
| ||||||||||||
| Late Surgery | ||||||||||||
|
| ||||||||||||
| SH | 44.8 (3.2) | 23.8 (3.2) | 37.0 (3.5) | 16.9 (2.5) | 3.1 (0.7) | 0.8 (0.3) | 6.6 (1.6) | 1.1 (0.6) | 2.6 (0.7) | 1.7 (0.4) | 3.4 (1.0) | 1.0 (0.5) |
| GX | 43.6 (4.2) | 22.7 (2.2) | 31.9 (3.0) | 12.4 (2.4) | 6.1 (2.0) | 3.1 (1.7) | 5.5 (0.8) | 1.0 (0.5) † | 3.2 (1.0) | 1.2 (0.5) | 2.5 (0.7) | 0.6 (0.3) |
|
| ||||||||||||
| NM | 47.5 (3.8) | 21.0 (3.7) | 32.0 (2.5) | 18.4 (2.5) | 4.2 (0.7) | 0.8 (0.4) | 3.7 (0.7) | 0.2 (0.1) | 3.3 (0.9) | 1.1 (0.4) | 1.4 (03) | 0.5 (0.2) |
Ethanol (EtOH)-challenged animals significantly less than saline-challenged animals collapsed across sex, surgery age, and surgery condition
Females significantly greater than males collapsed across surgery age, surgery condition and dose
GX animals significantly greater than SH animals collapsed across sex, surgery age and dose
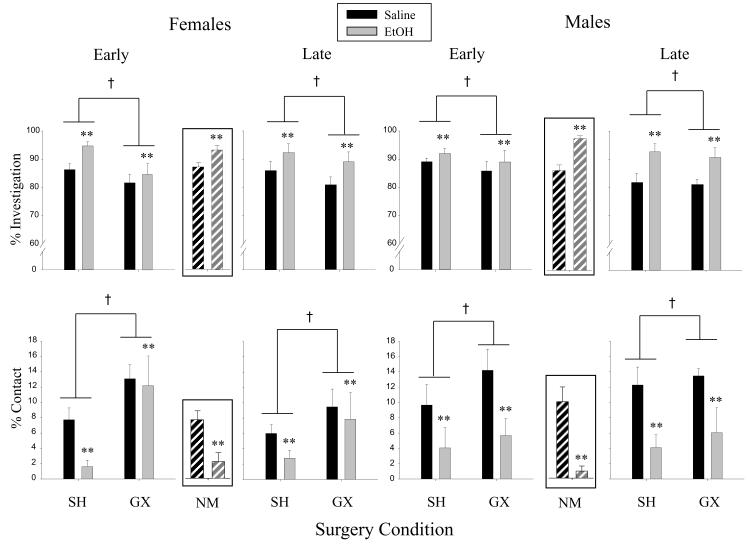
When each component social behavior was analyzed as a percentage of total social behavior, effects of surgery condition emerged, with GX animals (when collapsed across sex and age at the time of gonadectomy) showing significantly lower percentage investigation and greater percentage contact than SH animals. Again, both of these GX effects appear to be driven more strongly by females (see Fig. 2) although sex did not significantly interact with surgery condition in these analyses. Ethanol also was found to change the relative percentage of time spent in investigation and contact, increasing percentage investigation while conversely decreasing percentage contact.
Fig. 2.
The percentage of total social behavior represented by social investigation (top figure) and contact behavior (bottom figure) is shown for early and late gonadectomized (GX) and sham (SH) males and females (mean ± SEM). Although the non-manipulated (NM) groups were analyzed in a separate analysis with the early and late SH groups, for the sake of comparison, NM data are shown in the inserts between the early and late groups. The † symbol indicates significant differences between GX and SH groups when collapsed across surgery age, sex and drug. The ** symbol represents significant main effects of drug, with ethanol (EtOH)-challenged animals differing from their saline-challenged counterparts (when collapsed across surgery condition, surgery age and sex).
In the second set of ANOVAs focused on surgical manipulation effects, only typical ethanol-induced decreases in frequency of social investigation, contact and play behavior emerged, with no main effect or interactions of surgical manipulation emerging in the analyses of these behaviors when represented either as frequencies or as percentages of total social behavior.
