Abstract
The pathophysiology of obstructive sleep apnea (OSA) has been associated with dysregulation of the hypothalamic pituitary adrenal (HPA) axis; however a relationship between OSA and altered cortisol levels has not been conclusively established. We conducted a systematic review using the PRISMA Guidelines based on comprehensive database searches for (1) studies of OSA patients compared to controls in whom cortisol was measured and (2) studies of OSA patients treated with continuous positive airway pressure (CPAP) in whom cortisol was measured pre and post treatment. Five electronic databases were searched along with the reference lists of retrieved studies. The primary outcomes were (1) differences in cortisol between OSA and control subjects and (2) differences in cortisol pre-post CPAP treatment. Sampling methodology, sample timing and exclusion criteria were evaluated. Fifteen studies met the inclusion criteria. Heterogeneity of studies precluded statistical pooling. One study identified differences in cortisol between OSA patients and controls. Two studies showed statistically significant differences in cortisol levels pre-post CPAP. The majority of studies were limited by assessment of cortisol at a single time point. The available studies do not provide clear evidence that OSA is associated with alterations in cortisol levels or that treatment with CPAP changes cortisol levels. Methodological concerns such as infrequent sampling, failure to match comparison groups on demographic factors known to impact cortisol levels (age, body mass index; BMI),and inconsistent control of variables known to influence HPA function may have limited the results.
Keywords: Obstructive Sleep Apnea, Cortisol, Continuous Positive Airway Pressure, Systematic Review
Obstructive sleep apnea (OSA) is a prevalent sleep disorder characterized by repeated episodes of complete or partial obstruction of the upper airway, leading to disrupted breathing throughout the night (nocturnal hypoxia). Intermittent upper airway obstruction and consequent hypoxia lead to autonomic arousal during sleep of sufficient intensity to prompt transient wakening leading to a clearing of the airway and reversal of asphyxia(1). The clinical importance of OSA is highlighted by the finding of a causal link, independent of obesity, between OSA and the development of hypertension and cardiovascular disease (CVD)(2,3).
Reports suggest that nocturnal awakenings in OSA are associated with alterations in hypothalamic-pituitary-adrenal (HPA) activity, specifically, increased pulsatile cortisol release (4). Cortisol is the primary human glucocorticoid product of the HPA axis and major functions include metabolic (gluconeogenesis) and blood pressure regulation and immune suppression. Excess cortisol secretion is associated with numerous adverse consequences throughout the body. Cortisol's fluctuation throughout the night is intricately related to sleep, and dysregulation of cortisol has been proposed as a mechanism through which sleep disorders manifest some of their physiologic effects (5,6). Since nocturnal awakenings are associated with HPA axis activation and associated sympathetic activation, it is expected that concentrations of cortisol would be higher in patients with OSA. However, the empirical data does not seem to support this hypothesis; many studies have failed to find differences in cortisol between OSA subjects and normal controls. Further, several studies have reported that continuous positive airway pressure (CPAP), the gold standard treatment for OSA, does not reduce cortisol levels in patients with OSA. Finally, the removal of CPAP in OSA patients has not been shown to result in immediate increases in cortisol levels(7,8).
Methodological concerns, such as infrequent sampling and inconsistent timing of sample collection, may have contributed to null findings for some studies(1). Recent studies using more extensive circadian sampling have reported differences in cortisol levels between OSA patients and normal controls (9,10). Additional inconsistencies across studies, such as age and body composition of comparison groups, and control for variables known to be related to HPA activity (ie, smoking), may also contribute to mixed findings.
The aim of the present review was to evaluate whether HPA function differs in patients with OSA versus controls using measures of cortisol. The two hypotheses of the study were that (1) cortisol levels would be elevated in individuals with OSA versushealthy controls and that elevations would be particularly evident at night and (2) treatment of OSA with CPAP would result in reductions in cortisol levels.
Methods
A systematic review of peer-reviewed studies of adult human subjects (age 18+) that have examined the relationship between cortisol and OSA was conducted. (1) Existing literature comparing cortisol levels in patients with OSA and controls was reviewed and (2) studies examining the impact on cortisol levels of successful treatment of OSA using CPAP were reviewed. Careful attention was paid to the group compositions and methodology and timing of sample collection used to assess cortisol, and to inclusion and exclusion criteria applied to samples. The review is reported according to the PRISMA Statement guidelines (11).
