Abstract
Purpose
Portable CT scanners are beneficial for diagnosis in the intensive care unit, emergency room, and operating room. Portable fixed-base versus translating-base CT systems were evaluated for otologic image-guided surgical (IGS) applications based on geometric accuracy and utility for percutaneous cochlear implantation.
Methods
Five cadaveric skulls were fitted with fiducial markers and scanned using both a translating-base, 8-slice CT scanner (CereTom®) and a fixed-base, flat-panel, volume-CT (fpVCT) scanner (Xoran xCAT®). Images were analyzed for: (a) subjective quality (i.e. noise), (b) consistency of attenuation measurements (Hounsfield units) across similar tissue, and (c) geometric accuracy of fiducial marker positions. The utility of these scanners in clinical IGS cases was tested.
Results
Five cadaveric specimens were scanned using each of the scanners. The translating-base, 8-slice CT scanner had spatially consistent Hounsfield units, and the image quality was subjectively good. However, because of movement variations during scanning, the geometric accuracy of fiducial marker positions was low. The fixed-base, fpVCT system had high spatial resolution, but the images were noisy and had spatially inconsistent attenuation measurements; while the geometric representation of the fiducial markers was highly accurate.
Conclusion
Two types of portable CT scanners were evaluated for otologic IGS. The translating-base, 8-slice CT scanner provided better image quality than a fixed-base, fpVCT scanner. However, the inherent error in three-dimensional spatial relationships by the translating-based system makes it suboptimal for otologic IGS use.
Keywords: portable CT scanners, CereTom, xCAT, translating-base scanner, fixed-base scanner, image-guided surgery, fpVCT
INTRODUCTION
Shortly after Roentgen invented the x-ray machine in 1895, the desire for portable machines led to their invention in 1919 by Frederick Jones. Among their first uses were imaging on the battlefield during World War I. Today, portable x-ray machines are used on a daily basis, allowing physicians to acquire two-dimensional images of patients without moving them from their place of treatment (e.g. intensive care unit, operating room). With the invention of computerized tomography by Sir Godfrey Hounsfield in 1967 and clinical introduction in 1971, the desire for portability led to the development and to the clinical introduction of a portable 8-slice CT scanner by NeuroLogica Corporation (Danvers, MA) in 2005 and a portable flat-panel, volume, CT scanner (fpVCT) by Xoran Technologies®, Inc. (Ann Arbor, Michigan) in 2006—both for head-based applications. Because it is likely that other companies will soon enter the market and clinicians and researchers will have increased access to them, we sought to understand the pros and cons of such systems, especially as applied to image-guided otologic surgery.
There are two fundamental differences between Neurologica’s portable 8-slice CT scanner, the CereTom®, and Xoran’s portable fpVCT, the xCAT®. First, while both are CT scanners, the method by which images are generated is different. The CereTom® is a traditional CT scanner comprising a fan-beam radiation source and collimators creating 8-slices of slice thickness that is equal to the distance between two adjacent collimators. The xCAT® is an fpVCT with a cone-beam radiation source and flat-plate radiation detector. Second, to cover the entire field of view (FOV), the scanners utilize different techniques. The CereTom® uses a translating base to cover the FOV. In clinical use, large wheels are used to move the CereTom® along hospital corridors to the patient’s bedside, following which the wheels are locked and tank treads are used to move the scanner between individual 8-slice captures. For a 1.25 mm slice thickness, each scan covers 10 mm following which the treads move the entire scanner 10 mm, and another scan is acquired (Figure 1). In this incremental fashion the entire FOV is covered. The xCAT® uses a fixed-base system with a gantry that rotates around a horizontal axis through the center of the FOV. As with the CereTom®, large wheels are used to move the xCAT® along hospital corridors to the patient’s bedside, following which the wheels are locked and the scanner remains stationary while its gantry rotates around the patient’s anatomy (Figure 2).
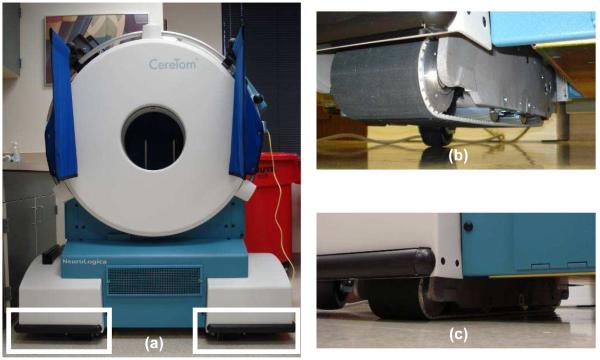
Figure 1.
