Abstract
Background
Negative affect is an important predictor of smoking behavior, and many smokers believe that smoking reduces negative affect. However, it is unclear whether such beliefs, known as negative reinforcement smoking outcome expectancies (NRSOE), are associated with changes in negative affect in response to nicotine deprivation and administration.
Methods
Smokers (N = 114) participated in 4 sessions that balanced overnight smoking deprivation (12-hr deprived vs. ad lib) and nasal spray administration (nicotine vs. placebo). Corrugator supercilii (COR) EMG, skin conductance (SCR), and in-session ratings were collected while the participants viewed affective, cigarette-related, and neutral slides. Retrospective questionnaire data were collected prior to slide viewing. NRSOE were determined using the Smoking Consequences Questionnaire-Adult Nicotine Affect Reduction scale (SCQ-NAR).
Results
High scores on the SCQ-NAR were associated with smaller COR EMG to unpleasant slides following nicotine nasal spray administration compared to placebo spray, regardless of overnight deprivation. Smokers who had high scores on the SCQ-NAR had smaller SCR, following nicotine nasal spray administration compared to placebo spray, but only after overnight deprivation. The in-session ratings and retrospective questionnaire measures indicated that smokers who had high scores on the SCQ-NAR experienced greater negative affect and craving, and less positive affect, than smokers with low scores on the SCQ-NAR, regardless of nicotine exposure.
Conclusions
Our questionnaire results suggest that while smokers who have high NRSOE self-report greater overall levels of negative affect and craving, while the psychophysiological data suggest that such smokers may experience negative affect reduction when blindly administered a dose of nicotine.
Keywords: smoking, expectancy, negative affect, reinforcement, corrugator EMG, skin conductance
1. Introduction
All smokers have smoking outcome expectancies involving beliefs that smoking will bring about desired or undesired personal consequences, such as beliefs that smoking will improve one’s mood, make one more sociable, control weight, or harm one’s health (Brandon et al., 1999). Such expectancies are thought to result from associative learning (Colwill and Rescorla, 1986). However, far from being a harmless epiphenomenon of dependence, expectancies can influence drug seeking behavior, because outcome desirability influences the probability of engaging in behavior associated with the outcome (Bandura, 1977). Of particular importance are expectancies that nicotine will produce desirable emotional consequences, particularly negative affect reduction, given that negative affect has been found to be a significant contributor to relapse following smoking cessation attempts (Kenford et al., 1994; Shiffman et al., 1996).
Negative reinforcement smoking outcome expectancies (NRSOE) have been found to be important predictors of smoking withdrawal severity (Tate et al., 1994) and cessation relapse (Brandon et al., 1999). For example, Wetter et al. (1994) found that the negative reinforcement scale of the Smoking Consequences Questionnaire (SCQ) was positively associated with smoking withdrawal severity and negatively associated with cessation outcome success. Thus, NRSOE may be a marker of vulnerability: smokers who report greater expectation that smoking will alleviate negative affect may be more vulnerable to experiencing negative affect in response to deprivation and thus be more likely to relapse.
NRSOE are also likely to influence both the subjective response to the drug (i.e., the pharmacological effect) and the response to a perceived drug (i.e., a placebo effect). For example, Juliano and Brandon (2002) examined the impact of drug (nicotine vs. placebo cigarette) and stimulus expectancy (told nicotine vs. told placebo cigarette) on anxiety reduction. Overall, nicotine cigarettes reduced anxiety compared to placebo cigarettes, but participants scoring high on the SCQ negative reinforcement scale reported reduced anxiety when told they received nicotine, regardless of the actual nicotine content of the cigarette, compared to participants low on this scale. Thus, the subjective effects of nicotine are likely moderated, at least in-part, by NRSOE.
Despite evidence linking NRSOE with withdrawal, relapse, and acute drug response, some issues remain to be addressed. First, are the effects of NRSOE on smoking behavior due to self-fulfilling prophecy (Kirsch and Lynn, 1999) or do these expectancies reflect cognizance of an actual drug effect on negative affect reduction? Using less subjective measures of affective response, such as psychophysiology, in conjunction with blindly administered nicotine and placebo might address this issue because response to drug would less likely be cognitively mediated than self-report ratings following unblinded drug administration. Second, is NRSOE an independent construct or simply a proxy for other predictors of smoking behavior, such as smoking dependence or gender? Measuring and controlling for such factors would allow for the determination of whether NRSOE independently predict affective response to nicotine deprivation and administration.
