Abstract
The ability to store and manipulate online information may be enhanced by an inner speech mechanism that draws upon motor brain regions. Neural correlates of this mechanism were examined using event-related functional magnetic resonance imaging (fMRI). Sixteen participants completed two conditions of a verbal working memory task. In both conditions, participants viewed one or two target letters. In the “storage” condition, these targets were held in mind across a delay. Then a probe letter was presented, and participants indicated by button press whether the probe matched the targets. In the “manipulation” condition, participants identified new targets by thinking two alphabetical letters forward of each original target (e.g., f-->h). Participants subsequently indicated whether the probe matched the newly derived targets. Brain activity during the storage and manipulation conditions was examined specifically during the delay phase in order to directly compare manipulation versus storage processes. Activations that were common to both conditions, yet disproportionately greater with manipulation, were observed in the left inferior frontal cortex, premotor cortex, and anterior insula, bilaterally in the parietal lobes and superior cerebellum, and in the right inferior cerebellum. This network shares substrates with overt speech and may represent an inner speech pathway that increases activity with greater working memory demands. Additionally, an inverse correlation was observed between manipulation-related brain activity (on correct trials) and test accuracy in the left premotor cortex, anterior insula, and bilateral superior cerebellum. This inverse relationship may represent intensification of inner speech as one struggles to maintain performance levels.
Keywords: working memory, speech, Sternberg, fMRI, phonological loop, cerebellum, premotor, frontal, parietal, insula
1. Introduction
An influential model of working memory includes a sub-component known as the phonological loop, (Baddeley, 1992). The phonological loop is comprised of 1–2 seconds of passive storage of phonological content (i.e., sounds, words, and phrases), which is followed by a secondary active rehearsal process that retains this information beyond 1–2 seconds. The passive and active phases of the phonological loop enable everyday tasks, such as following navigational commands while driving or adhering to a written recipe while cooking. The phonological loop may have evolved from speech systems as a way to enhance language acquisition (Aboitiz, Garcia, Bosman, & Brunetti, 2006; Baddeley, Gathercole, & Papagno, 1998). For example, a simple mechanism for the rehearsal of phonological utterances could have developed into a more complex system to incorporate several items at once, leading to an ability to create specific and meaningful sound combinations. According to Baddeley (Baddeley et al., 1998), the modern day function of the phonological loop is to create a reliable representation of a novel speech event (e.g., a new word). This concept has direct implications for language skills because the ability to hold unfamiliar phonemes in mind is a critical aspect of learning and expanding one’s vocabulary. The ability to immediately recall non-words, for example, has been correlated to vocabulary skills in young children (Gathercole & Baddeley, 1989). This same ability was found to be specifically impaired in language disordered children, even after perceptual processing and articulation rates were considered (Gathercole & Baddeley, 1990). Thus, from an evolutionary and developmental perspective, the phonological loop may be intrinsically involved in the acquisition and refinement of language skills.
The act of vocalization -- even merely mouthing or whispering-- while learning verbal content (e.g., letters) improves the immediate recall of that information, relative to silent reading without any mouth movements (Murray, 1965). Moreover, producing speech responses that conflict with the acoustic nature of the verbal content (e.g., repeating “the, the, the” while reading) impairs immediate recall performance, relative to reading the verbal content aloud (Levy, 1971; Murray, 1967). It seems, therefore, that engaging speech mechanisms can enhance verbal learning, as long as the motor and auditory patterns are consistent with the phonological form of the content. Internalization of this vocalization process (inner speech) may provide similar benefits. For example, one study found that the covert repetition of pseudowords subsequently improved the pronunciation accuracy for pseudowords that had been presented multiple times relative to those that had been presented only once (Rauschecker, Pringle, & Watkins, 2008). The authors speculated that subjects had learned to articulate pseudowords using inner speech mechanisms in conjunction with the phonological loop. A separate experiment showed that studying a picture of an object speeded subsequent word reading if the object-word pair began with the same phoneme (Roelofs, Ozdemir, & Levelt, 2007). Thus, inner speech may interact with working memory in order to enhance the encoding of new material.
The term inner speech has been defined variably in the literature (for an informal, yet lengthy review, see Conrad, 1971). One common feature is that inner speech is inaudible. In this report, inner speech is broadly defined as internalized, inaudible verbal thought that may or may not reach conscious awareness and may or may not be accompanied by subliminal vocal activity. To a certain extent, our views concur with those of Vygotsky (Vygotsky, 1986) who posited that inner speech would not resemble spoken language as we know it, but would be compressed. Thus, inner speech may represent a variant of external speech, but is not necessarily a direct emulation of it (i.e., speech without sound). Conceivably, though, inner speech engages a verbal code, drawing upon motor planning and preparatory brain regions that precede overt speech (Ackermann, Mathiak, & Riecker, 2007). An internal code for motor sequences related to the vocalization (even if not executed) may serve as a memory trace that enhances verbal working memory (Marvel & Desmond, 2010b; Ravizza, Delgado, Chein, Becker, & Fiez, 2004). A motor memory trace would provide redundancy with visual and auditory traces also involved in the encoding process of verbal content. Presumably, without this redundancy, working memory would still be possible, but more effortful. The first step in creating a motor trace would begin during encoding with the creation of an articulatory trajectory for the phonological information that is then entered into a phonological loop (Desmond, Gabrieli, Wagner, Ginier, & Glover, 1997). From there, information can be rehearsed, refreshed, and held in mind. Based on this model, the creation of an articulatory trajectory would be directly involved in verbal encoding but would be less important for phonological rehearsal. Accordingly, dissociative neural systems for phonological encoding versus rehearsal have been demonstrated in several event-related fMRI studies (Chang, Crottaz-Herbette, & Menon, 2007; Chein & Fiez, 2001; Chen & Desmond, 2005a, 2005b; Marvel & Desmond, 2010a).
There is considerable overlap between the neurobiology of speech (overt and covert) and verbal working memory. Neuroimaging studies of overt speech implicate primary motor areas, such as the motor cortex (M1) and the medial anterior cerebellum (Lobule IV–V, rostral to the primary fissure) (Bohland & Guenther, 2006; Ghosh, Tourville, & Guenther, 2008; Turkeltaub, Eden, Jones, & Zeffiro, 2002). However, these studies have also identified secondary motor areas as part of the process. Such regions include the left inferior frontal cortex (IFC--Broca’s area), ventral premotor cortex, supplementary motor area (SMA), pre-SMA, striatum, and lateral superior cerebellum (Lobule VI and Crus I, between the primary and horizontal fissures). These secondary motor areas have been shown to support motor functions (such as a button press) by activating prior to motor execution (Hulsmann, Erb, & Grodd, 2003), suggesting that their role is supportive but not directly responsible for overt motor execution. In studies of verbal working memory, activations in many of these secondary motor areas have also been observed when sensorimotor variables were controlled (Chang et al., 2007; Chein & Fiez, 2001; Chen & Desmond, 2005a, 2005b; Desmond et al., 1997; Durisko & Fiez, 2010; Marvel & Desmond, 2010a; Ravizza et al., 2004). Therefore, it appears that a secondary motor system may contribute to both overt speech and verbal working memory via motor planning and preparatory mechanisms, which would be consistent with the notion of an inner speech process that creates a motor trace of internally verbalized content to support working memory.
