Abstract
Non-steroidal anti-Inflammatory drugs (NSAIDs) exhibit antineoplastic properties, but conventional NSAIDs do not fully meet safety and efficacy criteria for use as anti-cancer agents. In this study, we evaluated the chemotherapeutic efficacy of five novel phospho-NSAIDs, each of which includes in addition to the NSAID moiety a diethylphosphate linked through a butane moiety. All five compounds inhibited the growth of human breast, colon and pancreatic cancer cell lines with micromolar potency. In vivo investigations confirmed the antitumor activity of phospho-aspirin (PA) and phospho-sulindac (PS) in inhibiting tumor growth in established human xenograft models, where cell proliferation was suppressed and apoptosis enhanced in the absence of detectable animal toxicity. Notably, all of the phospho-NSAIDs tested induced reactive oxygen and nitrogen species in cultured cells, with PA and PS inducing detectable levels of oxidative stress in vivo that were associated positively with apoptosis and negatively with proliferation. Potentially explaining these effects, all of the phospho-NSAIDs tested also inhibited the thioredoxin system and the redox sensitive transcription factor NF-κB. Taken together, our findings demonstrate the strong anticancer efficacy and promising safety of phospho-NSAIDs in preclinical models of breast, colon and pancreatic cancer, suggesting further evaluation as anticancer agents..
Keywords: Phospho-sulindac, phospho-ibuprofen, phospho-aspirin, phospho-flurbiprofen, cancer treatment, chemotherapy, thioredoxin, thioredoxin reductase, NF-κB
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most widely used anti-inflammatory compounds, with aspirin, the prototypical NSAID, being one of the oldest and still most extensively used medications in the world (1, 2). NSAIDs have a significant antineoplastic effect, which is currently viewed in the context of the recently appreciated role of inflammation in cancer (3). Interventional studies have established aspirin and sulindac as chemopreventive agents against colon cancer (4, 5). For the remaining NSAIDs, the evidence of their chemopreventive properties strong as it is, is mainly based on epidemiological studies (4, 6, 7). For example, a meta-analysis of 91 epidemiological studies showed a significant exponential decline with increasing intake of NSAIDs in the risk for 7–10 malignancies including the four major types: colon, breast, lung, and prostate cancer (8, 9). However, there is no chemotherapeutic application for aspirin or other NSAIDs in humans or animals. Only sulindac when combined with other anticancer agents is reported to have a chemotherapeutic effect in mice (10).
NSAIDs prevent cancer likely through pleiotropic effects reviewed in (11–13)). Although their best-recognized molecular target is the enzyme cyclooxygenase (COX), there is considerable evidence that such an effect may not be required for their anticancer actions (14, 15). Regardless of mechanistic issues, it is clear that conventional NSAIDs do not meet the criteria of safety and efficacy for their application as anti-cancer agents. NSAIDs are associated with considerable side effects, and their chemoprevention efficacy is rather limited, at best not exceeding 50% (16). Thus, there is a need to develop compounds with improved efficacy and safety.
Prompted by these considerations, we synthesized a series of compounds based on four representative NSAIDs, aspirin, sulindac (2 derivatives, differing in the structure of the sulindac moiety), ibuprofen, and flurbiprofen (Suppl. Fig. 1). Each one of these NSAIDs is chemopreventive against at least one major type of cancer based either on preclinical or epidemiological data (7, 8, 17, 18). Structurally, these five compounds belong to a broader class of novel compounds that we have synthesized, which conform to the general chemical formula A-aliphatic linker-DEP, where A can be any compound, the linker can vary in size and/or structure as long as it is aliphatic, and DEP= diethylphosphate. Here, we report on compounds where A=NSAID moiety and the linker is a moiety derived from 1,4-butane diol. Of note, the enhanced safety of the phospho-NSAIDs is attributed in part to this chemical modification, since the carboxylic group, present in nearly all NSAIDs, mediates much of their gastrointestinal toxicity (19).
Phospho-sulindac (PS; OXT-328), phospho-aspirin (PA; MDC-118) and phospho-ibuprofen (PI; MDC-917) are highly effective against inflammation, as shown in a rat arthritis model (20). All three had a favorable safety profile, especially concerning gastrointestinal toxicity (20, 21). Recently, PS was found in preclinical models of colon cancer to be strongly chemopreventive and chemotherapeutic. For example, in combination with difluoromethylornithine, PS prevented 91% of intestinal tumors in Min mice (21) and PS alone inhibited the growth of human colon xenografts in nude mice by 70% (22).
In the present study, we evaluated the chemotherapeutic potential of these five compounds against breast, colon, and pancreatic cancer using human cancer xenografts. All displayed a significant anticancer effect, which was mediated, at least to some extent, by a redox effect that likely affected major downstream signaling pathways.
MATERIALS AND METHODS
Reagents
Phospho-NSAIDs, synthesized following the methodology of Penning et al (23), were provided by Medicon Pharmaceuticals, Inc, Setauket, NY. The lipids for the preparation of liposomes were from Avanti Phospholipids (Alabaster, AL). For each compound we prepared a 100 mM stock solution in DMSO. In all cell culture media, the final DMSO concentration was adjusted to 1%. All general solvents and reagentswere of HPLC grade or of the highest grade commercially available.
Cell culture
Human breast (MCF-7 and MDA-MB 231), colon (HT-29, and SW480) and pancreatic (MIA PaCa-2 and BxPC-3) cell lines were obtained from AmericanType Culture Collection (Manassas, VA) and were grown in the specific medium and conditions suggested by ATCC. All the cell lines were passaged in our laboratory for less than 6 months after receipt.
Cell viability assay
Following treatment with various concentrations of phospho-NSAIDs for 24 h, the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye (MTT), was determined according to the manufacture’s protocol (Promega, Madison, WI, USA).
Cell kinetics analysis
5-bromo-2′-deoxyuridine (BrdU) incorporation into newly synthesized cellular DNA was used to determine cell proliferation as described (24). For apoptosis, 1.0×105 cells/well were treated with phospho-NSAIDs for 24 h, trypsinized, stained with Annexin V-FITC (100X dilution, Invitrogen) and PI (0.5 μg/ml, Sigma) and analyzed by FACScaliber (BD Bioscience). Cell cycle progression was analyzed by flow cytometry as described (24).
Determination of reactive oxygen and nitrogen species (RONS)
After treatment, cells were collected by trypsinization, resuspended in 10 μM of 5-(and-6)-carboxy-2′,7′-dichlorofluorescine diacetate (DCFDA; Invitrogen, Carlsbad, CA), incubated at 37 °C for 30 min in the dark and their fluorescence intensity was determined by flow cytometry (Beckman Coulter Inc., Fullerton, CA). We analyzed a minimum of 10,000 events using the WinMDI software and expressed the data as the fluorescent intensity versus events.
