Abstract
Physiological investigations of snakes have established the importance of heart position and pulmonary structure in contexts of gravity effects on blood circulation. Here we investigate morphological correlates of cardiopulmonary physiology in contexts related to ecology, behavior and evolution. We analyze data for heart position and length of vascular lung in 154 species of snakes that exhibit a broad range of characteristic behaviors and habitat associations. We construct a composite phylogeny for these species, and we codify gravitational stress according to species habitat and behavior. We use conventional regression and phylogenetically independent contrasts to evaluate whether trait diversity is correlated with gravitational habitat related to evolutionary transitions within the composite tree topology. We demonstrate that snake species living in arboreal habitats, or which express strongly climbing behaviors, possess relatively short blood columns between the heart and the head, as well as relatively short vascular lungs, compared to terrestrial species. Aquatic species, which experience little or no gravity stress in water, show the reverse – significantly longer heart–head distance and longer vascular lungs. These phylogenetic differences complement the results of physiological studies and are reflected in multiple habitat transitions during the evolutionary histories of these snake lineages, providing strong evidence that heart–to–head distance and length of vascular lung are co–adaptive cardiopulmonary features of snakes.
Keywords: heart, lung, circulation, phylogeny, hydrostatic pressure, aquatic, arboreal
1. Introduction
Gravity profoundly affects the biomechanics and body fluid dynamics of terrestrial vertebrates that are long, tall, and especially upright in posture. Successful living on land, therefore, requires adaptation of form and function in gravity-sensitive systems such as the blood circulation and musculoskeletal anatomy of larger species (Lillywhite, 1996; Vogel, 2003). Cardiovascular adaptations to gravity have evolved over millions of years and are more pronounced in taller or elongate terrestrial species having greater gradients of blood pressure between their heart, head, and limb or tail extremities (Hargens, 2007). Humans, snakes and giraffes have provided important animal models in which gravitational adaptations can be appreciated moreso than in animals of short stature. These species exhibit mechanisms to provide adequate blood flow to their heads, while restricting blood flow and swelling in dependent tissues (Hargens et al., 1987, 2007; Rowell, 1993; Lillywhite, 2005). On the other hand, similar functional adaptations are not present in fully aquatic snakes, which succumb quickly to the effects of gravity when tilted vertically outside of water (Lillywhite and Pough, 1983). In water, however, the external gradient in hydrostatic pressure nearly matches the gravitational gradient of the blood column, effectively producing a near-weightless environment.
In vertical blood columns such as the vasculature of tall animals, the gravity vector acts parallel to the vertical height of the body and uniformly increases pressure at progressively lower points in the fluid column. This influence tends to elevate capillary filtration pressures that promote tissue edema. Blood also tends to pool in compliant vessels, especially veins, but can be opposed by a tight tissue space and muscular, low compliance arteries. If, however, pooling of blood in dependent vasculature reduces venous return to the heart significantly, decreased cardiac filling may reduce cardiac outflow and arterial pressure required to move blood against gravity (Seymour et al., 1993; Lillywhite, 1996). On the uphill side of the heart, diminished arterial pressure can, at worst, lead to collapse of vessels and diminished, or zero, blood flow to the head (Seymour et al., 1993; Lillywhite and Donald, 1994; Seymour 2000). Natural selection for countermeasures to gravity stress on blood circulation largely involves character states that are associated with these linked problems. These countermeasures appear to be convergent in elongate vertebrates such as snakes and upright mammals and include levels of arterial pressure, neural regulation of heart function and vascular resistance, gradients of structural and functional features of blood vessels, a morphological “antigravity suit” involving tight perivascular tissues, and behavioral assistance of the return of venous blood to the heart (Hargens et al., 1987, 2007; Rowell, 1993; Lillywhite, 1995, 1996, 2005).
Snakes are elongate in body form and therefore potentially subject to shifts of blood volume and disturbance to cardiovascular function when they assume upright or vertical postures. More than 3,000 species of snakes are recognized, and the earliest fossil species date from the mid-Cretaceous approximately 100 million years ago (Caldwell, 2007). Extant species of snakes occupy a diversity of habitats, and several lineages have evolved fully or semi-aquatic forms while others have become highly arboreal (Greene, 1997). Therefore, the evolutionary radiation of snakes provides living material from a natural experiment in long-term responses to different gravitational environments. From a comparative and phylogenetic perspective, snakes include forms that routinely experience gravity stress, such as in climbing (scansorial) species, especially climbing in trees (arboreal), as well as others that might be regarded as evolutionarily “deconditioned” to gravity, such as fully aquatic species. Thus, within a single taxon that has a body form sensitive to gravity, it is possible to sample a range of gravitational adaptations attributable to diversification of habitat demands and behaviors (Lillywhite, 2005).
Cardiopulmonary function has been shown to be gravity–sensitive in mammals and other vertebrates, especially snakes (Lillywhite, 2005). A relation between gravity and anatomic position of the heart has been demonstrated in comparative studies of cardiovascular function in snakes: the hearts of aquatic species are significantly closer to the body center than are those of arboreal and scansorial species, while semi–aquatic and relatively non–climbing terrestrial species appear intermediate (Seymour and Lillywhite, 1976; Lillywhite, 1987; Seymour, 1987). At extremes, the heart occupies positions as little as 15% of the total body length from the head in some arboreal species, whereas the position is nearly 45% of the total body length from the head in aquatic species. The relative heart position is independent of body mass and length, and there is no apparent tendency for a reduction of heart–head distance in longer snakes within a habitat category (Seymour, 1987; Gartner et al., 2010). In terrestrial snakes, the increased hydrostatic pressure of the blood column is compensated by increased systemic arterial blood pressure that leads to greater heart work; conversely in aquatic species, a heart position near the body center potentially reduces the work necessary to perfuse the body, and working against gravity is not an issue (Seymour, 1987).
The lungs of snakes are elongated structures with a single, large axial chamber in most species (Wallach, 1998). They are divisible into anatomically and functionally discrete segments. The respiratory region is highly vascular and is termed the vascular lung for purposes of the present discussion. The remainder of the lung, which has a lumen continuous with the vascular segment, virtually lacks respiratory parenchyma and continues for variable distances as a saccular lung. While the entire pulmonary structure extends for much of the length of the body cavity, the relative lengths of the vascular and saccular segments vary considerably among species. In arboreal and scansorial species, the vascular segment has been shown always to be very short, beginning usually near the heart and extending less than 20% of the total body length. In some species, the vascular segment is less than 9% of the total body length (Wallach 1998). On the other hand, vascularized parenchyma in lungs of totally aquatic species extends a much greater length of the body cavity, and in some species occupies virtually the entire body cavity. This includes a vascular portion anterior to the heart, called the tracheal lung (Cope, 1894). The differences in vascular segments appear to represent an absolute constraint on the length of pulmonary vasculature in the scansorial species, for gravitational pressures develop in the pulmonary vasculature in proportion to the absolute height of the blood column and can easily exceed the threshold for creating serious pulmonary edema (Lillywhite, 1987; Smits, 1989). Filtration of fluid into the reptilian lung has been estimated to exceed that of mammals by two orders of magnitude (Smits, 1989), and the leakiness of reptilian lungs has been characterized as a “wet lung” syndrome (Burggren, 1982). When sea snakes are tilted head-up outside of water, the lung develops severe edema and even capillary rupture in the dependent segment (Lillywhite, 2005).
Recently Gartner et al. (2010) analyzed a large data set relating heart position with habitat and phylogeny in 155 snake species. Their phylogenetic analysis indicated that aspects of both habitat and clade predicted heart position, but their analysis did not include fully aquatic species which represent an important end of a gravitational habitat spectrum. Here we examine the evolution and correlation of gravity–sensitive characters related to heart and lung function in a sample of snake species representing a broad range of taxa, behaviors, and habitats, including at least six transitions from terrestrial to aquatic environments. We test the hypothesis that anterior heart position and short vascular lungs evolve together in contexts of gravity stress. We also use comparative phylogenetic methods to examine whether these two characters are correlated with gravitational habitat, independent of phylogeny. The analysis represents a robust example of character state evolution under strong influence of the environment in context of correlated structure and function. For clarity of presentation, enhanced insight, and robust analysis, we evaluate data using both conventional regression and comparative phylogenetic methods.
2. Materials and Methods
2.1 Data on heart position and lung morphology
Data on heart and lung position were obtained opportunistically during numerous prior investigations into the physiology and anatomy of snakes, attributable to H. B. Lillywhite and R. S. Seymour. Some of these data have been published earlier (Seymour, 1987), whereas roughly 75% of the data are previously unpublished. The data represent 588 measurements of heart position in 154 species representing 8 families of snakes, and 304 measurements of lung position in 70 species representing 6 families (Table 1). We calculated mean values for each species, based on numbers of individuals that ranged from 1 to 29 (mean = 4 ± 0.35 SE). We used data only for adult animals to minimize bias related to possible ontogenetic allometry.
Table 1.
Measurements of heart–to–head distance and length of vascular lung, each expressed as a decimal fraction of the total body length. The behavioral rank specifies the gravitational habitat as described in the text.
