Summary
The major known risk factors for retinopathy of prematurity (ROP) are extremely low gestational age, exposure to high levels of oxygen early after birth (phase I) and relatively lower oxygen levels later (phase II). In this review, we summarize recent data suggesting that exposure to perinatal infection/inflammation is associated with an increased risk for ROP. Part of this effect might be due to direct exposure of the developing retina to circulating products of infection and/or inflammation. Another potential mechanism that deserves exploration is that inflammation and/or oxidative stress can modify the known increased risk of oxygen-associated ROP. Taken together, accumulating evidence suggests that prenatal, perinatal, and postnatal systemic inflammation contribute to a ‘pre-phase’, sensitizing the pre-ROP retina for subsequent insults, setting the stage for what are now called phase I and phase II of ROP pathogenesis. Strategies targeting inflammatory responses might help reduce the risk for ROP in extremely low gestational age newborns.
Keywords: Infection, Inflammation, Retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP) is a vasoproliferative disorder of the developing retina that mainly occurs in preterm newborns.1 The long term visual outcome among children with ROP includes a marked increased risk for blindness2 and visual disability.3 Identifying the antecedents and clarifying the pathogenesis is crucial for preventing and treating ROP.
Current pathogenetic model
ROP develops in two sequential phases. The first phase involves the cessation of normal retinal vascularization in the setting of hyperoxia. The second phase results in abnormal neovascularization of the retinal vessels.4 The current understanding of the etiology of ROP is that ‘only low birth weight, low gestational age, and supplemental oxygen therapy following delivery have consistently been associated with disease.’5 Indeed, the major pathogenic factor appears to be exposure of the immature retina to a succession of changing levels of oxygen, culminating in abnormally low/high levels of retinal growth factors, such as vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF). These, in turn, lead to a dysregulation of retinal vascularization.4
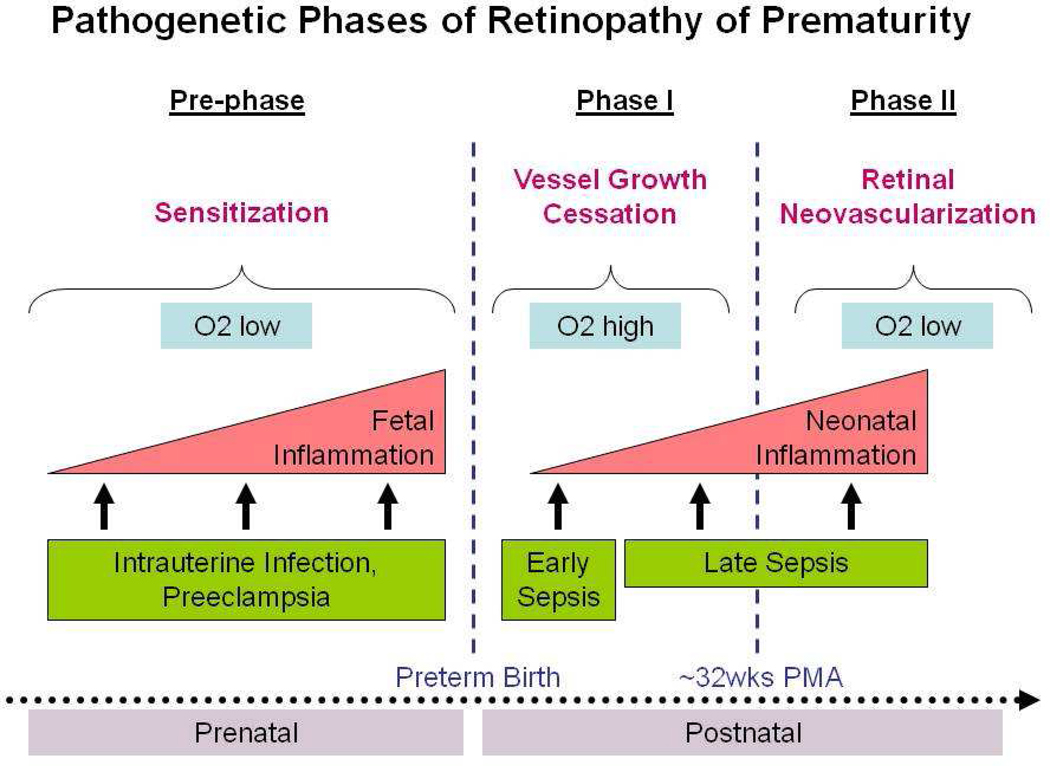
This two-phase model of ROP pathogenesis has been studied extensively, but does not include the important influence of the in-utero environment before birth. Below, we suggest that a prenatal ‘pre-phase’ of ROP sensitizes the developing retina via multiple maternal and fetal inflammatory factors produced in the setting of conditions such as severe pre-eclampsia and/or chorioamnionitis (Fig. 1).
