Abstract
Successful restoration of vision in human patients with gene therapy affirmed its promise to cure ocular diseases and disorders. The efficacy of gene therapy is contingent upon vector and mode of therapeutic DNA introduction into targeted cells/tissues. The cornea is an ideal tissue for gene therapy due to its ease of access and relative immune-privilege. Considerable progress has been made in the field of corneal gene therapy in last 5 years. Several new gene transfer vectors, techniques and approaches have evolved. Although corneal gene therapy is still in its early stages of development, the potential of gene-based interventions to treat corneal abnormalities have begun to surface. Identification of next generation viral and nanoparticle vectors, characterization of delivered gene levels, localization, and duration in the cornea, and significant success in controlling corneal disorders, particularly fibrosis and angiogenesis, in experimental animal disease models, with no major side effects have propelled gene therapy a step closer towards establishing gene-based therapies for corneal blindness. Recently, researchers have assessed the delivery of therapeutic genes for corneal diseases and disorders due to trauma, infections, chemical, mechanical, and surgical injury, and/or abnormal wound healing. This review provides an update on the developments in gene therapy for corneal diseases and discusses the barriers that hinder its utilization for delivering genes in the cornea.
Keywords: Cornea, gene therapy, AAV, Nanoparticles, decorin, corneal scarring, corneal neovascularization
1. Introduction
Gene therapy has advanced in leaps and bounds since its introduction to medicine almost 20 years ago as a query on gene therapy in the United States National Library of Medicine database PubMed yields over 124,000 articles from almost all medical disciplines. In the field of ophthalmology, gene therapy has shown astounding success. Recently, a breakthrough was attained in the restoration of vision in human patients suffering from Leber’s congenital amaurosis using gene replacement therapy in the retinal pigment epithelium (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008; Maguire et al., 2009; Simonelli et al., 2010). This plainly gives credence to the potential of gene therapy to cure diseases of the ocular system and prevent blindness.
Current treatments for corneal disorders include various pharmacological agents and surgical approaches depending on the cause, extent, and type of corneal damage. Conventional drug therapies to treat corneal disorders provide only short-term relief, require repeated application, cause side effects, and are often ineffective. More importantly they do not correct the cause of the problem but merely suppress symptoms. Conversely, gene therapy fixes the root of the problem, provides long-term cure, and does not require repeated applications or clinic visits. However, at present no gene therapy modalities are available for corneal diseases. The cornea is an ideal tissue for gene therapy due to its ease of access and relative immune-privilege. As it is a transparent tissue and devoid of blood vessels, it can be readily monitored visually. These properties also allow for the administration of therapeutic genes into corneal cells with relative ease. The cornea is well suited for conducting ex vivo gene therapy strategies because it can be maintained in culture for a long time. The arduous task of developing novel gene-based modalities for cornea has greatly improved due to increased comprehension of acquired and inherited corneal diseases in terms of molecular mechanisms and pathogenesis. Numerous approaches employing different viral and non-viral vectors and techniques to introduce genes into the cornea in vitro, ex vivo, and in vivo have been tested. Among viral vectors, adenovirus, adeno-associated virus (AAV), retrovirus, and lentivirus vectors have been found to efficiently transport genes into corneal tissue. However, concerns over safety and immunogenicity have limited their use. Nonviral vectors including plasmid DNA, lipids, polymers, and nanoparticles are generally safe but often found less efficient than their viral counterparts. Various physical techniques such as topical administration, gene gun, electroporation, intrastromal injection, and iontophoresis have been used to augment delivery of both viral and nonviral vectors. However, none of these vectors or techniques is ideal, and each has its benefits and shortcomings. Herein we provide a comprehensive review of corneal gene therapy approaches tested in the past six years and a brief general overview of vectors. A detailed overview of gene therapy vectors and their mode of action can be found in our previous corneal gene therapy review (Mohan et al., 2005).
2. Gene therapy vehicles for the cornea
2.1 Viral vectors
Viruses have been used since the dawn of gene transfer technology to deliver genes into different cells and tissues. Viruses were used as a vector in about 70% of gene therapy clinical trials (Young et al., 2006). Adenovirus (AV), adeno-associated virus (AAV), retrovirus, and lentivirus have been found to efficiently transport genes into the cornea. Nevertheless, each of these vectors has its own limitations. Adenovirus and retrovirus can successfully deliver genes into the cornea for short periods of time with mild-to-severe inflammatory responses. However, both of these vectors are of limited use for corneal gene therapy because of their inability to transduce low/non-dividing cells such as corneal endothelium and keratocytes, and induction of immune reactions. AAV and disabled lentivirus vectors offer better alternatives for delivering genes into corneal keratocytes and endothelium because of their ability to transduce slow/non-dividing cells and ability to provide long-term transgene expression. The origin of lentivirus vectors (equine infectious anemia virus and HIV) remains a major concern and significantly dampens enthusiasm for its use in human patients. Among viral vectors, AAV appears to be a good choice for corneal gene therapy because of their potency and safety profile. Recombinant AAV vectors have shown great promise for ocular gene therapy and restoring vision in patients with no major side effects.
2.1.1 Adenovirus
Recombinant forms of AV have been engineered and utilized in gene transfer studies in the past (Mohan et al., 2005). In sum, first-generation AV vectors lack the E1 gene region rendering them unable to replicate although they can proliferate in cell lines that provide E1 gene product. Second-generation AV vectors lack E1, E2, and E4 viral genes leading to less immunogenicity than first-generation vectors. In third-generation AV vectors, the AV viral genome is absent and only ITR sequences and packaging genes are present thus giving the name gutless or high-capacity vectors. Third generation AV vectors are able to carry larger gene inserts and are less immunogenic but require a helper virus. In order to reduce helper virus contamination, Cre/lox-P system and 293 cells stably transfected with Cre recombinase are utilized and have been shown to decrease helper virus contamination to <0.01% (Palmer and Ng, 2005). However, even this minimal level of contamination may be detrimental in clinical scenarios where large doses are required.
Multiple studies have examined the efficiency of AV vectors for corneal gene therapy (Mohan et al, 2005). AV vector was found most efficient in transducing murine cornea compared to equine immunodeficiency, lenti and bacculovirus vectors (Beutelspacher et al., 2005). Recently, AV vectors have been shown to deliver genes successfully into mouse corneal stroma and endothelium under control of cytomegalovirus immediate-early promoter (CMV) (Yu et al., 2007). The Ritter group has also reported cytokine or growth factor gene transfer with AV vector in rodent corneas (Ritter et al., 2007; Gong et al., 2007a; Gong et al., 2007b). AV vectors provide short-lived transgene expression and also demonstrate moderate to severe immune reaction apart from other side effects and (Yu et al., 2007; Ritter et al., 2007; Gong et al., 2007a; Gong et al., 2007b; Sharma et al., 2010a). Recombinant AV vectors have potential use in corneal gene therapy to over-express proteins in the corneal epithelium particularly in scenarios such as diabetes mellitus where transient gene expression is desirable. However, the ephemeral nature of AV vectors discourages its application in gene-based treatment for inherited gene defects (Saghizadeh et al., 2010).
2.1.2 Adeno-associated virus
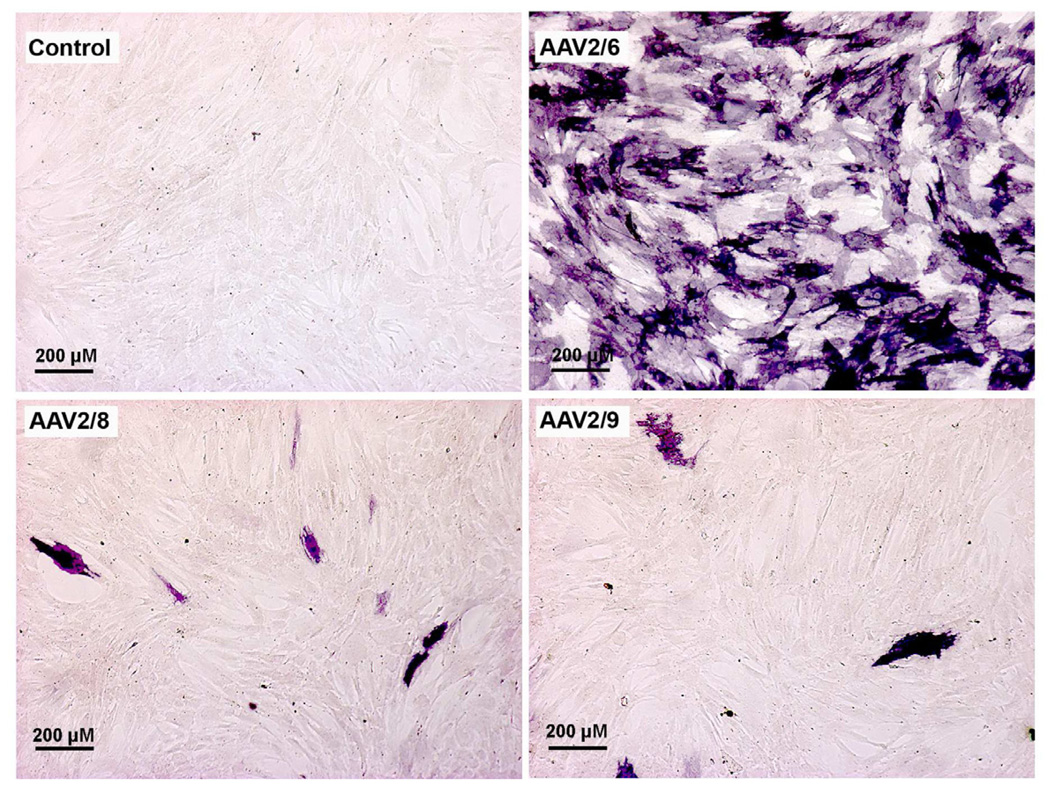
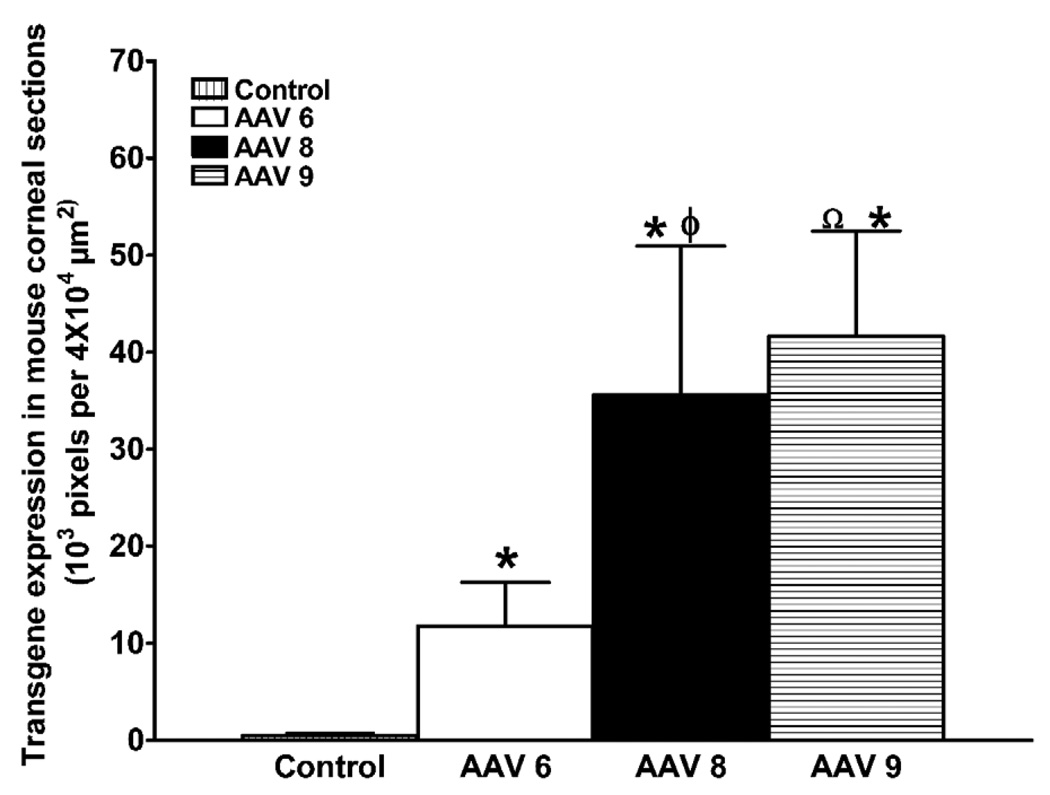
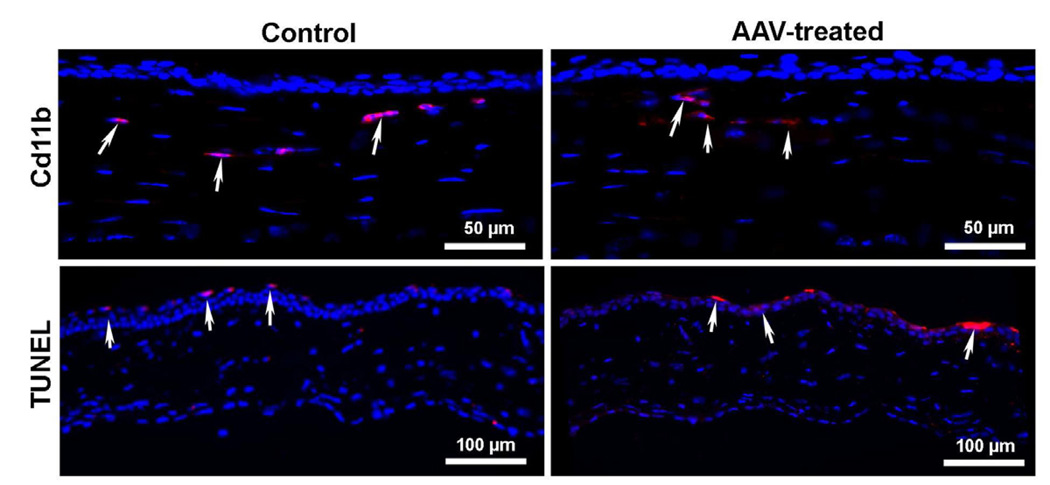
Among 110 identified AAV serotypes, to date only serotypes 1–10 have been used for gene therapy. AAV vectors have demonstrated high transduction efficiency and long-term transgene expression in the retina, cornea, and many non-ocular tissues in vivo (Alexander and Hauswirth, 2008; Gao et al., 2005; Surace and Auricchio, 2008 and references therein). AAV2 has been tested more in-depth compared to other AAV serotypes for gene therapy although each serotype has shown unique transduction patterns for various cells/tissues. AAV6, -8 and -9, especially, have the ability to mediate whole-body gene transfer efficiently (Ghosh et al., 2007; Pacak et al., 2006). The variation in gene transfer by different AAV serotypes is likely due to the interactions between host-cell receptor and viral capsid (Van Vliet et al., 2008). Structural variations in the capsid region of different AAV serotypes enable each serotype to bind different cell surface receptors. For instance, AAV serotypes 4, 5, and 6 use sialic acid (Wu et al., 2006) whereas AAV 8 and 9 use laminin receptors to enter cells (Akache et al., 2006). This led to the development of hybrid AAV vectors as a variety of pseudopackaged vectors were produced using transencapsidation of the AAV2 genome into the capsid of AAV1–9. These next generation hybrid vectors AAV2/1–9, displayed much greater transduction efficiency compared to AAV2/2 in ocular tissues of various animal models (Surace and Auricchio, 2008). It is important to note that a great deal of variation in transduction efficiency, tissue preference, first transgene appearance timing, and duration was observed in studies performed with various hybrid recombinant AAV vectors (Surace and Auricchio, 2008). Lebherz et al. (2008) also evaluated the gene delivery efficiency and expression stability of AAV2/7, 2/8, 2/9, with AAV2/1, 2/2, 2/5 using enhanced green fluorescent protein (EGFP) and rhesus erythropoietin as transgenes. After vector introduction into the eye via intravitreal and subretinal injection, the authors demonstrated that AAV2/7 and 2/8 possess superior long-term transduction ability (up to 6 months) in both retinal and anterior chamber tissues including the iris, trabecular meshwork, and cornea, (Lebherz et al., 2008). Subsequent studies with AAV2/6, AAV2/7, AAV2/8, and AAV2/9 vectors further reinforced these finding for retinal tissue (Surace and Auricchio, 2008). In a similar fashion, AAV2/8 and 2/9 demonstrated 5–100 fold superior transduction efficiency in non-ocular tissues such as heart (Wang et al., 2005), brain (Broekman et al., 2006), skeletal muscle (Wang et al., 2005), lung (Limberis and Wilson, 2006), and liver (Gao et al., 2006). We compared the efficiency of AAV2/6, AAV2/8, and AAV2/9 serotypes for corneal gene delivery using in vitro, in vivo, and ex vivo models to test a hypothesis that the relative efficiency of AAV-mediated gene transfer in the cornea depends on serotype. In our first study, the cellular tropism and transduction efficiency of AAV2/6, AAV2/8, and AAV2/9 vectors encoding alkaline phosphatase (AP) were tested using primary human corneal fibroblasts cultures. We found the tested serotypes efficiently transduce human corneal fibroblasts but that they differed in transduction efficiency. AAV2/6 displayed 30–50 fold higher transduction efficiency compared to AAV2/8 or AAV2/9 (Fig. 1). A remarkably positive and important finding was that none of the tested AAV serotypes induced significant cell death or loss of cellular viability reaffirming that AAV vectors are safe for the cornea (Sharma et al., 2010b). Interestingly, our subsequent studies with these vectors using mouse cornea in vivo and human cornea ex vivo showed very different transduction profiles compared to our in vitro investigations (Sharma et al., 2010c). Contrary to in vitro findings, the order of transduction efficiency for the three tested vectors was found to be AAV2/9 ≥ AAV2/8 > AAV2/6 (Fig. 2). These findings are not completely surprising as past literature reports imply that in vitro gene delivery may differ from in vivo transduction (Lipkowitz et al., 1999, Richter et al., 2000). However, the important take home message from these studies is that in vivo testing of gene therapy vectors for the cornea is important before making any conclusive inferences about vector efficacy. The one thing that remained unchanged during in vitro, ex vivo and in vivo testing was the safety profile of tested AAV serotypes. None of the three tested serotypes cause any significant side effects such as cell death, loss of cellular viability, inflammation and/or noticeable immune reaction in the cornea as measured with TUNEL and CD11b or F4/80 immunostaining (Fig. 3). Recently, transgene expression in the corneal epithelium was detected up to 8 months with AAV2 in the mouse eye suggesting that rAAV-delivered transgenes can persist for several months, and possibly years, in vivo (Alexander and Hauswirth, 2008; Lai et al., 2007). Thus, using AAV2/5 vector and a mouse model we performed time-dependent long-term animal experiments to characterize delivered transgene expression and duration for the cornea in vivo. Our ongoing studies showed different levels of transgene expression in the cornea in vivo up to 10 months following topical vector application (Mohan et al., unpublished data).
Fig. 1.
Representative cytochemical staining images showing transduction efficiency of AAV2/6, AAV2/8 and AAV2/9 vectors for human corneal fibroblasts. The alkaline phosphatase marker gene (stained purple) and 30 h time point were used for comparison.
Fig. 2.
Quantification of AAV2/6, AAV2/8 or AAV2/9 mediated transgene delivery in mouse stroma in vivo. The delivered alkaline phosphatase gene expression in the corneal tissue sections was quantified digitally by measuring pixels of purple stained tissue in 4 × 104 µm2 tissue area. * p < 0.05 AAV6/AAV8/AAV9 compared to control, φ = p < 0.05 AAV8 to compared to AAV6, Ω = p < 0.01 AAV9 compared to AAV6.
Fig. 3.
