Abstract
Background
Corticosteroid regimens that stimulate both mineralocorticoid and glucocorticoid pathways consistently reverse vasopressor-dependent hypotension in septic shock, but have variable effects on survival.
Objective and Methods
To determine if exogenous mineralocorticoid and glucocorticoid stimulation have distinct effects, and whether the timing on such stimulation alters their effects, in septic shock. Desoxycorticosterone (DOC), a selective mineralocorticoid agonist; dexamethasone (DEX), a selective glucocorticoid agonist; and placebo were administered either several days before (prophylactic) or immediately after (therapeutic) infectious challenge and continued for 96 h in 74 canines with staphylococcal pneumonia.
Measurements and Main Results
Effects of DOC and DEX were different and opposite depending on timing of administration for survival (p=0.05); fluid requirements (p=0.05); central venous pressures (p≤0.007); indicators of hemoconcentration [i.e. sodium (p=0.0004), albumin (p=0.05), and platelet counts (p=0.02)]; IL-6 levels (p=0.04); and cardiac dysfunction (p=0.05). Prophylactic DOC treatment significantly improved survival, shock, and all the other outcomes above, but therapeutic DOC did not. Conversely, prophylactic DEX was much less effective for improving these outcomes compared to therapeutic DEX, with the exception of shock reversal. Prophylactic DEX given before sepsis induction also significantly reduced serum aldosterone and cortisol levels and increased body temperature and lactate levels compared to therapeutic DEX (p≤0.05), consistent with adrenal suppression.
Conclusions
In septic shock, mineralocorticoids are only beneficial if given prophylactically, while glucocorticoids are most beneficial when given close to the onset of infection. Prophylactic mineralocorticoids should be further investigated in patients at high risk to develop sepsis, whereas glucocorticoids should only be administered therapeutically to prevent adrenal suppression and worse outcomes.
Keywords: sepsis, infection, corticosteroids, models, animal
Introduction
Sepsis is a substantial cause of morbidity and mortality worldwide. In the United States alone, 751,000 hospitalizations (3.0 per 1,000 population) and 215,000 deaths annually are attributable to this syndrome (1). Despite numerous clinical trials over the past half century, new drug therapies have been largely unsuccessful in reducing sepsis-related deaths (2, 3). One possible exception is corticosteroid administration (4-7). Over the last 35 years, the doses, regimens, and extent of corticosteroid use have varied, likely in response to inconsistent results from clinical trials. Most recently, use of physiologic doses equivalent to the stress cortisol response (i.e., most commonly hydrocortisone in doses of 200 to 300 mg/day for 7-10 days) have been advocated, in part because of clinical trials demonstrating shock reversal with such use (7). However, this approach is not universally accepted because effects on survival have varied and some studies have reported an increased risk of secondary infection and myopathy (8, 9).
Corticosteroid administration in septic shock has been based on at least three different rationales: 1) suppression of an excessive inflammatory response; 2) direct reversal of sepsis-induced vascular hyporeactivity; and 3) treatment of relative adrenal insufficiency (8). However, serum levels of cortisol, the main endogenous corticosteroid, generally increase with sepsis severity and the entity of relative adrenal insufficiency, or “critical illness-related corticosteroid insufficiency,” has been difficult to define or characterize clinically (10, 11). Likewise, the benefits of anti-inflammatory therapy were not confirmed in studies using high-dose glucocorticoids or other agents that specifically target components of the innate immune response (12, 13). Further advances in this field may require more precise knowledge of the mechanisms underlying the purported benefits and risks of corticosteroids in septic shock.
Hydrocortisone, the corticosteroid used most commonly for sepsis, has both glucocorticoid and mineralocorticoid activity (14). In contrast to the strong anti-inflammatory effects of glucocorticoids (15), mineralocorticoid receptor-specific ligands have relatively weaker anti-inflammatory effects (16) and have not been associated with overt immune suppression. However, the regulation of sodium metabolism, intravascular volume and endothelial function by mineralocorticoid receptors (17) may be more important in the treatment of septic shock than suppression of the inflammatory response (18). Regimens of pure mineralocorticoid agonists devoid of glucocorticoid activity have not been investigated in clinical trials (5, 19), and a literature review did not reveal any animal studies examining mineralocorticoid therapy alone in septic shock. Therefore, to our knowledge, the safety and efficacy of mineralocorticoid agonists alone in septic shock have not been tested, and the therapeutic contribution of mineralocorticoid activity, if any, to corticosteroid regimens with mixed activity is unclear.
Based on the different activity of mineralocorticoid and glucocorticoid ligands, we hypothesized that the effects of corticosteroids in sepsis would be different depending on agonist activity and timing of administration. Using intrapulmonary challenges of Staphylococcus aureus to produce septic shock in canines (20, 21), stress doses of dexamethasone (DEX), a specific glucocorticoid agonist, or desoxycorticosterone (DOC), a specific mineralocorticoid agonist, were compared for effects on pathophysiology and survival. To simulate therapeutic interventions commonly employed in human septic shock, animals were managed with standardized sedation, antibiotic therapy, mechanical ventilation support adjusted to correct gas exchange abnormalities, and need-based cardiovascular support using vasopressors and fluids to normalize intravascular pressures. Glucocorticoid- and mineralocorticoid-specific regimens were both evaluated using two different time frames of administration. Prophylactic therapy was started several days before the onset of infection to fully establish any phenotypic changes that may result from downstream gene regulation. Therapeutic treatment was started immediately after the onset of infection to simulate the traditional manner in which corticosteroids have been used to treat septic shock. We demonstrate that the timing and activity of different corticosteroid regimens fundamentally affect the therapeutic benefits of glucocorticoids and mineralocorticoids in septic shock.