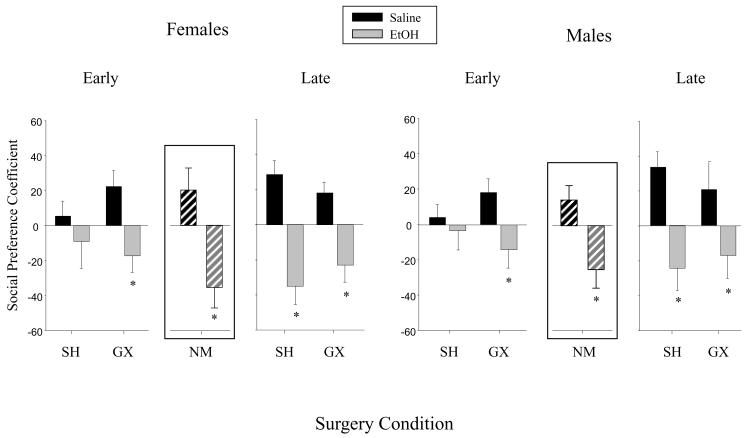
3.3 Ethanol effects on social motivation
Analysis of the social preference coefficient revealed an effect of ethanol that was influenced by an interaction of both surgery condition and surgery age. As can be seen in Fig. 3, post-hoc analyses of the data collapsed across sex revealed typical ethanol-induced decreases in social preference in late SH animals as well as in GX animals regardless of their age at surgery. It was the early SH animals that did not show an ethanol-related decrease in social preference. This effect was due both to attenuated levels of social preference after saline challenge in early SH relative to late SH animals, as well as less social aversion after ethanol challenge in early SH animals relative to late SH animals. These effects were specific to social behavior, with no significant effects revealed in the ANOVA of overall activity (indexed via total number of chamber crosses) during the social interaction session.
Fig. 3.
Social preference of gonadectomized (GX) and sham (SH) males and females are shown by drug and surgery age (mean ± SEM). Although the non-manipulated (NM) groups were analyzed in a separate analysis with the early and late SH groups, for the sake of comparison, NM data are shown in the inserts between the early and late groups. The * symbol represents significant differences between ethanol (EtOH)- and saline-challenged animals within a given group when collapsed across sex as determined by post-hoc analyses of the surgery age × surgery condition × drug interaction.
The ANOVA focused on surgical manipulation effects in the social preference data confirmed that the difference in social motivation seen between early and late SH animals was driven by effects of early surgical manipulation. That is, post-hoc analyses of a significant interaction between drug and surgical manipulation in this analysis revealed similar ethanol-related decreases in social preference in late SH and NM animals, whereas no ethanol effect was evident in the early SH group.
3.4. Blood ethanol concentration
No significant effects emerged in the analyses of post-test BECs in animals challenged with ethanol, although there was a trend in the ANOVA focused on gonadectomy effects for females overall to show slightly lower (mean mg/dl ± SEM) BECs (70.90 ± 0.96) than males (74.34 ± 1.43)(F(1, 68) = 3.76, p=.06).
3.5. Hormone levels
Testosterone levels (mean ng/ml ± SEM) were found to be significantly lower in GX males (0.0 ± 0.0) than their SH counterparts (1.60 ± 0.12). Estradiol levels (mean pg/ml ± SEM) were modestly, but significantly decreased in GX females (63.53 ± 1.49) compared to SH females (76.10 ± 2.55) regardless of surgery age, with progesterone likewise significantly lower in GX (39.52 ± 3.01) than SH (81.56 ± 6.75) females, again regardless of age at surgery.
4. Discussion
In general, typical ethanol-induced decreases in social behavior [e.g., 17, 18, 20] were observed in both males and females, regardless of surgery condition or age of surgery. Although gonadectomy did not influence ethanol-induced social inhibition in either sex, removal of the gonads did alter the microstructure of social behavior, with GX males and females exhibiting proportionally less time in social investigation and proportionally more time in contact behavior than SH animals regardless of age of gonadectomy. The early sham surgical manipulation process itself, however, did influence ethanol-associated social behavior, with early SH surgery eliminating ethanol-induced decreases in social preference, an effect not observed in early GX or either of the late surgery groups. Response to novelty was little affected by gonadectomy, but it was influenced by age of surgical manipulation, with early surgical manipulations, regardless of surgery type (i.e. GX or SH), suppressing novelty-directed behaviors in both sexes.
