Abstract
Microglia and astrocytes play complex roles following spinal cord injury (SCI), contributing to inflammatory processes that both exacerbate injury and promote functional recovery by supporting neuro-protection and neuroplasticity. The crossed phrenic phenomenon (CPP) is an example of respiratory plasticity in which C2 cervical hemisection (C2HS) strengthens crossed-spinal synaptic pathways to phrenic motor neurons ipsilateral to injury. We hypothesized that microglia and astrocytes are activated in the phrenic motor nucleus caudal and ipsilateral to C2HS, suggesting their potential for involvement in the CPP. To test this hypothesis, an incomplete cervical spinal hemisection (C2 lateral injury; C2LI) was performed, and rats were allowed to recover for 1, 3, 14 or 28 days before collecting perfused spinal tissues. Microglia (via OX42) and astrocytes (via GFAP) were visualized with immunofluorescence microscopy in the C4-C5 ventral horn, the region encompassing most of the phrenic motor nucleus. OX42-occupied fractional area ipsilateral to injury increased with C2LI (vs. sham) at 1 (12.5 +/- 1.8%, p<0.001), 3 (29.0 +/- 1.9%, p<0.001), 14 (26.1 +/- 3.1%, p<0.001) and 28 (19.2 +/- 2.0%, p<0.001) days post-C2LI. GFAP-occupied fractional area also increased with C2LI at 3 (24.4 +/- 3.2%, p<0.001) and 14 (16.8 +/- 8.3%, p=0.012) days, but not at 1 (6.2 +/- 3.9%, p=0.262) or 28 (10.6 +/- 3.9%, p=0.059) days post-C2LI. Thus, microglia and astrocytes are activated in the phrenic motor nucleus caudal to C2LI, suggesting that they play a role in functional deficits and/or recovery following spinal injury.
Keywords: astrocyte, microglia, phrenic, inflammation, crossed-phrenic
1. Introduction
Strengthening surviving neural pathways following spinal cord injury (SCI) via spontaneous or induced neuroplasticity is an important therapeutic strategy (Winslow and Rozovsky, 2003). However, factors that enable or disenable plasticity-induced recovery from SCI must be understood before we can maximize success in restoring function. One important factor that may modulate plasticity is widespread and long-lasting spinal inflammation associated with spinal injuries (Ankeny and Popovich, 2008; Fleming et al., 2006; Popovich et al., 1997; Sroga et al., 2003; Wu et al., 2005). Microglia, the resident CNS immune cells, are activated following spinal injury and are thought to induce neuropathic pain (Coull et al., 2005; Hulsebosch, 2008). On the other hand, microglia may also promote functional recovery (Rabchevsky and Streit, 1997; Rapalino et al., 1998; Schwartz and Yoles, 2006). For example, microglia release trophic substances, such as brain-derived neurotrophic factor (BDNF), vascular endothelial growth factor (VEGF) and tumor necrosis factor α (TNFα), that often promote plasticity (Bessis et al., 2007; Coull et al., 2005; Di Filippo et al., 2008; Skold et al., 2000; Yan et al., 2001).
Astrocytes are also involved in inflammatory processes, and may enhance or suppress functional recovery following SCI. For instance, inhibition of NfκB-mediated transcription in the astrocytes of spinally injured mice increases the density of translesion neural projections and improves behavioral recovery (Brambilla et al., 2005; Brambilla et al., 2009). Spinal hemisection at T13 activates astrocytes in lumbar dorsal horn for at least 28 days post-surgery in rats (Gwak and Hulsebosch, 2009). Further, treatments that suppress astrocyte activation improve recovery from SCI and decrease signs of neuropathic pain.
Little is known concerning the role of glial activation in the recovery of respiratory motor function following cervical injury. High cervical spinal hemisection at spinal segment C2 (C2HS) strengthens latent bulbospinal pathways that cross the spinal midline to phrenic motor neurons below the injury (Porter, 1895). This spontaneous functional recovery is known as the crossed phrenic phenomenon (CPP; Goshgarian, 2003). Plasticity in bulbospinal and propriospinal pathways underlies the CPP (Lane et al., 2008; Moreno et al., 1992; Vinit et al., 2008), and the CPP underlies partial recovery of diaphragmatic function ipsilateral to C2HS (Goshgarian, 2003; Vinit and Kastner, 2009). Although phrenicotomy causes long-lasting activation of microglia and astrocytes in the phrenic motor nucleus, and this activation is exacerbated by C2HS (Gould and Goshgarian, 1997), no previous studies describe specific reactions of microglia and astrocytes to C2HS alone in this critical region for respiratory motor control.
An understanding of spatial and temporal patterns of glial activation following C2HS is necessary before we will appreciate the potential roles of these cells in enhancing or diminishing plasticity that is vital for functional recovery of breathing capacity. Since the histological changes underlying the CPP likely occur at the level of the phrenic motor nucleus, we chose to examine microglia and astrocyte activation in the C4-C5 ventral horn. Glia may suppress or enhance spontaneous compensatory plasticity necessary for full recovery of breathing capacity via production of pro-inflammatory/toxic and/or pro-survival/pro-plasticity molecules. However, this can only occur if the cells are in close proximity to respiratory-related motor neurons.
In this study, we hypothesized that microglia and astrocytes are activated below a C2 lateral injury (C2LI) in the phrenic motor nucleus. To test this hypothesis, we examined temporal profiles of neurochemical/morphological activation of microglia and astrocytes after C2 lateral injury in adult rats. Increased OX42 (microglia) and GFAP labeling (astrocytes) is observed bilaterally in the C4 ventral horn 1-28 days post-C2LI. Thus, glia are activated in temporal and spatial domains appropriate to play a role in compensatory respiratory plasticity following cervical spinal injury.