| Familyspecies | n | Behavioral rank1 | Heart–to–head distance | Length of vascular Lung | Body length, cm |
|---|---|---|---|---|---|
| Acrochordidae | |||||
| Acrochordus arafurae | 14 | 0 | .37 | --- | 131 |
| Acrochordus granulatus | 10 | 0 | .43 | .74 | 60.8 |
| Acrochordus javanicus | 1 | 0 | .39 | .728 | 135 |
| Boidae | |||||
| Boa constrictor | 5 | 1 | .267 | --- | 100.5 |
| Candoia carinata | 2 | 2 | .26 | --- | 78 |
| Corallus caninus | 4 | 2 | .232 | --- | 69.6 |
| Corallus hortulanus | 3 | 2 | .252 | --- | 126.7 |
| Epicrates cenchria | 3 | 1 | .226 | --- | 99 |
| Colubridae | |||||
| Ahaetulla nasuta | 2 | 2 | .24 | .07 | 105 |
| Ahaetulla prasinus | 3 | 2 | .23 | --- | 138 |
| Arizona elegans | 1 | 1 | .19 | .13 | 107 |
| Boiga dendrophila | 6 | 2 | .17 | --- | 126 |
| Boiga irregularis | 6 | 2 | .19 | --- | 132 |
| Carphophis amoenus | 1 | 1 | .195 | .128 | 27.2 |
| Chrysopelea ornata | 1 | 2 | .16 | --- | 105 |
| Coelognathus radiata | 2 | 2 | .18 | .097 | 117 |
| Coluber constrictor | 2 | 2 | .14 | .095 | 122 |
| Dendrelaphis pictus | 1 | 2 | .16 | --- | 76 |
| Dendrelaphis punctulatus | 6 | 2 | .15 | --- | 124 |
| Dendrelaphis calligastra | 3 | 2 | .15 | --- | 103 |
| Dendrelaphis caudolineata | 2 | 2 | .185 | --- | 84.75 |
| Dendrophidion vinitor | 3 | 2 | .147 | --- | 99.8 |
| Diadophis punctatus | 8 | 1 | .16 | .171 | 25.6 |
| Drymarchon corais | 4 | 2 | .156 | --- | 124.9 |
| Drymobius margaritiferus | 2 | 2 | .147 | --- | 90.5 |
| Drymobius chloroticus | 3 | 2 | .134 | --- | 102.3 |
| Farancia erytrogramma | 1 | 1 | .189 | .168 | 98 |
| Farancia abacura | 2 | 1 | .178 | .248 | 87.8 |
| Gonyosoma oxycephalum | 4 | 2 | .184 | .067 | 167 |
| Heterodon platirhinos | 1 | 1 | .229 | .221 | 24 |
| Imantodes cenchoa | 4 | 2 | .28 | --- | 117.9 |
| Lampropeltis extenuatum | 1 | 1 | .188 | .129 | 48 |
| Lampropeltis getula | 2 | 1 | .15 | .12 | 101.8 |
| Leptophis ahaetulla | 1 | 2 | .151 | .044 | 192 |
| Masticophis flagellum | 7 | 1 | .166 | .112 | 110.7 |
| Nerodia rhombifer | 1 | 1 | .159 | .20 | 132 |
| Nerodia sipedon | 1 | 1 | .15 | --- | 101 |
| Nerodia taxispilota | 4 | 1 | .19 | .086 | 93 |
| Opheodrys aestivus | 11 | 2 | .146 | .067 | 65 |
| Oxybelis aeneus | 4 | 2 | .178 | --- | 136.3 |
| Oxybelis fulgidus | 4 | 2 | .172 | --- | 158.3 |
| Pantherophis obsoletus | 19 | 2 | .172 | .10 | 139.2 |
| Philodryas baroni | 3 | 2 | .166 | .09 | 148.3 |
| Pituophis melanoleucus | 5 | 1 | .189 | .116 | 142 |
| Pseustes poecilonotus | 3 | 2 | .144 | .069 | 145 |
| Ptyas carinata | 1 | 2 | .16 | .104 | 198 |
| Ptyas korros | 1 | 2 | .18 | --- | 93 |
| Regina alleni | 2 | 1 | .184 | .175 | 53.7 |
| Spilotes pullatus | 8 | 2 | .156 | .099 | 232.8 |
| Storeria dekayi | 1 | 1 | .134 | .077 | 26 |
| Tantilla relicta | 2 | 1 | .187 | .121 | 16.6 |
| Tropidonophis mairii | 4 | 1 | .14 | --- | 48.9 |
| Uromacer frenatus | 1 | 2 | .146 | .06 | 151 |
| Uromacer oxyrhynchus | 1 | 2 | .15 | .056 | 116.5 |
| Xenocrophis flavipunctatus | 1 | 1 | .145 | .08 | 69 |
| Cylindrophiidae | |||||
| Cylindrophis ruffus | 1 | 1 | .123 | .152 | 101.8 |
| Elapidae | |||||
| Acalyptophis peronii | 3 | 0 | --- | .69 | 85.2 |
| Acanthophis antarcticus | 11 | 1 | .20 | --- | 56.7 |
| Acanthophis pyrrhus | 2 | 1 | .18 | --- | 58.5 |
| Aipysurus eydouxii | 2 | 0 | .23 | --- | 73.3 |
| Aipysurus laevis | 28 | 0 | .233 | .592 | 92.7 |
| Astrotia stokesii | 3 | 0 | .275 | .47 | 133.7 |
| Austrelaps superbus | 15 | 2 | .16 | --- | 55.3 |
| Cacophis squamulosus | 2 | 1 | .15 | --- | 72.6 |
| Demansia olivacea | 1 | 1 | .15 | --- | 122 |
| Denisonia devisi | 3 | 1 | .16 | --- | 36.8 |
| Disteira major | 1 | 0 | .36 | --- | 91 |
| Echiopsis curta | 3 | 1 | .17 | --- | 42.5 |
| Emydocephalus annulatus | 4 | 0 | --- | .607 | 80.7 |
| Emydocephalus ijimae | 1 | 0 | .26 | .57 | 43 |
| Furina diadema | 6 | 1 | .19 | --- | 38.8 |
| Furina tristis | 2 | 1 | .23 | --- | 86 |
| Hemiaspis signata | 2 | 1 | .16 | --- | 52.9 |
| Hoplocephalus stephensii | 2 | 2 | .17 | --- | 87.8 |
| Hydrelaps darwiniensis | 4 | 0 | .26 | --- | 39.1 |
| Chitulia belcheri | 6 | 0 | .38 | .78 | 96.5 |
| Hydrophis cyanocinctus | 6 | 0 | .42 | --- | 56.8 |
| Hydrophis elegans | 1 | 0 | .31 | --- | 198 |
| Hydrophis fasciatus | 1 | 0 | .34 | --- | 45.5 |
| Chitulia inornatus | 5 | 0 | .29 | --- | 61.4 |
| Chitulia ornatus | 29 | 0 | .33 | --- | 64.5 |
| Lapemis hardwickii | 4 | 0 | .28 | --- | 63.5 |
| Laticauda colubrina | 3 | 0 | .36 | .67 | 67 |
| Laticauda laticaudata | 4 | 0 | .22 | .44 | 90.8 |
| Laticauda semifasciata | 4 | 0 | .21 | .41 | 103 |
| Micrurus fulvius | 3 | 1 | .212 | .148 | 63.2 |
| Micrurus narduccii | 2 | 1 | .21 | --- | 70.8 |
| Micrurus spixii | 3 | 1 | .198 | --- | 98.7 |
| Naja atra | 1 | 1 | .186 | .093 | 118.2 |
| Naja haje | 1 | 2 | .144 | --- | 90 |
| Naja kaouthia | 1 | 1 | .179 | .112 | 134 |
| Notechis scutatus | 12 | 2 | .16 | .18 | 87.9 |
| Oxyuranus microlepidotus | 3 | 1 | .17 | --- | 166 |
| Oxyuranus scutellatus | 3 | 1 | .20 | --- | 210 |
| Pelamis platurus | 9 | 0 | .31 | .788 | 64 |
| Pseudechis australis | 3 | 1 | .18 | --- | 144 |
| Pseudechis porphyriacus | 9 | 1 | .19 | .161 | 80.1 |
| Pseudonaja affinis | 5 | 1 | .17 | --- | 107 |
| Pseudonaja nuchalis | 5 | 1 | .18 | --- | 138 |
| Pseudonaja textilis | 10 | 1 | .17 | .139 | 142.6 |
| Rhinoplocephalus bicolor | 2 | 1 | .18 | --- | 34 |
| Rhinoplocephalus nigrescens | 4 | 1 | .16 | --- | 48.1 |
| Simoselaps fasciolatus | 1 | 1 | .19 | --- | 29.1 |
| Simoselaps incinctus | 1 | 1 | .17 | --- | 27.8 |
| Simoselaps littoralis | 3 | 1 | .15 | --- | 21.5 |
| Suta flagellum | 2 | 1 | .19 | --- | 25.6 |
| Suta suta | 1 | 1 | .20 | --- | 40 |
| Tropidechis carinatus | 4 | 1 | .16 | --- | 82.1 |
| Vermicella annulata | 4 | 1 | .19 | --- | 48.2 |
| Homalopsidae | |||||
| Cerberus australis | 3 | 1 | .25 | --- | 50 |
| Cerberus rhynchops | 11 | 0 | .24 | .283 | 64.2 |
| Enhydris enhydris | 2 | 0 | .37 | .442 | 55 |
| Enhydris polylepis | 4 | 1 | .29 | --- | 66.4 |
| Fordonia leucobalia | 5 | 1 | .20 | --- | 54.5 |
| Homalopsis buccata | 1 | 0 | .29 | .377 | 34.5 |
| Loxocemidae | |||||
| Loxocemis bicolor | 3 | 1 | .241 | --- | 85 |
| Pythonidae | |||||
| Antaresia childreni | 5 | 1 | .23 | --- | 76.9 |
| Aspidites melanocephalus | 5 | 1 | .23 | --- | 131 |
| Aspidites ramsayi | 3 | 1 | .22 | --- | 195 |
| Liasis fuscus | 4 | 1 | .23 | --- | 149 |
| Liasis olivaceus | 1 | 1 | .24 | --- | 240 |
| Morelia spilota | 9 | 2 | .25 | .09 | 137 |
| Morelia viridis | 1 | 2 | .16 | --- | 155 |
| Python molurus | 1 | 1 | .259 | .068 | 73.5 |
| Broghammerus reticulatus | 2 | 1 | .23 | .09 | 103 |
| Viperidae | |||||
| Agkistrodon contortrix | 3 | 1 | .29 | .28 | 80.7 |
| Agkistrodon piscivorus | 12 | 1 | .32 | .324 | 100.8 |
| Bitis arietans | 3 | 1 | .352 | .242 | 109.7 |
| Bitis nasicornis | 2 | 1 | .303 | .225 | 104 |
| Bitis gabonica | 3 | 1 | .339 | .222 | 113 |
| Bothrops atrox | 1 | 1 | .27 | --- | 175 |
| Bothriechis schlegelii | 3 | 2 | .278 | .26 | 70.2 |
| Cerastes sp. | 1 | 1 | .28 | --- | 43.3 |
| Crotalus atrox | 1 | 1 | .382 | --- | 123 |
| Crotalus cerastes | 1 | 1 | .35 | --- | 50 |
| Crotalus horridus | 3 | 1 | .358 | .297 | 86.6 |
| Crotalus mitchelli | 1 | 1 | .39 | .487 | 115 |
| Crotalus ruber | 3 | 1 | .39 | .36 | 101.4 |
| Crotalus viridis | 17 | 1 | .39 | .36 | 88.8 |
| Cryptelytrops albolabris | 3 | 2 | .31 | .276 | 56 |
| Cryptelytrops macrops | 1 | 2 | .29 | .24 | 58.5 |
| Cryptelytrops purpureomaculatus | 3 | 2 | .319 | .278 | 88.9 |
| Daboia russellii siamensis | 1 | 1 | .35 | .22 | 35.5 |
| Echis carinatus | 1 | 1 | .25 | --- | 44.5 |
| Lachesis muta | 2 | 1 | .20 | --- | 189 |
| Macrovipera lebetina | 4 | 1 | .335 | .28 | 91 |
| Montivipera xanthina | 4 | 1 | .341 | --- | 36.7 |
| Popeia popeorum | 1 | 2 | .338 | .284 | 37 |
| Rhinocerophis alternatus | 1 | 1 | .335 | .229 | 68.7 |
| Sistrurus miliarius | 3 | 1 | .327 | .313 | 45.3 |
| Typhlopidae | |||||
| Austrotyphlops australis | 2 | 1 | .35 | --- | 32.8 |
| Austrotyphlops bituberculatus | 2 | 1 | .29 | --- | 25.6 |
| Ramphotyphlops braminus | 6 | 1 | .294 | --- | 12.9 |
| Ramphotyphlops polygrammicus | 2 | 1 | .31 | --- | 51 |
Behavioral ranks:
Aquatic or highly aquatic, stenotopic = 0
Amphibious, terrestrial, eurytopic = 1
Arboreal/scansorial, stenotopic = 2
In all cases measurements were made on fresh tissue with the body laid out straight, either during the performance of surgical procedures in anesthetized snakes or on freshly euthanized snakes. Total body length measurements were made from the tip of the snout or eye to the tip of the tail. Heart position was measured as the distance from the tip of the snout to the center of the ventricle. With respect to pulmonary measurements, the left lung is greatly reduced, vestigial or absent in most snake species, and the right lung is elongated as the prominent single functional lung. Thus, in a gravitational context we used measurements for the right lung, which is the functional elongated structure of interest. The vascular lung position was measured from the anterior extension of vascularized tissue to the most posterior extension of vascularized tissue. In many cases these measurements corresponded to the visible presence of respiratory faveoli, but in other cases these gradually gave way to increasingly simple wall tissue with diminishing vascularization. In cases where there was evident gradation from vascular to saccular regions, the visible termination of the pulmonary artery, or its associated branches, was taken as the termination of vascular lung. The termination of the saccular lung was determined by following the structure to the most posterior aspect, evident as a rounded tip but sometimes difficult to see because of the extremely thin and transparent nature of the tissue. In many cases involving euthanized animals, the termination was confirmed by introducing a blue dye into the pulmonary lumen and following its course by gentle palpation and gravity to the posterior termination, which became plainly evident by presence of the dye.