Search Strategy
Five electronic databases (PubMed, EMBASE, PsycINFO, Biosis and Cochrane Library Central Register of Controlled Trials databases) were searched from January 1966 to November 2010. All database searches were conducted by the first author (LT). A free-text search was performed using the following search terms: obstructive sleep apnea AND cortisol, hydrocortisone*, corticosteroid*, cortison*, glucocorticoid. To supplement the electronic searches, the bibliographies/reference lists of included studies were reviewed as well as the content of recentissues of selected journals (Sleep, Journal of Biological Rhythms, Journal of Sleep Research, Sleep Medicine, Sleep and Breathing, Behavioral Sleep Medicine, Journal of Clinical Sleep Medicine).
Study Selection
Two authors (LT and KE) independently assessed each retrieved study and disagreements with respect to inclusion were resolved through discussion with the senior author (JD). All published papers that reported data on: (1) controlled studies, (2) measurements of cortisol, and (3) populations of adult patients with OSA versus adult persons without OSA or (4) adult patients with OSA who were treated with CPAP were considered. To determine eligibility, the titles and abstracts of each article were reviewed and marked as “no”, “yes” and “maybe”. Those marked “no” were excluded. The text of those articles marked “yes” or “maybe” were reviewed in full. Articles that did not meet inclusion/exclusion criteria restrictions were omittedfrom the review.
Inclusion/Exclusion Criteria
To be included, studies must have been published in the English language and in peer-reviewed journals. Case reports, comments, letters to the editor, replies and articles not published in English were excluded. Animal studies and non-peer reviewed sources were excluded. The diagnosis of OSA must have been based on an AHI cut-off of ≥ 5 and diagnostic criteria must have been clearly described and reported within the text. The OSA control group must have had an AHI < 5(12). Studies that did not use standard polysomnography to diagnose OSA were excluded. Subjects must not have been treated with CPAP prior to participation. Cortisol levels had to be determined with a standard biological assay and biological cortisol levels had to be described either by presentation of mean values or graphic presentation of the data. Studies were excluded if the HPA axis was pharmacologically challenged (e.g. by dexamethasone) before cortisol measurement. Studies were limited to an emphasis on patients with OSA. Studies that evaluated both OSA and other sleep disordered individuals were included as long as data for OSA subjects could be analyzed separately from that of other subject populations. In the case of multiple papers from a single study, the publication with the largestnumber of cortisol measurements was included.
Data Extraction
Details from each of the selected articles were logged into a standard form to characterize each study. Of greatestinterest in each article was the study design, approach used to assess cortisol, and the significance of associations between OSA and cortisol. The following data were extracted: (1) sample size, demographic and clinical characteristics of patients and controls, (2) information about the diagnosis of OSA and the mean, standard deviation and/or standard error of the mean for AHI in the patient sample, (3) level of detail describing cortisol assessment (via saliva or venous blood draw), (4) the time points for obtaining cortisol, (5) p-values for differences in mean cortisol levels or diurnal rhythm of cortisol between OSA patients and controls and pre to post CPAP, (6) characteristics of cortisol assessment (e.g., RIA).
In consideration of the potential confounders of OSA cortisol associations, we also collected study exclusion criteria (smoking and depression) and data on matching of participants and controls on demographic variables (age and BMI).
Synthesis of Results
Studies were heterogeneous in terms of methodologies employed and populations sampled making a meta-analysis inappropriate, thus we decided to conduct a systematic qualitative review of the literature. Summary results are presented in two parts: (1) differences in cortisol levels between OSA patients and controls, and (2) changes in cortisol levels in OSA patients before and after treatment with CPAP.
Results
Study Flow
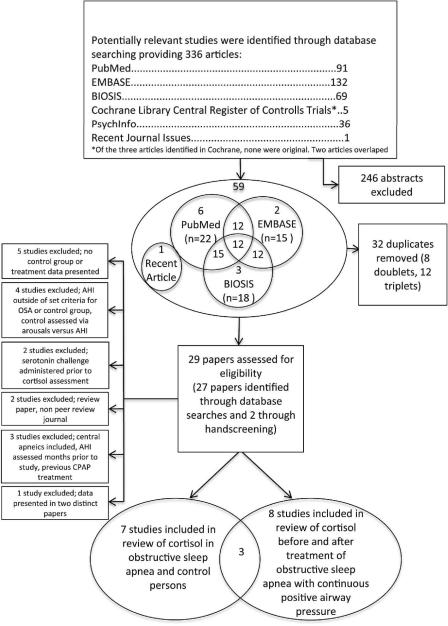
Figure 1 shows the PRISMA flow diagram. In relation to aims 1 and 2, the literature search identified: (1) seven studies on patients with OSA versus healthy controls fulfilling the inclusion criteria (9,13–18), (2) eight studies on patients with OSA who were treated with CPAP (9,10,13,15,19–22). Three studies fit into both categories (e.g., data was presented comparing OSA versus healthy controls followed by treatment of the OSA group with CPAP) (9,13,15).