2. Materials and Methods
2.1 Rats
Adult (aged 3-5 months) male Lewis rats (n = 41) were obtained from Harlan (300-350g) and housed individually with free access to food and water and access to one enrichment item (a small PVC tube standard to all rats housed at facility). The University of Wisconsin, School of Veterinary Medicine Animal Care and Use Committee approved all procedures.
2.2 Surgery
Rats were administered buprenorphine (0.1mg/kg), carprofen (4mg/kg), enrofloxacin (5mg/kg) and medetomidine (500ug/kg) prior to delivery of isoflurane by orotracheal intubation. Rats were ventilated with 1.5-2% isoflurane (balance oxygen) for the duration of surgery. After surgery, rats were administered atipamezole (500μg/kg). All drugs were delivered subcutaneously. The spinal cord was approached dorsally for lateral injury surgery (C2LI). A 2-3 cm incision was made in the skin from the rostral side of the C1 spinous process in the caudal direction. All underlying muscle layers and connective tissues over the C2 spinous process were retracted from the C2 vertebra. A complete dorsal C2 laminectomy was performed and the left-side C2 dorsal root was visualized. A 1-mm gap was created in the spinal cord just caudal to the C2 dorsal root with a blunt-tipped 25-gauge needle connected to a suction pump after cutting the dura over C2 with microdissection scissors. The surgery created a lesion that extended from the spinal midline to the ventral edge of the spinal cord. Performing all procedures except for C2 durotomy and lateral injury created sham subjects. Following surgery, deep muscles of the back were not sutured, but both intermediate and superficial muscle layers were sutured together in a simple continuous pattern using 3-0 absorbable sutures. The incised skin was held together with wound clips for 14 days following surgery before removal. Rats were treated, twice daily, with buprenorphine (0.1mg/kg), carprofen (4mg/kg) and enrofloxacin (5mg/kg) for 3 days following surgery.
2.3 Immunohistochemistry
Rats were perfused transcardially, under isoflurane, with 4% formaldehyde after surgery and recovery. Spinal segments C1-C3 and C4-C6 were collected and sectioned to 40μm using a sliding frozen microtome. Sections from the site of injury were visualized with Cresyl Violet to confirm the extent of injury (Figure 1). For immunohistochemistry, 4 sections from the spinal cord immediately caudal to the site of injury (C2) and 4 sections taken from spinal segments C4-C5 were stained from each animal. Sections were labeled with antibodies raised against glial fibrillary acidic protein (GFAP, Millipore), a protein expressed by reactive astrocytes (Eng et al., 2000), and CD11b (OX42, Santa Cruz Biotechnology), a protein expressed by microglia and invading macrophages in the central nervous system (Robinson et al., 1986). It was not possible to differentiate between resident microglia and invading macrophages by morphology or CD11b expression, therefore the cells present at the site of injury may represent a mixture of both populations. However, the cells present in the area of the phrenic motor nucleus are most likely composed of resident microglia (David and Kroner, 2011). A fluorescent Nissl counterstain (Neurotrace 435/455, Invitrogen) was applied to sections for 20 minutes to locate presumptive phrenic motor neurons in the C4-C5 ventral horn (Bagley et al., 2007; Windelborn and Lipton, 2008). Sections were mounted on slides with ProLong Gold Antifade Mounting Medium (Invitrogen) and imaged using a Nikon wide-field epifluorescent system with MetaMorph 7 imaging software.
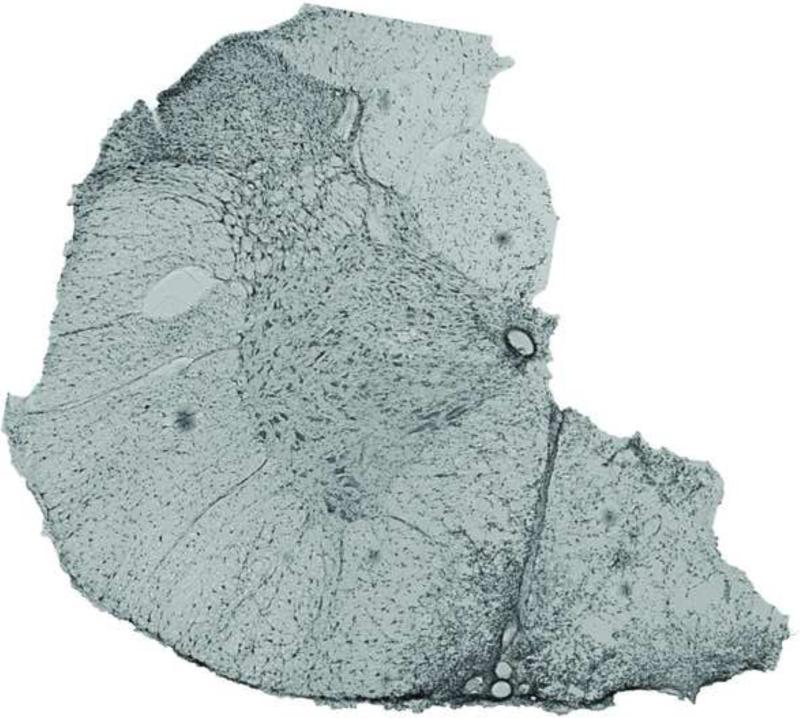
Figure 1.
A coronal section taken from the site of C2 lateral section shows a typical extent of injury. C2 lateral section was incomplete in these studies, with the ventromedial funiculus remaining intact. These images are similar in many respects to many previous studies concerning spontaneous recovery of respiratory motor function following cervical spinal hemisection (Fuller et al., 2009; Goshgarian, 1981). The micrograph shows a full coronal section and was obtained using phase-contrast microscopy.