Figure 1.
Proposed multi-phase model of retinopathy of prematurity (ROP) pathogenesis. In the pre-phase, prenatal pro-inflammatory events such as intrauterine infection and pre-eclampsia contribute to fetal inflammation and sensitization to subsequent postnatal insults. In phase I, preterm infants’ developing retinas are exposed to hyperoxia relative to in-utero oxygen levels, resulting in retinal vessel growth attenuation. Prenatal exposure to inflammation seems to exacerbate effects of postnatal factors. In phase II, retinal growth leaves part of the retina hypoxic, thereby stimulating intense neovascularization around 32 weeks of postmenstrual age (PMA), which in turn leads to high grade ROP.
Current therapy
Current therapeutic strategies include tight control of environmental oxygen6,7 and laser treatment.8 Intravitreal injection of anti-VEGF (Avastin®) is still considered experimental therapy.9,10 Although limiting oxygen exposure is non-invasive and apparently effective in reducing ROP risk, some are hesitant to adopt low oxygen strategies due to the lack of long term developmental outcome studies. An impressive reduction of ROP (8 vs 19%) co-occurred with a slight increase in mortality (20 vs 16%) in one recent trial.11 However, it is uncertain whether these two phenomena are causally related and what the long term developmental outcome will be in both groups. Other treatments currently being investigated include those that have the potential to prevent phase I ROP (birth to ≤32 weeks of PMA) as well as those targeting proliferative ROP development in phase II (infants >32–34 weeks of PMA).12 Therapies such as retinal erythropoietin and serum IGF-1, tight serum glucose control, and omega-3 supplements may decrease phase I ROP. A small observational study has suggested that intravenous fish-oil might reduce ROP risk.13 Interventions for phase II ROP include vitamin E supplements, increased thresholds for transfusions in infants with stage III ROP, photopic adaptation, and omega-3 supplements.14 Given the limited success of these interventions, new therapeutic alternatives should be investigated.
Infection and inflammation as a novel pathogenetic pathway
Whereas immaturity at birth and exposure to supplemental oxygen unquestionably play important etiologic roles in ROP,15–17 a novel line of evidence focusing on infection/inflammation is gradually receiving attention.
Retina
It has become evident that the VEGF164 isoform leads to leukocyte recruitment in pathologic hypoxia-induced retinal neovascularization.18 Indeed, oxidative stress, which can be a consequence of inflammation,19 has been implicated in ROP etiology.20 Moreover, the reduction of pathological retinal angiogenesis by omega-3-polyunsaturated fatty acids may be regulated, in part, through suppression of tumor necrosis factor-alpha (TNF-α),21 one of the major pro-inflammatory cytokines. Taken together, these data suggest that inflammatory processes might be part of the retinal neovascularization process, the hallmark of ROP.