Figure 1.
PRISMA trial flow used to identify studies for detailed analysis of cortisol in (1) patients with obstructive sleep apnea and healthy controls and (2) patients with obstructive sleep apnea before and after treatment with continuous positive airway pressure.
AHI = Apnea hypopnea index; CPAP = Continuous positive airway pressure
Study Characteristics
OSA Versus Controls
The seven studies of cortisol in patients with OSA compared to controls are presented in Table 1. As per the inclusion criteria, healthy controls each had an AHI of less than 5. In threestudies patients were diagnosed with OSA if AHI ≥ 5 (13,16,17) and in two studies patients were diagnosed with OSA if AHI ≥ 10 (15,18), and in two studies, patients were diagnosed with OSA if AHI ≥ 20 (9,14). The median AHI score within the OSA group was 50 and the range was 22.3 to 65.7. According to the American Academy of Sleep Medicine Criteria (AASM), six studies had patients whose mean AHI scoreswerein the severe range of OSA and one study had patients whose mean AHI scoreswerein the moderate range (12).
Table 1.
The 7 included studies of cortisol in patients with OSA versus controls
| Study | Female-male, n | BMI, mean ± SE | Age, mean ± SE | AHI, mean ± SE | Timing of samples | Comparison Method and P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal Weight Control | Obese Control | OSA | Normal Weight Control | Obese Control | OSA | Normal Weight Control | Obese Control | OSA | OSA | |||
| Barcelo etal.13 | 0–23 | --- | w EDS 0–22 |
25 ± 0.6 | ---- | 32 ± 0.6 | 48 ± 1.2 | --- | 49 ± 1.3 50 ± 1.1 |
52 ± 4.1 | 0800–1000 | Comparison of mean value w EDS p= .45 |
| wo EDS 0–22 |
31 ± 0.9 | 48 ± 3.4 | wo EDS p=.38 |
|||||||||
| Carneiro etal.15 | --- | 0–13 | 0–16 | --- | 42.8 ± 1.3 | 46.9 ± 2.0 | --- | 38.8 ± 3.3 | 40.1 ± 2.8 | 65.7 ± 9.9 | 0800 2300 |
Comparison of mean values 0800 p = .72 |
| 2300 p= .39 |
||||||||||||
| Dadoun etal.14 | 0–10 | 0–8 | 0–9 | 22.4 ± 0.7 | 38.4 ± 2.1 | 38.0 ± 1.5 | 35.3 ± 2.3 | 33.0 ± 5.1 | 42.0 ± 4.3 | 44.8 ± 6.4 | Every 30 minutes 1200-1200 | Comparison of 24-hour mean p > .05 |
| Lam et al. 16 | 0–25 | --- | 0–69 | 24.6 ± 0.5 | --- | 27.9 ± 0.5 | 39.6 ± 1.7 | --- | 45.2 ± 1.1 | 36.8 ± 2.9 | Morning | Comparison of mean value p > .05 |
| Lanfranc o et al. 17 | 0–15 | 0–15 | 0–15 | 21.2 ± 0.8 | 41.2 ± 2.0 | 39.2 ± 3.1 | 38.2 ± 1.4* | 39.7 ± 1.2 | 43.5 ± 1.6 | 53.4 ± 8.7 | Morning | Comparison of mean value p > .05 |
| Panaree etal. | 0–24 | --- | 15–39 | 21.5 ± 0.5 | --- | 28.5 ± 0.8 | 32.3 ± 2.4 | --- | 47.2 ± 1.6 | 22.3 ± 4.6 | 0800 | Comparison of mean value p > .05 |
| Vgontzas et al. | 0–16 | 0–13 | 0–16 | 26.0 ± 0.7 | 36.2 ± 2.2 | 38.4 ± 1.5 | 40.9 ± 3.2 | 45.6 ± 2.3 | 48.1 ± 1.4 | 53.3 ± 7.0 | Every 30 minutes for 24 hours | Comparison of obese OSA and obese controls on 8-hour mean sleeping value p < .05 |
Na = no information; OSA = Obstructive Sleep Apnea; BMI = Body mass index; AHI = Apnea hypopnea index; EDS = Excessive daytime sleepiness; w = with; wo = without.