2.4 Image Analysis
Raw images were analyzed using Image J (NIH, Bethesda, MD). Analysis was performed using images taken at 400x magnification of fields surrounding putative phrenic motor neurons at spinal segment C4-C5 (i.e. phrenic motor nucleus) or in the vicinity of large Neurotrace-positive neurons in the area immediately caudal to the C2 ventral horn. Location of putative phrenic motor neurons was based on previous studies using retrograde tracers from the diaphragm (Guenther et al., 2010). The full microscopic field of each image was analyzed, except in cases that bubbles or dust were eliminated from the analysis. The fractional area occupied by GFAP or OX42 label in raw image files was computed by Image J (as the percentage of the total field occupied by label-positive pixels) and interpreted as the fractional area of reactive astrocyte or microglia, respectively. Increased fractional area of OX42 staining indicates greater microglial activity (Ling et al., 1990; Ling and Wong, 1993; Robinson et al., 1986; Roy et al., 2006). This analysis does not allow a distinction between greater cell sizes versus greater cell number. Background staining was subtracted from each image using Image J's “set threshold from background” plug-in, choosing a cell-free area from each image for selection of a background region of interest. Images were then processed for publication using Photoshop CS3 (Adobe). Processing consisted of adjustments to an image's brightness and contrast and was applied equally across all images.
2.5 Statistics
Analyses were performed using ANOVAs indicated in figure legends; individual differences were detected with Fisher's LSD post hoc test.
3. Results
3.1 Sham surgery elicits microglial and astrocytic activation
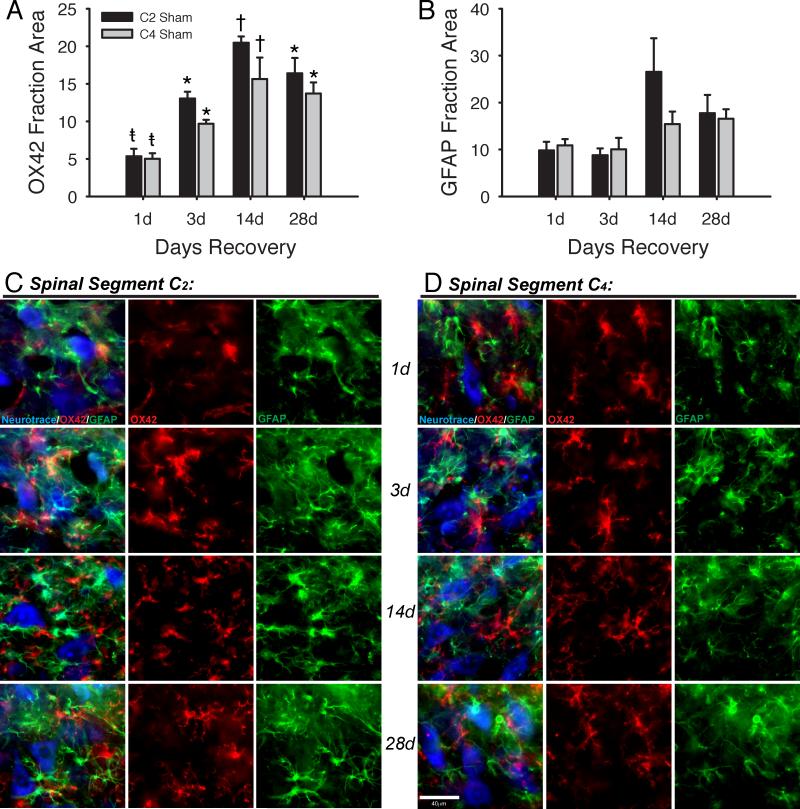
Baseline measurements of the fractional areas of microglial (OX42) and astrocytic (GFAP) fluorescence were made in spinal cord sections from sham-treated rats. Microglial activation was estimated based on the fractional area of a microscopic field occupied by OX42 staining in the C2 or C4 ventral horn. Because a visual trend appeared in sham-operated tissues, analyses were performed on microglial and astrocytic staining across recovery times in both spinal segments C2 and C4. The fractional area of sham C2 ventral horn occupied by OX42 fluorescence was significantly increased at 3, 14 and 28 days post-surgery vs. the fractional area at 1-day post-surgery (Figure 2A, black bars). OX42 fractional area peaked at 14-days post-surgery; values at 14-days were significantly greater than those at either 1-day or 3-days post-surgery.
Figure 2.
Immunohistochemistry revealed significant differences between sham tissues after different post-surgical durations. The fraction of C2 and C4 ventral horn occupied by OX42 (A) was significantly different between days. The fraction of C2 and C4 ventral horn occupied by GFAP (B) did not differ significantly between days. A representative image from each day post-surgery is provided in rows for both C2 (C) and C4 (D). All images were taken from sham tissues. The first column in each panel is a merged image showing Neurotrace (blue), OX42 (red) and GFAP (green). OX42 and GFAP are shown individually in panels to the right of the merged image. One-Way ANOVA run for each antigen separately within each spinal segment; p<0.05: *vs. 1d, †vs. 1d and 3d, ‡vs. 3d. Scale bar is 40μm.
The fractional area of sham C4 ventral horn occupied by OX42 fluorescence was also significantly increased at 3, 14 and 28 days post-surgery vs. fractional area at 1-day post-surgery (Figure 2A, grey bars). OX42 fractional area peaked at 14-days post-surgery, when values were significantly greater than those at either 1-day or 3-days recovery. Representative images for OX42 are shown from spinal segments C2 (Figure 2C) and C4 (Figure 2D).