Determination of TrxR reductase activity
After treatment, cells were lysed and TrxR activity was determined in the protein lysate using a commercially available kit, following the instructions of the manufacturer (Cayman Chemical). In this assay, TrxR uses NADPH to reduce 5,5′-dithiobis-(2-nitrobenzoic acid) to 5-thio-2-nitrobenzoic acid (TNB).
Protein extraction from whole cell lysates
After treatment, cells were scraped on ice, washed with ice-cold phosphate-buffered saline (PBS) and lysed in RIPA lysis buffer (Sigma, St Louis, MO). Protein concentration was determined using the Bradford method (Bio-Rad, Hercules, CA).
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear fractions were isolated from 2×106 cells (treated and controls) and subjected to EMSA as previously described (24).
Liposome-PI
PI was loaded onto PEGylated liposomes, following standard protocols (25, 26). Briefly, 50 mg phosphatidylcholine, 19 mg 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] and 40 mg of PI were dissolved in chloroform. The free drug was removed by dialyzing the liposomal solution against PBS, and PI concentration was determined by HPLC.
Efficacy in nude mouse breast, colon and pancreatic xenografts
Female Balb/C nude mice (Charles River Laboratories, Wilmington, MA) were inoculated subcutaneously into each of their flanks with 2.5×106 MDA-MB-231 human breast cancer cells in Matrigel (BD Biosciences, Franklin Lakes, NJ) or 1.5×106 HT-29 colon cancer cells or 2×106 BxPC-3 human pancreatic cancer cells in PBS (final volume 100 μl). Once the tumor reached approximately 100–150 mm3, animals were randomized into the control group, which received corn oil and the treatment groups which received PA 110 mg/kg or PS 160 mg/kg in corn oil or PI or liposome-PI 300 mg/kg (n=10/group). The treatment was administered once daily x5d/wk by oral gavage for PA and PS or by intraperitoneally for PI and liposome-PI (Lipo-PI). Tumor volume was calculated as [length × width × (length + width/2) × 0.56]. Tumor growth inhibition was calculated by dividing the percent increase from baseline of the tumor volume of the treated group over the corresponding percent volume increase of the control group. At the end of treatment, animals were sacrificed and tumors were removed and weighed.
Immunohistochemistry
Immunohistochemical staining for Ki-67, TrxR and phospho-NF-κB (activated form of NF-κB) was performed on human breast, colon and pancreatic xenograft tissue samples as previously described (27). Apoptosis was determined by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end-labeling (TUNEL) assay (28). Positive control: treatment of samples with DNAse I. Scoring: At least 10 fields/sample (at magnification 200x) were scored independently by investigators blinded to their identity. Cells with a blue nucleus (or blue cytoplasma in the case of TrxR) were considered unlabelled, while those with a brown nucleus (or brown cytoplasma in the case of TrxR) were considered labeled. We calculated the percentage of proliferating and apoptotic cells by dividing the number of labeled cells by the number of cells in each field and multiplying by 100.
Statistical analyses
Results are expressed as Mean ± SEM. Differences between groups were determined by one-factor analysis of variance followed by Tukey’s test for multiple comparisons and the association between data sets by correlation analysis and computing the correlation coefficient (R). p<0.05 was statistically significant.
RESULTS
Phospho-NSAIDs inhibit the growth of human breast, colon and pancreatic cancer in vitro and in xenografts
Initially, we evaluated the growth inhibitory effect of the five phospho-NSAIDs in six human cancer cell lines, two each from breast, colon and pancreatic cancers. The members of each pair differ significantly from each other. Table 1 shows key genotypic features of each cell line and the 24-h IC50 of these compounds, which range from 17 to 303 μM. It seems that the growth inhibition potency of these compounds was independent of the tissue of origin, receptor status or COX expression of these cell lines.
Table 1.
The effect of phospho-NSAIDs on the growth of human cancer cell lines
| 24h-IC50, μM | ||||||
|---|---|---|---|---|---|---|
| Breast | Colon | Pancreas | ||||
| Compound | MCF-7 | MDA-MB-231 | HT-29 | SW480 | BxPC-3 | MIA Paca-2 |
| ER+, PR+ | ER−, PR−, HER2/Neu− | COX-2+ | COX-2− | COX-2+, K-ras WT | COX-2−, K-ras mutant | |
| PS | 62 | 17 | 65 | 98 | 62 | 88 |
|
| ||||||
| PDS | 38 | 18 | 70 | 73 | 32 | 92 |
|
| ||||||
| PF | 65 | 17 | 80 | 104 | 34 | 135 |
|
| ||||||
| PI | 79 | 28 | 82 | 75 | 53 | 104 |
|
| ||||||
| PA | 32 | 199 | 54 | 83 | 63 | 303 |
Values are representative of at least three independent experiments performed in quintuplicate; results were within ~15%.