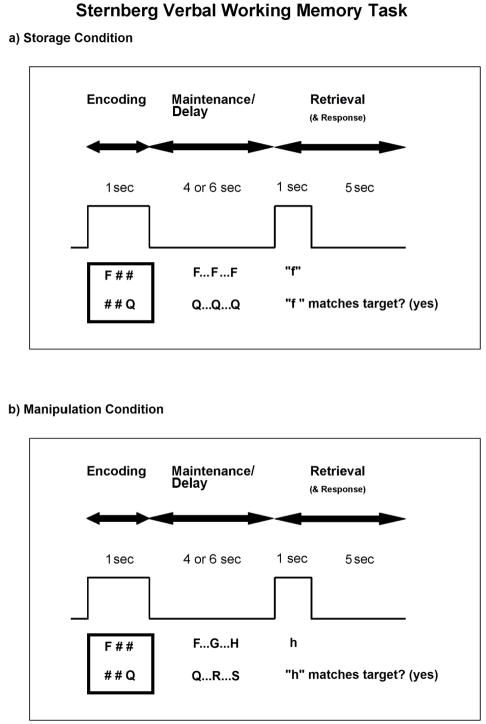
Verbal working memory is often tested in the laboratory using a delayed item recognition paradigm known as the Sternberg task (Sternberg, 1966). Although many variations have been used, the Sternberg task generally consists of a list of items, such as letters, that are briefly presented for study, or encoding (Figure 1a) (encoding phase). This is followed by a delay period in which the items are rehearsed and maintained (maintenance phase). Finally, a probe is presented and a comparison is made between the probe and target items presented at the beginning of the trial (retrieval phase).
Figure 1.
A variation of the Sternberg working memory task was given under two conditions. a) In the storage condition, subjects encoded 1 or 2 targets (encoding phase). Note that in the example, 2 targets are shown, but on half the trials, only 1 target was shown. Then, subjects silently rehearsed these targets across a delay (traditionally called the maintenance phase, but here we use the term “delay” phase). Finally, at the presentation of a letter probe, subjects indicated whether the probe matched either of the targets (retrieval phase). b) In the manipulation condition, the encoding phase was identical to that in the storage condition. However, in the delay phase, instead of simply rehearsing the targets, subjects counted two alphabetical letters forward of each. Then they rehearsed these newly identified targets. In the retrieval phase, subjects indicated whether the probe matched either of the newly identified targets (rather than the original targets). In both figures, the box wave indicates when stimuli were visible on the screen.
Coupling functional magnetic resonance imaging (fMRI) with the Sternberg task allows researchers to examine the neural correlates of the cognitive mechanisms associated with each phase of the task (Chang et al., 2007; Chen & Desmond, 2005b; Marvel & Desmond, 2010a). In response to high target load, the encoding phase tends to elicit activity in the left IFC and in secondary motor regions, such as the pre-SMA, premotor cortex, and bilateral superior cerebellum. Activation of these motor planning areas is consistent with a requirement to translate orthographically presented information into a phonological representation and initiating an articulatory trajectory for subsequent phonological rehearsal (Chen & Desmond, 2005b; Murray, 1967). During the maintenance phase, activation continues in the pre-SMA and ventral premotor cortex, and is accompanied by additional activations in the bilateral anterior insula, left inferior parietal lobe (IPL: BA 40), and right inferior cerebellum. The anterior insula has been associated with interoception, which involves detecting, integrating, and filtering relevant information when that information originates from inside the body (Craig, 2009; Dosenbach et al., 2006). Interoception would logically intensify when verbal content is mentally rehearsed, especially while using an internal voice. The IPL and inferior cerebellum tend to activate together, to operate independent from motor functions, and may be involved in phonological storage (Chen & Desmond, 2005a; Marvel & Desmond, 2010b). During retrieval, activations related to target load, in which a button press is required for both high and low loads, have been reported in the anterior cingulate, bilateral anterior insula, and left premotor cortex (Chen & Desmond, 2005b).
Of the three phases within the Sternberg task, it is presumed that the maintenance phase requires the most phonological rehearsal. Therefore, all regions related to inner speech would be expected to maintain or even increase activity, throughout the maintenance phase. However, this appears to be the case only for certain brain regions, suggesting dissociable contributions to verbal working memory within the system. As noted, the pre-SMA, premotor cortex, anterior insula, IPL, and inferior cerebellum remain active during the maintenance phase. Yet, previous studies have shown that the left IFC and the superior cerebellum, which are involved early in the task during encoding, actually decrease activity throughout the maintenance phase (Chein & Fiez, 2001; Chen & Desmond, 2005b). One reason for this decrease may be that the rehearsal of information that has already been entered into an articulatory trajectory does not require ongoing recruitment of brain regions related to creating a trajectory, as long as that information remains unchanging, or static. If so, then it should be possible to disturb this process by manipulating online content during the maintenance phase, which would effectively : 1) eliminate the static nature of the content, 2) require ongoing initiation of an articulatory trajectory for new and changing online information, and 3) actively engage inner speech brains regions involved in articulatory trajectory that would otherwise return to baseline during phonological rehearsal.
The present study used an event-related fMRI design of a novel variant of the Sternberg paradigm to examine brain regions involved in inner speech processes related to manipulating versus storing verbal content during working memory. An additional advantage to using an event-related fMRI design is that motor-related processes involved in either the manipulation or storage of verbal content can be examined in isolation, without contamination from a subsequent motor response (e.g., button press) that may draw upon similar neural mechanisms. For this study, immediately following the presentation of target letters, subjects were asked to identify two alphabetical letters forward of each target (e.g., “f” to “h”) and to hold these newly identified targets in mind until a probe was presented (Figure 1b). If the subsequent probe matched one of the newly identified targets, then subjects would indicate a match via button press. The control condition required subjects to compare the targets and probe directly, similar to standard Sternberg paradigms. Thus, it was hypothesized that the “manipulation” condition would prolong the process of initiating an articulatory trajectory beyond the initial encoding period as subjects identified new letter targets and, consequently, prolong the activation of brain regions specifically related to this process (e.g., the left IFC and superior cerebellum). This would be in contrast to previous studies that have demonstrated decreased activity in these regions during phonological rehearsal.
The manipulation condition in this task occurred during a delay period that is often referred to as the “maintenance phase”. However, this term could become confusing in the current context given that subjects sometimes manipulated the information and sometimes (as in the control condition) simply maintained the information. Therefore, for clarity, the maintenance phase will be referred to as the “delay phase” for the duration of this report. Although data were analyzed from all three phases, this report focuses on the delay phase. Information regarding the encoding and retrieval phases are available in the online supplement.
