Abstract
Background
A dysregulation of the corticotropin-releasing hormone (CRH) system has been implicated in the development of excessive alcohol consumption and dependence. The aim of the present study was to evaluate whether the CRH system is also recruited when non-dependent Wistar rats escalate to high alcohol intake in the intermittent (alternate days) model of drinking.
Methods
We compared intermittent and continuous access to 20% (v/v) alcohol in a two-bottle free choice drinking paradigm. Following a total of twenty 24-hour exposures for every experimental group, we assessed signs of alcohol withdrawal, including anxiety-like behavior and sensitivity to stress. The selective CRH1 receptor (CRH1R) antagonist antalarmin (0, 10, 20 mg/kg, i.p.) was tested on alcohol consumption.
Results
Intermittent access to 20% alcohol led non-selected Wistar rats to escalate their voluntary intake to a high and stable level, whereas continuously exposed animals maintained a lower consumption. These groups did not differ in physical withdrawal signs. In addition, no differences were found when anxiogenic-like behavior was studied, neither under basal conditions or following restraint stress. Nevertheless, sensitivity to the treatment with the CRH1R antalarmin was observed since a reduction of 20% alcohol intake was found in both groups of animals regardless of the regimen of alcohol exposure. In addition, antalarmin was effective when injected to animals exposed to intermittent 10% (v/v) alcohol whereas it failed to suppress 10% continuous alcohol intake.
Conclusions
Pharmacological blockade of CRH1R reduced alcohol drinking when sustained high levels of intake were achieved suggesting that the CRH system plays a key role when high doses of ethanol are consumed by non-dependent subjects. This supports the notion that CRH system not only maintains the dependent state but also engages the transition to dependence.
Keywords: Intermittent, Alcohol, CRH1R, Intake, Withdrawal, Antalarmin
1. INTRODUCTION
Reduction of heavy drinking and relapse prevention are main therapeutic objectives in alcoholism treatment. These aspects of alcoholism can be modeled in animals. For example, to assess excessive alcohol consumption, rodent lines obtained by selective breeding for high and low alcohol consumption and other rodent strains exhibiting innately high alcohol drinking characteristics have been extensively used (McBride and Li, 1998). However, a procedure that leads to escalation of alcohol consumption in unselected Wistar rats has also been described (Wise, 1973). When alcohol solutions were made available on alternate days, voluntary ethanol intake was much greater than with continuous availability. Motivation to consume alcohol in this model was dissociated from physical dependence (Wise, 1975), while subsequent behavioral studies have established that in order to reach a dependent state, intermittent exposure to repeated cycles of forced intoxication and withdrawal are more effective than constant exposure (Breese et al., 2005; O'Dell et al., 2004; Rimondini et al., 2002; Sommer et al., 2008).
The past decade has seen an expansion of research aimed at developing pharmacotherapy for alcoholism. Two agents, acamprosate and naltrexone, have been approved for the treatment of alcoholism, while baclofen, topiramate and ondansetron have shown promise in clinical trials (Egli, 2005; Heilig and Egli, 2006; Johnson, 2008). In addition, other drug mechanisms are under evaluation for the development of novel alcohol addiction treatment. One of the target systems that preclinical pharmacology has recently pointed out as a candidate for new medications is the corticotropin-releasing hormone (CRH) system (Ciccocioppo et al., 2009; Heilig et al., 2010; Lowery and Thiele, 2010). CRH integrates many of the endocrine, behavioral, and autonomic responses to stress and has been implicated in alcohol addiction in part because stress is a key factor that contributes to the vulnerability to relapse to alcohol seeking (Sarnyai et al., 2001; Shaham et al., 2003; Sinha, 2001), and in part because there is evidence that neuroadaptive changes triggered by a prolonged history of alcohol exposure lead to a persistent dysregulation of the CRH system. That, in turn may drive excessive and uncontrolled alcohol consumption and would motivate subjects to continue drinking despite detrimental consequences (Heilig and Koob, 2007; Koob, 2003, 2010). Compelling evidence supports a role of the brain stress system in core symptoms of alcohol dependence such as excessive alcohol self-administration, stress-induced relapse to alcohol seeking, dependence and withdrawal (Breese et al., 2011). Consistent with this notion, CRH1R antagonists have been shown to be particularly efficacious in blocking alcohol abuse-related behaviors in animals with a history of alcohol dependence, while little effect was observed in non-dependent subjects (Funk et al., 2006; Gehlert et al., 2007; Hansson et al., 2006) In recent studies, however, it was shown that CRHR signaling may also modulate alcohol intake in non-dependent rodents when the level of alcohol intake is high. For example, in the “drinking in the dark” procedure in mice, treatment with CRH1R antagonists significantly attenuated binge-like drinking whereas it was ineffective in altering consumption of moderate amounts of alcohol (Lowery et al., 2010; Sparta et al., 2008).
The intermittent model of 20% alcohol drinking has been pharmacologically validated as both naltrexone and acamprosate were demonstrated to decrease alcohol consumption of high alcohol drinking rats subjected to the alternate days exposure (Simms et al., 2008). Given the important role of brain stress system following intermittent exposure to alcohol (Breese et al., 2005; Breese et al., 2011; Sommer et al., 2008; Zhang et al., 2007) and other drugs of abuse including morphine (Houshyar et al., 2003), the aim of the present study was to examine whether the CRH system is engaged when non-dependent Wistar rats escalate to high alcohol intake following a prolonged exposure to different alcohol concentrations in the intermittent (alternate days) model of drinking. To this end, we first studied alcohol drinking in animals exposed to a 20-day two-bottle free choice procedure in which a 20% solution was offered under intermittent or continuous regimen. Then, these drinking patterns were compared to those resulting from an identical intermittent or continuous exposure to a lower, 10% alcohol concentration. Third, we assessed the presence of physical and emotional signs of withdrawal in animals drinking higher alcohol concentrations. Lastly, we tested the effect of the selective CRH1R antagonist antalarmin on both 20% and 10% intermittent versus continuous alcohol access.
2. MATERIAL AND METHODS
2.1 Animals
Male Wistar rats, purchased from Charles River Laboratories (Wilmington, MA), were used. Animals were single housed in a temperature and humidity controlled vivarium and maintained on a reverse 12 h light/dark cycle, with the light switched off at 8:00 A.M. Food and water were available ad libitum. Prior to initiating experiments, animals (body weight 325–350 g) were repeatedly handled. Experiments were conducted during the dark cycle. All experiments were conducted according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism. All animals were only used once except where specified. Two batches of naïve Wistar animals were used in the present study. The first batch underwent the 20-day exposure to 20% alcohol as described below. Of these animals one group (N=16) was used to test the effects of antalarmin on 20% alcohol drinking under the intermittent (8 rats) vs continuous regimen (8 rats). The second group (N=20) was used to assess differences between intermittent (N=10) and continuous (N=10) access in somatic and emotional (anxiety) symptoms of alcohol withdrawal as well as in sensitivity to stress. A second batch of rats (N=16) was also divided into continuous (N=8) and intermittent (N=8) access but exposed to 10% alcohol concentration. In these animals the effect of antalarmin was also evaluated.