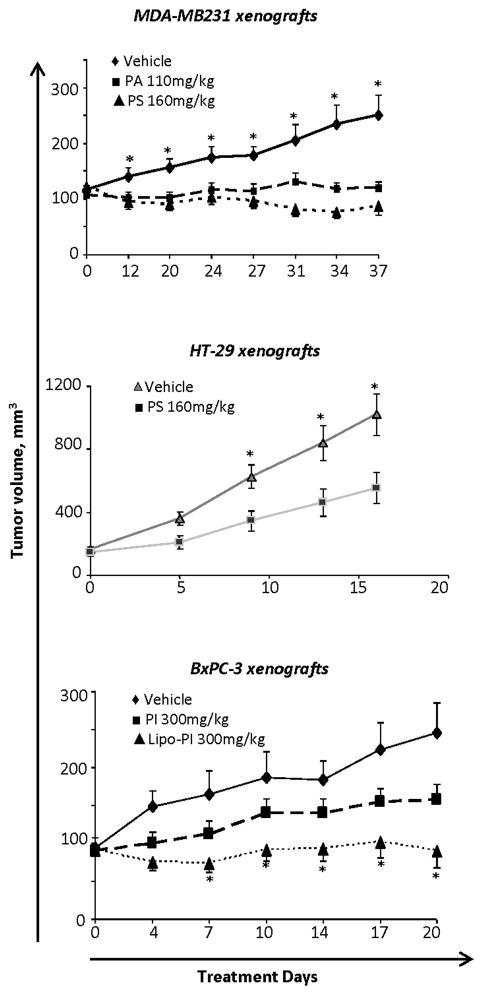
We then determined the effect of PA, PS and PI on the growth of breast, colon and pancreatic human cancer xenografts in athymic nude mice (Fig. 1). Our results are as following: a) MDA-MB-231 human breast cancer xenografts: PA 110 mg/kg and PS 160 mg/kg were both given orally daily 5d/wk, starting when the tumor volume reached approximately 100 mm3 and continued to day 37 when mice were euthanized. Compared to controls, both drugs inhibited tumor growth statistically significantly between day 12 and the end of the study. On day 37, the tumor volume of each study group was as follows: control = 250±48 mm3 (mean ± SEM for this and all subsequent values), PA = 119±50 mm3 and PS = 87±32 mm3, representing growth inhibition of 87.3% (tumor stasis) by PA and 108.6% (tumor regression) by PS (p<0.01–0.05 for both). b) HT-29 human colon cancer xenografts: PS 160 mg/kg was given orally daily, 5d/wk, starting when the tumor volume was approximately 150 mm3. Tumor growth inhibition became statistically significant staring on treatment day 9. After 17 days of treatment, the tumor volume of the control group was 1,020±130 mm3 and of the PS-treated group 554±96 mm3 (mean ± SEM for both groups), representing a 54.0% tumor growth inhibition by PS (p<0.05). c) BxPC-3 human pancreatic cancer xenografts: PI 300 mg/kg, plain or incorporated in liposomes (Lipo-PI), was given intraperitoneally daily, 5d/wk for 20 days, starting when the tumor volume was ~100 mm3. At sacrifice, the tumor volume of each study group was as follows: control = 246±40 mm3 (mean ± SEM for this and all subsequent values), PI = 157±20 mm3 and Lipo-PI = 90±23 mm3. After 20 days of treatment, PI reduced tumor growth by 57% (not statistically significant), whereas Lipo-PI completely prevented tumor growth (100% inhibition, p<0.01–0.05) maintaining tumor stasis throughout the treatment period. Underscoring the significance of the drug delivery method, the mouse plasma level of PI was 3.5-fold higher in Lipo-PI compared to PI (data not shown). Of note, the liposomal formulation of PI had a minimal effect on its in vitro IC50 (Suppl Fig. 2).
Fig. 1. Phospho-NSAIDs inhibit the growth of breast, colon and pancreatic xenografts.
Nude mice bearing xenografts of MDA-MB-231 breast or HT-29 colon or BxPC-3 pancreatic cancer cells were treated with phospho-NSAIDs or vehicle as indicated doses. Values are mean ± SEM, *, p<0.01–0.05, compared to the respective control; n=10–16 tumors/group.
Consistent with our previous findings (20, 21), none of the animals showed any signs of toxicity from the phospho-NSAIDs, including weight loss or distress. In addition, on autopsy at sacrifice there were no signs of GI bleeding, or gastrointestinal, pancreatic, renal or liver damage.
The cytokinetic effect of phospho-NSAIDs in vitro and in vivo
To understand the mechanism by which these compounds inhibited cell and tumor growth, we evaluated their effect on cell kinetics in both cancer cells and xenografts.
Inhibition of cell proliferation
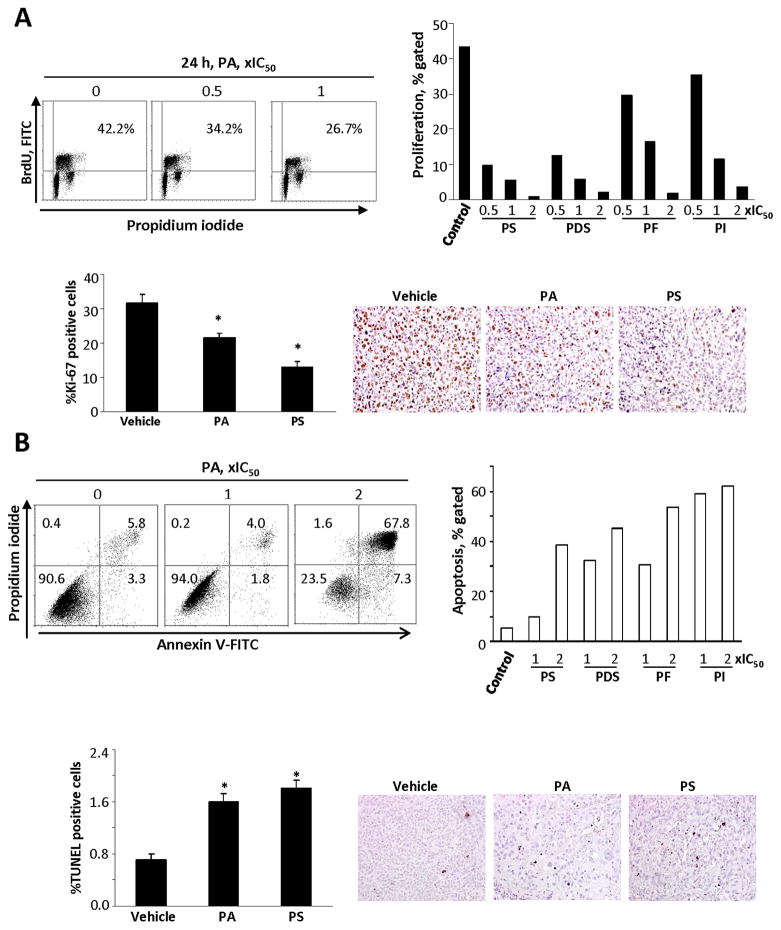
At their IC50, the five phospho-NSAIDs inhibited the proliferation of MDA-MB-231 breast cancer cells 37–87% compared to controls, with PS being the most and PA the least potent. Similar results were obtained in BxPC-3 human pancreatic cells. The phospho-NSAIDs also inhibited cell proliferation in xenografts from these cell lines by 29% – 59%, except for plain PI, whose effect (13% reduction) was not statistically significant and contrasted to that of Lipo-PI. These results are shown in Table 2A, Fig. 2A, Suppl. Table 1 and Suppl. Fig. 3.
Table 2.