The median number of participants in the studies was 45 (range 27 to 94). Samples were overwhelmingly male with only one investigation reporting women in the OSA group(18). There was considerable variability in approaches to matching patients and controls. OSA patients and controls were matched for age in four studies (9,14,15,17). One study compared OSA patients with and without excessive daytime sleepiness (EDS); the group without EDS (but not those with EDS) was significantly older than the control group (13). In the remaining two studies, subjects in the control group were significantly younger than subjects in the OSA group (16,18). Four studies utilized a control group with a similar BMI to the OSAgroup;in three of these studies a normal weight control group was also employed (9,14,15,17). In the remaining studies, participants with OSA had significantly higher BMIs than participants in the control group (13,16,18).
Six studies assessed cortisol using serum from samples obtained through venous blood (9,13,14,16–18) and one assessed cortisol via saliva (15). Of those studies using venous blood, four used a single measurement taken in the morning; however the description of the sample timing differed across studies. One study specified that samples were obtained at 0800 (18), one specified samples were obtained between 0800 and 1000 (13) and two simply noted that the samples were obtained in the `morning' (16,17). Two studies utilizing venous blood draws used more extensive sampling, obtaining samples every 30 minutes over a 24 hour period (9,14). One study assessed cortisol via saliva obtained by the participants chewing a cotton swab (Salivette)at two time points (0800 and 2300)(15).
Pre and Post CPAP Intervention
Eight studies were identified that assessed cortisol levels in OSA patients before and after CPAP treatment;data are presented in Table 2 (9,10,13,15,19–22). As per the inclusion criteria, AHI was ≥ 5 in OSA patients in each of the studies. According to the AASM Criteria, the mean AHI score of each sample was in the severe range (12). The median number of patients in the studies was 13 (range 9 to 52). Consistent with studies comparing OSA patients to controls, samples were overwhelmingly male, with only one study reporting three women in their sample (22). Of the five studies that reported CPAP compliance, the average nightly use was above 4 hours per night.
Table 2.
The 8 included studies of cortisol in patients with OSA treated with CPAP
| Study | Female-male, n | Age, mean ± SE | BMI, mean ± SE | AHI, mean ± SE | Treatment Length | CPAP Compliance (Hours) mean ± SE | Timing of samples | Comparison method & p value | |
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | ||||||||
| Barcelo et al. 13 | wEDS 0–20 |
49±1.3 | 32±.7 | 52±4.2 | na | 3 months | 5.5±.3 5.7±.3 |
0800–1000 | Comparison of mean value |
| woEDS 015 |
50±1.3 | 31±1.0 | 48±4.1 | p > .05 | |||||
| Carneiro etal. 15 | 0–9 | na | 44.3±2.4 | 92±7.6 | 21.1±9.7 | 3 months | na | 0800 | Comparison of mean value p= .86 |
| Chin et al.19 | 0–15 | 52.2±3.3 | 29.3±1.1 | 52.2±3.3 | 2.9±0.8 | 3–4 days | na | 0815 | Comparison of mean value p > .05 |
| Follenius etal.20 | 0–7 | 40±1 | 35.8±2.9 | 91±9 ‡ | na | 1 night | na | Every 10 minutes 2200-0630 | Comparison of mean values p > .05 |
| Henley et al. 10 | 0–10 | 51.4±3.0 | 36.8±1.7 | 60.2±7.9 | 4.2±0.6 | 3-months | 5.3±0.3 | Every 10 minutes 1900-1900 | Comparison of mean value of total cortisol p < .001 |
| Meston et al.21 | 0–52 | 57±2.8 | 32±1.4 | Na | Na | 1 month | 4.6±0.3 | “midmorning” | Comparison of mean value p = 0.40 |
| Schmoller etal.22 | 3–47 | 55.1±1.6 | 35.4±0.9 | 59.6±2.0 | 5.8±1.4 | 3 months | 5.9±0.4 | 0600, 0700, 0800, 1130, 1230,2230 | Repeated measures ANOVA p < .05 |
| Comparison of 1130 and 2230 time point p < .05 | |||||||||
| Vgontzas et al.9 | 0–16 | 46.6±2.8 | 38.4±1.5 | 53.3±7.0 | 4.5±1.9 | 3 months | 4.6±0.4 | Every 30 minutes for 24 hours. | Comparison of mean value of nighttime cortisol p= .10 |
Apnea Index: Based on the severity of the index in this sample, the study was included.