The aim of this study was to determine whether NRSOE were associated with psychophysiological (corrugator supercilii electromyography and skin conductance) and self-report measures of affect following within-subjects acute nicotine deprivation (12-hr overnight deprived vs. ad lib smoking) and administration (nicotine vs. placebo nasal spray) manipulations. We hypothesized that smokers holding high levels of NRSOE, as measured by the Smoking Consequences Questionnaire-Adult (Copeland et al., 1995) Nicotine Affect Reduction scale (SCQ-NAR), would show greater negative affect in response to overnight nicotine deprivation and to placebo administration, compared to ad lib smoking and to nicotine nasal spray, respectively. We also hypothesized that low SCQ-NAR smokers' levels of negative affect would not differ by these nicotine dose manipulations. The secondary aim of this study was to determine whether NRSOE were associated with affect following acute nicotine deprivation and administration independently of baseline demographics and smoking behavior possibly linked with affective response to smoking. We hypothesized that associations between SCQ-NAR and negative affect in response to acute nicotine deprivation and administration would be maintained while covarying smokers' baseline smoking (cigs/day), expired carbon monoxide, FTND scores, gender, and age. To our knowledge, this is the first study to examine the association between baseline NRSOE and negative affect experienced following nicotine deprivation and administration manipulations using psychophysiological measures of affect.
2. Method
2.1. Participants
One hundred thirty-nine cigarette smokers were recruited from the Houston metropolitan area and were paid $125 for attending one screening and four laboratory sessions. Participants were between the ages of 18 and 59, smoked 10 or more cigarettes per day, produced an expired carbon monoxide level greater than 8 ppm (or produced saliva cotinine >30 ng/ml), were fluent in English, had no uncontrolled medical illness, were not taking psychotropic or narcotic medication, did not meet criteria for a current psychiatric disorder as measured by the PRIME-MD (Spitzer et al., 1994), did not report hearing loss, and were not involved in current smoking cessation activity. Twenty-five participants met inclusionary criteria but were completely excluded from analysis due to experimental noncompliance, technical failure, or completing only a single lab session, leaving 114 included in the analyses. Participants provided informed consent and the protocol was approved by the University of Texas MD Anderson Cancer Center’s Institutional Review Board.
2.2. Procedures
2.2.1. Phone Screening and Orientation Session
Potential participants were initially screened by telephone to establish their eligibility for the study. Eligible participants attended an orientation session and completed baseline questionnaires, including the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991), the Center for Epidemiologic Studies’ Depression Scale (CES-D; Radloff, 1977), and the Smoking Consequences Questionnaire - Adult (SCQ-A; Copeland et al., 1995). The FTND measures nicotine dependence by assessing various components of smoking behavior such as daily intake, difficulty in refraining from smoking, and time to first cigarette. The CES-D assesses depressive symptoms in community (nonclinical) populations. The SCQ-A is a measure of smoking expectancies that includes 10 scales: Negative Affect Reduction, Stimulation/State Enhancement, Health Risks, Taste/Sensorimotor Manipulation, Social Facilitation, Weight Control, Craving/Addiction, Negative Physical Feelings, Boredom Reduction, and Negative Social Impression.
2.2.2. Laboratory Sessions
Participants attended four laboratory sessions, approximately one week apart, following the orientation visit. The laboratory sessions counterbalanced overnight deprivation (ad lib smoking vs. 12 hr. deprived) with nasal spray administration (nicotine vs. placebo spray). At each laboratory session, participants completed smoking/deprivation assessment, retrospective questionnaires, and the slide viewing task during which psychophysiology and self-report ratings were collected.
2.2.2.1. Smoking assessment
Smoking during the last 12 hrs was assessed with a questionnaire and confirmed using expired CO (Vitalograph Breath CO monitor model 29.700; Vitalograph Inc., Lenexa, KS). Nonabstinent participants in the deprived condition were rescheduled. Smoking was unrestricted before the nondeprived sessions, and participants in the non-deprived condition smoked one cigarette preceding the retrospective questionnaires and slide viewing task during these sessions to ensure similar conditions of nondeprivation.
2.2.2.2. Retrospective Questionnaires
Participants completed the Positive and Negative Affect Scale (PANAS; Watson et al., 1988) and the Wisconsin Smoking Withdrawal Scale (WSWS; Welsch et al., 1999) at each laboratory session following the smoking assessment (and cigarette smoking, if a nondeprived session). The PANAS measures positive and negative affect over the past week. The WSWS is a self-report measure of nicotine withdrawal that consists of 7 subscales: Anger, Anxiety, Concentration, Craving, Hunger, Sadness, and Sleep.