We expressed the relative heart position as the percentage of total body length attributable to the distance from the center of ventricle to the tip of the snout. We expressed the relative vascular lung as the percentage of total body length occupied by the vascular segment as measured from anterior to posterior margins. All data are presented in Table 1.
2.2 Construction of a phylogeny for snakes
A consensus phylogenetic tree of snake interrelationships does not currently exist. Therefore, in order to examine our data within a phylogenetic context we constructed a fully–resolved (e.g., completely bifurcating) composite tree from multiple published molecular phylogenies. Morphology was used, however, for relationships within the genus Laticauda (see below). The composite tree constituted a base phylogeny (Wiens et al., 2008) to which certain additional groups and species were added or expanded (see below and Fig. 1). This base phylogeny is consistent with most other studies in that the relative placement of major higher-level taxonomic groups such as Scolecophidia, Alethinophidia and Colubroidea, as well as many family and subfamily-level relationships, are strongly supported with both molecular (e.g. Lawson et al., 2005; Vidal et al., 2007; Pyron et al., 2011) and morphological (e.g., Lee et al., 2007) characters. Phylogenetic resolution was achieved by selecting branches with highest nodal support (e.g., posterior probability), and by using well–resolved trees from different studies. For different trees containing similar species with the same number of characters (genes), we used the tree with the strongest nodal support and, when available, the more extensive taxon sampling. However, we chose the tree that incorporated more characters to decide between trees with the same number of taxa. Genera were assumed to represent monophyletic groups unless a study suggested otherwise (e.g., Enhydris). Thus, two congeneric taxa were assumed to be sister taxa even if only one of them was included in a phylogenetic study. In three instances (i.e., Dendrelaphis, Chitulia and Micrurus), a genus was placed on the tree but the relationships among the three or four species within that genus were unknown. For these cases, the conspecific relationships were in the end resolved based on biogeographic contiguity. These groupings contain congeners categorized within the same gravitational habitat and results were not affected when all possible alternative polytomy resolutions for conspecific relationships were tested (results not shown).
Figure 1.
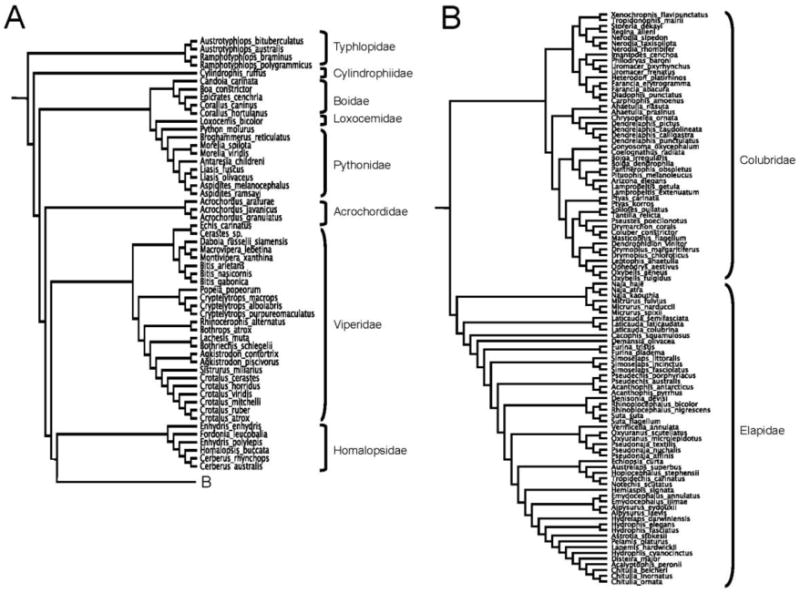
Phylogeny of 154 ophidian taxa that contributed morphological data to this study. The composite tree topology is from multiple sources, as described in the text.
The following studies were used to add taxa to the base phylogeny to construct the composite tree (Fig. 1). The phylogenies of Pyron et al., (2011) and Weins et al., (2008) were used for the relative placement of snake families and subfamilies. The scolecophidian clade was replaced using the taxonomy of Wallach (2006). The phylogeny of Wilcox et al. (2002) was used for placement of Loxocemus. The phylogenies of Rawlings et al. (2008) and Burbrink (2005) were used for relationships within the Pythonidae and Boidae, respectively. The phylogeny of Wüster et al. (2008) was used for placement of true vipers. The phylogenies of Malhotra and Thorpe (2004) and Castoe and Parkinson (2006) were used for Asian pitviper relationships, whereas the phylogeny of Castoe and Parkinson (2006) was used for New World pitviper relationships, and the phylogeny of Fenwick et al. (2009) was used to place Bothrops and related taxa. The phylogeny of Alfaro et al. (2008) was used for homalopsid relationships. Within the Elapidae, the phylogeny of Castoe et al. (2007) was used for placement of the Elapinae, phylogenies of Lukoschek and Keogh (2006) and Metzger et al. (2010) were used for relationships within the Hydrophiinae, and the morphological study by Kharin and Czeblukov (2006) was used for relationships within the genus Laticauda. Within the family Colubridae, the phylogeny of Pyron et al. (2011) was used for colubrine relationships and for placement of Xenochrophis and Dendrelaphis, the phylogeny of Zaher et al. (2008) was used for dipsadine and xenodontine relationships, the phylogeny of Alfaro and Arnold (2001) was used for Thamnophiine snake relationships (i.e., Nerodia, Regina, and Storeria), and the phylogeny of Highton et al. (2002) was used to estimate the position of Pseustes. The composite tree was constructed using MacClade 4.08 (Maddison and Maddison, 2005).
2.3 Designating gravitational habitats
To determine whether heart and lung morphology correlate with gravity stress imposed upon these organs, we categorized species according to three states of what we call “gravitational habitat.” Traditional habitat designations based on environmental characteristics alone (e.g., aquatic, terrestrial, arboreal) do not always well represent the gravitational stresses experienced by snakes. These gravitational stresses arise from behaviors associated with posture and movement, especially climbing, that change the attitude of the lung and the position of the head with respect to the heart. Moreover, detailed natural history information is lacking for many snake species, so we do not divide gravitational habitats too finely. Information on the habits of species was compiled from extensive literature and from observations of snakes in nature by the authors and others.
As a consequence of the foregoing considerations, we assigned species to one of three categories of gravitational habitat. We assigned “0” to species that are stenotopically aquatic or very highly aquatic. We assigned a “1” to species that are eurytopically amphibious or terrestrial. This category included fossorial and ground-dwelling terrestrial species. We assigned a “2” to species that are stenotopically arboreal or scansorial. This category included species that are terrestrial but also climb, either frequently or with fully vertical posture. Adoption of a fully vertical position imposes maximum gravity stress on the blood columns, and such species possess potent neurogenic adjustments of blood flow and pressure in context of such stress (e.g., Lillywhite and Gallagher, 1985; Lillywhite and Donald, 1994).
The multistate coding scheme we employed in this study is novel, i.e. categorizing behavioral traits along a continuum of gravitational habitats (from low-stress aquatic, through mid-stress terrestrial, to potentially high-stress arboreal), and also the range of gravitational stresses (stenotopic vs. eurytopic) commonly experienced. Behavioral traits have long been used in phylogenetic analyses (e.g., de Queiroz and Wimberger, 1993; Goicoechea et al., 2010), and some behavioral traits have been shown to have a heritable component in squamate taxa (Blomberg et al., 2003). The use of habitat breadth to characterize snake ecology, and the recognition of discrete aquatic, terrestrial, and arboreal habitats for individual snake taxa is not without precedent (e.g., Rabb and Marx, 1973; Seymour and Lillywhite, 1976; Gartner et al. 2010).
2.4 Statistical analyses
We used conventional regression and Phylogenetically Independent Contrasts (PIC) analyses to quantify the relationships between phenotypic traits of cardiopulmonary anatomy in snakes. Regression analysis can be used to infer causal relationships between the independent and dependent variables under restricted conditions. A widely recognized problem with conventional regression analysis of data from different species is that phylogenetic relationships among species can inflate the degrees of freedom for traits that evolve deeper in the tree (i.e. exhibit phylogenetic signal) and are at least to some extent phylogenetically conserved. Thus, we used PIC to transform data for each species (at terminal tips of the phylogenetic tree) into values that are statistically independent and identically distributed, using phylogenetic information and a Brownian motion-like model of trait evolution (Felsenstein, 1985; Garland et al., 1992).
The use and appropriateness of PIC is widely debated, owing in part to the necessary assumptions required to perform the analysis. Other approaches are being applied to effect phylogenetic corrections of comparative data, but these methods involve yet further assumptions and fitting procedures that specify regressors and response variables. PIC remains an important and widely used method for phylogenetic correction, and it demonstrates advantages over some recently applied model-fitting approaches (Sieg et al., 2009). We do not take a model fitting approach here because the available methods do not seem biologically relevant, and because they require the assertion of even more as yet unsubstantiated assumptions. In simulations where the exact timings of evolutionary divergences (ancestral nodes) and character states were known, and different rates of evolution were applied, the Felsenstein (1985) PIC method was found to correlate most closely with true contrast values (O'Connor et al., 2007; Sieg et al., 2009).
We first analyzed relationships among variables using ANOVA and conventional regression analysis performed in SAS StatView version 5.0.1. Heart and lung measurements expressed as relative values were analyzed using both log–transformed and non–transformed data. Log transformation did not affect the statistical outcome, and non-transformed data are presented in the graphic figures which illustrate fundamental patterns of trait variation.
Bivariate analyses were also performed in Excel 11.3.6 using both the uncorrected species values (i.e., those of the terminal taxa), and the values of PIC standardized by the standard deviation of branch–lengths (Felsenstein, 1985; Harvey and Pagel, 1991). Analyses of species data were performed using all species for which we had data on relative heart position or relative length of the vascular lung, in addition to gravitational habitat. This allowed us to maximize the power of each separate analysis despite different sample sizes between analyses. All variables were loge–transformed prior to analysis. Analyses of PIC were calculated using the PDAP: PDTREE v. 1.74 module of Mesquite 2.72 (Maddison and Maddison, 2007; Midford et al., 2008), following log–transformation. PIC values were calculated between all sister lineages on the tree, including the assumption that all branch lengths are equal (Grafen, 1989). The null hypothesis of no relationship between absolute values of the standardized phylogenetically independent contrasts and their standard deviations was tested with regressions performed through the origin (Felsenstein, 1985; Garland et al., 1992). We tested for correlations between the contrasts and their standard deviations (SD) to check whether branch length transformations were needed to control type I error rates (Diaz-Uriarte and Garland, 1998). The correlations between absolute values of contrasts and their SD were not significant for either parameter: PDAP diagnosis for heart values, n = 153 contrasts, R2 = 0.004, t = −2.52, F = 6.37, P = 0.13 (2-tailed); for lung values n = 69 contrasts, R2 = 0.006, t = 0.68, F = 0.47, P = 0.49 (2-tailed). Therefore, no branch length transformations were needed. The three gravitationally–defined habitat categories (i.e., stenotopically aquatic, eurytopically amphibious–terrestrial, and stenotopically arboreal–scansorial) were treated as states of a continuous variable, not discrete variables, under the assumption that these states lie along a single environmental gradient of gravity stress. Insofar as our assignment of the gravitational habitat is based on careful consideration of both habitat and behavior, in addition to conservative consideration of the level 1 “terrestrial” category, treating this trait as a continuous (vs. discrete) variable arguably yields a more realistic assignment based on information that is available to us at this time.