In contrast to OX-42, no significant differences were measured across post-surgical times for GFAP fractional area in sham rats at either spinal segment (Figure 2B). Representative images for GFAP are shown from spinal segments C2 (Figure 2C) and C4 (Figure 2D).
Negative controls were created for non-specific fluorescence by omitting primary antibody or by omitting primary and secondary antibodies from the immunohistochemical procedures. Detectable fluorescence in these cases was minimal (data not shown).
3.2 Increased microglial and astrocytic markers at injury site (C2)
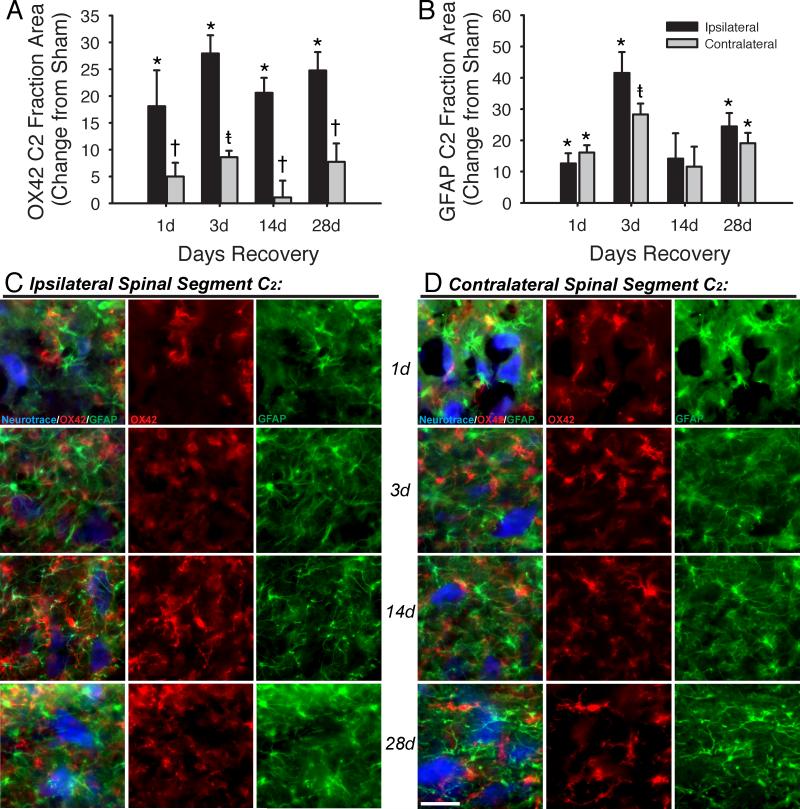
Following C2LI, ventral horn regions ipsilateral and contralateral to injury were examined for changes in microglia and astrocyte activation. The fractional area of ipsilateral C2 ventral horn occupied by OX42 fluorescence was significantly increased in injured over sham animals at 1-day (23.5 +/- 6.7% vs. 5.4 +/- 1.0%, p<0.001), 3-days (41.0 +/- 3.4% vs. 13.1 +/- 0.9%, p<0.001), 14-days (41.1 +/- 2.8% vs. 20.5 +/- 0.8%, p<0.001) and 28-days (41.2 +/- 3.4% vs. 16.4 +/- 2.1%, p<0.001) post-surgery (Figures 3A, black bars, and 3C, red). Contralateral to injury, the fractional area of C2 ventral horn occupied by OX42 fluorescence was not significantly different from sham at 1 (10.4 +/- 2.6%, p=0.245), 14 (21.6 +/1 3.1%, p=0.826) or 28 (24.1 +/- 3.4%, p=0.075) days post-C2LI (Figures 3A, grey bars, and 3D, red). At 3 days post-C2LI, contralateral OX42 fractional area was significantly greater than sham (21.6 +/- 3.1%, p=0.049). Comparisons between ipsilateral and contralateral tissues were made by two-way ANOVA based on fractional areas normalized to time-matched shams (Figure 3) because of the shifting baseline fraction areas in sham tissues (Figure 2). The normalized OX42 fluorescence in ipsilateral C2 ventral horn was significantly greater than contralateral C2 ventral horn at 1 (p=0.017), 3 (p<0.001), 14 (p=0.002), and 28 (p=0.001) days post-C2LI. Thus, microglial activation ipsilateral to injury was already significant at 1-day, and reached a peak by 3-days but then remained elevated for at least 28-days post-C2LI.
Figure 3.
Microglial and astrocytic staining is increased in the ventral horn immediately caudal to the site of C2 lateral section. OX42 staining (A) ipsilateral to injury is increased vs. sham at all times post-injury. Contralateral to injury, increases are observed at only 3 days post-injury. Additionally, increases contralateral to injury are significantly lower than increases measured ipsilateral to injury. GFAP staining (B) was increased vs. sham at 1, 3, and 28 days post-injury. In contrast to OX42 staining, no difference was observed between ipsilateral and contralateral GFAP except 3 days after injury. Images taken from the C2-C3 ventral horn of spinally injured rats are shown for sites ipsilateral (C) and contralateral (D) to injury, with each row containing a field from 1, 3, 14, or 28 days after injury. The left-hand column in each panel shows a merged image, with Neurotrace in blue, OX42 in red and GFAP in green; the central column shows only OX42; the right-hand column shows only GFAP. Scale bar is 40μm. Three-way ANOVA tested differences between sham, contralateral and ipsilateral area fractions across all recovery times. Two-way ANOVA tested normalized differences between contralateral and ipsilateral changes from sham across all recovery times. *p<0.05 vs. sham; †p<0.05 vs. ipsilateral; ‡p<0.05 vs. sham and ipsilateral.