Na = no information; OSA = Obstructive Sleep Apnea; BMI = Body mass index AHI = Apnea hypopnea index; EDS = Excessive daytime sleepiness
Six studies assessed cortisol using serum from samples obtained through venous blood (9,10,13,19–21) and two assessed cortisol via saliva (Salivette)(15,22). Of those studies using venous blood, three used a single measurement taken in the morning, one study specified that samples were obtained at 0815 (19), one specified samples were obtained between 0800 and 1000 (13) and one simply noted that the samples were obtained in the “midmorning” (21). Two studies utilizing blood draws employedmore extensive sampling, obtaining samples every 10 minutes over an 8.5 (20) and 24 hour period (10)(10,10,20) and every 30 minutes over a 24 hour period (9). Of the studies that assessed cortisol via saliva, one study obtained a single morning measurement (15) and one assessed cortisol at six time points throughout the day (22).
All Studies
Seven studies recruited both OSA patients and controls from clinical populations, primarily sleep disorder clinics(10,14–17,21,22). In one study, patients with OSA and obese controls were recruited from a clinical population, while normal weight controls were recruited from staff members (17). One study noted that OSA and control subjects were recruited both from a clinical population and through advertisement within the community (9). One study recruited OSA and control subjects “randomly”, population unspecified (18) and three studies did not note how patients and or controls were recruited (13,19,20,23). Authors reporting on the storage of saliva samples gave information on saliva stored in a freezer at temperatures ranging from −20 to −80 degrees Celsius until processing. Diverse assay approaches were used to analyze cortisol.
Exclusionary criteria
Studies were inconsistent in whether or not they excluded participants with clinical depression or who smoked.
Smoking
Two studies noted smoking as exclusion criterion(10,15) and one reported that smoking was an exclusionary criterionin their control group, but that over 40% of the OSA group smoked(13). The remaining studies did not exclude potential participants for smoking, nor was data reported about participant smoking status.
Depression
One study excluded patients with depression (10) and another specified that patients with severe depression (15) were excluded. Three studies excluded patients with any major psychiatric disorder (13,18,22), and one excluded patients with major and minor psychiatric disorders (9); the remaining investigations did not consider depression in their exclusion criteria.
Qualitative Data Synthesis and Analyses
OSA versus controls
Amongthe four studies that assessed cortisol througha single morning venous blood draw sample, none reported significant differences between the OSA and control groups (13,16–18). Differences were also not detected in the study that assessed salivary cortisol via a morning and evening sample (15).
Mean 24 hour cortisol levels (sampling every 30 minutes)obtained through venous blood draw were reported in two studies. One investigationfound no differences between controls (obese and normal weight) and OSA patients on measures of 24-h mean cortisol. Differences were also not detected in regards to morning maximum, trough-period mean, or peak –period mean of plasma cortisol(14). In contrast, differences in cortisol between obstructive sleep apneics and controls were found in the second study, which reported that OSA patients had higher cortisol levels than obese controls during sleep(9).
Pre and Post CPAP Intervention
Two studies assessed single morning measurements of cortisol after brief (1–4 days) CPAP intervention; neither found cortisol differences pre-post treatment (19,20). Similarly, of the studies that investigated cortisolusing a single, morning measurement no differences were detected after 1-month (21)or 3-months of CPAP therapy (13,15).
Schmoller et al., (2009) assessed salivary cortisol at 6 time points throughout the day and detected a significant effect of 3-months of CPAP therapy on diurnal cortisol profiles. Individual tests of specific time points pre-post CPAP showed that post-CPAP, patients evidenced a decrease in evening cortisol levels and an increase in cortisol at the time point before lunch(22).