2. Materials and Methods
2.1 Subjects
Sixteen healthy male (n=8) and female (n=8) subjects were recruited from the Baltimore community. All subjects were native English speakers, right-handed, with no known psychological or neurological conditions, or history of head trauma. The subjects’ mean age was 25.82 years (range: 20–30), and mean educational attainment was 16.50 years (range: 14–18). This research was approved by the Johns Hopkins School of Medicine Institutional Review Board. All subjects gave their written informed consent prior to inclusion in the study, and all were paid for their participation.
2.2 Task Procedures
Subjects performed two tests of working memory, a “storage” condition and a “manipulation” condition. In the storage condition, subjects viewed either one or two black uppercase consonants centered on a white background, as depicted in Figure 1. These target letters were presented for one second (encoding phase). Then subjects viewed a blank screen for four or six seconds while silently rehearsing the target letters (delay phase). Finally, a single lowercase letter was presented for one second. Subjects indicated via a button press whether the single probe matched the targets (retrieval phase). In the manipulation condition, the presentation of the task was visually identical to that of the storage condition. However, the instructions of the task were different. Upon presentation of the target letters, subjects were asked to count two alphabetical letters forward of each target letter. For example, if the letters “f” and “q” were presented, subjects would count forward to the letter “h” and to the letter “s”. Subjects were then instructed to silently rehearse these newly identified letters (“h” and “s”) until a probe appeared. Therefore, the two conditions differed in the way that the target letters were processed following encoding, i.e., during the delay phase. In the storage condition, the target letters were simply rehearsed.
Subjects were instructed to respond as quickly and accurately as possible while conducting all manipulations silently “in your head”. They were given up to six seconds to respond with a button press (yes = right index finger; no = right middle finger) following probe onset. Failure to respond did not inhibit the start of the next trial. Trials were jittered with an inter-trial interval (ITI) that lasted six to nine seconds. Response time (RT) and accuracy were recorded for each trial. In order to familiarize subjects with the rules of the task, subjects practiced 10 trials of each condition prior to entering the MRI environment.
Storage and manipulation conditions were performed during two separate blocks (i.e., two EPI-sequence runs). The order of block condition was counterbalanced across subjects. Each block contained 64 trials and lasted approximately 16 minutes. Consonant target letters were randomly generated prior to testing to create a “storage” list and “manipulation” list. In each list, letters were unique within a trial and randomly placed across six possible spatial positions, in concordance with a prior study (Marvel & Desmond, 2010a). As shown in Figure 1, pound (“#”) placeholders were displayed in the unused positions within a trial. Sixteen trials (25%) did not include any probe at all in order to allow the hemodynamic response to fully return to baseline following the delay phase. In those instances, subjects viewed a blank screen throughout the retrieval phase, and no response was expected. For the remaining 48 trials, probes corresponded to a target on half of all trials. In such trials, the location of the target letter was counterbalanced across spatial positions. The probe did not directly match the target in manipulation trials. This reduced the possibility that, in the manipulation condition, a subject might reflexively indicate a match between the probe and targets instead of comparing the probe to the newly identified targets. The number of studied letters (one or two), length of the maintenance phase (four or six seconds), expected response (yes or no), and duration of ITI (six to nine seconds) were pseudorandomized so that presentation of identical parameters was limited to three consecutive trials.
Individualized hemodynamic response functions (HRFs) were obtained using an event-related finger tapping task that consisted of a button press with the right index finger every 29–31 sec for 10 min. This task reliably elicits activation from the left motor cortex, and right anterior superior cerebellar cortex (Desmond et al., 1997). Two HRFs were used because of subtle timing differences between neocortical and cerebellar HRFs (Chen & Desmond, 2005b).
Stimuli were delivered using E-Prime 1.1 software (Psychology Software Tools, 2002) on a Hewlett Packard xw4300 workstation running Windows XP Pro. The computer-generated visual display was rear-projected onto a screen in the MRI scanner situated behind the participant’s head. The display was then reflected onto a mirror directly within the participant’s line of view just outside the head coil. Responses were collected using two velcro-connected fiber optic button boxes (MRA, Inc., Washington, PA) that were held in the subject’s right hand.
2.3 MRI Data Acquisition
All MRI data were acquired using a 3.0T Philips Intera scanner. The structural MRI protocol consisted of a T1-weighted MPRAGE (TR = 6.97 ms; TE = 3.3 ms; TI = 982 ms; flip = 8°, inplane resolution = 0.75 mm; slice thickness = 1 mm; 170 sagittal slices; FOV = 240 mm; 1 NEX). FMRI data were collected using a T2*-weighted gradient echo EPI pulse sequence (TR = 1000 ms; TE = 30 ms; flip = 61°; inplane resolution = 3.75 mm; slice thickness = 6 mm skip 1mm; 20 oblique-axial slices; FOV = 240 mm; 1 NEX). T2*-weighted images were acquired in the oblique-axial plane rotated 25° clockwise with respect to the AC-PC line in order to optimize imaging of the cerebellum and neocortex. The number of acquired volumes within each block ranged from 917–922 for the working memory task and was 600 for the tapping task. The start of the fMRI scan was triggered by E-prime software at the beginning of each block.
2.4 Functional Data Analysis
The SPM2 software package (Wellcome Department of Cognitive Neurology) was used for preprocessing and statistical computations. As described by Chen and Desmond (Chen & Desmond, 2005b), high temporal resolution fMRI in conjunction with neocortical- and cerebellar-specific HRFs was used to ensure maximum accuracy in characterizing phase-specific BOLD responses. Each of these individual HRF regressors was convolved with reference waveforms for the encoding (1 s), delay (4 or 6 s) and retrieval (6 s) phases of the task for each subject within the first-level analysis.
Standard image preprocessing steps were performed, including slice timing correction (reference = middle slice), motion correction, anatomical coregistration, normalization to the Montreal Neurological Institute (MNI) stereotaxic space, and spatial smoothing (FWHM = 5 mm). Individual statistical maps were computed for each subject using the general linear model approach as implemented in SPM2, with high pass filtering of 234 seconds. A random effects analysis was then performed to map the average responses to the encoding, delay, and retrieval phases of the task on correct trials only. Incorrect trials were not given a regressor and were considered as residual variance. This analysis was performed by computing a contrast volume per subject and using these volumes to calculate one-sample t-test values at every voxel. Of particular interest were contrasts comparing the BOLD signal difference in target load (2-target minus 1-target) and working memory condition (manipulation minus storage), as an interaction analysis during the delay phase of the task (note that the same analyses were applied to the encoding and retrieval phases, the results of which may be viewed in the online supplement).