Representative images showing AAV9 effects on immune reaction (measured by Cd11b) and cell death (measured by TUNEL) in mouse corneas. Like control corneas (no AAV treatment), few Cd11b+ and TUNEL+ cells were observed in AAV-treated mouse stroma. The TUNEL + cells detected in the corneal epithelium were because of its replenishment via apoptosis. The AAV6 and AAV8 showed similar results (data not shown). Nuclei are stained blue with DAPI.
The alterations in function, host range, and tissue tropism shown by different AAV serotypes may be due to a degree of sequence variation within the capsid region (Van Vliet et al., 2008). Furthermore, the differences in transduction profile are likely related to distinct uptake and intracellular trafficking mechanisms of the various serotypes. In addition, different serotype-specific ITR elements may also influence transgene expression. Various studies report that the use of different serotypes or cross-packaging expands the tissue tropism of rAAV vectors. Moreover, selective use of serotypes might allow for targeting specific tissues. For example, an rAAV2 genome packaged in an AAV5 capsid (AAV2/5) transduced photoreceptors and retinal pigment epithelial cells in the eye (Gao et al., 2005; Surace and Auricchio, 2008) whereas AAV2-encapsidated vector transduced primarily photoreceptors, and an AAV1-encapsidated vector transduced mainly retinal pigment epithelial cells. Additionally, the utilization of different AAV serotypes may overcome the problems associated with vector re-administration and preexisting immunity to rAAV2 in humans (Adriaansen et al., 2006).
AAV vectors have emerged to the forefront in ocular gene therapy as they have been used in a ground-breaking clinical trial to treat retinal diseases. Despite the proven potency and safety of AAV vectors, relatively few studies have been performed to evaluate the potential of AAV vectors for ocular surface gene delivery including the cornea. A recent study compared rAV and rAAV tropism to human corneal cells in vitro and rabbit corneas in vivo. It was noted that rAAV transduced more keratocytes while rAV transduction was detected mostly in the epithelium. Interestingly, rAAV-mediated gene delivery led to lower GFP expression compared to rAV. This may have specific clinical ramifications as choice of vector may be dependent on transgene expression level or number of corneal cells requiring gene transfer. For example, in corneal disorders of the epithelium like diabetes mellitus, rAV may be more useful whereas rAAV may be more beneficial in stromal keratopathies where keratocytes are targeted (Liu et al., 2008a). Using AAV2, RPE65 gene was delivered to the retina of patients with Leber’s congenital amaurosis and successfully restored vision (Bainbridge et al., 2008; Hauswirth et al., 2008; Maguire et al., 2008; Maguire et al., 2009; Simonelli et al., 2010). These studies clearly outline the safety and efficiency of AAV based gene therapy for the retina leading the way for gene therapy for other ocular tissues including the cornea.
2.1.3 Retrovirus and lentivirus
Limited progress has occurred in the development and/or testing of retro- and lenti-virus vectors. The “gutless” retroviral vectors were developed by deleting the majority of the viral genome not required for infection. Further, the risk of replication was curtailed by using a packaging cell line that employs gag/pol and env from separate constructs, and by replacing the 5’ long terminal repeat (LTR) U3 region in the viral genome with the CMV promoter (Lech and Somaia, 2008). LTRs, the control center for gene expression, are sequences of DNA that flank functional genes found in retroviral DNA and retrotransposons. Retroviruses such as HIV use LTRs to insert their genetic sequences into the host genome. Even though modifications in these sequences have been tested, the risk of insertional oncogenesis due to their random integration remains. During a clinical trial for X-linked severe combined immunodeficiency treatment, retroviral vector integration was noted near protooncogenes, and led to the development of T-cell leukemia in 4 patients (Hacein-Bey-Abina et al., 2008), and the death of one of those individuals (Herzog, 2010). This clearly reduces enthusiasm for adopting retrovirus for gene therapy.
The human immunodeficiency virus (HIV)-based lentiviral vectors have also been investigated for corneal gene transfer. Lentivirus appears to be less toxic to the cell’s genome than retrovirus (Montini et al., 2006). Current lentiviral vectors for gene therapy lack the entire viral genome, and contain only the packaging signal and the cis-acting elements, including the LTR. In an attempt to generate safer lentiviral vectors, the U3 region of the 3’ LTR has been deleted. In recently generated lentiviral vectors, the endogenous promoter has been replaced with a CMV/LTR hybrid promoter in an effort to improve safety. Currently, glycoprotein of vesicular stomatitis virus is the mostly commonly used env protein for lentiviral pseudotyping (Lech and Somaia, 2008). Not long ago it was reported that transcriptionally active LTRs are chief contributors to genotoxicity and that self-inactivating LTRs decrease the oncogenic potential thus improving the safety of lentiviral vectors (Montini et al., 2009). Clinically, lentiviral vectors have yielded no reports of serious adverse events (Williams and Coster, 2010). Selective studies have been performed to evaluate the efficacy of lentiviral vectors for corneal gene therapy during the past 6 years. Beutelspacher et al. (2005) compared self-inactivating HIV-1 and Equine Infectious Anaemia Virus (EIAV) transduction efficiency in murine, rabbit, and human corneal endothelial cells and found EIAV to be more effective than HIV-1 for corneal endothelial gene therapy. Another study reported that a single injection of HIV-based lentivirus into the anterior chamber of the rodent eye efficiently delivered transgene into the corneal endothelium (Challa et al., 2005). Parker et al. (2007) demonstrated 80–90% transduction of rat, bovine, and human corneal endothelial cells with lentiviral vector expressing enhanced yellow fluorescent protein under control of the Simian virus type 40 early promoter. Bemelmans et al. (2009) demonstrated efficient transduction of keratocytes up to 3 weeks in a pig model by injecting HIV-1 expressing GFP into a stromal pocket created with a femtosecond laser.
2.2 Nonviral vectors
The introduction of plasmid DNA expressing therapeutic genes into target cells without the use of viruses falls under the broad category of nonviral gene transfer methods. Nonviral gene therapy is considered safer than viral gene therapy due to low toxicity, immunogenicity, and pathogenicity. Additionally, plasmid vector production is straightforward and cost-effective. Nevertheless, low transfection efficiency is a major challenge. Many strategies have been developed and are in the preclinical pipeline to improve plasmid delivery in cells. A brief description of popular techniques is given below.
2.2.1 Microinjection technique
Introduction of plasmid via microinjection has led to successful delivery of genes including GFP, interleukin (IL)18, Flt23k, endostatin, MMP14, and vasohibin into various cells of the cornea (Kim et al., 2005; Jani et al., 2007; Lai et al., 2007; Galiacy et al., 2011; Zhou et al., 2010; Sharma A, et al. IOVS 2010;51:ARVO E-Abstract 2839,). Microinjections targeting different layers of the cornea have been performed at various anatomic locations and include intrastromal, subconjunctival, and directly into the anterior chamber (Singh et al., 2005; Kim et al., 2005; Lai et al., 2007; Zhou et al., 2010; Yu et al., 2007; Sharma A, et al. IOVS 2010;51:ARVO E-Abstract 2839). A recent study performed in mice demonstrated that bevacizumab treatment given via intraocular injection was more efficacious than the subconjunctival (periocular) route (Dratviman-Storobinsky et al., 2009). Intrastromal injection may be appropriate in the treatment of acute corneal diseases as short-lived gene expression was detected in the cornea following intrastromal injection (Hao et al., 2010). Conversely, subconjunctival injection showed long-term stable transgene expression and may help to avoid the endophthalmitis and cataract formation associated with intracameral injection (Kuo et al., 2009). Microsurgical techniques also offer a feasible method of exploring gene function in corneal disorders. For example, Kuo et al. (2008) showed successful transgene delivery in the cornea in vivo using intrastromal lamellar implantation of a partially dried p-bFGF–SAINT-18 complex composed of SAINT-18 and plasmid vector encoding reporter or FGF2 gene. This corneal gene transfer method permitted localized transgene delivery in the cornea. It is thought that except for topical and subconjunctival administration, all other gene delivery strategies to the cornea are invasive and compromise corneal integrity (Cheng et al., 2007).
2.2.2 Electroporation
Electroporation, also known as electrogenetherapy or electropermeabilization, makes use of high-intensity electrical pulses to form transient pores in the cell membrane and is useful for gene delivery in both cultured eye cells and ocular surface tissues in vivo. An advantage is large DNA constructs can be transported into cells although specialized equipment is necessary (Williams and Coster, 2010). However, very few studies have been reported in the cornea since 2005. Electroporation has the ability to deliver foreign genes into the corneal epithelium as well as keratocytes (Zhou and Dean, 2007). Electrical current of 200 V/cm did not cause trauma, corneal edema, or inflammation but introduced transgene at low levels. Higher electrical current resulted in enhanced gene transfer but also led to considerable corneal damage. Electrical current can cause irreversible tissue damage as a result of thermal heating or Ca2+ influx due to disruption of cell membranes. Electrically assisted gene delivery to the endothelium of ex vivo human corneas was recently described (He et al., 2010). Using custom-designed electrodes, two reporter genes, EGFP and beta-galactosidase (βgal), were successfully transported into human corneas in organ culture using eight 1-Hz 100-ms pulses of 125 mA square current. Although efficiency was much lower than viral vector, low cell death and no remarkable change in tight junction integrity of endothelial cells show its potential clinical application (He et al., 2010). The electrotransfer of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) small interfering RNA (siRNA) and dextran macromolecules into mice corneal epithelium in vivo using iontophoresis and electroporation individually and in combination has been reported (Hao et al., 2009). Although both iontophoresis and electroporation independently delivered macromolecules into the cornea, iontophoresis was found to be more efficient and the combination of iontophoresis followed by electroporation was more effective than both methods alone (Hao et al., 2009).
2.2.3 Sonoporation
Sonoporation employs ultrasound waves to create pores in the plasma membrane in order to deliver DNA to the nucleus. Ultrasound is effective for cell transfection in vitro and in vivo (Liu et al., 2006). Transfection efficiency of this approach is dependent on the transducer frequency, acoustic pressure, output strength, and pulse duration of ultrasound treatment in addition to the use of contrast agents such as microbubbles. Microbubbles were generated as contrast agents to not only enhance imaging but also improve gene delivery efficiency by boosting cell permeability (Mitragotri, 2005). Ultrasound-targeted microbubble destruction may hold great potential as a site-specific gene transfer approach and has been used successfully in AAV-mediated gene transfection of human RPE cells in vitro and rat retina in vivo (Li et al., 2009). Microbubbles generally measure approximately 3 micrometers in diameter and are composed of a shell which houses a gas core. Available commercially, microbubbles differ in shell composition (i.e. albumin, galactose, lipid, polymers) and gas core (i.e. air, perfluorocarbon, nitrogen) (Lindner 2004). A notable advantage of microbubbles is that they may be targeted to specific areas of interest. One way to accomplish this is the addition of a tiered polymer coat to the shell and subsequent covalent (i.e. carbodiimide-mediated amide) or noncovalent (i.e. biotin-avidin) coupling of targeting ligands to the polymer coat (Klibinov 2006). The precise mechanism by which gene transfection is enhanced by ultrasound-mediated destruction of carrier microbubbles is not clear but may include shell fragmentation-induced cellular microporation and micro-environment changes brought about by high-velocity pressure jets or heat and free radical production (Lindner, 2004). Ultrasound facilitates gene transfer by way of passive diffusion (Gao et al., 2007). The Sakamoto group demonstrated successful gene delivery into rabbit cornea in vitro and in vivo by combining ultrasound and microbubbles (Sonoda et al., 2006). The central cornea was injected with plasmid mixed with perflutren protein and a probe was placed on the ocular surface for ultrasound (1 MHz, 120 s, 50% duty cycle, 1 to 2 W/cm2) exposure. The ultrasound and microbubbles greatly increased transduction efficiency into keratocytes without tissue damage. GFP was expressed in the stroma for up to 30 days and was limited to the area exposed to ultrasound (Sonoda et al., 2006). However, multiple safety concerns of microbubble use including instability in serum and infection risk of components such as human albumin limit its use. Subsequent studies entailed the utilization of ultrasound with a newly developed contrast agent, a bubble liposome made of polyethylenglycol modified liposome containing perfluoropropane gas. Sonoporation coupled with the bubble liposome showed enhanced transgene delivery into rabbit corneal epithelial cells and rat subconjunctiva (Yamashita et al., 2007). Although these studies outline the potential of sonoporation for corneal gene therapy, many parameters remain to be investigated.
2.2.4 Gene gun
Gene gun is a ballistic (also called bioballistic) gene transfer method. It utilizes micron-sized biologically inert heavy metal (gold, silver or tungsten) particles and mechanical or macroprojectile (centripetal, magnetic or electrostatic) force. The bombarding of DNA-coated particles on cells/tissues with high velocity results in gene transfer. Gene delivery with gene gun depends on many factors such as amount of DNA-coated on particles, temperature, amount of cells, amount of force, number of DNA-coated particles, etc. Shallow penetration of particles, substantial cell damage, uncontrolled gene transfer, high cost, access to internal organs, etc. are few among many limitations of this method. The successful gene delivery in the cornea with gene gun has been reported (Hao et al. 2010). Genes such as IL4 and IL10 plasmid DNA and opioid growth factor receptor (OGFr) have been introduced into the corneal epithelium with this method (Bauer et al., 2006; Zagon et al., 2006). Zagon and cohorts showed delayed corneal abrasion healing after gene gun-mediated delivery of sense OGFr cDNA in rat eyes. Conversely, they found that antisense OGFr cDNA over-expression by the same delivery method led to accelerated corneal wound healing (Zagon et al., 2006). This study established the autocrine behavior of the OGF-OGFr axis, its regulatory role in ocular surface wound healing, and its potential in gene therapy approaches for corneal diseases where wound healing is impaired such as diabetic keratopathy. In addition, it highlighted the gene gun technique as a valuable tool in examining the role of genes in epithelial abnormalities (Zagon et al., 2006). Another research group targeted plasmids expressing IL10 and IL4 to the mouse cornea in order to determine immune modulation of HSV-1 infection. The expression of delivered genes was limited to corneal epithelial cells and attenuated the clinical course of infection although small numbers of F4/80+ and CD11b+ cells were noted with treatment (Bauer et al., 2006). Thus the gene gun method may be of great benefit in the focal treatment of corneal epithelial disorders as minimal transfection occurs in neighboring tissues. However, the technique, in its present form, is in need of additional optimization before being adopted for use in the clinic (Bauer et al., 2006). Furthermore, due to high corneal epithelial cell turnover, transgene expression is transient.
2.2.5 Controlled corneal dehydration
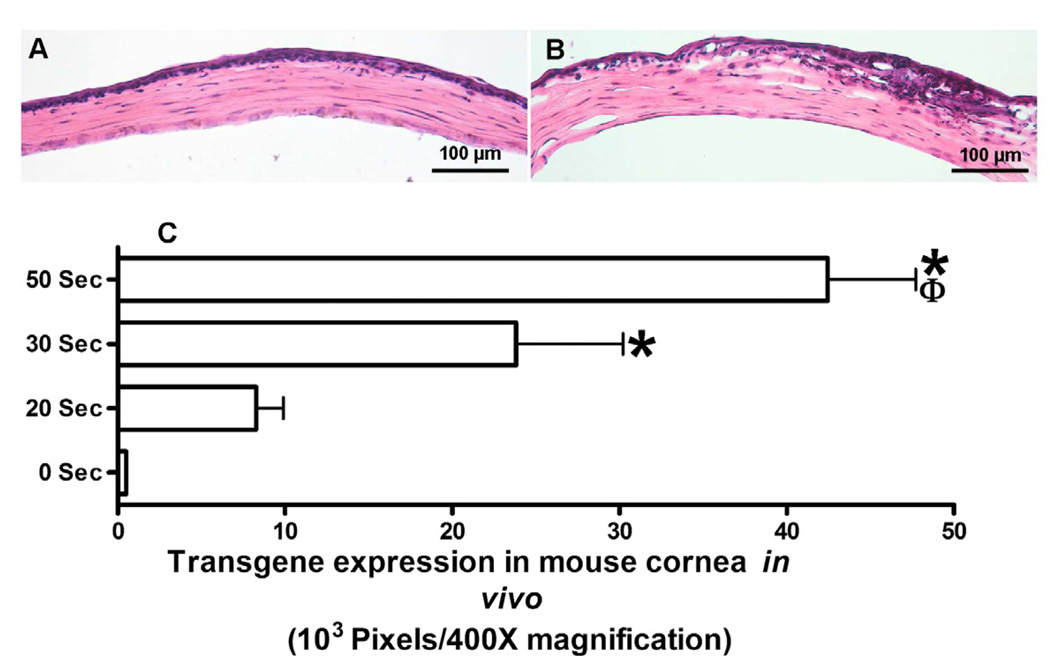
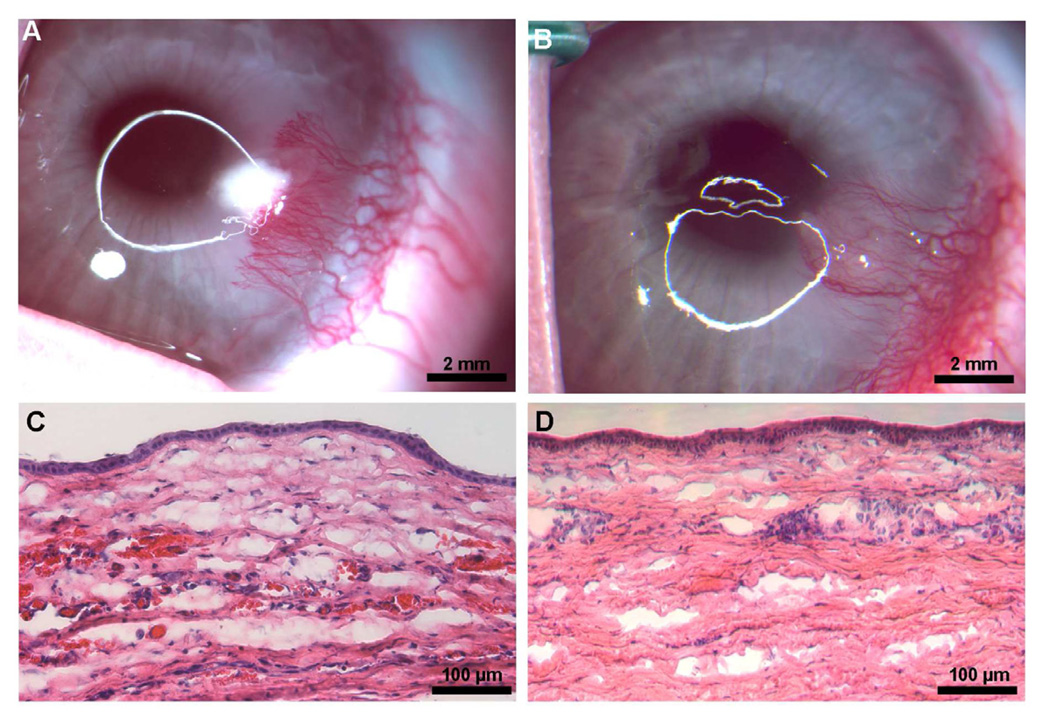
Recently we reported that controlled corneal dehydration increases vector absorption in mouse and rabbit corneas in vivo and human cornea ex vivo using a hairdryer following epithelial removal. Vectors expressing marker gene were used to study the effect of corneal drying on gene transfer. As evident from the data presented in Fig. 4, increased corneal drying with a hairdryer showed increased transgene delivery in the mouse cornea in vivo. However, excessive dehydration may have a negative impact on corneal integrity. Detection of significantly higher transgene delivery in vivo after 50 seconds of corneal drying with moderate changes in corneal morphology affirmed the promise of this simple technique for corneal gene therapy. High transgene delivery was also observed after 30 seconds of drying without jeopardizing corneal morphology and moderate gene delivery was noted after 10 or 20 seconds of drying with no altered corneal morphology. This study revealed that controlled corneal drying modulates gene delivery in the cornea presumably due to a change in corneal hydration. Thus, we postulated that administration of efficient vector employing simple minimally-invasive techniques is a novel approach for delivering therapeutic levels of genes in the cornea in vivo (Mohan et al., 2010b).