Two studies obtained multiple measure of cortisol via venous blood draw over a 24-hour period pre and post treatment with 3-months of CPAP. Henley et al. (2009) sampled blood at 10-minute intervals over 24-hours,findings from their investigationfound that cortisol levels were globally reduced after 3-months of CPAP therapy. Vgontzas et al. (2007) sampled blood at 30-minute intervals over 24-hours and also found a trend towards global reductionin 24-hour cortisol levels after 3-months of CPAP treatment.
Discussion
The present review summarizesdata from case-controlled studies comparing cortisol levels in OSA and controlsubjects and studies comparing cortisol levels in OSA patients before and after CPAP treatment. Of the studies reviewed, the majority assessed cortisol at only one or two time points. None of these investigations found differences in cortisol between obstructive sleep apneics and control subjects. Similarly, of treatment studies that assessed cortisol at only one time point, all failed to find differences in cortisol before and after CPAP treatment.
Two studies compared OSA subjects and controls using multiple measures of cortisol;one study found no differences in cortisol levels, while one found that OSA subjects had higher cortisol levels at night (9,14). Differences pre-post CPAP emerged in two studies that assessed cortisol at multiple time points(10,22)and one study reported a trend towards cortisol reduction after successful treatment with CPAP(9). Additionally, Henley et al. (2009) reported that untreated OSA was associated with a longer duration of cortisol secretory pulses which was corrected after CPAP treatment, resulting in the observed reductions of overall cortisol (10).
Cortisol Sampling Protocols
Cortisol is a hormone that exhibits a clear circadian rhythm. Sleep is associated with a decrease in cortisol with the nadir occurring at approximately 12pm. Cortisol levels remain low for several hours in the night and begin to rise approximately 2–3 hours after sleep onset. The peak, or acrophase in cortisol occurs at approximately 9am followed by a decrease in cortisol throughout the day(24). Hypoxia and nocturnal awakenings have been linked to transient increases in cortisol (4). Given that apneas and hypopneas only occur during sleeping hours in OSA subjects, assessment of the circadian rhythm, including nocturnal measurements,of cortisol is imperative when investigating the potential relationship between OSA and cortisol (1). The innate variation within the diurnal cycle of cortisol is of a magnitude that may obscure many, smaller, but still meaningful differences if sample timing is not tightly controlled. Measuring cortisol at undefined time points (i.e., “morning”) increases measurement variability and is a constraint limiting the information gathered from many investigations of the HPA axis(5). Notably, none of the studies using a single morning measure of cortisol identified significant group differences or pre-post CPAP changes.
Subject Matching
Age is associated with alterations in cortisol profiles (25,26) and cortisol response to challenge (27); yet, of the case controlled studies identified, OSA patients and controls were matched for age inonly four of seven studies. Additionally, obesity has been associated with alterations in cortisol(28,29); however several studies failed to match their control and OSA groups on measures of obesity. Matching OSA patients and controls on variables with known associations to cortisol, or at a minimum, controlling for group differences in these variables, is an important step for future investigations.
Exclusion Criteria
Investigations evidenced significant variability regarding control for variables known to be associated with the HPA axis. Smoking is associated with increases in cortisol levels and smoking cessation is associated with hypocortisolism(30,31). Despite associations between smoking and cortisol, few studies considered smoking or recent cessation as exclusionary criteria. Additionally, depression, a variable prevalent in OSA (32), andimplicated in dysregulation of the HPA axis (33,34)was considered an exclusionary criterion in less than half of the investigations.
Of note, many medications impact the HPA axis. Our review of the literature suggested that criteria for medication exclusion differed across studies with little consensus among exclusionary criteria. We recognize that there is always a dilemma in clinical research between studying samples free from comorbiditiesand medications versus those more representative of patients observed in clinical practice. We would recommend that in studies trying to establish relationships between OSA and cortisol that medications known to influence the HPA axis should serve as exclusionary criteria.
Future Directions
This paper reviewed existing literature on cortisol levels as they relate to OSA. There are other ways of characterizing HPA axis activation, such challenge tests (9,15,17) and they may well be fruitful topics for future research. For example, individuals with OSA evidence decreased cortisol suppression after low-dose dexamethasone, which is corrected after treatment with CPAP (15). Additionally, OSA patients also show hyperresponsiveness of adrenocorticotropic hormone (ACTH) in response to stimulation with corticotrophin releasing hormone (CRH) (17). However, at this point, there is limited work published probing HPA physiology as it relates to OSA other than by characterizing cortisol levels itself.
