Abstract
Objectives
To describe patterns of use for mannitol and hypertonic saline in children with traumatic brain injury (TBI), to evaluate any potential associations between hypertonic saline and mannitol use and patient demographic, injury, and treatment hospital characteristics, and to determine if the 2003 guidelines for severe pediatric TBI impacted clinical practice regarding osmolar therapy.
Design
Retrospective cohort study
Setting
Pediatric Health Information System (PHIS) database, January, 2001 to December, 2008
Patients
Children (age < 18 years) with TBI and head/neck Abbreviated Injury Scale (AIS) score ≥ 3 who received mechanical ventilation and intensive care
Interventions
None
Measurements and Main Results
The primary outcome was hospital billing for parenteral hypertonic saline and mannitol use, by day of service. Overall, 33% (2,069 of 6,238) of the patients received hypertonic saline and 40% (2,500 of 6,238) received mannitol. Of the 1,854 patients who received hypertonic saline or mannitol for ≥ 2 days in the first week of therapy, 29% did not have ICP monitoring. After adjustment for hospital-level variation, primary insurance payer, and overall injury severity, use of both drugs was independently associated with older patient age, intracranial hemorrhage (other than epidural), skull fracture, and higher head/neck injury severity. Hypertonic saline use increased and mannitol use decreased with publication of the 2003 guidelines, and these trends continued through 2008.
Conclusions
Hypertonic saline and mannitol are used less in infants than in older children. The patient-level and hospital-level variation in osmolar therapy use and the substantial amount of sustained osmolar therapy without ICP monitoring suggest opportunities to improve the quality of pediatric TBI care. With limited high-quality evidence available, published expert guidelines appear to significantly impact clinical practice in this area.
Keywords: Pediatrics, Craniocerebral Trauma, Brain Edema, Intracranial Hypertension, Mannitol, Hypertonic Saline Solution
Introduction
Pediatric traumatic brain injury (TBI) is an important public health problem in the United States, and is estimated to cause approximately 2,300 deaths, 42,000 hospitalizations, and 404,000 Emergency Department visits annually among children 0–14 years old.[1, 2] TBI also causes substantial and long-lasting disability in children.[3] Elevated intracranial pressure (ICP)/intracranial hypertension develops after TBI via several mechanisms including cellular swelling and blood-brain barrier disruption.[4]
The July, 2003 guidelines for the management of severe pediatric TBI support the use of mannitol and/or hypertonic saline (any concentration greater than 0.9% saline) to decrease ICP at the discretion of the treating physician, but neither osmolar agent has sufficient evidence for a higher-grade recommendation.[5, 6] Both broad clinical use and published studies support mannitol use in adult TBI patients with intracranial hypertension, but its pediatric use as of 2003 was supported by only two single-center retrospective analyses.[6–10] Use of hypertonic saline as a therapy for elevated ICP was supported by three prospective pediatric studies.[11–14] Choice of osmolar agent to treat intracranial hypertension and variation in care across patient and hospital characteristics have not been evaluated.
The purpose of this study was to describe patterns of mannitol and hypertonic saline use in children with TBI, to evaluate any potential associations of preferential mannitol and hypertonic saline use with demographic, injury type, injury severity, or treatment hospital characteristics, and to assess change in use after publication of the 2003 guidelines. Because of pediatric-specific studies supporting hypertonic saline, we hypothesized that, after adjustment for clustering by hospital and baseline trend, hypertonic saline use would increase after the guidelines were published.
Methods
Study Design
We conducted a retrospective cohort study of the Pediatric Health Information System (PHIS) database developed by the Child Health Corporation of America[15] (CHCA) (Shawnee Mission, KS). We studied children who received care for TBI including intensive care unit (ICU) admission and mechanical ventilation at a PHIS hospital.
Setting
CHCA is a collaboration of more than 40 children’s hospitals, and PHIS contains administrative data including demographics, diagnoses, procedures, and charges. In addition, a subset of PHIS hospitals submits “Level II” data including pharmacy, clinical services, imaging, and supply data.[16] As described by Conway et al, “[o]versight of PHIS data quality and accuracy is a joint effort between Child Health Corporation of America, Thomson Healthcare (the data manager), and participating hospitals. Data are de-identified at the time of data submission and subjected to 175 reliability and validity checks. Data are accepted into the database when classified errors occur in <2% of a hospital’s quarterly data.”[17] We obtained data from PHIS regarding patients admitted to the 26 hospitals submitting “Level II” data with patients that met our inclusion criteria. Our data use agreement prevents identification of hospitals, but inpatient data on many PHIS hospitals have been published previously.[16]
Selection of Participants
We identified children < 18 years of age treated at a PHIS hospital with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code for TBI (800.0–801.9, 803.0–804.9, 850–854.1, or 959.01) and clinical coding for mechanical ventilation and ICU care (see below) from January, 2001 to December, 2008 (Figure 1). These ICD-9-CM diagnosis codes are used by the Centers for Disease Control to track hospitalization and Emergency Department visits for TBI rates nationally.[18] Mechanical ventilation was identified using Clinical Transaction Classification™ (CTC) codes 521160, 521161, 521162, 521164, 521165, 521166, 521167, or 521169.[19] CTC codes reflect hospital billing, and can be used to identify services received by patients.[16, 17, 20] ICU care was identified using an “ICU Flag” coded by PHIS.
