Abstract
The stress response is a multifaceted physiological reaction that engages a wide range of systems. Animal studies examining stress and the stress response employ diverse methods as stressors. While many of these stressors are capable of inducing a stress response in animals, a need exists for an ethologically relevant stressor for female rats. The purpose of the current study was to use an ethologically relevant social stressor to induce behavioral alterations in adult female rats. Adult (postnatal day 90) female Wistar rats were repeatedly exposed to lactating Long Evans female rats to simulate chronic stress. After six days of sessions, intruder females exposed to defeat were tested in the sucrose consumption test, the forced swim test, acoustic startle test, elevated plus maze, and open field test. At the conclusion of behavioral testing, animals were restrained for 30 minutes and trunk blood was collected for assessment of serum hormones. Female rats exposed to maternal aggression exhibited decreased sucrose consumption, and impaired coping behavior in the forced swim test. Additionally, female rats exposed to repeated maternal aggression exhibited an increased acoustic startle response. No changes were observed in female rats in the elevated plus maze or open field test. Serum hormones were unaltered due to repeated exposure to maternal aggression. These data indicate the importance of the social experience in the development of stress-related behaviors: an acerbic social experience in female rats precipitates the manifestation of depressive-like behaviors and an enhanced startle response.
Keywords: stress, lactation, social defeat, depression, anxiety
1. Introduction
Stress disorders are multifaceted and capable of affecting males and females differently. Sex differences represent an important and understudied dichotomy in the pathogenesis of stress-related disorders. In humans and rats, sex differences span multiple systems to affect the hypothalamic-pituitary-adrenal (HPA) axis, negative feedback of the stress response, and behavioral modulation in response to stress [1–5]. Although our understanding of the effects of chronic stress has grown significantly in recent years, there exists a need for characterization of an ethologically relevant chronic stressor that works effectively in adult female rats.
Modulation of anxiety-like and depressive-like behavior between male and female rats is inherently dimorphic. Female rodents explore more than male rodents in adolescence [6] and adulthood [7]. Female rodents also engage in more coping/escape behavior at baseline [8]. The dichotomy in anxiety-like and depressive-like behaviors is theorized to arise from the cross-talk between HPA and hypothalamic-pituitary-gonadal axes [9]. While social defeat is a potent stressor and produces anxiety-like and depressive-like behavior in both sexes during adolescence [10], traditional social defeat in adult rodents has been shown to only affect males [11; 12]. Adult male rats are more aggressive than adult female rats, which may partially explain the problems with traditional social defeat models [13]. More studies in adult female rodents employing an ethologically-relevant stimulus are necessary to properly investigate stress-associated behavioral modulation.
Diverse methods to induce chronic stress have been previously explored. These often involve physical manipulation of the animal including restraint, damp bedding, cage tilt, and foot shock to induce behavioral alterations. Additionally, psychological manipulations have been employed such as predator odor, loud noise, overnight illumination, and isolation housing. Several studies using one or several of these stressors have shown HPA alterations and differential behavioral modulation [14; 15]. Social defeat is a potent stressor that dually alters the HPA axis and behavior.
The reaction to social stress is an evolutionarily-conversed reaction across species for the procurement of resources, making this form of stress ethologically relevant. Establishment of a social dominance hierarchy through social stress is necessary for the long term survival of an organism, but social stress may also induce depressive behaviors typified in a low rank order animal [16]. A previous study used a social defeat-housing manipulation stressor to induce sexually dimorphic effects on weight gain and adrenal weight, but anxiety-like and depressive-like behaviors have not been fully explored [17]. While female rats are less likely than male rats to engage in aggressive behavior, postpartum female rats are programmed to defend their pups from predators causing a cascade of hyperactivated neuroendocrine pathways and increased aggressive behavior [18]. We employed this aggressive phenotype to induce defeat of an intruding female rat by a lactating female rat. The purpose of the present study was to use a maternal defeat model consisting of isolation housing and social defeat by a lactating female as an ethologically-relevant stimuli to investigate changes in anxiety-like and depressive-like behaviors. We demonstrate here that exposure to a maternal defeat model is effective at altering affective-like behaviors in adult female rats.