3 Results
The mean species values for heart–to–head distance and length of vascular lung, relative to total body length, are given in Table 2 for each of the categories of gravitational habitat. Aquatic snakes have greater values for both relative heart–to–head distance and length of the vascular lung, compared to terrestrial species and arboreal–scansorial species. The vascularized lung extends both anterior and posterior to the heart, which has a relatively central position. In acrochordids the vascular pulmonary tissue extends from the anterior neck (and therefore includes “tracheal lung”) to the terminus of the body cavity, and there is no saccular lung segment. In the sea snakes (laticaudine and hydrophiine species), vascularized pulmonary tissue extends a considerable distance both directions from the heart and there is a relatively short and muscular saccular segment at the posterior terminus. Arboreal–scansorial species have shorter relative heart–head distance and length of the vascular lung, compared to terrestrial and aquatic species. In the former species, the vascular lung tissue is relatively short and located anteriorly in the body, usually at or immediately posterior to the heart. The same is generally true of many terrestrial species of snakes. Further details regarding segmental lung structure can be found in Wallach (1998). Correlation analysis reveals a strong and highly significant correlation between the heart and lung measurements when paired for all the species (r = 0.708, P < 0.0001) (Fig. 2). That is, snakes having short heart–to–head distances also possess short segments of vascular lung.
Table 2.
Mean values ± SE (n, species) for heart–to–head distance and length of vascular lung – both expressed as decimal fraction of the total body length – in three categories of gravitational habitat, as described in the text. ANOVA for log-transformed data indicate that each parameter is statistically different among and between G habitat categories (Fisher's PLSD, P = 0.0021 for differences between terrestrial and arboreal–scansorial snakes; P<0.0001 for all other comparisons).
| G Habitat | Heart–to–head distance Relative to body length | Length of Vascular Lung Relative to body length |
|---|---|---|
| Aquatic | 0.31 ± 0.014 (23) | 0.57 ± 0.041 (15) |
| Terrestrial | 0.23 ± 0.008 (86) | 0.19 ± 0.016 (37) |
| Arboreal–Scansorial | 0.19 ± 0.009 (43) | 0.13 ± 0.019 (20) |
Figure 2.
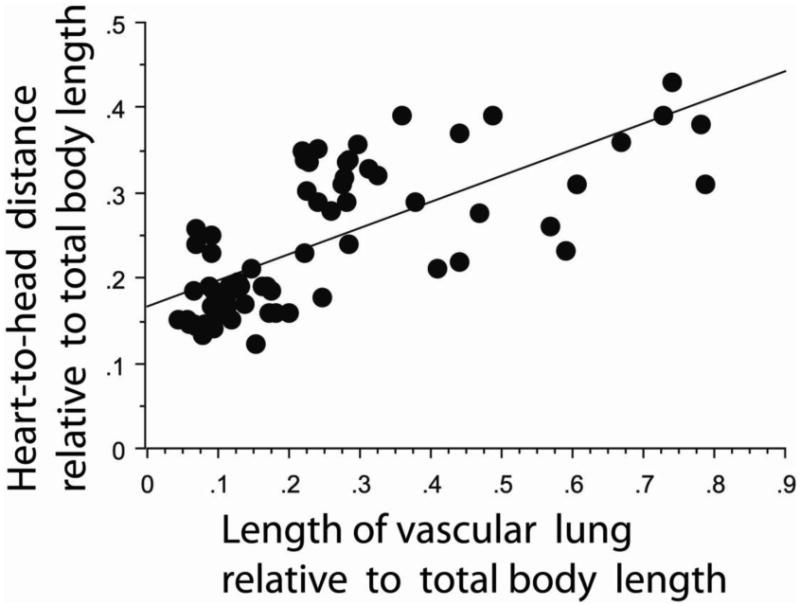
Correlation between heart–to–head distance (HH) and length of vascular lung (VL), both relative to total body length in snake species for which all three parameters were measured. The positive relationship shown is highly significant (HHrel = 0.166 + 0.308 VLrel; r = 0.708, P < 0.0001).
Considering all snakes, the absolute distance for heart position increased with increasing total body length (r2 = 0.602, P < 0.0001) whereas the absolute length of the vascular lung did not change with total body length (r2= 0.027, P = 0.1689) (Fig. 3). The pattern for heart position also occurred within each gravitational habitat (all P<0.0001). For the arboreal–scansorial species, the heart–to–head distance relative to total body length decreased (r2= 0.155, P = 0.0061), whereas this was not true for either the terrestrial or the aquatic groups of snakes (Fig. 4). Also within the scansorial group of snakes the relative length of vascular lung decreased significantly with increasing total body length (r2= −0.462, P = 0.0006), whereas this was not true for terrestrial or aquatic species (Fig. 5). Thus, changes in heart and lung morphology differ between arboreal–scansorial species and the other snakes, especially fully aquatic species. The tendencies for heart–to–head distance and length of vascular lung to elongate with increasing total body length was stronger in aquatic snakes (Figs. 4 and 5).
Figure 3.
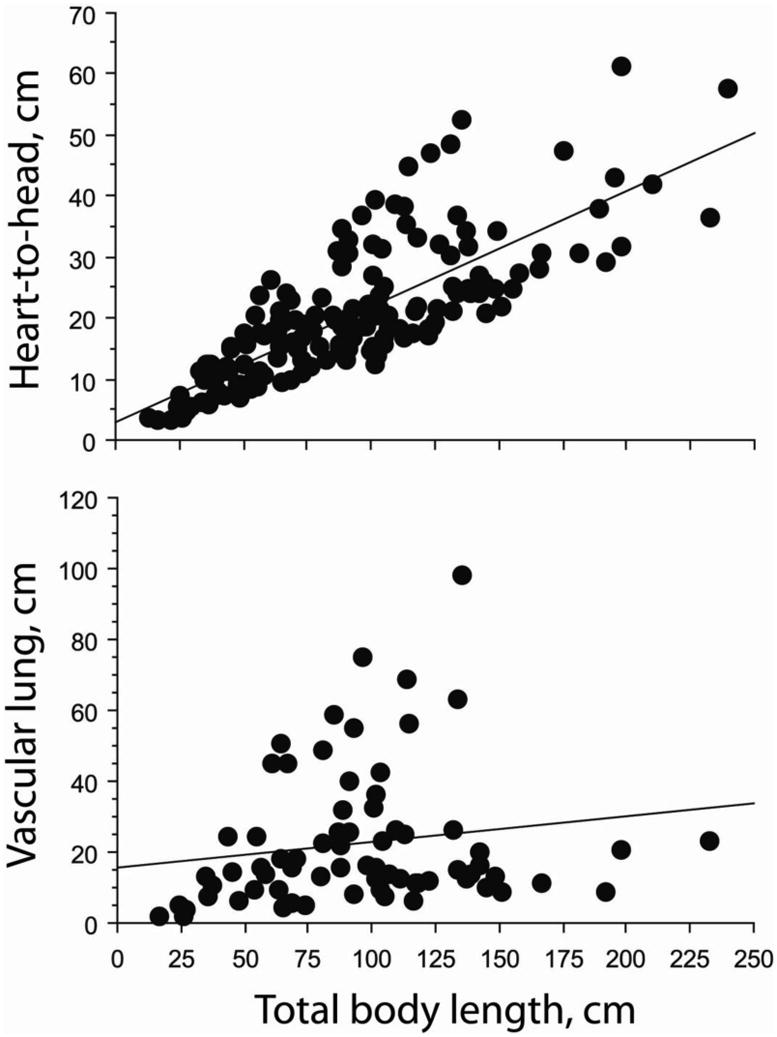
Scatter plot showing relationship between absolute heart–to–head distance (HH) and length of the vascular lung (VL), each plotted in relation total to body length (TL) for all species of snakes in this study. The regression for heart position is significant (HH = 2.983 + 0.189 TL; r2 = 0.602, P < 0.0001), whereas that for vascular lung is not (VL = 15.558 + 0.073 TL; r2 = 0.027, P = 0.169).
Figure 4.
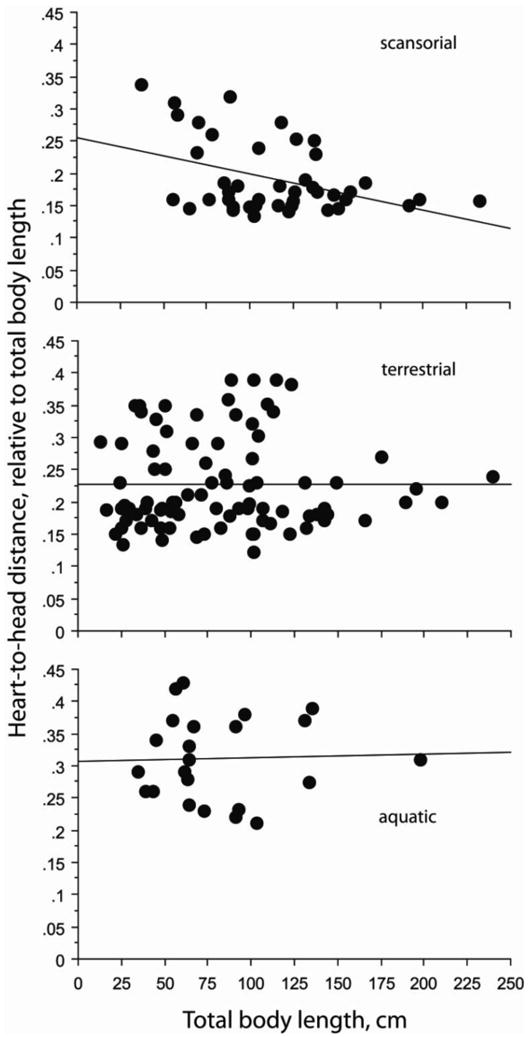
Relationships between heart–to–head position (HH), expressed as decimal fraction of total body length and plotted against total body length (TL) for species of snakes separated by habitat category. Regression equations are: Scansorial HHrel = 0.25 − 0.001 TL; r2 = 0.155, P = 0.0061; Terrestrial HHrel = 0.228 − 9.196 × 10−7 TL; r2 = 3.713 × 10−7, P = 0.996; Aquatic HHrel = 0.306 + 5.963 × 10−5 TL; r2 = 0.001, P = 0.873. Note that only the arboreal-scansorial snakes exhibit a tendency for significant reduction of HH with increasing total body length, and the aquatic species exhibit a tendency toward reversal of this pattern.
Figure 5.
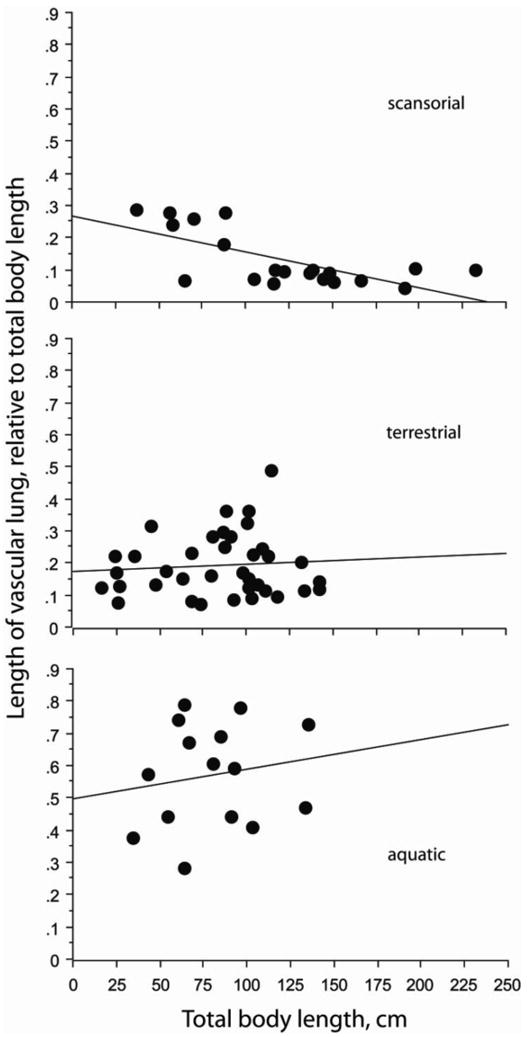
Relationships between the length of vascular lung (VL), expressed as decimal fraction of total body length and plotted against total body length (TL) for species of snakes separated by habitat category. Regression equations are: Scansorial VLrel = 0.267 − 0.001 TL; r2 = 0.462, P = 0.001; Terrestrial HHrel = 0.172 + 2.314 × 10−4 TL; r2 = 0.007, P = 0.621; Aquatic HHrel = 0.499 + 0.001 TL; r2 = 0.031, P = 0.516. Note that only the arboreal–scansorial snakes exhibit a tendency for significant reduction of VL with increasing total body length, and the aquatic species exhibit a tendency toward reversal of this pattern.
