Abstract
When Syrian hamsters (Mesocricetus auratus) are defeated by a larger, more aggressive hamster, they subsequently exhibit submissive and defensive behavior, instead of their usual aggressive and social behavior, even towards a smaller, non-aggressive opponent. This change in behavior is termed conditioned defeat, and we have found that the amygdala, bed nucleus of the stria terminalis, and ventral hippocampus, among others, are crucial brain areas for either the acquisition and/or expression of this behavioral response to social stress. In the present study, we tested the hypothesis that the nucleus accumbens is also a necessary component of the circuit mediating the acquisition and expression of conditioned defeat. We found that infusion of the GABAA agonist muscimol into the nucleus accumbens prior to defeat training failed to affect acquisition of conditioned defeat, but infusion prior to testing significantly decreased submissive behavior and significantly increased aggressive behavior directed toward the non-aggressive intruder. These data indicate that, unlike the basolateral complex of the amygdala, the nucleus accumbens is not a critical site for the plasticity underlying conditioned defeat acquisition, but it does appear to be an important component of the circuit mediating the expression of the behavioral changes that are produced in response to a previous social defeat. Of note, this is the first component of the putative “conditioned defeat neural circuit” wherein we have found that pharmacological manipulations are effective in restoring the territorial aggressive response in previously defeated hamsters.
Keywords: conditioned fear, social defeat, aggression, aggressive behavior, submissive behavior
1. Introduction
Social conflict is a ubiquitous factor in human and non-human animal relationships[1], and this conflict affects a wide range of organisms both physically and emotionally. Determining the neurobiology of social stress can help lead to new treatments for disorders that arise from or are exacerbated by this stress, such as post-traumatic stress disorder[2], schizophrenia[3], and depression[4]. Social stress can have many physical effects, including increased activation of the hypothalamic-pituitary-adrenal (HPA) axis[5, 6], decreased testosterone in males [1, 7], and effects on body mass [8, 9]. These physical effects are often harmful in humans and are known to exacerbate heart disease, obesity, and other ailments that cost society millions of dollars in terms of health care and lost productivity every year[10, 11].
Our lab has used a model to study the neurobiology of social stress that we term conditioned defeat. In this model, a hamster that has been defeated, even a single time, by a larger, more aggressive opponent subsequently exhibits a striking behavioral change. Instead of producing the species-typical territorial aggression toward a novel intruder, previously defeated hamsters instead exhibit submissive and defensive behavior, even when the intruder is smaller and non-aggressive[12]. This is an excellent model of social stress because the defeat by the larger, aggressive hamster does not result in wounding (and therefore the stress is more psychological than physical), it is very simple to elicit, and defeated hamsters exhibit unambiguous behavior that is identified and quantified easily. By contrast, many other rodent models of social stress involve chronic exposure to an aggressor, often involving up to 2 weeks wherein the subject is defeated each day and then spends the next 24 hours within the same cage as the aggressor but protected by a wire mesh divider[13–19]. This sort of social stress experience, while also extremely useful for studying the physiological concomitants of and putative treatments for the effects of social stress, is much more severe than that studied in our laboratory. We maintain that conditioned defeat is an ethologically relevant form of fear conditioning that can be used to explore how even a single, brief exposure to social stress can lead to long-term changes in future behavior.
We have used the conditioned defeat model to begin to define a neural circuit that underlies acute defeat-induced behavioral plasticity (i.e., how a single social experience can dramatically “switch” an animal’s behavior). We have found that the basolateral amygdala[20] and the ventral hippocampus[21] are necessary for both the acquisition and expression of conditioned defeat. We have also shown that the bed nucleus of the stria terminalis is necessary for the expression of conditioned defeat[22]. To date, the only brain area that we have determined to be critical for conditioned defeat-induced neural plasticity is the basolateral amygdala, as inhibition of protein synthesis within this area before training with the resident aggressor will block expression of social stress behaviors the following day[23]. The other brain areas mentioned above (ventral hippocampus, bed nucleus of the stria terminalis) seem to modulate this memory or its output, but they may not be crucial for storage of its memory. Another brain area that might play a role in conditioned defeat is the nucleus accumbens. It is a brain area that is most commonly associated with reward and drug addiction[24–28], but prior research has also demonstrated that the nucleus accumbens is necessary for both the acquisition [29] and expression[30] of conditioned fear (but see also[31]) and is activated during fear recall in humans[32]. It is also known that neurotrophic factors in the nucleus accumbens are necessary for acquisition of social avoidance in reaction to prolonged social stress[15, 16] in mice. Therefore, the purpose of the present study was to test the hypothesis that the nucleus accumbens is necessary for the acquisition and the expression of conditioned defeat in hamsters.