The fractional area occupied by GFAP fluorescence was used as a measure of astrocyte activation. The fractional area of ipsilateral C2 ventral horn occupied by GFAP fluorescence was significantly increased in injured over sham animals at 1-day (22.4 +/- 3.2% vs. 9.8 +/- 1.9%, p=0.012), 3-days (50.3 +/- 6.7% vs. 8.8 +/- 1.4%, p<0.001), and 28-days (42.2 +/- 4.3% vs. 17.8 +/- 3.9%, p<0.001) post-surgery (Figures 3B, black bars, and 3C, green). GFAP fractional area 14 days post-surgery was not significantly different from sham (40.7 +/- 8.1% vs. 26.5 +/- 7.2%, p=0.056). Contralateral to injury, the fractional area of C2 ventral horn occupied by GFAP fluorescence was also significantly different from sham at 1 (25.9 +/- 2.3%, p=0.047), 3 (37.1 +/- 3.5%, p<0.001) and 28 (36.8 +/- 3.3%, p=0.003) days post-C2LI (Figures 3B, grey bars, and 3D, green). At 14 days post-C2LI, contralateral GFAP fractional area was not significantly greater than sham (36.8 +/- 6.4%, p=0.116). The normalized GFAP fluorescence in ipsilateral C2 ventral horn was significantly greater than contralateral C2 ventral horn at 3 (p=0.03) days post-C2LI, but did not differ from ipsilateral fraction area at 1 (p=0.591), 14 (p=0.725) or 28 (p=0.368) days. Thus, the timecourse and side-to-injury characteristics of astrocyte activation differed from microglia activation following C2LS.
3.3 Increased microglial and astrocytic markers caudal to injury
Microglia and astrocyte activation were also estimated caudal to injury in the C4-C5 ventral horn, segments that contain most of the phrenic motor nucleus. Image fields were chosen based on the presence of presumptive phrenic motor neurons (large Neurotrace-positive cells often localized in a centralized cluster of the ventral horn; Guenther et al., 2010).
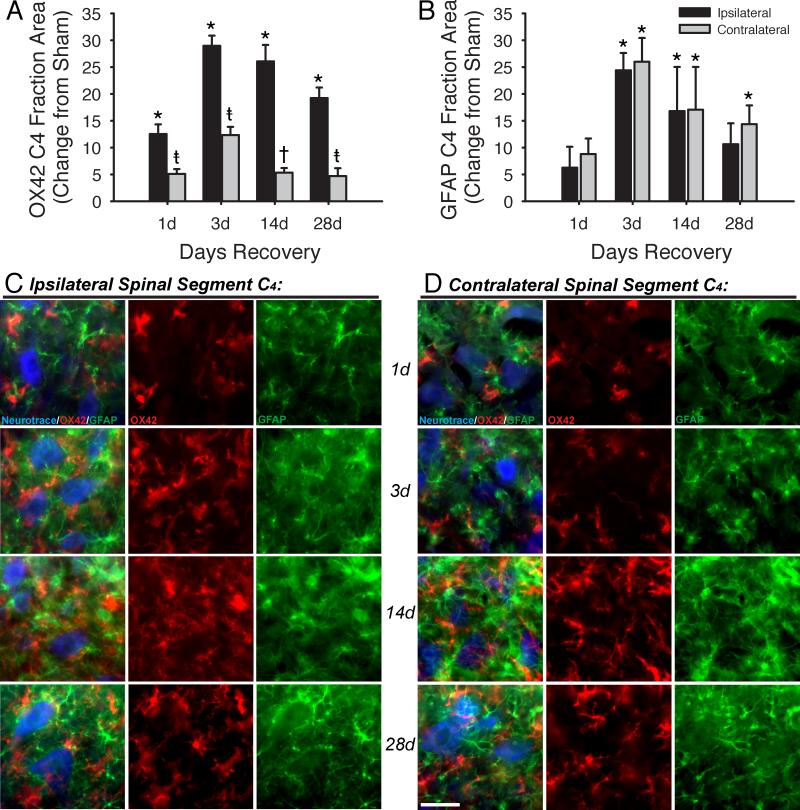
The fractional area occupied by OX42 fluorescence in the ipsilateral C4 ventral horn significantly increased in injured vs. sham rats at 1-day (17.6 +/- 1.8% vs. 5.0 +/- 0.7%, p<0.001), 3-days (38.6 +/- 1.9% vs. 9.7 +/- 0.5%, p<0.001), 14-days (41.7 +/- 3.1% vs. 15.6 +/- 2.9%, p<0.001) and 28-days (32.9 +/- 2.0% vs. 13.7 +/- 1.5%, p<0.001) post-C2LI (Figures 4A, black bars, and 4C, red). OX42-positive cells appeared thick and condensed in injured rats. This morphological state is indicative of activated microglia, providing further evidence that individual microglia were activated in the phrenic motor nucleus (i.e. caudal to injury) at all times tested. The large number of glial cells and overlapping processes made it difficult to differentiate one cell from another, so cell numbers were not determined. Contralateral to injury, the fractional area of C4 ventral horn occupied by OX42 fluorescence was significantly greater than sham at 1 (10.1 +/- 0.9%, p=0.032), 3 (22.0 +/- 1.5%, p<0.001) and 28 (18.4 +/- 1.5%, p=0.048) days post-C2LI (Figures 4A, grey bars, and 4D, red). OX42 fractional area did not differ significantly from sham 14 days post-C2LI (20.9 +/- 0.9%, p=0.054). Sham-normalized ipsilateral C4 OX42 fractional area was significantly greater than the contralateral side at 1 (p=0.007), 3 (p<0.001), 14 (p<0.001) and 28 (p<0.001) days post-C2LI.
Figure 4.