MNI coordinates were transformed into the coordinate system of the Talairach and Tourneaux stereotaxic atlas (Talairach & Tournoux, 1988) using the MNI to Talairach transformation described by Lancaster et al. (Lancaster et al., 1997) in order to make anatomical determinations of the activations. However, MNI coordinates are reported in the tables and figures. For the cerebellum, MNI coordinates were referenced with the cerebellar atlas of Schmahmann et al. (Schmahmann, Doyon, Petrides, Evans, & Toga, 2000) and with a supplemental probabilistic atlas of human cerebellar nuclei (Dimitrova et al., 2006).
3. Results
3.1 Behavioral Results
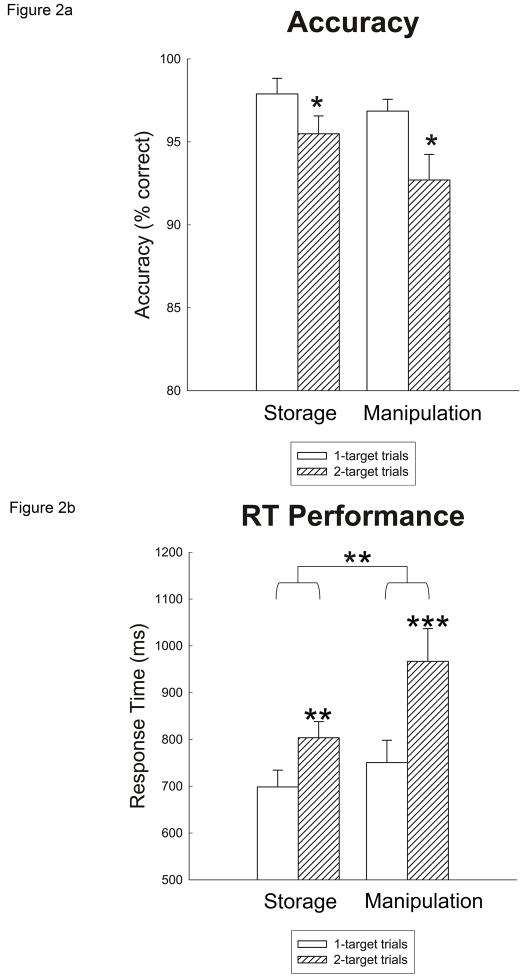
Mean accuracy and RT were calculated for the following trial types: 1-target storage condition, 2-target storage condition, 1-target manipulation condition, and 2-target manipulation condition. As shown in Figure 2a, mean accuracy differed across trial types. A 2 (load: 1 target vs. 2 targets) × 2 (condition: storage vs. manipulation) repeated-measures analysis of variance (ANOVA) yielded a main effect of target load, F(1,15) = 9.346, p < .01, indicating that subjects committed more errors on 2-target trials than on 1-target trials. Post-hoc t-tests revealed that the effect of target load on performance was significant for both conditions [storage: t(15) = 2.366, p < .05; manipulation: t(15) = 2.230, p < .04]. There was no main effect of working memory condition, F(1,15) = 2.663, p = .12, suggesting that accuracy was equated, overall, between the storage and manipulation trials. There was also no interaction of load × condition, F(1,15) = .701, p = .42. The RT measure was more sensitive to the subjects’ performance differences across trial types. Mean RTs were computed for accurate trials only, and are graphed in Figure 2b. A 2 (load) × 2 (condition) repeated-measures ANOVA on RT scores yielded two main findings. First, there was a main effect of target load, F(1,15) = 59.501, p < .001, indicating that subjects responded more slowly to 2-target trials than to 1-target trials. Post-hoc t-tests revealed a significant RT difference between target loads for both conditions [storage: t(3.901) p < .01; manipulation: t(5.081), p < .001]. Second, there was a main effect of condition, indicating that subjects responded more slowly to trials in the manipulation condition relative to the storage condition, F(1,15) = 10.429 p < .01. As depicted in Figure 2b, the RT difference in target load in the manipulation condition appeared to be larger than in the storage condition. There was a trend towards an interaction of load × condition, F(1,15) = 3.694, p = .07. Taken together, these results indicated that subjects experienced the most difficulty with the manipulation condition, especially when two targets were presented.
Figure 2.
Performance results are presented by target load and working memory condition. a) Accuracy is shown as percent correct. Subjects committed more errors in the 2-target condition, regardless of the working memory condition (storage or manipulation). * indicates p-value < .05. B) RT for accurate trials only in milliseconds. RTs slowed during the 2-target load condition and, generally, in the manipulation condition. A marginal interaction suggested that the 2-target manipulation condition was slowest. ** indicates p-value < .01; *** indicates p-value < .001. Error bars in both graphs represent standard error.
3.2 Imaging Results
3.2.1 Manipulation versus Storage Condition Activations During the Delay Phase
The imaging analysis of the present study focused on the following interaction contrast: manipulation (2-target minus 1-target) minus storage (2-target minus 1-target) during the delay phase of the task. This contrast, therefore, considered the possibility that brain activity was differentially affected by a combination of both target load and working memory condition. Given that behaviorally the 2-target load and manipulation conditions were most difficult for subjects, the positive BOLD signals in this particular contrast have been interpreted to represent a greater 2- versus 1-target differential in the manipulation condition relative to that in the storage condition. Results for this contrast are shown in Figure 3 and described below. [Results for the encoding and retrieval phases are presented in the online supplement Tables 1 and 2.] We used a cluster threshold of 17 voxels, based on a criterion that corresponded to a .05 cluster significance level for structures that are relatively spatially large, and a p-value threshold of .001. Thus, activations smaller than 17 voxels, and p > .001 were excluded from the results.
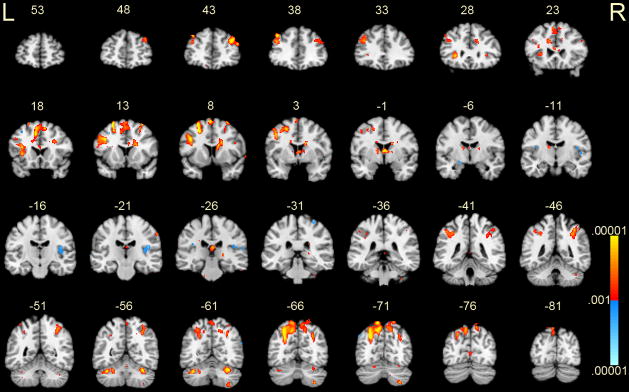
Figure 3.
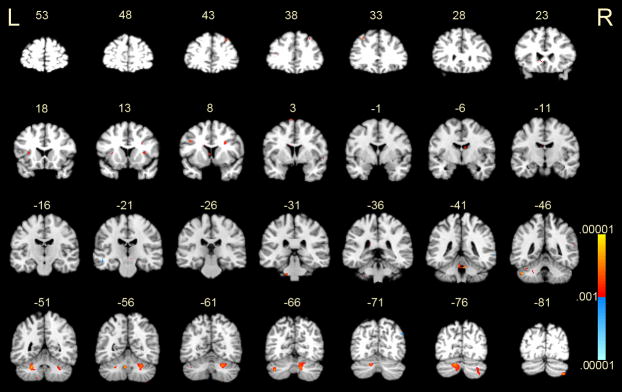
Activations during the delay phase for manipulation (2-target minus 1-target) versus storage (2-target minus 1-target) conditions. Coronal slices from MNI y = +53 mm to −81 mm are depicted. Positive activations, shown in red-yellow, represent greater BOLD signal associated with the manipulation condition. Negative activations, shown in blue, represent greater BOLD signal associated with the storage condition. P-values ranged from .001 – .00001. R = right; L = left.