2. Methods and Procedures
2.1 Animals and housing conditions
Subjects were adult male Syrian hamsters (Mesocricetus auratus; Charles River Laboratories, Wilmington, MA) that weighed 110–135 grams and were about 9 weeks old at the time of testing. All subjects were individually housed for at least one week prior to the start of testing in a temperature (20° ± 2°C) and humidity-controlled room with ad libitum access to food and water. Animals were kept on a 14:10 light:dark cycle (lights out at 10:00 h). Resident aggressors (RA) used for defeat training were older (>6 mo), singly housed males weighing between 160 and 180 g. Younger males (~2 mo) that weighed between 100 and 110 grams were group-housed (four to five per cage) and were used as non-aggressive intruders (NAI). The cages of the experimental animals and the resident aggressors were not changed for 1 week prior to testing so that animals could scent mark their cages and establish residence. No RA or NAI was used more than twice on a single training or testing day, respectively. All procedures and protocols were approved by the Georgia State University Institutional Care and Use Committee and conform to PHS guidelines.
2.2 Surgical procedures
Subjects were anesthetized with sodium pentobarbital (90 mg/kg, i.p.), placed into a stereotaxic frame, and bregma and lambda were leveled within the same plane. Stainless steel guide cannula (26-gauge, 4.0 mm long below the pedestal) were implanted bilaterally into the brain and aimed at the nucleus accumbens (2.4 mm rostral and ±3.2 mm lateral relative to bregma and at a 20° angle toward the midline). In order to prevent damage to the area of interest, the guide cannula was lowered to only 2.7 mm below dura. On the day of injection, a 33-gauge injection needle was used that projected 2.3 mm below the guide cannula, reaching a final depth of 5 mm below dura. Following surgery, dummy stylets were placed in the guide cannula to help maintain patency. Hamsters were allowed 7–10 days to recover from surgery prior to the start of behavioral testing. Beginning two days after surgery, the hamsters were handled each day by gently restraining them and removing and then replacing the dummy stylets in order to maintain patency and to habituate the subjects to the injection procedure.
2.3 Social defeat and behavioral testing
The conditioned defeat model has been extensively described elsewhere[33]. Briefly, on the day of social defeat training, animals were transported to the testing suite within the vivarium and were allowed to acclimate to the environment for 1 h. All training and testing sessions were performed under dim red illumination during the first 3 h of the dark phase of the light–dark cycle[34]. Training consisted of one 15-min exposure to the RA in the aggressor’s home cage, upon which time the RA reliably attacked the experimental hamsters within 60 sec. The following day, animals were again transported to the same testing suite, and a NAI was placed into the subject’s home cage for 5 min. An animal was considered to show conditioned defeat if it exhibited no aggressive behavior and displayed an increase in submissive and defensive behavior when the NAI was introduced into its home cage. Conversely, a reduction in conditioned defeat was operationally defined as a significant reduction in the duration of submissive and defensive behavior. In contrast, non-defeated animals typically exhibit minimal submissive behavior and show high levels of territorial aggression directed toward the NAI. All training and testing sessions were videotaped via a CCD camera mounted overhead. These videos were scored by an experimentally blind observer using the behavioral scoring program Noldus ObserverPro. A second observer scored a random subset of these videos and interrater reliability for scored behavior between the two observers was above 90%. The total duration of four classes of behavior were measured during the test session: (1) social behavior (stretch, approach, sniff, nose touching, and flank marking); (2) non-social behavior (locomotion, exploration, grooming, nesting, feeding, and sleeping); (3) submissive/defensive behavior (flight, avoidance, tail up, upright, side defense, full submissive posture, stretch attend, head flag, attempted escape from cage); and (4) aggressive behavior (upright and side offense, chase and attack, including bites).
2.4 Drug infusion
Initially, we infused a dose of 1.1 nmol muscimol into the nucleus accumbens before defeat. This dosage had been used successfully to significantly reduce conditioned defeat after microinjection in the ventral hippocampus[21] and BNST[22]. In the nucleus accumbens, however, this dose produced a confounding stereotypy (in the form of a marked mouthing or chewing response) that increased the aggression of the resident aggressor toward the experimental animal forcing us to reduce the dosage to 0.55 nmol, which caused no stereotypy. Infusion of this dose into other brain areas has been shown to be effective in altering behavior. For example, 0.5 nmol of muscimol has been infused unilaterally into the dorsomedial hypothalamus to significantly decrease escape from the open arms in the elevated T-maze[35], a measure of anxiety. In addition, a bilateral infusion of 0.5 nmol muscimol into the amygdala is also effective in reducing intake of palatable food in rats[36]. Therefore, in the present study muscimol (Sigma, 0.55 nmol in 150 nl of saline) or vehicle control (150 nl of saline) was infused bilaterally into the nucleus accumbens over a 1-min period using a 1-μl syringe and a PHD 2000 Harvard Apparatus microinfusion pump connected to a 33-gauge injection needle via polyethylene tubing. The injection volume of 150 nl was chosen to minimize spread to adjacent structures (such as the BNST, which we have also found is necessary for expression of conditioned defeat) and to maximize anatomical specificity. The needle was kept in place for an additional minute before being removed to ensure diffusion of the drug or vehicle after which the dummy stylet was replaced. A successful injection was indicated by movement of an air bubble separating drug and water down the tubing and/or patency of the needle before and after the injection. Training or testing began 5 minutes after drug or vehicle control infusion.