The effect of phospho-NSAIDs on cytokinetics, F2-isoprostane, and NF-κB
| A. Cytokinetic effect on xenografts
| ||||
|---|---|---|---|---|
| MDA-MB-231 | HT-29 | BxPC-3 | ||
| PA | PS | PS | PI | Lipo-PI |
| Inhibition of proliferation, % over control
| ||||
| 32% | 59% | 29% | 13% | 50% |
| p<0.05 | p<0.05 | p<0.01 | NS* | p<0.01 |
|
| ||||
| Induction of apoptosis, % over control
| ||||
| 128% | 157% | 125% | 71% | 171% |
| p<0.01 | p<0.01 | p<0.005 | p<0.01 | p<0.01 |
| B. Effect on urinary F2-isoprostane levels in mice
| |||
|---|---|---|---|
| Xenograft | Control | PA | PS |
| ng/mg creatinine (% increase) | |||
| MDA-MB-231 | 4.2±1.3 | 9.4±2.2 (124%) | 18.4±3.7 (338%) |
| p<0.05 | p<0.05 | ||
| HT-29 | 18.7±1.3 | ND** | 28.6±4.2 (53%) |
| p<0.01 | |||
| C. Effect on NF-κB activation in xenografts
| ||||
|---|---|---|---|---|
| MDA-MB-231 | HT-29 | BxPC-3 | ||
| PA | PS | PS | PI | Lipo-PI |
| Inhibition of NF-κB, % over control
| ||||
| 44% | 56% | 21% | 35% | 70% |
| p<0.04 | p<0.04 | p<0.03 | p<0.05 | p<0.002 |
, NS=Not significant;
, ND=Not determined.
Each statistical comparison is to the respective control group
Fig. 2. The cytokinetic effect of phospho-NSAIDs on MDA-MB-231 cells in vitro and in vivo.
A. Upper panel: Cell proliferation assay based on BrdU incorporation into DNA during the S-phase of the cell cycle. MDA-MB-231 cells were grown overnight and treated with various phospho-NSAIDs as shown. The percentage of BrdU positive cells after treatment with PA is shown in the right upper corner of each panel. The bar graph summarizes the finding with each phospho-NSAID. The study was repeated at least once generating results within 11%. Lower panel: Cell proliferation was determined by Ki-67 expression in tissue sections of MDA-MB-231 xenografts harvested at sacrifice. The bar graph shows the percentage of Ki-67 positive cells. Values are mean ± SEM; *, p<0.01 compared to control. Three representative tissue sections are shown; magnification 200X. B. Upper panel: Flow cytometric analysis of cells stained with propidium iodide and Annexin-V (A). A(−)/PI(−) cells are viable cells; A(+)/PI(−) are early apoptotic; A(+)/PI(+) are late apoptotic; and A(−)/PI(+) are necrotic. The numbers inside each panel represent the percentage of cells in each category. The bar graph summarizes the finding with each phospho-NSAID. The study was repeated at least once generating results within 10%. Lower panel: Apoptosis was determined by the TUNEL assay in tissue sections of MDA-MB231 xenografts harvested at sacrifice. The bar graph shows the mean ± SEM values of each group; *, p<0.01 compared to control. Three representative tissue sections are shown; magnification 200X.
Induction of apoptosis
We distinguished two stages of apoptosis, discernible by flow cytometry, namely “early apoptosis,” in which the cells are viable (they exclude propidium iodide) and “late apoptosis,” known also as secondary necrosis, in which the cells cannot exclude propidium iodide. The main findings in MDA-MB-231 cells are: a) late apoptosis predominates; b) PI had the strongest and PA the weakest pro-apoptotic effect; and c) despite their minimal structural differences, PDS was more potent than PS. Of note, PI evaluated in BxPC-3 cells had similar effects. In breast, colon and pancreatic xenografts PA, PS and PI induced apoptosis significantly, ranging between 71% and 171% over controls. These results are shown in Table 2A, Fig. 2B, Suppl. Table 1 and Suppl. Fig. 4.
Induction of necrosis
All five compounds induced necrosis modestly, not exceeding 7.2% of the total number of MDA-MB-231 cells (Suppl. Table 1). As with apoptosis, PI was the most potent inducer of cell necrosis, generating similar results in BxPC-3 cells (Suppl. Fig. 4A).
Induction of cell cycle arrest
All compounds inhibited the G1 to S cell cycle phase transition in MDA-MB-231 cells, except for PS, which arrested the G2/M phase transition (Suppl. Fig. 5A, B). Similar effects were obtained in BxPC-3 cells treated with PI (Suppl. Fig. 5C).
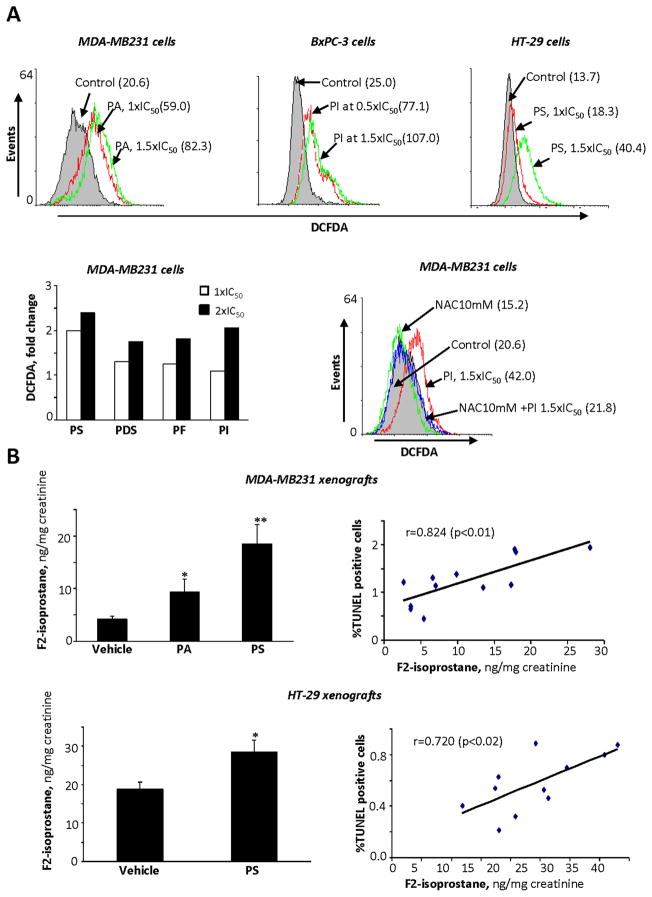
Induction of reactive oxygen and nitrogen species (RONS): Correlation with cytokinetic changes
RONS play a crucial role in the mechanism of action of several anticancer agents (29–31). We determined the effect of phospho-NSAIDs on the intracellular levels of RONS using the general RONS probe, DCFDA. Compared to control by 1 h and at 1.5xIC50, RONS levels were enhanced 3.3-fold by PI (BxPC-3 cells), 3-fold by PA (MDA-MB-231 cells) and 1.9-fold by PS (HT-29 cells); this effect was concentration-dependent (MDA-MB-231 cells) (Fig. 3A). Interestingly, pretreatment of the cells with 10 mM NAC abrogated RONS induction (completely in MDA-MB-231 cells and by 59% in BxPC-3 cells treated with PI; Fig. 3A and Suppl. Fig. 6A).