Increased BOLD signals associated with the manipulation condition were observed in several brain regions (Figure 3 and Table 1). A rather large region of increased signal was observed in the left inferior frontal gyrus, which included BA 8, 9, the anterior insula and claustrum. The premotor cortex (BA 6) was activated bilaterally, along with the pre-SMA. The right superior frontal gyrus (BA 9) and cingulate were also activated. Posteriorly, increased signals were observed bilaterally in the IPL and in precuneus. In the cerebellum, the superior cerebellum (Lobule VI/Crus I) was activated bilaterally. Activity increased in the inferior cerebellum (Lobule VIII) on the right side only.
Table 1.
Brain regions, listed anterior to posterior, that showed significant activation in response to the manipulation (2-target minus 1-target) versus storage (2-target minus 1-target) condition during the delay phase of the task.
| Brain Region (Peak Activation) | Brodmann’s Area (BA) | MNI Coordinates | SPM Z max | NVox | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Positive Activations | ||||||
| Left Side: | ||||||
| L Anterior Cingulate | BA 33 | −4 | 22 | 12 | 3.8 | 31 |
| L Inferior Frontal Gyrus | BA 9 | −42 | 10 | 30 | 4.7 | 1160 |
| L Middle Frontal Gyrus | BA 8 | −40 | 40 | 38 | 4.7 | |
| L Claustrum | −30 | 26 | −2 | 4.3 | ||
| L Insula | BA 13 | −36 | 18 | 2 | 4.1 | |
| L Precentral Gyrus | BA 6 | −46 | 4 | 40 | 4.5 | |
| L Superior Frontal Gyrus (pre-SMA) | BA 6 | −4 | 8 | 66 | 4.4 | 663 |
| L Superior Frontal Gyrus | BA 6 | −8 | 20 | 52 | 4.2 | |
| L Medial Frontal Gyrus | BA 32 | −10 | 18 | 42 | 4.4 | |
| R Superior Frontal Gyrus | BA 6 | 10 | 18 | 52 | 4.0 | |
| L Caudate Body | −14 | 0 | 26 | 3.6 | 27 | |
| L Thalamus | −14 | −12 | 14 | 3.6 | 61 | |
| L Inferior Parietal Lobule | BA 40 | -44 | −44 | 44 | 3.8 | 236 |
| L Cerebellum Crus I | −38 | −60 | −32 | 4.5 | 228 | |
| L Precuneus | BA 19 | −28 | −72 | 38 | 5.1 | 1735 |
| L Precuneus | BA 7 | −10 | −70 | 52 | 4.5 | |
| Right Side: | ||||||
| R Superior Frontal Gyrus | BA 9 | 38 | 42 | 26 | 4.4 | 268 |
| R Middle Frontal Gyrus | BA 8 | 48 | 34 | 38 | 3.3 | 23 |
| R Cingulate Gyrus | BA 32 | 14 | 26 | 26 | 3.7 | 57 |
| R Anterior Cingulate | BA 33 | 6 | 24 | 10 | 3.5 | 24 |
| R Middle Frontal Gyrus | BA 9 | 52 | 24 | 28 | 3.5 | 17 |
| R Cingulate Gyrus | BA 32 | 26 | 18 | 26 | 3.7 | 30 |
| R Superior Temporal Gyrus | BA 38 | 64 | 14 | −38 | 3.8 | 19 |
| R Cingulate Gyrus | BA 24 | 18 | 6 | 27 | 4.5 | 383 |
| R Caudate | 12 | 8 | 18 | 4.3 | ||
| Thalamus | 0 | −2 | 8 | 4.5 | ||
| Thalamus | 0 | −28 | 14 | 3.9 | 78 | |
| Thalamus | 20 | −30 | 2 | 3.9 | 19 | |
| R Superior Parietal Lobule | BA 7 | 36 | −54 | 54 | 4.1 | 593 |
| R Inferior Parietal Lobule | BA 40 | 36 | −46 | 46 | 3.9 | |
| R Precuneus | BA 7 | 28 | −66 | 38 | 4.0 | |
| R Cerebellum Lobule VI | 32 | −60 | −30 | 4.6 | 203 | |
| R Cerebellum Lobule VIII | 32 | −70 | −54 | 4.1 | 108 | |
| Lingual Gyrus | BA 18 | 0 | −76 | 4 | 3.4 | 40 |
| Negative Activations | ||||||
| Left Side: | ||||||
| L Superior Parietal Lobule | BA 7 | −30 | −46 | 76 | −3.6 | 19 |
| L Angular Gyrus | BA 39 | −52 | −72 | 42 | −3.7 | 64 |
| Right Side: | ||||||
| R Insula | BA 13 | 44 | −24 | 16 | −3.6 | 203 |
| R Postcentral Gyrus | BA 5 | 30 | −32 | 66 | −3.7 | 22 |
| R Middle Temporal Gyrus | BA 39 | 58 | −64 | 24 | −3.7 | 56 |
Note: Each brain region listed in the table refers to the location of the peak activation. Items with no voxel number listed represent local maxima within the primary cluster. All p-values < .001.
The manipulation condition was more difficult than the storage condition, as indicated by longer RTs. This suggested that some activation increases in the manipulation condition were related to processes not directly involved in manipulation, such as attention or retrieval from long term-memory for alphabetical letters. To identify activations that were unique to the manipulation condition, we conducted a conjunction analysis (Chen & Desmond, 2005a; Friston, Holmes, Price, Buchel, & Worsley, 1999; LaBar, Gitelman, Parrish, & Mesulam, 1999) between the delay phase of the manipulation condition and the encoding or delay phase of the storage condition, following Boolean operations of AND (conjunction), OR (union), and NOT (negation) applied to the 2- minus 1-target contrasts. This comparison directly addressed our hypothesis that manipulation relied on inner speech processes involved in the creation of motor memory traces. Thus, brain regions that were shared by manipulation (delay phase) and storage (encoding and/or delay phase) were computed from the Boolean computation [manipulation AND (storage encoding OR storage delay)]. Regions that were unique to manipulation were computed from the Boolean computation [manipulation AND NOT (storage encoding OR storage delay)]. The p-threshold in both analyses was set to .001 for the manipulation contrast and to .05 for the storage contrast mask. Setting the threshold to .05 in the mask yielded a conservative approach that identified--for the manipulation AND NOT storage conjunction--voxels with strong activation during manipulation (at the .001 level) but not even weak activation during storage (at the .05 level). The net results of combining these two Boolean computations, shown in Figure 4, revealed substantial overlap in activations, notably in speech and motor-related regions of the left IFC, left premotor cortex, pre-SMA, SMA and superior cerebellum [represented by red (overlap with storage encoding) and yellow (overlap with storage delay)]. Regions that were unique to manipulation (shown in blue) included the right middle frontal gyrus (BA 9) and the left inferior cerebellum (Lobule VIIb/VIIIa).