2.2 Drugs
Both 20% (v/v) and 10% (v/v) alcohol solutions was prepared by diluting 95% alcohol in tap water. The selective CRH1 receptor antagonist antalarmin (N-butyl-N-ethyl-[2,5,6-trimethyl-7-(2,4,6-trimethylphenyl)-7H-pyrrolo[2,3-d]pyrimidin4-yl]-amine (Webster et al., 1996)) was suspended in a vehicle composed by 10% Tween 80 and distilled water and given intraperitoneally (i.p.) at the doses of 10 and 20 mg/kg in a volume of 1 ml/kg.
2.3 20% (v/v) alcohol: intermittent vs continuous access to two-bottle choice drinking paradigm
All fluids were presented in 100 ml graduated plastic cylinders equipped with a stainless-steel drinking spouts inserted through two grommets in front of the cage. Fluids were changed daily at 3 hours into the dark period of the light/dark cycle. According to the method described by Wise (1973) animals were trained to drink 20% (v/v) alcohol on alternate days. Briefly, after 1 week acclimatization period, all the animals were provided with two bottles of water for three days. The fourth day, rats were divided into two groups. In one of them (intermittent access group), one bottle of 20% ethanol solution replaced one bottle of water and was available for the next 24 hours. The next day, the 20% alcohol bottle was removed and replaced with a bottle of water. This procedure took place across 40 days for a total of 20 days of alcohol exposure. Meanwhile, a second group of rats (continuous access group) had access to water only (2 bottles of water) until the intermittent group reached ten exposures. At that point, one bottle of 20% alcohol replaced one of the water bottles, and exposure to one bottle of alcohol and one of water was maintained for 20 consecutive days. The placement of the alcohol bottle was alternated daily to control for side preferences in continuous exposed animals whereas it was alternated every other day in the intermittent exposed rats.
2.4 Blood alcohol concentrations (BACs)
Blood samples (N=8 per group) were taken from the rat tail vein one, four, and twenty-four hours following the onset of the last day of 20% ethanol exposure and assayed as previously described (Cippitelli et al., 2010). Blood was collected in 300 µl vials containing EDTA dipotassium salt (Sarstedt, Nümbrecht, Germany) and stored at −20 °C. Quantitative gas chromatography was used to assess blood alcohol concentrations.
2.5 Effect of antalarmin on 20% alcohol consumption in intermittent vs continuous access drinking rats
At the end of 20 alcohol exposures, 8 animals subjected to intermittent 20% alcohol access and 8 belonging to the continuous group were tested with the selective CRH1R antagonist antalarmin (0, 10, 20 mg/kg) given i.p. 30 min prior to alcohol exposure of the intermittent access group. Each rat received all doses with a four-day wash-out period between injections using a counter-balanced Latin square design. Alcohol was provided every day or every other day between drug testing days. The effect of the treatment was evaluated at the 6 and 24 hours time points. Water and total fluid intake were also measured throughout the experiment.
2.6 Measurement of physical alcohol withdrawal signs
Following 20 alcohol exposures, the remaining 20 animals (10 of the intermittent group and 10 of the continuous group) were given only water. Animals were, then, examined for physical signs of withdrawal right before and 12 hours following removal of alcohol by an experimenter blind to treatment conditions. Using a withdrawal rating scale adapted from Macey and colleagues (Macey et al., 1996) and described by Braconi and colleagues (Braconi et al., 2010), ethanol withdrawal signs, including ventro-medial limb retraction, irritability to touch (vocalization), tail rigidity, and body tremors, were scored. To each sign was assigned a score of 0 to 2, based on the following severity scale: 0 = no sign, 1 = moderate, 2 = severe. The sum of the 4 observation scores (0 to 8) was used as a quantitative measure of withdrawal severity. For these behavioral observations, animals were individually transferred from their home cages to a quiet observation room to avoid excessive stimulation.
2.7 Elevated plus-maze (EPM)
This experiment was conducted as described previously (Cippitelli et al., 2011). The apparatus was elevated 50 cm above the floor and consisted of two black plastic open arms (50 × 10 cm) and two enclosed arms (50 × 10 × 45 cm), which were arranged so that the similar arms were opposite to each other. The arms were connected by a central area measuring 10 × 10 cm where the animal was placed facing a closed arm when the 5-min test procedure began. The maze was cleaned with water and dried after each trial. Behavior was scored by the EthoVision video tracking system for automation of behavioral studies. The percent of time spent in open arms and the percent of open arm entries were used as measures of anxiety-like behavior, while the number of entries into the closed arms was used as an indicator of general motor activity (Cruz et al., 1994). Animals (N=10 intermittently and 9 continuously exposed) were tested under dim red light 30 hours following the removal of the bottles corresponding to the time at which animals drinking under the intermittent regimen would have resumed access to alcohol. To further examine the possible difference in anxiety-like behavior under more stressful conditions, the same animals (N=9 intermittently and 9 continuously exposed) were subjected to an additional 20-day long 20% alcohol exposure and then again tested for the EPM performance following the completion of a 15 min restraint stress (Valdez et al., 2003). This time EPM was conducted under 120 lux 30 hours following removal of the bottles.
2.8 Effect of antalarmin on 10% alcohol consumption in intermittent vs continuous access drinking rats
A separate group (N=16, 8 intermittently and 8 continuously exposed) of rats were subjected to a 20-day exposure to 10% alcohol instead of 20%. At this point in a counter-balanced Latin square design with a four day wash-out period between each drug administration antalarmin (0, 10, 20 mg/kg) was tested. The drug was given i.p. 30 min prior alcohol exposure to the intermittent access group. The effect of the treatment was evaluated at 6 and 24 hours time points. Water and total fluid intake were also measured.
2.9 Statistics
Statistical analyses were performed using analysis of variance (ANOVA). When appropriate, analyses were followed by Newman Keuls post hoc tests. Drinking patterns of the 20-day intermittent vs. continuous exposure to both 20% and 10% ethanol were conducted separately by using a two-way ANOVA with days of exposure (“day”) as the within subject variable and groups (“group”) as the between factor. Two-way ANOVA was also used for assessment of the overall physical withdrawal sign score. Here one between factor (exposure group) and one within factor (time, T=0 and T=12 hours) were used. Analysis of EPM-related variables was carried out by one-way ANOVA, which also was used to determine the average in the intake of 20% and 10% intermittent vs. continuous access groups. To analyze the effects of antalarmin on alcohol drinking, water and total fluid intake a three-way ANOVA was used separately for the two concentrations. Factors for the three-way ANOVA were 2 within subjects (time point, “time” and antalarmin doses, “treatment”) and one between factor (intermittent vs. continuous, “group”). Determination of BACs was carried out by a linear regression analysis.