Microglial and astrocytic staining is increased in the region of the phrenic motor nucleus after spinal injury. OX42 staining (A) ipsilateral to injury is increased vs. sham at all times post-injury. Contralateral to injury, increases are observed at 1, 3 and 28 days, but not at 14 days post-injury. Additionally, increases contralateral to injury are significantly lower than increases measured ipsilateral to injury. GFAP staining (B) did not increase vs. sham at 1 day post-injury, but was significantly increased vs. sham at 3, 14 and 28 days post-injury. In contrast to OX42 staining, no difference was observed between ipsilateral and contralateral GFAP after injury. Images taken from the C4 ventral horn of spinally injured rats are shown for sites ipsilateral (C) and contralateral (D) to injury, with each row containing a field from 1, 3, 14, or 28 days after injury. The left-hand column in each panel shows a merged image, with Neurotrace in blue, OX42 in red and GFAP in green; the central column shows only OX42; the right-hand column shows only GFAP. Scale bar is 40μm. Three-way ANOVA tested differences between sham, contralateral and ipsilateral area fractions across all recovery times. Two-way ANOVA tested normalized differences between contralateral and ipsilateral changes from sham across all recovery times.*p<0.05 vs. sham; †p<0.05 vs. ipsilateral; ‡p<0.05 vs. sham and ipsilateral.
The fractional area occupied by GFAP fluorescence in the ipsilateral C4 ventral horn was increased in injured vs. sham rats at 3-days (34.4 +/- 3.2% vs. 10.0 +/- 2.5%, p<0.001) and 14-days (32.2 +/- 8.3% vs. 15.4 +/- 2.7%, p=0.012) post-C2LI (Figures 4B, black bars, and 4C, green). GFAP-positive cells appeared hypertrophied in spinally injured versus sham rats, indicating a more reactive state following C2LI. GFAP fractional area did not differ significantly from sham on 1 (17.1 +/- 3.9% vs. 10.9 +/- 1.4%, p=0.262) or 28 (27.2 +/- 3.9% vs. 16.6 +/- 2.0%, p=0.059) days post-C2LI. Contralateral to injury, the fractional area of C4 ventral horn occupied by GFAP was significantly greater than sham at 3 (36.0 +/- 4.5%, p<0.001), 14 (32.4 +/- 8.0%, p=0.011) and 28 (30.9 +/- 3.5%, p=0.012) days post-C2LI (Figures 4B, grey bars, and 4D, green). At 1-day post-C2LI, contralateral GFAP did not differ significantly from sham fractional area (19.7 +/- 2.9%, p=0.117). When normalized to time-matched shams, ipsilateral GFAP fractional areas did not differ from contralateral at any time post-C2LI (p>0.5). These results indicate a rapid and persistent inflammatory response at sites remote from spinal injury, and that this response occurs in areas critically involved in the control of inspiration. However, unlike microglia, astrocytes were activated without significant differences between ipsilateral and contralateral GFAP following C2LI.
4. Discussion
4.1 Significance of findings
We demonstrated that glial activation occurs at sites caudal to cervical spinal injury, including the phrenic motor nucleus, a site critical for respiratory motor control. Previous reports have demonstrated rapid inflammatory responses at the site of spinal injury, as well as at distant sites caudal to injury (Byrnes et al., 2006; Carlson et al., 1998; Fleming et al., 2006; Gwak and Hulsebosch, 2009; Popovich et al., 1997). We extend those findings here by focusing on the inflammatory response in or near the phrenic motor nucleus. C2 hemisection is frequently studied as a model of respiratory insufficiency and functional recovery following cervical spinal injury. C2 hemisection eliminates 90% of bulbospinal pathways to phrenic motor neurons, and induces spontaneous respiratory plasticity and partial functional recovery below the site of injury (Goshgarian, 2003; Vinit and Kastner, 2009). This is the first report of chronic glial activation (and possible inflammation) in the phrenic motor nucleus following C2 lateral injury.
The injury performed in this manuscript would have previously been grouped into the broad category of C2 hemisection. However, a recent report described several variations of the C2 hemisection, and the surgery performed most closely matches what was termed ‘C2 hemilesion with moderate ventromedial sparing’ (Fuller et al., 2009). In that report, the term ‘C2 hemisection’ is reserved for injuries in which a complete ablation of one-half of the spinal cord is performed at spinal segment C2.
4.2 Sham surgery
Increased OX42 staining was measured over time following sham surgeries. This finding suggests that some damage was done to the spinal cord by laminectomy and/or durotomy. On the other hand, measurements of GFAP over time remained stable following sham surgery. Unfortunately, naïve rats were not run at the time of these experiments. Thus, additional statistical analyses were necessary including the comparison of both raw fraction areas and fraction areas normalized to sham values.
4.3 Sidedness of activation
C2 hemisections cause lateralized effects on the spinal cord and on animal physiology. For instance, expression of long-term facilitation is observed in the ipsilateral (Golder et al., 2005), but not contralateral, phrenic nerve of rats 1-2 months post-surgery (Doperalski and Fuller, 2006). It was suggested that this differential response is a result of a compensatory increase of respiratory drive to contralateral phrenic motor neurons that limits their ability to express LTF. Identifying mechanisms by which ipsilateral respiratory drive can be heightened is, thus, an important step towards inducing functional recovery via mechanisms of plasticity. Thus, will be important to identify if the glial activation described here is pro-plasticity versus pro-inflammatory since inflammation may be a formidable barrier to full expression of spinal respiratory plasticity (Huxtable et al., 2011).