Patterns of heart and lung morphology in terrestrial species were intermediate but more similar to those in arboreal–scansorial snakes (Figs. 4 and 5), with exception of viperid species as described below. These tendencies were more pronounced for lung morphology than for heart–to–head distance, and the differences become clear when viperid species are removed from data plots (Fig. 6).
Figure 6.
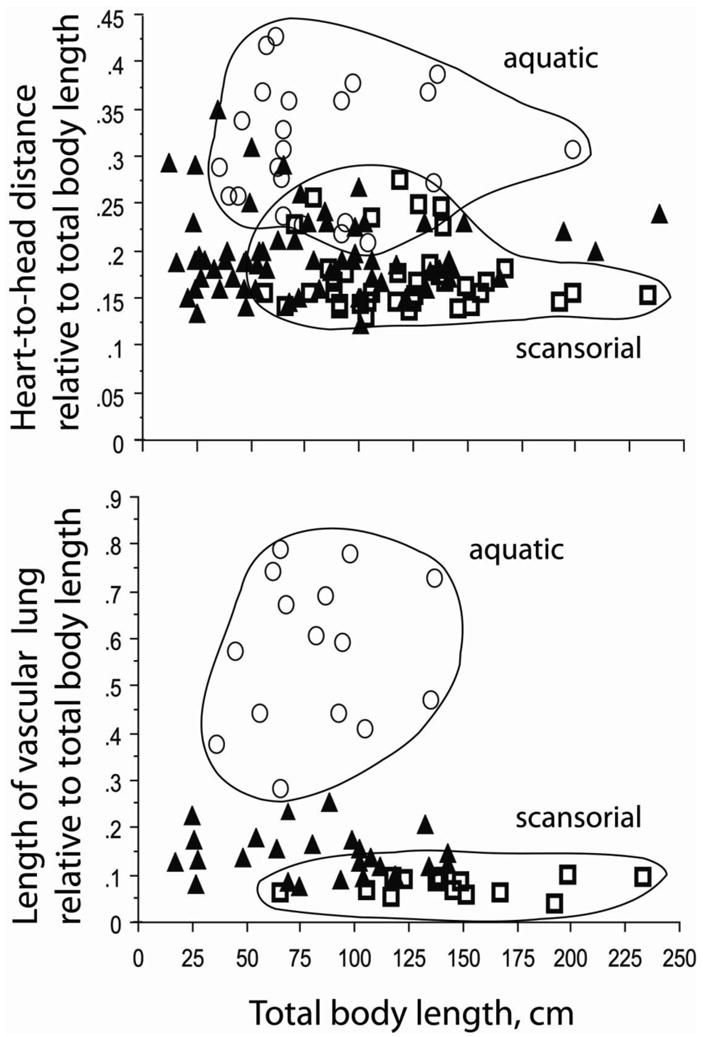
Scatter plots of heart–to–head distance and vascular lung length, expressed as percentage of total body length and plotted in relation to total body length. Envelopes have been drawn around aquatic (○) and scansorial species (□), respectively, to illustrate the separation of data for these two groups. Values for terrestrial species (▲) are intermediate. Data are for all snake taxa, except for viperids as explained in the text.
Considering all species in context of phylogeny, both heart position and the relative length of the vascular lung are positively correlated with gravitational habitat, both in standard regression (HHrel = 0.289 − 0.055 G habitat; r2 = 0.235, P < 0.0001; VLrel = 0.477 − 0.209 G habitat; r2 = 0.539, P < 0.0001; VLrel = 0.477 − 0.209 G habitat; r2 = 0.539, P < 0.0001), and as plots of independent contrasts (HHrel contrasts = 0.001 − 0.029 G habitat; r2 = 0.051, P = 0.006; VLrel contrasts = 0.006 − 0.144 G habitat; r2 = 0.249, P < 0.0001).
Figure 7 illustrates the composite phylogenetic tree with taxonomic distributions of heart position, vascular lung length, and gravitational habitat. Continuous trait data for heart and lung were optimized using squared–change parsimony (Maddison, 1991). There are at least six independent transitions to aquatic habitats wherein the heart position is never near the head, and the vascular lung is never short. There are at least nine independent transitions to arboreal–scansorial habitats wherein the heart position is never at midbody and the lung is never long.
Figure 7.
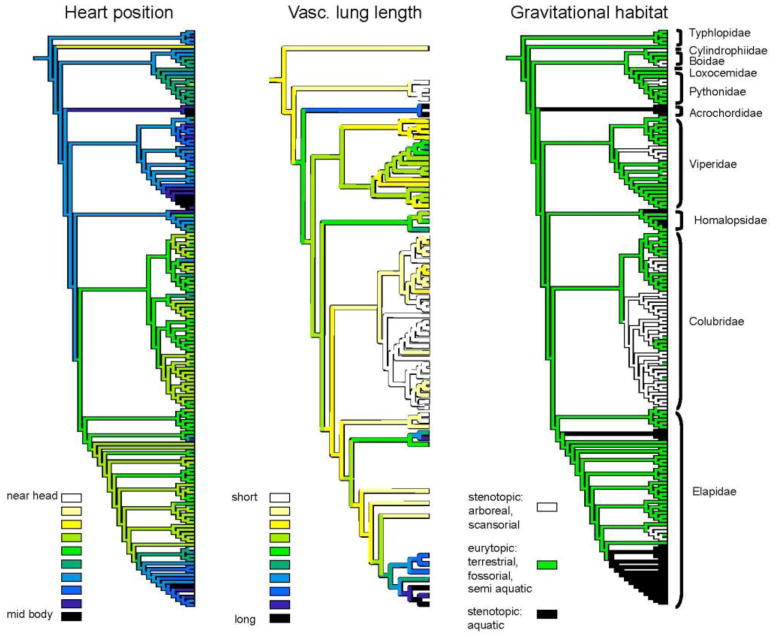
Evolution of physiological traits as optimized using least-squared parsimony on the topology of Fig. 1. See text for further explanation.
The viperids are a special case as a taxonomic group. There is no statistically significant trend of relative heart position with increasing total body length, as is true for non–viperid, terrestrial taxa (Fig. 8), and the trend for relative vascular lung in relation to changes in total body length is opposite to that for combined non–viperid taxa (Fig. 9).
Figure 8.
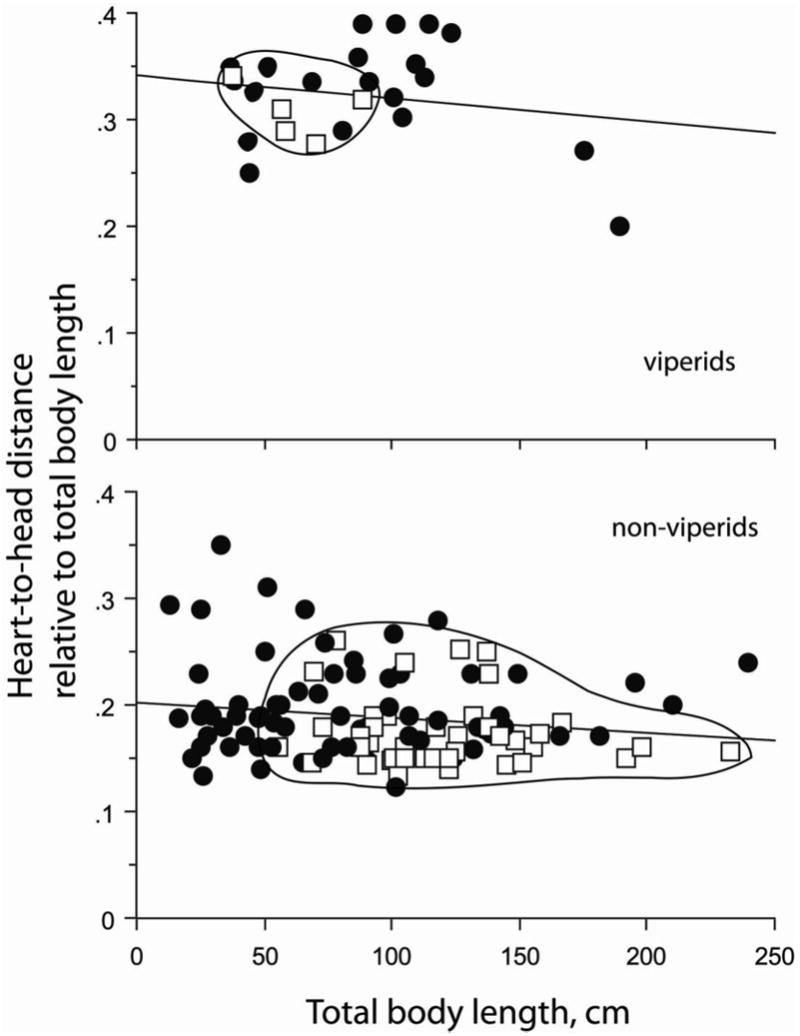
Heart–to–head distance (HH), expressed as a decimal fraction of total body length (TL), for terrestrial viperid and non–viperid species. Arboreal–scansorial species of each group are included, represented by the open squares (□) within envelopes to emphasize separation. The regression equation for viperid species is HHrel = 0.342 − 2.189 × 10−4 TL; r2 = 0.036, P = 0.362. That for the non-viperid species is HHrel = 0.203 − 1.328 × 10−4 TL; r2 = 0.023, P = 0.127.
Figure 9.

Relative length of the vascular lung (VL), expressed as a decimal fraction of total body length (TL), for terrestrial viperid and non–viperid species. Arboreal–scansorial species of each group are included, represented by the open squares (□) within envelopes to emphasize separation. The regression for viperid species is not significant (VLrel = 0.221 + 0.001TL; r2 = 0.104, P = 0.362, P = 0.192), whereas the relative length of vascular lung in other terrestrial species decreases with increasing total body length (VLrel = 0.154 − 3.589 × 10−4 TL; r2 = 0.144, P = 0.017). Note that trends for this character are opposite in viperid compared with non–viperid species of snakes.
4. Discussion
Here we report a new analysis of heart and lung position in snakes. We demonstrate that heart–to–head distance covaries with the length of vascular lung in a broad range of taxa, and we provide strong evidence that both characters are adaptive with respect to gravitational demands related to postural behaviors and environment. Our phylogenetically–informed analysis demonstrates that heart position is related to gravitational habitat and is located farther away from the head in aquatic species and closer to the head in arboreal–scansorial species, compared to terrestrial species (Table 2, Fig. 6). This result reaffirms previous conclusions regarding heart position in snakes (Seymour and Lillywhite, 1976; Seymour, 1987; Lillywhite, 1987, 2005). In a recent study based on phylogenetic methods, it was reported that arboreal species of snakes have relatively posterior hearts compared with terrestrial species (Gartner et al., 2010). However, the conclusions were based on data for heart position relative to snout–vent length, not total body length, which is the appropriate variable because it represents the true length of the cardiovascular system. When the data were corrected to total body length, the difference was not significant, and no valid physiological explanation for posterior hearts could be made (see Lillywhite and Seymour, 2011). Most importantly, that study employed a different data set, one that was taxonomically biased to terrestrial xenodontines and viperids and totally lacking in aquatic species that should be most affected by natural selection due to gravity.