Methods
Study Design
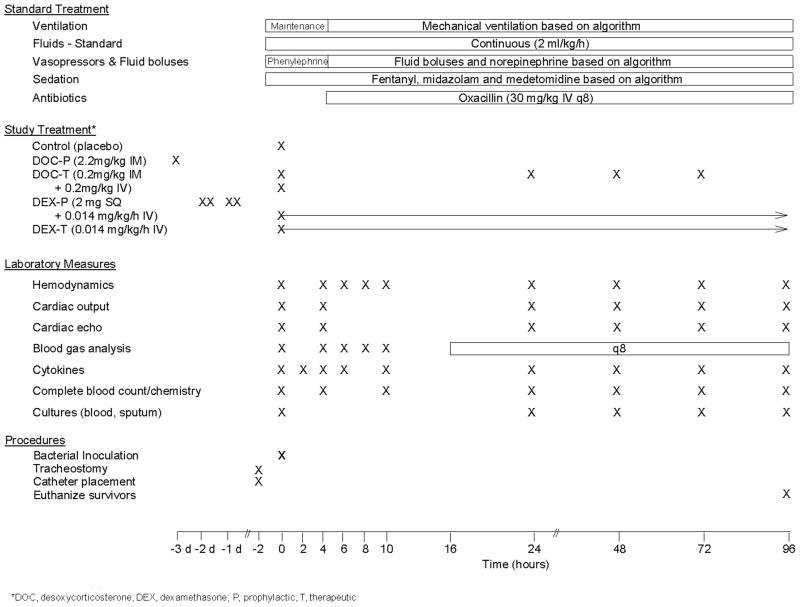
All experiments were performed under protocol approved by the Animal Care and Use Committee of the Clinical Center at the National Institutes of Health. Seventy-four purpose-bred beagles (12-18 mo, 10-12 kg) were studied prospectively for 96 h using a canine S. aureus pneumonia model of sepsis separated in one of four sets of experiments designed to examine and compare the individual effects of DOC and DEX given prophylactically (P) or therapeutically (T) (Figure 1). To study DOC-P, animals were randomized to receive DOC subcutaneously (SQ) 72 h prior to bacterial inoculation in a depot preparation that releases drug throughout the experiment (DOC-P; n = 14) or placebo (control; n = 14). For DOC-T, animals were randomized to receive an infusion of DOC or placebo immediately after bacterial challenge, followed by daily SQ injections of DOC for the duration of the experiment (DOC-T; n = 6), or placebo (controls; n = 6). To study DEX-P, animals were randomized to receive DEX twice daily SQ or placebo for 48 h prior to bacterial inoculation, followed by continuous infusions of DEX (DEX-P; n = 13), or placebo (control; n = 13) for the duration of study. For DEX-T, animals were randomized to receive a continuous infusion of either DEX (DEX-T; n = 12) or placebo (controls; n = 6) starting immediately after bacterial challenge and continuing for the duration of study. DOC was administered in a dose equivalent to that used clinically to treat adrenal insufficiency in canines (22). DEX was administered in a dose that is comparable to the stress dose cortisol therapy (hydrocortisone 300 mg/day) used to treat sepsis clinically (5). For a more detailed description of the treatment regimens and dose selection, see Corticosteroid Dosing in the Supplementary Methods.
Figure 1. Study Protocol.
Treatments, laboratory measures, and procedures performed during the course of the 96 h study.
In each set of experiments, animals were allocated such that 2 treatment animals were always studied with 1-2 concurrent controls each study week. Technicians and veterinarians responsible for randomizing and caring for animals and for performing all treatments, making clinical decisions, and recording and laboratory measures (Supplementary Methods) were unaware of (blinded to) the study design. Due to intensive care unit resources constraints, a maximum of four animals were enrolled in any study week.
Study Protocol
The protocol followed in these experiments has been previously described (20, 21, 23) (Figure 1 and Supplementary Methods). At time 0 (T0), baseline blood samples and hemodynamic profiles were obtained (Supplementary Methods), and then animals received an inoculation of S. aureus (1.5 – 7.5 × 109 cfu/kg) into the right caudal lobe via bronchoscopy(20). During the first 4 h after S. aureus inoculation, phenylephrine was titrated to maintain mean arterial pressure (MAP) > 80 mmHg as sedation was optimized and sepsis developed. After 4 h, treatment for sepsis was initiated based on algorithms to maintain pressures by titrating norepinephrine (NE), oxygenation by adjusting fractional inspired oxygen concentration (FiO2) and positive end expiratory pressure (PEEP) levels and acid-base status by adjusting respiratory rate (RR) measured by arterial blood gas. Preload was maintained with fluid boluses based on scheduled pulmonary artery occlusion pressure (PAOP) measures (20). Oxacillin (30 mg/kg IV q8 h) was started 4 h after bacterial inoculation and administered every 8 h thereafter. Conventional intensive care unit support employed during the ventilation of critically ill large animals was administered as previously described (20). Animals alive at 96 h were considered survivors and subsequently euthanized (Beuthanol; 75 mg/kg IV).
Statistical Methods
Data was analyzed using a Cox proportional hazards model and stratified log rank tests (survival effects); principal component analyses (shock reversal score and pulmonary function score); and linear mixed models (Supplementary Methods). All p-values are two-tailed and considered significant if p ≤ 0.05. We report interactions based on two-tailed p-values as large as p = 0.06 to limit type II errors.
Results
Effects of DOC and DEX Given Prophylactically or Therapeutically on Survival
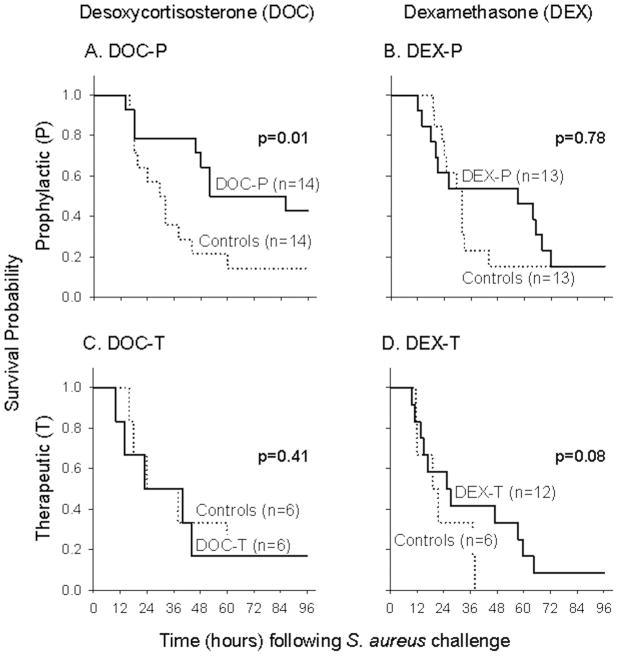
DOC given prophylactically improved survival compared to controls (stratified log-rank p = 0.01; Figure 2A), but lost its benefit if given therapeutically (stratified log-rank p = 0.41; Figure 2C). In contrast, DEX given prophylactically (DEX-P) had no significant effect on survival compared to controls (stratified log-rank p = 0.78; Figure 2B), but given therapeutically had a survival effect that approached significance for benefit (stratified log-rank p = 0.08; Figure 2D). Notably, when comparing timing of treatment (prophylactic and therapeutic), mineralocorticoids and glucocorticoids had significantly different and opposite effects on survival (p = 0.05 for interaction; Table 1).