Further, because the effect of OSA on cortisol is relatively small, studies that use a dichotomization of apneicversusnonapneic may be inadvertently limiting their power. Indeed, a recent paper by Edwards et al., (2011) reported no cortisol differences between those classified as apneics vs. nonapneics, but did observe a significant correlation between cortisol levels and oxygen desaturation index measured continuously(35).
Finally, theseverity of apnea in patient groups varied substantially across studies and it strikes us that apnea severity may influencefindings. For example,studies whose OSA patients suffer from severe apnea may draw different conclusions than those compared of OSA patients suffering from mild or moderate apnea. Whether apnea severity is associated with cortisol secretion may be an important question in future research.
Conclusions
Given the heterogeneity of subject compositionacross studies, inconsistent sampling times and techniques and variable exclusion criteria, it is difficult to draw definitive conclusions about the relationship between OSA and cortisol levels. If there is are alterations in cortisol concentrations or rhythms associated with OSA, they are small enough that one has to be precise regarding the timing of blood draws and accounting for confounds such as obesity, age, smoking and depression.
Acknowledgements
This work was supported, in part, by Grants HL36005, HL44915 and HL091848. We would like to thank Dr. Linda Gallo for her thoughtful comments on the initial draft of this manuscript. We also thank the anonymous reviewers for their encouraging comments and recommendations.
Abbreviations
- OSA
obstructive sleep apnea
- HPA
hypothalamic pituitary adrenal
- CPAP
continuous positive airway pressure
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- * (1).Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Shamsuzzaman ASM, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- (3).Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- (4).Späth-Schwalbe E, Gofferje M, Kern W, Born J, Fehm HL. Sleep disruption alters nocturnal ACTH and cortisol secretory patterns. Biol Psychiatry. 1991;29:575–584. doi: 10.1016/0006-3223(91)90093-2. [DOI] [PubMed] [Google Scholar]
- *(5).Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocr & Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- (6).Lanfranco F, Motta G, Minetto M, Baldi M, Bablo M, Ghigo E, et al. Neuroendocrine Alterations in Obese Patients with Sleep Apnea Syndrome. Int J Endocrin. 2010;2010:1–11. doi: 10.1155/2010/474518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Nakamura T, Chin K, Shimizu K, Kita H, Mishima M, Nakamura T, et al. Acute effect of nasal continuous positive airway pressure therapy on the systemic immunity of patients with obstructive sleep apnea syndrome. Sleep. 2001;24:545–553. doi: 10.1093/sleep/24.5.545. [DOI] [PubMed] [Google Scholar]
- (8).Grunstein RR, Stewart DA, Lloyd H, Akinci M, Cheng N, Sullivan CE. Acute withdrawal of nasal CPAP in obstructive sleep apnea does not cause a rise in stress hormones. Sleep. 1996;19:774–782. doi: 10.1093/sleep/19.10.774. [DOI] [PubMed] [Google Scholar]
- * (9).Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bentley CM, Bixler EO, et al. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2007;92:4199–4207. doi: 10.1210/jc.2007-0774. [DOI] [PubMed] [Google Scholar]
- * (10).Henley DE, Russell GM, Douthwaite JA, Wood SA, Buchanan F, Gibson R, et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab. 2009;94:4234–4242. doi: 10.1210/jc.2009-1174. [DOI] [PubMed] [Google Scholar]
- (11).Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- (12).Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- (13).Barceló A, Barbé F, De la Pena M, Martinez P, Soriano JB, Piérola J, et al. Insulin resistance and daytime sleepiness in patients with sleep apnoea. Thorax. 2008;63:946–950. doi: 10.1136/thx.2007.093740. [DOI] [PubMed] [Google Scholar]
- * (14).Dadoun F, Darmon P, Achard V, Boullu-Ciocca S, Philip-Joet F, Alessi MC, et al. Effect of sleep apnea syndrome on the circadian profile of cortisol in obese men. Am J Physiol Endocrinol Metab. 2007;293:E466–E474. doi: 10.1152/ajpendo.00126.2007. [DOI] [PubMed] [Google Scholar]