2. Material and Methods
2.1 Animals
Timed pregnant Wistar rats (Charles River, Wilmington, MA) arrived on gestational day 12 (n = 24). Pregnancy was determined at the Charles River facility by the presence of a vaginal plug. Rats were housed on a 14:10 reverse light:dark cycle in a facility controlled for humidity (60%) and temperature (20°C–23°C). Rodent diet 5001 chow (Purina Mills, Richmond, IN) and water were available ad libitum throughout the study. Estrous cycle was tracked throughout the study using vaginal lavage. All animal procedures were approved by Emory University’s IACUC and were carried out to minimize pain and suffering to the animal in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Maternal Aggression Stress
Maternal defeat stress (Stress) was defined as daily exposure to maternal defeat and isolation housing beginning in adulthood (postnatal day 90). The cohort of rats consisted of the following groups: 1) single housed females (Control female) and 2) single housed females exposed to maternal defeat (Stress female). The maternal defeat stress was adapted from previous studies [19]. Rats in the stress group were placed in the home cage of a lactating female. Initially, the two rats were separated by a wire mesh screen. Female residents were adult Long Evans lactating females (post parturition day 5–11) actively nursing their pups. Lactating female Long Evans rats demonstrate the territorial aggression necessary for the social defeat stressor and lactation accentuates this behavior in females [20]. On the first day of social defeat, after five minutes, the barrier was removed for five minutes or until the intruder was pinned five times. On the second day, the barrier was replaced after three pins or five minutes. All subsequent bouts of defeat interactions consisted of only one pin or a maximum of five minutes of interaction. At the conclusion of the physical interaction, the wire mesh screen was replaced. The maternal aggression stress procedure was administered over six days, with the last four days consisting of the one pin or five minute interaction condition. Lactating resident females in this study pinned the intruder 46.3% of the time. Intruders and residents remained separated by the wire mesh screen for an additional 30 minutes and subsequently the intruder was returned to its home cage. Separation with the wire mesh was used to pair all intruders and residents for the same amount of time without subjecting the intruder to repeated bouts of defeat. Intruder and resident pairings were randomly assigned each day to prevent stabilization of a dominance hierarchy. All sessions occurred during the light cycle to coincide with the nadir of the circadian cycle of corticosterone to maximally increase corticosterone.
2.3 Behavioral Testing
Behavioral testing began after the final maternal aggression stress. These behavioral tests are not intended to recapitulate exact human psychopathology but rather have a degree of predictive validity for various neurobehavioral traits in human psychopathology [21]. Behavioral testing for each cohort consisted sequentially of the sucrose consumption test, the open field test, the elevated plus maze, the acoustic startle test, and the forced swim test. Order of subjects tested each day was randomized to minimize any effect due to testing time. One behavioral test was conducted per day with the exception of the sucrose consumption test, which was administered over two days. The elevated plus maze was conducted two hours after onset of the dark cycle while all other tests were conducted in the light cycle.
2.4.1 Sucrose Consumption Test
The sucrose consumption test measures the rat’s consumption of a sucrose solution (0.8%) versus water as a measure of hedonic state. The sucrose consumption test began during the last day of maternal aggression stress, after conclusion of the stress procedure. Male and female rats subjected to chronic social defeat consume less sucrose than control rats and this behavior has been described as a depressive-like state [10, 22]. Rats were given free access to one bottle of tap water and one bottle of a 0.8% sucrose solution in tap water. In order to prevent any effect due to side bias, bottle location was reversed after 24 hours. After 24 and 48 hours, the bottles were weighed to determine sucrose and water consumption. Data reported are the average consumption of day 1 and day 2 of the sucrose consumption test.
2.4.2 Elevated Plus Maze
As an index of anxiety-like behavior, animals were tested in the elevated plus maze. Four days after conclusion of the maternal aggression stress, rats were placed in the center of a plastic plus maze and recorded for five minutes. Testing took place two hours after lights out and was conducted under dim red light. Tapes were scored for number of entries and time spent in the closed or open arms [23]. Percent of time spent in the open arm was calculated as time spent in the open arm divided by total testing time (300 seconds). To measure other exploratory behavior, rearing behavior was counted [24].
2.4.3 Acoustic Startle Response
The acoustic startle reflex test was performed six days after the last maternal aggression stressor as previously described [25]. Briefly, during the light cycle, rats were placed in a ventilated startle chamber (San Diego Instruments, San Diego, CA) using an accelerometer to measure the startle reflex. After a white-noise (65 db) acclimatization period in the chamber lasting five minutes, individual rats were exposed to 16 trials consisting of a short pulse of noise (115 db). Between each trial, there was a period of white noise which lasted 2, 5, 25, or 60 seconds.
2.4.4 Forced Swim Test
In order to assess motor activity in an inescable environment, the forced swim test was administered seven days after conclusion of the maternal aggression stressor. The forced swim test, while traditionally a screen for antidepressant drug efficacy, has been used to assess tendency to respond actively or inactively to a challenge [26; 27]. Rats were placed in a clear acrylic beaker (40 cm high×18 cm in diameter) filled with 30°C water for ten minutes during the light cycle. Following this training session, rats were tested the following day for five minutes. Two observers blind to treatment group scored the latency to the first float, time spent struggling, and the number of dives which previous studies have described as escape or active coping behavior [8; 28]. Latency to first float was defined as the rat's limbs remaining motionless for at least two seconds. Active swimming was defined as the rat breaking the surface of the water with all four limbs in motion. Diving was defined as the rat swimming below the surface of the water. Passive swimming was the remaining time not spent floating, actively swimming, or diving.