Fig. 3. The effect of phospho-NSAIDs on RONS levels in breast, colon and pancreatic cancer cells and xenografts.
A. Upper panel: MDA-MB231, BxPC-3 and HT-29 cells were preloaded with DCFDA, a general probe for RONS, and treated with PA, PI or PS for 1 h as shown. The values in parentheses are the geometric mean of the respective histograms. Lower left panel: RONS levels, measured with DCFDA, were quantified in MDA-MB-231 cells after treating them with 1x or 2xIC50 phospho-NSAIDs for 1 h. Lower right panel: RONS levels, detected by DCFDA flow cytometry, were decreased following pre-treatment with NAC. B: The levels of F2-isopostane were determined using an ELISA kit, as in Methods. in 24-hr urine of nude mice bearing MDA-MB-231 or HT-29 xenografts. Values are mean ± SEM; *, p<0.05 and **, p<0.01, compared to control. Right panels: The association between apoptosis (TUNEL(+) cells) in xenografts and urinary F2-isoprostane levels.
A similar effect was documented in vivo, by measuring the urinary levels of F2-isoprostane in mice; F2-isoprostane is a marker of oxidative stress (32, 33). As shown in Fig. 3B and Table 2B, PA and PS increased the levels of urinary F2-isoprostane in mice with MDA-MB-231 and HT-29 xenografts between 53 and 338% compared to the corresponding controls (all changes were statistically significant). We then assessed whether the induction of oxidative stress by phospho-NSAIDs correlated with the cytokinetic changes induced by these compounds in the xenografts (Fig. 3B). In both MDA-MB-231 and HT-29 xenografts, the urinary F2-isoprostane levels were positively associated with the percentage of apoptotic cells (R=0.824, p<0.01 and R=0.720, p<0.02, respectively) and negatively associated with the percentage of proliferating cells (R=−0.78, p<0.01 and R=−0.60, p<0.05, respectively; data not shown). These findings suggest a potential etiological connection between oxidative stress and the cytokinetic changes in tumor xenografts that underlie the antitumor effect of the phospho-NSAIDs.
Phospho-NSAIDs inhibit the thioredoxin system
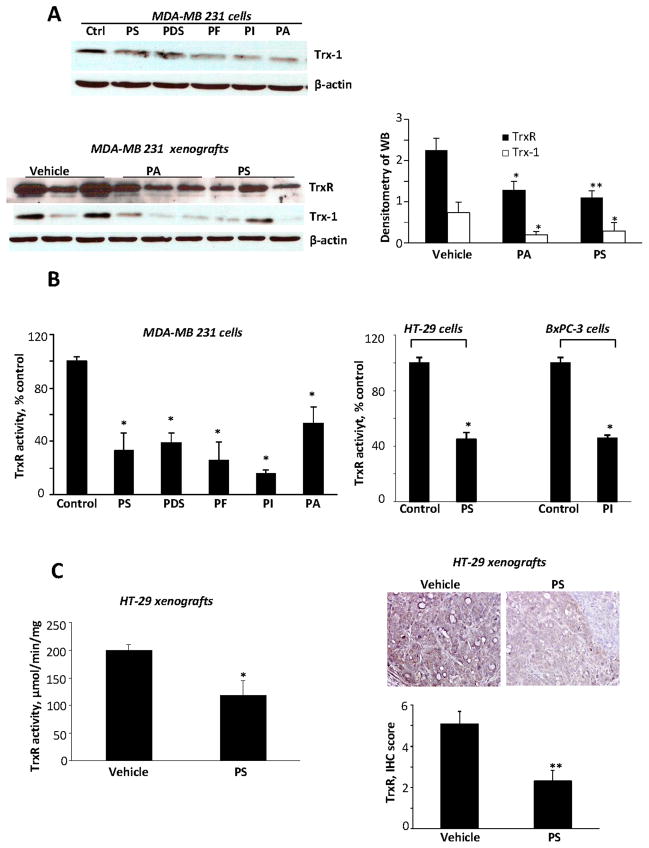
A critical redox modulator in the cells is the thioredoxin system (34). We have shown that the thioredoxin system could mediate redox-induced cell death in response to various anticancer agents (31). Trx-1, overexpressed in human cancers, has been linked to aggressive tumor growth, inhibition of apoptosis, and decreased patient survival (35). In cultured MDA-MB-231, the phospho-NSAIDs decreased the expression of Trx-1 (Fig. 4A). The effect of phospho-NSAIDs on Trx-1 expression in xenografts was variable. In BxPC-3 xenografts, Lipo-PI decreased Trx-1 expression levels by 61.6% (p<0.001) but plain PI failed to do so, in keeping with its lack of antitumor effect (Suppl. Fig. 6B). PA and PS also decreased Trx-1 expression in MDA-MB-231 xenografts by 75% and 63%, respectively (p<0.05 for both) (Fig. 4A). However, in HT-29 xenografts PS did not decrease Trx-1 levels, determined both by immunoblotting of tissue extracts and immunohistochemistry (Suppl. Fig. 6C).
Fig. 4. Phospho-NSAIDs inhibit Trx-1 and TrxR levels, and inhibit TrxR activity.
A: Upper panel: Immunoblots of Trx-1 in MDA-MB-231 cells treated with the indicated phospho-NSAIDs for 2 h, each at 1.5xIC50. Lower panel: Immunoblots of TrxR and Trx-1 in protein extracts from MDA-MB-231 xenografts treated with vehicle or with PA or PS for 37 days, as in Fig. 1. Band density, quantified using Image J software. Results are shown in the bar graph. Values are mean ± SEM; *, p<0.05 and **, p<0.01, compared to control. B: TrxR activity was determined as in Methods in MDA-MB231, HT-29 and BXPC-3 cells treated with phospho-NSAIDs 1.5xIC50 for 1 h. Values are mean ± SEM; *, p<0.01 compared to control. C: Left: TrxR activity was determined in protein lysates from HT-29 xenografts from animals treated with vehicle or PS for 17 days, as in Fig. 1. Right: Tumor tissue sections were immunohistochemically stained for TrxR and the number of TrxR-positive cells was determined and a score value was calculated (bar graph). Values are mean ± SEM; *, p<0.02, **, p<0.01, compared to control. Representative images are shown; magnification 200X.