2.2.2.3. Psychophysiological measurement
After the retrospective questionnaires were completed, our laboratory staff applied sensors to the face and hands of the participants that were used to record responses to the slide viewing task. Corrugator supercilii electromyography (COR EMG), a facial muscle located above the eye that activates during negative affect states (Lang et al., 1993; Witvliet and Vrana, 1995), was measured using Ag-AgCl electrodes filled with saline gel that were attached to the right side of the face in a bipolar configuration (Fridlund and Cacioppo, 1986). Skin conductance response (SCR) amplitude, a measure of sympathetic nervous system activation that is associated with emotional arousal (Lang et al., 1998), was collected by placing an electrodermal response transducer on the fore and ring fingers of the participant’s nondominant hand. The psychophysiological data were recorded and displayed by a BIOPAC Systems’ MP100WSW bioamplifier and AcqKnowledge III data acquisition software (version 3.5.3; Goleta, CA) sampling at 1000 Hz. Further information about the psychophysiological recording and data reduction procedures can be found elsewhere (Cinciripini et al., 2006; Robinson et al., 2007a). Following sensor attachment, participants sat quietly for 5 minutes to allow for initial habituation to the sensors.
2.2.2.4. Slide viewing task
The slide viewing task was divided into two consecutive blocks, the habituation and test blocks. The initial habituation block always followed placebo nasal spray and is not included in these analyses because it was designed to habituate the participants to the nasal spray. The subsequent test block followed either nicotine or placebo spray, and is the subject of these analyses. During each block, participants viewed a pseudo-random series of 24 slides, each for 6 s, followed by a randomly determined inter-slide interval that varied from 10 to 20 s. The slides were comprised of affective (pleasant, unpleasant), neutral, and cigarette-related content. The pleasant, unpleasant, and neutral slides were selected from the International Affective Picture System (IAPS; Center for the Study of Emotion and Attention, 1999) and the cigarette-related slides were compiled by our laboratory and are validated elsewhere (Carter et al., 2006). Acoustic startle probes were presented during 16 of 24 slides in each block. Slides containing startle probes resulted in larger COR EMG compared to slides without probes, but the presence of startle probes had no effect on SCR. When we covaried for the presence or absence of startle probes during the slides, the pattern of results of our physiological or in-session ratings analyses were not altered, so all slides were included in the analyses below. The startle results are detailed, along with further information about the slide selection, counterbalancing, and presentation, in a previous manuscript (Cinciripini et al., 2006).
2.2.2.5. In-session ratings
During the slide viewing task, the participants completed three mood and craving ratings after every third slide based on how they felt "at that moment." The ratings, which used a five-point scale, asked about the participant's positive mood state, negative mood state and craving to smoke. Each of the slide categories was followed by the in-session ratings an equal number of times, twice per block. More specific information about the in-session ratings items are reported elsewhere (Robinson et al., 2007b).
2.2.2.6. Nasal spray administration
Prior to the test block of the slide viewing task, participants blindly received either identically packaged nicotine (Nicotrol NS) or placebo nasal spray (Pharmacia and Upjohn, Inc., Peapack, NJ). However, prior to the initial habituation block, participants always received placebo nasal spray to become accustomed to its aversiveness (the active ingredient of the placebo was piperine). Because peak arterial levels of nicotine, about 10 ng/ml, occur within five minutes of a single application of the nicotine nasal spray (0.5 mg/nostril; Gourlay and Benowitz, 1997), participants sat quietly for five minutes after receiving the nasal spray before commencing the slide viewing task.
2.3. Data Reduction and Analysis
Participants were divided into high and low NRSOE groups by median split on the SCQ-NAR. For each of the in-session ratings, grand means were calculated by averaging the two ratings following each slide category for each block of each laboratory session. The psychophysiology data were scored as means over the 6 s following the onset of each slide, except for SCR, which was calculated from 2–7 s after slide onset due to the latency of the sweating response (Stern et al., 2001). Grand means were then calculated from the six slides comprising each slide category for each block of each laboratory session. Finally, z-scores were calculated across all sessions for each participant to facilitate comparison of the within-subjects nicotine deprivation by nasal spray manipulations while minimizing the large between-subjects variability in the means and in dispersion from the means typically found in physiology.