Suppression of all three components of social behavior (i.e., social investigation, contact behavior and play fighting) following a moderate dose of ethanol (1 g/kg) was observed in both sexes, regardless of surgery condition or surgery age (see Table 1). Previous studies conducted in our laboratory have yielded similar ethanol-related decreases in social interactions, with adult rats of both sexes showing less social investigation, contact behavior and play fighting when tested in a familiar environment after 0.75 and 1.0 g/kg ethanol challenges when compared to saline controls [33, 17]. Sex differences have occasionally been seen in this adult-typical response to moderate doses of ethanol. In a study examining the impact of chronic stress on ethanol-induced social behavior, adult females were found to be less sensitive than their male counterparts to the social inhibitory effects of ethanol regardless of stress condition, with females showing less impairment in social preference and play fighting behavior than males after challenge with 1g/kg ethanol [18], a sex effect not observed in the present study. While it is possible that the surgical manipulation process itself could have masked sex differences in ethanol-induced social inhibition in the present study, this possibility seems unlikely given the lack of sex differences among NM animals. Perhaps more likely are power differences across studies, with data from 12 animals of each sex represented when data were collapsed across stress condition in the Varlinskaya et al. [18] study, whereas 8-10 animals per group were tested here. Certainly, it is possible that potential sex-related differences in ethanol sensitivity may have emerged in the present study if multiple intermediate doses of ethanol had been examined.
While gonadectomy did not influence the impact of ethanol on social behavior in males or females, it did generally impact some components of social behavior regardless of drug condition. For example, when raw frequencies were analyzed, contact behavior was greater among GX than SH animals regardless of sex or surgery age, although this effect tended to be more pronounced in females than males, whereas social investigation and play fighting remained unaffected by GX. However, when each component behavior was analyzed as a percentage of the total amount of social behavior, changes in the microstructure of social behavior emerged, with GX animals showing relatively less of the most prominent social behavior normally seen in adults in this test situation - social investigation (social sniffing) - than SH animals, but proportionally more contact behavior, with no changes in proportion of play fighting. These effects were seen regardless of age at GX, despite the confound across early and late GX groups in length of gonadal hormone deprivation (an inherent confounding variable in this and many similar developmental studies). Findings that the age of gonad removal did not impact ethanol-induced social behavior among GX groups suggests that unequal hormone deprivation periods did not influence the measures examined here and supports the suggestion of a modest activational role of gonadal hormones in the microstructure of adult-typical social behavior.
Although it is difficult to compare social behaviors across the test situations used in different laboratories, the present results bear some similarities to those observed in a previous study examining the impact of castration on the social behavior of adult male rats in familiar and unfamiliar environments where castrates were found to exhibit less “curiosity” behavior (i.e., sniffing and following) when compared to sham-manipulated males, whereas no change in “physical” behavior (i.e., pushing, jumping, wrestling or grooming) was seen [47]. This earlier study, however, also showed a general reduction in overall social behavior following castration in adulthood, an effect not evident in the present study, perhaps due to methodological differences such as the use of slightly brighter lighting and a larger testing arena in that study [47] relative to that used here. The overall shift in the microstructure of social behavior induced by gonadectomy towards proportionally more contact behavior and less social investigation in the present study is reminiscent of a more immature social behavior profile. For example, although play fighting is the most predominant form of social behavior in both pre-pubertal and peri-pubertal adolescent rats, with this behavior greater in adolescents than adults, contact behavior also appears to be higher in adolescent rats compared to their adult counterparts [e.g., 48, 49]. Social investigation or sniffing, however, is more characteristic of adult social behavior, with adults showing greater levels of this behavior than adolescents [e.g. 49].