Fig. 4.
Representative H&E staining images showing histology of mouse corneas subjected to air drying and collected 14 days after AAV8 application. A: 0 s air-drying. B: 50 s air-drying. Mouse corneas subjected to 50 s drying showed moderate or higher levels of morphological changes in anterior stroma. C: shows digital quantification of delivered marker gene expression detected at day 14 in mouse corneas in vivo. These corneas received 2 µl of AAV8 vector immediately after 0, 20, 30, or 50 s of drying. *p<0.05 as compared to 20 s; ϕ p<0.05 as compared to 30 s.
2.2.6 Laser
Recently, a stromal pocket technique involving the use of the femtosecond laser to introduce genes into the pig cornea ex vivo was reported (Bemelmans et al., 2009). A stromal pocket 110 microns in depth was produced with a femtosecond laser and lentiviral vector expressing GFP was injected. Histology of corneal tissue performed 5 days after vector application showed wound closure and marker gene expression in the cells (most likely keratocytes) around the corneal pocket. Interestingly, the levels of transgene expression noted at day-5 remained up to 3 weeks (Bemelmans et al., 2009). This method facilitates gene delivery into cells in a specific, targeted corneal region. However, femtosecond laser is known to induce intense wound healing and infiltration of inflammatory cells in the rabbit cornea (Netto et al., 2007). Thus, the next step would entail the translation of this procedure from an ex vivo model to an in vivo model and the establishment of a safety profile.
2.2.7 Chemicals
Scores of natural and synthetic chemicals have been tested to introduce genes into corneal cells. In this review we provide a short description of more widely tested lipids and polymers for delivering genes in the cornea. Among many lipids dioleoyltrimethylammonium chloride (DOTMA), dioleoylphosphatidyl-ethanolamie (DOPE), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), dimethyl dioctadecyl ammonium bromide (DDAB), 3β-[N-(N’,N’-dimethylaminoethane) carbamoyl}cholesterol (DC-cholesterol), and N-methyl-4(dioleyl) methylpyridinium chloride (SAINT-2) showed promise for corneal gene therapy. A transfection solution prepared from five different lipids showed 7–17% transgene delivery in corneal endothelial cells in vitro (Dannowski et al., 2005). Our laboratory formulated transfection solutions manipulating ratios of DOPE and DDAB, and observed 12–15% gene delivery in human corneal fibroblasts in vitro, and rodent and rabbit stroma in vivo after corneal administration via a defined delivery technique (Mohan et al., unpublished data). Gene delivery in cultured human epithelial cells and in rabbit corneal epithelium in vivo has also been reported with DOTAP/DOPE transfection mixture (Toropainen et al., 2007). A formulation of liposomes and transferrin was shown to modulate chemokine expression with viral macrophage inflammatory protein II in a murine model of corneal allograft rejection (Pillai et al., 2008). Transferrin was used to enhance transfection efficiency by promoting endocytosis. Among cationic lipids/polymers, polyethyleneimine (PEI), poly-lactide-co-glycolide (PLGA), polylactic acid (PLA), and chitosan have been evaluated for corneal gene transfer. PEI showed significant transgene delivery in cultured human corneal epithelial and rabbit cornea in vivo (Hornof et al., 2008; Yuan et al., 2006). Chitosan, a naturally occurring aminopolysaccharide resulting from alkaline deacetylation of chitin, has been examined widely for gene transfer because of its non-toxic nature at high concentrations, high availability, low cost, and biodegradable character. Several studies have reported successful delivery of genes in ocular tissues, including the cornea, using chitosan (de la Fuente et al., 2008a, b). Nonetheless, many challenges such as poor transfection, significant immune reaction, cell-targeting etc. remain to be resolved for its wider application as a useful delivery system (Xu et al., 2010).
2.3 Next generation AAV and nanoparticle vectors
To overcome the obstacles presented by conventional viral and nonviral vehicles many new vectors considered next generation were developed. Discussing all of these is beyond the scope of this review. Herein we focus our attention on tyrosine mutant AAV, nanoparticles, and dendrimers which have shown promise for corneal gene therapy in preclinical animal studies.
2.3.1 Tyrosine mutant AAV vectors
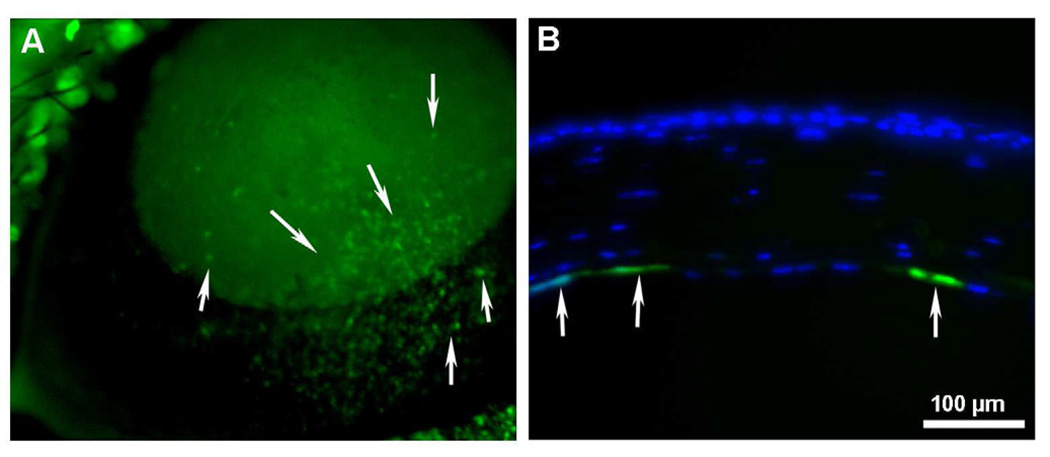
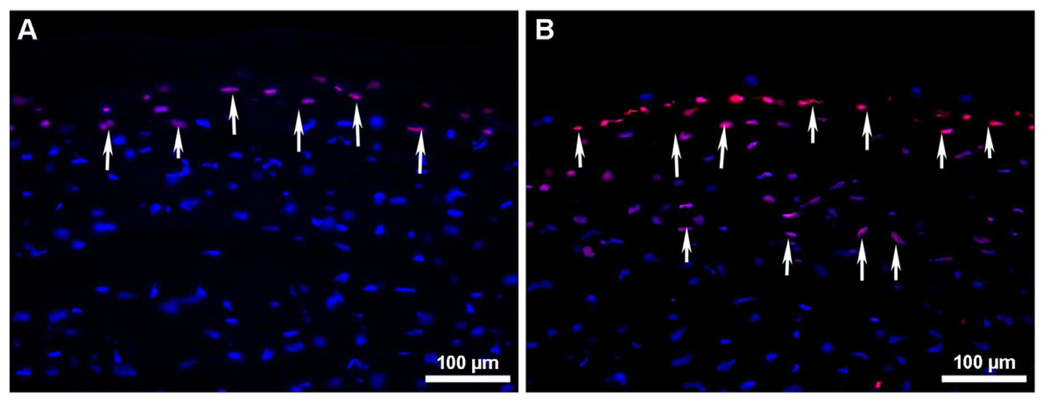
The AAV capsid surface is a fundamental element involved in host receptor binding, cellular uptake, and intracellular trafficking (Van Vliet et al., 2008) thus affecting transduction efficiency. Trafficking within the target cell renders the AAV vector susceptible to natural cellular degradation mechanisms such as the ubiquitin-proteasome pathway (Douar et al., 2001). Phosphorylation of AAV surface exposed tyrosine residues marks the vector for ubiquitination and subsequent proteasome-mediated degradation prior to entering the nucleus (Petrs-Silva et al., 2009). A recent study presented evidence that phosphorylation of surface exposed tyrosine residues by epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK) significantly reduces transduction efficiency of both single-stranded and self-complementary AAV2 vectors by ~68% and ~74%, respectively. In addition, intracellular trafficking is retarded due to AAV ubiquitination followed by degradation mediated by proteasomes (Zhong et al., 2008). Site-directed mutagenesis of each of the seven AAV2 capsid tyrosine residues (Y252, Y272, Y444, Y500, Y700, Y704, and Y730) by phenylalanine residue substitution leads to increased vector transduction and transgene expression by circumventing EGFR-PTK phosphorylation and the ubiquitin-proteasome pathway in human cells in vitro and murine hepatocytes in vivo (Zhong et al., 2008). These so-called next generation tyrosine mutant AAV2, AAV8, and AAV9 vectors have several-fold higher transgene delivery in retinal cells after subretinal or intravitreal injection compared to their wild-type counterparts (Petrs-Silva et al., 2009). These results are also clinically applicable in the context of vector dose. AAV2 vectors have been used in many Phase I/II clinical trials (Zhong et al., 2008). However, relatively large vector doses are required to attain therapeutic levels and may trigger an immune reaction. Neutralizing antibody levels are thought to be proportional to virus dose and preexisting antibodies may reduce transduction efficiency upon vector re-administration (Petry et al., 2008). Tyrosine mutant AAV vectors were shown to decrease vector dosage needed for transduction. A potent tyrosine mutant AAV2 Y444F vector showed superior transduction even at 10,000-fold lower titer compared to wild type AAV2. The lower AAV dose also leads to a decreased immune response (Petrs-Silva et al., 2009). Double, triple, and quadruple tyrosine-mutants have been generated to evaluate potential augmentation of AAV transduction efficiency. In bone marrow-derived primary murine cells and human mesenchymal stem cells, a triple AAV2 mutant consisting of Y444F, Y500F, and Y730F mutations increased transduction efficiency by almost 130-fold and significantly improved viral intracellular trafficking (Li et al., 2010). Our laboratory examined the efficacy of tyrosine-mutant AAV2, AAV8, and AAV9 vectors in delivering genes to the stroma and tyrosine-mutant AAV8 to the endothelium of the mouse cornea in vivo. In our studies, we utilized hairdryer-based topical application technique for achieving targeted delivery into the mouse stroma (Mohan et al., unpublished data) and specialized microinjection techniques to introduce the AAV8 vector encoding GFP into the anterior chamber of mice eyes (Sharma A, et al. IOVS 2010;51:ARVO E-Abstract 2839). All the three tested tyrosine-mutant AAV serotypes showed significantly higher transgene delivery in the mouse stroma in vivo compared to their corresponding wild type in preliminary studies (Mohan et al., unpublished data). The tyrosine-mutant AAV8 vector demonstrated substantially greater marker gene delivery than the wild type AAV8 vector (Fig. 5) into the corneal endothelium of mouse eye in vivo with no apparent adverse effects. Stereomicroscopy, immunocytochemistry and slit lamp biomicroscopy were used to analyze the efficacy and safety of screened vectors. Our recent studies showing substantial targeted gene delivery into corneal endothelium are particularly exciting as it has potential as an effective therapy for endothelial dystrophies in the future.
Fig. 5.
Representative stereomicroscopy (A) and immunocytochemistry in tissue sections (B) showing tyrosine-mutant AAV8 mediated transgene delivery in corneal endothelium of mouse eye in vivo. Dispensing of vector via custom delivery-technique fetched selective gene delivery into corneal endothelium. Cell nuclei were stained blue with DAPI while cells expressing GFP gene stained green.
2.3.2 Nanoparticle vectors
The National Institutes of Health coined the term nanoparticles (NPs) for materials 1–100 nm in size used in medicine for disease diagnosis and therapy. The advances in nanotechnology have supplied a large reservoir of NPs that may well revolutionize the future of medicine. Smaller than 100 nm in size, NPs are minuscule and thus able to access anywhere within the in vivo realm, as living cells are 10,000–20,000 nm in diameter (Sharma et al., 2010a). Additionally, the ability of NPs to multiplex and incorporate a myriad of ligands such as DNA, antibodies, peptides, and probes can give rise to an array of therapeutic modalities. Internalization of NPs in mammalian cells is carried out by phagocytosis, macropinocytosis, clathrin- or caveolae-mediated endocytosis, and other clathrin- and caveolae-independent endocytic pathways (Ragusa et al., 2007; Hillaireau and Couvreur, 2009).
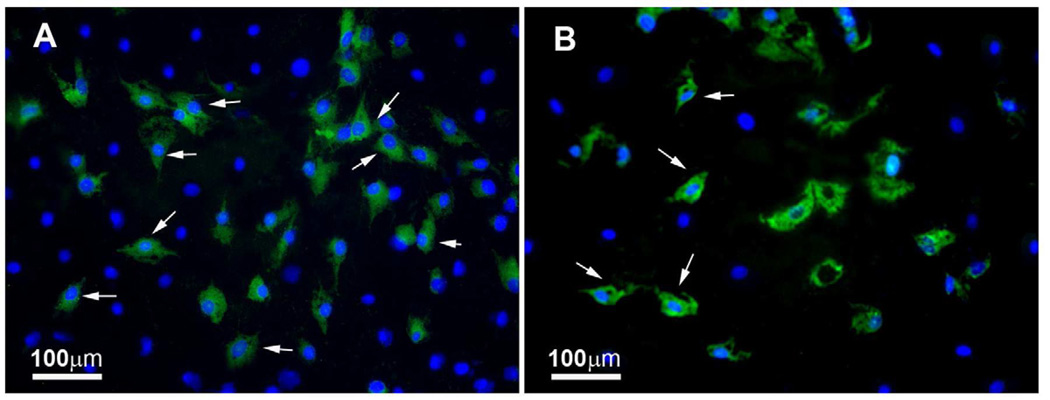
Many studies reported gene transfer in non-ocular cells with metallic (gold), polymeric (PEI, poly-L-lysine, PLGA), or hybrid (metallic-polymeric) NPs (Ragusa et al., 2007; Ghosh et al., 2008). Limited studies investigated the prospective of NPs for delivering genes in the eye, including the cornea (Cai et al., 2008; de la Fuente 2008a, 2008b; Sharma et al., 2011). An in vivo study showed marker gene delivery in the retina, lens, trabecular meshwork, and cornea with PEG-substituted-30mer lysine NPs (Cai et al., 2008). These NPs provided short-term transgene expression, and may necessitate repeated applications for sustained effect. NPs made from PLGA have been shown to deliver significant transgene in cultured rabbit conjunctival epithelial cells (Qaddoumi et al., 2004). Chitosan NPs have been shown to transfect up to 15% of human corneal epithelial and normal human conjunctival cells in vitro without affecting cellular viability (de la Fuente 2008a, 2008b). Recently, a nanodendritic compound was used as an in situ polymerizable adhesive in repairing a variety of corneal wounds in the porcine and human eyes ex vivo without major side effects (Grinstaff MW. IOVS 2008;49:ARVO E-Abstract 4801). A follow up in vivo study using chicken eyes reaffirmed biodendrimer efficacy and suggested that it may be superior to suturing (Berdahl et al., 2009). Our ongoing experiments with DOTAP NPs showed significant delivery of GFP marker gene in cultured human corneal fibroblasts (up to 20%) and human corneal endothelial cells (up to 14%), without compromising cellular viability and proliferation (Fig. 6; Mohan RR, et al. IOVS 2007;48:ARVO E-Abstract 2733). One in vitro study investigated a nanocarrier, comprised of biocompatible and bioadhesive compounds hyaluronic acid (HA) and chitosan, as a vehicle for gene delivery to human corneal epithelial and conjunctival cells (de la Fuente et al., 2008b). The NPs loaded with plasmid encoding reporter gene, were internalized by ocular cells via hyaluronan receptor CD44-mediated endocytosis as visualized by confocal microscopy and showed high levels of EGFP expression (up to 15%) with undetectable negative impacts on cellular viability. Reporter gene expression was directly proportional to HA content possibly due to improved internalization, trafficking, and transcription brought about by the polysaccharide. Interestingly, NPs composed of oligomeric HA yielded lower transfection than polymeric HA NPs, although the opposite effect was noted for chitosan. The HA quantity in NPs also affected cytotoxicity as increased HA levels were associated with a reduction in cellular toxicity. Thus NPs made of high HA content combined with chitosan oligomers may greatly improve gene transfer in ocular surface tissues. Later this group demonstrated transgene expression in the cornea and conjunctiva in vivo for 7 days with HA-chitosan NPs using a rabbit model (de la Fuente et al., 2008b). NPs were found to transfect more peripheral corneal cells possibly as a result of NP accumulation in those regions. The results of this study may have clinical application in eye drops for ocular surface abnormalities in the near future. Recently marketed ultrapure chitosan oligomers (NOVAFECT) were used by Klausner et al. (2010) as a blueprint in designing novel chitosan-based NPs with different polycation nitrogen to DNA phosphate group (N/P) ratios as gene carriers for the cornea. The chitosan-based NPs were intrastromally injected into rat corneas and successfully transfected keratocytes in vivo (Klausner et al., 2010). The transfection efficiency of chitosan and its derivatives is highly dependent on degree of deacetylation, molecular weight of chitosan, pH, serum, cell type, and N/P ratio (Kim et al., 2007). The utility of PEI nanometallic-conjugates for gene delivery has also been reported (Thomas and Klibanov, 2003). Gold nanoparticles (GNPs) are highly attractive as biocarriers because they are nontoxic, inert, easy to synthesize, and can condense DNA efficiently (Pissuwan et al., 2011). As gene transporters, GNPs have displayed significant marker gene delivery and expression into many mammalian cells (Ghosh et al., 2008; Li et al., 2009; Zhou et al., 2008; Thomas and Klibanov, 2003). PEI-gold nanoconjugate transfection efficiency and toxicity largely depends on PEI molecular weight. These authors used 2kDa PEI (PEI2) conjugated to GNPs and found that low molecular weight PEI increased nanoconstruct transfection efficiency of COS-7 cells six times more than PEI25 (Thomas et al., 2005). In addition, the synergistic action of PEI2-GNPs with N-dodecyl-PEI2 was also demonstrated and showed increased gene delivery by 11 fold compared to PEI25. An observation of paramount importance was the transfection of up to 50% cells with ternary complex, whereas unmodified PEI2 and PEI25 showed 4% and 8% transfection, respectively. The use of PEI2-GNPs also resulted in higher cytotoxicity and was linked to the ability of PEI to enter the nucleus. Subsequently these authors suggested that optimal N/P ratio is required for high transfection and is dependant on several factors such as polycation molecular weight, hydrophobicity, and degradability. The lower N/P ratio values are indicative of a polycation’s superior ability to condense DNA and better in vivo gene delivery (Thomas et al., 2005). PEI has been found to be toxic in vivo (Tiyaboonchai et al., 2003) and research is underway to minimize its toxicity by chemical modifications (Lungwitz et al., 2005).
Fig. 6.
Representative images showing GFP gene delivery by DOTAP nanoparticles in cultured human corneal fibroblasts (A) and HPV16-E6/E7 transformed human corneal endothelial cells (B). Cell nuclei were stained blue with DAPI while cells expressing GFP gene stained green.