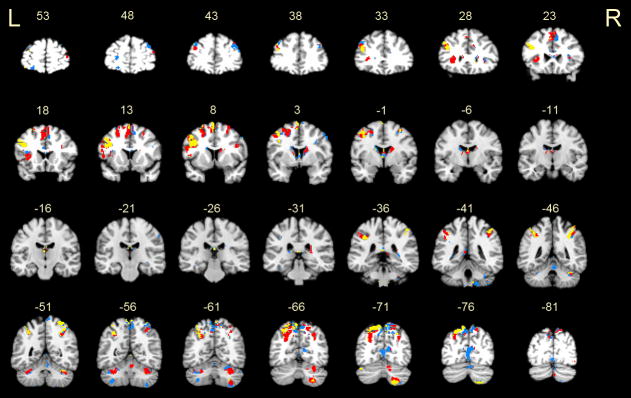
Figure 4.
A conjunction analysis shows overlapping activations between the manipulation (delay phase) and storage (encoding or delay phase) conditions. Regions in red represent activations common to manipulation and storage encoding. Regions in yellow represent activations common to manipulation and storage delay. An overwhelming amount of overlap was observed between the manipulation and storage conditions, indicating that manipulation re-engages substrates specific to the encoding phase and also those specific to phonological rehearsal. Areas unique to manipulation, shown in blue, were notably located in the right prefrontal cortex (slice y = +43) and left inferior cerebellum (slice y = −56). Slice labels are as described in Figure 3.
3.2.2 Relation Between BOLD Signal and Accuracy
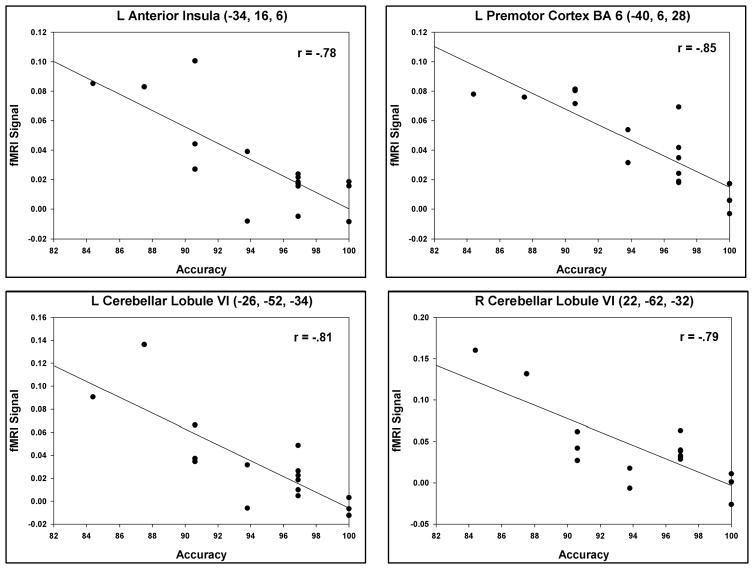
In order to more closely examine how the brain activity during the delay phase contributed to performance on the task, we conducted additional analyses to correlate BOLD signal contrast values to accuracy scores. Specifically, using SPM2, we conducted a whole-brain simple regression analysis that correlated each subject’s BOLD signal contrast during the delay phase (2-target manipulation minus 2-target storage) with his or her accuracy on the 2-target manipulation condition. Only accurate trials were considered in the contrast measures to ensure the measurement of a valid process. As with the contrast comparison above, threshold values of p < .001 and 17 voxel-size clusters were used. Two overall trends emerged from the findings: 1) the majority of correlations were located in the cerebellum, and 2) correlations were negative (see Figure 5 and Supplement Table 3). Notably, negative correlations were observed in the bilateral superior cerebellum (Lobule VI and Crus I). In the cerebrum, negative correlations were found in the left anterior insula (seen in Figure 5, slice y = 18) and in the left premotor cortex (BA 6) (Figure 5, slice y = 8). Figure 6 highlights this correlational relationship by depicting four scatterplots of each subject’s accuracy (x-axis) by the mean fMRI contrast value within the region of interest (ROI) (y-axis). These four regions of interest contained regional overlap with activations generated in the manipulation versus storage contrast (compare with Figure 3) and therefore underscore the relevance of these regions’ contributions to working memory: 1) left anterior insula (−34, 16, 6), 2) left premotor cortex (−40, 6, 28), 3) left cerebellar Lobule VI (−26, −52, −34), and 4) right cerebellar Lobule VI (22, −62, −32). For each region depicted, the range of accuracy scores indicated that the greater one’s manipulation-related activity relative to storage (on correct trials), the lower one’s overall accuracy.
Figure 5.
Brain regions in which the BOLD signal contrast value of 2-target manipulation minus 2-target storage conditions (on correct trials only) correlated with accuracy scores in the 2-target manipulation condition. Negative correlations, shown in red-yellow, indicated that greater manipulation-related activity correlated with lower overall accuracy. MNI coordinates of peak values are shown in Supplement Table 3. Note that clusters smaller than 17 voxels are not reported in the table, though they may be visible in the figure. P-values ranged from .001 – .00001. R = right; L = left.
Figure 6.
Scatterplots are shown for selected brain regions in which manipulation-related activity negatively correlated with accuracy. These regions may be part of an inner speech network that becomes hyperactive as one struggles to perform the task under high verbal working memory demands. Activations can be cross-referenced with those shown in Figure 5 and Supplement Table 3.
4. Discussion
This study examined the neural activity associated with the manipulation versus storage of verbal content during working memory. It was hypothesized that inner speech brain regions, namely those motor regions that tend to support motor preparation and planning but not overt motor execution, would increase activity during the manipulation of verbal content across a delay. This hypothesis was supported by the data.
Secondary motor-related brain regions, such as the premotor cortex, pre-SMA, and superior cerebellum, showed a relative increase in activity during the delay phase in response to the manipulation of information over and above what was seen in these brain regions during the storage condition. The speech-related IFC also activated in response to the manipulation condition. This overall pattern of activity contrasts previous reports showing an initial activation increase (during encoding) followed by a decrease (during delay) in the IFC and superior cerebellum while subjects rehearsed static (i.e., non-manipulated) information (Chein & Fiez, 2001; Chen & Desmond, 2005b). The current results effectively demonstrated that the IFC and superior cerebellum will remain active as long as information requires ongoing manipulation, and presumably the ongoing creation of new motor traces.