Our first hypothesis was addressed by separately modeling (a) SCQ-NAR × Deprivation Status × Nasal Spray interactions for COR EMG and SCR during unpleasant slides, (b) SCQ-NAR × Deprivation Status × Nasal Spray × Preceding Slide Type interactions for in-session ratings, and (c) SCQ-NAR × Deprivation Status interactions for retrospective questionnaires. This allowed us to examine whether the impact of deprivation status and/or nasal spray on multiple modalities of negative affect varied by SCQ-NAR group. We addressed our second hypothesis by covarying baseline smoking (cigs/day), expired carbon monoxide, FTND scores, gender, and age in the above models. If the interaction models involving SCQ-NAR maintained significance with these added covariates, then we could conclude that SCQ-NAR is associated with the observed negative affect changes above and beyond the effects of the covariates. These interaction models were assessed using mixed models analysis (SAS 9.2 Proc Mixed; SAS Institute, Carey, NC), with subject modeled as a random effect. All descriptions of differences between means following a significant mixed model effect were the result of contrast comparisons of least-square means (LSM) and standard errors (SE) of fixed effects using tests of simple effects. To correct for the effects of multiple comparisons on type I error rate, the family-wise α levels of post hoc contrasts were adjusted using the Holm-Bonferroni correction (Seaman et al., 1991). The comparisons of demographics and smoking behavior by SCQ-NAR scale median split and analyzed using t-tests and chi-square analyses.
3. Results
3.1. Demographics and Smoking Behavior Differences by SCQ-NAR
Demographic and smoking behavior for the sample are presented, by SCQ-NAR scale median split, in Table 1. In terms of demographics, participants differed by SCQ-NAR group on gender and age, such that those in the high SCQ-NAR group were more likely to be female and younger than those in the low SCQ-NAR group. Baseline smoking behavior did not differ by SCQ-NAR group. The high SCQ-NAR group had a significantly larger SCQ-NAR scores (M = 7.66, SD = 0.78) than the low SCQ-NAR group (M = 4.60, SD = 1.32), t(93.35) = 15.11, p < .0001 (Satterthwaite's approximation for degrees of freedom was used because variance was unequal).
Table.
Participant demographic and baseline smoking data, by SCQ-NAR group
| Low SCQ-NAR (n=58) |
High SCQ-NAR (n=56) |
Total (n=114) |
||
|---|---|---|---|---|
| Categorical Measure | N (%) | N (%) | N (%) | Chi-square |
| Gender, Female | 17 (29.3) | 41 (70.7)* | 58 (50.9) | 12.65 *** |
| Ethnicity/Race | 2.21 | |||
| African-American1 | 31 (53.5) | 25 (44.6) | 56 (49.1) | |
| Euro-American1 | 24 (41.4) | 24 (42.9) | 48 (42.1) | |
| Other2 | 3 (5.2) | 7 (12.5) | 10 (9.8) | |
| Continuous Measure | M (SD) | M (SD) | M (SD) | t-value |
| Age | 39.93 (11.0) | 34.64 (10.4) | 37.35 (10.9) | 2.63 ** |
| Cigs | 22.72 (12.1) | 21.57 (9.1) | 22.44 (10.7) | 0.57 |
| CO | 20.49 (10.2) | 19.84 (9.5) | 20.31 (9.7) | 0.35 |
| FTND | 4.54 (2.3) | 5.24 (1.9) | 4.96 (2.2) | 1.74 |
| CES-D | 10.52 (7.9) | 12.89 (10.3) | 11.59 (9.1) | 1.38 |
| BMI | 26.68 (5.9) | 27.14 (6.5) | 26.90 (6.1) | 0.39 |
Note.
Non-Hispanic.
Includes individuals of self-identified Hispanic, Asian-American, and multiethnic backgrounds.
p<.05,
p<.01,
p<.001
3.2. Corrugator EMG
We found a significant SCQ-NAR × Deprivation Status × Nasal Spray interactions for COR EMG during unpleasant slides, F(1,91) = 6.58, p < .02 (see Fig. 1). High SCQ-NAR participants produced smaller COR EMG to unpleasant slides after receiving nicotine nasal spray compared to placebo nasal spray, following both overnight nicotine deprivation, F(1,91) = 60.83, p < .0001, and ad lib smoking, F(1,91) = 56.47, p < .0001. For low SCQ-NAR participants, nicotine spray resulted in larger COR EMG to unpleasant slides compared to placebo, but only following overnight deprivation, F(1,91) = 20.90, p < .0001. Covarying baseline smoking, expired CO, FTND scores, gender, and age did not alter the significance of these findings.
Figure 1.