In phylogenetically–informed analyses, the length of the pulmonary vascular column is correlated with gravitational habitat and is relatively shorter in arboreal species as compared with aquatic species. We also demonstrate that snakes having relatively short vascularized respiratory segments of their lungs also have more anteriorly positioned hearts (Fig. 2). Of the two variables, the length of pulmonary vasculature appears to be more sensitive to the effects of gravity (Fig. 6). The allometric pattern for pulmonary vasculature in aquatic species is, in fact, opposite to that of snakes with scansorial behavior in arboreal environments (Fig. 5). That is, relative to total body length, the length of pulmonary vasculature decreases markedly with size increase in arboreal–scansorial species of snakes, but it actually increases with size in aquatic species of snakes (see also Fig. 6). There is a good functional explanation for the more dramatic effects of gravity on the lung. The explanation relies on the knowledge that gravity affects the systemic and pulmonary circulations in different ways that depend on the environment. In air, gravity affects both circuits similarly, by imposing a gravitational hydrostatic pressure on the columns. Blood pooling and increasing transmural pressure gradients occur in the dependent parts. In water, gravity does not affect the systemic circuit much, because the gravitational component of intravascular pressure is approximately matched to the external hydrostatic pressure. Blood is slightly denser than water, and tissues intervening between the blood and water can affect the pressure exerted on the vascular system, but these effects are apparently small. Immersion in water is thought to be nearly equivalent to weightlessness by space physiologists (Norsk, 2005). However, the blood in the pulmonary circuit is exposed to the pulmonary gas through thin gas exchange surfaces. There is a negligible gravitational hydrostatic gradient imposed on the gas along the length of the lung, since the gas space is continuous and the density of air is so low. Because the pulmonary blood column has a hydrostatic component, there is a possibility of blood pooling and higher transmural gradients in the dependent parts of the lung, even in aquatic species. It appears reasonable that the length of the lung in both terrestrial and aquatic environments be kept short to avoid this effect.
However, there appears to be selection for lengthening, not shortening, of the pulmonary vascular column in the aquatic environment. Elongate lungs are characteristic of fully aquatic taxa (e.g., sea snakes and acrochordids). These species use the lung to regulate buoyancy and attitude in the water (Seymour, 1982; Graham et al., 1987). Observations of the gas in sea snake (Pelamis platurus) lungs with isotopic imaging of 133Xe gas showed that head-up tilting in water causes complete collapse of the dependent lung (Seymour et al., 1981). Blood going to this gas-free space would therefore not be exposed to a low pulmonary luminal pressure and no excess transmural pressure gradient could develop. Luminal pressure would be influenced by higher hydrostatic pressure imposed through the body wall by the ambient water at that level. In head-down tilting, some gas was retained in the anterior (tracheal) lung, but it was clearly separated from the pulmonary gas as a whole and therefore also under higher ambient hydrostatic pressure. Note that this separation and isolation of pulmonary gas occurs only in water and cannot occur during tilting in air. It therefore provides a mechanism that permits longer vascularized lungs in aquatic snakes.
4.1 Are the two variables adaptive?
In the absence of direct links between a character state and fitness, there are two important lines of evidence for a trait being adaptive (sensu Endler, 1986). One is the demonstration that a character state confers a functional advantage in context of its hypothesized importance. Second is the demonstration that a character state evolved independently in two or more lineages having similar ecological pressures related to the hypothesized functional advantage (Futuyma, 1998). The latter is considered to be very powerful evidence for adaptation and is clearly applicable to our data reported here.
4.1.1 Functional considerations
Regarding the first line of evidence, there are ample publications that document the importance of heart position for homeostasis of cephalic blood pressure and blood flow during head-up posture (Lillywhite, 1987, 2005; Seymour et al., 1993; Seymour, 2000; Seymour and Arndt, 2004). Two results are especially noteworthy. One is the heart must generate pressure at least equivalent to the gravitational column above it in order to produce flow in the carotid artery (Lillywhite and Donald, 1994). Therefore, an anterior heart position reduces the pressure requirement if the brain is to be perfused during head-up posture, especially in longer snakes.
Second, pooling of blood in dependent, posterior vessels can compromise venous return of blood to the heart and thereby reduce cephalic blood pressure due to reduced cardiac output. Experimental data have demonstrated that heart–head distance is two times more important than blood pooling to maintain cephalic circulation in a terrestrial snake (Liasis fuscus), and four times more important in an aquatic snake (Acrochordus arafurae), when tilted in air (Seymour and Arndt 2004). These results emphasize the significance of heart position in snakes relative to other factors such as tissue or vessel compliance, which might mitigate the tendencies to blood pooling (Lillywhite, 1985, 1993, 1996; Lillywhite and Gallagher, 1985).
Arboreal species of snakes generally have higher levels of systemic arterial pressure than do aquatic species of snakes, quite possibly due to differences in levels of systemic peripheral resistance (Seymour and Lillywhite, 1976; Lillywhite and Donald, 1994). This is also clearly advantageous to brain perfusion when the head is up, so both heart position and arterial pressure act in concert to assist cephalic circulation during posture change (see discussion in Seymour and Lillywhite, 1976; Lillywhite, 2005). Clearly from previous literature arterial pressure and heart position are correlated, but there are insufficient data of the former for inclusion in a comparative phylogenetic analysis.
Few studies have addressed hemodynamic issues related to the ophidian lung. However, it seems clear that increased gravitational pressure associated with head–up postures induces serious edema and even hemorrhage in the elongate lung of sea snakes when they are tilted in air (Lillywhite, 2005). The capillaries of reptilian lungs are relatively “leaky” in comparison with mammals (Smits, 1989), and therefore might be especially sensitive to intravascular pressure gradients attributable to gravity. The maximal gravity head associated with fully vertical posture in the arboreal–scansorial species we sampled here is about 15 mm Hg (Fig. 10). Assuming there is flow in the pulmonary artery, this would contribute to a total intrapulmonary blood pressure of about 35 to 45 mm Hg (Lillywhite and Donald, 1994), which corresponds to levels thought to produce serious pulmonary edema (Smits, 1989). The extent to which these pressures are realized depends on the central pulmonary blood pressure, and the neural control of pulmonary blood flow attributable to vascular smooth muscle located in the extrinsic pulmonary artery or its branches proximal to the lung (Smits, 1989; Lillywhite and Donald, 1994). Reptiles with high systemic blood pressure, such as varanids and pythons, are able to reduce central pulmonary blood pressure by functional valving within the heart (Jensen et al., 2010). Pressure can be further reduced by exquisite neural control of pulmonary vasomotor tone that has been demonstrated in various species of amphibians and reptiles and can produce variations in pulmonary blood flow ranging from zero to over 90% of cardiac output (e.g., Lillywhite and Donald, 1989). We note that within our data set there is not a single exception of a snake species that is arboreal–scansorial and possesses a vascular lung of sufficient length to exceed an internal gravitational pressure threshold of about 15 mm Hg (Fig. 10). This includes viperid species that possess a tracheal lung and relatively elongate pulmonary vasculature (Lillywhite and Smits, 1992).
Figure 10.

Linear regressions illustrating differences between viperid and non–viperid species of snakes with respect to gravitational pressures associated with pulmonary blood columns (G column) in the vascular lung, assuming snakes are in a fully vertical position. Viperid and non–viperid species are represented by squares and circles, respectively, and arboreal–scansorial species are distinguished by open symbols and dashed regression lines. The heavy dashed line to the extreme left represents the gravitational pressure column assuming the vascular lung remained a constant 30% of the total body length. Note that the pulmonary vascular G column for arboreal and non–arboreal viperids increases similarly with total body length and represents the steepest slope in comparison with non–viperid species. Note also the arboreal viperid species are relatively short, so the G column remains within the range of that in other arboreal species. In the non–viperid species, pulmonary vascular G column of arboreal species increases with total body length along a flatter regression slope than does that of non–arboreal species. Considering viperid and other species combined, the G column does not exceed 20 mm Hg, regardless of length. Regression equations are: arboreal vipers G = −0.291 + 0.211 TL; r2 = 0.962, P = 0.0031; arboreal non-vipers G = 1.093 + 0.0.057 TL; r2 = 0.416, P = 0.0095; terrestrial vipers G = 0.63 + 0.209 TL; r2 = 0.661, P = 0.0013; terrestrial non-vipers G = 0.225 + 0.095 TL; r2 = 0.647, P = 0.0001.
4.1.2 Viperids: an instructive case
As a group, viperid snakes (vipers and pit vipers) invite special interest when the heart and lung data are examined in further detail (Figs. 10, 11). Many species are almost exclusively ground-dwelling and restricted to largely non-scansorial habits. Thus, viperid species exhibit cardiopulmonary characters that are more like aquatic snakes than arboreal–scansorial, or even some terrestrial, species in other taxa (Lillywhite and Smits, 1992; Lillywhite, 1993). The heart–to–head distance varies from 20 to 39% of total body length, and the length of vascular lung varies from 22 to 49% of total body length (Table 1).
Figure 11.

Linear regressions illustrating differences between viperid (open squares) and non–viperid species (closed circles) of arboreal–scansorial snakes with respect to the gravitational hydrostatic pressure component of the blood pressure that develops in the column of blood between the head and heart (G column) when snakes are in a fully vertical position. The heavy dashed line at the extreme left depicts the G column if the heart–to–head distance remained a constant 30% of total body length. Note that the arboreal viperid species are relatively short, and there appears to be a ceiling of G column for all species at about 27 mm Hg. Regression equations are: arboreal–scansorial vipers G = 0.363 + 0.23 TL; r2 = 0.946, P = 0.0054; non-viperid arboreal–scansorial species G = 1.625 + 0.121 TL; r2 = 0.658, P < 0.0001.
The heart of viperid species occupies a comparatively central position within the body cavity, presumably related to predominantly horizontal posture attributable to stout, massive bodies, passive foraging, and static defense behaviors (Lillywhite and Smits, 1992). Heart position in viperids is statistically indistinguishable from that in aquatic species, but differs significantly from that in arboreal species belonging to non–viperid taxa (Seymour, 1987). The limited data for arboreal pit vipers suggest that anterior migration of the heart has not evolved appreciably in these snakes. However, this conclusion must await further confirmation by examination of a greater range of arboreal species.
The lung morphology of viperid snakes differs characteristically from most other snakes, and the vascularized parenchyma is anterior in location. The trachea is a vascularized, tracheal lung, as in many aquatic species. This pulmonary morphology is described in detail by Frenkel and Kochva (1970) and Kardong (1972). The vascular portion of the viperid lung begins anteriorly at the 20–25th tracheal ring and extends as a spongy parenchyma to several centimeters posterior to the heart. The advantages of the anterior respiratory location are not known, although several ideas have been proposed including avoidance of prolonged compression of functional lung tissue during gastric digestion of large prey (Lillywhite and Smits, 1992).
Given that the viperid species we sampled all possess a tracheal lung which occupies about one–third of the total body length, it seems significant that arboreal species within this clade are all relatively short in overall body size (Fig. 12). Arboreal habits in this group possibly constrain total body length for many reasons, including the biomechanics related to heavy-bodied climbing or support on small branches, as well as possible selection based in physiology, predation, or camouflage. Regardless of such possibilities, the evolutionary association between short body length and arboreal habits maintains the absolute length of heart–to–head and pulmonary vascular columns, and therefore the correlated intravascular pressures attributable to gravity, within the range of those in longer species (both within and outside the family) which might have shorter vascular columns relative to total body length (Figs. 10, 11). Furthermore, the slope describing increasing length of these vascular columns with increasing total body length decreases in all terrestrial species, and further decreases in all arboreal–scansorial species, relative to the trend in viperid species (illustrated for the pulmonary vasculature in Fig. 10). These patterns collectively illustrate how body size and cardiovascular structure both evolve to limit the gravitational stress associated with vertical blood columns in scansorial species of snakes.