Figure 2. Survival.
The treatment effects on survival were different and opposite for mineralocorticoids (DOC) and glucocorticoids (DEX) depending on timing of administration (see also Table 1; survival log hazards ratio): DOC-P improved survival compared to controls (panel A), whereas DOC-T had no survival benefit (panel C). In contrast, DEX-P had no significant effect on survival compared to controls (panel B), but there was a survival benefit with DEX-T that approached significance (panel D).
Table 1. Parameters where treatment effects after S. aureus challenge changed in a different manner with mineralocorticoid vs. glucocorticoid therapy depending on timing of administration (prophylactically vs. therapeutically).
| Desoxycorticosterone (DOC) | Dexamethasone (DEX) | All Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| Parameter mean ± SEM or 95% CI | Prophylactic (DOC-P) | Therapeutic (DOC-T) | Mean difference | Prophylactic (DEX-P) | Therapeutic (DEX-T) | Mean difference | P-value interaction | Mean value |
| Survival log hazards ratio | - 0.96 ± 0.55 | 0.25 ± 0.72 | - 1.21 | - 0.17 ± 0.54 | - 1.17 ± 0.71 | + 1.00 | p = 0.05 | N/A |
| Pulmonary function score 12 h to 96 h | 0.95 ± 1.35 | 2.21 ± 1.18 | - 1.26 | 2.21 ± 0.98 | 1.89 ± 1.10 | + 0.32 | p = 0.06 | 2.08 ± 1.22 |
| Fluid Input (mL/kg/hr) over 96 h | 5.7 ± 0.3 | 6.5 ± 0.7 | - 0.8 | 6.0 ± 0.4 | 4.8 ± 0.5 | + 1.2 | p = 0.05 | 6.0 ± 0.3 |
| Central venous pressure (mmHg) 4 h to 12 h | 5.1 ± 0.4 | 3.9 ± 0.3 | + 1.2 | 4.2 ± 0.3 | 5.4 ± 0.4 | - 1.2 | p = 0.007 | 4.6 ± 0.3 |
| Central venous pressure (mmHg) 12 h 30 h | 5.6 ± 0.5 | 4.4 ± 0.6 | + 1.2 | 4.6 ± 0.6 | 6.4 ± 0.7 | - 1.8 | p = 0.005 | 4.7 ± 0.4 |
| Serum sodium (mEq/L) at 10 h | 142.9 ± 1.2 | 147.3 ± 0.5 | - 4.4 | 145.3 ± 0.8 | 141.9 ± 0.7 | + 3.4 | p = 0.0004 | 143.0 ± 0.5 |
| Lactate (mmol/L) at 10 h | 1.21 ± 0.24 | 0.72 ± 0.11 | + 0.49 | 2.74 ± 0.44 | 1.02 ± 0.10 | + 1.72 | p = 0.03 | 1.00 ± 0.10 |
| Hemoglobin (g/dL) at 10 h | 14.7 ± 0.8 | 16.9 ± 1.6 | - 2.2 | 15.4 ± 0.6 | 14.3 ± 1.6 | + 1.1 | p = 0.06 | 16.8 ± 0.6 |
| Albumin (g/dL) at 10 h | 1.96 ± 0.08 | 1.98 ± 0.012 | - 0.02 | 2.18 ± 0.05 | 1.96 ± 0.05 | + .22 | p = 0.05 | 2.04 ± 0.05 |
| Platelets (cells × 103) at 10 h | 245 ± 27 | 313 ± 31 | - 68 | 248 ± 16 | 170 ± 30 | + 78 | p = 0.02 | 231 ± 14 |
| Left ventricular ejection fraction (%) at 10 h | 0.45 ± 0.04 | 0.39 ± 0.03 | + 0.06 | 0.39 ± 0.02 | 0.49 ± 0.02 | - 0.10 | p = 0.05 | 0.41 ± 0.2 |
| IL-6 (pmol/L, log10 scale) at 10 h | 3.91 ± 0.08 | 4.0 ± 0.03 | - 0.09 | 4.21 ± 0.06 | 3.94 ± 0.13 | + 0.27 | p = 0.04 | 4.06 ± 0.04 |
Effects on Shock Reversal
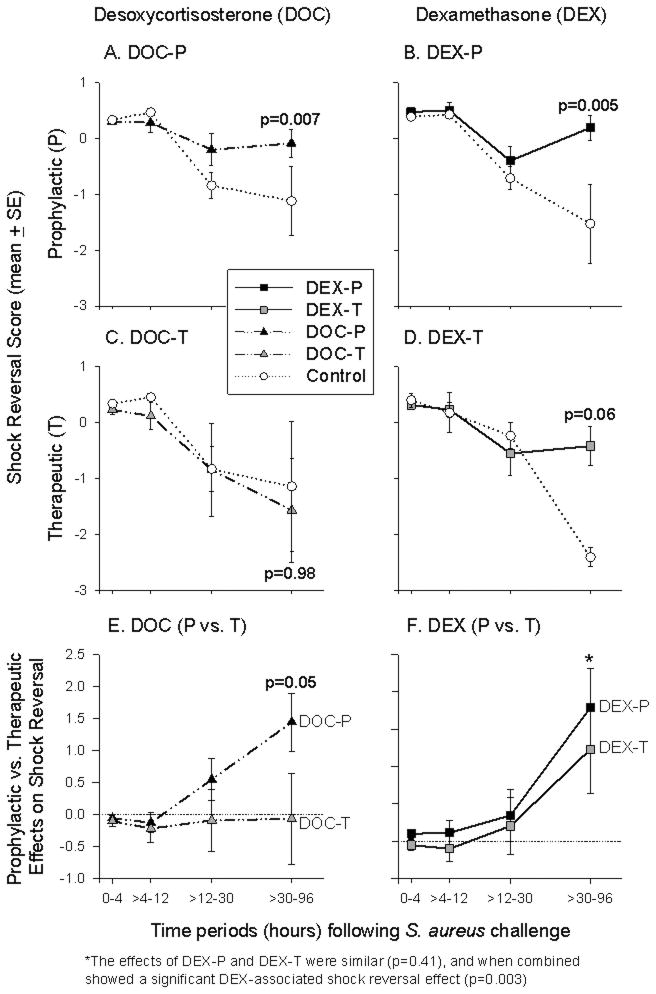
From baseline (0 h) to 32 h after S. aureus challenge, there were no significant differences in mean shock reversal score (MAP and NE requirements; see Statistical Methods for details) comparing all treatment groups and controls (all, p = ns; Figure 3). However, from 32 to 96 h after S. aureus challenge, DOC-P improved the mean shock reversal score compared to controls (p = 0.007; Figure 3A) and compared to DOC-T-treated animals (p = 0.05; Figure 3E). DOC-T had no significant effect compared to controls on shock reversal during that time period (p = ns; Figure 3C).