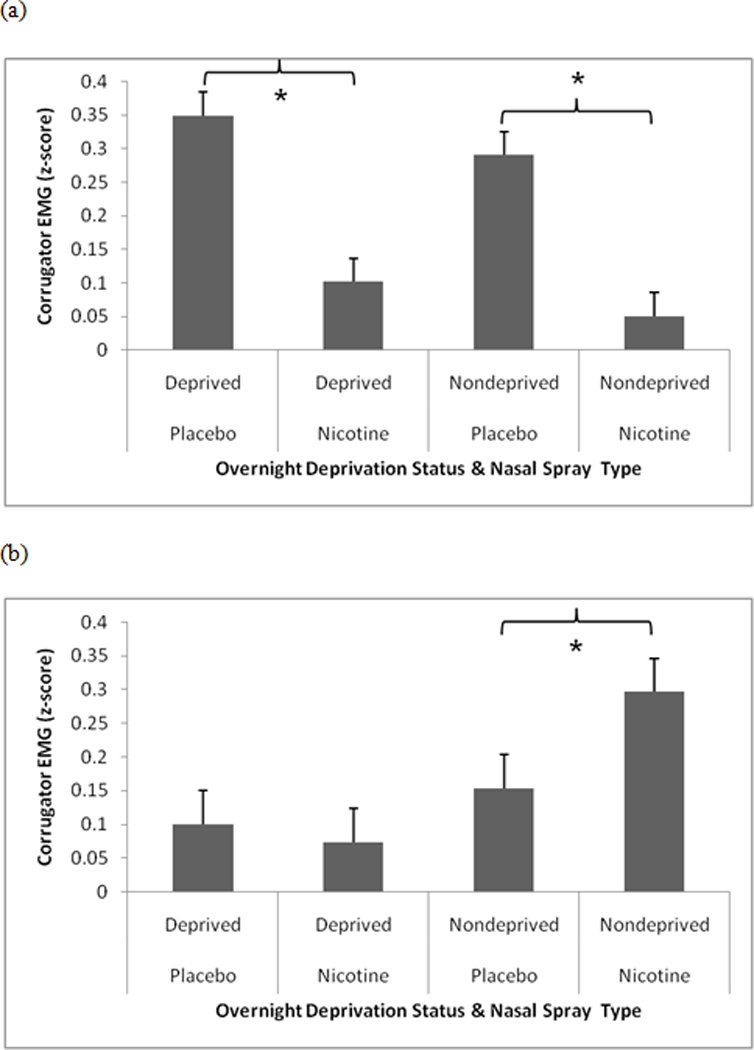
Significant SCQ-NAR × Deprivation Status × Nasal Spray interactions for corrugators EMG during unpleasant slides. (a) high SCQ-NAR, (b) low SCQ-NAR. Pairwise comparisons indicated by an asterisk are significant at the p < .0001 level.
3.3. Skin Conductance
We found a significant SCQ-NAR × Deprivation Status × Nasal Spray interactions for SCR during unpleasant slides, F(1,96) = 49.42, p < .0001 (see Figure 2). For high SCQ-NAR participants, nicotine spray resulted in smaller SCR to unpleasant slides compared to placebo, but only following overnight deprivation, F(1,96) = 78.24, p < .0001. All other post hoc contrasts were nonsignificant. Covarying baseline smoking, expired CO, FTND scores, gender, and age did not alter the significance of these findings.
Figure 2.
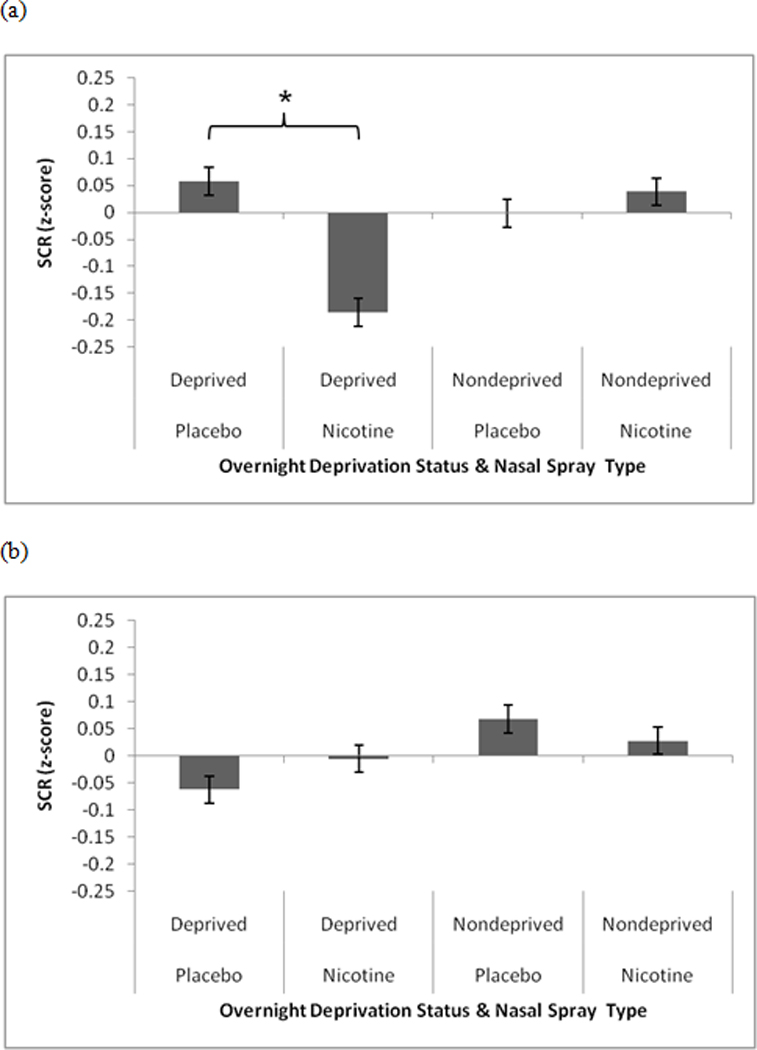
Significant SCQ-NAR × Deprivation Status × Nasal Spray interactions for skin conductance during unpleasant slides. (a) high SCQ-NAR, (b) low SCQ-NAR. Pairwise comparisons indicated by an asterisk are significant at the p < .0001 level.
3.4. In-Session Ratings
The in-session ratings (negative mood, positive mood, and craving) were administered after every third slide. We examined the interaction of SCQ-NAR and its interactions with deprivation status, nasal spray, and preceding slide type on each rating, but none of the interactions were significant. However, we found main effects of SCQ-NAR for negative mood, F(1,220) = 5.62, p < .02, positive mood, F(1,220) = 8.79, p < .003, and craving ratings, F(1,220) = 7.15, p < .008. Participants high in SCQ-NAR reported greater negative mood (M = 2.44 vs. M = 2.26) and craving (M = 3.19 vs. M = 2.98), and less positive mood (M = 3.12 vs. M = 3.35), than those low in SCQ-NAR (all SE's =.05), regardless of slide type preceding the ratings. Covarying baseline smoking, expired CO, FTND scores, gender, and age did not alter the significance of these findings.
3.5. Session Questionnaires
We examined the impact of SCQ-NAR and deprivation status on the session questionnaires using a 2 (SCQ-NAR) × 2 (Deprivation status) mixed models analysis on the questionnaires given prior to each lab session, the PANAS and WSWS. There was a main effect for SCQ-NAR on the PANAS Negative scale, F(1,110) = 4.61, p < .04, as participants who scored high on the SCQ-NAR