3. RESULTS
3.1 The 20% intermittent access group escalated whereas the continuously exposed group decreased alcohol consumption across the 20-day exposure
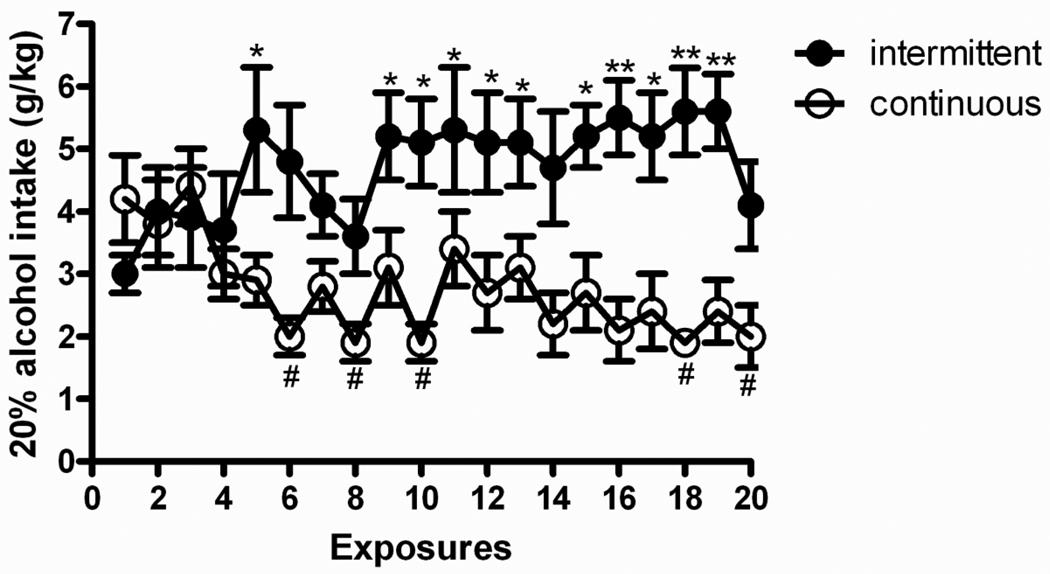
Figure 1 shows patterns of alcohol intake for intermittent and continuous 20% alcohol exposure groups. Overall ANOVA revealed a main effect of “group” [F(1,14)=10.3, p<0.01] as well as a main effect of “day” [F(19,266)=1.9, p<0.05], accompanied by a group × day interaction [F(19,266)=4.9, p<0.001] to suggest different drinking patterns between groups across the 20-day exposure. Post hoc analysis showed escalation of alcohol consumption for the intermittent access group, as a significantly different intake was found on day 5 and from days 9 to 20 (with the exception of days 14 and 20) compared with the intake of the first exposure. In contrast, the continuous access group showed attenuated consumption, as intake on days 6, 8, 10, 18 and 20 were decreased when compared to the first day of exposure.
Figure 1.
Intermittent access to 20% (v/v) alcohol in a two-bottle free choice drinking paradigm produces a robust escalation in alcohol intake. The values are expressed as mean ± SEM alcohol intake (g/kg/day). Statistical difference from the first drinking session: In the intermittent access group *p<0.05 and **p<0.01; in the continuous access group #p<0.05.
3.2 Antalarmin attenuated 20% alcohol consumption in both intermittent and continuous access groups
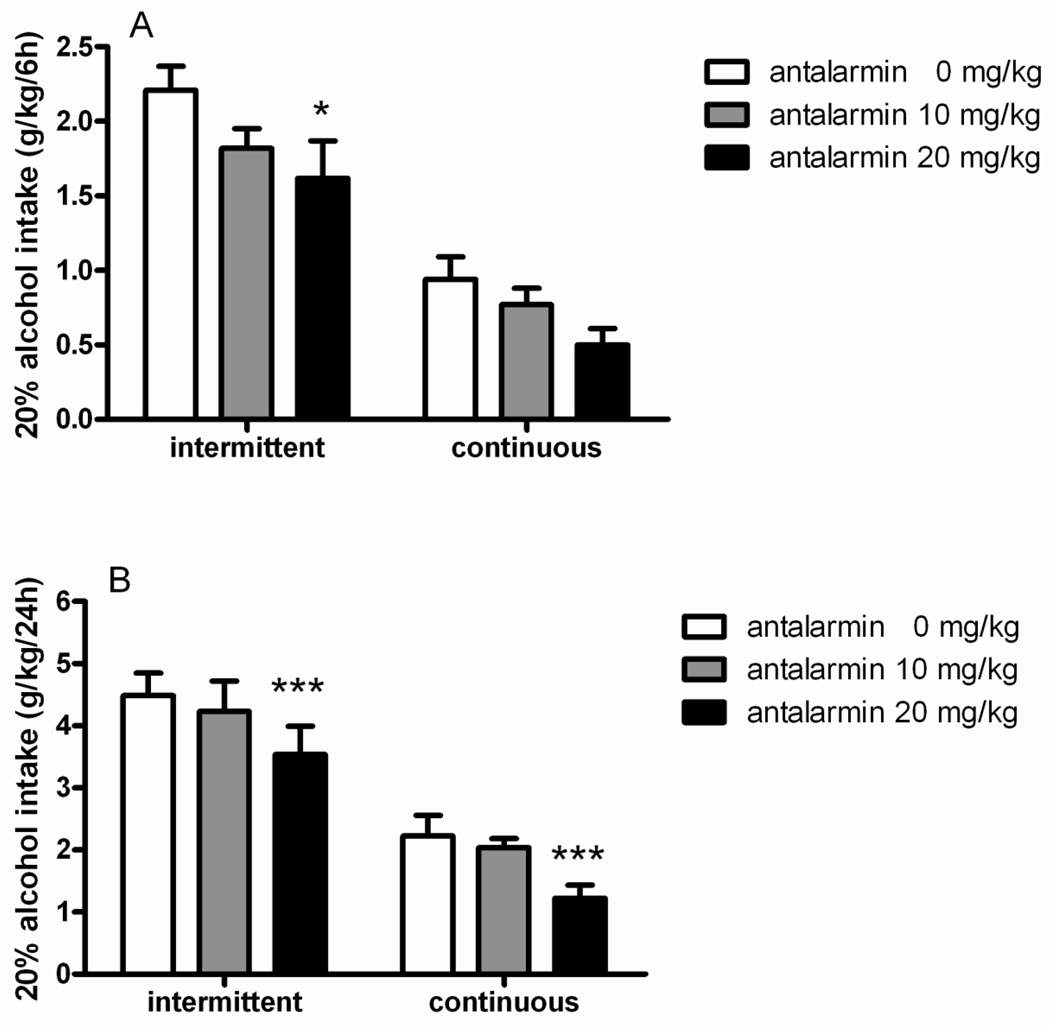
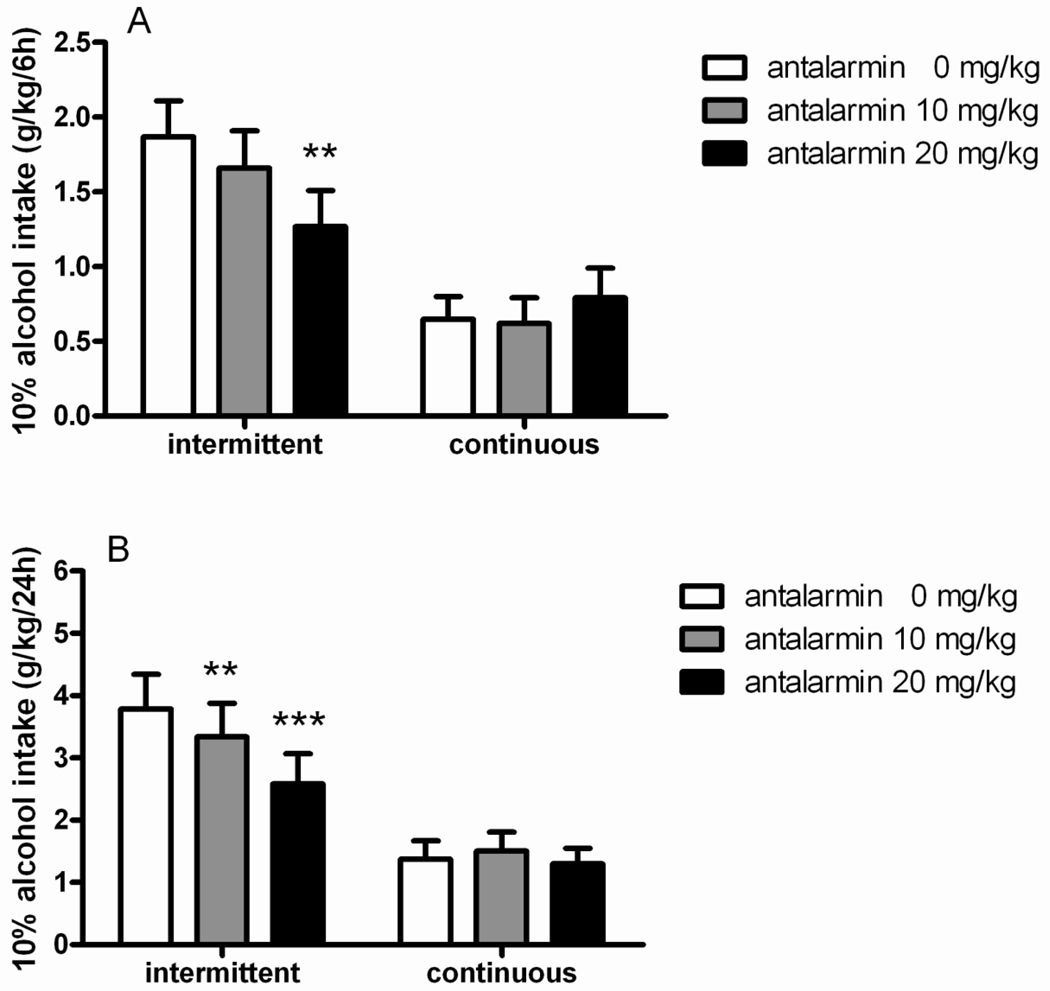
In agreement with the increased intake of rats exposed to intermittent access shown in Figure 1, ANOVA revealed a main effect of “group” [F(1,14)=35.5, p<0.001] to suggest that difference in baseline of consumption was maintained between groups during the drug testing. Treatment with the CRH1R antagonist antalarmin had an overall main effect on alcohol consumption (main effect of “treatment” [F(2,28)=10.9, p<0.001]), which did not differ between groups (interaction “treatment × group” [F(2,28)=0.1, NS]), to indicate that the drug was effective in both intermittent and continuous 20% alcohol exposed rats. Finally, as shown by the significant interaction “treatment × time” [F(2,28)=4.2, p<0.05], this reduction in alcohol intake was greater at the 24-hour time