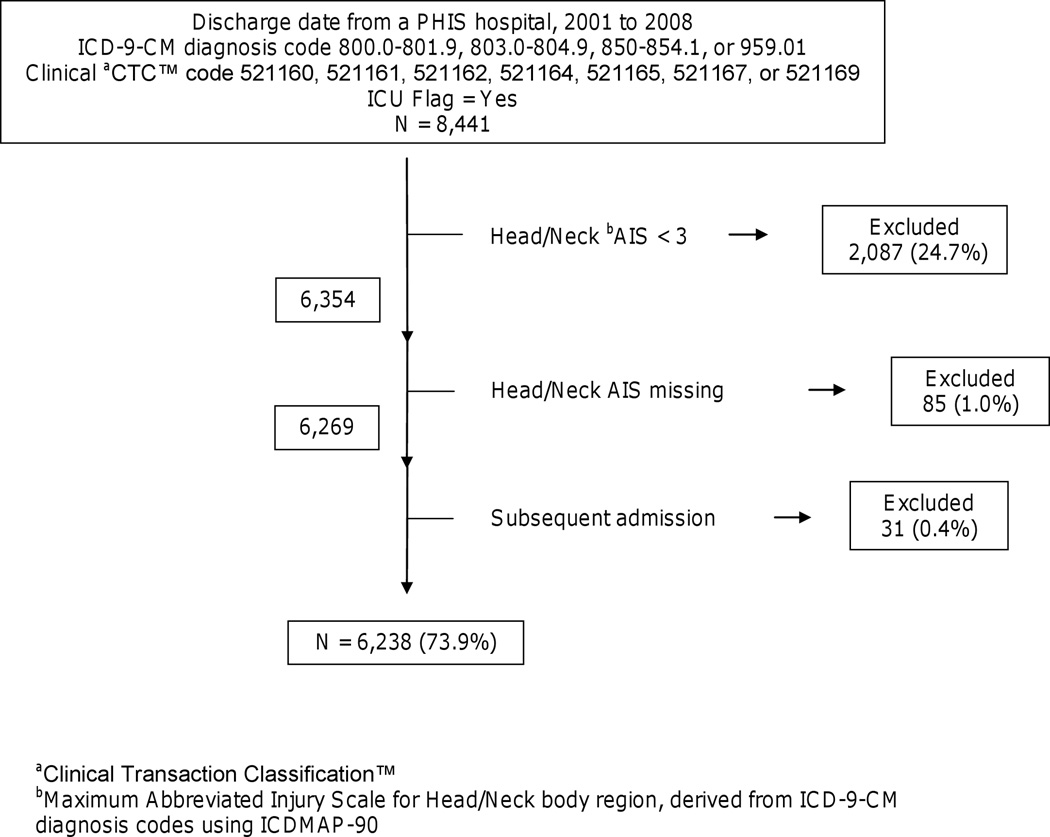
Figure 1.
Patient selection method for children < 18 years old with traumatic brain injury
We calculated injury severity score (ISS, or specifically, ICD/ISS) and maximum abbreviated injury scale (AIS) body region scores from ICD-9-CM diagnosis codes using ICDMAP-90 software (Johns Hopkins University and Tri-Analytics, Inc., Baltimore, MD).[21] We chose maximum AIS scores by ISS body region (6 regions). In order to select for patients with severe TBI, we excluded patients with maximum head/neck body region AIS scores of less than 3 (“Serious”) and the few patients with missing head/neck AIS scores (Figure 1). In addition, we excluded subsequent admissions.
Methods and Measurements/Outcome Measures
We analyzed the study population by demographic characteristics, insurance status, injury characteristics and severity, and admission date. We dichotomized admission date at July, 2003, when the guidelines were published.[5] We also analyzed the patients by hospital American College of Surgeons (ACS) Pediatric Trauma designation[22] and percent of study patients with a government primary payer.
The primary outcomes of interest were pharmacy billing (CTC) codes for parenteral hypertonic saline or mannitol. Each drug has an associated “day of service” code indicating the hospital day on which that drug was ordered. Hypertonic saline and mannitol may be used only rarely to treat imminent herniation, intermittently to treat intracranial hypertension, or as part of a treatment strategy to minimize intracranial pressure.[6] To identify patients given osmolar therapy as a treatment strategy, we selected patients who received hypertonic saline or mannitol for at least two days during the first week after hospital admission. In all analyses, we determined the proportion of patients who received a particular drug for×or more days using only those patients who had a length of stay of at least×days.
In order to evaluate other indications for hypertonic saline, we identified patients with a diagnosis of hyponatremia using ICD-9-CM diagnosis code 276.1.
Primary Data Analyses
We compared hypertonic saline and mannitol use across categories defined by patient and hospital variables using the chi-square test.
We used interrupted time series analyses to assess whether the July, 2003 guidelines were associated with changes in the use of hypertonic saline and mannitol. We built segmented multivariate linear regression models with separate terms for the baseline trend, the change in level with guideline publication, and the change in trend after guideline publication. We suspected a priori that substantial variation in hypertonic saline and mannitol use between hospitals existed, and used robust standard errors adjusted for clustering by hospital. We excluded July–December, 2003 from the time series analyses (these patients remained in the dataset for all other analyses) as a lag period to allow for uptake of the new guidelines.[23] We built a similar model for changes in the diagnosis of hyponatremia over time. Model fit and autocorrelation were assessed using visual inspection of model residuals.
We used multivariate logistic regression models with clustering by hospital, an exchangeable correlation structure, and robust standard errors calculated using generalized estimating equations (GEE) to estimate the effect of candidate predictors on osmolar therapy use. Variables with bivariate associations with hypertonic saline or mannitol use were candidates for inclusion, and we parameterized the variables to maximize clinical interpretability. Because an ICP monitor is required for ICP-directed therapy, we did not include the presence of an ICP monitor in the GEE models.
We used random effects logistic regression[16, 17] and the intraclass correlation coefficient to quantify variation in mannitol and hypertonic saline use between hospitals. Those variables identified in the GEE models as having independent associations with osmolar therapy use were included as covariates.
We defined statistical significance as p < 0.05 in all analyses. Statistical analyses were performed using Intercooled STATA™, version 10 (StataCorp LP, College Station, TX). The Institutional Review Board at the University of Utah School of Medicine waived the need for approval.
Results
Characteristics of Study Subjects
We identified 8,441 patients in the PHIS database and 6,238 remained in the dataset after exclusions (Figure 1). In each study month, a median of 64 (interquartile range (IQR) 53–76) patients were discharged from 26 PHIS hospitals. In-hospital mortality was 18.8% (1,173/6,212), and 26 patients (0.42%) were missing disposition data.