Figure 3. Shock Reversal.
DOC-P improved the mean shock reversal score (calculated from MAP and NE requirements; see Statistical Methods for details) compared to both controls (panel A) and DOC-T-treated animals (panel E) from 32 to 96 h after S. aureus challenge, whereas DOC-T had no significant effect compared to controls on shock reversal during that time period (panel C). DEX-P improved the mean shock reversal score from 32 to 96 h compared to controls (panel B), and there was also an improvement in the shock reversal score with DEX-T that approached significance compared to controls during this time period (panel D). Shock reversal with DEX-P compared to DEX-T was markedly similar from 32 to 96 h, and when combined was significantly improved compared to controls (panel F). (See supplementary Figure 1 for the individual components of shock reversal score over time by treatment group.)
Like DOC-P, DEX-P improved the mean shock reversal score from 32 to 96 h compared to controls (p = 0.005; Figure 3B). Unlike DOC-T, DEX-T also tended to improve shock reversal from 32 to 96 h, an effect that approached significance compared to controls (p = 0.06; Figure 3D). Shock reversal with DEX-P compared to DEX-T was markedly similar from 32 to 96 h (p = 0.41; Figure 3F), and when combined, shock reversal attributable to DEX regardless of time administered was significantly improved compared to controls (p = 0.003; Figure 3F).
For completeness, the effects of each corticosteroid treatments on individual components of the score are shown in Figure E1 in the online data supplement.
Effects on Pulmonary and Cardiac Function
Pulmonary
Before S. aureus challenge (0 h) to 12 h after, there were no significant differences in mean lung injury score (A-aO2, plateau pressure, PAP, SaO2, and RR; see Statistical Methods for details) comparing all treatment groups and controls (all, p = ns; data not shown). However, from 12 to 96 h after S. aureus challenge, the effect of corticosteroids on the mean lung injury score changed when given prophylactically vs. therapeutically (p = 0.06 for interaction; Table 1). Specifically, DOC-P-treated animals had less severe lung injury from 12 to 96 h after S. aureus challenge compared to DOC-T-treated animals, whereas DEX-P-treated animals had worse lung injury than DEX-T-treated animals during that same time period. For completeness, the effects of each corticosteroid treatment for individual components of the score are shown in Figure E2 in the online data supplement.
Cardiac
The effects of corticosteroids on mean LVEF were also different given prophylactically compared to therapeutically by 10 h after S. aureus challenge (p = 0.05 for interaction; Table 1). Specifically, DOC-P-treated animals had a higher mean LVEF than DOC-T-treated animals at 10 h, whereas DEX-P-treated animals had a lower mean LVEF than DEX-T-treated animals at this time point. There were no other significant differences on mean LVEF throughout the study (all, p = ns; data not shown).
Effects on Fluid Status and Hemoconcentration
Fluid Status
Mean fluid requirements and CVP changed when corticosteroids were given prophylactically vs. therapeutically (p = 0.05 and p ≤ 0.005 for interaction, respectively; Table 1): DOC-P-treated animals had higher mean CVPs from 4 to 24 h and required less fluids throughout the study to maintain systemic and cardiac filling pressures at pre-determined levels than DOC-T, whereas DEX-P-treated animals had lower CVPs and required more fluids than DEX-T to maintain these pressures. DOC-P-treated animals also retained more fluids than both controls and DOC-T-treated animals over the 96 h after S. aureus challenge (101.5 ± 16.4 vs. 63.4 ± 11.3 and 19.0 ± 1.6 mL/kg/24 h, respectively; p = 0.05 and p = 0.03, respectively), but there were no other significant differences in mean fluid retention throughout the study (all, p = ns; data not shown). Since PAOP was to be maintained using fluid boluses at ≥10 mmHg in all animals throughout the 96 h experiment study per protocol, as expected there were few differences in this measure throughout the study.
Hemoconcentration
Consistent with the above fluid status findings, DOC-P-treated animals had lower mean hemoglobin, sodium and albumin concentrations by 10 h after infection compared to DOC-T-treated animals, whereas DEX-P-treated animals had higher concentrations of hemoglobin, sodium and albumin compared to DEX-T-treated animals at this time point (p = 0.06, p = 0.0004, and p = 0.05 for interaction, respectively; Table 1). In addition, DOC-P-treated animals had a lower mean hemoglobin concentration compared to controls at 24 h (14.8 ± 0.9 vs. 17.5 ± 0.7 g/dL; p = 0.03), and DEX-P-treated animals had lower mean serum sodium concentrations than controls from 12 to 96 h after infection (139.6 ± 1.0 vs. 143.3 ± 1.0 mEq/L; p = 0.02). There were no other significant differences in mean hemoglobin, sodium, or albumin concentrations throughout the study (all, p = ns; data not shown).
