Abstract
Dissociable prototype learning systems have been demonstrated behaviorally and with neuroimaging in younger adults as well as with patient populations. In A/not-A (AN) prototype learning, participants are shown members of category A during training, and during test are asked to decide whether novel items are in category A or are not in category A. Research suggests that AN learning is mediated by a perceptual learning system. In A/B (AB) prototype learning, participants are shown members of category A and B during training, and during test are asked to decide whether novel items are in category A or category B. In contrast to AN, research suggests that AB learning is mediated by a declarative memory system. The current study examined the effects of normal aging on AN and AB prototype learning. We observed an age-related deficit in AB learning, but an age-related advantage in AN learning. Computational modeling supports one possible interpretation based on narrower selective attentional focus in older adults in the AB task and broader selective attention in the AN task. Neuropsychological testing in older participants suggested that executive functioning and attentional control were associated with better performance in both tasks. However, nonverbal memory was associated with better AN performance, while visual attention was associated with worse AB performance. The results support an interactive memory systems approach and suggest that age-related declines in one memory system can lead to deficits in some tasks, but to enhanced performance in others.
Keywords: aging, categorization, prototypes, memory systems
Introduction
The ability to quickly and accurately classify objects in our surrounding is essential to maintaining a functional lifestyle across the lifespan. An extensive body of research suggest that the learning of different types of classification tasks are mediated by functionally and neurally distinct category learning systems (Ashby, et al., 1998; Ashby & Maddox, 2005; Koenig, et al., 2005; Poldrack & Foerde, 2008; Smith, Patalano, & Jonides, 1998; Seger, 2008). For example, different neural circuits have been shown to underlie rule-based and information-integration classification learning (Ashby & Maddox, 2005). Recent research suggests an age-related deficit in rule-based and information-integration category learning, but that the processing locus of each deficit is unique (Maddox, et al., 2010, and see Filoteo & Maddox, 2004).
Another important type of classification learning is prototype learning (Homa, Sterling, & Trepel, 1981; Posner & Keele, 1968; Reed, 1974; Smith & Minda, 1998). Prototype learning has also been examined in normal aging. In an elegant series of studies, Hess and colleagues examined the effects of normal aging on prototype learning. In a typical prototype learning task a single prototype is constructed and category exemplars are generated by distorting the features of the prototype. Hess and colleagues utilized this task and found that age differences were greater when there was an emphasis on active hypothesis generation and testing (Hess & Slaughter, 1986a, 1986b). Older adults were also less likely to use information about specific category exemplars and showed reduced retention processes (Hess, 1982).
Two Prototype Learning Tasks
Prototype learning is a type of category learning that involves the classification of objects created by distorting one or more prototypes (see Posner & Keele, 1968; Rosch, 1973; Rosch & Mervis, 1975; Smith & Minda, 1998). In a typical prototype learning task, the participant is presented with a series of objects that are each drawn from one or more structured categories. During this training period, the participant is asked to classify each object into one of several categories, and receives corrective feedback regarding their responses. Through trial-by-trial feedback, the participant learns to discriminate amongst the categories. Following training, the participant is generally presented with a series of test items that are used to evaluate the participant’s category knowledge. The participant is required to generate a classification response, but receives no feedback. These items are also members of the trained categories, but are often novel members, not presented during training. Both tasks in the present study use this training-test format.
A/Not-A (AN) prototype learning involves training on exemplars distorted from one prototype, and formed the basis of much of the early work on prototype learning, while A/B (AB) prototype learning involves training on exemplars distorted from two distinct prototypes. In AN prototype learning task, participants are shown members of category A during training, and during test are asked to decide whether novel items are in category A or are not in category A. In AB prototype learning, participants are shown members of category A and B during training, and during test are asked to decide whether novel items are in category A or category B. Critically, the same stimuli are used in the test phase for both the AN and AB tasks. Thus, any differences observed in AN and AB performance cannot be attributed to differences between the structures of non-A versus B category, nor to any stimulus-specific differences.
Differing Views of Prototype Learning
Prototype learning may be accomplished by one underlying system, or by multiple systems. While the literature does not always agree on the number of systems, we believe that testing different forms of prototype learning in normal aging may shed light on this debate. Some recent investigations of prototype learning in young adults suggest that two different types of prototype learning exist and that each might rely on distinct neural circuits (Palmeri & Flannery, 1999; Zeithamova, Maddox, & Schnyer, 2008). Evidence for a functional dissociation between AN and AB prototype learning comes from neuroimaging and patient data (Bozoki, et al., 2006; Kéri et al., 2001; Knowlton & Squire, 1993; Reber & Squire, 1999; Zaki, et al., 2003; Zeithamova, et al., 2008). These data suggest that AN learning is mediated by lateral occipital and striatal regions associated with a perceptual learning system, whereas AB learning is mediated by parahippocampal, inferior parietal, and orbitofrontal regions associated with a declarative rule learning system. It is important to note that single system approaches have also been advanced and have been shown to model certain forms of prototype learning very well (Nosofsky & Zaki, 1998; Palmeri & Nosofsky, 2001; for an excellent review see Palmeri & Flanery, 2002). Single system approaches argue that prototype learning may be subserved by declarative memory systems and that prototype learning and explicit memory performance dissociations might be captured by differences in a single component of declarative memory.