Most patients were male, white, and had government-funded insurance (Table 1). Infants (less than one year of age) accounted for 20% of the patients. Most patients (71%) had an intracranial hemorrhage (ICH), and 55% had a skull fracture. The median ISS score was 16 (IQR 10-25). Most of the hospitals did not have an ACS Pediatric Trauma designation[22]. The median number of study participants per hospital was 221 (range 34–557, IQR 133-363). All 26 hospitals submitted data both before and after 2003. Approximately two-thirds of the patients were admitted after the July, 2003 guidelines were published.
Table 1.
Patient and hospital characteristics of pediatric traumatic brain injury cohort
| Patients, n(%) | ||
|---|---|---|
| N = 6,238 | ||
| Age | ||
| 0 to 364 days | 1,273(20) | |
| 1 to <5 years | 1,603(26) | |
| 5 to <13 years | 2,177(35) | |
| 13 to <18 years | 1,185(19) | |
| Gender | ||
| Female | 2,239(36) | |
| Male | 3,996(64) | |
| Missing | 3(0) | |
| Race | ||
| White | 3,858(62) | |
| Black | 1,467(24) | |
| Other | 607(10) | |
| Missing | 306(5) | |
| Insurance status | ||
| Government | 2,964(48) | |
| Private | 1,944(31) | |
| Other | 368(6) | |
| Missing | 962(15) | |
| Admission date | ||
| 2000-7/2003 | 2,194(35) | |
| 8/2003-2008 | 4,044(65) | |
| Injuries | ||
| Any ICHa | 4,449(71) | |
| Any skull fracture | 3,429(55) | |
| ICPb Monitor | ||
| No | 3,967(64) | |
| Yes | 2,271(36) | |
| Head/Neck AISc | ||
| 3 (Serious) | 3,055(49) | |
| 4 (Severe) | 1,949(31) | |
| 5 (Critical) | 1,227(20) | |
| 6 (Unsurvivable) | 7(0) | |
| ISSd | ||
| < 15 | 2,313(37) | |
| ≥ 15 | 3,925(63) | |
| ACS Trauma Level (# hospitals)e | ||
| I (n = 8) | 2,024(32) | |
| II (n = 2) | 534(9) | |
| None (n = 16) | 3,680(59) | |
| % Government Payerf | ||
| 15–49.9% | 3,209(51) | |
| 50–76% | 3,029(49) | |
Column percentages may not add to 100 because of rounding
ICH = Intracranial Hemorrhage
ICP = Intracranial Pressure
AIS = Abbreviated Injury Scale score, Head/Neck body region, derived using ICDMAP-90
ISS = Injury Severity Score, as derived from diagnosis codes using ICDMAP-90
American College of Surgeons Pediatric Trauma Designation, Level I, Level II, or None
By hospital, number of patients in this cohort with a government plan as the primary payer
Main Results
Overall, 33% (2,069 of 6,238) of the patients received hypertonic saline and 40% (2,500 of 6,238) received mannitol, with 22% (1,347 of 6,238) receiving both, 18% (1,153 of 6,238) only mannitol, and 12% (722 of 6,238) only hypertonic saline. Approximately 26% of all patients received 3% saline, 13% received 10–25% saline, and <1% received 5% saline (several patients received multiple concentrations, not shown). Osmolar therapy use was substantial on the first day of admission and peaked on the second day of admission (Figure 2). Mannitol use decreased throughout the first week of therapy, but hypertonic saline administration continued at a fairly constant rate. Of those patients still hospitalized on the seventh day after admission, approximately 27% of those who had received any hypertonic saline and 16% of those who had received any mannitol were still receiving those agents.
Figure 2.
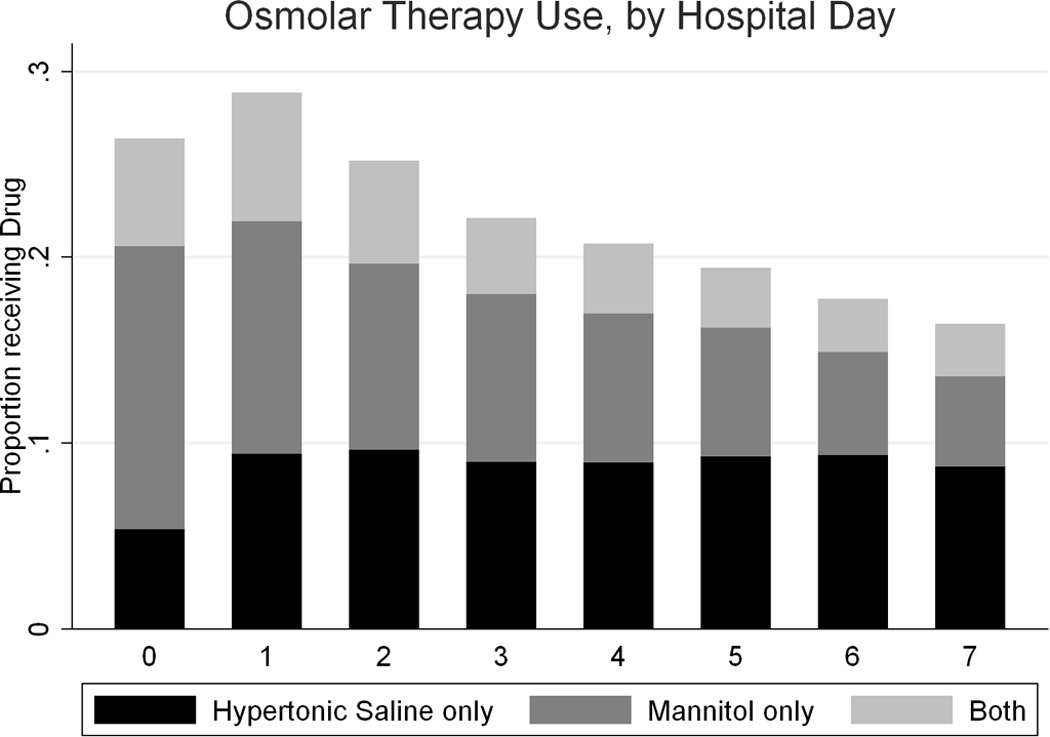
Hypertonic saline and mannitol use, by hospital day
Almost 5 percent of patients (4.6%) had a diagnosis of hyponatremia. Hypertonic saline use for ≥ 2 days was more common in patients with hyponatremia than without hyponatremia (43.4% versus 18.4%, p < 0.001). There was no gender difference in the diagnosis of hyponatremia (5.0% girls versus 4.4% boys, p = 0.590).