Oxidative stress
A delicate balance exists between the production of reactive oxygen species (ROS) and antioxidant defenses. This balance might be disturbed by increased ROS production or inadequate antioxidant defenses. Increased generation of ROS in newborn infants can occur as a result of many conditions, including hyperoxia, reperfusion, and/or infection. The premature infant has inadequate concentrations of antioxidants at birth, as well as an impaired synthesis of antioxidants in response to hyperoxia and is, therefore, especially susceptible to ROS-induced damage. This can lead to an increased risk for the development of ROS-induced diseases of the newborn, such as ROP.22
Infection
Low birth weight preterm infants are particularly susceptible to infection, as rates of infection increase with decreasing birth weight and gestational age. Postnatal infection is associated with significant morbidity and neonatal complications, prolonged hospitalization, and death.23 It has been suggested that exposure of the preterm newborn to infection and inflammatory mediators is associated with an increased risk for ROP. In a large cohort study, infants who developed early onset sepsis had an increased risk for severe ROP.24 In the ELGAN study, the increased risk for ROP was primarily observed in association with late onset neonatal sepsis.25 Multiple other studies support the concept that neonatal sepsis26–29 is a risk factor for severe ROP. Fungal infection has also been linked to chorioretinitis and ROP.30,31 Candida is known to interact with vascular endothelial cells via multiple mechanisms, including release of pro-inflammatory cytokines which injure the developing retinal blood vessels.32 Candida sepsis has been independently associated with increased severity of ROP as well as the need for surgical therapy for advanced ROP.30 A systematic review and meta-analysis of eight studies found that systemic fungal infection in very low birth weight infants was significantly associated with ROP and severe ROP.33
Systemic inflammation
Studies investigating histologic chorioamnionitis as a risk factor for ROP have been inconclusive; an association with ROP is present only in univariate analyses.34 Other studies reveal higher rates of ROP in infants born to mothers with evidence of histologic chorioamnionitis compared to those born to mothers without signs of inflammation.35,36 Infants born to mothers with clinical chorioamnionitis as well as maternal leukocytosis have also been reported to be at increased risk of ROP. Maternal systemic inflammation might also contribute to the development of ROP in preterm infants by decreasing levels of IGF-1.37 Low levels of IGF-1 have been associated with an increased risk of ROP and IGF-1 levels vary inversely with the severity of ROP.
At least part of the sepsis-associated risk increase for ROP might be due to circulating products of inflammation. In preterm infants with early onset sepsis, studies suggest there is a relationship between high plasma levels of cytokines IL-6, IL-8, and TNF-α in the first days of life with the later development of ROP requiring treatment.38 Findings from a very large cohort study suggest that levels of circulating proinflammatory cytokines are elevated at multiple time-points after birth in preterm infants who later develop ROP when compared to controls.39
Our own series of epidemiologic studies in three distinct patient populations provides strong support for the hypothesis that prenatal, perinatal, and postnatal systemic inflammation is an additional risk factor for ROP beyond immaturity and/or hyperoxemia.6,40,41 First, in trivariate analyses exploring the effects of gestational age <29 weeks, clinical chorioamnionitis (CAM) and neonatal systemic inflammatory response syndrome (SIRS) on ROP occurrence, low gestational age was the most important antecedent; additional individual or joint exposure to SIRS and CAM add appreciably to this risk of progression to high grade disease.40 Second, neonatal sepsis, oxygen exposure, and low gestational age are not only independently associated with a significantly increased risk of ROP, but also interact beyond additive and even multiplicative patterns.6 Third, while antenatal exposure to infection or inflammation alone does not appear to convey risk information for severe ROP, their co-occurrence does.41
A pre-phase of ROP?
Taken together, these data suggest that ROP pathogenesis begins in the prenatal period. Indeed, it might be possible that exposure to intrauterine infection and inflammation contribute to a ‘prephase’ of ROP, sensitizing the pre-ROP retina for subsequent insults, thereby setting the stage for what is now called phase I and phase II of ROP pathogenesis (Fig. 1).
Maternal pre-eclampsia
This has been associated with an increased risk for severe ROP in preterm infants in some studies,28 whereas others have noted a decreased risk.42 Maternal pre-eclampsia appears to have inflammation-like characteristics in the absence of obvious infection.43 Although the exact pathogenesis is still unknown, maternal systemic inflammation in response to pregnancy likely plays a major role in the development of pre-eclampsia as well as in fetal complications such as ROP. Many placental factors such as inflammatory cytokines, corticotropin-releasing hormone, free radical species and activin A are increased in pre-eclampsia.44 During placentation, trophoblast cell invasion into the decidua is associated with a massive leukocyte infiltration.45 Monocytes and neutrophils bind to syncytiotrophoblast microparticles resulting in increased production of TNF and IL-12 and superoxide radicals, all of which likely contribute to the overall inflammatory response in pre-eclampsia.46 The placental ischemia also induces the release of many bioactive factors including VEGF, cytokines, reactive ROS, hypoxia-inducible factors, and matrix metalloproteases (MMPs), leading to further inflammation.47 In concert, all of these pre-eclampsia-related factors can alter vascular development and may promote development of ROP.