2.5 Endocrine Analyses and Estrous Cycle Tracking
Rats were decapitated one day after the last behavioral test, two hours prior to the dark cycle. Following a 30 minute restraint session used to stimulate the HPA axis, rats were rapidly decapitated without anesthesia and trunk blood was collected immediately in BD Vacutainer EDTA collection tubes (BD, Franklin Lakes, NJ). Blood was spun down at 1,800 rcf and the plasma fraction was collected. Plasma corticosterone was assayed using 10 µL of plasma with the ImmuChem 125I Corticosterone RIA Kit with an intra-assay variability of 5.55%, an inter-assay variability of 6.97% and a sensitivity of 1 ng/mL (MP Biomedicals, Solon, OH). Plasma estradiol was assayed using 50 µL of plasma with the ImmuChem 125I 17β-Estradiol RIA Kit with an intra-assay variability of 4.02%and a sensitivity of 10 pg/mL (MP Biomedicals, Solon, OH). All estradiol samples were run in one assay, therefore no inter-assay variability was calculated. Samples were run in duplicate for all endocrine assays. Body mass was recorded throughout the study. Estrous cycle was tracked throughout the study by vaginal lavage.
2.6 Statistical Analyses
GraphPad Prism (GraphPad Software, La Jolla, CA) was used to conduct Student's t test statistical analyses. In the case of the acoustic startle response, a two-way ANOVA was used for statistical comparisons (time×stress). The alpha value was set to 0.05 for one-tailed t-tests.
3. Results
3.1 Physiological Outcomes in Adult Females Were Unaltered by Maternal Aggression Stress
Gross anatomical and endocrine measurements were conducted to determine any changes due to maternal aggression stress. Maternal aggression stress in adult females did not alter weight gain over the course of the study (F1,20 = 0.3, p = 0.30, Terminal Weight: Control: 278.3 ± 10.0 g, Stress: 274.3 ± 6.0 g). After an acute 30 minute restraint session designed to induce an HPA response, control and maternal aggression stress females did not display differences in plasma corticosterone (Control: 1,192 ± 153 ng/mL, Stress: 1,305 ± 79 ng/mL, t10 = 0.7, p = 0.26) or plasma estradiol (Control: 25.00 ± 8.66 pg/mL, Stress: 29.78 ± 4.54 pg/mL, t10 = 0.5, p = 0.32). Estrous phase did not predict behavioral test session, indicating that the estrous cycle did not account for the behavioral effects observed in this study (F1,368 = 0.60, p = 0.44).
3.2 Maternal Aggression Stress in Adult Females Decreased Sucrose Consumption
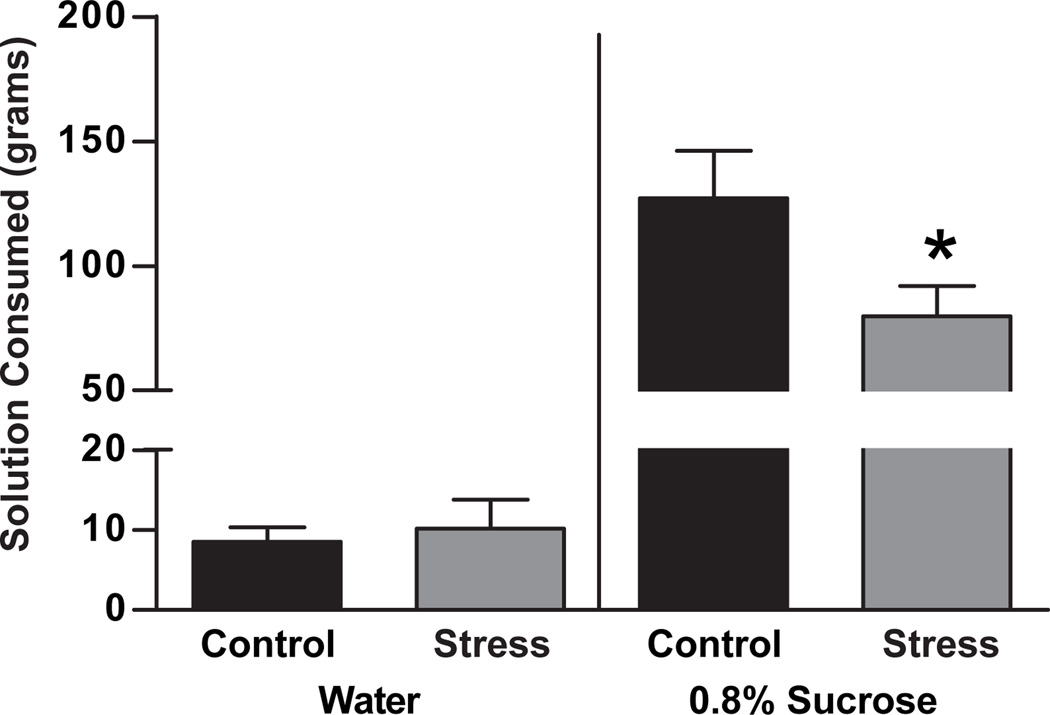
Sucrose consumption in a two bottle choice test was measured as a rough index of anhedonic behavior. There was a decrease in sucrose consumption in females exposed to maternal aggression stress compared to controls (t10 = 2.1, p = 0.03, Figure 1). Water consumption was unaltered in maternal aggression stress and control groups, showing no change in overall consumption behavior (t10 = 0.4, p = 0.35, Figure 1).