point. Post hoc analysis revealed significant difference when antalarmin was given at the dose of 20 mg/kg in the intermittent as well as in the continuous access groups (p<0.001) at 24 hours. In the intermittent group a significant (p<0.05) reduction of alcohol intake was observed also following higher antalarmin at the 6-hour time point (Figure 2A–B).
Figure 2.
Antalarmin (0, 10, 20 mg/kg, i.p.) decreases alcohol consumption in both intermittent (N=8) and continuous (N=8) groups with access to 20% (v/v) alcohol drinking. The values are expressed as mean ± SEM alcohol intake (g/kg) at (A) 6 hours and (B) 24 hours. Statistical difference from controls (0):*p<0.05 and ***p<0.001.
ANOVA revealed that basal water intake was higher in continuous compared to intermittent group [F(1,14)=7.2, p<0.05]. In addition, reduction of alcohol intake was accompanied by an increased water consumption following treatment with antalarmin [F(2,28)=6.8, p<0.01] regardless of the regimen of alcohol exposure [F(2,28)=2.5, NS]. Post hoc comparisons revealed that the heightened water intake was generally observed in the experimental groups where 20% alcohol consumption was shown to decrease (Table 1A). Conversely, the overall total fluid intake was not altered by the drug treatment ([F(2,28)=2.2, NS], Table 2A).
Table 1.
Water intake following administration of the CRH1 receptor antagonist antalarmin (0, 10, 20 mg/kg, i.p.) in unselected Wistar rats subjected to intermittent (alternate days) and continuous exposure to (A) 20% (v/v) alcohol or (B) 10% (v/v) alcohol in a two-bottle free choice procedure.
| A | ||||
|---|---|---|---|---|
| antalarmin (mg/kg) |
Water intake (g/kg) | |||
| 20% intermittent | 20% continuous | |||
| 6hrs | 24hrs | 6hrs | 24hrs | |
| 0 | 14.5±3.3 | 33.1±4.4 | 30.7±3.5 | 65.2±6.9 |
| 10 | 21.9±2.6* | 46.0±5.9*** | 28.9±5.2 | 66.6±8.7 |
| 20 | 23.4±3.1* | 44.4±6.0** | 35.5±6.7 | 77.6±11.0*** |
| B | ||||
|---|---|---|---|---|
| antalarmin (mg/kg) |
Water intake (g/kg) | |||
| 10% intermittent | 10% continuous | |||
| 6hrs | 24hrs | 6hrs | 24hrs | |
| 0 | 18.1±6.7 | 40.2±11.3 | 29.1±7.2 | 73.2±13.3 |
| 10 | 23.6±6.0 | 48.2±10.8* | 32.6±3.0 | 81.4±10.2** |
| 20 | 23.5±4.0 | 52.0±7.3** | 41.2±3.9 | 87.3±10.5*** |
Significant difference from controls (0)
p<0.05,
p<0.01,
p<0.001.