Pathogenesis of ROP
If infection and inflammation are causally involved in ROP pathogenesis, their effects could be direct, indirect, or both. Systemic inflammation could increase ROP risk by directly affecting retinal angiogenesis. Moreover, inflammation might exert its major effect on ROP incidence and severity by sensitizing the developing retina to O2-induced changes in growth factor availability and subsequent neovascularization, thereby modifying ROP risk. We consider it likely that the mechanisms are not mutually exclusive.
Direct effect
Infectious organisms and/or their microbial products can stimulate the production of proinflammatory cytokines.23 The evidence summarized above suggests that increased systemic levels of cytokines are associated with an increased risk for ROP.32,38,39 One way in which pro-inflammatory cytokines might exert a direct effect on neovascularization could be via inflammation-regulated VEGF availability.48 In this context, we suggest that an anti-inflammatory intervention should reduce oxygen-induced ROP risk in the presence of systemic inflammation, and perhaps even in its absence.
Indirect effect
Newborns with infection are at risk for circulatory and/or respiratory insufficiency with decreased systemic blood pressure, hypoxemia, and pathologic alterations in blood flow.23 The hypotension and fluctuation of oxygen saturation following sepsis might affect the retinal perfusion and lead to increased retinal ischemia.29 Frequent intermittent hypoxemic events have been associated with severe ROP requiring laser therapy, independent of gestational age or severity of early systemic illness.49
In animal models, the immature retina is very susceptible to hypoxic–ischemic insults. In rats exposed to hypoxia, retinal ganglion cell death was induced, resulting in the development of retinopathy.50 Hypoxia alone can stimulate the production of pro-inflammatory cytokines such as TNF-α in the retina, which results in the breakdown of the blood–retinal barrier. Increased expression of TNF-α and IL-1β is present in the retina of rats for up to three days after hypoxic exposure, inducing retinal ganglion cell death.50
Conclusion
Based on current knowledge about ROP etiology and pathogenesis, it seems likely that the O2/VEGF-related ROP risk is modified by systemic inflammation and cytokine release. The pathogenic mechanisms leading to ROP might begin long before birth. The role for anti-inflammatory drugs to prevent high grade ROP in the presence of systemic inflammation (and possibly even without systemic inflammation) should be investigated.
Practice points.
Known risk factors for ROP include prematurity, hyperoxia after birth (phase I) followed by relative hypoxia (phase II).
Exposure to perinatal infection/inflammation is associated with an increased risk for ROP.
Prenatal, perinatal, and postnatal systemic inflammation contribute to a ‘pre-phase’ of ROP, sensitizing the pre-ROP retina for subsequent insults.
Acknowledgments
Funding sources
O.D. is supported by the National Institutes of Health (EY019253) and the European Union (HEALTH-F2-2009-241778).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared.