Effects on Adrenal Function
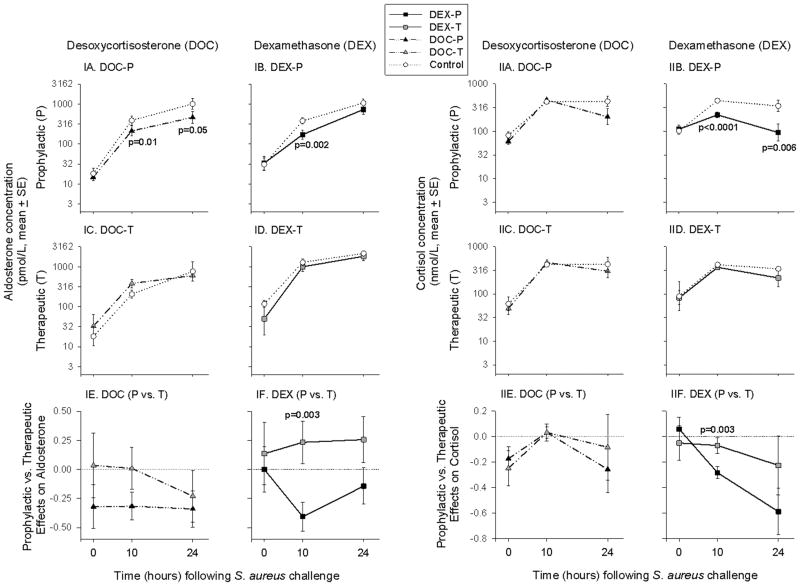
Before S. aureus challenge (0 h), there were no significant differences in mean aldosterone or cortisol levels comparing all treatment groups and controls (all, p = ns; Figure 4).
Figure 4. Aldosterone and Cortisol.
DOC-P-treated animals had lower mean aldosterone levels at 10 h and 24 h after S. aureus challenge compared to controls (panel IA), whereas DOC-T-treated animals had no significant differences in mean aldosterone levels compared to both controls (panel C) and DOC-P-treated animals (panel E) throughout the study. DOC-P- and DOC-T-treated animals had no significant differences in mean cortisol concentrations compared to controls or to each other throughout the study (panel IIA, C, E). DEX-P significantly reduced mean aldosterone concentration at 10 h after S. aureus challenge compared to both controls (panel IB) and DEX-T-treated animals (panel IF), whereas DEX-T had no significant effects on mean aldosterone concentration compared to controls throughout the study (panel ID). Similarly, DEX-P reduced mean cortisol levels compared to controls at 10 h and 24 h after S. aureus challenge (panel IIB), and also compared to DEX-T-treated animals at 10 h after S. aureus challenge (panel IIF). DEX-T had no significant effects on mean cortisol levels compared to controls throughout the study (panel IID).
Aldosterone
DOC-P-treated animals had lower mean aldosterone levels at 10 h (p = 0.01) and 24 h (p = 0.05) after S. aureus challenge compared to controls (Figure 4IA). In contrast, DOC-T-treated animals had no significant differences in mean aldosterone levels compared to both controls and DOC-P-treated animals throughout the study (all, p = ns; Figure 4IC, E). DEX-P significantly reduced mean aldosterone concentration at 10 h after S. aureus challenge compared to both controls (p = 0.002; Figure 4IB) and DEX-T-treated animals (p = 0.003; Figure 4IF). DEX-T had no significant effects on mean aldosterone concentration compared to controls throughout the study (all, p = ns; Figure 4ID).
Cortisol
DOC-P- and DOC-T-treated animals had no significant differences in mean cortisol concentrations compared to controls (all, p = ns; Figure 4IIA, C, E). In contrast, DEX-P reduced mean cortisol levels compared to controls at 10 h (p < 0.0001) and 24 h (p = 0.006) after S. aureus challenge (Figure 4IIB), and also compared to DEX-T-treated animals at 10 h after S. aureus challenge (p = 0.003; Figure 4IIF). DEX-T had no significant effects on mean cortisol levels compared to controls throughout the study (all, p = ns; Figure 4IID).
Effects on Platelet Counts, Temperature, Lactate, and Cytokines
Mean platelet counts, serum lactate concentrations and IL-6 levels changed when corticosteroids were given prophylactically vs. therapeutically (p = 0.02, p = 0.03, and p = 0.04 for interaction, respectively; Table 1): DOC-P-treated animals had lower platelet counts and IL-6 levels and similar lactate concentrations compared to DOC-T-treated animals at 10 h after infection, whereas DEX-P-treated animals had higher platelet counts and IL-6 levels and markedly increased mean serum lactate concentrations compared to DEX-T-treated animals at this time point. DEX-P-treated animals also had higher mean body temperatures compared to controls at 10 h (p = 0.05) and DEX-T-treated animals at 10 h and 24 h (both, p ≤ 0.03); and higher mean IL-10 levels compared to DEX-T at 10 h (p = 0.01). However, both DEX-P- and DEX-T-treated animals had lower mean IL-10 concentrations than controls at 24 h (both, p = 0.05). For the sake of brevity, a more detailed explanation of the treatment effects on platelet counts and inflammatory measures (i.e. lactate concentrations, body temperature, and IL-6 and IL-10 levels), as well as a presentation of WBC counts, can be found in the Supplementary Results.
Effects on Bacteriology
During the 96 h after S. aureus challenge, the number of positive blood cultures over the number collected for each treatment group were as follows: DEX-P: 2/34 (6%); DEX-T: 7/25; (28%); DOC-P: 5/44 (11%); DOC-T: 0/12 (0%); and controls: 11/53 (21%). DEX-T-treated animals had a higher rate of positive blood cultures compared to DEX-P (p = 0.03), but there were no other significant differences in the rate of positive blood cultures or the number of colony forming units (CFUs) in the blood over the 96 h study. There were also no significant differences in the probability of positive sputum cultures for S. aureus comparing all treatment groups and controls throughout the study (p = ns for all).
Discussion
In a canine model of bacterial pneumonia and septic shock, two different corticosteroid therapies were tested – a selective mineralocorticoid agonist and a selective glucocorticoid agonist - each administered both prophylactically and therapeutically. The mineralocorticoid was only beneficial when administered prophylactically, while the glucocorticoid was primarily beneficial when given therapeutically, close to the onset of infection.