2.5 Site verification
At the end of each experiment, hamsters were administered an overdose of sodium pentobarbital and infused with 150 nl of India ink bilaterally into the nucleus accumbens to verify the placement of the needle. The brains were post-fixed in 10% buffered formalin for at least 3 d before being sectioned on a cryostat. Thirty-μm sections were taken and stained with cresyl violet and coverslipped with DPX. Sections were then examined by two raters who were blind to experimental condition under a light microscope for placement verification. Only animals with ink injections that were 0.3 mm or less from the nucleus accumbens core or shell were included in the statistical analysis. Animals with one or both ink injections outside of the nucleus accumbens or with ink solely within the anterior commissure were included in a site control group (anatomical controls).
2.6 Role of the nucleus accumbens in the acquisition of conditioned defeat
The goal of this experiment was to determine whether the temporary inactivation of the nucleus accumbens using the GABAA receptor agonist muscimol would significantly reduce the acquisition of conditioned defeat. Animals (n=38) were matched by weight and randomly assigned to vehicle or drug conditions. Hamsters received either muscimol or saline injections bilaterally into the nucleus accumbens 5 minutes prior to being placed into the home cage of a resident aggressor for 15 minutes. On the following day, animals were tested drug-free in their own cage against a non-aggressive intruder for 5 minutes.
2.7 Role of the nucleus accumbens in the expression of conditioned defeat
The goal of this experiment was to determine whether the temporary inactivation of the nucleus accumbens using muscimol would significantly reduce the expression of conditioned defeat. Animals (n=42) were matched by weight and randomly assigned to vehicle or drug conditions. Hamsters were placed drug-free in the home cage of a resident aggressor for 15 minutes of defeat training. The next day, they received either muscimol or vehicle injections into the nucleus accumbens 5 minutes prior to the 5-minute test with the non-aggressive intruder (NAI).
2.8 No-defeat control experiment
The goal of this experiment was to determine whether the temporary inactivation of the nucleus accumbens using muscimol before testing would increase aggressive behavior in animals (n=22) that had not been defeated. Stereotaxic surgery to implant cannula guides was performed as described previously, and following recovery subjects were placed in the empty cage of a resident aggressor for 15 minutes as a control for the effect of exposure to a novel conspecific’s cage. On the following day, subjects were administered either 0.55 nmol muscimol in 150 nl saline or vehicle 5 minutes before a 5 min test with a NAI.
2.8 Statistical analysis
All data was analyzed using SPSS and tested for homogeneity of variance. Data that did not demonstrate homogeneity of variance were analyzed with non-parametric Mann-Whitney U statistical tests. Data that met the criteria for homogeneity of variance were analyzed using a one-way ANOVA. Statistical results given below that contain the F statistic met the criteria for homogeneity of variance, while results containing the U statistic did not meet this criteria and therefore were analyzed with the non-parametric Mann-Whitney U test. Significant differences for all analyses were set at p < 0.05.
3. Results
3.1: The nucleus accumbens is not necessary for the acquisition of conditioned defeat
Histology
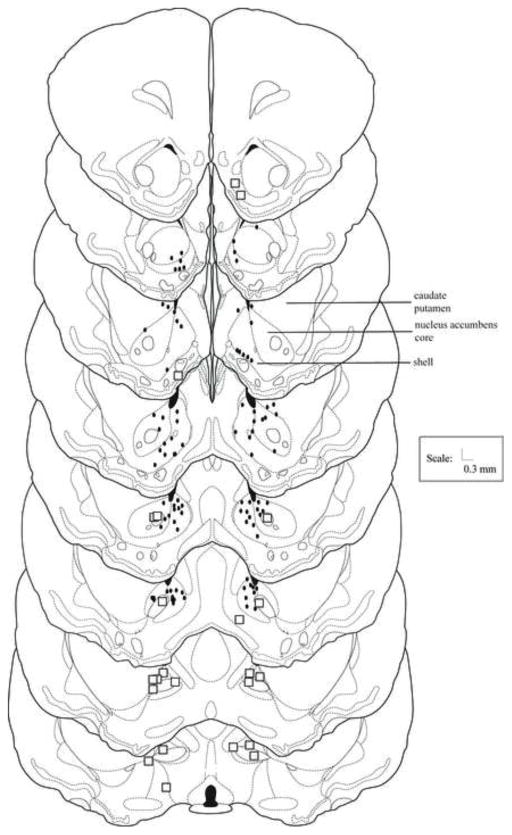
Figure 1 shows the location of ink injection sites for animals in Experiment 1 and 2. Misses for Experiment 1 largely were in the bed nucleus of the stria terminalis, the caudate putamen, and the lateral ventricle. Data from animals with bilateral placements in the nucleus accumbens core were compared with animals with placements in the accumbens shell. Ink injections that were all or mostly in the shell were counted as shell injections, whereas ink injections that were all or mostly in the core were counted as core injections. As there were no significant differences between these groups, data for the nucleus accumbens core and shell were pooled for statistical analyses.