Previous research in our laboratory [30, 32] and others [31] has shown that castrated males drink more ethanol compared to their sham-manipulated counterparts. Based on the results of the present study, it appears that gonadectomy does not alter sensitivity to the aversive effects of ethanol - at least when indexed via ethanol-induced social inhibition at the dose examined. It is possible that effects of GX might have emerged had another dose been examined or if other behaviors had been used to index the aversive consequences of ethanol. Yet, a perhaps a more likely possibility is that the increases in ethanol intake seen after GX in adult males may reflect alterations in the rewarding rather than the aversive properties of ethanol, with a testosterone-related suppression of ethanol reinforcement in intact adult males possibly contributing to the notable sex differences in ethanol intake typically observed in adulthood.
One prominent finding in this study was the impact exerted by the process of early surgical manipulation on novelty-directed behaviors and social motivation. During the novel object test, animals receiving early (P23) surgery showed decreased sniffing of and contact with the novel object when compared to animals receiving late (P70) surgical manipulations, regardless of sex or surgery condition (i.e., GX or SH), with follow-up comparisons between these SH groups and NM animals suggesting that these differences are driven in part by surgery effects at both ages (see Fig. 1). In contrast, the effects of surgical manipulation on ethanol-related social avoidance were restricted to early SH animals, with ethanol-induced attenuations in social preference observed in all but the early SH group, and early SH animals showing both less social preference when challenged with saline and less ethanol-associated social avoidance than the other groups, including NM controls.
It is possible that changes in ethanol sensitivity as a result of the process of early SH surgical manipulation on measures such as social motivation may be associated with the stress of this manipulation among juvenile animals. Indeed, other early life stressors have been shown to influence adult ethanol-related behavior [e.g., 23, 50, 51, 52]. Animals in the early SH group in the current study were surgically manipulated at P23 – i.e., during the juvenile period between conventional weaning (P21) and adolescence (P28-42) [see 53]. While few studies have focused specifically on the influence of stress during this period on later ethanol sensitivity, our results combined with the results of others suggest that early exposure to stressors may alter ethanol sensitivity in adulthood. Indeed, these effects of early surgery on social preferences are reminiscent of that seen after acute restraint stress, with stressed adolescent rats (but not their adult counterparts) showing attenuated social preferences following saline challenge and resistance to the socially-suppressing effects of ethanol under similar test circumstances [54]. Intriguingly, these effects of the stress of early surgery on social motivation may be mediated in part by gonadal hormones, given that early GX animals did not show the alterations in ethanol-related social motivation seen in early SH animals. That gonadectomy may protect against these effects induced by early stress or other effects of the prepubertal surgical process could prove to be a promising area for further inquiry.
Novelty-directed behavior was not found to be influenced by removal of the gonads pre or postpubertally, nor was it affected by sex. The lack of sex differences in response to a novel object in the current study is similar to the results of other work using the same test procedure in our laboratory where time spent sniffing a novel object was not found to differ by sex at any age across a wide age range spanning from prepubertal to adult rats [38]. However, others have observed sex differences in both adolescent and adult male Listar-hooded rats, with males showing increased preference for a novel object compared to their female counterparts [40]. It is possible that methodological differences such as rat strain or novelty paradigm could explain these variations found across studies.
Taken together, the results of this experiment suggest that both novelty-directed behavior and adult-typical responses to ethanol (as indexed via ethanol-induced social inhibition) are little influenced by the removal of gonadal hormones either prior to puberty or in adulthood. Removal of the gonads did, however, impact the microstructure of behavior expressed during social interaction, resulting in a pattern of social behavior more reminiscent of an adolescent than an adult animal. In contrast to the relatively nuanced effects of gonadal removal, the process of early surgical manipulation itself markedly influenced novelty-directed behavior and ethanol sensitivity (as indexed via social motivation), suggesting that the experience of surgical manipulation per se during this sensitive developmental period may have long lasting behavioral and pharmacological consequences.
Research Highlights.
Male and female rats were gonadectomized pre-pubertally and in adulthood.
Prepubertal sham surgical manipulation altered ethanol sensitivity and decreased response to novelty.
Gonadectomy did not alter sensitivity to ethanol or response to novelty.
Pubertal hormones do not influence certain adolescent-typical behaviors.
The experience of surgical manipulation during development exerts lasting effects.
Acknowledgements
Funding acknowledgements: This research was supported by NIAAA grant R01-AA017355.