TrxR, overexpressed in many cancers and associated with drug resistance, represents a key drug target (36). Thus, we determined the activity of TrxR in cultured breast, colon and pancreatic cancer cells treated with our phospho-NSAIDs. As shown in Fig. 4B, in MDA-MB-231, BxPC-3 and HT-29 cells, our five phospho-NSAIDs reduced TrxR activity by 53%–84%, compared to controls (statistically significant for all). PA and PS also decreased the expression of TrxR in MDA-MB-231 xenografts by 43%, and 51%. (p<0.03, and p<0.02, respectively; Fig. 4A). Furthermore, in HT-29 xenografts, PS reduced TrxR activity by 40% (p<0.02) and TrxR levels determined immunohistochemically by 55% (p<0.01; Fig. 4C).
Phospho-NSAIDs inhibit the activation of NF-κB
To further assess the biological relevance of the induction of oxidative stress by phospho-NSAIDs, we explored their effect on an intracellular redox-dependent signaling pathway. Thus we analyzed their effect on NF-κB, whose transcriptional activity is sensitive to redox changes (37). NF-κB modulates tumor cell growth, with the outcome depending on biological context (38).
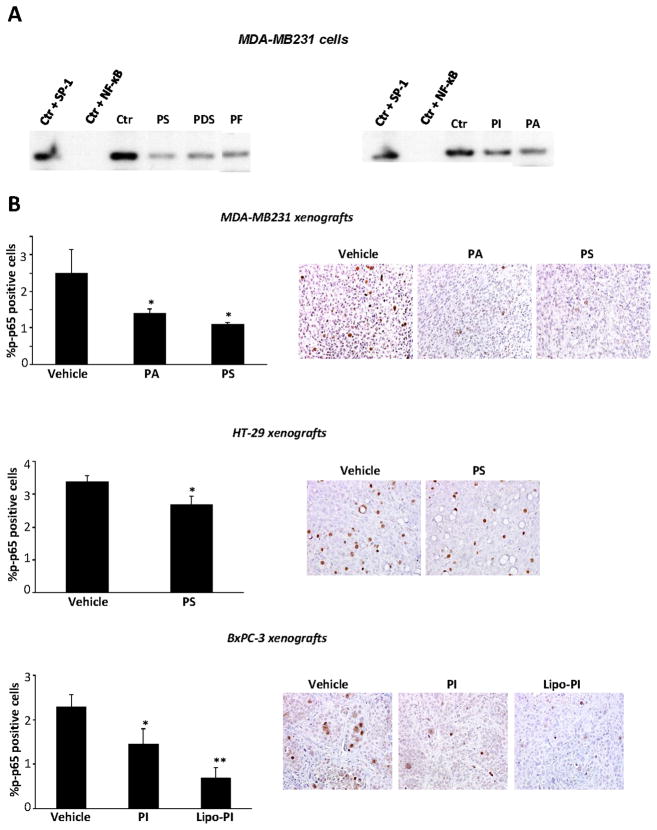
In vitro all five compounds inhibited NF-κB activation determined by the electrophoretic mobility shift assay (EMSA) in MDA-MB-231 breast cancer cells (Fig. 5A). A similar inhibitory effect was observed in vivo; we assessed in xenografts NF-κB activation by determining immunohistochemically the levels of p-p65 (Fig. 5B). As shown in Table 2C, in MDA-MB-231, HT-29 and BxPC-3 xenografts, PA and PS, PI and Lipo-PI reduced the percentage of p-p65 positive cells by 21% – 70 % (all statistically significant). Of note the effect of the more effective Lipo-PI was nearly double that of plain PI.
Fig. 5. Phospho-NSAIDs inhibit NF-κB activation in breast, colon and pancreatic cancer cells and in xenografts.
A: Phospho-NSAIDs inhibit constitutive NF-κB activation. EMSA for NF-κB of nuclear fractions isolated from MDA-MB-231 cells after 4 h-treatment without or with each phospho-NSAID at 1.5xIC50. To determine the specificity of the NF-κB transcription factor-DNA complex, the control nuclear fraction was incubated in the presence of 100-fold molar excess of unlabeled oligonucleotide containing the consensus sequence for either the specific (+ NF-κB) or an unspecific (+ SP-1) transcription factor. A representative image is shown. B: NF-κB (p-p65) levels from MDA-MB-231, HT-29 or BxPC-3 tumors were determined by immunohistochemistry using an anti-p-p65 antibody. The percentage of p-p65-positive cells is randomly selected fields was determined and averaged for each xenograft. Values are mean ± SEM; *, p<0.05, **, p<0.002, compared to control. Representative images are shown; magnification 200X.
DISCUSSION
Our data demonstrate in preclinical models of breast, colon and pancreatic cancer the significant anti-cancer properties of phospho-NSAIDs, representing a broader class of compounds. Phospho-NSAIDs exert their anticancer effect by inducing a state of oxidative stress in tumors that then modulates intracellular signaling pathways, such as NF-κB, an effect that culminates in enhanced apoptosis and suppressed cancer cell renewal.
The inhibition of cancer growth by phospho-NSAIDs has five features: First, it is quantitatively significant, as evidenced by the effect of the three compounds tested in xenograft tumor models. Second, the degree of tumor inhibition differs between compounds, with PS being the most efficacious. Third, the response of tumors to a given compound depends on the origin of the tumor, as demonstrated with PS, which caused tumor stasis in breast cancer xenografts but only modest growth inhibition in colon cancer xenografts. Fourth, the mode of drug delivery seems to influence substantially drug efficacy, as shown in the case of PI whose efficacy was significantly increased by its incorporation into liposomes. Finally, in contrast to conventional NSAIDs, the phospho-NSAIDs tested in mice appear to be safe, a finding consistent with our previous results (20, 21).
An interesting observation concerns the relatively high IC50 values of our compounds in vitro that contrast with their in vivo efficacy. Although counterintuitive, there are numerous examples of such discordant behavior, including DFMO, aspirin and valproic acid that have IC50 values in the mM range but are quite effective in animals and humans (5, 39, 40). What underlies this difference is not readily apparent, but the differential complexity of the two systems impacts the ability of in vitro IC50 values to predict in vivo responses.
As might be anticipated, tumor growth inhibition by these compounds is associated with a cytokinetic effect. All phospho-NSAIDs induced apoptosis and inhibited proliferation to a quantitatively significant but varying degree; neither effect, however, appeared dominant. Of interest, our cell culture studies mirror to some extent the in vivo cytokinetic effect of these compounds.