Bivariate Analyses
Use of osmolar therapy for ≥ 2 days in the first week showed statistically significant differences across age groups and insurance status, with older children and those with commercial insurance receiving more osmolar agents (not shown). Patients cared for at ACS Level I hospitals and hospitals without an ACS designation were more likely to receive osmolar therapy for ≥ 2 days in the first week than those cared for at ACS Level II hospitals (Table 2).
Table 2.
Hypertonic saline or mannitol use for ≥ 2 days (N = 6,093 with length of stay ≥ 2 days), by hospital and injury characteristics and ICP monitoring
| Hypertonic Saline, n(row%) | X2p | Mannitol, n(row%) | X2p | ||
|---|---|---|---|---|---|
| n = 1,191 of 6,093 | n = 1,204 of 6,093 | ||||
| Hospital ACSa Trauma level | <0.001 | < 0.001 | |||
| I (n = 1,983) | 388(20) | 454(23) | |||
| II (n = 520) | 59(11) | 84(16) | |||
| none (n = 3,590) | 744(21) | 666(19) | |||
| Injury Type | |||||
| Any ICHb vs. none | 931(21) | < 0.001 | 936(22) | < 0.001 | |
| (n = 4,347) | |||||
| Any skull fracture vs. none | 695(21) | 0.009 | 707(21) | 0.004 | |
| (n = 3,350) | |||||
| EDHc, no fracture (n = 220) | 28(13) | 0.009 | 24(11) | 0.001 | |
| SAHd, no fracture (n = 536) | 102(19) | 0.752 | 100(19) | 0.502 | |
| SDHe, no fracture (n = 1,232) | 238(19) | 0.821 | 273(22) | 0.018 | |
| Head/Neck AISf | < 0.001 | < 0.001 | |||
| 3 (n = 3,040) | 532(18) | 521(17) | |||
| 4 (n = 1,931) | 385(20) | 369(19) | |||
| 5–6 (n = 1,122) | 274(24) | 314(28) | |||
| ISSg | < 0.001 | < 0.001 | |||
| < 15 (n = 2,302) | 389(17) | 401(17) | |||
| ≥ 15 (n = 3,791) | 802(21) | 803(21) | |||
| ICPh Monitor | < 0.001 | < 0.001 | |||
| Yes (n = 2,248) | 893(40) | 899(40) | |||
| No (n = 3,845) | 298(8) | 305(8) | |||
ACS = American College of Surgeons, Pediatric Trauma Designation
ICH = Intracranial hemorrhage
EDH = epidural hematoma without skull fracture (ICD-9-CM diagnosis codes 852.4 and 852.5)
SAH = subarachnoid hemorrhage without skull fracture (ICD-9-CM diagnosis codes 852.0 and 852.1)
SDH = subdural hemorrhage without skull fracture (ICD-9-CM diagnosis codes 852.2 and 852.3)
AIS = Abbreviated Injury Scale score, Head/Neck body region, derived using ICDMAP-90
ISS = Injury Severity Score, derived using ICDMAP-90
Children with any ICH or any skull fracture received more osmolar therapy than children without those injuries (Table 2). Children with recorded epidural hemorrhage (EDH) without skull fracture tended to receive mannitol for one day (41%, not shown), but few received hypertonic saline or mannitol for two days in the first week (13% and 11%, respectively, Table 2). Both hypertonic saline and mannitol use increased with increasing head/neck injury severity (AIS) and overall injury severity (ISS). Children with ICP monitoring were more likely to receive both hypertonic saline and mannitol (Table 2). Of the 1,854 children who received ≥ 2 days of hypertonic saline (650 patients), mannitol (663), or both (541), 543 (29%) did not have an ICP monitor (not shown). Of the 372 infants who received ≥ 2 days of osmolar therapy, 192 (52%) did not have an ICP monitor.
Interrupted Time Series Analyses
Using segmented multivariate linear regression, excluding patients admitted July–December, 2003, and adjusting for clustering by hospital, we found that hypertonic saline use was increasing slowly and mannitol use was increasing fairly quickly before the guidelines were published (Figure 3 and Table 3). Following guideline publication, hypertonic saline use increased and mannitol use decreased substantially. Thereafter, hypertonic saline use continued to increase and mannitol use continued to decrease. Each of the predictors (baseline trend, change with guidelines, and post-guidelines change in trend) was independently associated with osmolar agent use in each model.
Figure 3.