Like physiological measures of recovery, at least some cell types involved in lateralized effects are expected to exhibit some degree of sidedness following C2 lateral injury. Microglial activation was distinct ipsilateral to injury, making it a candidate for a cell that contributes to the lateralized effects of C2 lateral injury. Measurements of contralateral OX42 were, for the most part, greater than sham, but they were significantly less than ipsilateral measurements at all times investigated. In contrast, astrocytic GFAP staining did not show sidedness following C2 lateral injury at any time studied, suggesting that microglia may play a more important role in this respect.
4.4 Timecourse vs. physiological literature
A previous report indicated that neither microglia nor astrocyte fractional areas are increased in the C4 ventral horn within 24 hours post-C2 lateral injury (Gould and Goshgarian, 1997). Our results were only partially consistent with those findings. Microglial staining in the area of the phrenic motor nucleus was significantly increased ipsilateral to injury after 1 day. Staining peaked at 3-days post-C2LI and remained elevated for at least 28 days. The pattern contralateral to injury was similar, but OX42 staining was considerably less than the ipsilateral levels response. Although astrocytic staining in the area of the phrenic motor nucleus was not significantly greater than sham at 1 day post-C2LI, it was significantly increased by 3-days post-C2LI, and remained elevated for at least 28 days. It is possible that the difference between results in this study and those from the earlier study are due to the gender of rats used. Gould and Goshgarian (1997) used female rats, while we used males. Lower estrogen levels in male rats may allow a greater inflammatory response following spinal injury (Sribnick et al., 2005), potentially explaining earlier activation of microglia in the phrenic motor nucleus of our male rats.
Neurochemical responses related to inflammation wane by 2-4 weeks post-surgery in other models of spinal injury (e.g. TNFα and p75 neurotrophin receptor expression), despite the fact that morphological signs of glial activation persist (Gwak and Hulsebosch, 2009; Popovich et al., 1997). Thus, our results are consistent with earlier reports from other spinal regions using different models of spinal injury since cervical (C3-5) microglia and astrocytes remained morphologically activated 28 days post-C2LI. The potential role of these cells in functional recovery at extended time points is an interesting topic for further investigation. However, since microglia and astrocytes produce a variety of growth factors following spinal injury (Brown et al., 2004; Dougherty et al., 2000; Skold et al., 2000), these cells may affect respiratory motor plasticity in either positive or negative ways by producing pro-inflammatory/toxic and pro-plasticity/pro-survival molecules.
Phrenic motor plasticity induced by intermittent hypoxia is suppressed by lipopolysaccharide (LPS) induced inflammation (Vinit et al., 2011). Similarly, injury-induced inflammation may at least transiently impair plasticity following cervical spinal injury. For example, intermittent hypoxia-induced phrenic motor facilitation is minimal below a C2 hemisection at 2-weeks post-surgery, is marginal at one month (depending on the rat strain), but is fully expressed by two months post-surgery (Golder and Mitchell, 2005). Although an argument was made that these dynamics reflect the loss of and then partial recovery of serotonergic innervation below the injury, the present data suggest an unrecognized factor with the potential to modulate the capacity for intermittent hypoxia induced plasticity following spinal injury, namely time-dependent changes in the inflammatory activities of microglia and astrocytes near phrenic motor neurons. Further experiments assessing production of pro-plasticity and pro-inflammatory factors by glia in the phrenic motor nucleus at various times after injury would help to test this possibility.
4.5 Conclusions
Microglia and astrocytes release many factors in the vicinity of phrenic motor neurons that could enhance or suppress respiratory motor plasticity. In the early stages post-injury, inflammatory molecules (e.g. cytokines and prostaglandins) may undermine the capacity for plasticity (Byrnes et al., 2006; Pineau and Lacroix, 2007; Yang et al., 2005; Zhao et al., 2007), whereas production of pro-plasticity molecules (e.g. BDNF and VEGF) may become more prominent with time post-injury (Krenz and Weaver, 2000; Peng et al., 2006; Sieck and Mantilla, 2009). Each of these growth/trophic factors has the potential to induce and/or potentiate plasticity. The net effects of glial activation will presumably depend on their balance of pro-plasticity vs. pro-inflammatory molecules.
Here we show that glial activation is rapid and persistent, suggesting that these cells likely participate in complex time-dependent responses following C2 hemisection. Future investigations must explore the timing and identity of glial protein expression. Such information will provide a better understanding of the cellular and molecular environment in which any new/potential treatments for SCI must operate and may help define times of greatest efficacy.
Highlights.
> We examined microglia and astrocyte activation in the phrenic motor nucleus after C2 hemisection. > Microglia activation was measured by OX42 immunofluorescence. > Astrocytic activation was measured by GFAP immunofluorescence. > Microglial activation increased at 1-28 days after injury. > Astrocytic activation increased at 1-28 days after injury.
Acknowledgements
Supported by National Institutes of Health R37 HL69064. J.A. Windelborn was supported by NIH T32HL007654.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
J.A. Windelborn Present Address: Boston Biomedical Research Institute 64 Grove Street Watertown, MA 02472
Author Disclosure Statement:
No competing financial interests exist.