A key feature of the mechanism of action of phospho-NSAIDs was their ability to induce oxidative stress. This effect was noted in both cultured cells and animals. In cultured cells, the induction of oxidative stress levels was significant, concentration-dependent and inhibitable by the antioxidant NAC. Again, there were individual variations, depending on the cell line and the compound. In animals, oxidative stress was assayed by the urinary levels of F2-isoprostane. This metabolite of PGF2α provides a validated measure of oxidative stress in the animal over some period of time (usually hours). As we have demonstrated recently, in the case of PI, the xenograft is indeed the source of the increased F2-isoprostane (41). Thus we can extrapolate that the oxidative stress that we observed in these studies originated in the xenografts. The induction of oxidative stress by the phospho-NSAIDs was a consequential event. There is a clear association between oxidative stress and cytokinetic changes (enhanced apoptosis, suppressed proliferation) both in vitro and, importantly, in vivo. To a first approximation, these changes underlie the tumor inhibitory effect of these compounds, making the redox homeostatic mechanism their prime molecular target.
Although cell signaling tends to be complex, the effect of the phospho-NSAIDs on the NF-κB pathway illustrates a possible link between drug-induced oxidative stress and cancer growth inhibition. It is conceivable that the anticancer effect of phosphor-NSAIDs is related in part to their redox effect on NF-κB, a prototypical redox-sensitive signaling pathway with relevance to cancer. Constitutive NF-κB activation is a hallmark of various types of cancer, including breast, colon and pancreatic (42–45). NF-κB, activated by oncogenes, carcinogens and inflammatory stimuli, promotes carcinogenesis and consequently has become a therapeutic target in cancer. When the strategically located Cys62 of NF-κB is oxidized, the NF-κB dimmer is rendered incapable of binding to DNA thus losing its extensive transcriptional effects. Phospho-NSAIDs inhibited NF-κB activation both in vitro and in vivo. This is a broader property of phospho-NSAIDs, which we recently showed to inhibit NF-κB activation in arthritis-associated inflammation (20). It is plausible that the effect of phospho-NSAIDs mediates part of their anticancer effect.
Equally complex is the mechanism by which these compounds induce oxidative stress. The central role of ROS as an apoptotic trigger in response to phospho-NSAIDs was documented recently (41). In the present work we focused on the thioredoxin system that consists of Trx, TrxR and NADPH. A pivotal component of cellular redox homeostasis, the thioredoxin system is involved in carcinogenesis and also in the response of tumors to chemotherapy (46–48). When RONS oxidize a cellular protein, thioredoxin reduces it, being itself oxidized in the process; TrxR restores the oxidized Trx to its normal status. Phospho-NSAIDs had a profound effect on the two protein members of this system, Trx-1 and TrxR. The expression of both was suppressed by phospho-NSAIDs and, in addition, the activity of TrxR (the only one of the two that was assayed for) was suppressed, generating a substantial cumulative effect. In only one case (HT-29 xenografts) the expression of Trx-1 was not suppressed by a phospho-NSAID (PS), but both the activity and expression of TrxR was suppressed, likely inactivating the thioredoxin system. Of note, PS did suppress Trx-1 expression in HT-29 xenografts treated under a different drug administration protocol (22). It is, therefore, clear, that based on the role of the thioredoxin system this effect contributes to the induction of the all-important state of oxidative stress with its direct repercussion on tumor mass (loss of cells through apoptosis and dampened cell renewal). Indeed between the two, TrxR was the one that was suppressed the most by the phospho-NSAIDs. Consistently, we have shown in an effective combination study of P-S and DFMO that TrxR correlated with tumor growth, stressing the importance of the Trx system as a molecular target of these drugs (22).
In conclusion, our findings indicate that these five phospho-NSAIDs, belonging to the broader pharmacological category of modified-NSAIDs, show strong anticancer efficacy and promising safety. Of particular interest is their shared ability to induce oxidative stress, an early event that appears to trigger cancer inhibitory cascades. Taken together, our data indicate that phospho-NSAIDs merit further evaluation as anticancer agents.
Supplementary Material
Acknowledgments
Grant support: NIH grants R01-CA139453, R01CA13945402 and 1N01CN43302WA22 and HHSN261201000109C
Footnotes
Competing interests
The authors have nothing to disclose except for BR, who has an equity position in Medicon Pharmaceuticals, Inc. and DK who works at the same
Refrences
- 1.Stanley P, Hegedus R. Aspirin--the first hundred years. Biologist (London) 2000;47:269–71. [PubMed] [Google Scholar]
- 2.Rinsema TJ. One hundred years of aspirin. Med Hist. 1999;43:502–7. doi: 10.1017/s0025727300065728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron JA. What now for aspirin and cancer prevention? J Natl Cancer Inst. 2004;96:4–5. doi: 10.1093/jnci/djh027. [DOI] [PubMed] [Google Scholar]
- 5.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–15. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 7.Thun MJ, Henley SJ, Gansler T. Inflammation and cancer: an epidemiological perspective. Novartis Found Symp. 2004;256:6–21. discussion 2–8, 49–52, 266–9. [PubMed] [Google Scholar]
- 8.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–83. [PubMed] [Google Scholar]
- 9.Ratliff TL. Aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) J Urol. 2005;174:787–8. doi: 10.1016/s0022-5347(01)68393-9. [DOI] [PubMed] [Google Scholar]
- 10.Yip-Schneider MT, Wu H, Ralstin M, Yiannoutsos C, Crooks PA, Neelakantan S, et al. Suppression of pancreatic tumor growth by combination chemotherapy with sulindac and LC-1 is associated with cyclin D1 inhibition in vivo. Mol Cancer Ther. 2007;6:1736–44. doi: 10.1158/1535-7163.MCT-06-0794. [DOI] [PubMed] [Google Scholar]
- 11.Babbar N, Gerner EW. Targeting polyamines and inflammation for cancer prevention. Recent Results Cancer Res. 2011;188:49–64. doi: 10.1007/978-3-642-10858-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Din FV, Theodoratou E, Farrington SM, Tenesa A, Barnetson RA, Cetnarskyj R, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–9. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- 13.Kwan ML, Habel LA, Slattery ML, Caan B. NSAIDs and breast cancer recurrence in a prospective cohort study. Cancer Causes Control. 2007;18:613–20. doi: 10.1007/s10552-007-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila Pa) 2009;2:572–80. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Chen J, Rigas B. Chemopreventive agents induce oxidative stress in cancer cells leading to COX-2 overexpression and COX-2-independent cell death. Carcinogenesis. 2009;30:93–100. doi: 10.1093/carcin/bgn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rayyan Y, Williams J, Rigas B. The role of NSAIDs in the prevention of colon cancer. Cancer Invest. 2002;20:1002–11. doi: 10.1081/cnv-120005917. [DOI] [PubMed] [Google Scholar]