Figure 1.
Nucleus accumbens histology. Histological reconstruction of injection sites of animals receiving infusions into the nucleus accumbens shell or core 5 min before defeat (Experiment 1) or testing (Experiment 2). Black dots represent the site of injection of one or more animals. Boxes represent one or more anatomical misses. Drawings adapted from [59].
Behavioral results
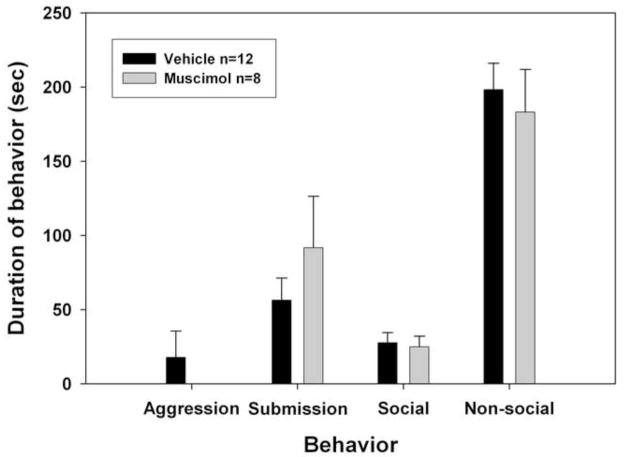
As shown in Figure 2, animals with vehicle or muscimol infusions into the nucleus accumbens before defeat were not significantly different in their interactions with the non-aggressive intruder the following day, including expression of submissive/defensive behavior(p>0.05).
Figure 2.
Muscimol in nucleus accumbens acquisition. Experiment 1 examined the effect of muscimol infusion on acquisition of conditioned defeat. Animals received infusions of vehicle or muscimol 5 minutes before being defeated by a resident aggressor for 15 min. Bar graph shows total duration (mean ± S.E.M.) of submissive/defensive, aggressive, social, and non-social behavior exhibited by defeated hamsters during a subsequent 5-min test with a non-aggressive intruder. No significant differences were observed between any of the groups.
3.2: The nucleus accumbens is necessary for the expression of conditioned defeat
Histology
Figure 1 also shows the location of ink injection sites for animals in Experiment 2. Misses for Experiment 2 largely went into the anterior commissure, caudate putamen, and the bed nucleus of the stria terminalis. Animals given muscimol that also had misplaced cannulae did not differ from vehicle controls in the duration of submissive/defensive or other behavior measured, indicating that the effect of muscimol in the nucleus accumbens group was anatomically specific (see Table 1 for means ± SEM).
Table 1.
Experiment 2 examined the effect of muscimol infusion into nucleus accumbens on expression of conditioned defeat. Asterisks indicate significant difference from vehicle. The lack of effect observed in unilateral and bilateral misses infused with muscimol demonstrates that the effect of muscimol in the nucleus accumbens is anatomically specific.
| Group | Aggression (Mean ± SEM) | Submission (Mean ± SEM) | Social (Mean ± SEM) | Nonsocial (Mean ± SEM) |
|---|---|---|---|---|
| Vehicle Bilateral Hit (n =11) | 0 ± 0 | 80.73 ± 15.16 | 39.82 ± 7.27 | 179.45 ± 12.64 |
| Muscimol Bilateral Miss (n=4) | 0 ± 0 | 56.5 ± 27.55 | 98.5 ± 52.02 | 104.0 ± 50.35 |
| Muscimol Unilateral Miss(n=5) | 0 ±0 | 78.6 ± 16.01 | 24.6 ± 11.05 | 196.8 ± 22.07 |
| Muscimol Bilateral Hit (n=14) | 35.57 ± 17.86* | 36.57 ± 10.46* | 113.64 ± 23.77* | 114.07 ± 18.77* |
Animals with nucleus accumbens core hits were compared with animals with shell hits. There were no significant differences between these groups (see Table 2 for exact means and SEM), so all bilateral hits within the nucleus accumbens core and/or shell pooled and were included in analyses.
Table 2.
Muscimol in nucleus accumbens expression core versus shell results. No significant differences in behavior were seen between drug animals with hits within the nucleus accumbens core or shell. Thus, bilateral hits in either core or shell were pooled for statistical analyses.