Figure 1.
The effects of maternal aggression on sucrose consumption were determined using a 48 hour sucrose consumption test. Rats were given free access to identical bottles which contained either tap water or 0.8% sucrose in tap water. Consumption of each liquid was measured at 24 hours and 48 hours and the values were averaged for each rat. Female rats exposed to repeated maternal aggression consumed less sucrose water than control female rats. Water consumption in females exposed to repeated maternal aggression was unaltered compared to female controls. * p < 0.1. Data are presented as mean ± SEM, N = 6/group.
3.3 Passive Coping Behavior in the Forced Swim Test Increased After Maternal Aggression Stress
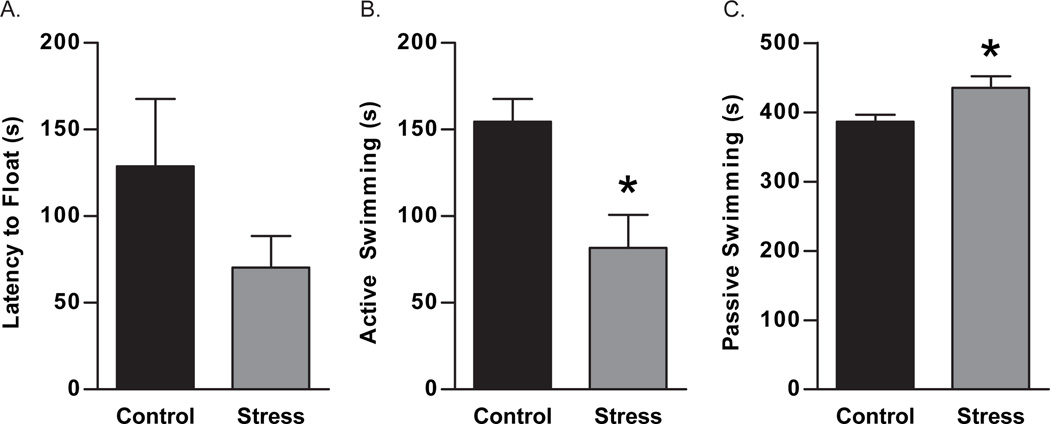
The forced swim test, although typically a test for antidepressant efficacy, was used to assess active coping behavior in an inescapable environment. Latency to first float was unaltered in adult females by maternal aggression stress (t10 = 1.2, p = 0.13, Figure 2A). Actual time spent floating was unaltered by maternal aggression stress (Control: 75.40 ± 17.56 s, Stress: 84.00 ± 12.83 s; t7 = 0.38, p = 0.72). Active swimming behavior, indicative of an active coping response, was decreased in females previously exposed to maternal aggression stress (t8 = 2.8, p = 0.01, Figure 2B). Maternal aggression stress in adult females increased passive swimming behavior (t7 = 2.7, p = 0.02, Figure 2C).
Figure 2.
The effects of repeated maternal aggression on behavior in the forced swim test were assessed and compared to control females. Females in the maternal aggression stress group exhibited no changes in the latency to float compared to control females (A). Exposure to repeated maternal aggression resulted in decreased active swimming behavior compared to control females (B). Passive swimming was increased due to repeated maternal aggression in females (C). * p < 0.05. Data are presented as mean ± SEM, N = 5–6.