Figure 12.
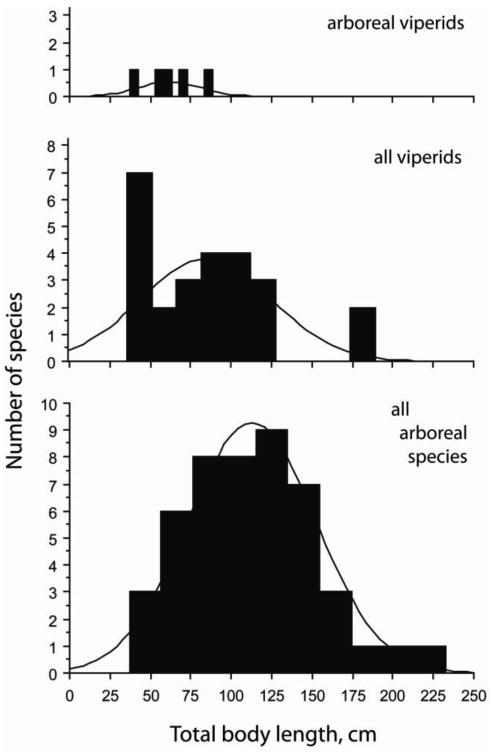
Histograms showing the distribution of total body length in various snakes. Arboreal viperid species (which have comparatively mid–body heart positions and elongate vascular lungs) are less than one meter in mean length and significantly shorter than arboreal species in other taxa (ANOVA, Fisher's PLSD, mean difference = 56.72 cm, P = 0.0025). The shorter total body length of the arboreal viperids limits the absolute length of the pulmonary vasculature and superior systemic arteries between the heart and head.
4.1.3 Phylogeny and the evolutionary transitions of cardiovascular characters
Inspection of Fig. 7 illustrates multiple instances of evolutionary transitions involving correlated changes in heart and lung characters with changes in gravitational habitat. Relatively short vascular lungs and systemic blood columns between the heart and head are associated with scansorial–arboreal habits in multiple boid, elapid, colubrid, and viperid lineage transitions, and the opposite is associated with aquatic habits in multiple acrochordid, elapid, and homalopsid lineage transitions. Clearly, the multiplicity of such correlated evolutionary changes provides strong supporting evidence that both heart position and pulmonary structure have evolved in response to changes in gravitational habitat. We believe that a breadth of physiological studies (see Lillywhite, 2005) contribute equally to understanding the influences that have operated in the natural selection for variation of cardiopulmonary characters in snakes and other vertebrates. Nonetheless, behavior, environment, and physiology interact in complex ways to produce the natural variation that is observed among ophidian taxa. Moreover, variability of characters is no doubt influenced by the evolutionary history of any particular clade, or individual species, and the length of time that each has spent in association with a particular habitat. However, we have little information that allows us to appropriately quantify the time scale for changes of characters relative to behavioral evolution and the respective histories of geographic distribution.
The viperids illustrate the importance of trait variation in context of phylogeny. While arboreal viperid species have retained heart and pulmonary characters similar to those of ground–dwelling relatives (hence, ‘phylogenetic signal’; see Blomberg and Garland, 2002), the assumption of arboreal habits can be understood within the context of gravitational constraint on body length. Thus, viperids with shorter bodies avoid by ‘default’ the gravitational stresses on fluid columns that might prevail if the animals were longer. Put another way, viperid snakes simply have not evolved long body size in arboreal habitats where length would otherwise be advantageous.
The details of behavior also are important. For example, there are anecdotes and some reports of rattlesnakes and other crotaline species being found occasionally in trees or other above–ground structures (e.g., Klauber, 1997; Beaupre and Roberts, 2001). It is, of course, possible for a longer snake to climb without gravitational insult to body fluid columns if the climbing is either very brief or occurs by means of switchback movements which minimize the angular departure from horizontal. Thus, a phylogenetic analysis of traits alone can be misleading without a finer dissection of evolutionary detail concerning the functional attributes of organisms (Westoby et al., 1995; Losos, 1999; McNab, 2003, 2008; Albert, 2007; Lillywhite and Albert, 2008).
Conclusions and perspectives
The importance of both heart position and pulmonary vascular column to arboreal–scansorial behaviors is emphasized by the high degree of correlation between these two characters (Fig. 2). Snakes with anterior hearts possess comparatively short vascular lungs, both serving to minimize the length of associated blood columns during head–up postures (Figs. 10, 11). The functional significance of each is different, but in both cases gravity stress seems to be the likely causative agent of natural selection which acts on these anatomical features in contexts of behavior and environment. Especially instructive is the reversed pattern (elongation) of heart–head and pulmonary vascular blood circuits in strictly aquatic species of snakes, which have had long evolutionary histories in a virtually weightless environment. Our results reaffirm previous conclusions related to what is perhaps nature's best possible experiment involving gravity and snakes (Lillywhite, 2005).
Acknowledgments
We thank various funding sources including the National Institutes of Health, National Science Foundation, National Research Council, and the Australian Research Council for support of prior work that contributed morphological data used in this study. All data were collected within institutional animal care and use guidelines of the University of Kansas, University of Florida, University of Adelaide, University of Armidale, and NASA Ames Research Center. We thank Craig R. White for advice, as well as numerous individuals who assisted with our research over many years and helped to generate, as a byproduct, the dataset we examine here. We are also grateful to two anonymous reviewers whose comments helped to improve the manuscript. This research was supported in part by NSF grant IOS-0926802 to HBL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert JS. Phylogenetic character reconstruction. In: Kaas JH, editor. Evolution of Nervous Systems Volume I: History of Ideas, Basic Concepts, and Developmental Mechanisms. Academic Press; Oxford: 2007. pp. 41–54. [Google Scholar]
- Alfaro ME, Arnold SJ. Molecular systematics and evolution of Regina and the Thamnophiine snakes. Mol Phylogenet Evol. 2001;21:408–423. doi: 10.1006/mpev.2001.1024. [DOI] [PubMed] [Google Scholar]
- Alfaro ME, Karns DR, Voris HK, Brock CD, Stuart BL. Phylogeny, evolutionary history, and biogeography of Oriental–Australian rear-fanged water snakes (Colubroidea: Homalopsidae) inferred from mitochondrial and nuclear DNA sequences. Mol Phylogenet Evol. 2008;46:576–593. doi: 10.1016/j.ympev.2007.10.024. [DOI] [PubMed] [Google Scholar]
- Beaupre SJ, Roberts KG. Agkistrodon contortrix contortrix (Southern Copperhead). Chemotaxis, arboreality and diet. Herpetol Rev. 2001;32:44–45. [Google Scholar]
- Blomberg SP, Garland T, Jr, Ives AR. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution. 2003;57:717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T., Jr Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J Evol Biol. 2002;15:899–910. [Google Scholar]
- Burbrink FT. Inferring the phylogenetic position of Boa constrictor among the Boinae. Mol Phylogenet Evol. 2005;34:167–180. doi: 10.1016/j.ympev.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Burggren WW. Pulmonary blood plasma filtration in reptiles: A “wet” vertebrate lung. Science. 1982;215:77–78. doi: 10.1126/science.215.4528.77. [DOI] [PubMed] [Google Scholar]
- Caldwell MW. Snake phylogeny, origins, and evolution: the role, impact, and importance of fossils (1869–2006) In: Anderson JS, Sues HD, editors. Major Transitions in Vertebrate Evolution. Indiana University Press; Bloomington and Indianapolis: 2007. pp. 253–302. [Google Scholar]
- Castoe TA, Parkinson CL. Bayesian mixed models and the phylogeny of pitvipers (Viperidae: Serpentes) Mol Phylogenet Evol. 2006;39:91–110. doi: 10.1016/j.ympev.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Castoe TA, Smith EN, Brown RM, Parkinson CL. Higher-level phylogeny of Asian and American coralsnakes, their placement within the Elapidae (Squamata), and the systematic affinities of the enigmatic Asian coralsnake Hemibungaris calligaster (Wiegmann, 1834) Zool J Linn Soc. 2007;151:809–831. [Google Scholar]
- Cope ED. On the lungs of the Ophidia. Proc Am Phil Soc. 1894;33:217–224. [Google Scholar]
- de Queiroz A, Wimberger P. The usefulness of behavior for phylogeny estimation: Levels of homoplasy in behavioral and morphological characters. Evolution. 1993;47:46–60. doi: 10.1111/j.1558-5646.1993.tb01198.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Uriarte R, Garland T. Effect of branch length errors on the performance of phylogenetically independent contrasts. Syst Biol. 1998;47:654–672. doi: 10.1080/106351598260653. [DOI] [PubMed] [Google Scholar]
- Endler JA. Natural Selection in the Wild. Princeton University Press; Princeton, New Jersey: 1986. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- Fenwick AM, Gutberlet RL, Jr, Evans JA, Parkinson CL. Morphological and molecular evidence for phylogeny and classification of South American pitvipers, genera Bothrops, Bothriopsis, and Bothrocophias (Serpentes: Viperidae) Zool J Linn Soc. 2009;156:617–640. [Google Scholar]
- Frenkel G, Kochva E. Visceral anatomy of Vipera palaestinae: an illustrated presentation. Israel J Zool. 1970;19:145–163. [Google Scholar]
- Futuyma DJ. Evolutionary Biology. 3rd. Sinauer Associates; Sunderland, Massachusetts: 1998. [Google Scholar]
- Garland T, Jr, Harvey PH, Ives AR. Procedures for the analysis of comparative data using phylogenetically independent contrasts. Syst Biol. 1992;41:18–32. [Google Scholar]
- Gartner GEA, Hicks JW, Manzani PR, Andrade DV, Abe AS, Wang T, Secor SM, Garland T., Jr Phylogeny, ecology, and heart position in snakes. Physiol Biochem Zool. 2010;83:43–54. doi: 10.1086/648509. [DOI] [PubMed] [Google Scholar]
- Goicoechea N, De La Riva I, Padial JM. Recovering phylogenetic signal from frog mating calls. Zoologica Scripta. 2010;39:141–154. [Google Scholar]
- Grafen A. The phylogenetic regression. Proc R Soc Lond B. 1989;326:119–156. doi: 10.1098/rstb.1989.0106. [DOI] [PubMed] [Google Scholar]
- Graham JB, Gee JH, Motta J, Rubinoff I. Subsurface buoyancy regulation by the sea snake Pelamis platurus. Physiol Zool. 1987;60:251–261. [Google Scholar]
- Greene HW. Snakes The Evolution of Mystery in Nature. University of California Press; Berkeley: 1997. [Google Scholar]
- Hargens AR. Commentary on Viewpoint “Human experimentation: No accurate, quantitative data? J Appl Physiol. 2007;102:1291. doi: 10.1152/japplphysiol.01393.2006. [DOI] [PubMed] [Google Scholar]
- Hargens A, Pettersson RK, Millard RW. Giraffe cardiovascular adaptations to gravity. In: Aird WC, editor. Endothelial Biomedicine. Cambridge University Press; Cambridge: 2007. pp. 99–106. [Google Scholar]
- Hargens AR, Millard RW, Pettersson K, van Hoven W, Gershuni DH, Johansen K. Gravitational haemodynamics and oedema prevention in the giraffe. Nature. 1987;329:59–60. doi: 10.1038/329059a0. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford University Press; Oxford: 1991. [Google Scholar]