- (15).Carneiro G, Togeiro SM, Hayashi LF, Ribeiro-Filho FF, Ribeiro AB, Tufik S, et al. Effect of continuous positive airway pressure therapy on hypothalamic-pituitary-adrenal axis function and 24-h blood pressure profile in obese men with obstructive sleep apnea syndrome. Am J Physiol - Endoc M. 2008;295:E380–E384. doi: 10.1152/ajpendo.00780.2007. [DOI] [PubMed] [Google Scholar]
- (16).Lam JCM, Yan CSW, Lai AYK, Tam S, Fong DYT, Lam B, et al. Determinants of Daytime Blood Pressure in Relation to Obstructive Sleep Apnea in Men. Lung. 2009;187:291–298. doi: 10.1007/s00408-009-9161-7. [DOI] [PubMed] [Google Scholar]
- (17).Lanfranco F, Gianotti L, Pivetti S, Navone F, Rossetto R, Tassone F, et al. Obese patients with obstructive sleep apnoea syndrome show a peculiar alteration of the corticotroph but not of the thyrotroph and lactotroph function. Clin Endocrinol. 2004;60:41–48. doi: 10.1111/j.1365-2265.2004.01938.x. [DOI] [PubMed] [Google Scholar]
- (18).Panaree B, Chantana M, Wasana S, Chairat N. Effects of obstructive sleep apnea on serum brain-derived neurotrophic factor protein, cortisol, and lipid levels. Sleep Breath. doi: 10.1007/s11325-010-0415-7. in press. [DOI] [PubMed] [Google Scholar]
- (19).Chin K, Shimizu K, Nakamura T, Narai N, Masuzaki H, Ogawa Y, et al. Changes in intra-abdominal visceral fat and serum leptin levels in patients with obstructive sleep apnea syndrome following nasal continuous positive airway pressure therapy. Circulation. 1999;100:706–712. doi: 10.1161/01.cir.100.7.706. [DOI] [PubMed] [Google Scholar]
- (20).Follenius M, Krieger J, Krauth MO, Sforza F, Brandenberger G. Obstructive sleep apnea treatment: peripheral and central effects on plasma renin activity and aldosterone. Sleep. 1991;14:211–217. [PubMed] [Google Scholar]
- (21).Meston N, Davies RJO, Mullins R, Jenkinson C, Wass JAH, Stradling JR. Endocrine effects of nasal continuous positive airway pressure in male patients with obstructive sleep apnoea. J Intern Med. 2003;254:447–454. doi: 10.1046/j.1365-2796.2003.01212.x. [DOI] [PubMed] [Google Scholar]
- * (22).Schmoller A, Eberhardt F, Jauch-Chara K, Schweiger U, Zabel P, Peters A, et al. Continuous positive airway pressure therapy decreases evening cortisol concentrations in patients with severe obstructive sleep apnea. Metab. 2009;58:848–853. doi: 10.1016/j.metabol.2009.02.014. [DOI] [PubMed] [Google Scholar]
- (23).Chin K, Ohi M, Kita H, Noguchi T, Otsuka N, Tsuboi T, et al. Effects of NCPAP therapy on fibrinogen levels in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;153:1972–1976. doi: 10.1164/ajrccm.153.6.8665063. [DOI] [PubMed] [Google Scholar]
- (24).Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, Von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- (25).Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–868. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- (26).Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- (27).Otte C, Hart S, Neylan TC, Marmar CR, Yaffe K, Mohr DC. A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- (28).Travison TG, O'Donnell AB, Araujo AB, Matsumoto AM, McKinlay JB. Cortisol levels and measures of body composition in middle-aged and older men. Clin Endocrinol. 2007;67:71–77. doi: 10.1111/j.1365-2265.2007.02837.x. [DOI] [PubMed] [Google Scholar]
- (29).Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II Study. J Clin Endocrinol Metab. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Steptoe A, Ussher M. Smoking, cortisol and nicotine. In J Psychophysiol. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- (31).Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J Clin Endocrinol Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- (32).Harris M, Glozier N, Ratnavadivel R, Grunstein RR. Obstructive sleep apnea and depression. Med Rev. 2009;13:437–444. doi: 10.1016/j.smrv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- (33).Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- (34).Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Edwards KM, Tomfohr LM, Mills PJ, Bosch JA, Ancoli-Israel S, Loredo JS, et al. Macrophage migratory inhibitory factor (MIF) may be a key factor in inflammation in obstructive sleep apnea. Sleep. 2011;34:161–163. doi: 10.1093/sleep/34.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]