CereTom® CT scanner (NeuroLogica Corporation, Danvers, MA), a translating-base system. The base of the scanner marked by the two rectangles in (a) are the wheels during the transportation as shown in (b) and are on tank threads during the scanning as shown in (c).
Figure 2.
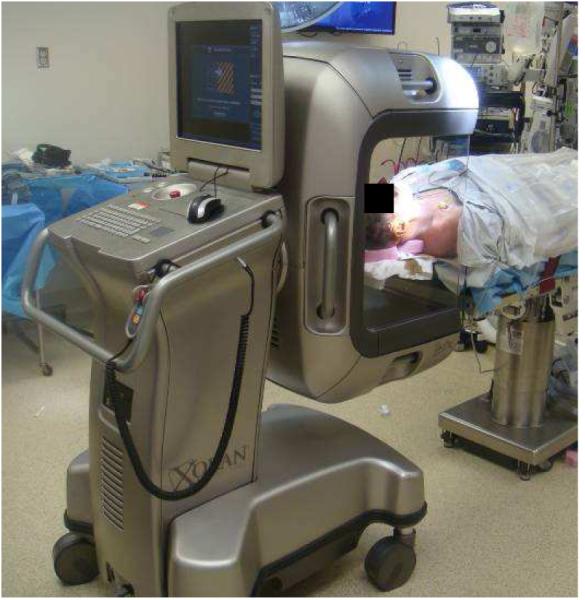
xCAT® ENT scanner (Xoran Technologies, Ann Arbor, MI), a fixed-base system, locked in position for scanning a patient in the operating room.
Image-guided surgery (IGS) involves using the patient’s radiological images (e.g. CT scans) as references to guide surgical interventions. The image of the patient provides a three-dimensional view of the anatomy before the actual surgery and enables better understanding of the patient’s unique anatomy. When real-time tracking of the surgical tool during the surgery is required, the CT image is usually registered to the patient in the physical space using commercially-available navigation (e.g. Vector Vision Navigation System; Brainlab Inc., Westchester, IL, USA). Surface-based registrations are commonly used to register the physical space to the image space. This registration is done by finding the best fit that minimizes the sum of squares of the distances from the points on the surface of the face extracted in the CT image and the points on the patient’s face scanned using a handheld laser scanner. Submillimetric accuracy is not routinely achieved by systems based on surface-based registration [1]. When submillimetric accuracy is required, systems that use bone-implanted fiducial markers for registration are needed [2, 3]. If the markers are not rigidly attached to the patient and the markers shift between imaging and operating (e.g. skin-affixed markers that become displaced), the accuracy of IGS is compromised. Thus, for high accuracy applications (e.g. percutaneous cochlear implantation and placement of deep brain stimulators), fiducial markers are rigidly attached to the bone of the patient before imaging allowing identification in both the radiographic image and on the patient in the operating room.
Bone-implanted fiducial markers are not commonly used in otologic applications because of the inconvenience to both the patient and the surgeon caused by the need for a separate invasive procedure to implant the markers prior to radiographic imaging. Portable CT scanners remove this inconvenience because fiducial marker implantation and imaging can take place in the operating room just prior to, or while, operating with the patient under general anesthesia. This intraoperative marker implantation and imaging also decreases the chance of displacement of fiducial markers between imaging and operating.
In an effort to achieve maximal accuracy during IGS, we have employed such work flow to target the inner ear for minimally-invasive cochlear implant surgery, a procedure that we term “percutaneous cochlear implant surgery” (PCI).
The traditional way of performing cochlear implant surgery is via wide field excavation to allow access to the inner ear via a mastoidectomy and facial recess approach. Through incremental drilling, vital structures in close proximity to the inner ear, such as the facial nerve that controls ipsilateral facial motion and the chorda tympani that controls taste on the ipsilateral tip of the tongue, are visually identified and avoided [4]. PCI involves drilling a linear path from the skull directly to the cochlea via the facial recess. The facial recess, which is bounded by the facial nerve posteriorly and the chorda tympani anteriorly can be as small as 2.4 mm wide [5]. Hence, submillimetric accuracy is a necessity to ensure safety during a PCI. In 2005, we demonstrated the concept of using IGS to access the cochlea in this minimally-invasive fashion [6]. Recognizing the difficulty in maintaining accuracy along a specific path while drilling freehand, we adapted the use of rigid microstereotactic frames that rely on bone-implanted markers. After cadaveric testing [7], this method progressed to a clinical trial using IGS to design and fabricate customized microstereotactic frames guiding a drill to the cochlea while avoiding vital structures [8, 9]. To achieve maximal accuracy and minimize patient inconvenience, we have employed portable CT scanners since 2008 [9] so that bone-implanted fiducial markers can be placed within the operating room with the patient under general anesthesia following which image-guided intervention takes place.