DOC given for 3 days prior to infection not only increased overall survival but also lowered fluid requirements, improved CVP, reversed shock, lessened pulmonary and cardiac dysfunction, and caused greater fluid retention. Moreover, prophylactic mineralocorticoid therapy reduced signs of hemoconcentration, lowering serum sodium, hemoglobin, platelet, and albumin concentrations. Taken together, these data suggest that DOC-P-treated animals were better able maintain intravascular volume compared to those that received DOC later, indicating that important changes may have occurred prior to bacterial challenge as a result of early DOC pretreatment.
Several known effects of mineralocorticoids may explain the benefits of early mineralocorticoid treatment observed in the present study. Mineralocorticoid receptor signaling regulates sodium channels and other genes in the vasculature (17) that maintain endothelial function and integrity (24), possibly reducing capillary leak and increasing intravascular volume in severe sepsis. In addition, DOC may increase venous smooth muscle tone through actions on endothelin-1 expression, calcium channel signaling, and sympathetically-mediated venoconstriction (25, 26). Since intravascular volume depletion is a potent stimulus for aldosterone production and secretion, DOC-P-associated reductions in this hormone is consistent with increased intravascular volume. Improved lung function may also reflect a mineralocorticoid-stimulated increase in alveolar sodium and water clearance (27); improving alveolar sodium clearance has been proposed as a therapeutic strategy for treating acute lung injury (28). DOC-P also had modest anti-inflammatory effects, manifested by reduced IL-6 concentrations, which may have contributed to improved hemodynamics and organ function. These findings are consistent with in vitro work demonstrating mineralocorticoid receptor-mediated suppression of NFκB signaling (16), a major transducer of the innate immune response, and aldosterone-mediated inhibition of neutrophil-induced ICAM-1 expression on endothelial cells (29). Such targeted anti-inflammatory effects may have limited endothelial injury and organ dysfunction, thereby contributing to improved cardiovascular function and survival.
Lack of benefit with DOC starting late after infectious challenge may relate to the length of time required to establish some mineralocorticoid effects in the vasculature. Importantly, continuous intravenous and intracerebroventricular aldosterone infusions do not produce significant blood pressure increases in healthy canines until 72 h after administration (30, 31), indicating that mineralocorticoid-induced changes in vascular function take time to develop. Of note, the onset of shock in our canine sepsis model is rapid, developing within hours of bacterial challenge. The short latency to severe illness, together with the prolonged time required for mineralocorticoids to increase MAP in normal animals, suggest that starting DOC after bacterial challenge would likely be too late to affect outcome.
In contrast to our findings with DOC, treatment with DEX significantly reversed shock regardless of timing of administration. Further, directly opposite to our mineralocorticoid findings, glucocorticoid therapy improved survival and reduced pulmonary and cardiac dysfunction when given therapeutically rather than prophylactically. DEX-T-treated animals required less fluids to maintain predetermined systemic pressures, had higher cardiac filling pressures (i.e. CVP), and showed reduced signs of hemoconcentration (i.e. lower hemoglobin, platelet, and albumin concentrations) compared to control animals or those that received DEX-P. Adrenal suppression with loss of mineralocorticoid activity (32) may in part explain why prophylactic DEX failed to provide benefit. Starting almost immediately after bacterial challenge, animals that received DEX-P had significantly lower total basal cortisol and aldosterone concentrations compared to controls. In contrast, these stress hormones were not significantly affected by DEX given immediately after bacterial challenge. Although giving DEX provides full glucocorticoid activity, it cannot replace the profound loss of mineralocorticoid activity caused by the suppression of both cortisol and aldosterone (33). Notably, clinical trials have demonstrated that critically ill patients with hypoaldosteronism suffer from persistent hypotension and higher mortality rates both with and without corticosteroid therapy (34-36). Animals pretreated with DEX not only were less able to maintain intravascular volume, but also had significantly higher serum lactate levels and body temperatures compared to controls at 10 h after infection, all consistent with suppressed adrenal function and worse shock. Thus, the suppression of endogenous mineralocorticoid activity after infection as a result of prolonged glucocorticoid therapy may have counteracted the benefits of DEX observed with DEX-T therapy.
Unexpectedly, the differential effects of DEX given prophylactically compared to therapeutically were also evident with regards to measures of anti-inflammatory activity: DEX given after infection somewhat lowered circulating IL-6 and IL-10 concentrations compared to DEX given prophylactically. Accordingly, DEX-T-treated animals had higher rates of positive blood cultures compared to DEX-P-treated animals, which may be a reflection the immunosuppressive activity of this treatment. However, despite the increase in positive blood cultures, overall outcomes were still better with DEX-T compared to DEX-P. During septic shock, the anti-inflammatory effects of glucocorticoids have been reported to play a significant role in their beneficial effects (37), and DEX-T may likewise have had a similar benefit in our study.
Similar to our shock reversal findings with DEX given pre- versus post-infectious challenge, Mansart et al. found that dexamethasone given early or late was associated with reversal of hypotension in a cecal ligation and perforation murine model of sepsis. Also similar to our study, the improvement of blood flow in this sepsis model was more substantial with late treatment (38). In contrast, Ottoson et al. showed that single doses of either dexamethasone given 2 h before Escherichia coli challenge in a rat model increased survival time, and this effect decreased linearly with later administration of dexamethasone up to 8 h after bacterial challenge (39). More recently, in a rat subcutaneous group B streptococcal-challenged model, Tran et al. showed a significant mortality benefit for animals receiving dexamethasone 24 h before bacterial challenge, but no survival benefits with dexamethasone given either 30 min. or 24 h after challenge (40). However, pretreatment in these studies was given closer to bacterial challenge than in our study and the type of pathogen used, site of infection, ancillary therapies given, and animal species studied was also different.