3.4 Behavior in the Elevated Plus Maze Was Unaltered by Maternal Aggression Stress
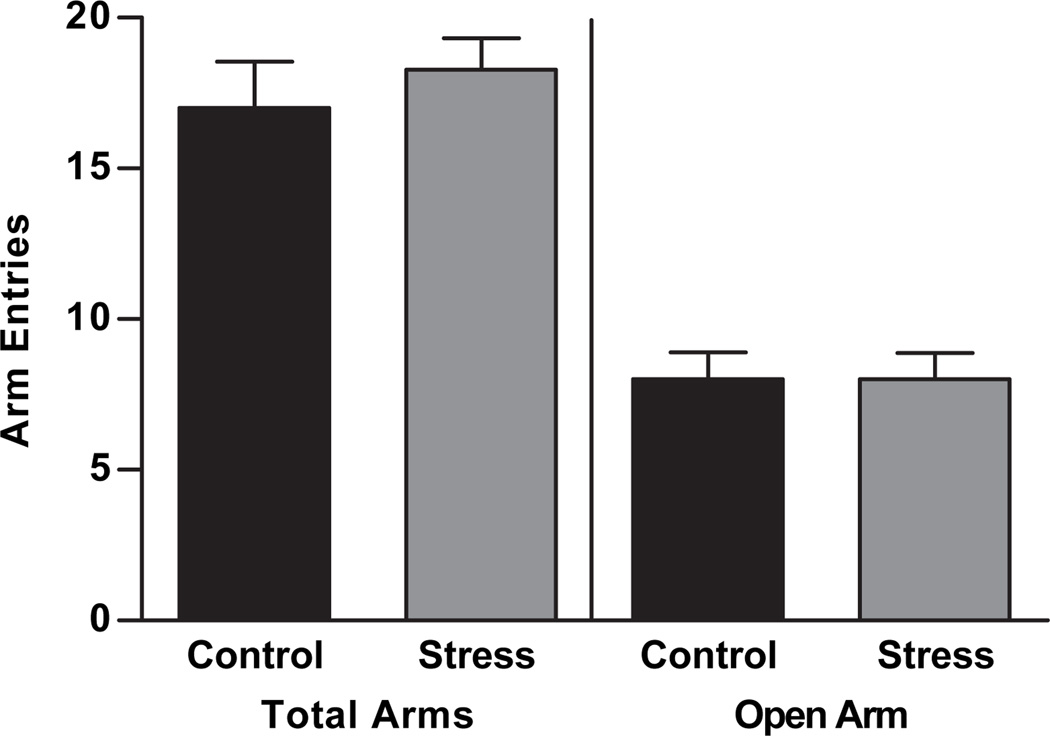
Exploratory behavior was measured in the elevated plus maze to assess anxiety-like behavior. Maternal aggression stress in females produced no changes in locomotion as assessed by total arm entries (t11 = 0.7, p = 0.25, Figure 3). Exploratory and risk assessment behavior was unaltered by maternal aggression stress: there were no changes due to maternal aggression stress in open arm entries (t10 = 0.0, p = 0.50, Figure 3).
Figure 3.
Repeated maternal aggression did not alter behavior in the elevated plus maze for females. Total arm entries and open arm entries were not altered by repeated maternal aggression stress in female adult rats compared to control females (p > 0.05). Data are presented as mean ± SEM, N = 6–7.
3.5 Startle Reflex Increased Following Maternal Aggression Stress in Adult Females
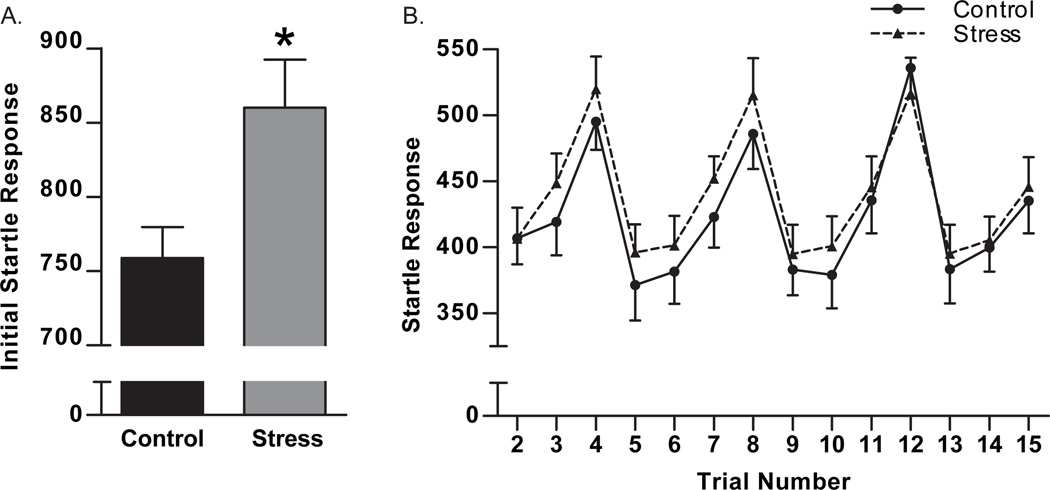
Acoustic startle response was measured in 16 trials of varying pulse intervals. Prior to any habituation, the initial startle response in trial 1 was increased due to maternal aggression stress (t11 = 2.5, p = 0.02, Figure 4A). Overall, acoustic startle response was higher in adult females exposed to maternal aggression stress (F1,155 = 5.4, p = 0.01, Figure 4B).
Figure 4.
Acoustic startle response was increased due to repeated exposure to maternal aggression compared to control females. Acoustic startle response was measured with startle boxes in 16 trials separated by different pulse interval lengths. The initial startle response in the first trial was increased due to maternal aggression stress compared to control females (A). Overall startle response was increased in adult females due to maternal aggression stress compared to control females (B). * p < 0.05, t-test. Data are presented as mean ± SEM, N = 6–7.