It is for this PCI application that we have used portable CT scanners clinically. Our experience led to bench-top testing, described below, in an effort to better define the pros and cons of portable CT scanners as used for IGS requiring submillimetric accuracy.
METHODS
Quality Analysis
To mimic the IGS procedures we perform in the operating room, the mastoid region of five cadaveric temporal bone specimens were fitted with bone-implanted, spherical fiducial markers (Figure 3) and scanned using both a modified CereTom® with 0.625 mm slice thickness and a modified xCAT® with its reconstruction algorithms tailored by the manufacturer for tissue differentiation within the temporal bone. The following criteria were tested.
Ability to localize fiducial markers – The center of the sphere in each fiducial marker was localized automatically using image processing techniques [10].
Ability to automatically segment regions of the ear and plan a drill path – Previously reported algorithms were employed to automatically segment the following structures:—the cochlea, the facial nerve, the chorda tympani, the semicircular canals, the ossicles, and the external auditory canal. All structures were segmented completely using an atlas-based approach [11] except for the facial nerve and the chorda tympani, which also involved additional processing to match the intensity-model of each structure [12]. A drill trajectory was then planned from the lateral skull to the cochlea that avoids damage to other structures [13].
Subjective impression of the quality of scan as compared to the scan of the bone from a traditional multi-slice CT scanner.
Figure 3.
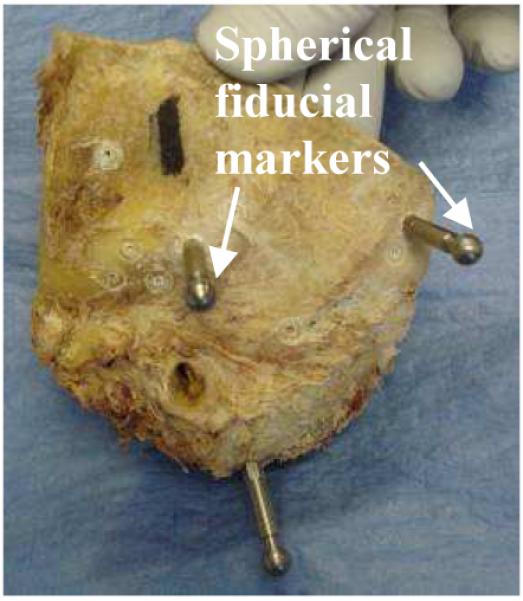
Temporal bone cadaveric specimen with bone-implanted spherical fiducial markers.
Clinical Validation
Based on the results of the quality analysis, we created research study protocols for scanning patients in the OR during clinical validation of PCI [9]. Patients were consented before surgery using institutional review board (IRB)-approved protocols and consent forms. The steps performed in the OR were: (1) implantation of three bone-anchored markers surrounding the surgical target, (2) acquisition of a CT scan, (3) path planning, (4) design and construction of a customized microstereotactic frame to target the cochlea, (5) sterilization of the microstereotactic frame, (6) mounting of the microstereotactic frame after performing traditional cochlear implant surgery, and (7) photodocumentation of the trajectory of the drill bit as guided by the frame and grading of the level of success regarding accurate targeting of the cochlear and avoidance of nearby vital anatomy including the facial nerve and the chorda tympani.
RESULTS
Analysis of images from CereTom® scanner
Initial analysis of the CereTom® was performed using the machine in its FDA-approved configuration comprising eight 1.25 mm detectors (i.e. slice thickness = 1.25 mm), spaced at 1.25 mm (i.e., zero gap). Other scanning parameters were 140 kVp, 7 mA, 2 second exposure, and in-plane pixel size of 0.5-by-0.5 mm. With this set-up we were able to localize the centers of the fiducial markers. The quality of images appeared to be similar to those from traditional scanners with homogeneous Hounsfield units. However, the large slice thickness was inadequate to identify very thin structures, such as the chorda tympani.