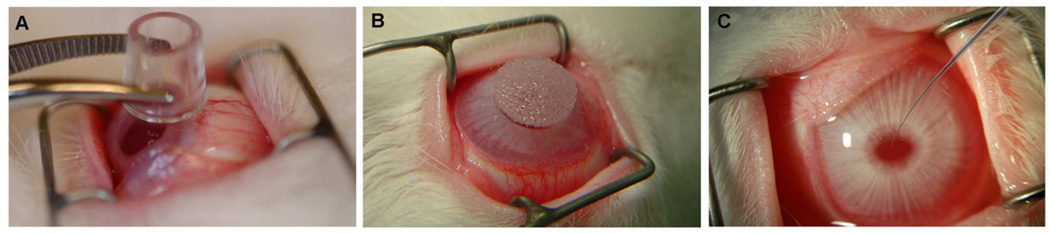
Recently we assessed the efficiency of PEI2-GNPs for delivering genes in the human cornea using an in vitro model. This was the first time hybrid GNPs were investigated for delivering therapeutics in the cornea. In these studies PEI2-GNPs with N/P ratios of 60, 90, 120, and 180 showed significant gene transport in human corneal fibroblasts with no negative effects on cellular viability or phenotype (Mohan et al., unpublished data). Sensing the potential of this nanoconstruct for corneal nanomedicine, we performed an elaborate study to evaluate PEI2-GNPs toxicity and safety for the cornea in vivo using a rabbit model. PEI2-GNPs were topically applied onto the cornea using a cloning cylinder with and without removing corneal epithelium once for a brief period of time ranging from 2–5 minutes (Sharma et al., 2011). Cloning cylinders are available commercially and are made in various sizes from a variety of materials such as glass, porcelain, stainless steel, etc (see Fig. 8A for appearance). The cloning cylinder was used based on our previous experience as it enhances vector delivery to corneal cells in a targeted region limiting its contact with neighboring ocular tissues, and thus preventing unwanted NP entry into adjacent ocular cells. The rabbit corneal tissues collected at 12 h, 72 h, or 7 d post-PEI2-GNP application displayed substantial gold uptake in the rabbit cornea with a slow clearance of GNP over time (Sharma et al., 2011). Transmission electron microscopy detected GNP in the extracellular matrix (ECM) and keratocytes of rabbit corneas. Slit lamp biomicroscopy performed 7 days after topical PEI2-GNP application revealed no corneal inflammation, redness, or edema in rabbit eyes in vivo although some degree of discoloration in the eye was noted due to the presence of GNPs (Fig. 7A). The effect of this discoloration on optical properties of the eye is still unknown and requires further investigation. The rabbit corneas collected 12h after PEI2-GNP application without removing corneal epithelium showed few TUNEL+ cells in the stroma (Fig. 7B) whereas corneas that received GNPs after removing corneal epithelium showed significant TUNEL+ cells (Fig. 7C). These results suggest that PEI2-GNPs are safe for the cornea and have potential for delivering gene therapy to the cornea but certainly demand more research in the area of GNPs clearance from the cornea and its effects on the optical properties of the eye (Sharma et al., 2011). Past reports point to renal excretion as the chief route of GNP clearance from the body (Hainfeld et al., 2006; De Jong et al., 2008). Since the cornea lacks a vascular supply, this route of clearance is not available. We postulate that GNPs are cleared from the cornea via slow diffusion into the tear fluid or aqueous humor (Sharma et al., 2011).
Fig. 8.
Representative image showing various minimally invasive defined vector-delivery techniques developed using cloning cylinder (A), soaked circular sponge (B), and glass-capillary (C) for targeted gene therapy approaches for the cornea.
Fig. 7.
Representative slit-lamp biomicroscopy (A) and TUNEL assay images (B and C) showing PEI2-GNP toxicity to rabbit cornea in vivo. No opacity, redness, or inflammation was seen but PEI2-GNP-treated corneas exhibited mild purple coloration (pointed with arrowhead) due to GNP uptake. The rabbit corneas in which PEI2-GNP was applied after epithelial removal (B) showed many TUNEL+ cells compared to the corneas that received transfection solution without epithelial removal (C). Nuclei are stained blue with DAPI and TUNEL+ cells in red.
NPs composed of human serum albumin (HSA), the most abundant plasma protein, have been studied for gene delivery in the cornea (Jani et al., 2007). HSA complexed with plasmid encoding a soluble trap for vascular endothelial growth factor (VEGF) was injected into the mouse stroma three weeks prior to alkali injury. HSA NPs were detected in keratocytes, showed transgene expression up to 5 weeks post injection, and reduced corneal neovessel formation. Furthermore, no corneal inflammation, edema, opacity, or other toxicity in corneas injected with HSA NPs was noticed during a three week observatory period (Jani et al., 2007). It was reported that HSA NPs enter cells via caveolae- or clathrin-mediated endocytosis, safeguard plasmid from DNase I- and vitreous humor-mediated biodegradation, permit plasmid escape from lysosome entrapment, and lead to sustained release of plasmid (>6 d in vitro) resulting in increased gene expression and activity (Mo et al., 2007).
2.3.3 Dendrimer vectors
Dendrimers, as the name suggests, are highly branched molecules with the ability to envelope or complex other molecules such as DNA (Mintzer and Simanek, 2009). The potential of dendrimers as drug delivery systems, antibacterial agents, and bioadhesives in wound repair for the cornea have been investigated (Durairaj et al., 2010; Degoricija et al., 2007; Berdahl et al., 2009; Calabretta et al., 2007). Activated polyamidoamine dendrimers complexed with plasmid DNA have been shown to transfect 6–10% of corneal endothelial cells after direct application to the cornea (Hudde et al., 1999). Marano and colleagues reported inhibition of laser-induced new blood vessel formation in rat eyes with no adverse effects by dendrimer-mediated delivery of anti-VEGF oligonucleotide (Marano et al., 2005). However, the potential of dendrimers for corneal gene transfer has not been investigated extensively.
3. Tissue-selective gene therapy tools
3.1 Vector engineering
Different vectors have been engineered to attain tissue-selective targeted gene delivery to corneal cells. A logical approach is to develop corneal cell specific vectors. However, such vector engineering is a challenging undertaking and demands active research. Ideally, cornea cell specific (epithelial, keratocyte, endothelial) vectors drive therapeutic gene expression in a controlled and targeted fashion in the desired cells of the cornea making them safe and effective for clinical applications of gene therapy. The use of tissue-specific promoters cloned into viral and nonviral vectors can accomplish tissue selectivity (Klausner et al., 2007). Although epithelial-specific (Shiraishi et al., 1998) and keratocyte-specific (Carlson et al., 2004) promoters for the cornea have been described earlier in murine and rabbit models, promoters with corneal endothelial cell specificity for gene therapy in vivo have yet to be defined. The keratin 12 (epithelial-specific) and keratocan (keratocyte-specific) promoters in conjunction with polymeric micelles were tested to deliver β-galactosidase marker gene into the corneas of mice and rabbits in a non-invasive manner using eye drops (Tong et al., 2007). This approach of using cornea-specific promoters in plasmids has significantly enhanced transgene expression in targeted corneal cells (Tong et al., 2007). Another approach for targeted gene therapy in the cornea includes usage of an inducible strong promoter with a switch-on and -off mechanism (Williams and Coster, 2010). These inducible promoters regulate activation and duration of gene expression, which is triggered in the presence of a supplementary factor that is either released endogenously under particular physiological conditions (e.g. ischemia) or administered exogenously (Bainbridge et al., 2006). Bitransgenic mouse lines that over-express β-galactosidase in the corneal epithelium upon induction with doxycycline have been characterized in the past (Chikama et al., 2005). A gene-targeting construct with an internal ribosomal entry site–reverse tetracycline transcription activator cassette was introduced into the keratin 12 gene (Krt12) to generate a knock-in Krt12rtTA/+ mouse line. These knock-in mice were then bred with tet-O-LacZ reporter mice to produce Krt12rtTA/+/tet-O-LacZ bitransgenic mice. Doxycycline ingestion by these bitransgenic mice increased corneal reporter gene expression 15-fold. In addition, β-galactosidase enzyme activity was detected 24 hrs post-antibiotic induction, leveled out at 2 d, and returned to basal levels 4 wks after doxycycline was eliminated from the diet (Chikama et al., 2005). Not only are studies of this nature extremely useful in expounding signaling mechanisms of different growth factors and cytokines, but also in clarifying the roles of various corneal genes under homeostatic and pathologic conditions (Chikama et al., 2005). More recently, Parker et al. (2009) studied a glucocorticosteroid-inducible promoter (GRE5) in a lentiviral vector encoding IL10 in A549 cells, and ovine and human corneas in vitro (Parker et al., 2009). A549 cells cultured with dexamethasone displayed a 30- to 40-fold increase in IL10 compared to controls while ovine and human corneas demonstrated 9- to 10-fold increase as quantified with enzyme-linked immunosorbent assay. This study outlines the efficacy of a steroid-inducible promoter in corneal gene transfer and paves the way for its application in future studies involving the modulation of transgene expression in donor corneal allografts (Parker et al., 2009). Even now, no ideal non-leaky cell-specific or inducible vectors are available for corneal gene therapy although considerable progress has been made in this area.
3.2 Custom vector-delivery techniques
Clinicians routinely perform simple surgical procedures such as epithelial scrape, microinjection, etc in the cornea in the general eye clinic to treat corneal abnormalities. We postulated a few years ago that an appropriate combination of vector and vector-delivery techniques could be used for developing targeted gene therapies. The ideal vector-delivery technique would involve minimal corneal injury during and/or following vector application and have the ability to introduce the vector to a precise location in a controlled manner. Methods such as intracameral delivery, intrastromal application, topical, and subconjunctival administration have all shown promising outcomes. However, with the exception of topical application and subconjunctival injection, all corneal gene transfer approaches have been deemed invasive with damaging impacts on the integrity of the ocular surface (Cheng et al., 2007). Even though topical application is the most acceptable approach to deliver therapeutics to the eye due to its non-invasive nature, preservation of corneal integrity, and minimal systemic side effects, the use of eye drops containing macromolecules including genes for corneal delivery is ineffective without opening tight junctions to enhance epithelial layer permeability (Cheng et al., 2007; Hao et al., 2010). Our recent studies demonstrated that vector-delivery techniques play a role in gene delivery in the cornea, and could be used as a tool to reduce contact of vector to unwanted tissue and enhance target specificity (Mohan et al., 2010b). A few strategies we have used for this purpose are shown in Fig. 8 and include cloning cylinder (8A) outlined previously, soaked circular sponge (8B), and glass-capillary (8C). It is well known that corneal permeability and hydration affect corneal transparency (Maurice, 1984). The dehydration of the cornea with a hair dryer is a conventional method to treat Fuchs’ dystrophy in human patients (Bainbridge 2008). We incorporated this technique currently in clinical use to dehydrate the cornea in a controlled fashion to increase vector absorption (Mohan et al., 2010b). After removing the epithelium via gentle scraping with a beaver blade, a technique commonly used in refractive laser surgery, a state of corneal dehydration was promoted by applying a hair dryer once, twice, thrice, or five times to blow warm air on the ocular surface for 10 s with 5 s intervals. Balanced salt solution (BSS) or AAV8 vector encoding alkaline phosphatase gene was then immediately applied topically for 2 mins with a custom-designed cloning cylinder to mouse and rabbit corneas in vivo and human cornea ex vivo. We found a statistically significant BSS/vector absorption ranging from 14–27% in corneas dried for 20, 30, and 50 s. AAV8-mediated transgene delivery was also increased with longer exposure to the hairdryer. However, corneas that underwent prolonged drying for 50 s demonstrated amplified infiltration of activated granulocytes as detected with CD11b immunostaining whereas 30 s of hair dryer-supplied warm air did not trigger severe immune reaction or jeopardize corneal morphology (Mohan et al., 2010b).
It has been long known that corneal epithelial injury induces apoptosis of keratocytes, inflammation, and wound healing in the cornea (Mohan et al., 2003; Wilson et al., 2002; Jester et al., 1999). Several researchers have demonstrated a cascade of cellular events including cytokine and growth factor release following epithelial injury leading to varied levels of corneal wound healing response (Mohan et al., 2003; Wilson et al., 2002; Jester et al., 1999). Scores of clinical and animal studies have found distinctly different wound healing responses in the cornea after surgical, mechanical, or chemical injury. Many investigators including us have reported that injury to the corneal epithelium causes keratocyte apoptosis in the anterior stroma. However, no studies were performed to examine whether the intensity of mechanical injury dictates the amount of keratocyte death in the anterior stroma. Recently, we hypothesized that careful, gentle removal of the corneal epithelium with a #64 surgical blade under controlled conditions induces clinically insignificant keratocyte apoptosis and wound healing in the cornea in vivo contrary to rough corneal epithelium removal with a #64 blade under undefined conditions. To test the hypothesis we performed a very simple experiment using a rabbit model. In the same rabbit eye, 5mm central corneal epithelial was gently removed following defined condition and 4mm peripheral corneal epithelium was removed in a rough manner with no defined conditions with a #64 surgical blade. Fig.9 shows the TUNEL assay of the rabbit cornea collected 24h after euthanasia. The detection of a few TUNEL+ cells in the central cornea where the epithelium was removed by defined gentle technique and several TUNEL+ cells in the peripheral cornea where the epithelium was removed by undefined rough technique strongly support our hypothesis. Additional support to our hypothesis comes from our recent hair dryer study in which we observed almost no inflammatory response in corneas subjected to up to 3 rounds of 10s dehydration as a comparable quantity of inflammatory cells were seen in murine corneas that did not experience hair dryer-assisted dehydration (Fig. 4 Mohan et al., 2010b). Thus we hold that the administration of an efficient vector via defined vector-delivery technique can successfully deliver therapeutic genes in the corneal stroma in vivo without compromising corneal integrity or function. More research is required to fully define parameters and techniques that minimize side effects, improve safety, and augment targeted delivery of therapeutic genes into keratocytes or the stroma in vivo with AAV vectors.
Fig. 9.
Representative TUNEL assay images showing effects of epithelial removal on keratocyte death by custom (A) and non-custom (B) epithelial scrape techniques in the rabbit corneas. Few TUNEL+ cells were noted in customized-gentle scrape technique (A) compared to non-optimized epithelial removal technique (B). Nuclei are stained blue with DAPI and TUNEL+ cells in red.
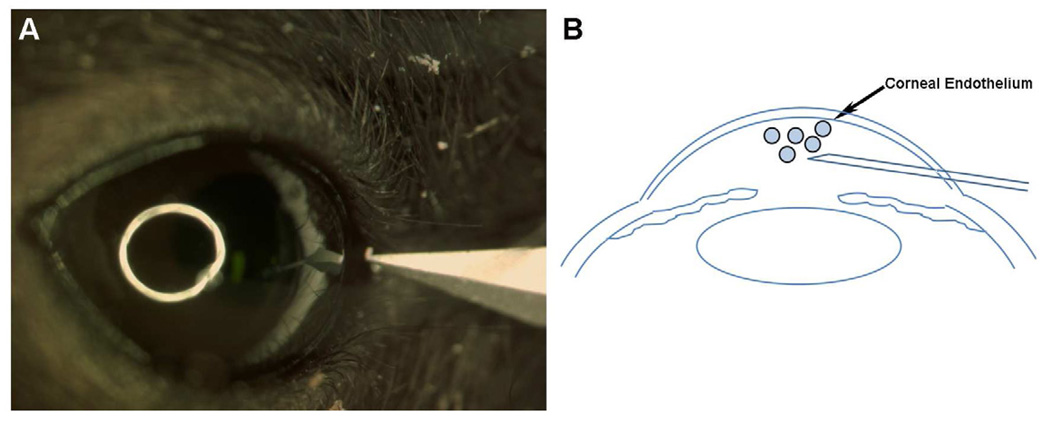
Delivering genes precisely into the corneal endothelium is a huge challenge and a major limitation for developing gene therapy for corneal dystrophies such as Fuchs’ dystrophy, a leading cause of corneal blindness with no effective treatments available. To address this challenge we developed minimally invasive microsurgical techniques based on the principle of air pressure force like a water gun as shown in Fig. 10. In our studies we found these techniques improved target-specificity significantly potentiating transgene delivery into mouse corneal endothelium in vivo. The newly optimized microsurgical procedures derived from microinjection technique are simple and may be used routinely in ophthalmology but may require practice to attain proficiency like all other surgical techniques.
Fig. 10.
Representative images showing technique utilized for achieving preferential gene delivery into mouse corneal endothelium in vivo.
4. Gene therapy updates in various corneal disorders
Trauma, injury and/or infections to the eye lead to corneal dysfunction and vision impairment. Corneal disorders are the 3rd leading cause of blindness in the world according to the World Health Organization. Eight million people in the world, including 1.5 million children, are blind due to corneal abnormalities (Whitcher et al., 2001). Corneal disease and disorders have a broad range of pathology leading to diverse outcomes. Haze, scarring, abnormal wound healing, new blood vessel ingrowth, conjunctivitis, dry eye, graft failure, endothelial defects, and Fuchs’ dystrophy are prevalent corneal problems among many acquired and genetic corneal diseases. Gene therapy holds great promise for treating as well as preventing corneal diseases and disorders. In the field of corneal gene therapy emphasis is more on treating common corneal disorders instead of curing genetic defects. Here we provide an overview of the progress made in the field of corneal gene therapy with a brief description about the disease condition/cause.
4.1. Corneal graft rejection
Keratoplasty is currently used for treating many corneal diseases. According to the Eye Bank Association of America more than 42,600 corneal transplants were performed in the United States in 2010 alone. Although perhaps the most successful transplant procedure, it is not without blemish. Corneal graft rejection due to immunological reaction is the chief cause of failure despite the cornea enjoying an immune privilege status (Williams and Coster, 2010). Post-operative complications and lack of good quality donor corneas are the other concerns. Gene therapy approaches have been tested to improve allograft survival by delivering various therapeutic genes to modulate cellular transport, apoptosis, angiogenesis, and wound healing in vivo (Williams and Coster, 2010). In 2006, the Larkin group reported the delivery of indoleamine 2,3-dioxygenase (IDO) in murine corneal endothelium with lentivirus vector (Beutelspacher et al., 2006). An increase in IDO mRNA and protein levels was detected and IDO expression was found to be intracellular and restricted to the endothelial layer of the cornea. Ironically corneal endothelial pathology accounts for almost 48% of penetrating keratoplasty. Once activated, IDO, an intracellular enzyme, induced by pro-inflammatory cytokines like interferon (IFN) and TNF, leads to the immunoregulatory catabolism of tryptophan via opening of the essential amino acid’s indole ring forming N-formylkynurenine and subsequently L-kynurenine (Higuchi and Hayaishi, 1967). The IDO-mediated diminution of tryptophan, in addition to the extracellular release of tryptophan catabolites, is believed to halt activated T cells in the cell cycle’s G1 phase encouraging immune tolerance (Munn et al., 1999) and apoptosis essential to the survival of transplanted corneas. Full thickness corneal grafts were also used by the Larkin group to study vector-mediated IDO over-expression (Beutelspacher et al., 2006). The study demonstrated significant prolongation of corneal allograft survival after IDO transduction of excised donor cornea ex vivo prior to transplantation compared to GFP and non-IDO-transduced control corneas (Beutelspacher et al., 2006). This demonstrates the potential of IDO gene therapy to thwart corneal allograft rejection. A year later in 2007, the Dana group reported the use of a lentivirus vector to over-express four anti-apoptotic genes (bcl-xL, bcl-2, survivin and p35) in a rodent corneal transplantation model. They found that lentivirus-mediated transfer of bcl-xL gene was not only effective at inhibiting apoptosis in the corneal endothelium in vitro, but also significantly enhanced corneal graft survival in vivo as a 90% success rate was observed at 8 weeks post-transplantation compared to 40% in uninfected controls and 30% in reporter gene controls (Barcia et al., 2007). More recently, the same group studied lentivirus-driven bcl-xL and p35 gene delivery in both primary human endothelial cells and human corneas, and found both genes displayed anti-apoptotic properties in the corneal endothelium although p35 had greater anti-apoptotic efficiency than bcl-xL (Fushsluger et al., 2011). It is also believed that gene therapy at earlier steps like the sensitization of host to foreign antigen may be more efficacious (Williams and Coster, 2010). Other potential gene therapy approaches include utilizing IL4, IL10, CTLA4-Ig, p40-IL12, viral MIPII, and nerve growth factor (Willaims and Coster 2010).