Our finding that DEX given therapeutically demonstrated a trend toward a survival benefit is different from the results of CORTICUS, the largest and most recent sepsis steroid trial to date (9). The CORTICUS trial (n = 499) reported no overall survival benefit of hydrocortisone for sepsis. However, meta-regression analysis has suggested that corticosteroids have a survival benefit only in septic patients at high risk of death (7). The control mortality of the CORTICUS study was only 31% within 28 days, compared to an expected 40% based on clinical sepsis trials reporting salutatory effects of corticosteroids and 90% within 4 days in the current study. The CORTICUS study presents strong evidence that, in low-risk sepsis patients, physiologic-dose corticosteroids provide no benefit and may increase risk of morbidity. The effect of corticosteroids in high-risk sepsis patients is unresolved. Of note, the CORTICUS trial did report a shorter time to shock reversal among all patients receiving hydrocortisone (9), which is consistent with the shock reversal effects that we observed with DEX.
The limitations of this study warrant discussion. Only two agents were tested, one for each corticosteroid receptor, at fixed doses, and only one pre- and one post-infectious timing of administration were utilized. Different doses of DEX or DOC or other glucocorticoid or mineralocorticoid receptor ligands might alter the efficacy of these approaches. Notably, different ligands can produce selective conformational changes in steroid receptors that alter their gene targets and biological activity (41). Likewise, the activation of glucocorticoid and mineralocorticoid receptors simultaneously might have effects different than expected from studying either in isolation. Regardless, the efficacy of selective mineralocorticoid and glucocorticoid agonists in septic shock differ in regards to optimal timing.
In conclusion, in a canine model of S. aureus pneumonia-induced septic shock utilizing pulmonary and cardiovascular support measures similar to those used clinically, mineralocorticoids lessened fluid requirements, increased central venous pressures and fluid retention, prevented hemoconcentration, reversed shock, and improved survival and cardiopulmonary dysfunction, but only if given prophylactically. In contrast, glucocorticoids reversed shock independent of timing of administration, but had markedly less beneficial effects on survival and other measures of organ function if they were given prior to rather than after infectious challenge. Applied clinically, our data suggest that selective mineralocorticoids should be investigated as a potential prophylactic agent for patients at high risk for septic shock (e.g. neutropenic cancer patients receiving chemotherapy, high risk surgery patients with abdominal infections, etc.) to improve salt and water metabolism, prevent shock, and lessen cardiopulmonary dysfunction and mortality. In contrast, glucocorticoids appear to be essential for reversing shock in septic patients. However, prolonged early glucocorticoid therapy should be avoided due to a risk of adrenal suppression-associated worse outcomes. Furthermore, supplemental therapy with mineralocorticoid agonists should be considered for patients at high risk to develop sepsis that are on glucocorticoids for other reasons.
Supplementary Material
Acknowledgments
We acknowledge the contributions of the following people for their assistance with this manuscript: Robert Wesley PhD, Melinda Fernandez, Allen Hilton, Hollie Lee, Catherine Roberts, Chris Romines DVM, and Margaret Tropea MS.
This study was funded, in part, by the National Institutes of Health and the Howard Hughes Medical Institute.
List of Abbreviations
- DEX-P
Dexamethasone given prophylactically
- DEX-T
Dexamethasone given therapeutically
- DOC-P
Desoxycorticosterone given prophylactically
- DOC-T
Desoxycorticosterone given therapeutically
- IL-6
Interleukin-6
- IL-10
Interleukin-10
Footnotes
All work pertaining to this manuscript was performed at the National Institutes of Health (NIH). The study was supported by NIH intramural funds, but the opinions expressed herein do not necessarily represent the opinions of the US Government.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney DA, Danner RL, Eichacker PQ, Natanson C. Once is not enough: clinical trials in sepsis. Intensive Care Med. 2008;34:1955–1960. doi: 10.1007/s00134-008-1274-6. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Bellissant E, Bollaert PE, Briegel J, Keh D, Kupfer Y. Corticosteroids for severe sepsis and septic shock: a systematic review and meta-analysis. BMJ. 2004;329:480. doi: 10.1136/bmj.38181.482222.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Meta-analysis: the effect of steroids on survival and shock during sepsis depends on the dose. Ann Intern Med. 2004;141:47–56. doi: 10.7326/0003-4819-141-1-200407060-00014. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Bellissant E, Bollaert PE, Briegel J, Confalonieri M, De Gaudio R, Keh D, Kupfer Y, Oppert M, Meduri GU. Corticosteroids in the treatment of severe sepsis and septic shock in adults: a systematic review. JAMA. 2009;301:2362–2375. doi: 10.1001/jama.2009.815. [DOI] [PubMed] [Google Scholar]
- 7.Minneci PC, Deans KJ, Eichacker PQ, Natanson C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin Microbiol Infect. 2009;15:308–318. doi: 10.1111/j.1469-0691.2009.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sprung CL, Goodman S, Weiss YG. Steroid therapy of septic shock. Crit Care Clin. 2009;25:825–34. x. doi: 10.1016/j.ccc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, Weiss YG, Benbenishty J, Kalenka A, Forst H, Laterre PF, Reinhart K, Cuthbertson BH, Payen D, Briegel J CORTICUS Study Group. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358:111–124. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 10.Marik PE. The diagnosis of adrenal insufficiency in the critically ill patient: does it really matter? Crit Care. 2006;10:176. doi: 10.1186/cc5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesh B, Cohen J. Sick adrenal or sick euadrenal? Crit Care Resusc. 2009;11:301–304. [PubMed] [Google Scholar]
- 12.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9:1651–1663. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 13.Eichacker PQ, Parent C, Kalil A, Esposito C, Cui X, Banks SM, Gerstenberger EP, Fitz Y, Danner RL, Natanson C. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am J Respir Crit Care Med. 2002;166:1197–1205. doi: 10.1164/rccm.200204-302OC. [DOI] [PubMed] [Google Scholar]