To overcome this problem of insufficient resolution, Neurologica provided a “thin-slice” machine with 0.625 mm detectors spaced at 0.625 mm. Other scanning parameters used were 120 kVp, 4 mA, 1 second exposure. These scanning parameters were chosen for the study from the list of protocols provided by the manufacturer because it produced images of acceptable image quality and also resulted in a radiation dose less than the 50 mrem limit per scan allowable under our IRB-approved protocol. With these settings, the machine produced CT images with voxel size 0.25-by-0.25-by-0.625 mm. On cadavers, we were able to identify both the fiducial markers and the chorda tympani in every case. The scanner allowed easy acquisition within the OR after fiducial marker implantation. However, it resulted in a failure with inaccurate targeting of the cochlea and a drill trajectory that, had it been followed, would have resulted in violation of a vital structure, the facial nerve.
After the surgery, the cause of the failure was analyzed. It was confirmed, by measuring dimensions using a FARO Gage-Plus measuring system (FARO Technologies, INC., Atlanta, GA), that there was no error in the manufacturing of the customized microstereotactic frame. Upon careful analysis of the scanning method used by the CereTom®, we realized that the scanner can cause small deviations in the relative positions of anatomic features because the tank-track drive system is dependent upon a perfectly flat floor to accurately reconstruct three-dimensional anatomy. Since the CereTom® that was used had eight 0.625-mm detectors, a 5 mm (8 × 0.625 mm) slab is imaged during each exposure. The scanner then moves along the floor by 5 mm to prepare for the scanning of the next slab. If the floor is not perfectly planar, then the movement of the scanner relative to the patient may be off-axis. Because of imperfections in the floor (e.g. dips or humps), translation of the scanner might produce yaw and pitch leading to rotational and translational error in the anatomy being covered between the two scanner positions. Any inconsistency caused during this movement, if not corrected in the image reconstruction, may cause misrepresentation of relative positions in the CT scan.
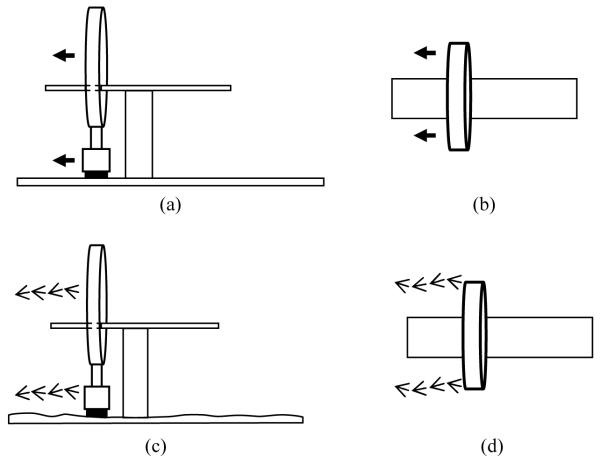
This effect is graphically shown in Figure 4, where (a) and (b) show the ideal movement desired for the CereTom® scanner and (c) and (d) show the movement that can occur in a non-ideal setting. Imperfections in planarity of the floor and/or debris encountered during the travel of the scanner may cause off-axis travel. This error was confirmed by scanning highly-accurate phantoms and measuring the lengths between two movements of the scanner. We found small deviations, some as large as 0.2 mm, between each step. While this level of distortion of three-dimensional anatomy may not be crucial in certain clinical cases (e.g. identification of intracranial masses), it may be disastrous for IGS cases.
Figure 4.
CereTom® scanner movement during image acquisition. (a) and (b) show the ideal movement as seen from the side and the top. (c) and (d) show the erroneous movement. Because of the uneven surface of the floor, varying pitch (c) and yaw (d) angles are observed during the scanning.