| Group | Aggression (Mean ± SEM) | Submission (Mean ± SEM) | Social (Mean ± SEM) | Nonsocial (Mean ± SEM) |
|---|---|---|---|---|
| Vehicle bilateral hit (n = 11) | 0 ± 0 | 80.73 ± 15.16 | 39.82 ± 7.27 | 179.45 ± 12.64 |
| Muscimol bilateral core (n = 3) | 39.33 ± 39.33 | 34.67 ± 17.33 | 129.67 ± 8.67 | 97.0 ± 29.82 |
| Muscimol one side core, one side shell (n= 3) | 45.67 ± 44.18 | 10.67 ± 6.49 | 172.0 ± 69.96 | 71.67 ± 30.75 |
| Muscimol bilateral shell (n = 8) | 30.38 ± 25.53 | 47.0 ± 16.34 | 85.75 ± 31.56 | 136.38 ± 27.67 |
Behavioral results
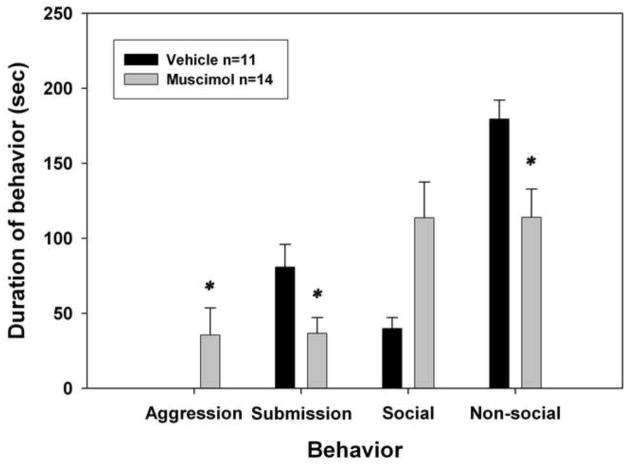
As shown in Figure 3, there were significant differences between animals that received muscimol and animals that received vehicle before testing in their behavior toward the NAI. Animals that received muscimol exhibited significantly less submission during testing than did animals receiving vehicle injections (F(1,23)=6.114, p=0.021). Animals that received muscimol also exhibited significantly more aggressive behavior (U= 38.5, p = 0.03) and less nonsocial behavior than did vehicle controls (U = 35.0, p = 0.021). There were no significant differences between vehicle and muscimol animals for social behavior (U = 43.0, ns, p = 0.066), though animals receiving muscimol did show a trend for increased social behavior (see Figure 3).
Figure 3.
Muscimol in nucleus accumbens expression. Total duration (mean ± S.E.M.) of submissive/defensive, aggressive, social, and non-social behavior exhibited by defeated hamsters during a 5-min test with a non-aggressive intruder in Experiment 2 (expression study). Animals received infusions of vehicle or muscimol 5 minutes before being tested with a non-aggressive intruder. *Significantly different from vehicle control (p < 0.05).
There was no evidence of stereotypy exhibited by the animals that received muscimol. The fact that nonsocial behavior decreased while aggressive behavior increased in these individuals suggests that there was not a non-specific effect of muscimol on behavior (i.e., lethargy or ataxia). The increase in aggressive and social behavior (and the decrease in submission was pervasive across the drug group; 8 of the 15 animals infused with muscimol showed aggressive or social behavior toward the non-aggressive intruder. The other six animals infused with muscimol did show some submission, but overall the average submission expressed by these animals was significantly less than that exhibited by vehicle animals.
3.3: No-defeat control study
Histology
Of the 22 animals used for the no-defeat control study, 1 animal lost its cap. Of the remaining 21 animals, there were 14 animals with bilateral hits (6 muscimol, 8 saline), 3 animals with unilateral hits, and 4 animals with bilateral misses. The behavioral results include only those animals with ink injections that were bilateral hits into the nucleus accumbens shell or core.
Behavioral results
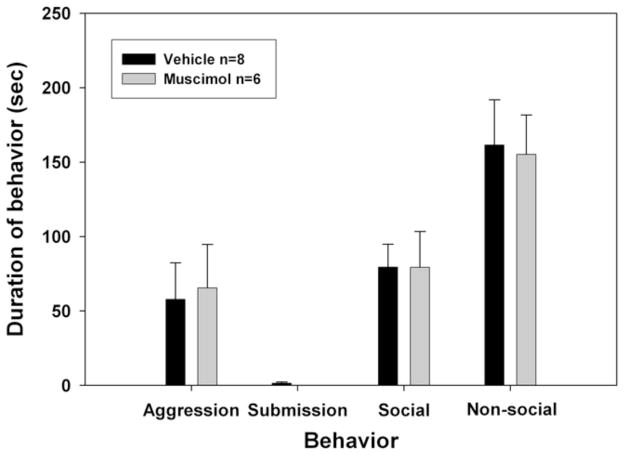
As shown in Figure 4, there were no significant differences between animals that received muscimol or saline before testing in their expression of social, non-social, submission, or aggression behavior toward the NAI.
Figure 4.
No-defeat control results. Effect of muscimol infusion into the nucleus accumbens on expression of behavior in non-defeated hamsters. Total duration (mean ± S.E.M.) of submissive/defensive, aggressive, social, and non-social behavior exhibited by non-defeated hamsters during a 5-min test with a non-aggressive intruder. No significant differences were found between groups receiving vehicle or muscimol.