Table 2.
Total fluid intake following administration of the CRH1 receptor antagonist antalarmin (0, 10, 20 mg/kg, i.p.) in unselected Wistar rats subjected to intermittent or continuous exposure to (A) 20% alcohol or (B) 10 % alcohol in a two-bottle free choice procedure.
| A | ||||
|---|---|---|---|---|
| antalarmin (mg/kg) |
Total fluid intake (ml) | |||
| 20% intermittent | 20% continuous | |||
| 6hrs | 24hrs | 6hrs | 24hrs | |
| 0 | 16.0±1.8 | 34.7±2.0 | 20.6±1.6 | 42.6±2.1 |
| 10 | 18.2±1.7 | 42.0±2.7 | 18.4±2.4 | 43.2±3.1 |
| 20 | 19.4±2.0 | 37.2±2.3 | 21.8±2.9 | 46.4±3.8 |
| B | ||||
|---|---|---|---|---|
| antalarmin (mg/kg) |
Total fluid intake (ml) | |||
| 10% intermittent | 10% continuous | |||
| 6hrs | 24hrs | 6hrs | 24hrs | |
| 0 | 20.2±3.9 | 42.7±5.8 | 18.5±3.2 | 45.1±6.5 |
| 10 | 21.5±3.1 | 43.5±4.6 | 20.1±2.3 | 49.8±6.3 |
| 20 | 19.1±1.8 | 40.9±2.9 | 23.4±2.9 | 51.8±6.6 |
No significant differences were detected between groups.
3.3 Intermittent exposure to 20% alcohol did not produce overt signs of alcohol withdrawal
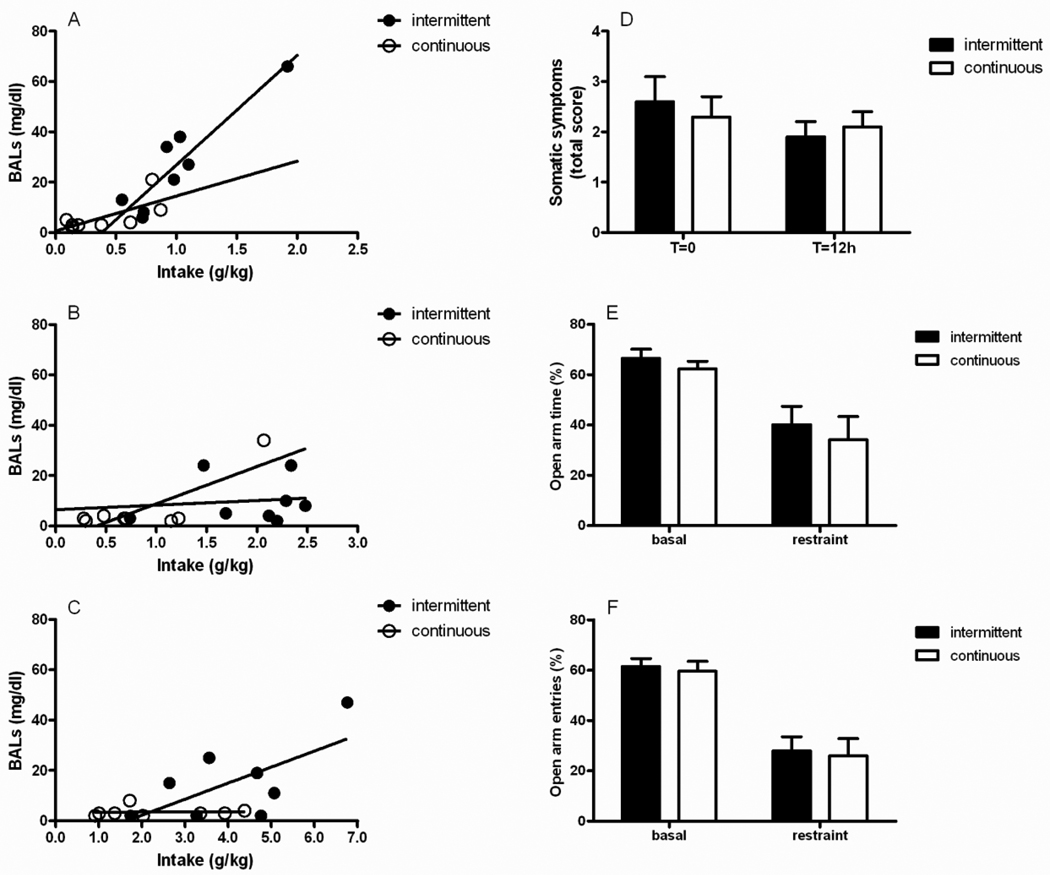
Blood alcohol concentrations were markedly below the threshold (~150 mg/dl) that is thought to induce alcohol dependence (Rimondini et al., 2002). Highest peaks were reached 1 hour following the onset of drinking by rats given access to 20% alcohol under the intermittent procedure. The amount of alcohol consumed after 1 hour significantly correlated with the measured BACs in the intermittent group (r2= 0.85; P=0.001) whereas correlation was weak in the continuous access group (r2= 0.48, P=0.05; Figure 3A). Correlation between alcohol consumed and BACs decreased over time in the group exposed every other day (4 hours: r2= 0.01, Figure 3B; 24 hours: r2= 0.001, Figure 3C) to suggest that these rats voluntarily drank ethanol in bouts of sufficient size and frequency particularly during the first hour following exposure.
Figure 3.
Rats subjected to intermittent drinking consumed alcohol in bouts which were markedly below the threshold known to produce dependence. Blood ethanol concentrations (BACs, mg/dl) were monitored at (A) 1, (B) 4 and (C) 24 hours of the last 20% alcohol exposure in both the intermittent (N=8) and the continuous access (N=8) groups. (D) Absence of physical signs of withdrawal in the intermittent group (N=10) compared with the continuous one (N=10) measured at 12 hours (T=12h) following the removal of the alcohol bottles (T=0h). The affective component (anxiety) of withdrawal was also absent. Elevated plus maze (EPM)-related variables, including (E) percent (%) open arm time and (F) % open arm entries did not differ between the intermittent and the continuous exposed groups.