had larger values (M = 17.59, SE = 0.77) than those who scored low on the SCQ-NAR (M = 15.20, SE = 0.76). Similarly, there was a main a main effect for SCQ-NAR on the PANAS Positive scale, F(1,108) = 4.82, p < .03, as participants scoring high on the SCQ-NAR had smaller values (M = 32.43, SE = 1.01) than those scoring low on the SCQ-NAR (M = 35.64, SE = 1.00). There were neither significant main effects of SCQ-NAR on the WSWS nor signification interactions of SCQ-NAR with deprivation status on either the WSWS or the PANAS. Covarying baseline smoking, expired CO, FTND scores, gender, and age did not alter the significance of these findings.
4. Discussion
Our results provide evidence that while both high SCQ-NAR and low SCQ-NAR smokers reported greater levels of negative affect in two of our subjective response categories (in-session ratings and session questionnaires), psychophysiological indices associated with negative affect (COR EMG) were selectively reduced among the high SCQ-NAR group by the presence of nicotine, regardless of deprivation. The fact that smokers who had high NRSOE experienced significant decreases in COR EMG to nicotine compared to placebo spray suggests that acute nicotine administration reduces negative affect in this group of smokers. In addition, smokers who had high NRSOE experienced decreased sympathetic nervous system activation (SCR) when viewing unpleasant slides following nicotine compared to placebo spray, but only when overnight nicotine deprived. While our first hypothesis was supported by our physiological data, discrepant findings were noted for our in-session and retrospective measures of negative affect. Our in-session ratings showed that smokers who had high NRSOE experienced greater negative affect than smokers who had low NRSOE regardless of nicotine deprivation, nasal spray condition, or preceding slide type. In addition, high SCQ-NAR smokers reported greater negative affect, and less positive affect on the PANAS, than low SCQ-NAR smokers, regardless of nicotine deprivation. Our second hypothesis, that associations between SCQ-NAR and negative affect in response to acute nicotine deprivation and administration would be maintained while covarying demographics and baseline smoking behavior potentially related to affective response to smoking, was confirmed across measurement modality.