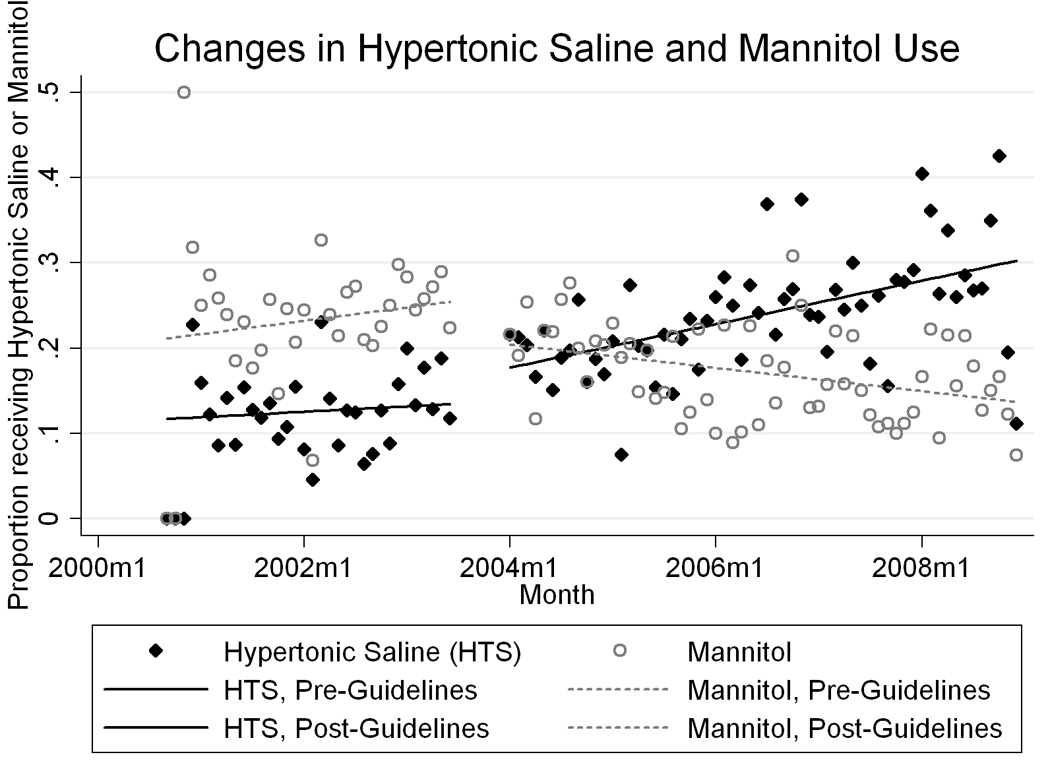
Changes in the proportion of children given hypertonic saline and mannitol ≥ 2 days in the first week after injury, with fitted segmented multivariate linear regression model. “2000m1” indicates January of 2000.
Table 3.
Interrupted time series models for osmolar agent use for ≥ 2 days in the first week after injury
| Hypertonic Saline | Mannitol | |
|---|---|---|
| % (95% CI) | % (95% CI) | |
| Before 9/2000 | 11.69 (11.20 to 12.17) | 21.14 (20.57 to 21.70) |
| Slope, per month (9/00-6/03) | 0.053 (0.031 to 0.075) | 0.13 (0.11 to 0.16) |
| Change with guidelines | 2.97 (2.49 to 3.44) | −4.47 (−4.99 to −3.95) |
| Slope, per month (1/04-12/08) | 0.16 (0.13 to 0.19) | −0.24 (−0.27 to −0.22) |
A similar model for the diagnosis of hyponatremia had a baseline rate of 2.3% of patients and was slowly increasing (0.07% per month, p < 0.001) prior to the guidelines, increased 0.6% with the guidelines (p = 0.001) and slowly decreased thereafter (−0.06% per month, p < 0.001), with little net change from the beginning to the end of the study.
Visual inspection of residuals for the hypertonic saline and mannitol time series models suggested a good fit and no significant autocorrelation (not shown).
GEE Models
Using multivariate logistic regression models with clustering by hospital, exchangeable correlation structure, and robust standard errors calculated using generalized estimating equations, we found that older age, admission after July, 2003, any ICH, any skull fracture, SDH (mannitol only), higher head/neck AIS score, were independently associated with hypertonic saline and mannitol use for ≥ 2 days in the first week (Table 4). As expected, children with EDH were less likely to receive osmolar therapy for ≥ 2 days. Government primary insurance payer and ISS were significant in the bivariate analyses but not in the multivariate models. In an exploratory fashion, we added an interaction term between SDH and age to the mannitol model, and it was not statistically significant. ACS Trauma designation, although significant in the bivariate analyses, is a parameterization of treatment hospital and is the cluster variable in the GEE models.
Table 4.
Multivariate logistic models for osmolar agent use ≥ 2 days in the first week after injury
| Hypertonic Saline | Mannitol | ||
|---|---|---|---|
| aORa (95% CI) | aOR (95% CI) | ||
| Age | |||
| 0 to 364 days | 1.00 (ref.)b | 1.00 (ref.) | |
| 1 to <5 years | 0.93 (0.74–1.16) | 1.26 (1.03–1.54) | |
| 5 to <13 years | 1.00 (0.80–1.25) | 1.53 (1.28–1.83) | |
| 13 to <18 years | 1.40 (1.06–1.87) | 1.81 (1.42–2.30) | |
| Insurance status | |||
| Other | 1.00 (ref.) | 1.00 (ref.) | |
| Government | 1.04 (0.90–1.19) | 1.04 (0.86–1.26) | |
| Admission date | |||
| 2000-7/2003 | 1.00 (ref.) | 1.00 (ref.) | |
| 8/2003-2008 | 2.08 (1.43–3.01) | 0.69 (0.52–0.91) | |
| Any ICHc | |||
| No | 1.00 (ref.) | 1.00 (ref.) | |
| Yes | 1.50 (1.13–1.99) | 1.62 (1.30–2.01) | |
| Any skull fracture | |||
| No | 1.00 (ref.) | 1.00 (ref.) | |
| Yes | 1.36 (1.10–1.67) | 1.48 (1.18–1.86) | |
| EDHd, no fracture | |||
| No | 1.00 (ref.) | 1.00 (ref.) | |
| Yes | 0.54 (0.36–0.80) | 0.49 (0.28–0.84) | |
| SDHe, no fracture | |||
| No | 1.00 (ref.) | ||
| Yes | 1.34 (1.03–1.75) | ||
| Head/Neck AISf | |||
| 3 | 1.00 (ref.) | 1.00 (ref.) | |
| 4 | 1.26 (0.92–1.73) | 1.06 (0.76–1.48) | |
| 5 or 6 | 1.64 (1.17–2.30) | 1.95 (1.47–2.60) | |
| ISSg | |||
| < 15 | 1.00 (ref.) | 1.00 (ref.) | |
| ≥ 15 | 1.12 (0.84–1.48) | 1.01 (0.80–1.28) | |
Odds Ratio, adjusted for clustering by hospital using generalized estimating equations
ref. = reference category
ICH = Intracranial hemorrhage
EDH = epidural hematoma without skull fracture (ICD-9-CM diagnosis codes 852.4 and 852.5)
SDH = subdural hemorrhage without skull fracture (ICD-9-CM diagnosis codes 852.2 and 852.3)
note: “SDH, no fracture” was not significant in the bivariate analysis for hypertonic saline
AIS = Abbreviated Injury Scale score, Head/Neck body region, derived using ICDMAP-90
ISS = Injury Severity Score, derived using ICDMAP-90
Random-effects Models
Using random-effects logistic regression adjusted for clustering by hospital and the independent predictors from the GEE models (age, any ICH, any skull fracture, no EDH [both], SDH [mannitol only], AIS, and admission after July, 2003), we estimated from the intraclass correlation coefficient that 12.2% (95% CI 7.0% to 20.4%) of the variance in hypertonic saline use for ≥ 2 days in the first week was between-hospital variance. In a similar fashion, we estimated that 14.8% (95% CI 8.4% to 24.7%) of the variance in mannitol use for ≥ 2 days in the first week was between-hospital variance.