Intermittently and continuously exposed rats were examined for expression of physical signs of withdrawal at 12 hours (T=12) following the removal of alcohol bottles. Somatic symptoms were compared with those shown right before removing the bottles (T=0). The sum of the 4 observation scores (vocalization, ventromedial limb retraction, tail rigidity and body tremor) were 2.6±0.5 and 2.3±0.4 on T=0 and 1.9±0.3 and 2.1±0.3 on T=12 for intermittent and continuous access groups, respectively. ANOVA revealed a significant effect of “time” [F(1,18)=4.4, p<0.05] with no effect of the exposure (“group”, intermittent vs. continuous [F(1,18)=0.0, NS]) or interaction “time × group” [F(1,18)=1.2, NS], Figure 3D). These results clearly ruled out the presence of significant withdrawal signs attributable to the intermittent or the continuous procedure.
Intermittently and continuously exposed rats showed comparable EPM performance under basal conditions (% open arm time [F(1,17)=0.8, NS], % open arm entries [F(1,17)=0.1, NS]) as well as following a restraint stress (% open arm time [F(1,16)=0.2, NS], % open arm entries [F(1,16)=0.0, NS], Figure 3E–F) to suggest absence of emotional signs of alcohol withdrawal.
3.4 The 10% intermittent access group escalated alcohol consumption across the 20-day exposure
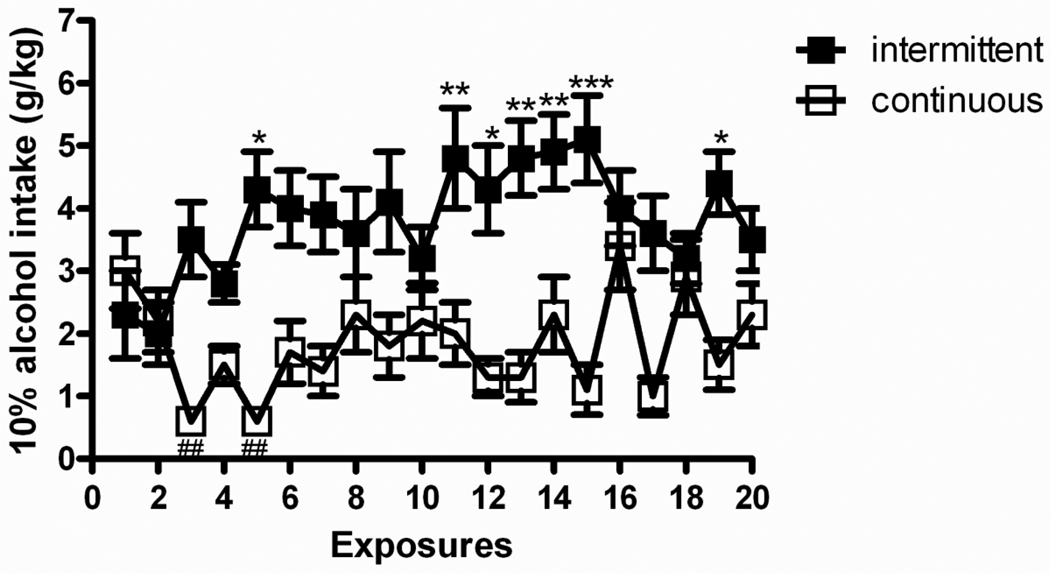
Figure 4 shows patterns of intake for both groups of the intermittent and continuous 10% alcohol exposure. Similarly to the 20% alcohol procedure, overall ANOVA revealed a main effect of “group” [F(1,14)=13.4, p<0.01] and a main effect of “day” [F(19,266)=2.4, p<0.01], accompanied by group × day interaction [F(19,266)=5.2, p<0.001] to suggest different drinking patterns between groups across the 20-day exposure. Post hoc analysis showed escalation of alcohol consumption for the intermittent access group as a significantly different intake was found between the first exposure and day 5 as well as days 11 to 15 and day 19. On the other hand, the continuous access group showed daily fluctuations throughout the procedure so that consumption did not change significantly over time.
Figure 4.
The intermittent access to 10% (v/v) alcohol in a two-bottle free choice drinking paradigm produces a robust escalation in alcohol intake. The values are expressed as mean ± SEM alcohol intake (g/kg/day) at each drinking session. Statistical difference from the first drinking session: In the intermittent access group (N=8) *p<0.05 and **p<0.01; in the continuous access group (N=8) ##p<0.01.
3.5 Antalarmin attenuated 10% alcohol consumption in the intermittent but not in continuous group
Baseline of alcohol consumption for both intermittent and continuous access to 10% alcohol were different, as shown by a main effect of “group” [F(1,14)=11.1, p<0.01]. Treatment with the CRH1R antagonist antalarmin had an overall main effect on alcohol consumption (main effect of “treatment” [F(2,28)=8.1, p<0.01]). Importantly, there was a significant treatment × group interaction [F(2,28)=9.0, p<0.001] to suggest a change in the intake occurring selectively in the intermittent but not in the continuous group. As shown by a significant treatment × time interaction [F(2,28)=4.8, p<0.05], the effect of the CRH1R antagonist was greater at the 24-hour time point. In the intermittent group post hoc analysis revealed significant reduction of drinking by antalarmin 20 mg/kg at both time points (6 hours: p<0.01; 24 hours: p<0.001). At the low dose (10 mg/kg) antalarmin decreased 10% alcohol intake only at the 24-hours time point. Intake of the continuous access group was not affected by antalarmin (Figure 5 A–B).
Figure 5.
Antalarmin (0, 10, 20 mg/kg, i.p.) decreases alcohol consumption in rats with intermittent (N=8) but not with continuous (N=8) exposure to 10% alcohol. The values are expressed as mean ± SEM alcohol intake (g/kg) at (A) 6 hours and (B) 24 hours. Statistical difference from controls (0): **p<0.01 and ***p<0.001.
A significant difference in basal water intake was found between the intermittent and the continuous groups [F(1,14)=5.5, p<0.05]. Treatment with antalarmin increased water intake (main effect of “treatment” [F(2,28)=3.4, p<0.05]) whereas interaction “treatment × group” did not reach significance [F(2,28)=0.2, NS]. Post hoc comparisons revealed a robust increment of the intake at all doses examined, particularly at the 24-hour time point (Table 1B). Furthermore, overall consumption of fluids was not altered following drug treatment ([F(2,28)=0.8, NS], Table 2B).