References
- 1.McGregor ML, Bremer DL, Cole C, et al. Retinopathy of prematurity outcome in infants with prethreshold retinopathy of prematurity and oxygen saturation >94% in room air: the high oxygen percentage in retinopathy of prematurity study. Pediatrics. 2002;110:540–544. doi: 10.1542/peds.110.3.540. [DOI] [PubMed] [Google Scholar]
- 2.Paysse EA, Lindsey JL, Coats DK, Contant CF, Jr, Steinkuller PG. Therapeutic outcomes of cryotherapy versus transpupillary diode laser photocoagulation for threshold retinopathy of prematurity. J AAPOS. 1999;3:234–240. doi: 10.1016/s1091-8531(99)70008-x. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 5.Wheatley CM, Dickinson JL, Mackey DA, Craig JE, Sale MM. Retinopathy of prematurity: recent advances in our understanding. Arch Dis Child Fetal Neonatal Ed. 2002;87:F78–F82. doi: 10.1136/fn.87.2.F78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen M, Citil A, McCabe F, et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology. 2010;99:125–132. doi: 10.1159/000312821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saugstad OD. Oxygen and retinopathy of prematurity. J Perinatol. 2006;26 Suppl 1:S46–S50. doi: 10.1038/sj.jp.7211475. discussion S63-44. [DOI] [PubMed] [Google Scholar]
- 8.Salvin JH, Lehman SS, Jin J, Hendricks DH. Update on retinopathy of prematurity: treatment options and outcomes. Curr Opin Ophthalmol. 2010;21:329–334. doi: 10.1097/ICU.0b013e32833cd40b. [DOI] [PubMed] [Google Scholar]
- 9.Mintz-Hittner HA, Best LM. Antivascular endothelial growth factor for retinopathy of prematurity. Curr Opin Pediatr. 2009;21:182–187. doi: 10.1097/MOP.0b013e32832925f9. [DOI] [PubMed] [Google Scholar]
- 10.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–615. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlo WA, Finer NN, Walsh MC, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mataftsi A, Dimitrakos SA, Adams GG. Mediators involved in retinopathy of prematurity and emerging therapeutic targets. Early Hum Dev. 2011 June 21; doi: 10.1016/j.earlhumdev.2011.05.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Pawlik D, Lauterbach R, Turyk E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics. 2011;127:223–228. doi: 10.1542/peds.2010-2427. [DOI] [PubMed] [Google Scholar]
- 14.Raghuveer TS, Bloom BT. A paradigm shift in the prevention of retinopathy of prematurity. Neonatology. 2011;100:116–129. doi: 10.1159/000322848. [DOI] [PubMed] [Google Scholar]
- 15.Campbell K. Intensive oxygen therapy as a possible cause of retrolental fibroplasia; a clinical approach. Med J Aust. 1951;2:48–50. [PubMed] [Google Scholar]
- 16.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326:1050–1054. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 17.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 18.Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saugstad OD. Oxidative stress in the newborn – a 30-year perspective. Biol Neonate. 2005;88:228–236. doi: 10.1159/000087586. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan JL. Retinopathy of prematurity and iron: a modification of the oxygen hypothesis. Pediatrics. 1986;78:1171–1172. [PubMed] [Google Scholar]
- 21.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JW, Davis JM. Future applications of antioxidants in premature infants. Curr Opin Pediatr. 2011;23:161–166. doi: 10.1097/MOP.0b013e3283423e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 24.Klinger G, Levy I, Sirota L, Boyko V, Lerner-Geva L, Reichman B. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125:e736–e740. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 25.Washburn Tolsma KA, EN, Chen M, Duker J, Leviton A, Dammann O. Neonatal bacteremia and retinopathy of prematurity: The ELGAN Study. Arch Ophthalmol. doi: 10.1001/archophthalmol.2011.319. (in press) [DOI] [PubMed] [Google Scholar]
- 26.Maheshwari R, Kumar H, Paul VK, Singh M, Deorari AK, Tiwari HK. Incidence and risk factors of retinopathy of prematurity in a tertiary care newborn unit in New Delhi. Natl Med J India. 1996;9:211–214. [PubMed] [Google Scholar]
- 27.Chye JK, Lim CT, Leong HL, Wong PK. Retinopathy of prematurity in very low birth weight infants. Ann Acad Med Singapore. 1999;28:193–198. [PubMed] [Google Scholar]
- 28.Shah VA, Yeo CL, Ling YL, Ho LY. Incidence, risk factors of retinopathy of prematurity among very low birth weight infants in Singapore. Ann Acad Med Singapore. 2005;34:169–178. [PubMed] [Google Scholar]