Sensitivity Analyses
Because the insurance payer variable had missing data which necessarily excluded those records from the logistic GEE models, we constructed otherwise identical models without a payer term and obtained similar point estimates and precision for the other predictors (not shown).
Our patient selection criteria reliably identified acute rather than rehabilitative TBI care, as only 2 of 6,238 patients had a billing code for a pediatric rehabilitation floor on the day of admission. Both also had billing codes for mechanical ventilation and intensive care unit stays.
The lag period from July–December, 2003 excluded 444 patients from the interrupted time series models. Hospital mortality for those patients was 19.1%, not different from those not admitted during the lag period (p = 0.664).
Exploratory time series models for hypertonic saline or mannitol use on ≥ 3 days in the first week had similar results to the ≥ 2 day models (not shown).
We also analyzed hypertonic saline and mannitol use for ≥ 3 days (restricted to the N=5,607 patients with length of stay ≥ 3 days) in patients with and without ICP monitoring. Of the 1,283 children who received ≥ 3 days of hypertonic saline (528 patients), mannitol (478), or both (279), 303 (24%) did not have an ICP monitor (not shown). Of the 233 infants who received ≥ 3 days of osmolar therapy, 49% did not have an ICP monitor.
In GEE models restricted to those with ICP monitors, older age, AIS category 4, any skull fracture, and admission after the guidelines were independently associated with hypertonic saline use for ≥ 2 days. Older age, any skull fracture, and SDH were independently associated with mannitol use when restricted to those with ICP monitors.
Discussion
In this large, multi-center database, we found that hypertonic saline and mannitol for pediatric TBI are used more often in older children, those with skull fractures and non-epidural intracranial hemorrhages, and those with more severe head injuries; however, substantial variation between hospitals was present. In addition, the 2003 guidelines for the care of children with severe TBI appear to have impacted practice, with increased hypertonic saline use and decreased mannitol use after their publication.
To our knowledge, associations of osmolar therapy use with head injury severity and age have not been previously reported. It seems logical that osmolar agents are used more often in patients with more severe head injuries, as these patients are more likely to have intracranial hypertension. Osmolar therapy was also much more common in children with ICP monitors, likely the more severely injured patients. Interestingly, approximately one-quarter of the sustained (≥ 2–3 days in the first week) osmolar therapy we found was in children without ICP monitoring. In infants, approximately half of the sustained osmolar therapy was without ICP monitoring. Morris et al also found that first-tier ICP-targeted therapy (CSF drainage, osmolar therapy, or mild hyperventilation) was common (35%) in children with severe TBI and no ICP monitor.[24]
The association of osmolar therapy use with age after adjustment for the effect of the published guidelines, between-hospital variation, and injury severity suggests that providers are treating infants as if they are at lower risk for intracranial hypertension, despite a clear statement in the 2003 guidelines that “[t]he presence of open fontanels and/or sutures in an infant with severe TBI does not preclude the development of intracranial hypertension...”[25] This effect persisted when the analysis was restricted to patients with ICP monitors.
It is impossible to determine which factors caused the changes in hypertonic saline and mannitol use at approximately the time of guideline publication, but interrupted time series analysis is among the most rigorous available quasi-experimental methods to study interventions at a particular time in the past.[23, 26–29] Although chosen a priori, cluster adjustment for variation across hospitals was necessary given the substantial hospital-level variation we found. Our interrupted time series models, as is typical, did not include patient-level covariates because of the low likelihood that, for example, injury type, changed in July, 2003 at the time of guideline publication.[23]
The peak of osmolar therapy use on the second day of admission fit with our understanding of the mechanisms and timing of cerebral edema and intracranial hypertension in children with TBI. However, we were surprised at the relatively constant rate of hypertonic saline use across the first week of hospital care. Prolonged tapers of hypertonic saline to avoid hyponatremia or malignant intracranial hypertension may explain this finding. It is possible that the mannitol use for only one day in patients with EDH reflects rapid medical stabilization prior to operative intervention or adoption in the pediatric community of adult recommendations for high-dose emergency department mannitol use based on now-retracted papers by Cruz et al.[30–32]
Hypertonic saline may be given to treat hyponatremia from cerebral salt wasting (CSW). We analyzed the diagnosis of hyponatremia (there is no ICD-9-CM diagnosis code for CSW) over the same time period, because increased awareness of CSW might increase the use of hypertonic saline. Hyponatremia was diagnosed at a relatively constant rate, and had a slightly decreasing trend after TBI guideline publication, making it unlikely that the increase in hypertonic saline use was due to treatment of hyponatremia. This may also mean that increasing hypertonic saline use has not resulted in an increase in rebound hyponatremia.