These findings suggest that NRSOE may reflect cognizance of an actual drug (physiological) effect on negative affect reduction, and that the increased negative affect found during reduced nicotine exposure conditions among those who have high NRSOE is not simply due to a self-fulfilling prophecy. The psychophysiology measures of negative affect, which are presumably less sensitive to the biases inherent to self-report measures (Robinson and Clore, 2002), were sensitive to the overnight deprivation and nasal spray manipulations. Moreover, psychophysiology's sensitivity to the nasal spray manipulation was found despite it being blindly administered to the participants.
However, with this design, we were unable to determine whether NRSOE reflect a sensitivity to the mood altering properties of nicotine or a chronic affective deficit that is modulated by nicotine. Our in-session ratings and retrospective questionnaire measures indicated that high SCQ-NAR smokers experienced greater negative affect and craving, and less positive affect, than smokers with low SCQ-NAR scores, regardless of nicotine exposure. However, our participants did not differ by SCQ-NAR group on baseline CES-D, a population measure of depression, which argues against NRSOE being an indicator of a chronic affective deficit.
Our psychophysiological results support previous findings that smokers who have high NRSOE may be more vulnerable to experiencing negative affect in response to deprivation (Tate et al., 1994; Wetter et al., 1994). Additionally, our self-report in-session ratings and session questionnaires suggest that high SCQ-NAR smokers experience a greater day-to-day level of negative affect than low SCQ-NAR smokers. Thus, smokers who have high NRSOE are likely to be at increased risk for relapse, as both increased negative affect in response to nicotine deprivation and increased trait negative affect have been linked with smoking relapse (Kassel et al., 2003).
Several limitations of our current study prevented us from addressing additional questions about the relationship between NRSOE and negative affect. First, our study tested associations between baseline NRSOE and negative affect by nicotine exposure. Future studies might manipulate NRSOE to better determine their predictive value. Second, our use of nasal spray meant that we were unable to account for the sensory aspects of smoking, which have been found to reduce negative affect regardless of nicotine dose (Perkins et al., 2008). Using nicotine and placebo cigarettes would address the sensory issue. Third, our use of smokers not desiring to quit prevents generalization of our findings to those seeking to quit smoking. Further research should evaluate the relationship between NRSOE and negative affect among treatment seekers. Finally, we excluded smokers with current psychiatric disorders and were unable to examine the relationship between affective disorders and NRSOE. Future studies should evaluate NRSOE among smokers predisposed to affective disturbances, such as depression or anxiety. Using such populations would address whether NRSOE reflect a tendency to "self-medicate" using nicotine (Williams and Ziedonis, 2004).
These results have several implications on our understanding the relationship between expectancies, negative affect, and smoking. The psychophysiological results suggest that NRSOE, as measured by SCQ-NAR, appears to be a valid predictor of negative affect in response to nicotine and nicotine deprivation. Smokers high on NRSOE might have difficulty quitting due to a reliance on nicotine to reduce negative affect. These individuals might benefit from cognitive-behavioral or pharmacological smoking-cessation interventions that target negative affect. The discrepancy between the psychophysiological and self-report measures of negative affect suggests that the former may be more sensitive to changes in negative affect in response to nicotine and nicotine deprivation.
In conclusion, this study was the first to use psychophysiological measures of affect to examine the association between baseline NRSOE and negative affect experienced following nicotine deprivation and administration manipulations. Our psychophysiological results suggest that smokers who have high NRSOE experience negative affect reduction when blindly administered a dose of nicotine, suggesting that some smokers are sensitive to the mood altering properties of nicotine. This association remains even when covarying variables such as degree of smoking dependence, gender, and age. Additional questions about the role of NRSOE might be addressed by studies manipulating NRSOE, using naturalistic placebo cigarettes, and including smokers prone to experiencing affective disturbance.
Acknowledgments
We thank Cathy Sanders, Renata Benjamin, Deena Martinez, and Dr. Tracy Long for their assistance in data collection.
Role of Funding source
This project was supported by State of Texas Tobacco Settlement Funds and a National Cancer Institute grant (P50CA70907) awarded to Paul M. Cinciripini, a career development grant (K23DA024697) and an MD Anderson Education Program in Cancer Prevention Postdoctoral Fellowship Grant (R25CA57730) to Jason D. Robinson, and a career development grant to Brian L. Carter (K07CA92209). Pharmacia & Upjohn, Inc. provided the nicotine and placebo nasal spray. None of the funding sources had a role in the design, collection, analysis, interpretation, or write-up of the data, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors participated in the design of the study and the study protocol. Drs. Robinson, Lam, Carter, and Cinciripini collected, scored, and analyzed the data. Dr. Robinson wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All of the authors declare that they have no conflicts of interest.