4. Discussion
The current study utilized a modified social defeat stressor in the form of maternal aggression to cause stress-induced behavioral changes in female rats. The purpose of the present study was to show that an ethologically-relevant stressor was capable of producing behavioral deficits in females. In our study of females, we utilized the aggressive properties in female rats that accompany lactation to stimulate aversive encounters. Adult female rats exposed to maternal aggression stress displayed depressive-like behaviors and hyperactive acoustic startle response that may indicate a fear-like and/or anxiety-like phenotype. These data indicate that stress-induced behaviors can be elicited by maternal aggression and this stressor may be a useful experimental paradigm for studying the effects of stress in females.
The maternal aggression stress induced a shift from active to passive coping behavior in the forced swim test. Other groups have observed a shift from passive to active coping behavior in the forced swim test after administration of a neurokinin receptor 1 antagonist, a potential antidepressant [29]. A similar shift from passive to active coping behavior was observed after chronic administration of paroxetine in rats bred for high anxiety-related behavior [30]. Additionally, we have reported that chronic stress in adolescence causes decreased active coping behavior in adolescent and adult female rats [10]. Current evidence documenting the shift in coping behavior during the forced swim test support our interpretation that maternal aggression stress induces decreased active coping behavior.
Several groups have postulated that an acerbic social experience is capable of inducing anxiety-like behaviors. The importance of the social experience is illustrated early in life: maternal separation is a potent and reproducible stressor used by multiple groups to affect the glucocorticoid receptor system, anxiety-like behaviors, and mediators of the HPA axis [31]. Blanchard and colleagues have used the visual burrow system, a psychosocial stressor, to show the marked impact of social stress on physiological processes, neurotransmitter systems, and behavior [32–34]. Others have used social defeat stress to induce behavioral alterations [35–37]. Our results are in agreement with the extant literature indicating that an acerbic social experience can induce behavioral alterations.
Studies have frequently documented the positive effects of "social buffering", where the stress experience is mitigated by the presence of a companion [38]. In humans, the social experience also places a critical role in etiology of mood disorders: social factors correlate with the psychopathology of anxiety and depressive disorders [39]. The social unit, therefore, seems to have a profound impact on the perception and manifestation of the stress response. Consistent with previous work in males, we demonstrate that an acerbic social experience can precipitate depressive-like behaviors and enhanced startle in adult female rats.
The importance of the current study pertains to the nature of the stress involved. As previously mentioned, several groups have used intense physical stressors such as restraint, foot shock, damp bedding, loud noise, or cage tilt to induce anxiety-like and depressive-like behaviors in female rats [40; 41]. Other groups use psychological stressors such as predator odor, overnight illumination, or isolation housing to induce behavioral dysfunction [40; 42]. Social stress dually acts on these modes of manipulation but also incorporates the social nature of the stress experience [16]. The importance of the social nature of the stress experience has been demonstrated by Nephew and colleagues who have utilized a social stressor consisting of exposure to a male to increase maternal aggression [43]. The nature of social stress highlights the importance of the social experience and incorporates an ethologically-relevant stressor for behavioral studies in female rats.
Animal studies that employ an ethologically-relevant stressor in females are vital to the understanding of the underlying neurobiology of the stress response. Stress and anxiety are multi-faceted disorders that can be evaluated from several different modes of stress and phenotypic endpoints in animal studies. Chemical manipulations such as corticosterone or corticotropin-releasing factor injections aid in understanding neurobiological mechanisms, but the clinical relevance of these manipulations is dubious as they typically hyper-activate select systems. In contrast, the stress response is multifaceted and an ethologically relevant stressor activates several diverse neurotransmitter and hormonal systems that act to regulate the stress response.
Induction of social defeat in females is difficult due to the nature of the stressor involved. Social hierarchy in rats typically causes larger males to defeat smaller males. However, at baseline, adult female rats are less likely to engage in defeat bouts than male rats. Conversely, the high levels of oxytocin, vasopressin, and progesterone that are present during lactation serve to increase aggression [20; 44–46]. Albers and colleagues have examined the aggressive response in female Syrian hamsters and demonstrated an inhibitory role of arginine vasopressin centrally administered to the anterior hypothalamus [47]. The role of arginine vasopressin in the regulation of aggression has been shown to be pronounced during pregnancy: centrally administered arginine vasopressin increases maternal aggression [48]. The increased aggression demonstrated by lactating rats was used in this study to induce social stress in female conspecifics. This model of maternal aggression provides a paradigm in which the effects of ethologically relevant social stress can be assessed in female rats using unstressed females as the control group.
5. Conclusions
The current study demonstrates that repeated exposure to maternal aggression is sufficient to induce depressive-like behavior and enhanced startle in adult female rats. The social stress paradigm described here provides an ethologically relevant stressor, which can be used to induce stress-related pathophysiology in adult female rats. Introduction of this stress paradigm provides a laboratory condition that can be used to facilitate the study of mechanisms underlying stress-induced pathophysiology in adult female rats.