Post-resuscitation Glasgow Coma Scale (GCS) score, pupillary exam, and CT results, the most predictive measures of TBI severity, are not present in the PHIS database. We restricted our analysis using head/neck AIS scores and mechanical ventilation and ICU billing codes as proxies for severity, but they may not completely represent GCS-based severity of TBI. ICDMAP-90, the software package we used to calculate AIS and ISS scores from ICD-9-CM diagnosis codes, has been validated overall[33] and in children[34] for its ability to determine injury severity, and has been successfully used in several studies of children with TBI, including one using the PHIS database.[35–37] Coates et al and Di Gennaro et al also defined their study populations using head AIS scores (≥3) in analyses of children with TBI.[38, 39]
Despite the absence of GCS scores, pupillary exam, and CT results in our dataset, our patients were similar to those in other studies. The in-hospital mortality of our study population (18.8%) compared well with published single-center estimates (22–24%) and with a multicenter trial without an apparent treatment effect (16.4%, with 8.9% of patients lost to follow-up).[40–43] Some older studies reported higher mortality[44], and a recently published study reported lower mortality.[45] The rates of skull fracture (55% versus 52%) and intracranial hematoma (71% versus 69%) in our sample compared well with those reported by Hutchison et al.[43] The median ISS in our sample was lower (16 versus 25) than in the work of Coates et al and Zebrack et al.[38, 40] Hutchison et al reported higher rates of hypertonic saline and mannitol use (50–60% for both osmolar agents), but the “usual care” arm of clinical trials may be different than usual care in other settings.[43, 46]
There are several other potential limitations to this study, primarily related to our use of an existing database. The PHIS database has advantages over a strictly administrative database, but pharmacy and clinical billing data are only available by day of service, and not, for example, by hour. In addition, the available ICD-9-CM diagnosis codes for TBI do not allow ideal categorization of injury type, and those diagnosis codes were used to derive both injury scores (AIS, ISS) and specific injury types (SDH, EDH, etc).
In conclusion, hypertonic saline and mannitol use in pediatric TBI is higher in older children, those with skull fractures and intracranial hemorrhage, and those with more severe head injuries, with substantial variation between hospitals. The patient-level and hospital-level variation and the significant amount of sustained osmolar therapy without an ICP monitor, particularly in infants, suggest opportunities to improve the quality of pediatric TBI care. The apparent effect of published guidelines on clinical practice in this area suggests that, with limited available high-quality evidence on how to use hypertonic saline and mannitol in children with TBI, providers attempt to follow expert guidelines.
ACKNOWLEDGEMENTS
We would like to thank Dr. Matthew Hall at CHCA for his guidance regarding CTC coding, Dr. Larry Cook for assistance with ICDMAP-90, and Drs. Tamara Simon and Raj Srivastava for study design assistance.
Financial Disclosures: Dr. Bennett is partially supported by the Mentored Scholars Program for Translational Comparative Effectiveness Research, NIH/NCI Grant Number 1KM1CA156723.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have not disclosed any potential conflicts of interest.
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Anderson VA, Catroppa C, Haritou F, Morse S, Rosenfeld JV. Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J Neurol Neurosurg Psychiatry. 2005;76(3):401–408. doi: 10.1136/jnnp.2003.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochanek PM, et al. Severe Traumatic Brain Injury in Infants and Children. In: Fuhrman BP, Zimmerman Jerry, editors. Pediatric Critical Care. 3rd edn. Philadelphia, PA: Mosby Elsevier; 2006. pp. 1596–1617. [Google Scholar]
- 5.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr Crit Care Med. 2003;4 3 Suppl:S2–S4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 6.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 11. Use of hyperosmolar therapy in the management of severe pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4 3 Suppl:S40–S44. [PubMed] [Google Scholar]
- 7.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, et al. Guidelines for the management of severe traumatic brain injury. II. Hyperosmolar therapy. J Neurotrauma. 2007;24 Suppl 1:S14–S20. doi: 10.1089/neu.2007.9994. [DOI] [PubMed] [Google Scholar]
- 8.Wakai A, Roberts I, Schierhout G. Mannitol for acute traumatic brain injury. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD001049.pub4. CD001049. [DOI] [PubMed] [Google Scholar]
- 9.James HE. Methodology for the control of intracranial pressure with hypertonic mannitol. Acta Neurochir (Wien) 1980;51(3–4):161–172. doi: 10.1007/BF01406742. [DOI] [PubMed] [Google Scholar]
- 10.Miller JD, Piper IR, Dearden NM. Management of intracranial hypertension in head injury: matching treatment with cause. Acta Neurochir Suppl (Wien) 1993;57:152–159. doi: 10.1007/978-3-7091-9266-5_22. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Thomas D, Peterson B. Hypertonic saline lowers raised intracranial pressure in children after head trauma. J Neurosurg Anesthesiol. 1992;4(1):4–10. doi: 10.1097/00008506-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Khanna S, Davis D, Peterson B, Fisher B, Tung H, O'Quigley J, Deutsch R. Use of hypertonic saline in the treatment of severe refractory posttraumatic intracranial hypertension in pediatric traumatic brain injury. Crit Care Med. 2000;28(4):1144–1151. doi: 10.1097/00003246-200004000-00038. [DOI] [PubMed] [Google Scholar]
- 13.Simma B, Burger R, Falk M, Sacher P, Fanconi S. A prospective, randomized, and controlled study of fluid management in children with severe head injury: lactated Ringer's solution versus hypertonic saline. Crit Care Med. 1998;26(7):1265–1270. doi: 10.1097/00003246-199807000-00032. [DOI] [PubMed] [Google Scholar]
- 14.Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28(4):1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 15.Child Health Corporation of America. [Accessed January 4th, 2011]; Available at http://www.chca.com/index_no_flash.html.
- 16.Weiss PF, Klink AJ, Hexem K, Burnham JM, Leonard MB, Keren R, Localio R, Feudtner C. Variation in inpatient therapy and diagnostic evaluation of children with Henoch Schonlein purpura. J Pediatr. 2009;155(6):812–818. doi: 10.1016/j.jpeds.2009.05.030. e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789–796. doi: 10.1016/j.jpeds.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Langlois JA, Rutland-Brown W, Thomas KE. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Division of Injury Response, Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 19.Child Health Corporation of America. CTC™ 2010 Code Structure: Module 5 Clinical Services. 2010 January 4; Available at https://sharepoint.chca.com/CHCAForums/PerformanceImprovement/PHIS/Reference Library/CTC Resources/Forms/AllItems.aspx Version: Modified.