4. Discussion
The present experiments indicate that infusion of the GABAA receptor agonist muscimol into the nucleus accumbens blocks the expression but not the acquisition of conditioned defeat. These data are the first to suggest that the nucleus accumbens is a necessary component of the neural circuit underlying the expression of conditioned defeat and thus plays an important role in behavioral responses to social stress in hamsters. These data also indicate, however, that neural activity in the nucleus accumbens during defeat training is not necessary for the formation of defeat-induced behavioral changes in this species. Finally, these data are particularly important in that they identify for the first time a component of the neural circuit mediating conditioned defeat wherein pharmacological manipulation both reduces social avoidance and restores territorial aggression in previously defeated hamsters.
The finding that the acquisition of conditioned defeat is not blocked by temporary inactivation of the nucleus accumbens is perhaps surprising based on findings from the conditioned fear literature indicating that the nucleus accumbens (specifically the shell region) is required for acquisition of conditioned fear [29], including fear-potentiated startle [37] (but see also [31]). It has also been shown that activation of neurons by neurotrophins[15] or transcription factors[14] is required in the nucleus accumbens to elicit social avoidance after social defeat, suggesting that activation of the nucleus accumbens during the defeat experience is necessary for subsequent defeat-induced behavioral changes to occur. There are several possible explanations for the inconsistencies in the data. First, the dose of muscimol used in the present study could have been too low to effectively inactivate the nucleus accumbens during acquisition. We were limited to the lower dose, however, because the higher dose caused stereotyped responses that in turn elicited greater aggression from the resident aggressor. Future studies could use an alternative method (such as lidocaine or an NMDA antagonist, which was effective in reducing acquisition of conditioned defeat in the BLA[38]) to inhibit the nucleus accumbens in order to reexamine the possibility that this area is involved in the acquisition of CD in hamsters. The dose used in the acquisition experiment, however, was the same dose that was shown to effectively inhibit the expression of CD, suggesting that this dose of muscimol was sufficient to inhibit the nucleus accumbens. In addition, there are also previous studies indicating that a similar dose of muscimol alters panic/anxiety behavior or feeding behavior after infusion into the hypothalamus [35] or the amygdala [36], respectively.
Another explanation for this inconsistency is that the nucleus accumbens may not govern acquisition of social stress-induced behavioral changes in hamsters as it does in other species. There are other instances in which differences in the importance of certain brain areas in rats and mice versus hamsters[39, 40] have been noted, though this was observed in aggression, not in fear or social stress-induced behavior. A third reason for the conflicting acquisition results could be the nature of the stimuli leading to conditioned defeat. Previous studies have suggested that the nucleus accumbens may be necessary for learning of cued fear stimuli, while contextual fear learning occurs elsewhere. It is not clear at this point what the critical stimuli are that cause conditioned defeat as the behavior generalizes to very different testing conditions. This is certainly an interesting problem for future study. Finally, this inconsistency might be due to differences in the stressors used (i.e., physical stress versus psychological stress) or in the duration of the stressor (i.e., repeated defeat vs. acute defeat). Differences in the importance of brain areas mediating physical stress versus psychological stress responses are frequently noted in the literature[41–44], as are differences in the brain areas that are affected by or required for responses to repeated/chronic versus acute stressors[45].
The finding that the nucleus accumbens is required for the expression of conditioned defeat is exciting given that this is the first demonstration that manipulation of the nucleus accumbens can alter defeat-induced behavior. Whereas some studies have indicated that the nucleus accumbens is activated during social stress[46] and other studies have indirectly found that neuronal activation of this brain area is required during a defeat experience for social stress-induced behavioral changes to occur [15, 16], no prior study to our knowledge has demonstrated that the nucleus accumbens is necessary for the expression of behavioral responses to social stress. Our findings are congruent with previous data, however, demonstrating that the expression of fear-potentiated startle requires a functional nucleus accumbens[37] and that expression of conditioned fear is impaired following a nucleus accumbens lesion[30].
The demonstration that inactivation of the nucleus accumbens during conditioned defeat testing causes an increase in aggressive behavior in Syrian hamsters is an important and novel finding that extends our previous model of the neural circuit underlying conditioned defeat. It is particularly important to note that our previous studies have demonstrated that inactivation of other brain areas in the putative conditioned defeat neural circuit, such as the amygdala[20], bed nucleus of the stria terminalis[22], and the ventral hippocampus[21] before conditioned defeat testing lead to a reduction in submission, but this occurs without a concomitant increase in aggression. Thus, our putative conditioned defeat neural circuit has previously lacked a site in which the defeat-induced reduction in aggression might be mediated. The current results thus represent the first experimental manipulation that has led to the resumption of territorial aggression toward an NAI by a previously defeated animal. This finding is consistent with data indicating that inactivation of the nucleus accumbens [47–49] can stimulate aggression in rats and that increased numbers of androgen receptors within the nucleus accumbens are expressed following winning an aggressive bout[50]. It is very important to note, however, that in the current study the nucleus accumbens selectively stimulated aggression in defeated hamsters and that there was no increase in aggression in non-defeated animals that received muscimol before testing.