Another avenue for the utilization of gene therapy with regard to corneal transplantation involves corneal endothelial cell density maintenance in corneal tissue procured and stored at eye banks. Recombinant adenovirus-driven over-expression of transcription factor E2F2 ex vivo demonstrated increased endothelial cell count in rabbit and human corneas (Joyce et al., 2004; McAlister 2005). Endothelial cells, normally amitotic, progressed through the cell cycle from G1 to S phase in significant quantities accounting for the boost in the monolayer cell density. E2F2 expression was short lived with expression dropping off 7 d following a 2 hr vector exposure (McAlister et al., 2005). This has great potential in not only the long-term preservation of corneas but also the donation of corneas with low endothelial density previously considered unsuitable for keratoplasty involving endothelial replacement. In addition, the benefits of the vector-mediated transfer of E2F2 may prove beneficial in the treatment of corneal endotheliopathies.
4.2 Corneal scarring and wound healing
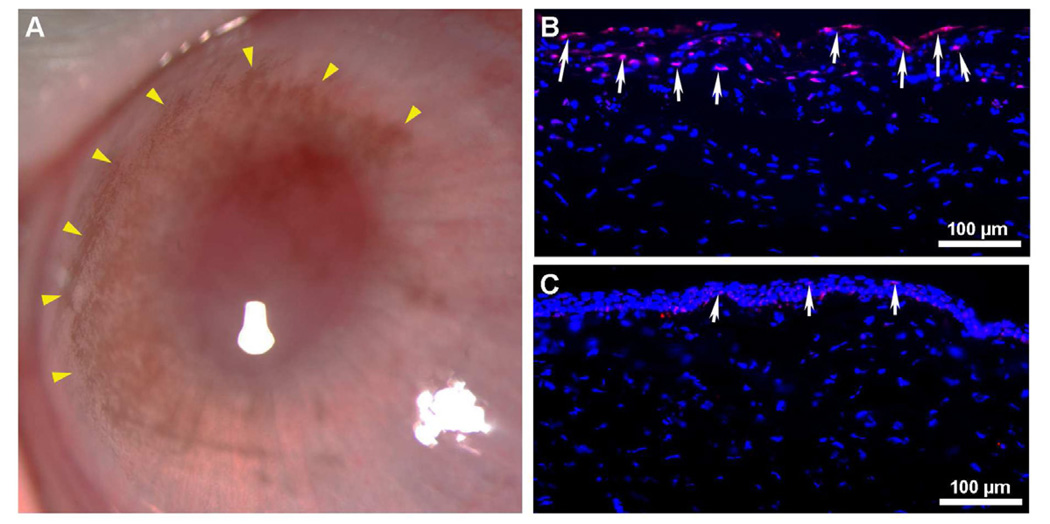
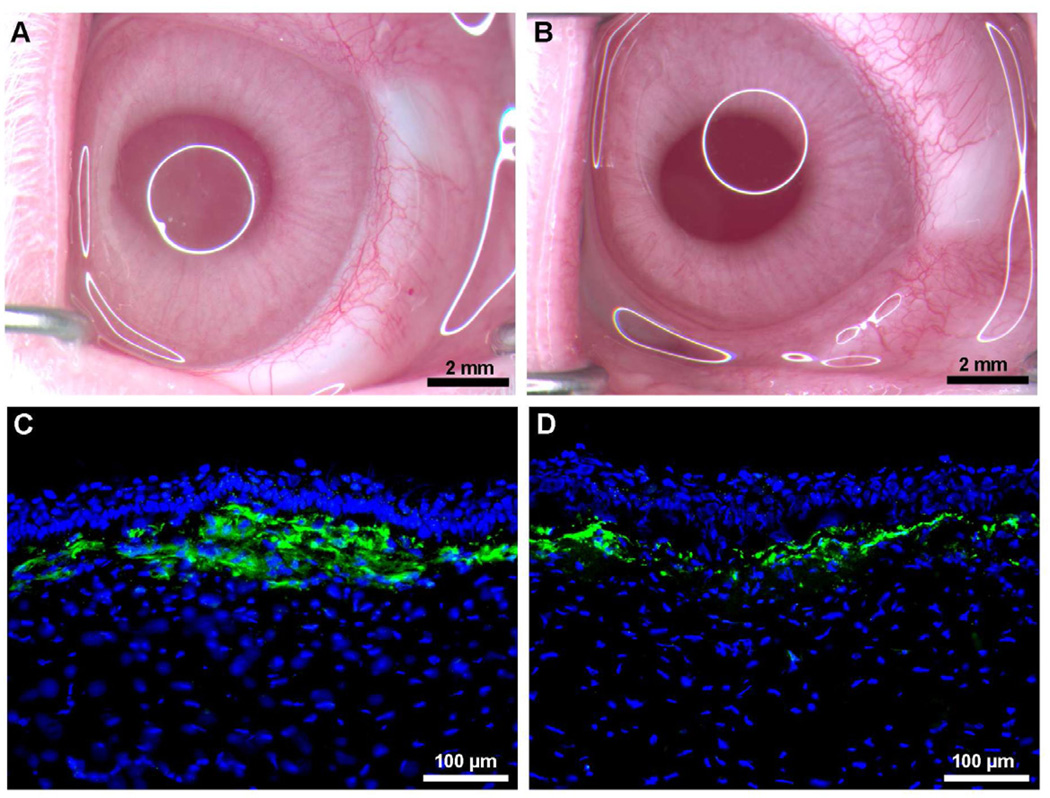
Wound healing plays a central part in the maintenance of corneal transparency and thus normal vision. Corneal injury, regardless of cause, may ignite a dysregulated wound healing response that commonly leads to corneal fibrosis and loss of visual function. Corneal wound healing is an extremely intricate process under the regulation of several cytokines and growth factors. Among several cytokines transforming growth factor beta (TGFβ) has been identified as a chief player in the formation of a myofibroblasts and opacity in the cornea. Thus, it has become a prime target for gene therapy aimed at preventing corneal scarring and other corneal disorders caused by TGFβ. Many investigators share the thought that impeding TGFβ or its signal transduction represents a powerful strategy to modulate uncontrolled corneal healing and prevent or even cure corneal scarring. Recently, using an in vitro model our laboratory demonstrated that decorin over-expression in human corneal fibroblasts significantly inhibits TGFβ-driven keratocyte differentiation to myofibroblasts without compromising cellular viability (Mohan et al., 2010a). Decorin, a member of the small leucine-rich proteoglycan family, is expressed in the corneal stroma and plays an important role in wound healing and structural support in the cornea (Mohan et al., 2011a). Using laser capture technique, we found that photorefractive keratectomy-induced laser injury to the cornea does not alter decorin significantly but dramatically increases TGFβ levels in the rabbit cornea, in vivo (Tandon et al., 2010). This observation concurs with our hypothesis that AAV-mediated decorin gene therapy can effectively reduce corneal scarring in vivo. Indeed, in a very recent study we detected significant inhibition of corneal scarring in the rabbit eye (59–73%; p <0.001 or <0.01) with no adverse effects at one-month time point with AAV5-mediated decorin gene therapy (Mohan et al., 2011b). This study was the first to demonstrate the therapeutic potential of decorin for treating corneal diseases via targeted gene therapy (Fig. 11). Sensing the translational potential of AAV-mediated decorin gene therapy for corneal scarring we are studying whether long-term targeted over-expression of decorin cause any complication to the eye or changes in corneal function or transparency. The clinical and slit lamp examination in the eyes of live rabbit at up to 4 months showed no sign of adverse effects indicating that decorin gene therapy is safe for patients (Mohan et al unpublished data). The pending histological, immunochemical, molecular and transmission electron microscopy studies will validate this notion.
Fig. 11.
Representative stereomicroscopy (A, B) and α-smooth muscle actin immunostaining (C, D) images showing corneal haze inhibition by targeted AAV5-decorin gene therapy in rabbits in vivo. AAV5 viral titer (6.5×1012 vg/ml) was topically applied onto the cornea once for 2 minutes after PRK.
Venturing to block specific targets in TGFβ signaling is another approach used by scientists to develop corneal fibrosis treatment. Numerous studies have shown that TGFβ uses the Smad-pathway to relay its signal to the nucleus leading to fibrosis (Tandon et al., 2010). The translocation of Smad2 and Smad4 proteins during corneal wound healing in vivo has been demonstrated by performing keratectomy and epithelial debridement wounds in Sprague-Dawley rats (Hutcheon et al., 2005). This study motivated us to perform in vitro studies with Smad2, Smad3, Smad4, and Smad7 siRNA to examine whether TGFβ in human corneal fibroblast cells relays its signal via the Smad pathway. The results of our study showed that TGFβ-mediated keratocyte transdifferentiation to myofibroblasts occurs via Smad signaling and that Smad7 is an attractive target to prevent myofibroblast formation. Our findings are in accord with many literature reports as Smad7 gene therapy has been reported to inhibit fibrosis in many non-ocular tissues such as kidneys, peritoneum, liver, etc. (Chen et al., 2011; Dooley et al., 2003). Recently, Galiacy et al. (2011) used gene therapy strategies to hinder excessive collagen deposition in corneal scar development. Using a recombinant AAV-based vector (AAV2/8) the researchers overexpressed a specific fibril collagenase, matrix metalloproteinase (MMP) 14, to prevent collagen deposition and subsequent scarring following incisional injury to the cornea in mice. After microdissecting a 0.75 mm full-thickness button from the central corneal region, a single injection of 10 ul PBS into the stroma in control mice was performed (vector was injected 10 hours prior to incisional injury in the case of AAV2/8-GFP and AAV2/8-MMP14). This previously outlined model encourages the fibroproliferative response during repair including the development of alpha smooth muscle actin (αSMA)-expressing myofibroblasts from stromal keratocytes (Stramer et al., 2003). The investigators demonstrated that a single intrastromal injection with recombinant AAV-MMP14 mediated murine stromal keratocyte gene expression. This was evident with decreased mRNA expression of αSMA and type III collagen, two key gene markers of corneal fibrosis (Galiacy et al., 2011). MMP, like TGFβ, also has important implications in other processes such as neovessel formation.
4.3 Corneal neovascularization
Neovascularization may occur in any of the layers of the cornea following ocular trauma, infection, injury, etc. and leads to corneal opacity. The sprouting of new blood vessels in the cornea from the limbus is closely linked to the inflammatory response and poses a major risk for corneal allograft rejection (Klausner et al, 2007). The mechanism of neovascular formation and regression is tremendously complex involving a slew of cytokines, growth factors, and cell types. However, it is widely accepted that vascular endothelial growth factor (VEGF) plays a pivotal role in the development of new blood vessels. A well-designed study performed by the Ambati group reported that the avascular phenotype of the cornea is due, at least in part, to soluble VEGF receptor-1(sVEGFR-1) or sflt-1, an endogenous trap for VEGF (Ambati et al., 2006). Thus patients with aniridia and mutations in Paired box gene 6 (PAX6) lack sflt-1 and display spontaneous corneal neovascularization (CNV). Gene therapy with sflt-1 may greatly benefit patients with these types of corneal disturbances. Five years earlier in 2001, Lai and cohorts conducted a study in a rat model showing the inhibition of CNV with recombinant adenovirus-mediated delivery of sflt-1 (Lai et al., 2001). Gene therapy utilizing sflt-1 may certainly prove beneficial in averting VEGF-induced neovascularization in the setting of corneal graft rejection and other corneal disorders. A variety of gene therapy methodologies have been probed to stem corneal angiogenesis using experimental models and different transgenes that target VEGF such as decorin, angiostatin, PEDF, vasohibin-1 and VEGF receptors Flt-1 and Flk-1 (Cheng et al., 2007; Jani et al., 2007; Lai et al., 2007; Oh et al., 2010; Kuo et al., 2009; Yu et al., 2007; Zhou et al., 2010; Mohan et al. 2011b). Adenovirus-mediated gene transfer of soluble VEGF receptor-2, sFlk-1 was shown to inhibit VEGF-stimulated proliferation of murine and human umbilical vein endothelial cells (Yu et al., 2007). The same study demonstrated that CNV development in rats in vivo following cauterization is suppressed by anterior chamber injection of adenovirus delivery of sFlk-1 (Yu et al., 2007). Immunohistochemistry studies of corneas from these animals showed localization of protein production in the stroma and endothelium 14 days following cauterization compared to absent sFlk-1 expression in controls (adenovirus-null and adenovirus-GFP). This strongly suggests that adenovirus gene therapy has the ability to transduce corneal cells of the endothelial and stromal layers with sFlk-1, a vital element in the development of angiogenesis (Yu et al., 2007).
Gene therapy strategies using endostatin have also shown promise in treating CNV. Endostatin, a naturally occurring fragment of collagen type XVIII, blocks endothelial cell adhesion, migration and proliferation, and inhibits apoptosis (O'Reilly et al., 1997). Mice eyes treated via single subconjunctival injection with recombinant AAV encoding endostatin following silver nitrate cauterization to induce CNV showed significant inhibition of corneal angiogenesis. Endostatin released from the conjunctiva suppressed the migrating limbal vasculature, was detected at 4 days post-injection, and retained stable levels for more than 8 months. Interestingly no difference in immune response (measured by CD4 and CD8 lymphocyte staining) was noticed in mice treated with recombinant AAV-GFP injection, PBS injection, and no injection (Lai et al., 2007).
Another gene therapy approach to obstruct VEGF-induced CNV development utilized intrastromal injection of intraceptors linked to the endoplasmic reticulum retaining peptide KDEL. Plasmids encoding for specific VEGF binding domains of sFlt-1, Flt23K and Flt24K, coupled to KDEL were delivered via pCMV vector and were shown to interrupt VEGF signaling in vitro and in vivo. These peptides offset intracellular VEGF as they are not liberated from the cell into the extracellular milieu. Interestingly a variation in VEGF downregulation was seen between the two intraceptors with Flt23K suppressing injury induced CNV by 66.8% and Flt24K by almost 50%, possibly due to differences in protein configuration (Singh et al., 2005). The same group then tested the ability of Flt23K to impede corneal neovessel growth using albumin nanoparticles and showed CNV regression up to 40% at 5 weeks post intrastromal injection in a murine model (Jani et al., 2007).
Recently Chen and cohorts evaluated gene therapy with multiple genes known to have antivasculogenic activity in a murine model of CNV (Chen et al., 2010). Retrovirus encoding murine sFlk-1, sTie2, and endostatin genes was used in cell cultures of human umbilical vein endothelial cells (HUVEC) as well as in vivo studies involving subconjunctival injection of vector into corneas of C57B1/6 mice with chemically induced CNV. Studies in vitro showed decreased HUVEC proliferation with obvious additive effects when two or three genes were combined (i.e. sFlk-1/sTie2, msFlk-1/endostatin, sTie2/endostatin, sFlk-1/sTie2/endostatin). However, although HUVEC migration was also decreased by these genes individually, the additive effect seen in proliferation was absent highlighting the various pathways involved in these intricate processes of CNV. For the in vivo segment of the study, gene combination showed efficient CNV inhibition specifically downregulating levels of IL-1β, MMP-9, and VEGF as measured by western blotting and RT-PCR. In addition, the combinations of sFlk-1/endostatin and sFlk-1/sTie2/endostatin were found to significantly decrease nuclear factor (NF)-κB phosphorylation and super repressor inhibitor kappa Bα (IkBα) expression without regard to total NF-κB quantity (Chen et al., 2010). This has direct implications to CNV as NF-κB leads to VEGF expression while IkBα inhibits NF-κB by maintaining it in an inactive complex in the cytoplasm (Josko and Mazurek, 2004; Karin, 1999).
Combining genes to thwart CNV is not a new concept. For example, an almost decade old report from Casey Eye Institute evaluated lentivirus transfer of a fusion protein in an allogenic transplantation model of CNV. Lentivirus was employed to transfect rabbit corneal buttons ex vivo with a fusion gene made up of endostatin and the kringle 5 domain of plasminogen. This small specific plasminogen fragment is known to display robust inhibition of angiogenesis. Corneas transduced with the endostatin and kringle 5 fusion protein showed decreased proliferation and migration of endothelial cells and subsequently less postoperative neovascularization and inflammation in comparison to controls (Murthy et al., 2003). Other kringle elements have also been assessed for their antiangiogenic properties. Angiostatin is a product of proteolytic cleavage that comprises the first four kringle domains of plasminogen. One group examined the role of subconjunctival injection of AAV carrying angiostatin in a rat model of alkali-induced CNV. The researchers illustrated corneal gene transduction of angiostatin and a post-operative day 7 mean CNV area of 15.83±0.55 mm2 in untreated controls and 6.87±2.23 mm2 in angiostatin treated animals reflecting 56.6% abatement of CNV due to angiostatin (Cheng et al., 2007).
In another study, mouse corneas were transfected with DNA plasmid encoding IL18 using intrastromal injection. The angiostatic property of IL18 is well known making it potentially useful in the treatment of ocular disorders associated with CNV such as herpes keratitis. The investigators showed a decrease in VEGF production in IL18 treated eyes and subsequently a weakened immunoinflammatory response compared to controls. Furthermore, bFGF induced MBE cell lines treated with IL18 demonstrated diminished VEGF production and gene expression in contrast to cells incubated with OVA (Kim et al., 2005).
Vasohibin-1, an endothelium-derived negative feedback modulator of neovessel formation characterized by Watanabe, has also been studied in gene therapy studies as a means to inhibit CNV (Watanabe et al., 2004; Zhou et al., 2010). A 2010 report by Zhou and colleagues described the subconjunctival injection of adenovirus encoding vasohibin-1 that resulted in significant CNV reduction. It was speculated that the antiangiogenic nature of vasohibin-1 may be related to the downregulation of VEGF type 2 receptor (Zhou et al., 2010).
Other gene transfer studies have assessed the utility of CD36 (Mwaikambo et al., 2006), pigment epithelium derived factor (Kuo et al., 2009), and GA binding protein (Yoon et al., 2009) among other approaches to treating CNV. Future experiments may include vector mediated delivery of inhibitors of angiogenesis derived from basement membrane such as neostatin, restin, arresten, canstatin, and tumstatin (Ellenberg et al., 2010). Recently, our laboratory detected significant CNV reduction in rabbit eyes after targeted decorin gene therapy using AAV5 vector (Mohan et al., 2011c). CNV in rabbit eyes was induced by VEGF implantation and AAV expressing decorin or GFP gene was topically applied for two minutes on the rabbit cornea one day after VEGF implantation. As shown in Fig. 12, targeted decorin delivery in the stroma showed (52–66% p<0.05, p<0.001 or p<0.01) reduction at various stages of CNV in rabbit model (Mohan et al., 2011c).
Figure 12.
Representative stereomicroscopy (A, B) and H & E (C, D) images showing VEGF-induced corneal neovascularization inhibition in rabbits in vivo by AAV5-decorin gene therapy. Decorin gene was delivered in the rabbit stroma by a single topical application of AAV5 titer (5×1012 vg/ml) on the cornea for 2 minutes.