3.6 The continuous access to 10% alcohol group showed the lowest intake
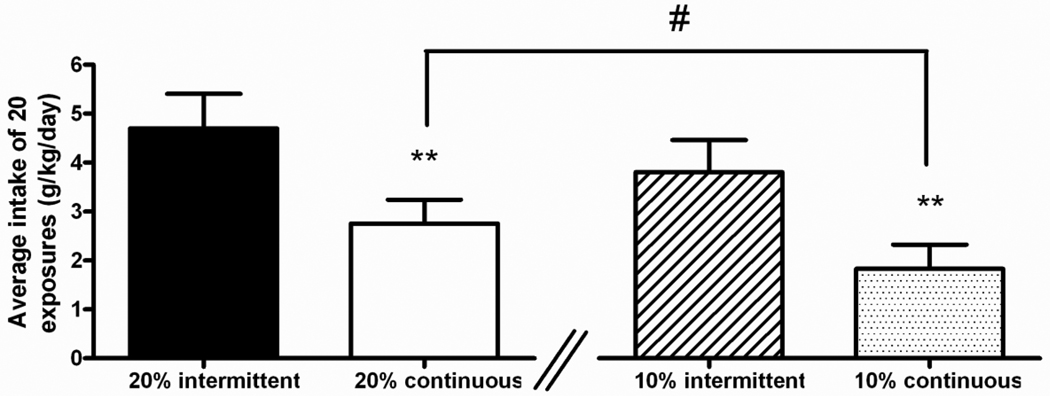
Further analysis of alcohol drinking was carried out to evaluate differences between rats exposed to 20% and 10% under both intermittent and continuous condition. As shown in Figure 6 in the 20% groups the mean ethanol intake per day were 4.7±0.7 and 2.7±0.5 g/kg in the intermittent and continuous access groups, respectively. ANOVA revealed a significant overall difference in alcohol consumption between groups [F(1,14)=10.3, p<0.01]. In the 10% groups the average daily alcohol intake were 3.8±0.6 and 1.8±0.5 g/kg/day in the intermittent and continuous groups, respectively. ANOVA revealed a significant overall difference in alcohol consumption between groups [F(1,14)=13.4, p<0.01]. Of note, ANOVA also revealed no difference in average alcohol intake between 20% and 10% intermittent groups [F(1,14)=1.7, NS]; while a significant lower level of alcohol consumption was observed in rats taking continuous 10% alcohol as opposed to the group of animals taking continuous 20% [F(1,14)=4.4, p=0.05].
Figure 6.
Both 20% and 10% intermittent access groups showed higher average alcohol intake compared to 20% and 10% continuous access groups. The intake values are reported as average of 20 daily exposures. Statistical difference between intermittent and continuous groups exposed to 20 or 10% alcohol respectively, **p<0.01. Statistical difference between goups exposed to 20% and 10% continuous access #p=0.05.
4. DISCUSSION
We show here that Wistar rats given intermittent access to 20% and 10% alcohol in a two-bottle choice drinking paradigm consume higher levels than animals exposed continuously. As shown by the absence of physical withdrawal signs and unaltered anxiety-like responses this escalation in alcohol intake is not associated with a dependence-like state, but is attenuated by the selective CRH1R antagonist antalarmin. In addition, antalarmin decreases drinking in the continuous group exposed to 20% alcohol while it failed to affect consumption of 10% alcohol under an unlimited 20-day alcohol access.
Our drinking data obtained allowing intermittent access to a 20% alcohol solution closely parallel those reported in previous studies where an escalation of alcohol intake was observed both in rats (Hopf et al., 2010; Loi et al., 2010; Simms et al., 2010; Simms et al., 2008; Wise, 1973) and mice (Melendez, 2011). Indeed, in the present study five 24-hour exposures to 20% alcohol led Wistar rats to escalate their intake up to 5–6 g/kg/day. This high consumption then persisted for most of the remaining exposures period raising the possibility that neuroadaptive changes occurred to maintain sustained high amounts of intake. Notably, this amount almost doubled that of animals exposed to 20% alcohol under a continuous regimen, which instead decreased their consumption across the 20-day procedure. The time course of BACs analyzed during the last 24-h exposure of the procedure suggested that correlation between alcohol consumed and BACs just occurred following the first hour of exposure, to indicate that rats intermittently exposed to the 20% alcohol solution voluntarily drank in bouts of sufficient size and frequency particularly during the first hour following the onset of the exposure. Then, BACs were maintained at lower levels throughout the remaining period of alcohol access. Animals continuously exposed to 20% alcohol showed a weak correlation between alcohol consumption and BACs that remained substantially low. However, it should be also pointed out that peaks of blood alcohol concentrations observed in the present study were generally lower than those showed by Wistar rats intermittently exposed to 20% alcohol in the study conducted by Simms et al (2008). This discrepancy may be due to the decreased amount of alcohol intake observed in our experiment on the last day of alcohol access (see Figure 1), possibly as a consequence of the three sampling collections carried out across the 24-h of that day. Alternatively, it is possible that blood samples for BAC analysis were here collected one hour following the onset of alcohol access, a time point which may not reflect the maximal peak of alcohol absorption in this alcohol drinking paradigm whereas a 30 min time appears to be more appropriate for blood sampling.
Following 20 daily exposures to this alternate 20% alcohol access, Wistar rats failed to show physical symptoms of alcohol withdrawal (Braconi et al., 2010; Economidou et al., 2011). Similarly, no increase in anxiety-like behavior, another marker of alcohol withdrawal (Breese et al., 2011; Heilig and Koob, 2007) was observed in the 20% intermittent access group when their elevated plus-maze performance was assessed under basal conditions or following a restraint stress (Valdez et al., 2003) and compared with that of the control group (20% continuous alcohol access). These findings are consistent with data on BACs which remained markedly below the threshold (~ 150 mg/dl) that is thought to induce alcohol dependence (Rimondini et al., 2002; Rimondini et al., 2003; Roberts et al., 2000; Spanagel, 2003). Hence, results clearly suggest that despite an elevated alcohol intake associated to intermittent 20% alcohol exposure unlikely animals develop dependence.