- 14.Becker KL. Principles and practice of endocrinology and metabolism. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 15.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 16.Liden J, Delaunay F, Rafter I, Gustafsson J, Okret S. A new function for the C-terminal zinc finger of the glucocorticoid receptor Repression of RelA transactivation. J Biol Chem. 1997;272:21467–21472. doi: 10.1074/jbc.272.34.21467. [DOI] [PubMed] [Google Scholar]
- 17.Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012. doi: 10.1621/nrs.05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Druce LA, Thorpe CM, Wilton A. Mineralocorticoid effects due to cortisol inactivation overload explain the beneficial use of hydrocortisone in septic shock. Med Hypotheses. 2008;70:56–60. doi: 10.1016/j.mehy.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 19.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, Capellier G, Cohen Y, Azoulay E, Troche G, Chaumet-Riffaud P, Bellissant E. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 20.Minneci PC, Deans KJ, Hansen B, Parent C, Romines C, Gonzales DA, Ying SX, Munson P, Suffredini AF, Feng J, Solomon MA, Banks SM, Kern SJ, Danner RL, Eichacker PQ, Natanson C, Solomon SB. A canine model of septic shock: balancing animal welfare and scientific relevance. Am J Physiol Heart Circ Physiol. 2007;293:H2487–500. doi: 10.1152/ajpheart.00589.2007. [DOI] [PubMed] [Google Scholar]
- 21.Solomon SB, Minneci PC, Deans KJ, Feng J, Eichacker PQ, Banks SM, Danner RL, Natanson C, Solomon MA. Effects of intra-aortic balloon counterpulsation in a model of septic shock. Crit Care Med. 2009;37:7–18. doi: 10.1097/CCM.0b013e31818727bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kintzer PP, Peterson ME. Primary and secondary canine hypoadrenocorticism. Vet Clin North Am Small Anim Pract. 1997;(27):349–357. doi: 10.1016/s0195-5616(97)50036-2. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney DA, Natanson C, Banks SM, Solomon SB, Behrend EN. Defining normal adrenal function testing in the intensive care unit setting: a canine study. Crit Care Med. 2010;38:553–561. doi: 10.1097/CCM.0b013e3181cb0a25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104:16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink GD, Johnson RJ, Galligan JJ. Mechanisms of increased venous smooth muscle tone in desoxycorticosterone acetate-salt hypertension. Hypertension. 2000;35:464–469. doi: 10.1161/01.hyp.35.1.464. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen Dinh Cat A, Griol-Charhbili V, Loufrani L, Labat C, Benjamin L, Farman N, Lacolley P, Henrion D, Jaisser F. The endothelial mineralocorticoid receptor regulates vasoconstrictor tone and blood pressure. FASEB J. 2010;24:2454–2463. doi: 10.1096/fj.09-147926. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki S, Tsubochi H, Suzuki T, Darnel AD, Krozowski ZS, Sasano H, Kondo T. Modulation of transalveolar fluid absorption by endogenous aldosterone in adult rats. Exp Lung Res. 2001;27:143–155. doi: 10.1080/019021401750069384. [DOI] [PubMed] [Google Scholar]
- 28.Guidot DM, Folkesson HG, Jain L, Sznajder JI, Pittet JF, Matthay MA. Integrating acute lung injury and regulation of alveolar fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2006;291:L301–6. doi: 10.1152/ajplung.00153.2006. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann A, Eulenberg C, Wellner M, Rolle S, Luft F, Kettritz R. Aldosterone abrogates nuclear factor kappaB-mediated tumor necrosis factor alpha production in human neutrophils via the mineralocorticoid receptor. Hypertension. 2010;55:370–379. doi: 10.1161/HYPERTENSIONAHA.109.141309. [DOI] [PubMed] [Google Scholar]
- 30.Pan YJ, Young DB. Experimental aldosterone hypertension in the dog. Hypertension. 1982;4:279–287. doi: 10.1161/01.hyp.4.2.279. [DOI] [PubMed] [Google Scholar]
- 31.Kageyama Y, Bravo EL. Hypertensive mechanisms associated with centrally administered aldosterone in dogs. Hypertension. 1988;11:750–753. doi: 10.1161/01.hyp.11.6.750. [DOI] [PubMed] [Google Scholar]
- 32.Corrigan AM, Behrend EN, Martin LG, Kemppainen RJ. Effect of glucocorticoid administration on serum aldosterone concentration in clinically normal dogs. Am J Vet Res. 2010;71:649–654. doi: 10.2460/ajvr.71.6.649. [DOI] [PubMed] [Google Scholar]
- 33.Barrett KE, Ganong WF. Ganong's review of medical physiology. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 34.Davenport MW, Zipser RD. Association of hypotension with hyperreninemic hypoaldosteronism in the critically ill patient. Arch Intern Med. 1983;143:735–737. [PubMed] [Google Scholar]
- 35.du Cheyron D, Lesage A, Daubin C, Ramakers M, Charbonneau P. Hyperreninemic hypoaldosteronism: a possible etiological factor of septic shock-induced acute renal failure. Intensive Care Med. 2003;29:1703–1709. doi: 10.1007/s00134-003-1986-6. [DOI] [PubMed] [Google Scholar]
- 36.Zipser RD, Davenport MW, Martin KL, Tuck ML, Warner NE, Swinney RR, Davis CL, Horton R. Hyperreninemic hypoaldosteronism in the critically ill: a new entity. J Clin Endocrinol Metab. 1981;53:867–873. doi: 10.1210/jcem-53-4-867. [DOI] [PubMed] [Google Scholar]
- 37.Yeager MP, Guyre PM, Munck AU. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol Scand. 2004;48:799–813. doi: 10.1111/j.1399-6576.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- 38.Mansart A, Bollaert PE, Seguin C, Levy B, Longrois D, Mallie JP. Hemodynamic effects of early versus late glucocorticosteroid administration in experimental septic shock. Shock. 2003;19:38–44. doi: 10.1097/00024382-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Ottosson J, Brandberg A, Erikson B, Hedman L, Dawidson I, Soderberg R. Experimental septic shock--effects of corticosteroids. Circ Shock. 1982;9:571–577. [PubMed] [Google Scholar]
- 40.Tran TV, Weisman LE. Dexamethasone effects on group B streptococcal infection in newborn rats. Pediatr Infect Dis J. 2004;23:47–52. doi: 10.1097/01.inf.0000105107.76541.ee. [DOI] [PubMed] [Google Scholar]
- 41.Phuc Le P, Friedman JR, Schug J, Brestelli JE, Parker JB, Bochkis IM, Kaestner KH. Glucocorticoid receptor-dependent gene regulatory networks. PLoS Genet. 2005;1:e16. doi: 10.1371/journal.pgen.0010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.