4.4 Corneal alkali burn
Alkali burn injury to the cornea is a major clinical entity that leads to sight-threatening sequelae such as corneal scarring, neovessel formation, and ulceration. Scores of preclinical studies have been dedicated to finding ways to halt the progression of these negative after effects of alkali burn. In 2007 the Saika group outlined the use of adenovirus to introduce peroxisome proliferator-activated receptor-γ (PPARγ) to keratocytes in a mouse alkali burn model (Saika et al, 2007). Gene therapy employing PPARγ, a nuclear hormone receptor with immunomodulatory functions, has been evaluated extensively for non-ocular tissues (Lewis et al., 2008; Klotz et al. 2009; Kulkarni et al., 2011). In the cornea, a faint expression of PPARγ was noted via immunohistochemistry in the undamaged corneal epithelium as well as corneas subjected to NaOH and treated with Cre-adenovirus. Conversely PPARγ in burned corneas treated with adenovirus-PPARγ showed strong protein expression in stromal and newly generated epithelial layers (Saika et al., 2007). PPARγ inhibited TGFβ1-induced fibroblast to myofibroblast transdifferentiation as well as the production of profibrogenic factors TGFβ1, TGFβ2, and connective tissue growth factor (CTGF) in addition to collagen type Iα2 and MMP2/9. Corneal epithelium recovery and reconstruction of the basement membrane were also encouraged by PPARγ gene delivery in healing mice corneas in vivo. This study also showed that in the cornea PPARγ blocks a critical step in Smad signaling, the nuclear entry of phosphorylated Smad2/3 (Saika et al., 2007). Another study from same authors reported that adenovirus-mediated Smad7 overexpression in a murine eye accelerated corneal wound healing and restricted corneal opacity (Saika et al., 2005a). Furthermore, adenovirus introduction of bone morphogenic protein 7 (BMP7) was found to promote corneal reepithelialization, inhibit myofibroblast production and monocyte/macrophage infiltration, as well as downregulate TGFβ, MCP-1, and collagen expression in the stroma (Saika et al., 2005b). BMP7 over-expression activated Smad1, 5, and 8 and partly inhibited phosphorylated Smad2. However, it was unsuccessful at suppressing levels of VEGF and the development of NaOH-induced CNV (Saika et al, 2005b). Apparently, none of the previously discussed studies investigated the downside of PPARγ, SMAD7, or BMP7 gene transfer in the cornea as no information regarding side effects or adverse reactions has been reported.
4.5 Other corneal disorders
Corneal inflammation due to ocular herpes simplex virus (HSV) infection, or herpes stromal keratitis (HSK), is a leading cause of infectious blindness worldwide particularly in more developed nations including the U.S. Recurrent infections often lead to corneal scarring, neovascularization, and thinning resulting in visual abnormalities and blindness (Kaye and Choudhary, 2006). Many studies have shared the common goal of developing a vaccine using gene transfer technology to enhance protection against ocular HSV. These studies have used individual HSV-1 glycoproteins (i.e. gD) as well as a cocktail of glycoproteins and have shown variable success in controlling HSV keratitis (Sharma et al., 2010a). The recent development of a humanized HLA-Tg rabbit model will hopefully assist in the design of future vaccines for HSV-1 keratitis as it demonstrates spontaneous HSV reactivation leading to recurrent HSK comparable to what is seen clinically. Moreover, this rabbit model expresses human, rather than rabbit, HLA class I proteins (Hu et al., 2006; Dasgupta et al., 2009).
In the past siRNAs and RNA enzymes have shown promise in cell culture experiments but their instability in vivo has been a barrier to their translation into clinical use. A group of researchers from different medical disciplines at the University of Florida converged to engineer a hammerhead ribozyme with a specific target, the mRNA of an essential late HSV-1 gene, UL20. Ribozymes are RNA molecules that possess the ability to catalyze the cleavage and formation of covalent bonds in RNA strands at specific sites. The “hammerhead” motif is the smallest (approximately 30-nucleotides long) and best characterized small catalytic ribozyme (Citti and Rainaldi, 2005, Mulhbacher et al., 2010). Gene-tailored ribozymes have been designed, produced, and given to cells to knock down the expression of specific genes (Citti and Rainaldi, 2005)..The investigators in the aforementioned study used iontophoresis and an adenovirus vector to deliver the ribozyme in rabbit skin cells in addition to rabbit eyes in vivo and discovered significant ribozyme-induced UL20 inhibition leading to a subsequent decrease in viral load in vitro and decreased ocular HSV-1 severity in vivo (Liu J, et al. IOVS 2006;47:ARVO E-Abstract 3080). A follow up study by the same group used the ribozyme to decrease HSV-1 severity in a murine footpad model (Liu et al., 2008b). These findings give credence to the value of RNA gene therapy for HSV-1 by targeting late genes required for HSV-1 survival and may find a niche in the treatment of ocular herpes including HSK in the future. Another group used gene gun method to deliver gold particles carrying plasmid DNA encoding specific interleukins to determine effect in HSK. A one time low dose (1 µg/shot) of IL4 or IL10 plasmid DNA was found to significantly abrogate the clinical course of HSK when administered at 300 psi helium pressure with minimal alterations in immune response systemically. IL6, shown to play a role in HSK development (Banerjee et al., 2004), was also downregulated. In addition, gene gun utilization did not lead to significant corneal damage as ascertained by histology and clinical examination (Bauer et al., 2006).
Diabetic disease of the cornea is a common occurrence in patients with type I and II diabetes mellitus and can lead to sight-threatening corneal neuropathy and epitheliopathy. Currently, no treatment exists to thwart the progression or cure the disease and treatment regimens are largely symptomatic. Saghizadeh et al. (2010) recently used recombinant AV to overexpress the hepatocyte growth factor (HGF) receptor c-met proto-oncogene in corneal epithelium of diabetic human corneas in vitro. Their past studies outlined disturbances in HGF/c-met signaling in diabetic corneas as c-met expression was reduced in the face of upregulated HGF expression (Saghizadeh et al., 2005). With the introduction of exogenous c-met via recombinant AV vector they reported improved reestablishment of HGF signaling and subsequent restoration of marker protein patterns and enhanced wound healing (Saghizadeh et al., 2010).
Mucopolysaccharidosis (MPS) is a set of metabolic disorders characterized by the inability to breakdown glycosaminoglycans (GAG) due to lysosomal enzyme deficiency. GAGs thus accumulate in lysosomes systemically as well as in the eyes (Ashworth et al., 2006). MPS VII, or Sly disease, is due to β-glucuronidase (GUSB) insufficiency leading to the stockpiling of GAGs (dermatan, heparan, and chondroitin sulphate) while MPS VI, or Maroteaux–Lamy syndrome, results from aryl N-acetylgalactosamine-4-sulfatase deficiency leading to accumulation of dermatan sulphate (Ashworth et al., 2006). Pathology is common in the ocular system and results in visual impairment due to opacification of the cornea and disorders of other ocular tissues (Ashworth et al., 2006). While lysosomal accumulation occurs in the corneal endothelium, it is their storage in keratocytes that causes corneal opacity (Williams and Coster, 2010). Systemic gene therapy using a retroviral vector has been shown to avert GAG accrual and ensuing corneal clouding due to MPS using neonatal canines (Wang et al., 2006; Traas et al., 2007; Ponder and Auricchio, 2010). Even 6.5–8 years following therapy, little or no corneal opacity was detected in dogs with MPS VII (Ponder and Auricchio, 2010). Gene therapy may have a role as an alternative therapeutic strategy for MPS disorders by introducing genes that encode for specific enzymes, such as GUSB, clearing lysosome build up in the eye and other tissues.
5. Lessons learned from corneal genetic studies: humans and animals
Advances in molecular biology have led to the identification of numerous genes and gene mutations associated with dystrophic diseases of the cornea. Different modes of Mendelian inheritance are evident in corneal dystrophies likely due to alterations in several genes including CHST6, KRT3, TGFBI, and UBIAD1 (Klintworth, 2009). The TGFβ induced gene (TGFBI), commonly known as βig-h3, encodes the TGFBI protein (TGFBIp or keratoepithelin). Changes in the genomic sequence of TGFBI have been linked to several dystrophies of the corneal stroma such as Lattice dystrophy type I, Reis-Buckler dystrophy, Thiel-Behnke corneal dystrophy, granular corneal dystrophy type I, and granular corneal dystrophy II (Avellino corneal dystrophy) (Runager et al., 2008; Klintworth, 2009). Indeed 38 different mutations in the TGFBI gene are responsible for various corneal dystrophies, the majority being missense mutations (Runager et al., 2008). TGFBIp functions as a cell adhesion molecule acting by way of integrins (α1β1, α3β1, αvβ3, etc) and elements of the ECM including proteoglycans (i.e. decorin), fibronectin, and collagens (Runager et al., 2008). The precise function of these interactions remains to be identified although TGFBIp may play a role as a regulatory modulator between the ECM and cells found in its supporting meshwork (Runager et al., 2008). Dystrophic corneas contain aggregates of unknown makeup although increased TGFBIp has been detected in addition to clusterin, a proaggregation protein, in Fuchs endothelial corneal dystrophy (Runager et al., 2008; Jurkunas et al., 2009). A recent report identified the two corneal dystrophies, gelatinous drop-like corneal dystrophy and spheroidal degeneration, in the same patient (Zhang and Yao, 2010). Upon molecular genetic examination a new missense mutation in the tumor associated calcium signal transducer 2 (TACSTD2) gene was unearthed (Zhang and Yao, 2010). Earlier it was found that alterations in the UbiA prenyltransferase domain containing 1 gene are responsible for Schnyder corneal dystrophy (Weiss et al., 2007). The corneal endothelial defect was found to be caused, at least in part, by variations in the transcription factor 4 (TCF4) gene (Baratz et al., 2010). The knowledge of gene localization is paramount in creating an animal model of disease and treatment approaches via gene therapy. Previously it was thought that type I and II macular corneal dystrophy were due to genetic aberrations of carbohydrate sulfotransferase 6 (CHST6) in the epithelial layer (Abbruzzese et al., 2004) and that limbal stem cell therapy might possibly offer benefits to patients in terms of therapy. It was recently shown however, that CHST6 along with keratan sulfate are chiefly the stromal products of keratocytes underscoring the need for therapeutic modalities, including gene therapy, to target stromal keratocytes (Di Iorio et al., 2010).
Nonetheless, the lack of in vivo animal models has sharply hindered progress in the development of gene therapy options for patients suffering from corneal dystrophies (Weiss, 2010). Furthermore, the pathogenesis and localization of genes responsible for many of these disorders has yet to be elucidated. Recently, an animal model mimicking human corneal dystrophy was reported by Chen and colleagues in 2010. A mouse model of congenital stromal corneal dystrophy, caused by a faulty decorin gene leading to corneal clouding, was created utilizing a Cre-on approach. It was also surmised that mutations in decorin accounting for the disorganized stromal architecture may be influenced by class II SLRPs lumican and keratocan (Chen S, et al. IOVS 2010; 51:ARVO E-Abstract 3832). It is conceivable that keratocytes may induce an increase in GAG chain and SLRP production to offset deficiencies in stromal ECM GAGs or SLRPs brought about by genetic causes. The tissue-selective targeted gene-based therapeutic approaches we are attempting to define for the cornea have great potential for corneal genetic dystrophies.
6. Ocular gene therapy clinical trials: current status
Currently 20 gene therapy trials for eye disease are listed on The Journal of Gene Medicine Clinical Trial website. Out of 20 gene therapy clinical trials, 18 are directed towards retinal disorders, 1 for glaucoma, and 1 for the cornea. The lone corneal gene therapy Phase I/II clinical trial is focused on evaluating the safety and efficacy of a matrix-targeted retroviral vector bearing a dominant negative cyclin G1 construct (Mx-dnG1) as adjunctive intervention for superficial corneal opacity/corneal scarring. The findings of the clinical trial assessing gene therapy for diseases of the cornea have not yet been published.
7. Future directions: regenerative medicine, nanomedicine and other approaches
The future of gene therapy for the cornea is very promising and involves different branches of medicine including regenerative medicine and nanotechnology. Indeed, nanotechnology will lead to the development of regenerative medicine, microsensors and feedback apparatus, improved imaging in vivo, and artificial vision (Zarbin et al., 2010a). Regenerative nanomedicine is a new field in medicine gaining widespread attention. It entails the use of nanoparticles containing regulatory molecules such as gene transcription factors that allow for cellular reprogramming in vivo (Zarbin et al., 2010a). Nanoparticles may not only serve as vectors for therapeutic gene delivery but may also act as biocarriers of genes which may assist in the diagnosis and monitoring of corneal disease. This process of coupling disease diagnosis with targeted treatment is termed theragnostics and has enormous potential in the ocular system including the cornea. For example, oxidative stress has been implicated in numerous inflammatory, metabolic, traumatic, and iatrogenic corneal diseases (Shoham et al., 2008). By coupling a therapeutic gene such as peroxidase, superoxide dismutase, or catalase (as well as a marker gene like GFP) to nanoparticles capable of activating antioxidant response element, one may produce a nano-device with dual diagnostic-therapeutic effects enabling corneal cells to treat themselves following oxidative damage (Zarbin et al., 2010b).
Fagerholm and colleagues recently reported the results of a clinical study in Sweden involving corneal regeneration with recombinant human collagen. Recombinantly produced human collagen type III was cross-linked with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) and fashioned into substitutes with human corneal dimensions. This biomimetic substitute was then transplanted into patients following anterior lamellar keratoplasty. The investigators found that a cell-free corneal substitute imitating the cornea’s unique ECM can successfully provide support for endogenous restoration of corneal epithelium, stroma, and nerves. Of note was the absence of inflammation, neovascularization, or rejection that plagues recipients of human donor corneas (Fagerholm et al., 2009). Although the study had a small patient population (n = 10), it clearly highlights the safety profile of recombinant human collagen and cross-linking methods (Fagerholm et al., 2009). Preliminary results from the study demonstrate improved visual acuity, ocular surface quality, and corneal sensitivity in patients with a biomimetic corneal substitute. These corneal substitutes may prove beneficial in circumstances necessitating short-term or emergency corneal replacements, where availability of human donor corneas is poor or nonexistent and where prostheses are unfavorable (Fagerholm et al., 2009). In the future these biomimetic corneas may serve as efficient permanent corneal replacements and a viable option to human corneal tissue in transplantation. These substitutes in combination with single or multiple gene therapy may find a niche in the treatment of recurrent CNV and other disorders of the ocular surface.
Cell-based therapy for corneal diseases has also been examined. A study from the Funderburgh group (Du et al., 2009) used stem cells isolated from adult human corneal stroma to rectify stromal opacity due to SLRP lumican deficiency in mice via intrastromal injection of cells. Stem cells subsisted in wild-type mice for months without host cell fusion or provoking an immune T-cell response. Human stromal stem cells introduced into the stroma of lumican-null mice with corneal opacity (similar in nature to that of scar tissue due to disorganized stromal collagen) led to rectification of fibrillar collagen defects and stromal thickness and restoration of corneal transparency (Du et al., 2009). Thus stem cell therapy shows proof of principle in treating corneal disease through the regeneration of corneal tissue in a safe and efficacious manner similar to organogenesis. The results of a remarkable clinical study in Italy involving autologous limbal cell treatment were recently reported (Rama et al., 2010). Currently limbal stem cells, a group of cells responsible for corneal epithelial renewal and regeneration, are transplanted as a treatment for limbal stem cell deficiency (Pellegrini et al., 2009). 112 patients with burn-related corneal destruction associated with limbal stem-cell deficiency were treated with autologous limbal stem cells cultivated on fibrin. Corneal transparency was restored in 76.6% of eyes up to 10 years after corneal transplantation with limbal stem-cell grafts identifying limbal stem cell cultures as a source of transplantable cells for treating corneal burns (Rama et al., 2010).
Future studies involving gene therapy for corneal diseases may include non-coding RNA (ncRNA). ncRNA includes several types of functional RNA molecules not translated into proteins that play key roles in several cellular processes such as transfer RNA, ribosomal RNA, small interfering RNAs, microRNAs, PIWI-associated RNAs, small nucleolar RNAs, promoter-associated RNAs, and telomere specific small RNAs. It has been shown that ncRNAs play a part in oncogenesis and may be a factor in molecular aberrations of other diseases (Galasso et al., 2010).
In the recent past, toll like receptors (TLRs) have gained attention in the cornea as their activation sets off a multifaceted signaling pathway instigating innate and adaptive immunity generating inflammatory cytokines and co-stimulatory molecules (Redfern and McDermott, 2010). The production of chemokines encourages corneal infiltration of macrophages and neutrophils resulting in the functional loss of corneal epithelium, stroma, and endothelium leading to the loss of corneal transparency and visual activity (Kanwar et al., 2010). The modulation of TLR signaling via gene therapy methods may play a vital role in blocking immune-mediated corneal graft rejection as well as aberrant wound healing following corneal infection.
The future will reveal animal models of human corneal disease in which to test highly efficient viral or nonviral vectors with low-immunogenicity and sustained transgene expression in combination with cell-based therapy and regenerative approaches. Presently the cornea lags behind the retina in gene therapy for various reasons including lack of animal models of inherited disease for experimentation. Currently available technologies are likely nontoxic and effective, at least in the short term, with little further development (Weiss, 2010).
8. Conclusions
The last five to six years have brought about great progress and advancement in the field of corneal gene therapy. The increased understanding of the molecular mechanisms and course of inherited and acquired corneal diseases have allowed for the submergence of targets for gene-based therapy. The testing of various viral and non-viral vectors and multiple delivery systems has been the key to the success observed in various models of corneal disease. We along with other investigators have examined the efficacy of gene therapy using in vitro, ex vivo, and in vivo animal (equine, murine, canine, etc) and human corneal tissues, identified several efficient AAV serotypes and nanoparticles, and defined simple minimally invasive vector delivery techniques for delivering genes precisely into targeted cells (keratocytes and endothelium) of the cornea in vivo. Furthermore, using a combination of vector and delivery techniques, we have defined tissue-selected targeted gene therapy approaches for the cornea. Although much of the corneal gene therapy investigations have been accomplished in animals, there is a dearth of research that has been translated fully from bench to bedside including the clinic. The progress in establishing corneal gene therapy clinical trials has been meager compared to gene therapy trials conducted in other ocular tissues. Even though the cornea is easily accessible for gene transfer, it is lagging far behind the retina in terms of clinical application of gene therapy for human patients. The future is bright however, as novel innovations and treatment approaches have proved effective in animal models (i.e. rabbits) that closely resemble the human cornea in terms of anatomy, wound healing response, and function.
Over the past few years, much effort has been made to develop strategies for effective delivery of DNA to the nucleus of target cells. The most pertinent question concerning the success of gene therapy is “how to introduce therapeutic genes into target cells/tissues?” Vectors are important tools for introducing foreign genes into cells and are one of the two major determinants for the success of gene therapy, the other being gene delivery technique. Several viral and non-viral vectors have been developed and each has its own advantages as well as limitations. In addition, many vector-delivery techniques have been developed to enhance vector efficiency and achieve target specificity. With the discovery of animal models of human corneal disease and the combination of gene therapy approaches with stem cell therapy and regenerative medicine, the cure for corneal blindness may well become a reality within the next decade.
Article highlights.
-
➢
Next generation AAV vectors for corneal gene therapy.
-
➢
Nanoparticles are promising nonviral vectors for corneal gene therapy.
-
➢
Delivery techniques role in tissue-targeted corneal gene delivery in vivo.
-
➢
Decorin gene therapy could be a potential treatment for corneal blindness.
-
➢
Translation of corneal gene therapy requires further research.