References
- Ankeny D, Popovich P. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2008;158:1112–1121. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley J, LaRocca G, Jimenez DA, Urban NN. Adult neurogenesis and specific replacement of interneuron subtypes in the mouse main olfactory bulb. BMC neuroscience. 2007;8:92. doi: 10.1186/1471-2202-8-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, Bethea JR. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–778. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Ricci M, Weaver L. NGF message and protein distribution in the injured rat spinal cord. Exp Neurol. 2004;188:115–127. doi: 10.1016/j.expneurol.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Byrnes K, Garay J, Di Giovanni S, De Biase A, Knoblach S, Hoffman E, Movsesyan V, Faden A. Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia. 2006;53:420–433. doi: 10.1002/glia.20295. [DOI] [PubMed] [Google Scholar]
- Carlson S, Parrish M, Springer J, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Coull J, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter M, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nature reviews. Neuroscience. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Sarchielli P, Picconi B, Calabresi P. Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol Sci. 2008;29:402–412. doi: 10.1016/j.tips.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Doperalski NJ, Fuller DD. Long-term facilitation of ipsilateral but not contralateral phrenic output after cervical spinal cord hemisection. Experimental neurology. 2006;200:74–81. doi: 10.1016/j.expneurol.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Dougherty K, Dreyfus C, Black I. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP- thirty-one years (1969-2000). Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fleming J, Norenberg M, Ramsay D, Dekaban G, Marcillo A, Saenz A, Pasquale-Styles M, Dietrich W, Weaver L. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, Lane MA, White TE, Bishop MD, Reier PJ. Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol. 2009;165:245–253. doi: 10.1016/j.resp.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25:2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Gould D, Goshgarian H. Glial changes in the phrenic nucleus following superimposed cervical spinal cord hemisection and peripheral chronic phrenicotomy injuries in adult rats. Exp Neurol. 1997;148:1–9. doi: 10.1006/exnr.1997.6556. [DOI] [PubMed] [Google Scholar]
- Guenther CH, Vinit S, Windelborn JA, Behan M, Mitchell GS. Atypical protein kinase C expression in phrenic motor neurons of the rat. Neuroscience. 2010;169:787–793. doi: 10.1016/j.neuroscience.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;161:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsebosch C. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008;214:6–9. doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxtable AG, Vinit S, Windelborn JA, Crader SM, Guenther CH, Watters JJ, Mitchell GS. Systemic inflammation impairs respiratory chemoreflexes and plasticity. Respiratory physiology & neurobiology. 2011;178:482–489. doi: 10.1016/j.resp.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenz N, Weaver L. Nerve growth factor in glia and inflammatory cells of the injured rat spinal cord. J Neurochem. 2000;74:730–739. doi: 10.1046/j.1471-4159.2000.740730.x. [DOI] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511:692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling EA, Kaur LC, Yick TY, Wong WC. Immunocytochemical localization of CR3 complement receptors with OX-42 in amoeboid microglia in postnatal rats. Anatomy and embryology. 1990;182:481–486. doi: 10.1007/BF00178913. [DOI] [PubMed] [Google Scholar]
- Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Peng X, Zhou Z, Glorioso J, Fink D, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;59:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- Pineau I, Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: multiphasic expression pattern and identification of the cell types involved. J Comp Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Popovich P, Wei P, Stokes B. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Porter WT. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J Physiol. 1895;17:455–485. doi: 10.1113/jphysiol.1895.sp000553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky A, Streit W. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 1997;47:34–48. [PubMed] [Google Scholar]
- Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, Hadani M, Schwartz M. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. The Journal of biological chemistry. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: autologous macrophages and beyond. J Neurotrauma. 2006;23:360–370. doi: 10.1089/neu.2006.23.360. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB. Role of neurotrophins in recovery of phrenic motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:218–225. doi: 10.1016/j.resp.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skold M, Cullheim S, Hammarberg H, Piehl F, Suneson A, Lake S, Sjogren A, Walum E, Risling M. Induction of VEGF and VEGF receptors in the spinal cord after mechanical spinal injury and prostaglandin administration. Eur J Neurosci. 2000;12:3675–3686. doi: 10.1046/j.1460-9568.2000.00263.x. [DOI] [PubMed] [Google Scholar]
- Sribnick E, Wingrave J, Matzelle D, Wilford G, Ray S, Banik N. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosci Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sroga J, Jones T, Kigerl K, McGaughy V, Popovich P. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- Vinit S, Darlot F, Stamegna JC, Sanchez P, Gauthier P, Kastner A. Long-term reorganization of respiratory pathways after partial cervical spinal cord injury. Eur J Neurosci. 2008;27:897–908. doi: 10.1111/j.1460-9568.2008.06072.x. [DOI] [PubMed] [Google Scholar]
- Vinit S, Kastner A. Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury. Respir Physiol Neurobiol. 2009;169:115–122. doi: 10.1016/j.resp.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respiratory physiology & neurobiology. 2011;176:130–135. doi: 10.1016/j.resp.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windelborn JA, Lipton P. Lysosomal release of cathepsins causes ischemic damage in the rat hippocampal slice and depends on NMDA-mediated calcium influx, arachidonic acid metabolism, and free radical production. J Neurochem. 2008;106:56–69. doi: 10.1111/j.1471-4159.2008.05349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Wu D, Miyamoto O, Shibuya S, Okada M, Igawa H, Janjua N, Norimatsu H, Itano T. Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med Okayama. 2005;59:121–127. doi: 10.18926/AMO/31950. [DOI] [PubMed] [Google Scholar]
- Yan P, Li Q, Kim G, Xu J, Hsu C, Xu X. Cellular localization of tumor necrosis factor-alpha following acute spinal cord injury in adult rats. J Neurotrauma. 2001;18:563–568. doi: 10.1089/089771501300227369. [DOI] [PubMed] [Google Scholar]
- Yang L, Jones N, Blumbergs P, Van Den Heuvel C, Moore E, Manavis J, Sarvestani G, Ghabriel M. Severity-dependent expression of pro-inflammatory cytokines in traumatic spinal cord injury in the rat. J Clin Neurosci. 2005;12:276–284. doi: 10.1016/j.jocn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Zhao P, Waxman SG, Hains BC. Extracellular signal-regulated kinase-regulated microglia-neuron signaling by prostaglandin E2 contributes to pain after spinal cord injury. J Neurosci. 2007;27:2357–2368. doi: 10.1523/JNEUROSCI.0138-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]