Prior reports have speculated on how the nucleus accumbens modulates a variety of forms of conditioned fear. One conception is that the nucleus accumbens is an important interface between limbic and motor areas of the brain [51] and is a site within which glutamatergic innervation from the BLA[52], which may be activated during expression of conditioned defeat, and enhanced dopaminergic activation from the ventral tegmental area[53] attributed to social stress[54] come together and synapse on projection neurons within the nucleus accumbens[52, 55, 56]. This convergence of glutamatergic and dopaminergic signaling seems to gate downstream inhibition [57, 58] of motor output areas, including those involved in aggression[48]. The nucleus accumbens may act to inhibit aggression following social defeat, as stated above, and when its activation is blocked by muscimol, as in this study, aggression is no longer inhibited and the experimental animal behaves aggressively toward an intruder.
In summary, the findings of this paper indicate that the nucleus accumbens is necessary for expression of conditioned defeat in Syrian hamsters, but not its acquisition. It also appears to be a particularly important part of the neural circuit mediating conditioned defeat expression in that the nucleus accumbens affects not just submissive and avoidant behavior but also their opposite, given that infusion of muscimol before conditioned defeat testing significantly increased aggression directed toward an intruder. This finding is a first for our lab, as we have not previously found a treatment that would rapidly and reliably restore territorial aggression after a previous social defeat.
Highlights.
The nucleus accumbens (NAcc) is part of the neural circuit for conditioned defeat
The NAcc is necessary for the expression of conditioned defeat
The NAcc is not necessary for the acquisition of conditioned defeat
Temporary inactivation of the NAcc reduces submissive behavior and social avoidance
Muscimol in the NAcc increases aggression in previously defeated hamsters
Acknowledgments
6. Funding source
This work was supported by NIH MH62044 to KLH and based on work supported in part by the Center for Behavioral Neuroscience under NSF agreement #IBN-9876754.
The authors extend special thanks to Daniel Erwin for his assistance with this research.
Footnotes
5. Conflict of Interest
The authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Alisa Norvelle, Email: anorvelle@gsu.edu.
Kim Huhman, Email: khuhman@gsu.edu.
References
- 1.Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–42. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- 2.Bonafons C, Jehel L, Coroller-Bequet A. Specificity of the links between workplace harassment and PTSD: primary results using court decisions, a pilot study in France. Int Arch Occup Environ Health. 2009;82:663–8. doi: 10.1007/s00420-008-0370-9. [DOI] [PubMed] [Google Scholar]
- 3.Yuii K, Suzuki M, Kurachi M. Stress sensitization in schizophrenia. Ann N Y Acad Sci. 2007;1113:276–90. doi: 10.1196/annals.1391.013. [DOI] [PubMed] [Google Scholar]
- 4.Hunter SC, Durkin K, Heim D, Howe C, Bergin D. Psychosocial mediators and moderators of the effect of peer-victimization upon depressive symptomatology. J Child Psychol Psychiatry. doi: 10.1111/j.1469-7610.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 5.Ebner K, Wotjak CT, Landgraf R, Engelmann M. Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm Behav. 2005;47:14–21. doi: 10.1016/j.yhbeh.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiol Behav. 1990;47:949–56. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- 7.Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm Behav. 1991;25:206–16. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- 8.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–93. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 9.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–42. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Agid O, Shapira B, Zislin J, Ritsner M, Hanin B, Murad H, et al. Environment and vulnerability to major psychiatric illness: a case control study of early parental loss in major depression, bipolar disorder and schizophrenia. Mol Psychiatry. 1999;4:163–72. doi: 10.1038/sj.mp.4000473. [DOI] [PubMed] [Google Scholar]
- 11.Agid O, Kohn Y, Lerer B. Environmental stress and psychiatric illness. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2000;54:135–41. doi: 10.1016/S0753-3322(00)89046-0. [DOI] [PubMed] [Google Scholar]
- 12.Huhman KL, Jasnow AM. Conditioned Defeat. In: Nelson RJ, editor. Biology of Aggression. New York: Oxford University Press, Inc; 2006. pp. 295–326. [Google Scholar]
- 13.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–25. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 14.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 13:745–52. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covington HE, 3rd, Maze I, Laplant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant Actions of Histone Deacetylase Inhibitors. J Neurosci. 2009;29:11451–60. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991;38:315–20. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 20.Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–50. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- 21.Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn Mem. 2010;17:109–16. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–59. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 25.Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- 26.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–52. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 27.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 28.Cardinal RN, Winstanley CA, Robbins TW, Everitt BJ. Limbic corticostriatal systems and delayed reinforcement. Ann N Y Acad Sci. 2004;1021:33–50. doi: 10.1196/annals.1308.004. [DOI] [PubMed] [Google Scholar]