Activation of the brain stress system is thought to play a pivotal role in alcohol dependence (Heilig and Koob, 2007; Koob, 2008, 2010) as a long-term upregulation of CRH1 receptors has been observed in structures of the extended amygdala of animals with a previous history of dependence and in rats genetically selected for high alcohol intake and preference (Ciccocioppo et al., 2006; Hansson et al., 2007; Hansson et al., 2006; Merlo Pich et al., 1995; Olive et al., 2002; Sommer et al., 2008). Consistently, dependence-induced excessive drinking was showed to be attenuated by treatment with CRH1R antagonists in alcohol dependent subjects at doses that had no effects in non-dependent Wistar rats (Funk et al., 2006; Gehlert et al., 2007) suggesting that such neuroadaptations may drive core symptoms of alcoholism including the increased motivation to take alcohol. However, the understanding of whether or not the CRH1R signaling is also engaged when non-dependent rats escalate their levels of drinking has received less attention. Our data clearly demonstrate that pretreatment with the selective CRH1R antagonist antalarmin, at doses previously shown to be selective for alcohol-related behaviors (Hansson et al., 2006; Lodge and Lawrence, 2003; Marinelli et al., 2007), decreased consumption of a 20% alcohol solution at both time points examined (6 and 24 hours). Importantly, this reduction occurred regardless of the regimen of exposure (intermittent vs. continuous) and amounts of alcohol consumed. Indeed, despite the fact that the average intake markedly differed between the groups, antalarmin attenuated both the elevated intake in 20% intermittent access as well as the modest intake of 20% continuous alcohol access group. The observation that antalarmin decreased intake of 20% alcohol both in the intermittent and continuous access groups may indicate that both groups managed to drink enough alcohol in the first hour of exposure to produce an engagement of the CRH system. This would be in accordance with the positive correlation observed at 1hour time point between alcohol consumed and BACs in both experimental groups. At a minimum, these data indicate that CRH1R signaling can modulate alcohol drinking in Wistar rats even in the absence of dependence. However, when the 20% alcohol solution was replaced with a lower concentration, antalarmin selectively reduced the elevated intake of the intermittent access group in a dose-dependent manner, while leaving the lower intake of the continuous access 10% alcohol group unaltered. Therefore, with 10% alcohol, the escalation seems to recruit CRH-dependence, presumably because of the increased amount of alcohol taken. Notably, the observation that the continuous access to 10% alcohol group showed a lower intake (expressed as average intake of 20 exposures) than that of the continuous access to 20% alcohol group may account for the described differential effects of antalarmin. Altogether these data indicate that inactivation at CRH1 receptors generally modulates alcohol drinking in Wistar rats even in the absence of dependence if sufficiently high doses of alcohol are consumed. Our data are, therefore, consistent with recent work showing that CRH1R antagonists reduce binge-like alcohol consumption in non-dependent C57BL/6J mice subjected to the “Drinking in the Dark“ procedure (Lowery et al., 2010; Lowery and Thiele, 2010; Sparta et al., 2008) where high amounts of alcohol are consumed in a limited period of time (Crabbe et al., 2011). Conversely, CRH1R antagonists were shown to be ineffective in altering alcohol consumption in mice drinking moderate amounts (Sparta et al., 2008). Altogether these observations may suggest that CRH system does not only play a role in maintaining the dependence state but it is also engaged during the transition to dependence.
5. CONCLUSIONS
We replicate here prior findings showing that intermittent 20% alcohol exposure leads to escalation of drinking and lead to high alcohol consumption in Wistar rats. We then extend these findings by presenting novel data demonstrating that neither 20 cycles of intermittent nor continuous access are followed by development of an overt dependence-like state. By evaluating the effects of the CRH1R antagonist antalarmin we also provided pharmacological evidence that CRH system may modulate consumption in non-dependent subjects, thus indicating that stress system may be engaged during the transition to dependence. Finally, these results strengthen the evidence that CRH1R antagonism may have pharmacotherapeutic potential in the treatment of alcohol addiction.
Highlights.
Wistar rats given intermittent access to 20% and 10% alcohol show escalation of drinking
Escalation in alcohol intake is not associated with a dependence-like state
CRH system may modulate consumption in non-dependent subjects
Stress system may be engaged during the transition to dependence
Abbreviations
- CRH
corticotropin-releasing hormone
- CRH1R
corticotropin-releasing hormone receptor 1
- BACs
blood alcohol concentrations
- EPM
elevated-plus maze
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting intragastric ethanol intubation as a dependence induction method for studies of ethanol reward and motivation in rats. Alcohol Clin Exp Res. 2010;34:538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a "kindling"/stress hypothesis. Psychopharmacology (Berl) 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addict Biol. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Gehlert DR, Ryabinin A, Kaur S, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Economidou D, Stopponi S, Cannella N, Braconi S, Kallupi M, de Guglielmo G, Massi M, George DT, Gilman J, Hersh J, Tauscher JT, Hunt SP, Hommer D, Heilig M. Stress-related neuropeptides and alcoholism: CRH, NPY, and beyond. Alcohol. 2009;43:491–498. doi: 10.1016/j.alcohol.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Rezvani AH, Robinson JE, Eisenberg L, Levin ED, Bonaventure P, Motley ST, Lovenberg TW, Heilig M, Thorsell A. The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 2011 Sep;45(6):567–576. doi: 10.1016/j.alcohol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Zook M, Bell L, Damadzic R, Eskay RL, Schwandt M, Heilig M. Reversibility of object recognition but not spatial memory impairment following binge-like alcohol exposure in rats. Neurobiol Learn Mem. 2010;94:538–546. doi: 10.1016/j.nlm.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict Biol. 2005;10:309–319. doi: 10.1080/13556210500314550. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Le AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl- imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Ciccocioppo R, Heilig M. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Bjork K, Soverchia L, Terasmaa A, Massi M, Heilig M, Ciccocioppo R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, Ramchandani VA, George DT, Hommer D, Barr CS. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neurosci Biobehav Rev. 2010;35:334–344. doi: 10.1016/j.neubiorev.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol Psychiatry. 2010;69:618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshyar H, Gomez F, Manalo S, Bhargava A, Dallman MF. Intermittent morphine administration induces dependence and is a chronic stressor in rats. Neuropsychopharmacology. 2003;28:1960–1972. doi: 10.1038/sj.npp.1300271. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol. 2008;75:34–56. doi: 10.1016/j.bcp.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Lawrence AJ. The CRF1 receptor antagonist antalarmin reduces volitional ethanol consumption in isolation-reared fawn-hooded rats. Neuroscience. 2003;117:243–247. doi: 10.1016/s0306-4522(02)00793-5. [DOI] [PubMed] [Google Scholar]
- Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Thiele TE. Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS Neurol Disord Drug Targets. 2010;9:77–86. doi: 10.2174/187152710790966605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Melendez RI. Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:652–658. doi: 10.1111/j.1530-0277.2010.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Lorang M, Yeganeh M, Rodriguez de Fonseca F, Raber J, Koob GF, Weiss F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995;15:5439–5447. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olive MF, Koenig HN, Nannini MA, Hodge CW. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002;72:213–220. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol. 2003;64:445–449. doi: 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z, Shaham Y, Heinrichs SC. The role of corticotropin-releasing factor in drug addiction. Pharmacol Rev. 2001;53:209–243. [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcohol addiction research: from animal models to clinics. Best Pract Res Clin Gastroenterol. 2003;17:507–518. doi: 10.1016/s1521-6918(03)00031-3. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Sparrow AM, Lowery EG, Fee JR, Knapp DJ, Thiele TE. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008;32:259–265. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]
- Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Morse AC, Koob GF, Schulteis G. Dose- and time-dependent expression of anxiety-like behavior in the elevated plus-maze during withdrawal from acute and repeated intermittent ethanol intoxication in rats. Alcohol Clin Exp Res. 2007;31:1811–1819. doi: 10.1111/j.1530-0277.2007.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]