Acknowledgements
The work was supported from the RO1EY17294 (R.R.M.) National Eye Institute, NIH, Bethesda, MD, NEI/NIH RO1EY17294S2 Diversity Supplement (J.C.K.T.), 1I01BX00035701 (R.R.M.) Veteran Health Affairs, Washington, DC, and an Unrestricted Research to Prevent Blindness, New York, NY grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbruzzese C, Kuhn U, Molina F, Rama P, De Luca M. Novel mutations in the CHST6 gene causing macular corneal dystrophy. Clin Genet. 2004;65:120–125. doi: 10.1111/j.0009-9163.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Adriaansen J, Vervoordeldonk MJ, Tak PP. Gene therapy as a therapeutic approach for the treatment of rheumatoid arthritis: innovative vectors and therapeutic genes. Rheumatology (Oxford) 2006;45:656–668. doi: 10.1093/rheumatology/kel047. [DOI] [PubMed] [Google Scholar]
- Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 2006;80:9831–9836. doi: 10.1128/JVI.00878-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv Exp Med Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, Kleinman ME, Caldwell RB, Lin Q, Ogura Y, Orecchia A, Samuelson DA, Agnew DW, St Leger J, Green WR, Mahasreshti PJ, Curiel DT, Kwan D, Marsh H, Ikeda S, Leiper LJ, Collinson JM, Bogdanovich S, Khurana TS, Shibuya M, Baldwin ME, Ferrara N, Gerber HP, De Falco S, Witta J, Baffi JZ, Raisler BJ, Ambati J. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth JL, Biswas S, Wraith E, Lloyd IC. Mucopolysaccharidoses and the eye. Surv Ophthalmol. 2006;51:1–17. doi: 10.1016/j.survophthal.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–1197. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL-6-induced neovascularization. J Immunol. 2004;172:1237–1245. doi: 10.4049/jimmunol.172.2.1237. [DOI] [PubMed] [Google Scholar]
- Baratz KH, Tosakulwong N, Ryu E, Brown WL, Branham K, Chen W, Tran KD, Schmid-Kubista KE, Heckenlively JR, Swaroop A, Abecasis G, Bailey KR, Edwards AO. E2-2 protein and Fuchs's corneal dystrophy. N Engl J Med. 2010;363:1016–1024. doi: 10.1056/NEJMoa1007064. [DOI] [PubMed] [Google Scholar]
- Barcia RN, Dana MR, Kazlauskas A. Corneal graft rejection is accompanied by apoptosis of the endothelium and is prevented by gene therapy with bcl-xL. Am J Transplant. 2007;7:2082–2089. doi: 10.1111/j.1600-6143.2007.01897.x. [DOI] [PubMed] [Google Scholar]
- Bauer D, Lu M, Wasmuth S, Li H, Yang Y, Roggendorf M, Steuhl KP, Heiligenhaus A. Immunomodulation by topical particle-mediated administration of cytokine plasmid DNA suppresses herpetic stromal keratitis without impairment of antiviral defense. Graefes Arch Clin Exp Ophthalmol. 2006;244:216–225. doi: 10.1007/s00417-005-0070-z. [DOI] [PubMed] [Google Scholar]
- Bemelmans AP, Arsenijevic Y, Majo F. Efficient lentiviral gene transfer into corneal stroma cells using a femtosecond laser. Gene Ther. 2009;16:933–938. doi: 10.1038/gt.2009.41. [DOI] [PubMed] [Google Scholar]
- Berdahl JP, Johnson CS, Proia AD, Grinstaff MW, Kim T. Comparison of sutures and dendritic polymer adhesives for corneal laceration repair in an in vivo chicken model. Arch Ophthalmol. 2009;127:442–447. doi: 10.1001/archophthalmol.2008.582. [DOI] [PubMed] [Google Scholar]
- Beutelspacher SC, Ardjomand N, Tan PH, Patton GS, Larkin DF, George AJ, McClure MO. Comparison of HIV-1 and EIAV-based lentiviral vectors in corneal transduction. Exp Eye Res. 2005;80:787–794. doi: 10.1016/j.exer.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Beutelspacher SC, Pillai R, Watson MP, Tan PH, Tsang J, McClure MO, George AJ, Larkin DF. Function of indoleamine 2,3-dioxygenase in corneal allograft rejection and prolongation of allograft survival by over-expression. Eur J Immunol. 2006;36:690–700. doi: 10.1002/eji.200535238. [DOI] [PubMed] [Google Scholar]
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision Res. 2008;48:319–324. doi: 10.1016/j.visres.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta MK, Kumar A, McDermott AM, Cai C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules. 2007;8:1807–1811. doi: 10.1021/bm0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson EC, Liu CY, Yang X, Gregory M, Ksander B, Drazba J, Perez VL. In vivo gene delivery and visualization of corneal stromal cells using an adenoviral vector and keratocyte-specific promoter. Invest Ophthalmol Vis Sci. 2004;45:2194–2200. doi: 10.1167/iovs.03-1224. [DOI] [PubMed] [Google Scholar]
- Challa P, Luna C, Liton PB, Chamblin B, Wakefield J, Ramabhadran R, Epstein DL, Gonzalez P. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol Vis. 2005;11:425–430. [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Huang XR, Wang W, Li JH, Heuchel RL, Chung AC, Lan HY. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes. 2011;60:590–601. doi: 10.2337/db10-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Yin H, Wang Y, Mi J, He W, Xie L, Wang Y. Multi-gene targeted antiangiogenic therapies for experimental corneal neovascularization. Mol Vis. 2010;16:310–319. [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Yeh SI, Tsao YP, Kuo PC. Subconjunctival injection of recombinant AAV-angiostatin ameliorates alkali burn induced corneal angiogenesis. Mol Vis. 2007;13:2344–2352. [PubMed] [Google Scholar]
- Chikama T, Hayashi Y, Liu CY, Terai N, Terai K, Kao CW, Wang L, Hayashi M, Nishida T, Sanford P, Doestchman T, Kao WW. Characterization of tetracycline-inducible bitransgenic Krt12rtTA/+/tet-O-LacZ mice. Invest Ophthalmol Vis Sci. 2005;46:1966–1972. doi: 10.1167/iovs.04-1464. [DOI] [PubMed] [Google Scholar]
- Citti L, Rainaldi G. Synthetic hammerhead ribozymes as therapeutic tools to control disease genes. Curr Gene Ther. 2005;5:11–24. doi: 10.2174/1566523052997541. [DOI] [PubMed] [Google Scholar]
- Dannowski H, Bednarz J, Reszka R, Engelmann K, Pleyer U. Lipid-mediated gene transfer of acidic fibroblast growth factor into human corneal endothelial cells. Exp Eye Res. 2005;80:93–101. doi: 10.1016/j.exer.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines. 2009;8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- de la Fuente M, Seijo B, Alonso MJ. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008a;15:668–676. doi: 10.1038/gt.2008.16. [DOI] [PubMed] [Google Scholar]
- de la Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest Ophthalmol Vis Sci. 2008b;49:2016–2024. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- Degoricija L, Johnson CS, Wathier M, Kim T, Grinstaff MW. Photo cross-linkable Biodendrimers as ophthalmic adhesives for central lacerations and penetrating keratoplasties. Invest Ophthalmol Vis Sci. 2007;48:2037–2042. doi: 10.1167/iovs.06-0957. [DOI] [PubMed] [Google Scholar]
- Di Iorio E, Barbaro V, Volpi N, Bertolin M, Ferrari B, Fasolo A, Arnaldi R, Brusini P, Prosdocimo G, Ponzin D, Ferrari S. Localization and expression of CHST6 and keratan sulfate proteoglycans in the human cornea. Exp Eye Res. 2010;91:293–299. doi: 10.1016/j.exer.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–191. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adenoassociated virus vectors: Routing to the late endosomal compartment and proteasome degradation. J Virol. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratviman-Storobinsky O, Lubin BC, Hasanreisoglu M, Goldenberg-Cohen N. Effect of subconjuctival and intraocular bevacizumab injection on angiogenic gene expression levels in a mouse model of corneal neovascularization. Mol Vis. 2009;15:2326–2338. [PMC free article] [PubMed] [Google Scholar]
- Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW, Funderburgh JL. Stem cell therapy restores transparency to defective murine corneas. Stem Cells. 2009;27:1635–1642. doi: 10.1002/stem.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durairaj C, Kadam RS, Chandler JW, Hutcherson SL, Kompella UB. Nanosized dendritic polyguanidilyated translocators for enhanced solubility, permeability, and delivery of gatifloxacin. Invest Ophthalmol Vis Sci. 2010;51:5804–5816. doi: 10.1167/iovs.10-5388. [DOI] [PubMed] [Google Scholar]
- Ellenberg D, Azar DT, Hallak JA, Tobaigy F, Han KY, Jain S, Zhou Z, Chang JH. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. 2010;29:208–248. doi: 10.1016/j.preteyeres.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eye Bank Association of America. Statistical Report. [Accessed January 26, 2011];2009 Available at: http://www.corneas.org/repository/images/pressimages/EBAA%202009%20Statistical%20Report%20-%20Final.pdf.
- Eye Bank Association of America. Statistical Report. 2010 [Google Scholar]
- Fagerholm P, Lagali NS, Carlsson DJ, Merrett K, Griffith M. Corneal Regeneration Following Implantation of a Biomimetic Tissue-Engineered Substitute. Clinical and Translational Science. 2009;2:162–164. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsluger TA, Jurkunas U, Kazlauskas A, Dana R. Corneal Endothelial Cells Are Protected from Apoptosis by Gene Therapy. Hum Gene Ther. 2011;22:549–558. doi: 10.1089/hum.2010.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasso M, Elena Sana M, Volinia S. Non-coding RNAs: a key to future personalized molecular therapy? Genome Med. 2010;2:12. doi: 10.1186/gm133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiacy SD, Fournié P, Massoudi D, Ancèle E, Quintyn JC, Erraud A, Raymond-Letron I, Rolling F, Malecaze F. Matrix metalloproteinase 14 overexpression reduces corneal scarring. Gene Ther. 2011;18:462–468. doi: 10.1038/gt.2010.159. [DOI] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Gao G, Lu Y, Calcedo R, Grant RL, Bell P, Wang L, Figueredo J, Lock M, Wilson JM. Biology of AAV serotype vectors in liver-directed gene transfer to nonhuman primates. Mol Ther. 2006;13:77–87. doi: 10.1016/j.ymthe.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Yue Y, Long C, Bostick B, Duan D. Efficient whole-body transduction with trans-splicing adeno-associated viral vectors. Mol Ther. 2007;15:750–755. doi: 10.1038/sj.mt.6300081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, Pleyer U, Vogt K, Anegon I, Flügel A, Volk HD, Ritter T. Local overexpression of nerve growth factor in rat corneal transplants improves allograft survival. Invest Ophthalmol Vis Sci. 2007a;48:1043–1052. doi: 10.1167/iovs.06-1084. [DOI] [PubMed] [Google Scholar]
- Gong N, Pleyer U, Volk HD, Ritter T. Effects of local and systemic viral interleukin-10 gene transfer on corneal allograft survival. Gene Ther. 2007b;14:484–490. doi: 10.1038/sj.gt.3302884. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, Clappier E, Caccavelli L, Delabesse E, Beldjord K, Asnafi V, MacIntyre E, Dal Cortivo L, Radford I, Brousse N, Sigaux F, Moshous D, Hauer J, Borkhardt A, Belohradsky BH, Wintergerst U, Velez MC, Leiva L, Sorensen R, Wulffraat N, Blanche S, Bushman FD, Fischer A, Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006;79:248–253. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- Hao J, Li SK, Liu CY, Kao WW. Electrically assisted delivery of macromolecules into the corneal epithelium. Exp Eye Res. 2009;89:934–941. doi: 10.1016/j.exer.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Li SK, Kao WW, Liu CY. Gene delivery to cornea. Brain Res Bull. 2010;81:256–261. doi: 10.1016/j.brainresbull.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL, Flotte TR, Byrne BJ, Jacobson SG. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Pipparelli A, Manissolle C, Acquart S, Garraud O, Gain P, Thuret G. Ex vivo gene electrotransfer to the endothelium of organ cultured human corneas. Ophthalmic Res. 2010;43:43–55. doi: 10.1159/000246577. [DOI] [PubMed] [Google Scholar]
- Herzog RW. Gene therapy for SCID-X1: round 2. Mol Ther. 2010;18:1891. doi: 10.1038/mt.2010.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, Hayaishi O. Enzymic formation of d-kynurenine from dtryptophan. Arch. Biochem. Biophys. 1967;120:397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Hillaireau H, Couvreur P. Nanocarriers' entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornof M, de la Fuente M, Hallikainen M, Tammi RH, Urtti A. Low molecular weight hyaluronan shielding of DNA/PEI polyplexes facilitates CD44 receptor mediated uptake in human corneal epithelial cells. J Gene Med. 2008;10:70–80. doi: 10.1002/jgm.1125. [DOI] [PubMed] [Google Scholar]
- Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006;177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- Hudde T, Rayner SA, Comer RM, Weber M, Isaacs JD, Waldmann H, Larkin DF, George AJ. Activated polyamidoamine dendrimers, a non-viral vector for gene transfer to the corneal endothelium. Gene Ther. 1999;6:939–943. doi: 10.1038/sj.gt.3300886. [DOI] [PubMed] [Google Scholar]
- Hutcheon AE, Guo XQ, Stepp MA, Simon KJ, Weinreb PH, Violette SM, Zieske JD. Effect of wound type on Smad 2 and 4 translocation. Invest Ophthalmol Vis Sci. 2005;46:2362–2368. doi: 10.1167/iovs.04-0759. [DOI] [PubMed] [Google Scholar]
- Jani PD, Singh N, Jenkins C, Raghava S, Mo Y, Amin S, Kompella UB, Ambati BK. Nanoparticles sustain expression of Flt intraceptors in the cornea and inhibit injury-induced corneal angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:2030–2036. doi: 10.1167/iovs.06-0853. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Jośko J, Mazurek M. Transcription factors having impact on vascular endothelial growth factor (VEGF) gene expression in angiogenesis. Med Sci Monit. 2004;10:RA89–RA98. [PubMed] [Google Scholar]
- Joyce NC, Harris DL, McAlister JC, Ali RR, Larkin DFP. Overexpression of the transcription factor E2F2 induces cell cycle progression in rabbit corneal endothelial cells. Invest Ophthalmol Vis Sci. 2004;45:1340–1348. doi: 10.1167/iovs.03-0335. [DOI] [PubMed] [Google Scholar]
- Jurkunas UV, Bitar M, Rawe I. Colocalization of increased transforming growth factor-beta-induced protein (TGFBIp) and Clusterin in Fuchs endothelial corneal dystrophy. Invest Ophthalmol Vis Sci. 2009;50:1129–1136. doi: 10.1167/iovs.08-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar JR, Mohan RR, Kanwar RK, Roy K, Bawa R. Applications of aptamers in nanodelivery systems in cancer, eye and inflammatory diseases. Nanomedicine (Lond) 2010;5:1435–1445. doi: 10.2217/nnm.10.115. [DOI] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Kaye S, Choudhary A. Herpes simplex keratitis. Prog Retin Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Kim B, Lee S, Suvas S, Rouse BT. Application of plasmid DNA encoding IL-18 diminishes development of herpetic stromal keratitis by antiangiogenic effects. J Immunol. 2005;175:509–516. doi: 10.4049/jimmunol.175.1.509. [DOI] [PubMed] [Google Scholar]
- Kim TH, Jiang HL, Jere D, Park IK, Cho MH, Nah JW, Choi UJ, Akaike T, Cho CS. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog Polym Sci. 2007;32:726–753. [Google Scholar]
- Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J Control Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Klausner EA, Zhang Z, Chapman RL, Multack RF, Volin MV. Ultrapure chitosan oligomers as carriers for corneal gene transfer. Biomaterials. 2010;31:1814–1820. doi: 10.1016/j.biomaterials.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Klibanov AL. Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 2006;41:354–362. doi: 10.1097/01.rli.0000199292.88189.0f. [DOI] [PubMed] [Google Scholar]
- Klintworth GK. Corneal dystrophies. Orphanet J Rare Dis. 2009;4:7. doi: 10.1186/1750-1172-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz L, Hucke S, Thimm D, Classen S, Gaarz A, Schultze J, Edenhofer F, Kurts C, Klockgether T, Limmer A, Knolle P, Burgdorf S. Increased antigen cross-presentation but impaired cross-priming after activation of peroxisome proliferator-activated receptor gamma is mediated by up-regulation of B7H1. J Immunol. 2009;183:129–136. doi: 10.4049/jimmunol.0804260. [DOI] [PubMed] [Google Scholar]
- Kulkarni AA, Thatcher TH, Olsen KC, Maggirwar SB, Phipps RP, Sime PJ. PPAR-γ ligands repress TGFβ-induced myofibroblast differentiation by targeting the PI3K/Akt pathway: implications for therapy of fibrosis. PLoS One. 2011;6:e15909. doi: 10.1371/journal.pone.0015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CN, Yang LC, Yang CT, Chen MF, Lai CH, Chen YH, Chen CH, Chen CH, Wu PC, Kou HK, Tsai JC, Hung CH. A novel vector system for gene transfer into the cornea using a partially dried plasmid expressing 18 basic fibroblast growth factor-synthetic amphiphile INTeraction-18 (SAINT-18) complex. Curr Eye Res. 2008;33:839–848. doi: 10.1080/02713680802382963. [DOI] [PubMed] [Google Scholar]
- Kuo CN, Yang LC, Yang CT, Lai CH, Chen MF, Chen CY, Chen CH, Wu PC, Kou HK, Chen YJ, Hung CH, Tsai CB. Inhibition of corneal neovascularization with plasmid pigment epithelium-derived factor (p-PEDF) delivered by synthetic amphiphile INTeraction-18 (SAINT-18) vector in an experimental model of rat corneal angiogenesis. Exp Eye Res. 2009;89:678–685. doi: 10.1016/j.exer.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Lai CM, Brankov M, Zaknich T, Lai YK, Shen WY, Constable IJ, Kovesdi I, Rakoczy PE. Inhibition of angiogenesis by adenovirus-mediated sFlt-1 expression in a rat model of corneal neovascularization. Hum Gene Ther. 2001;12:1299–1310. doi: 10.1089/104303401750270959. [DOI] [PubMed] [Google Scholar]
- Lai LJ, Xiao X, Wu JH. Inhibition of corneal neovascularization with endostatin delivered by adeno-associated viral (AAV) vector in a mouse corneal injury model. J Biomed Sci. 2007;14:313–322. doi: 10.1007/s11373-007-9153-7. [DOI] [PubMed] [Google Scholar]
- Lebherz C, Maguire A, Tang W, Bennett J, Wilson JM. Novel AAV serotypes for improved ocular gene transfer. J Gene Med. 2008;10:375–382. doi: 10.1002/jgm.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lech P, Somaia NV. Retrovirus vectors. In: Benigni A, Remuzzi G, editors. Gene Therapy for Renal Diseases and Transplantation. Karger, Basel: 2008. pp. 30–46. [Google Scholar]
- Lewis JD, Lichtenstein GR, Deren JJ, Sands BE, Hanauer SB, Katz JA, Lashner B, Present DH, Chuai S, Ellenberg JH, Nessel L, Wu GD Rosiglitazone for Ulcerative Colitis Study Group. Rosiglitazone for active ulcerative colitis: a randomized placebo-controlled trial. Gastroenterology. 2008;134:688–695. doi: 10.1053/j.gastro.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Li P, Li G, Wang J, Wang E. The effect of nocodazole on the transfection efficiency of lipid-bilayer coated gold nanoparticles. Biomaterials. 2009;30:1382–1388. doi: 10.1016/j.biomaterials.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Li HL, Zheng XZ, Wang HP, Li F, Wu Y, Du LF. Ultrasound-targeted microbubble destruction enhances AAV-mediated gene transfection in human RPE cells in vitro and rat retina in vivo. Gene Ther. 2009;16:1146–1153. doi: 10.1038/gt.2009.84. [DOI] [PubMed] [Google Scholar]
- Li M, Jayandharan GR, Li B, Ling C, Ma W, Srivastava A, Zhong L. High-efficiency transduction of fibroblasts and mesenchymal stem cells by tyrosine-mutant AAV2 vectors for their potential use in cellular therapy. Hum Gene Ther. 2010;21:1527–1543. doi: 10.1089/hum.2010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov. 2004;3:527–532. doi: 10.1038/nrd1417. [DOI] [PubMed] [Google Scholar]
- Liu J, Saghizadeh M, Tuli SS, Kramerov AA, Lewin AS, Bloom DC, Hauswirth WW, Castro MG, Schultz GS, Ljubimov AV. Different tropism of adenoviruses and adeno-associated viruses to corneal cells: implications for corneal gene therapy. Mol Vis. 2008a;14:2087–2096. [PMC free article] [PubMed] [Google Scholar]
- Liu J, Lewin AS, Tuli SS, Ghivizzani SC, Schultz GS, Bloom DC. Reduction in severity of a herpes simplex virus type 1 murine infection by treatment with a ribozyme targeting the UL20 gene RNA. J Virol. 2008b;82:7467–7474. doi: 10.1128/JVI.02720-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Yang H, Sakanishi A. Ultrasound: mechanical gene transfer into plant cells by sonoporation. Biotechnol Adv. 2006;24:1–16. doi: 10.1016/j.biotechadv.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lipkowitz MS, Hanss B, Tulchin N, Wilson PD, Langer JC, Ross MD, Kurtzman GJ, Klotman PE, Klotman ME. Transduction of renal cells in vitro and in vivo by adeno-associated virus gene therapy vectors. J Am Soc Nephrol. 1999;10:1908–1915. doi: 10.1681/ASN.V1091908. [DOI] [PubMed] [Google Scholar]
- Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- McAlister JC, Joyce NC, Harris DL, Ali RR, Larkin DF. Induction of replication in human corneal endothelial cells by E2F2 transcription factor cDNA transfer. Invest Ophthalmol Vis Sci. 2005;46:3597–3603. doi: 10.1167/iovs.04-0551. [DOI] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell'Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene Ther. 2005;12:1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- Maurice DM. Cornea and sclera. In: Davson D, editor. The eye. London: Academic Press; 1984. pp. 1–158. [Google Scholar]
- Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- Mo Y, Barnett ME, Takemoto D, Davidson H, Kompella UB. Human serum albumin nanoparticles for efficient delivery of Cu, Zn superoxide dismutase gene. Mol Vis. 2007;13:746–757. [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrósio R, Jr, Zieske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myofibroblast formation in human corneal fibroblasts. Exp Eye Res. 2010a;91:238–245. doi: 10.1016/j.exer.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Cebulko TC, Tandon A. Vector delivery technique affects gene transfer in the cornea in vivo. Mol Vis. 2010b;16:2494–2501. [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Gupta R, Sharma A, Tandon A. Decorin biology, expression, function and therapy in the cornea. Curr Mol Med. 2011a;11:110–128. doi: 10.2174/156652411794859241. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC. Significant Inhibition of Corneal Scarring In Vivo With Tissue-Selective Targeted AAV5 Decorin Gene Therapy. Invest Ophthalmol Vis Sci. 2011b doi: 10.1167/iovs.11-7357. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Tovey JC, Sharma A, Schultz G, Cowden J, Tandon A. Targeted decorin gene therapy delivered with adeno-associated virus effectively retards corneal neovascularization in vivo. PLoS One. 2011c doi: 10.1371/journal.pone.0026432. (Communicated) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C, Sergi Sergi L, Benedicenti F, Ambrosi A, Di Serio C, Doglioni C, von Kalle C, Naldini L. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol. 2006;24:687–696. doi: 10.1038/nbt1216. [DOI] [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhbacher J, St-Pierre P, Lafontaine DA. Therapeutic applications of ribozymes and riboswitches. Curr Opin Pharmacol. 2010;10:551–556. doi: 10.1016/j.coph.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy RC, McFarland TJ, Yoken J, Chen S, Barone C, Burke D, Zhang Y, Appukuttan B, Stout JT. Corneal transduction to inhibit angiogenesis and graft failure. Invest Ophthalmol Vis Sci. 2003;44:1837–1842. doi: 10.1167/iovs.02-0853. [DOI] [PubMed] [Google Scholar]
- Mwaikambo BR, Sennlaub F, Ong H, Chemtob S, Hardy P. Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Invest Ophthalmol Vis Sci. 2006;47:4356–4364. doi: 10.1167/iovs.05-1656. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Medeiros FW, Dupps WJ, Jr, Sinha S, Krueger RR, Stapleton WM, Rayborn M, Suto C, Wilson SE. Femtosecond laser and microkeratome corneal flaps: comparison of stromal wound healing and inflammation. J Refract Surg. 2007;23:667–676. doi: 10.3928/1081-597x-20070901-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- Oh JY, Roddy GW, Choi H, Lee RH, Ylöstalo JH, Rosa RH, Jr, Prockop DJ. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc Natl Acad Sci U S A. 2010;107:16875–16880. doi: 10.1073/pnas.1012451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Online Mendelian Inheritance in Man. [Accessed April 28, 2011]; Available at: http://www.ncbi.nlm.nih.gov/omim.
- Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–e9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- Palmer DJ, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16:1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- Parker DG, Kaufmann C, Brereton HM, Anson DS, Francis-Staite L, Jessup CF, Marshall K, Tan C, Koldej R, Coster DJ, Williams KA. Lentivirus-mediated gene transfer to the rat, ovine and human cornea. Gene Ther. 2007;14:760–767. doi: 10.1038/sj.gt.3302921. [DOI] [PubMed] [Google Scholar]
- Parker DG, Brereton HM, Klebe S, Coster DJ, Williams KA. A steroid-inducible promoter for the cornea. Br J Ophthalmol. 2009;93:1255–1259. doi: 10.1136/bjo.2009.159137. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Rama P, Mavilio F, De Luca M. Epithelial stem cells in corneal regeneration and epidermal gene therapy. J Pathol. 2009;217:217–228. doi: 10.1002/path.2441. [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, Zhong L, Zolotukhin S, Srivastava A, Lewin AS, Hauswirth WW. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry H, Brooks A, Orme A, Wang P, Liu P, Xie J, Kretschmer P, Qian HS, Hermiston TW, Harkins RN. Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice. Gene Ther. 2008;15:54–60. doi: 10.1038/sj.gt.3303037. [DOI] [PubMed] [Google Scholar]
- Pillai RG, Beutelspacher SC, Larkin DF, George AJ. Expression of the chemokine antagonist vMIP II using a non-viral vector can prolong corneal allograft survival. Transplantation. 2008;85:1640–1647. doi: 10.1097/TP.0b013e318172813f. [DOI] [PubMed] [Google Scholar]
- Pissuwan D, Niidome T, Cortie MB. The forthcoming applications of gold nanoparticles in drug and gene delivery systems. J Control Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Ponder KP, Auricchio A. Gene therapy for ocular problems in mucopolysaccharidosis: an experimental and promising approach with benefits in animal models – a review. Clin Exp Ophthalmol. 2010;38:43–51. [Google Scholar]
- Qaddoumi MG, Ueda H, Yang J, Davda J, Labhasetwar V, Lee VH. The characteristics and mechanisms of uptake of PLGA nanoparticles in rabbit conjunctival epithelial cell layers. Pharm Res. 2004;21:641–648. doi: 10.1023/b:pham.0000022411.47059.76. [DOI] [PubMed] [Google Scholar]
- Ragusa A, García I, Penadés S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans Nanobioscience. 2007;6:319–330. doi: 10.1109/tnb.2007.908996. [DOI] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- Redfern RL, McDermott AM. Toll-like receptors in ocular surface disease. Exp Eye Res. 2010;90:679–687. doi: 10.1016/j.exer.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL, Allen MD. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics. 2000;2:117–127. doi: 10.1152/physiolgenomics.2000.2.3.117. [DOI] [PubMed] [Google Scholar]
- Ritter T, Yang J, Dannowski H, Vogt K, Volk HD, Pleyer U. Effects of interleukin-12p40 gene transfer on rat corneal allograft survival. Transpl Immunol. 2007;18:101–107. doi: 10.1016/j.trim.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Runager K, Enghild JJ, Klintworth GK. Focus on molecules: Transforming growth factor beta induced protein (TGFBIp) Exp Eye Res. 2008;87:298–299. doi: 10.1016/j.exer.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Tajbakhsh J, Aoki AM, Wang C, Chai NN, Ljubimova JY, Sasaki T, Sosne G, Carlson MR, Nelson SF, Ljubimov AV. Proteinase and growth factor alterations revealed by gene microarray analysis of human diabetic corneas. Invest Ophthalmol Vis Sci. 2005;46:3604–3615. doi: 10.1167/iovs.04-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghizadeh M, Kramerov AA, Yu FS, Castro MG, Ljubimov AV. Normalization of wound healing and diabetic markers in organ cultured human diabetic corneas by adenoviral delivery of c-Met gene. Invest Ophthalmol Vis Sci. 2010;51:1970–1980. doi: 10.1167/iovs.09-4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Nakajima Y, Kao WW, Flanders KC, Roberts AB. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue after exposure to alkali. Am J Pathol. 2005a;166:1405–1418. doi: 10.1016/S0002-9440(10)62358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Flanders KC, Nakajima Y, Miyamoto T, Ohnishi Y, Kao WW, Muragaki Y, Ooshima A. Therapeutic effects of adenoviral gene transfer of bone morphogenic protein-7 on a corneal alkali injury model in mice. Lab Invest. 2005b;85:474–486. doi: 10.1038/labinvest.3700247. [DOI] [PubMed] [Google Scholar]
- Saika S, Yamanaka O, Okada Y, Miyamoto T, Kitano A, Flanders KC, Ohnishi Y, Nakajima Y, Kao WW, Ikeda K. Effect of overexpression of PPARgamma on the healing process of corneal alkali burn in mice. Am J Physiol Cell Physiol. 2007;293:C75–C86. doi: 10.1152/ajpcell.00332.2006. [DOI] [PubMed] [Google Scholar]
- Sharma A, Ghosh A, Siddappa C, Mohan RR. Ocular Surface: Gene Therapy. In: Besharse J, Dana R, Dartt DA, editors. Encyclopedia of the eye. Elsevier: 2010a. pp. 185–194. [Google Scholar]
- Sharma A, Ghosh A, Hansen ET, Newman JM, Mohan RR. Transduction efficiency of AAV 2/6, 2/8 and 2/9 vectors for delivering genes in human corneal fibroblasts. Brain Res. Bull. 2010b;81:273–278. doi: 10.1016/j.brainresbull.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tovey JC, Ghosh A, Mohan RR. AAV serotype influences gene transfer in corneal stroma in vivo. Exp Eye Res. 2010c;91:440–448. doi: 10.1016/j.exer.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Tandon A, Tovey JC, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR. Polyethylenimine-conjugated gold nanoparticles: Gene transfer potential and low toxicity in the cornea. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.01.006. PMID: 21272669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi A, Converse RL, Liu CY, Zhou F, Kao CW, Kao WW. Identification of the cornea-specific keratin 12 promoter by in vivo particle-mediated gene transfer. Invest Ophthalmol Vis Sci. 1998;39:2554–2561. [PubMed] [Google Scholar]
- Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM. Oxidative stress in diseases of the human cornea. Free Radic Biol Med. 2008;45:1047–1055. doi: 10.1016/j.freeradbiomed.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A. Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther. 2010;18:643–650. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Amin S, Richter E, Rashid S, Scoglietti V, Jani PD, Wang J, Kaur R, Ambati J, Dong Z, Ambati BK. Flt-1 intraceptors inhibit hypoxia-induced VEGF expression in vitro and corneal neovascularization in vivo. Invest Ophthalmol Vis Sci. 2005;46:1647–1652. doi: 10.1167/iovs.04-1172. [DOI] [PubMed] [Google Scholar]
- Sonoda S, Tachibana K, Uchino E, Okubo A, Yamamoto M, Sakoda K, Hisatomi T, Sonoda KH, Negishi Y, Izumi Y, Takao S, Sakamoto T. Gene transfer to corneal epithelium and keratocytes mediated by ultrasound with microbubbles. Invest Ophthalmol Vis Sci. 2006;47:558–564. doi: 10.1167/iovs.05-0889. [DOI] [PubMed] [Google Scholar]
- Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Molecular mechanisms controlling the fibrotic repair phenotype in cornea: implications for surgical outcomes. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359. doi: 10.1016/j.visres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr Mol Med. 2010;10:565–578. doi: 10.2174/1566524011009060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Journal of Gene Medicine Clinical Trial website. [Accessed 23 May 2011];2010 June; Updated, Available at: http://www.abedia.com/wiley/index.html.
- Thomas M, Klibanov AM. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc Natl Acad Sci U S A. 2003;100:9138–9143. doi: 10.1073/pnas.1233634100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res. 2005;22:373–380. doi: 10.1007/s11095-004-1874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiyaboonchai W, Woiszwillo J, Middaugh CR. Formulation and characterization of DNA-polyethylenimine-dextran sulfate nanoparticles. Eur. J. Pharm. Sci. 2003;19:191–202. doi: 10.1016/s0928-0987(03)00102-7. [DOI] [PubMed] [Google Scholar]
- Tong YC, Chang SF, Liu CY, Kao WW, Huang CH, Liaw J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J Gene Med. 2007;9:956–966. doi: 10.1002/jgm.1093. [DOI] [PubMed] [Google Scholar]
- Toropainen E, Hornof M, Kaarniranta K, Johansson P, Urtti A. Corneal epithelium as a platform for secretion of transgene products after transfection with liposomal gene eyedrops. J Gene Med. 2007;9:208–216. doi: 10.1002/jgm.1011. [DOI] [PubMed] [Google Scholar]
- Traas AM, Wang P, Ma X, Tittiger M, Schaller L, O'donnell P, Sleeper MM, Vite C, Herati R, Aguirre GD, Haskins M, Ponder KP. Correction of clinical manifestations of canine mucopolysaccharidosis I with neonatal retroviral vector gene therapy. Mol Ther. 2007;15:1423–1431. doi: 10.1038/sj.mt.6300201. [DOI] [PubMed] [Google Scholar]
- Van Vliet KM, Blouin V, Brument N, Agbandje-McKenna M, Snyder RO. The role of the adeno-associated virus capsid in gene transfer. Methods Mol Biol. 2008;437:51–91. doi: 10.1007/978-1-59745-210-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, O'Malley TM, Xu L, Vite C, Wang P, O'Donnell PA, Ellinwood NM, Haskins ME, Ponder KP. Expression in blood cells may contribute to biochemical and pathological improvements after neonatal intravenous gene therapy for mucopolysaccharidosis VII in dogs. Mol Genet Metab. 2006;87:8–21. doi: 10.1016/j.ymgme.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Hasegawa Y, Yamashita H, Shimizu K, Ding Y, Abe M, Ohta H, Imagawa K, Hojo K, Maki H, Sonoda H, Sato Y. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JS, Kruth HS, Kuivaniemi H, Tromp G, White PS, Winters RS, Lisch W, Henn W, Denninger E, Krause M, Wasson P, Ebenezer N, Mahurkar S, Nickerson ML. Mutations in the UBIAD1 gene on chromosome short arm 1, region 36, cause Schnyder crystalline corneal dystrophy. Invest Ophthalmol Vis Sci. 2007;48:5007–5012. doi: 10.1167/iovs.07-0845. [DOI] [PubMed] [Google Scholar]
- Weiss JS Fifth ARVO/Pfizer Ophthalmics Research Institute Conference Working Group. Corneal dystrophies: molecular genetics to therapeutic intervention—Fifth ARVO/Pfizer Ophthalmics Research Institute Conference; Invest Ophthalmol Vis Sci; 2010. pp. 5391–5402. [DOI] [PubMed] [Google Scholar]
- Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Org. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- Williams KA, Coster DJ. Gene therapy for diseases of the cornea - a review. Clin Experiment Ophthalmol. 2010;38:93–103. doi: 10.1111/j.1442-9071.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Hong J, Lee J, Choi R, Liu JJ, Mohan RR. Apoptosis in the cornea in response to epithelial injury: significance to wound healing and dry eye. Adv Exp Med Biol. 2002;506:821–826. doi: 10.1007/978-1-4615-0717-8_116. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Visual impairment and blindness. [Accessed January 26, 2011]; Available at: http://www.who.int/mediacentre/factsheets/fs282/en/
- Wu Z, Miller E, Agbandje-McKenna M, Samulski RJ. Alpha2,3 and alpha2, 6 N-linked sialic acids facilitate efficient binding and transduction by adenoassociated virus types 1 and 6. J. Virol. 2006;80:9093–9103. doi: 10.1128/JVI.00895-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang CH, Pack DW. Polymeric carriers for gene delivery: chitosan and poly(amidoamine) dendrimers. Curr Pharm Des. 2010;16:2350–2368. doi: 10.2174/138161210791920469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Sonoda S, Suzuki R, Arimura N, Tachibana K, Maruyama K, Sakamoto T. A novel bubble liposome and ultrasound - mediated gene transfer to ocular surface: RC-1 cells in vitro and conjunctiva in vivo. Exp Eye Res. 2007;85:741–748. doi: 10.1016/j.exer.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Yoon KC, Bae JA, Park HJ, Im SK, Oh HJ, Lin XH, Kim MY, Lee JH, Lee SE, Ahn KY, Kim KK. Subconjunctival gene delivery of the transcription factor GA-binding protein delays corneal neovascularization in a mouse model. Gene Ther. 2009;16:973–981. doi: 10.1038/gt.2009.50. [DOI] [PubMed] [Google Scholar]
- Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. J Pathol. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- Yu H, Wu J, Li H, Wang Z, Chen X, Tian Y, Yi M, Ji X, Ma J, Huang Q. Inhibition of corneal neovascularization by recombinant adenovirus-mediated sFlk-1 expression. Biochem Biophys Res Commun. 2007;361:946–952. doi: 10.1016/j.bbrc.2007.07.114. [DOI] [PubMed] [Google Scholar]
- Yuan J, Chen JQ, Zhou SY, Liu ZG, Wang ZC, Gu JJ. The transfection and expression of IL-1ra gene to the rabbit cornea in situ via cation polymer mediation Zhonghua Yan Ke Za Zhi. 2006;42:686–693. [PubMed] [Google Scholar]
- Zagon IS, Sassani JW, Malefyt KJ, McLaughlin PJ. Regulation of corneal repair by particle-mediated gene transfer of opioid growth factor receptor complementary DNA. Arch Ophthalmol. 2006;124:1620–1624. doi: 10.1001/archopht.124.11.1620. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanomedicine in ophthalmology: the new frontier. Am J Ophthalmol. 2010a;150:144–162.e2. doi: 10.1016/j.ajo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Zarbin MA, Montemagno C, Leary JF, Ritch R. Nanotechnology in ophthalmology. Can J Ophthalmol. 2010b;45:457–476. doi: 10.3129/i10-090. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yao YF. Gelatinous drop-like corneal dystrophy with a novel mutation of TACSTD2 manifested in combination with spheroidal degeneration in a Chinese patient. Mol Vis. 2010;16:1570–1575. [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, Herzog RW, Weigel-Van Aken KA, Hobbs JA, Zolotukhin S, Muzyczka N, Srivastava A. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Dean DA. Gene transfer of interleukin 10 to the murine cornea using electroporation. Exp Biol Med (Maywood) 2007;232:362–369. [PMC free article] [PubMed] [Google Scholar]
- Zhou SY, Xie ZL, Xiao O, Yang XR, Heng BC, Sato Y. Inhibition of mouse alkali burn induced-corneal neovascularization by recombinant adenovirus encoding human vasohibin-1. Mol Vis. 2010;16:1389–1398. [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Zhang X, Yu X, Zha X, Fu Q, Liu B, Wang X, Chen Y, Chen Y, Shan Y, Jin Y, Wu Y, Liu J, Kong W, Shen J. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials. 2008;29:111–117. doi: 10.1016/j.biomaterials.2007.09.007. [DOI] [PubMed] [Google Scholar]