The authors would like to thank Julia Shultz for performing the surgeries and Judy Sharp for analyzing the hormonal and blood ethanol concentration assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- [2].Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;194:469–98. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- [3].Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- [4].Primus RJ, Kellogg CK. Pubertal-related changes influence the development of environment-related social interaction in the male rat. Dev Psychobiol. 1989;22:633–43. doi: 10.1002/dev.420220608. [DOI] [PubMed] [Google Scholar]
- [5].Primus RJ, Kellogg CK. Developmental influence of gonadal function on the anxiolytic effect of diazepam on environment-related social interaction in the male rat. Behav Pharmacol. 1990;1:437–46. [PubMed] [Google Scholar]
- [6].Romeo RD, Schulz KM, Nelson AL, Menard TA, Sisk CL. Testosterone, puberty, and the pattern of male aggression in Syrian hamsters. Dev Psychobiol. 2003;43:102–8. doi: 10.1002/dev.10125. [DOI] [PubMed] [Google Scholar]
- [7].Schulz KM, Richardson HN, Zehr JL, Osetek AJ, Menard TA, Sisk CL. Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm Behav. 2004;45:242–9. doi: 10.1016/j.yhbeh.2003.12.007. [DOI] [PubMed] [Google Scholar]
- [8].Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254-255:120–6. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- [9].Chan KK, Neighbors C, Gilson M, Larimer ME, Marlatt G Alan. Epidemiological trends in drinking by age and gender: providing normative feedback to adults. Addict Behav. 2007;32:967–76. doi: 10.1016/j.addbeh.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [10].Greenfield SF. Women and alcohol use disorders. Harv Rev Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- [11].Fillmore MT, Weafer J. Alcohol impairment of behavior in men and women. Addiction. 2004;99:1237–46. doi: 10.1111/j.1360-0443.2004.00805.x. [DOI] [PubMed] [Google Scholar]
- [12].Mills KC, Bisgrove EZ. Body sway and divided attention performance under the influence of alcohol: dose-response differences between males and females. Alcohol Clin Exp Res. 1983;7:393–7. doi: 10.1111/j.1530-0277.1983.tb05492.x. [DOI] [PubMed] [Google Scholar]
- [13].Mumenthaler MS, Taylor JL, O’Hara R, Yesavage JA. Gender differences in moderate drinking effects. Alcohol Res Health. 1999;23:55–64. [PMC free article] [PubMed] [Google Scholar]
- [14].National Institute on Alcohol Abuse and Alcoholism Alcohol- An important women’s health issue. Alcohol Alert. 2004;62 (2004) [Google Scholar]
- [15].Wang GJ, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y. Alcohol intoxication induces greater reductions in brain metabolism in male than in female subjects. Alcohol Clin Exp Res. 2003;27:909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- [16].Varlinskaya EI, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33:991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varlinskaya EI, Spear LP. Chronic tolerance to the social consequences of ethanol in adolescent and adult Sprague-Dawley rats. Neurotoxicol Teratol. 2007;29:23–30. doi: 10.1016/j.ntt.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Varlinskaya EI, Doremus-Fitzwater TL, Spear LP. Repeated restraint stress alters sensitivity to the social consequences of ethanol in adolescent and adult rats. Pharmacol Biochem Behav. 2010;96(2):228–35. doi: 10.1016/j.pbb.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Middaugh LD, Frackelton WF, Boggan WO, Onofrio A, Shepherd CL. Gender differences in the effects of ethanol on C57BL/6 mice. Alcohol. 1992;9(3):257–60. doi: 10.1016/0741-8329(92)90062-f. [DOI] [PubMed] [Google Scholar]
- [20].Varlinskaya EI, Spear LP. Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague-Dawley rats. Alcohol Clin Exp Res. 2004;28(1):40–50. doi: 10.1097/01.ALC.0000108655.51087.DF. [DOI] [PubMed] [Google Scholar]
- [21].Substance Abuse and Mental Health Services Administration. National Survey on Drug Use and Health. U.S. Dept of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies 2008.