- 20.Goldin AB, Sawin RS, Garrison MM, Zerr DM, Christakis DA. Aminoglycoside-based triple-antibiotic therapy versus monotherapy for children with ruptured appendicitis. Pediatrics. 2007;119(5):905–911. doi: 10.1542/peds.2006-2040. [DOI] [PubMed] [Google Scholar]
- 21.Tri-Analytics, Inc. and Johns Hopkins University. ICDMAP-90 Software User's Guide. 1997 [Google Scholar]
- 22. [Accessed October 26, 2010];American College of Surgeons Committee on Trauma, Verified Trauma Centers. Available at http://www.facs.org/trauma/verified.html.
- 23.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 24.Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA. Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Med. 2006;32(10):1606–1612. doi: 10.1007/s00134-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 25.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, Kochanek PM, Miller HC, Partington MD, Selden NR, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S19–S24. [PubMed] [Google Scholar]
- 26.Soumerai SB, Ross-Degnan D, Gortmaker S, Avorn J. Withdrawing payment for nonscientific drug therapy. Intended and unexpected effects of a large-scale natural experiment. JAMA. 1990;263(6):831–839. [PubMed] [Google Scholar]
- 27.Madden JM, Soumerai SB, Lieu TA, Mandl KD, Zhang F, Ross-Degnan D. Effects of a law against early postpartum discharge on newborn follow-up, adverse events, and HMO expenditures. N Engl J Med. 2002;347(25):2031–2038. doi: 10.1056/NEJMsa020408. [DOI] [PubMed] [Google Scholar]
- 28.Meara E, Kotagal UR, Atherton HD, Lieu TA. Impact of early newborn discharge legislation and early follow-up visits on infant outcomes in a state Medicaid population. Pediatrics. 2004;113(6):1619–1627. doi: 10.1542/peds.113.6.1619. [DOI] [PubMed] [Google Scholar]
- 29.Shardell M, Harris AD, El-Kamary SS, Furuno JP, Miller RR, Perencevich EN. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45(7):901–907. doi: 10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 30.Cruz J, Minoja G, Okuchi K. Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial. Neurosurgery. 2001;49(4):864–871. doi: 10.1097/00006123-200110000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Cruz J, Minoja G, Okuchi K. Major clinical and physiological benefits of early high doses of mannitol for intraparenchymal temporal lobe hemorrhages with abnormal pupillary widening: a randomized trial. Neurosurgery. 2002;51(3):628–637. discussion 637-628. [PubMed] [Google Scholar]
- 32.Cruz J, Minoja G, Okuchi K, Facco E. Successful use of the new high-dose mannitol treatment in patients with Glasgow Coma Scale scores of 3 and bilateral abnormal pupillary widening: a randomized trial. J Neurosurg. 2004;100(3):376–383. doi: 10.3171/jns.2004.100.3.0376. [DOI] [PubMed] [Google Scholar]
- 33.Expert Group on Injury Severity Measurement. Discussion document on injury severity measurement in administrative datasets. Center for Disease Control and Prevention, U.S. Department of Health and Human Services; [Accessed January 5th, 2011]. Available at http://www.cdc.gov/nchs/data/injury/DicussionDocu.pdf. [Google Scholar]
- 34.Durbin DR, Localio AR, MacKenzie EJ. Validation of the ICD/AIS MAP for pediatric use. Inj Prev. 2001;7(2):96–99. doi: 10.1136/ip.7.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tilford JM, Aitken ME, Anand KJ, Green JW, Goodman AC, Parker JG, Killingsworth JB, Fiser DH, Adelson PD. Hospitalizations for critically ill children with traumatic brain injuries: a longitudinal analysis. Crit Care Med. 2005;33(9):2074–2081. doi: 10.1097/01.ccm.0000171839.65687.f5. [DOI] [PubMed] [Google Scholar]
- 36.Bowman SM, Bird TM, Aitken ME, Tilford JM. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics. 2008;122(5):988–993. doi: 10.1542/peds.2007-3511. [DOI] [PubMed] [Google Scholar]
- 37.Wood JN, Hall M, Schilling S, Keren R, Mitra N, Rubin DM. Disparities in the evaluation and diagnosis of abuse among infants with traumatic brain injury. Pediatrics. 2010;126(3):408–414. doi: 10.1542/peds.2010-0031. [DOI] [PubMed] [Google Scholar]
- 38.Coates BM, Vavilala MS, Mack CD, Muangman S, Suz P, Sharar SR, Bulger E, Lam AM. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit Care Med. 2005;33(11):2645–2650. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Gennaro JLMC, Malakouti A, Zimmerman JJ, Armstead W, Monica S Vavilala. Use and Effect of Vasopressors after Pediatric Traumatic Brain Injury. Developmental Neuroscience. 2010;32(5–6):420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zebrack M, Dandoy C, Hansen K, Scaife E, Mann NC, Bratton SL. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56–64. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 41.Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, Carli PA. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–467. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 42.White JR, Farukhi Z, Bull C, Christensen J, Gordon T, Paidas C, Nichols DG. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29(3):534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, Dirks PB, Doucette S, Fergusson D, Gottesman R, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–2456. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DL, Krishnamurthy S. Severe pediatric head injury: myth, magic, and actual fact. Pediatr Neurosurg. 1998;28(4):167–172. doi: 10.1159/000028643. [DOI] [PubMed] [Google Scholar]
- 45.Tasker RC, Fleming TJ, Young AE, Morris KP, Parslow RC. Severe head injury in children: intensive care unit activity and mortality in England and Wales. Br J Neurosurg. 2011;25(1):68–77. doi: 10.3109/02688697.2010.538770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dawson L, Zarin DA, Emanuel EJ, Friedman LM, Chaudhari B, Goodman SN. Considering usual medical care in clinical trial design. PLoS Med. 2009;6(9):e1000111. doi: 10.1371/journal.pmed.1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]