References
- Bandura A. Social Learning Theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- Brandon TH, Juliano LM, Copeland AL. Expectancies for tobacco smoking. In: Kirsh I, editor. How Expectancies Shape Experience. Washington, DC: American Psychological Association; 1999. pp. 263–299. [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Day SX, Tsan JY, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine Tob. Res. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Center for the Study of Emotion and Attention. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH-Center for the Study of Emotion and Attention, University of Florida; 1999. [Google Scholar]
- Cinciripini PM, Robinson JD, Carter BL, Lam CY, Wu X, De Moor CA, Baile WS, Wetter DW. The effects of smoking deprivation and nicotine administration on emotional reactivity. Nicotine Tob. Res. 2006;8:379–392. doi: 10.1080/14622200600670272. [DOI] [PubMed] [Google Scholar]
- Colwill RM, Rescorla RA. Associative structures in instrumental learning. In: Bower GH, editor. The Psychology of Learning and Motivation. New York: Academic Press; 1986. pp. 55–104. [Google Scholar]
- Copeland AL, Brandon TH, Quinn EP. The Smoking Consequences Questionnaire-Adult: measurement of smoking outcome expectancies of experienced smokers. Psychol. Assess. 1995;7:484–494. [Google Scholar]
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986;23:567–589. doi: 10.1111/j.1469-8986.1986.tb00676.x. [DOI] [PubMed] [Google Scholar]
- Gourlay SG, Benowitz NL. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin. Pharmacol. Ther. 1997;62:453–463. doi: 10.1016/S0009-9236(97)90124-7. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol. Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter DW, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Lynn SJ. Automaticity in clinical psychology. Am. Psychol. 1999;54:504–515. doi: 10.1037//0003-066x.54.7.504. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol. Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin CA, Milanak ME, Grottenthaler A, Sayette MA. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. J. Abnorm. Psychol. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Robinson JD, Cinciripini PM, Carter BL, Lam CY, Wetter DW. Facial EMG as an index of affective response to nicotine. Exp. Clin. Psychopharmacol. 2007a;15:390–399. doi: 10.1037/1064-1297.15.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JD, Cinciripini PM, Tiffany ST, Carter BL, Lam CY, Wetter DW. Gender differences in affective response to acute nicotine administration and deprivation. Addict. Behav. 2007b;32:543–561. doi: 10.1016/j.addbeh.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, Clore GL. Belief and feeling: evidence for an accessibility model of emotional self-report. Psychol. Bull. 2002;128:934–960. doi: 10.1037/0033-2909.128.6.934. [DOI] [PubMed] [Google Scholar]
- Seaman MA, Levin JR, Serlin RC. New developments in pairwise multiple comparisons: some powerful and practicable procedures. Psychol. Bull. 1991;110:577–586. [Google Scholar]
- Shiffman S, Paty J, Gnys M, Kassel J, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J. Consult. Clin. Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV, Hahn SR, Brody D, Johnson JG. Utility of a new procedure for diagnosing mental disorders in primary care: the PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological Recording. 2nd Ed. New York: Oxford University Press; 2001. [Google Scholar]
- Tate JC, Stanton AL, Green SB, Schmitz JM, Le T, Marshall B. Experimental analysis of the role of expectancy in nicotine withdrawal. Psychol. Addict. Behav. 1994;8:169–178. [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS Scales. J. Pers. Soc. Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp. Clin. Psychopharmacol. 1999;7:354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Smith SS, Kenford SL, Jorenby DE, Fiore MC, Hurt RD, Offord KP, Baker TB. Smoking outcome expectancies: factor structure, predictive validity, and discriminant validity. J. Abnorm. Psychol. 1994;103:801–811. doi: 10.1037//0021-843x.103.4.801. [DOI] [PubMed] [Google Scholar]
- Williams JM, Ziedonis D. Addressing tobacco among individuals with a mental illness or an addiction. Addict. Behav. 2004;29:1067–1083. doi: 10.1016/j.addbeh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Witvliet CVO, Vrana SR. Psychophysiological responses as indices of affective dimensions. Psychophysiology. 1995;32:436–443. doi: 10.1111/j.1469-8986.1995.tb02094.x. [DOI] [PubMed] [Google Scholar]