- 29.Bradfield LA, McNally GP. The role of nucleus accumbens shell in learning about neutral versus excitatory stimuli during Pavlovian fear conditioning. Learn Mem. 17:337–43. doi: 10.1101/lm.1798810. [DOI] [PubMed] [Google Scholar]
- 30.Antoniadis EA, McDonald RJ. Fornix, medial prefrontal cortex, nucleus accumbens, and mediodorsal thalamic nucleus: roles in a fear-based context discrimination task. Neurobiol Learn Mem. 2006;85:71–85. doi: 10.1016/j.nlm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Josselyn SA, Falls WA, Gewirtz JC, Pistell P, Davis M. The nucleus accumbens is not critically involved in mediating the effects of a safety signal on behavior. Neuropsychopharmacology. 2005;30:17–26. doi: 10.1038/sj.npp.1300530. [DOI] [PubMed] [Google Scholar]
- 32.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–26. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 33.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, et al. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–9. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Landau IT. Light-dark rhythms in aggressive behavior of the male golden hamster. Physiol Behav. 1975;14:767–74. doi: 10.1016/0031-9384(75)90068-2. [DOI] [PubMed] [Google Scholar]
- 35.Nascimento JO, Zangrossi H, Jr, Viana MB. Effects of reversible inactivation of the dorsomedial hypothalamus on panic- and anxiety-related responses in rats. Braz J Med Biol Res. 2010;43:869–73. doi: 10.1590/s0100-879x2010007500075. [DOI] [PubMed] [Google Scholar]
- 36.Minano FJ, Meneres Sancho MS, Sancibrian M, Salinas P, Myers RD. GABAA receptors in the amygdala: role in feeding in fasted and satiated rats. Brain Res. 1992;586:104–10. doi: 10.1016/0006-8993(92)91377-q. [DOI] [PubMed] [Google Scholar]
- 37.Schwienbacher I, Fendt M, Richardson R, Schnitzler HU. Temporary inactivation of the nucleus accumbens disrupts acquisition and expression of fear-potentiated startle in rats. Brain Res. 2004;1027:87–93. doi: 10.1016/j.brainres.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 38.Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–34. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Adams DB. Brain mechanisms of aggressive behavior: an updated review. Neurosci Biobehav Rev. 2006;30:304–18. doi: 10.1016/j.neubiorev.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Adams DB. Brain mechanisms for offense, defense, and submission. The Behavioral and Brain Sciences. 1979;2:201–13. [Google Scholar]
- 41.Canteras NS, Goto M. Fos-like immunoreactivity in the periaqueductal gray of rats exposed to a natural predator. Neuroreport. 1999;10:413–8. doi: 10.1097/00001756-199902050-00037. [DOI] [PubMed] [Google Scholar]
- 42.Motta SC, Goto M, Gouveia FV, Baldo MV, Canteras NS, Swanson LW. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci U S A. 2009;106:4870–5. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, et al. Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci Lett. 2003;345:145–8. doi: 10.1016/s0304-3940(03)00415-4. [DOI] [PubMed] [Google Scholar]
- 44.Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: effects on responsivity to a Cat, Cat odor, and nonpredator threat. Neurosci Biobehav Rev. 2005;29:1243–53. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 45.Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, et al. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–69. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–9. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 47.Albert DJ, Walsh ML, Ryan J, Siemens Y, White R. Mouse killing in rats induced by lesions of the medial hypothalamus or medial accumbens: short-term preoperative exposure to a mouse does not suppress the killing. Behav Neural Biol. 1983;38:113–9. doi: 10.1016/s0163-1047(83)90444-2. [DOI] [PubMed] [Google Scholar]
- 48.Lee SC, Yamamoto T, Ueki S. Characteristics of aggressive behavior induced by nucleus accumbens septi lesions in rats. Behav Neural Biol. 1983;37:237–45. doi: 10.1016/s0163-1047(83)91254-2. [DOI] [PubMed] [Google Scholar]
- 49.Albert DJ, Chew GL. The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav Neural Biol. 1980;30:357–88. doi: 10.1016/s0163-1047(80)91247-9. [DOI] [PubMed] [Google Scholar]
- 50.Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci U S A. 2010;107:12393–8. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mogenson GJ, Jones DL, Yim CY. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 52.Kiyatkin EA. Dopamine in the nucleus accumbens: cellular actions, drug- and behavior-associated fluctuations, and a possible role in an organism’s adaptive activity. Behav Brain Res. 2002;137:27–46. doi: 10.1016/s0166-4328(02)00283-8. [DOI] [PubMed] [Google Scholar]
- 53.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 54.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–7. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 56.West AR, Floresco SB, Charara A, Rosenkranz JA, Grace AA. Electrophysiological interactions between striatal glutamatergic and dopaminergic systems. Ann N Y Acad Sci. 2003;1003:53–74. doi: 10.1196/annals.1300.004. [DOI] [PubMed] [Google Scholar]
- 57.Groenewegen HJ, Wright CI, Beijer AV. The nucleus accumbens: gateway for limbic structures to reach the motor system? Prog Brain Res. 1996;107:485–511. doi: 10.1016/s0079-6123(08)61883-x. [DOI] [PubMed] [Google Scholar]
- 58.Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 59.Morin LP, Wood RJ. Atlas of the golden hamster brain. San Diego: Academic Press; 2001. [Google Scholar]