Predictions
To foreshadow, our main prediction is based on a dual system approach. In this approach, a dysfunctional declarative system in older adults could lead to impaired AB performance despite intact or enhanced AN performance. This is due to the perceptual learning system taking control of the task sooner in older adults than younger adults. In AB, the declarative system drives performance in both older and younger adults, and so younger adults would have an advantage. Other predictions are viable, and we outline both single and dual system predictions below.
In dual system approaches, AB prototype learning is thought to rely upon medial temporal lobe (MTL) and frontal regions (Zeithamova et al, 2008). While MTL regions may not experience decline in normal aging, large declines in white and gray matter in frontal regions have been shown (Davis et al, 2009; Head et al., 2004; Raz et al., 2005; Raz, Rodrigue, & Haacke, 2007; Sullivan, Marsh, & Pfefferbaum, 2005). Frontal volume decline suggests that age-related deficits in AB prototype learning are likely. Importantly for single system approaches, prefrontal cortex (PFC) decline is linked with declarative memory ability (Janowsky, Shimamura & Squire, 1989). Thus, single and dual system approaches would both predict declines in normal aging.
What is less clear are the predictions that dual system approaches make for the effects of normal aging on AN prototype learning. On the one hand, deficits in striatal processing (Filoteo & Maddox, 2004) as well as reductions in striatal volumes (Raz, et al., 1998; Raz, et al., 2003) have been shown to occur in normal aging.. Furthermore, reduced tonic dopamine levels and dopaminergic dysregulation are associated with normal aging (Martin, Palmer, Patlak, & Calne, 1989; Nieuwenhuis et al., 2002; van Dyck et al., 2002; Volkow et al., 2000; Volkow et al., 1998). These have been hypothesized to account for some of the cognitive impairments in normal aging (Braver & Barch, 2002; Braver et al., 2001). In addition, visual processing areas have been shown to reduce with normal aging. This reduction predicts performance on nonverbal working memory tasks (Raz, et al., 1998). On the other hand, age-related declines in striatal volumes are not correlated with changes in cognitive processing (Raz, et al., 1998), and although visual processing volumes predict nonverbal working memory performance, AN prototype learning involves a more implicit form of memory subserved by the perceptual learning system (Zeithamova, et al., 2008). A single system approach may suggest either deficits in AN due to overall declarative dysfunction, or perhaps enhanced AN performance due to increased generalizability resulting from less precise memory traces of individual category members.
Taken together, dual system approaches suggest that age-related deficits may not emerge, or may be small in magnitude and lead to the possibility for intact performance in AN prototype learning in normal aging. This follows from an interactive memory systems approach that is gaining favor in the literature (Ashby, et al.,1998; Maddox, et al., in press; Poldrack & Packard, 2003; Poldrack & Foerde, 2008). The idea is that declarative memory systems tend to dominate early with control being passed to other systems only when performance demands dictate; for example, when better performance can be achieved by another system (Ashby, et al., 1998; Poldrack & Packard, 2003). If the declarative memory system shows age-related declines, and the perceptual learning system shows smaller declines or no decline at all, then an interactive memory system approach would predict an age-related deficit in AB performance but intact AN performance. The age-related deficit in AB would emerge because declarative memory systems should dominate but operate less efficiently with normal aging. The age-related advantage in AN would emerge because performance would be passed more quickly from the deficient declarative memory system to the more optimal perceptual learning system.
Prior research supports the prediction for an age-related AN advantage and AB disadvantage. Gopie, Craik, and Hasher (2011) demonstrated that older adults performed better than younger adults in an implicit memory task which relies on perceptual processing. Conversely, the pattern reversed for an explicit memory task which was more conceptually driven. This pattern lends credence to the dual-systems based prediction since the AN task has been associated with the perceptual learning system, while the AB task is more of an explicit processing task.
However, it is possible that a single underlying system is responsible for both AN and AB prototype learning. If this were the case, we may still expect to see differences between these two tasks in older versus younger adults. For example, declarative memory deficits associated with normal aging may lead to overall difficulty for older adults in AB but not AN. This could arise, for example, if older adults acquired a better representation of the sole training category in AN due to fuzzier exemplar representations which lead to better generalizability of the category. Similarly, AB performance would decline due to problems in generalizing exemplars from two categories into distinct categories. Importantly, this would predict that older adults would have better accuracy for the A category in the AN task versus the B category.
In the current study, younger and older adults completed AN or AB prototype learning tasks. Behavioral analysis based on the accuracy of responding during the test phase, and computational modeling are employed to investigate the effect of normal aging on performance in these dissociable prototype learning systems. Furthermore, neuropsychological testing is used to investigate individual differences within the older adult group.