Analysis of images from xCAT® ENT scanner
For the xCAT®, two scanning protocols were available. Both the protocols used the same scanning parameters, 120 kVp, 6 mA, 20 seconds, but differed in the reconstruction of the image. The default “sinus” protocol produced a CT image that had a voxel size of 0.4 mm isotropic. The other research protocol, which was designed specifically for our PCI application, resulted in a higher spatial resolution with voxels of 0.3 mm isotropic. It also included post-acquisition image processing to reduce the intensity inhomogeneity. We chose this later research protocol for our testing. The scanner was used in the OR without any difficulties. We were able to identify the fiducial marker location satisfactorily. However, identification of anatomy was unsatisfactory for some structures. While images from the xCAT® were found to have high spatial resolution, they were noisy and had spatially inconsistent intensity. For example, while bone consistently exhibited Hounsfield values between 1000 and 2000 in both the traditional CT scanner and the CereTom, the xCAT gave values ranging from approximately 350 to 1300, and while air consistently exhibited Hounsfield values between −800 and −1100 in both the traditional CT scanner and the CereTom, the xCAT gave values ranging from −600 to −1000. Because of this inconsistency, our automatic anatomical identification algorithms, which were initially based on intensity thresholding, did not function in an acceptable manner on the xCAT® scans. With modification of the algorithms to lessen their dependence on consistent intensity the results improved, but automatic anatomical identification remained unacceptable for facial nerve and chorda tympani segmentation. It was possible to manually adjust segmentation of the facial nerve, but manual adjustment was very difficult for the chorda tympani, which, because it is a very thin structure, was hard to consistently visually identify on the xCAT® scans. To overcome this difficulty, we now use the xCAT® to identify fiducial markers intraoperatively, and we rigidly register them to a pre-operative multi-slice CT scan on which the pertinent anatomy has been identified and path planning performed using a standard mutual-information method [14].
The pros and cons of the two scanners are listed in the Table 1.
Table 1.
Pros and cons of the two portable CT scanners tested.
| Voxel Size | Spatial intensity consistency | 3-D representation | |
|---|---|---|---|
| CereTom® | 0.625 × 0.5 × 0.5 mm | Good | Unacceptable |
| xCAT® | 0.3 × 0.3 × 0.3 mm | Unacceptable | Good |
DISCUSSION
Portable CT scanners are beneficial in critical care units when patients cannot be transported to the radiological suite and an immediate diagnostics is required [15-17]. They are also beneficial for updating anatomical information real-time for image guided surgery as suggested by Das et al. [18]. In this paper, we tested the use of these scanners for image-guided surgical procedures that require submillimetric accuracy and use bone-anchors to achieve it, such as PCI.
We tested two portable CT scanners in both a cadaver laboratory and the operating room. These scanners differed in two fundamental ways. First, the CereTom® is a conventional 8-slice, fan-beam CT scanner, while the xCAT® is a cone-beam, flat-panel, volume CT. Second, the strategies to cover the field of view are different: The CereTom®, uses a translating-base system and the xCAT®, uses a fixed-base with the radiation source and emitters fixed in a gantry that rotates around the target tissue. Because of these differences, each scanner offered advantages and disadvantages.
The CereTom®, because of its collimated fan-beam radiation, produced homogeneous in-plane images with spatially consistent Hounsfield units whose values are typical for multi-slice CT scanners. However, because of its dependence on a flat floor to translate on-axis, it produced subtly distorted anatomy between scans such that three-dimensional relationships between points in different planes in the volume were inaccurate. Though this finding was based on our experience with PCI, it is also applicable to other image-guided surgical applications. Accuracy of any IGS system depends on the accuracy of the images that guide the surgery. If the CT images misrepresent the physical anatomy and a surgery is performed based on that misrepresented data, it could lead to unwanted results during the surgery. Because of errors in its three-dimensional representation of anatomy, this scanner is not recommended for IGS especially for those applications that require submillimetric accuracy, including intraoperative guidance. However, these scanners are still useful in the intensive care unit and emergency room where patients need immediate diagnostics without the need for submillimetric three-dimensional reconstruction [15, 17].
The xCAT®, because of its cone-beam radiation source and flat-panel detector, produced highly accurate geometric scans with isotropic voxels. However cone-beam technology is different from that associated with the collimated radiation sources used in multi-slice CT scanners, and as a result the Hounsfield units were spatially inconsistent. Areas of similar tissue (e.g. cortical bone) exhibited widely different Hounsfield units when located at different positions in the same image volume (e.g. range of 350 to 1300 for bone compared to a range of 1000 to 2000 for traditional multi-slice CT scanners). Though this intensity inhomogeneity does not affect the visual interpretation of the scans by the surgeons, it can affect computer processing that uses software that is dependent upon intensity values. Nevertheless, the intraoperative CT scans acquired using the xCAT scanner, when combined with pre-operative scans from traditional multi-slice CT scanners, allow submillimetric accuracy, such as that required for image-guided access to the cochlea (e.g., PCI). Other studies have also shown that the image quality of fpVCT scans, especially those of soft tissue, is worse than that of multi-slice CT scanners [16, 19, 20] and that scans from portable, cone-beam scanners should be augmented with information from corresponding multi-slice CT scanners [16].