Research Highlights.
Exposure to maternal aggression was used as a chronic social stressor.
Female rats exposed to maternal aggression exhibited decreased sucrose consumption.
Female rats exposed to maternal aggression displayed impaired coping behavior.
Female rats exposed to maternal aggression exhibited increased startle response.
Acknowledgments
Thanks to EE Hardy for animal care, behavioral testing, and behavioral analysis. Thanks to JC Ritchie and CH Ramsey for technical support. Special thanks to B Kinkead and SM Rogers for their assistance with animal handling and scoring.
Role of Funding Source
Funding for this study was provided by unrestricted funds provided by Emory University’s Comprehensive Neurosciences Center’s Child and Adolescent Mood Program and salary support for CH Bourke was provided by NIEHS Grant T32ES012870; neither funding source had a role in study design, data collection, analysis and interpretation of data, manuscript preparation, or the decision to submit the manuscript for publication.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
CH Bourke declares that he has no competing interests.
GN Neigh receives grant funding from NIMH, AHA, NARSAD, GSK, and Emory University.
References
- 1.Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int J Dev Neurosci. 2008;26(3–4):259–268. doi: 10.1016/j.ijdevneu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological. Psychoneuroendocrinology. 2009 Feb;34(2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Young EA. Sex differences and the HPA axis: implications for psychiatric disease. J Gend Specif Med. 1998;1(1):21–27. [PubMed] [Google Scholar]
- 4.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29(1):83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 5.Laviola G, Adriani W, Morley-Fletcher S, Terranova ML. Peculiar response of adolescent mice to acute and chronic stress and to amphetamine: evidence of sex differences. Behav Brain Res. 2002 Mar;130(1–2):117–125. doi: 10.1016/s0166-4328(01)00420-x. [DOI] [PubMed] [Google Scholar]
- 6.Toledo-Rodriguez M, Sandi C. Stress during Adolescence Increases Novelty Seeking and Risk-Taking Behavior in Male and Female Rats. Front Behav Neurosci. 2011 Jan;5:17. doi: 10.3389/fnbeh.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palanza P, Gioiosa L, Parmigiani S. Social stress in mice: gender differences and effects of estrous cycle and social dominance. Physiol Behav. 2001 Jun;73(3):411–420. doi: 10.1016/s0031-9384(01)00494-2. [DOI] [PubMed] [Google Scholar]
- 8.Campbell T, Lin S, DeVries C, Lambert K. Coping strategies in male and female rats exposed to multiple stressors. Physiol Behav. 2003 Mar;78(3):495–504. doi: 10.1016/s0031-9384(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 9.Mastorakos G, Pavlatou MG, Mizamtsidi M. The hypothalamic-pituitary-adrenal and the hypothalamic- pituitary-gonadal axes interplay. Pediatr Endocrinol Rev. 2006 Jan;3 Suppl 1:172–181. [PubMed] [Google Scholar]
- 10.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011 Jun;60(1):112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006 Nov;175(1):43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 12.Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001 May;25(3):219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- 13.DeBold JF, Miczek KA. Aggression persists after ovariectomy in female rats. Horm Behav. 1984 Jun;18(2):177–190. doi: 10.1016/0018-506x(84)90041-2. [DOI] [PubMed] [Google Scholar]
- 14.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987 Jan;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 15.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005 Jan;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 16.Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006 Nov;50(4):640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 17.Haller J, Fuchs E, Halász J, Makara GB. Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of a social stress model in female rats. Brain Res Bull. 1999 Sep;50(1):33–39. doi: 10.1016/s0361-9230(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 18.Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002 Dec;26(8):869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 19.Bolhuis JJ, Fitzgerald RE, Dijk DJ, Koolhaas JM. The corticomedial amygdala and learning in an agonistic situation in the rat. Physiol Behav. 1984 Apr;32(4):575–579. doi: 10.1016/0031-9384(84)90311-1. [DOI] [PubMed] [Google Scholar]
- 20.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005 Jul;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Staay FJ. Animal models of behavioral dysfunctions: basic concepts and classifications, and an evaluation strategy. Brain Res Rev. 2006 Aug;52(1):131–159. doi: 10.1016/j.brainresrev.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005 Jul;162(1):127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Pellow S, Chopin P, File S, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985 Aug;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 24.Van den Berg CL, Lamberts RR, Wolterink G, Wiegant VM, Van Ree JM. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats involvement of opioids. Brain Res. 1998 Jul;799(1):6–15. doi: 10.1016/s0006-8993(98)00397-7. [DOI] [PubMed] [Google Scholar]