- 29.Liu PM, Fang PC, Huang CB, et al. Risk factors of retinopathy of prematurity in premature infants weighing less than 1600 g. Am J Perinatol. 2005;22:115–120. doi: 10.1055/s-2005-837276. [DOI] [PubMed] [Google Scholar]
- 30.Mittal M, Dhanireddy R, Higgins RD. Candida sepsis and association with retinopathy of prematurity. Pediatrics. 1998;101(4 Pt 1):654–657. doi: 10.1542/peds.101.4.654. [DOI] [PubMed] [Google Scholar]
- 31.Manzoni P, Maestri A, Leonessa M, Mostert M, Farina D, Gomirato G. Fungal and bacterial sepsis and threshold ROP in preterm very low birth weight neonates. J Perinatol. 2006;26:23–30. doi: 10.1038/sj.jp.7211420. [DOI] [PubMed] [Google Scholar]
- 32.Filler SG, Pfunder AS, Spellberg BJ, Spellberg JP, Edwards JE., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bharwani SK, Dhanireddy R. Systemic fungal infection is associated with the development of retinopathy of prematurity in very low birth weight infants: a meta-review. J Perinatol. 2008;28:61–66. doi: 10.1038/sj.jp.7211878. [DOI] [PubMed] [Google Scholar]
- 34.Hendson L, Russell L, Robertson CM, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. J Pediatr. 2011;158:397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med. 2005;159:1032–1035. doi: 10.1001/archpedi.159.11.1032. [DOI] [PubMed] [Google Scholar]
- 36.Moscuzza F, Belcari F, Nardini V, et al. Correlation between placental histopathology and fetal/neonatal outcome: chorioamnionitis and funisitis are associated to intraventricular haemorrage and retinopathy of prematurity in preterm newborns. Gynecol Endocrinol. 2011;27:319–323. doi: 10.3109/09513590.2010.487619. [DOI] [PubMed] [Google Scholar]
- 37.Woo SJ, Park KH, Jung HJ, Kim SN, Choe G, Ahn J. Effects of maternal and placental inflammation on retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2011 doi: 10.1007/s00417-011-1648-2. [DOI] [PubMed] [Google Scholar]
- 38.Silveira RC, Filho JB, Procianoy RS. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest Ophthalmol Vis Sci. 2011;52:1297–1301. doi: 10.1167/iovs.10-6279. [DOI] [PubMed] [Google Scholar]
- 39.Sood BG, Madan A, Saha S, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dammann O, Brinkhaus MJ, Bartels DB, et al. Immaturity, perinatal inflammation, and retinopathy of prematurity: a multi-hit hypothesis. Early Hum Dev. 2009;85:325–329. doi: 10.1016/j.earlhumdev.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 41.Chen M, Allred E, Hecht J, et al. Placenta microbiology and histology, and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.11-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fortes Filho JB, Costa MC, Eckert GU, Santos PG, Silveira RC, Procianoy RS. Maternal preeclampsia protects preterm infants against severe retinopathy of prematurity. J Pediatr. 2011;158:372–376. doi: 10.1016/j.jpeds.2010.08.051. [DOI] [PubMed] [Google Scholar]
- 43.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 44.Hahn S, Holzgreve W. Fetal cells and cell-free fetal DNA in maternal blood: new insights into pre-eclampsia. Hum Reprod Update. 2002;8:501–508. doi: 10.1093/humupd/8.6.501. [DOI] [PubMed] [Google Scholar]
- 45.Aly AS, Khandelwal M, Zhao J, Mehmet AH, Sammel MD, Parry S. Neutrophils are stimulated by syncytiotrophoblast microvillous membranes to generate superoxide radicals in women with preeclampsia. Am J Obstet Gynecol. 2004;190:252–258. doi: 10.1016/j.ajog.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Benyo DF, Smarason A, Redman CW, Sims C, Conrad KP. Expression of inflammatory cytokines in placentas from women with preeclampsia. J Clin Endocrinol Metab. 2001;86:2505–2512. doi: 10.1210/jcem.86.6.7585. [DOI] [PubMed] [Google Scholar]
- 47.Tanbe AF, Khalil RA. Circulating and vascular bioactive factors during hypertension in pregnancy. Curr Bioact Compd. 2010;6:60–75. doi: 10.2174/157340710790711737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 49.Di Fiore JM, Bloom JN, Orge F, et al. A higher incidence of intermittent hypoxemic episodes is associated with severe retinopathy of prematurity. J Pediatr. 2011;157:69–73. doi: 10.1016/j.jpeds.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sivakumar V, Foulds WS, Luu CD, Ling EA, Kaur C. Retinal ganglion cell death is induced by microglia derived pro-inflammatory cytokines in the hypoxic neonatal retina. J Pathol. 2011;224:245–260. doi: 10.1002/path.2858. [DOI] [PubMed] [Google Scholar]