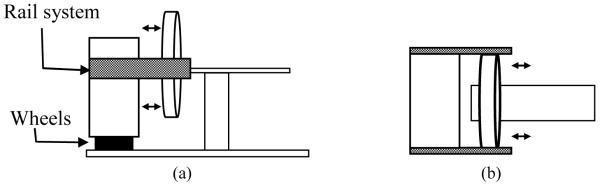
Until cone-beam technology can be rectified with collimated multi-slice CT scanner technology, we propose that the ideal portable CT scanner—in terms of accuracy and consistency with current Hounsfield units—would be a thin cut, multi-slice CT scanner that translates over the field of view independently of translational movement inconsistencies (e.g. debris or dips in the floor) as shown in Figure 5.
Figure 5.
Ideal design of a portable CT scanner. (a) and (b) show the side and top view of the system. The wheels at the bottom will enable transportation of the entire unit between rooms and will be locked during scanning. The rail system will enable movement of the scanner relative to the patient and independent of the floor during scanning.
CONCLUSION
We analyzed the use of two types of portable CT scanners for image-guided access to the cochlea, a.k.a. PCI, a surgical procedure that requires submillimetric accuracy. We found that availability of portable CT scanners allows placement of bone markers and acquisition of CT scan in the operating room just prior to the surgery, thus simplifying the workflow for PCI. We found the image quality of a translating-base, fan-beam CT scanner to be better than a fixed-base cone-beam volume scanner. However, limitations in the current design of the translating-base system lead to skewing of the data sets, which makes them suboptimal for use with image-guided surgical systems, especially those requiring submillimetric accuracy. The ideal system that minimizes skewing and maximizes accuracy would consist of a fixed-base, fan-beam CT scanner which translates on a rail system relative to the patient. Short of such a device, image-guided interventions that require submillimetric accuracy will depend upon either (i) traditional multi-slice CT scanning after bone-implanted fiducial markers are placed or (ii) rigidly registering a pre-operative traditional multi-slice CT scan to an intraoperatively obtained fpVCT scan after bone-implanted markers are placed. We have successfully used option (ii) in our continued testing and implementation of PCI.
Acknowledgement
The project described was supported by Award Numbers R01DC008408 from the National Institute on Deafness and Other Communication Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
Contributor Information
Ramya Balachandran, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center 1215 21st Avenue South, 10450 Medical Center, East South Tower Nashville, TN 37232.
Daniel Schurzig, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center 1215 21st Avenue South, 10450 Medical Center, East South Tower Nashville, TN 37232.
J Michael Fitzpatrick, Department of Electrical Engineering and Computer Science Vanderbilt University, Nashville, TN j.michael.fitzpatrick@vanderbilt.edu.
Robert F Labadie, Department of Otolaryngology-Head and Neck Surgery Vanderbilt University Medical Center, Nashville, TN robert.labadie@vanderbilt.edu.
REFERENCES
- 1.Schlaier J, Warnat J, Brawanski A. Registration accuracy and practicability of laser-directed surface matching. Comput Aided Surg. 2002;7:284Y90. doi: 10.1002/igs.10053. [DOI] [PubMed] [Google Scholar]
- 2.Maurer C, Fitpatrick J, Wang M, et al. Registration of head volume images using implantable fiducial markers. IEEE Trans Med Imaging. 1997;16:447Y62. doi: 10.1109/42.611354. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick JM. The role of registration in accurate surgical guidance. Journal of Engineering in Medicine. 2010 May;224(5):607–622. doi: 10.1243/09544119JEIM589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.House W. Surgical considerations in cochlear implantation. Ann Otol Rhinol Laryngol Supp. 1982;vol. 91(no. 2 Pt 3):15–20. [PubMed] [Google Scholar]