- 25.Kinkead B, Yan F, Owens MJ, Nemeroff CB. Endogenous neurotensin is involved in estrous cycle related alterations in prepulse inhibition of the acoustic startle reflex in female rats. Psychoneuroendocrinology. 2008 Feb;33(2):178–187. doi: 10.1016/j.psyneuen.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernal-Morales B, Contreras CM, Cueto-Escobedo J. Acute restraint stress produces behavioral despair in weanling rats in the forced swim test. Behav Processes. 2009 Oct;82(2):219–222. doi: 10.1016/j.beproc.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977 Apr;266(5604):730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 28.Tõnissaar M, Mällo T, Eller M, Häidkind R, Kõiv K, Harro J. Rat behavior after chronic variable stress and partial lesioning of 5-HT-ergic neurotransmission: effects of citalopram. Prog Neuropsychopharmacol Biol Psychiatry. 2008 Jan;32(1):164–177. doi: 10.1016/j.pnpbp.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Ebner K, Singewald GM, Whittle N, Ferraguti F, Singewald N. Neurokinin 1 receptor antagonism promotes active stress coping via enhanced septal 5-HT transmission. Neuropsychopharmacology. 2008 Jul;33(8):1929–1941. doi: 10.1038/sj.npp.1301594. [DOI] [PubMed] [Google Scholar]
- 30.Keck ME, Welt T, Müller MB, Uhr M, Ohl F, Wigger A, Toschi N, Holsboer F, Landgraf R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology. 2003 Feb;28(2):235–243. doi: 10.1038/sj.npp.1300040. [DOI] [PubMed] [Google Scholar]
- 31.Litvin Y, Tovote P, Pentkowski NS, Zeyda T, King LB, Vasconcellos AJ, Dunlap C, Spiess J, Blanchard DC, Blanchard RJ. Maternal separation modulates short-term behavioral and physiological indices of the stress response. Horm Behav. 2010 Jul;58(2):241–249. doi: 10.1016/j.yhbeh.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Blanchard RJ, McKittrick CR, Blanchard DC. Animal models of social stress: effects on behavior and brain neurochemical systems. Physiol Behav. 2001 Jun;73(3):261–271. doi: 10.1016/s0031-9384(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 33.McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000 May;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 34.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997 Jun;17(12):4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haller J, Leveleki C, Baranyi J, Mikics E, Bakos N. Stress, social avoidance and anxiolytics: a potential model of stress-induced anxiety. Behav Pharmacol. 2003 Sep;14(5–6):439–446. doi: 10.1097/01.fbp.0000087735.21047.e7. [DOI] [PubMed] [Google Scholar]
- 36.Scholtens J, Roozen M, Mirmiran M, van de Poll NE. Role of noradrenaline in behavioral changes after defeat in male and female rats. Behav Brain Res. 1990 Jan;36(3):199–202. doi: 10.1016/0166-4328(90)90057-l. [DOI] [PubMed] [Google Scholar]
- 37.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003 Sep;44(3):293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009 Oct;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Coyne JC, Downey G. Social factors and psychopathology: stress, social support, and coping processes. Annual Rev Psychol. 1991 Jan;42:401–425. doi: 10.1146/annurev.ps.42.020191.002153. [DOI] [PubMed] [Google Scholar]
- 40.Matuszewich L, Karney JJ, Carter SR, Janasik SP, O’Brien JL, Friedman RD. The delayed effects of chronic unpredictable stress on anxiety measures. Physiol Behav. 2007 Mar;90(4):674–681. doi: 10.1016/j.physbeh.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kavushansky A, Ben-Shachar D, Richter-Levin G, Klein E. Physical stress differs from psychosocial stress in the pattern and time-course of behavioral responses, serum corticosterone and expression of plasticity-related genes in the rat. Stress. 2009 Jan;12(5):412–425. doi: 10.1080/10253890802556081. [DOI] [PubMed] [Google Scholar]
- 42.Adamec R, Head D, Blundell J, Burton P, Berton O. Lasting anxiogenic effects of feline predator stress in mice: sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav. 2006 Jun;88(1–2):12–29. doi: 10.1016/j.physbeh.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011 Nov;14(6):677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010 Mar;31(5):883–891. doi: 10.1111/j.1460-9568.2010.07115.x. [DOI] [PubMed] [Google Scholar]
- 45.Bosch OJ, Pförtsch J, Beiderbeck DI, Landgraf R, Neumann ID. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J Neuroendocrinol. 2010 May;22(5):420–429. doi: 10.1111/j.1365-2826.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 46.de Sousa FL, Lazzari V, de Azevedo MS, de Almeida S, Sanvitto GL, Lucion AB, Giovenardi M. Progesterone and maternal aggressive behavior in rats. Behav Brain Res. 2010 Sep;212(1):84–89. doi: 10.1016/j.bbr.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 47.Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) Eur J Neurosci. 2010 May;31(9):1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- 48.Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol Biochem Behav. 2008 Nov;91(1):77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]