- Highton R, Hedges SB, Hass CA, Dowling HG. Snake relationships revealed by slowly-evolving proteins: further analysis and a reply. Herpetologica. 2002;58:270–275. [Google Scholar]
- Jensen B, Nielsen JM, Axelsson M, Pedersen M, Löfman C, Wang T. How the python heart separates pulmonary and systemic blood pressures and blood flows. J Exp Biol. 2010;213:1611–1617. doi: 10.1242/jeb.030999. [DOI] [PubMed] [Google Scholar]
- Kardong KV. Gegenbaurs Morph Jahrb. Vol. 3. Leipzig: 1972. Morphology of the respiratory system and its musculature in different snake genera. Part I Crotalus and Elaphe; pp. 285–302. [PubMed] [Google Scholar]
- Kharin VE, Czeblukov VP. A new revision of the sea kraits of family Laticaudidae Cope, 1879 (Serpentes: Colubroidea) Russian J Herpetol. 2006;13:227–246. [Google Scholar]
- Klauber LM. Rattlesnakes: Their Habits, Life Histories and Influence on Mankind. 2nd. University of California Press; Berkeley and Los Angeles: 1997. [Google Scholar]
- Lawson R, Slowinski JB, Crother BI, Burbrink FT. Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Mol Phylogenet Evol. 2005;37:581–601. doi: 10.1016/j.ympev.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Lee MSY, Hugall AF, Lawson R, Scanlon JD. Phylogeny of snakes (Serpentes): combining morphological and molecular data in likelihood, Bayesian, and parsimony analyses. Syst Biodiv. 2007;4:371–389. [Google Scholar]
- Lillywhite HB. Postural edema and blood pooling in snakes. Physiol Zool. 1985;58:759–766. [Google Scholar]
- Lillywhite HB. Circulatory adaptations of snakes to gravity. Amer Zool. 1987;27:81–95. [Google Scholar]
- Lillywhite HB. Orthostatic intolerance of viperid snakes. Physiol Zool. 1993;66:1000–1014. [Google Scholar]
- Lillywhite HB. Evolution of cardiovascular adaptation to gravity. J Grav Physiol. 1995;2:P1–P4. [PubMed] [Google Scholar]
- Lillywhite HB. Gravity, blood circulation, and the adaptation of form and function in lower vertebrates. J Exp Zool. 1996;275:217–225. [PubMed] [Google Scholar]
- Lillywhite HB. Cardiovascular adaptations to gravity: Lessons from comparative studies of snakes. In: Hargens A, Takeda N, Singal PK, editors. Adaptation Biology and Medicine, Vol 4 Current Concepts. Narosa Publishing House; New Delhi, India: 2005. pp. 68–81. [Google Scholar]
- Lillywhite HJ, Albert J. Evolutionary physiology, comparative data, and phylogenetic methods. In: Morris S, Vosloo A, editors. 4th CBP Meeting in Africa: MARA 2008, “Molecules to Migration: The Pressures of Life,” International Proceedings, Medimond S.r.l Monduzzi Editore International; Bologna, Italy. 2008. pp. 613–620. [Google Scholar]
- Lillywhite HB, Donald JA. Pulmonary blood flow regulation in an aquatic snake. Science. 1989;245:293–295. doi: 10.1126/science.2749262. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Donald JA. Neural regulation of arterial blood pressure in snakes. Physiol Zool. 1994;67:1260–1283. [Google Scholar]
- Lillywhite HB, Gallagher KP. Hemodynamic adjustments to head–up posture in the partly arboreal snake, Elaphe obsoleta. J Exp Zool. 1985;235:325–334. doi: 10.1002/jez.1402350303. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Pough FH. Control of arterial pressure in aquatic sea snakes. Am J Physiol Regul Integr Comp Physiol. 1983;244:R66–R73. doi: 10.1152/ajpregu.1983.244.1.R66. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Seymour RS. Heart position in snakes: Response to “Phylogeny, ecology and heart position in snakes. Physiol Biochem Zool. 2011;84:99–101. doi: 10.1086/658082. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Smits AW. The cardiovascular adaptations of viperid snakes. In: Campbell JA, Brodie ED Jr, editors. Biology of Pitvipers. Selva, Tyler; Texas: 1992. pp. 143–153. [Google Scholar]
- Losos JB. Uncertainty in the reconstruction of ancestral character states and limitations on the use of phylogenetic comparative methods. Animal Behaviour. 1999;58:1319–1324. doi: 10.1006/anbe.1999.1261. [DOI] [PubMed] [Google Scholar]
- Lukoschek V, Keogh S. Molecular phylogeny of sea snakes reveals rapidly diverged adaptive radiation. Biol J Linn Soc. 2006;89:523–539. [Google Scholar]
- Maddison WP. Squared-change parsimony reconstructions of ancestral states for continuous-valued characters. Syst Zool. 1991;40:304–314. [Google Scholar]
- Maddison WP, Maddison DR. MacClade Ver 4.08 Analysis of phylogeny and character evolution. Sinauer Associates; Sunderland, MA: 2005. Available at http://macclade.org. [DOI] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.01. 2007 Available at http://mesquiteproject.org.
- Malhotra A, Thorpe RS. A phylogeny of the Trimeresurus group of pit vipers: new evidence from a mitochondrial gene tree. Mol Phylogenet Evol. 2004;16:199–211. doi: 10.1006/mpev.2000.0779. [DOI] [PubMed] [Google Scholar]
- McNab BK. Standard energetics of phyllostomid bats: the inadequacies of phylogenetic contrast analyses. Comp Biochem Physiol A. 2003;135:357–368. doi: 10.1016/s1095-6433(03)00090-4. [DOI] [PubMed] [Google Scholar]
- McNab BK. Energy expenditure cannot be effectively analyzed with phylogenetically-based techniques. In: Morris W, Vosloo A, editors. 4th CBP Meeting in Africa, MARA 2008, “Molecules to Migration: The Pressures of Life.” International Proceedings, Medimond S.r.l Monduzzi Editore International; Bologna, Italy. 2008. pp. 621–625. [Google Scholar]
- Metzger GA, Kraus F, Allison A, Parkinson CL. Uncovering cryptic diversity in Aspidomorphus (Serpentes: Elapidae): evidence from mitochondrial and nuclear markers. Mol Phylogenet Evol. 2010;54:405–416. doi: 10.1016/j.ympev.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Midford PE, Garland T, Jr, Maddison W. PDAP: PDTREE package for Mesquite Version 1.11. 2008 Accessed at http://mesquiteproject.org/pdap_mesquite/index.html.
- Norsk P. Cardiovascular and fluid volume control in humans in space. Curr Pharm Biotechnol. 2005;6:325–330. doi: 10.2174/1389201054553734. [DOI] [PubMed] [Google Scholar]
- O'Connor MP, Agosta SJ, Hansen F, Kemp SJ, Sieg AE, McNair JN, Dunham AE. Phylogeny, regression, and the allometry of physiological traits. Am Nat. 2007;170:431–442. doi: 10.1086/519459. [DOI] [PubMed] [Google Scholar]
- Pyron RA, Burbrink FT, Colli GR, Montes de Oca AN, Vitt LJ, Kuczynski CA, Wiens JJ. The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Mol Phylogenet Evol. 2011;58:329–342. doi: 10.1016/j.ympev.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Rabb GB, Marx H. Major ecological and geographic patterns in the evolution of colubroid snakes. Evolution. 1973;27:69–83. doi: 10.1111/j.1558-5646.1973.tb05918.x. [DOI] [PubMed] [Google Scholar]
- Rawlings LH, Rabosky DL, Donnellan SC, Hutchinson MN. Python phylogenetics: inference from morphology and mitochondrial DNA. Biol J Linn Soc. 2008;93:603–619. [Google Scholar]
- Rowell LB. Human Cardiovascular Control. Oxford University Press; New York. USA: 1993. [Google Scholar]
- Seymour RS. Physiological adaptations to aquatic life. In: Gans C, Pough FH, editors. Biology of the Reptilia, Vol 13, Physiological Ecology. Academic Press; London and New York: 1982. pp. 1–51. [Google Scholar]
- Seymour RS. Scaling of cardiovascular physiology in snakes. Amer Zool. 1987;27:97–109. [Google Scholar]
- Seymour RS. Model analogues in the study of cephalic circulation. Comp Biochem Physiol. 2000;125:517–524. doi: 10.1016/s1095-6433(00)00180-x. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Arndt JO. Independent effects of heart–head distance and caudal blood pooling on blood pressure regulation in aquatic and terrestrial snakes. J Exp Biol. 2004;207:1305–1311. doi: 10.1242/jeb.00882. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Lillywhite HB. Blood pressure in snakes from different habitats. Nature. 1976;264:664–666. doi: 10.1038/264664a0. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Hargens AR, Pedley TJ. The heart works against gravity. Am J Physiol. 1993;265:R715–R720. doi: 10.1152/ajpregu.1993.265.4.R715. Regulatory Integrative Comp. Physiol. 34. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Spragg RG, Hartman MT. Distribution of ventilation and perfusion in the sea snake, Pelamis platurus. J Comp Physiol. 1981;145:109–115. [Google Scholar]
- Sieg AE, O'Connor MP, McNair JN, Grant BW, Agosta SJ, Dunham AE. Mammalian metabolic allometry: Do intraspecific variation, phylogeny, and regression models matter? Am Nat. 2009;174:720–733. doi: 10.1086/606023. [DOI] [PubMed] [Google Scholar]
- Smits AW. Fluid balance in vertebrate lungs. In: Wood SC, editor. Comparative Pulmonary Physiology Current Concepts. Marcel Dekker; New York: 1989. pp. 503–537. [Google Scholar]
- Vidal N, Delmas AS, David P, Cruaud C, Couloux A, Hedges SB. The phylogeny and classification of caenophidian snakes inferred from seven nuclear protein-coding genes. C R Biologies. 2007;330:182–187. doi: 10.1016/j.crvi.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Vogel S. Comparative Biomechanics Life's Physical World. Princeton University Press; Princeton, New Jersey: 2003. [Google Scholar]
- Wallach V. The lungs of snakes. In: Gans C, Gaunt AS, editors. Biology of the Reptilia Vol 19, Morphology G, Visceral Organs Soc Study Amphibians and Reptiles. Ithaca, New York: 1998. pp. 93–295. [Google Scholar]
- Wallach V. The nomenclatural status of Australian Ramphotyphlops (Serpentes: Typhlopidae) Bull Maryland Herp Soc. 2006;42:8–24. [Google Scholar]
- Westoby M, Leishman MR, Lord JM. On misinterpreting the ‘phylogenetic correction ’. J Ecol. 1995;83:531–534. [Google Scholar]
- Wiens JJ, Kuczynski CA, Smith SA, Mulcahy DG, Sites JW, Jr, Townsend TM, Reeder TW. Branch lengths, support, and congruence: testing the phylogenomic approach with 20 nuclear loci in snakes. Syst Biol. 2008;57:420–431. doi: 10.1080/10635150802166053. [DOI] [PubMed] [Google Scholar]
- Wilcox TP, Zwickl DJ, Heath TA, Hillis DM. Phylogenetic relationships of the dwarf boas and a comparison of Bayesian and bootstrap measures of phylogenetic support. Mol Phylogenet Evol. 2002;25:361–371. doi: 10.1016/s1055-7903(02)00244-0. [DOI] [PubMed] [Google Scholar]
- Wüster W, Peppin L, Pook CE, Walker DE. A nesting of vipers: Phylogeny and historical biogeography of the Viperidae (Squamata: Serpentes) Mol Phylogenet Evol. 2008;49:445–459. doi: 10.1016/j.ympev.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Zaher H, Grazziotin FG, Cadle JE, Murphy RW, de Moura-Leite JC, Bonatto SL. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papéis Avulsos de Zoologia. 2009;49:115–153. [Google Scholar]