Aside from the regions named above, manipulation-related activations were observed in areas that have been implicated in working memory: the IPL, dorsal prefrontal cortex (BA 9), anterior insula, and inferior cerebellum. The inferior aspect of the parietal lobe has been associated with storage functions (Becker, MacAndrew, & Fiez, 1999; Wager & Smith, 2003), it has more recently been implicated in the manipulation of information, such as re-ordering verbal and non-verbal information online, with the dorsal prefrontal region supporting information monitoring throughout the manipulation process (Champod & Petrides, 2007, 2010). Thus, the finding that the IPL and prefrontal cortex increased activity in tandem, in response to the manipulation condition, is consistent with the findings of others. Increased activity in the anterior insula, in association with manipulation, agrees with this region’s putative role in interoception (Craig, 2009; Dosenbach et al., 2006). As subjects silently counted two alphabetical letters forward of each target during the manipulation condition, they may have experienced heightened awareness of the internal speech associated with this process. Finally, the finding that activity increased in the right inferior cerebellum also agrees with findings from prior studies, but less attention has been paid to the functionality of this region in working memory. Under straightforward storage/rehearsal conditions, this region has shown heightened activity during the delay phase in Sternberg-type tasks (Chang et al., 2007; Chen & Desmond, 2005b; Marvel & Desmond, 2010a). Moreover, greater involvement of the inferior cerebellum has been observed while subjects silently rehearsed letters across a delay relative to a control condition in which subjects silently repeated letters that were continuously displayed throughout the delay (thereby diminishing working memory demands) (Chen & Desmond, 2005a; Desmond et al., 1997). These convergent results suggest that the inferior cerebellum may be particularly important for maintaining or updating information during working memory (Marvel & Desmond, 2010b). This specificity of function sets the inferior cerebellum apart from the superior cerebellum, the latter of which appears to be involved in (motor-related) aspects of encoding (Chein & Fiez, 2001; Chen & Desmond, 2005b; Marvel & Desmond, 2010a), and in this study, creating new motor traces while manipulating information across a delay.
Many of the manipulation-related activations overlapped with storage encoding activations (red activations in Figure 4). This relationship highlights the prolonged involvement of “encoding-like” processes during manipulation, and further supported our hypothesis that motor traces are created to support manipulation. Relatively few activations were uniquely tied to manipulation (blue activations in Figure 4), but it is likely that they contributed to performance. These areas may have been more generally involved in manipulation (e.g., attention, long-term retrieval, spatial memory for the letter arrangement) separate from the motor-related inner speech mechanism described here.
Support for the notion that speech and vocalization enhance working memory ability was demonstrated behaviorally decades ago in studies of immediate verbal recall (Levy, 1971; Murray, 1965, 1967). Developmentally, around age 5 children begin to use internal verbal coding to aid in memorization (Conrad, 1971). Moreover, disorders in the phonological loop have been specifically tied to language acquisition and language disorders (Gathercole & Baddeley, 1989, 1990). Thus, the concept that inner speech supports working memory, and that this process relies on a motor-related neural network, is plausible. The cerebellum may be a particularly important part of this process. Henrietta Leiner, a longtime proponent of this concept, noted that the dramatic enlargement of the cerebellum over the last million years of human evolution may have critically enabled the coupling of “fast thinking and fast action”, leading to the emergence of fluent speech and language (Leiner, 2010). Neuroimaging studies have consistently shown cerebellar involvement in tasks in which information is verbalizable (e.g., when computing numbers, rearranging syllables into words, or performing the n-back task for letters) (Hayter, Langdon, & Ramnani, 2007; Owen, McMillan, Laird, & Bullmore, 2005; Zago et al., 2001; Zago et al., 2008). Consequently, it has been proposed that inner speech, and the cerebellar involvement of inner speech, may be used to generally represent, maintain, and organize task-relevant information and conscious thoughts (Strick, Dum, & Fiez, 2009).
An exploratory analysis revealed that increased activity on accurate trials correlated to lower overall accuracy performance, and this was most notable in the cerebellum. This result may seem counterintuitive at first, if inner speech mechanisms truly represent an adaptive process that benefits working memory. Inner speech functions, however, may be more generalized in purpose. For example, it has been proposed that the cerebellum contributes to the temporal organization of words (or syllable strings) at the level of inner speech (Ackermann, Mathiak, & Ivry, 2004). Thus, the neural substrates that support inner speech (or the timing of it) may do so even if a person were struggling to complete the task. In the current paradigm, if a subject had trouble remembering the order of the alphabet, or had to get a “running start” leading up to a letter, or always needed to begin with the letter “A” when scanning the alphabet, this could lead to both excessive inner speech as well as an increased likelihood of making an error.
Corroborating this interpretation, inverse correlations between accuracy and BOLD signal were found in the left ventral premotor cortex (BA 6), left anterior insula, and bilateral superior cerebellum—regions related to motor-related aspects of inner speech and interoception. Notably, brain regions that may be involved in motor-independent processes of working memory storage and manipulation did not correlate with accuracy (e.g., the IPL and inferior cerebellum). It should be understood that the BOLD contrasts used in the correlation analysis included correct trials only. Because fewer than 15% of trials were incorrect, there was inadequate data to study the pattern of activity related to errors. However, the information available has been interpreted to mean that low-scoring subjects with relatively high regional fMRI manipulation-minus-storage contrasts represented those subjects who had a harder time in general with the task. Presumably, those subjects used inefficient strategies consistently throughout the task, even though they answered most trials correctly. This interpretation, though speculative, fits with observations of hyperactivity of the fronto-cerebellar pathway in a variety of clinical populations with known working memory impairments, such as alcoholism (Desmond et al., 2003), schizophrenia (Koch et al., 2010; Marvel et al., 2007), and individuals who have received chemotherapy (Silverman et al., 2007). It seems that the more effortful a task is, the more one engages an inner speech pathway, and this phenomenon may be more pronounced in clinical populations who represent the edge of the behavioral spectrum.
In summary, the results of the present study suggest that during working memory the process of manipulating verbalizable information elicits activity in brain regions related to secondary motor functions, language, and interoception. Activity in this neural pathway may represent the ongoing creation of internal motor representations associated with inner speech—an effect that is augmented when information is manipulated. However, intense recruitment of this neural system can also signify one’s struggle to keep up with working memory demands.
Supplementary Material
Highlights.
FMRI was used to examine inner speech mechanisms of working memory.
A speech-like neural network increased activity as working memory demands increased.
Higher network activity on correct trials correlated with lower test performance.
Inner speech may intensify as one struggles with high working memory demands.