- [22].Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- [23].Chester JA, Barrenha G de Paula, DeMaria A, Finegan A. Different effects of stress on alcohol drinking behaviour in male and female mice selectively bred for high alcohol preference. Alcohol Alcohol. 2006;41:44–53. doi: 10.1093/alcalc/agh242. [DOI] [PubMed] [Google Scholar]
- [24].Le AD, Israel Y, Juzytsch W, Quan B, Harding S. Genetic selection for high and low alcohol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2001;25:1613–1620. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- [25].Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44:547–54. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ford MM, Eldridge JC, Samson HH. Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res. 2002;26:635–643. [PubMed] [Google Scholar]
- [27].Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF. Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res. 1998;22:1564–1569. [PubMed] [Google Scholar]
- [28].Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–6. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- [29].Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [30].Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35 doi: 10.1111/j.1530-0277.2011.01555.x. DOI: 10.1111/j.1530-0277.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sherrill LK, Koss WA, Foreman ES, Gulley JM. Effects of pre-pubertal gonadectomy and binge-like ethanol exposure during adolescence on drinking behavior in adult male and female rats. Behav Brain Res. 2011;216:569–575. doi: 10.1016/j.bbr.2010.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Vetter-O’Hagen CS, Sanders KW. Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behav Brain Res. 2011;31:403–17. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26:1502–11. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- [34].Varlinskaya EI, Spear LP. Difference in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48:146–61. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- [35].Spear LP, Varlinskaya EI. Adolescence: Alcohol sensitivity, tolerance and intake. In: Galanter M, editor. Recent Developments in Alcoholism. Volume 17: Alcohol Problems in Adolescents and Young Adults. Kulwer Academic/Plenum Publishers; New York: 2005. pp. 143–159. [PubMed] [Google Scholar]
- [36].Douglas LA, Varlinskaya EI, Spear LP. Novel-object place conditioning in adolescent and adult male and female rats: effects of social isolation. Physiol Behav. 2003;80:317–25. doi: 10.1016/j.physbeh.2003.08.003. [DOI] [PubMed] [Google Scholar]
- [37].Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- [38].Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. doi: 10.1002/dev.20610. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- [40].Ontogeny of sex differences in response to novel objects from adolescence to adulthood in lister-hooded rats. Dev Psychobiol. 2011 doi: 10.1002/dev.20542. DOI: 10.1002/dev.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cyrenne DL, Brown GR. Effects of suppressing gonadal hormones on response to novel objects in adolescent rats. Horm Behav. 2011 doi: 10.1016/j.yhbeh.2011.08.015. DOI:10.1016/j.yhbeh.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Institute of Laboratory Animal Research. National Research Council . Guide for the Care and Use of Laboratory Animals. National Academies Press; Washington, DC: 2010. [Google Scholar]
- [43].Varlinskaya EI, Spear LP. Ontogeny of acute tolerance to ethanol-induced social inhibition in Sprague-Dawley rats. Alcohol Clin Exp Res. 2006;30:1833–44. doi: 10.1111/j.1530-0277.2006.00220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Varlinskaya EI, Spear LP, Spear NE. Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res. 2001;25:377–85. [PubMed] [Google Scholar]
- [45].File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–38. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- [46].Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- [47].Primus RJ, Kellogg CK. Gonadal hormones during puberty organize environment-related social interaction in the male rat. Horm Behav. 1990;24:311–23. doi: 10.1016/0018-506x(90)90012-m. [DOI] [PubMed] [Google Scholar]
- [48].Varlinskaya EI, Spear LP. Social interactions in adolescent and adult Sprague-Dawley rats: impact of social deprivation and test context familiarity. Behav Brain Res. 2008;188:398–405. doi: 10.1016/j.bbr.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Willey AR, Varlinskaya EI, Spear LP. Social interactions and 50 kHz ultrasonic vocalizations in adolescent and adult rats. Behav Brain Res. 2009;202:122–9. doi: 10.1016/j.bbr.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cruz FC, Quadros IM, Planeta Cda S, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–68. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001;158:366–73. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- [52].Rockman GE, Hall A, Markert L, Glavin GB. Early weaning effects on voluntary ethanol consumption and stress responsivity in rats. Physiol Behav. 1987;40:673–6. doi: 10.1016/0031-9384(87)90116-8. [DOI] [PubMed] [Google Scholar]
- [53].Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- [54].Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. doi: 10.1016/j.pbb.2011.10.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]