- 5.Su W, Marion MS, Hinojosa R, Matz GJ. Anatomical measurements of the cochlear aqueduct, round window membrane, round window niche, and facial recess. Laryngoscope. 1982;92:483–6. doi: 10.1288/00005537-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Labadie RF, Choudhury P, Cetinkaya E, Balachandran R, Haynes DS, Fenlon M, Juscyzk S, Fitzpatrick JM. Minimally-invasive, image-guided, facial-recess approach to the middle ear: Demonstration of the concept of percutaneous cochlear access in vitro. Otology and Neurotology. 2005 July;26:557–562. doi: 10.1097/01.mao.0000178117.61537.5b. [DOI] [PubMed] [Google Scholar]
- 7.Warren FM, Balachandran R, Fitzpatrick JM, Labadie RF. Percutaneous cochlear access using bone-mounted, customized drill guides: demonstration of concept in vitro. Otology and Neurotology. 2007 Apr;28(3):325–329. doi: 10.1097/01.mao.0000253287.86737.2e. [DOI] [PubMed] [Google Scholar]
- 8.Labadie RF, Noble JH, Dawant BM, Balachandran R, Majdani O, Fitzpatrick JM. Clinical Validation of Percutaneous Cochlear Implant Surgery: Initial Report. The Laryngoscope. 2008 June;118(6):1031–1039. doi: 10.1097/MLG.0b013e31816b309e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labadie RF, Balachandran R, Mitchell J, Noble JH, Majdani O, Dawant BM, Bennett M, Haynes DS, Fitzpatrick JM. Clinical Validation Study of Percutaneous Cochlear Access Using Patient Customized Micro-Stereotactic Frames. Otology and Neurotology. 2010 Jan;31(1):94–99. doi: 10.1097/MAO.0b013e3181c2f81a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MY, Maurer CR, Jr., Fitzpatrick JM, Maciunas RJ. An automatic technique for finding and localizing externally attached markers in CT and MR volume images of the head. IEEE Trans Biomed Eng. 1996;43:627–37. doi: 10.1109/10.495282. [DOI] [PubMed] [Google Scholar]
- 11.Noble JH, Dawant BM, Warren FM, et al. Automatic identification and 3D rendering of temporal bone anatomy. Otol Neurotol. 2009 Jun;vol. 30(no. 4):436–42. doi: 10.1097/MAO.0b013e31819e61ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble JH, Warren FM, Labadie RF, et al. Automatic segmentation of the facial nerve and chorda tympani in CT images using spatially dependent feature values. Med Phys. 2008 Dec;vol. 35(no. 12):5375–84. doi: 10.1118/1.3005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noble JH, Warren FM, Labadie RF, et al. Determination of drill paths for percutaneous cochlear access accounting for target positioning error. Progress in Biomedical Optics and Imaging - Proceedings of SPIE. 2007;vol. 6509:650925.1–650925.10. [Google Scholar]
- 14.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–98. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 15.Peace K, Wilensky EM, Frangos S, MacMurtrie E, Shields E, Hujcs M, Levine J, Kofke A, Yang W, Le Roux PD. The use of a portable head CT scanner in the intensive care unit. J. Neurosci Nurs. 2010 Apr;42(2):109–16. doi: 10.1097/jnn.0b013e3181ce5c5b. [DOI] [PubMed] [Google Scholar]
- 16.Rumboldta Z, Hudaa W, Allb JW. Review of Portable CT with Assessment of a Dedicated Head CT Scanner. AJNR. 2009 Oct;30(9):1630–6. doi: 10.3174/ajnr.A1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinreb DB, Stahl JE. Portable CT imaging of acute stroke patients in the emergency department. Radiol Manage. 2009 Mar-Apr;31(2):41–5. [PubMed] [Google Scholar]
- 18.Das S, Maeso PA, Figueroa RE, Senior BA, Delgaudio JM, Sillers MJ, Schlosser RJ, Kountakis SE. The use of portable intraoperative computed tomography scanning for real-time image guidance: a pilot cadaver study. Am J Rhinol. 2008 Mar-Apr;22(2):166–9. doi: 10.2500/ajr.2008.22.3152. [DOI] [PubMed] [Google Scholar]
- 19.Loubele M, Maes F, Jacobs R, et al. Comparative study of image quality for MSCTand CBCT scanners for dentomaxillofacial radiology applications. Radiat Prot Dosimetry. 2008;129:222–26. doi: 10.1093/rpd/ncn154. [DOI] [PubMed] [Google Scholar]
- 20.Majdani O, Thews K, Bartling S, Leinung M, Dalchow C, Labadie R, Lenarz T, Heidrich G. Temporal bone imaging: comparison of flat panel volume CT and multisection CT. AJNR. 2009 Aug;30(7):1419–24. doi: 10.3174/ajnr.A1560. [DOI] [PMC free article] [PubMed] [Google Scholar]