Acknowledgments
The authors would like to thank Deborah Ellis for her assistance with subject recruitment and data collection. MRI scans were obtained at the Kirby Center of the Kennedy Krieger Institute in Baltimore, MD. Parts of this research have been reported previously at the 37th Annual Meeting of Society for Neuroscience 2007, the 17th Annual Meeting of the Organization for Human Brain Mapping 2011, and the 19th Annual Meeting of the International Society of Behavioral Neuroscience 2011. This research was funded by NIMH R01 MH060234 (JED) and NIDA K01 DA030442 (CLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aboitiz F, Garcia RR, Bosman C, Brunetti E. Cortical memory mechanisms and language origins. Brain Lang. 2006;98(1):40–56. doi: 10.1016/j.bandl.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, Ivry RB. Temporal organization of “internal speech” as a basis for cerebellar modulation of cognitive functions. Behav Cogn Neurosci Rev. 2004;3(1):14–22. doi: 10.1177/1534582304263251. [DOI] [PubMed] [Google Scholar]
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6(3):202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105(1):158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Becker JT, MacAndrew DK, Fiez JA. A comment on the functional localization of the phonological storage subsystem of working memory. Brain and Cognition. 1999;41(1):27–38. doi: 10.1006/brcg.1999.1094. [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH. An fMRI investigation of syllable sequence production. Neuroimage. 2006;32(2):821–841. doi: 10.1016/j.neuroimage.2006.04.173. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104(37):14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociation within the frontoparietal network in verbal working memory: a parametric functional magnetic resonance imaging study. J Neurosci. 2010;30(10):3849–3856. doi: 10.1523/JNEUROSCI.0097-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Crottaz-Herbette S, Menon V. Temporal dynamics of basal ganglia response and connectivity during verbal working memory. Neuroimage. 2007;34(3):1253–1269. doi: 10.1016/j.neuroimage.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 2001;11(11):1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage. 2005a;24(2):332–338. doi: 10.1016/j.neuroimage.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005b;43(9):1227–1237. doi: 10.1016/j.neuropsychologia.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Conrad R. The Chronology of the Development of Covert Speech in Children. Developmental Psychology. 1971;5(3):398–405. [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19(4):1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. J Neurosci. 1997;17(24):9675–9685. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova A, Zeljko D, Schwarze F, Maschke M, Gerwig M, Frings M, et al. Probabilistic 3D MRI atlas of the human cerebellar dentate/interposed nuclei. Neuroimage. 2006;30(1):12–25. doi: 10.1016/j.neuroimage.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex. 2010;46(7):896–906. doi: 10.1016/j.cortex.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. NeuroImage. 1999;10(4):385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Gathercole S, Baddeley A. Evaluation of the Role of Phonological STM in the Development of Vocabulary in Children: A Longitudinal Study. Journal of Memory and Language. 1989;28:200–213. [Google Scholar]
- Gathercole S, Baddeley A. Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language. 1990;29:336–360. [Google Scholar]
- Ghosh SS, Tourville JA, Guenther FH. A neuroimaging study of premotor lateralization and cerebellar involvement in the production of phonemes and syllables. J Speech Lang Hear Res. 2008;51(5):1183–1202. doi: 10.1044/1092-4388(2008/07-0119). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayter AL, Langdon DW, Ramnani N. Cerebellar contributions to working memory. Neuroimage. 2007;36(3):943–954. doi: 10.1016/j.neuroimage.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Hulsmann E, Erb M, Grodd W. From will to action: sequential cerebellar contributions to voluntary movement. Neuroimage. 2003;20(3):1485–1492. doi: 10.1016/s1053-8119(03)00307-0. [DOI] [PubMed] [Google Scholar]
- Koch K, Wagner G, Schachtzabel C, Schultz C, Sauer H, Schlosser RG. Association between learning capabilities and practice-related activation changes in schizophrenia. Schizophr Bull. 2010;36(3):486–495. doi: 10.1093/schbul/sbq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10(6):695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated Labeling of the Human Brain: A Preliminary Report on the Development and Evaluation of a Forward-Transform Method. Human Brain Mapping. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiner HC. Solving the mystery of the human cerebellum. Neuropsychol Rev. 2010;20(3):229–235. doi: 10.1007/s11065-010-9140-z. [DOI] [PubMed] [Google Scholar]
- Levy BA. Role of Articulation in Auditory and Visual Short-Term Memory. Journal of Verbal Learning and Verbal Behavior. 1971;10:123–132. [Google Scholar]
- Marvel CL, Desmond JE. The contributions of cerebro-cerebellar circuitry to executive verbal working memory. Cortex. 2010a;46(7):880–895. doi: 10.1016/j.cortex.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Desmond JE. Functional topography of the cerebellum in verbal working memory. Neuropsychol Rev. 2010b;20(3):271–279. doi: 10.1007/s11065-010-9137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Turner BM, O’Leary DS, Johnson HJ, Pierson RK, Ponto LL, et al. The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology. 2007;21(6):761–777. doi: 10.1037/0894-4105.21.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray DJ. The effect of white noise upon the recall of vocalized lists. Can J Psychol. 1965;19(4):333–345. doi: 10.1037/h0082913. [DOI] [PubMed] [Google Scholar]
- Murray DJ. The role of speech responses in short-term memory. Can J Psychol. 1967;21(3):263–276. doi: 10.1037/h0082978. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools, I. E-Prime v1.1 (Version 1.1) Pittsburgh; 2002. [Google Scholar]
- Rauschecker AM, Pringle A, Watkins KE. Changes in neural activity associated with learning to articulate novel auditory pseudowords by covert repetition. Hum Brain Mapp. 2008;29(11):1231–1242. doi: 10.1002/hbm.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22(2):562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Roelofs A, Ozdemir R, Levelt WJ. Influences of spoken word planning on speech recognition. J Exp Psychol Learn Mem Cogn. 2007;33(5):900–913. doi: 10.1037/0278-7393.33.5.900. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, Petrides M, Evans AC, Toga AW. MRI Atlas of the Human Cerebellum. San Diego: Academic Press; 2000. [DOI] [PubMed] [Google Scholar]
- Silverman DHS, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5-1- years after chemotherapy. Breast Cancer Research and Treatment. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. In: Co-planar Stereotaxic Atlas of the Human Brain 3-D Proportional System, An Approach to Cerebral Imaging. Rayport M, translator. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16(3 Pt 1):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Vygotsky L. Thought and Language--Revised Edition. Cambridge, MA: The MIT Press; 1986. [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cognitive, affective & behavioral neuroscience. 2003;3(4):255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Zago L, Pesenti M, Mellet E, Crivello F, Mazoyer B, Tzourio-Mazoyer N. Neural correlates of simple and complex mental calculation. Neuroimage. 2001;13(2):314–327. doi: 10.1006/nimg.2000.0697. [DOI] [PubMed] [Google Scholar]
- Zago L, Petit L, Turbelin MR, Andersson F, Vigneau M, Tzourio-Mazoyer N. How verbal and spatial manipulation networks contribute to calculation: an fMRI study. Neuropsychologia. 2008;46(9):2403–2414. doi: 10.1016/j.neuropsychologia.2008.03.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.