- 17.Wechter WJ, Leipold DD, Murray ED, Jr, Quiggle D, McCracken JD, Barrios RS, et al. E-7869 (R-flurbiprofen) inhibits progression of prostate cancer in the TRAMP mouse. Cancer research. 2000;60:2203–8. [PubMed] [Google Scholar]
- 18.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 19.Piazza GA, Keeton AB, Tinsley HN, Gary BD, Whitt JD, Mathew B, et al. A novel sulindac derivative that does not inhibit cyclooxygenases but potently inhibits colon tumor cell growth and induces apoptosis with antitumor activity. Cancer Prev Res (Phila) 2009;2:572–80. doi: 10.1158/1940-6207.CAPR-09-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang L, Mackenzie G, Ouyang N, Sun Y, Xie G, Johnson F, et al. The novel phospho-non-steroidal anti-inflammatory drugs, OXT-328, MDC-22 and MDC-917, inhibit adjuvant-induced arthritis in rats. Br J Pharmacol. 2011;162:1521–33. doi: 10.1111/j.1476-5381.2010.01162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackenzie GG, Sun Y, Huang L, Xie G, Ouyang N, Gupta RC, et al. Phospho-sulindac (OXT-328), a novel sulindac derivative, is safe and effective in colon cancer prevention in mice. Gastroenterology. 2010;139:1320–32. doi: 10.1053/j.gastro.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackenzie GG, Ouyang N, Xie G, Vrankova K, Huang L, Sun Y, et al. Phospho-sulindac (OXT-328) combined with difluoromethylornithine prevents colon cancer in mice. Cancer Prev Res (Phila) 2011;4:1052–60. doi: 10.1158/1940-6207.CAPR-11-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–65. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Mackenzie GG, Murray OT, Zhang Z, Rigas B. Phosphoaspirin (MDC-43), a novel benzyl ester of aspirin, inhibits the growth of human cancer cell lines more potently than aspirin: a redox-dependent effect. Carcinogenesis. 2009;30:512–9. doi: 10.1093/carcin/bgp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madden TD, Bally MB, Hope MJ, Cullis PR, Schieren HP, Janoff AS. Protection of large unilamellar vesicles by trehalose during dehydration: retention of vesicle contents. Biochimica et biophysica acta. 1985;817:67–74. doi: 10.1016/0005-2736(85)90069-0. [DOI] [PubMed] [Google Scholar]
- 26.Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochimica et biophysica acta. 1986;858:161–8. doi: 10.1016/0005-2736(86)90302-0. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR){delta} expression in APCmin/+ mice proportionally to their tumor inhibitory effect: Implications for the role of PPAR{delta} in carcinogenesis. Carcinogenesis. 2006;27:232–9. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 28.Rigas B, Kozoni V. The novel phenylester anticancer compounds: Study of a derivative of aspirin (phoshoaspirin) Int J Oncol. 2008;32:97–100. [PubMed] [Google Scholar]
- 29.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–60. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun Y, Huang L, Mackenzie GG, Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-NSAIDs: Implications for the role of oxidative stress in the action of anticancer agents. The Journal of pharmacology and experimental therapeutics. 2011 doi: 10.1124/jpet.111.183533. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Rigas B. The thioredoxin system mediates redox-induced cell death in human colon cancer cells: implications for the mechanism of action of anticancer agents. Cancer Res. 2008;68:8269–77. doi: 10.1158/0008-5472.CAN-08-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008;10:1405–34. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 33.Tacconelli S, Capone ML, Patrignani P. Measurement of 8-iso-prostaglandin F2alpha in biological fluids as a measure of lipid peroxidation. Methods Mol Biol. 2010;644:165–78. doi: 10.1007/978-1-59745-364-6_14. [DOI] [PubMed] [Google Scholar]
- 34.Pennington JD, Jacobs KM, Sun L, Bar-Sela G, Mishra M, Gius D. Thioredoxin and thioredoxin reductase as redox-sensitive molecular targets for cancer therapy. Current pharmaceutical design. 2007;13:3368–77. [PubMed] [Google Scholar]
- 35.Raffel J, Bhattacharyya AK, Gallegos A, Cui H, Einspahr JG, Alberts DS, et al. Increased expression of thioredoxin-1 in human colorectal cancer is associated with decreased patient survival. J Lab Clin Med. 2003;142:46–51. doi: 10.1016/S0022-2143(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee A, Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. The British journal of radiology. 2008;81(Spec No 1):S57–68. doi: 10.1259/bjr/34180435. [DOI] [PubMed] [Google Scholar]
- 37.Matthews JR, Wakasugi N, Virelizier JL, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic acids research. 1992;20:3821–30. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2010 doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duenas-Gonzalez A, Candelaria M, Perez-Plascencia C, Perez-Cardenas E, de la Cruz-Hernandez E, Herrera LA. Valproic acid as epigenetic cancer drug: preclinical, clinical and transcriptional effects on solid tumors. Cancer Treat Rev. 2008;34:206–22. doi: 10.1016/j.ctrv.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Huang L, Mackenzie GG, Rigas B. Oxidative stress mediates through apoptosis the anticancer effect of phospho-NSAIDs: Implications for the role of oxidative stress in the action of anticancer agents. J Pharmacol Exp Ther. 2011 doi: 10.1124/jpet.111.183533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–59. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 43.Ahmed KM, Cao N, Li JJ. HER-2 and NF-kappaB as the targets for therapy-resistant breast cancer. Anticancer Res. 2006;26:4235–43. [PMC free article] [PubMed] [Google Scholar]
- 44.Algul H, Adler G, Schmid RM. NF-kappaB/Rel transcriptional pathway: implications in pancreatic cancer. Int J Gastrointest Cancer. 2002;31:71–8. doi: 10.1385/IJGC:31:1-3:71. [DOI] [PubMed] [Google Scholar]
- 45.Sakamoto K, Maeda S. Targeting NF-kappaB for colorectal cancer. Expert Opin Ther Targets. 2010;14:593–601. doi: 10.1517/14728221003769903. [DOI] [PubMed] [Google Scholar]
- 46.Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A. 2007;104:12288–93. doi: 10.1073/pnas.0701549104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sasada T, Nakamura H, Ueda S, Sato N, Kitaoka Y, Gon Y, et al. Possible involvement of thioredoxin reductase as well as thioredoxin in cellular sensitivity to cis-diamminedichloroplatinum (II) Free Radic Biol Med. 1999;27:504–14. doi: 10.1016/s0891-5849(99)00101-x. [DOI] [PubMed] [Google Scholar]
- 48.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–6. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.