Methods
Participants
Fifty-four younger adults from the University of Texas and Austin community participated for monetary compensation or class credit (28 male; AgeMean= 20.6; AgeSD= 0.33; range = 18 – 29 years). Fifty-six older adults from the local community were recruited and participated for monetary compensation (20 male; AgeMean= 69.4; AgeSD= 0.32; range = 60 – 84 years). Participants were paid $10 per hour for participating. Older adults were administered an extensive neuropsychological testing battery to identify any mental declines not due to normal aging. All older adults included in the study were consistently within 1 SD of normal performance across each domain on the neuropsychological measures. Younger and older adults were matched based upon the scaled WAIS-IV (Wechsler, 2008) vocabulary test [t(107)=1.88, p=0.06] and gender ratio [χ2(1, N = 109) = 0.39, p = 0.5]. Because the younger group was comprised of college aged individuals, older adults had on average 3.5 more years of education than the younger group. Scaled WAIS vocabulary score were never significant correlates of behavioral performance. Gender was also tested as an categorical independent variable, and was never significant. Three younger adults and six older adults were excluded for performing below chance in the test phase and were not included in the participant counts.
Stimuli
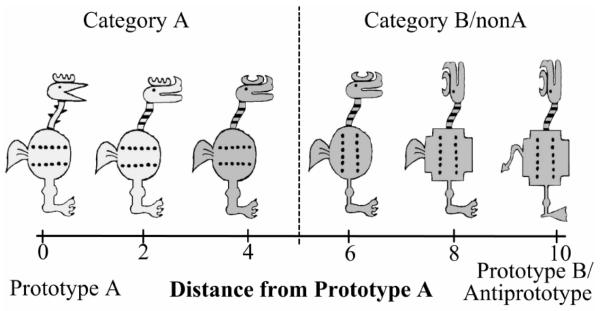
The stimuli were cartoon animals that varied along 10 binary dimensions, such as body shape (round or square), head position (facing forward or upward), tail shape (feathery or pointy), etc (see Figure 1). In total, the size of the set of all possible exemplars is 210 = 1,024. For each participant, a category A prototype was randomly generated by selecting values for each binary dimension. Next, a category B prototype was defined as the anti-prototype of the category A prototype. In other words, the category B prototype has values along the 10 binary dimension that are opposite of the category A prototype. In this way, the two prototypes are separated by maximum Hamming distance within the set of possible exemplars. An exemplar for a given category was generated by distorting the prototype by changing one or more of the binary valued dimensions. Thus, each exemplar could differ from its prototype by varying distance. If only one dimension differed, the exemplar had a distance of one. If two dimensions differed, the exemplar had a distance of two, etc. Items with a distance of five were ambiguous and were not included. In the AN task, only members from category A were shown in the training phase. In the AB task, members from both categories were shown in the training phase.
Figure 1.
Example stimuli from categories A and B, varying by distance
The test phase was identical for both tasks: members from both categories were shown, with no corrective feedback given. Test stimuli were 42 novel exemplars. For both categories 21 exemplars were shown: 5 exemplars at each of the four distance levels, along with 1 prototype stimulus. These were the same stimuli and task from Zeithamova, et al. (2008).
Procedure
In the AB condition, training consisted of 10 A items and 10 B items, presented in random order. On each trial, 2 seconds after stimulus onset, the participant was prompted to give an A or B response, followed by corrective feedback. Within each category, 2 training stimuli differed from the category prototype on 1 feature, 3 differed on 2 features, 3 differed on 3 features and 2 differed on 4 features. Across all 10 stimuli within each category, the category typical features were presented 7 or 8 times and the opposite category typical features were presented 2 or 3 times. Neither prototype was presented in the training phase.
In the AN condition, prior to training, participants were told that they would later need to categorize A members from non-A members. The training phase consisted of category A members only. Twenty category A members were shown in random order and passively for a minimum of 2 seconds, with a keystroke required to advance to the next item. Five items varied from the category A prototype on 1 feature, five differed on 2 features, five differed on 3 features, and five differed on 4 features. Across all 20 stimuli, the prototypical value on each dimension was presented 15 times and the non-prototypical value on each dimension was presented 5 times.
The testing phase was identical for both tasks, with only the label of the second category (B versus nonA) differing between the tasks. Participants were presented with 42 stimuli, one at a time that included both prototypes and five stimuli selected from each distance from the prototype (except distance 5 - ambiguous stimuli). None of the stimuli were previously used in the training phase. No feedback was provided. A fixation cross was presented between each stimulus onset lasting 2.5 seconds.
Neuropsychological Testing
All older adults completed a large battery of neuropsychological tests including the Wisconsin Card Sorting Test (WCST; Heaton, 1980), Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV; Wechsler, 2008), Stroop test (Stroop, 1935), Trail-making test (Corrigan & Hinkeldy, 1987), and Wechsler Memory Scale (WMS-IV). All results were normalized for age using standardized procedures and converted to Z-scores.
Results
Overall Accuracy
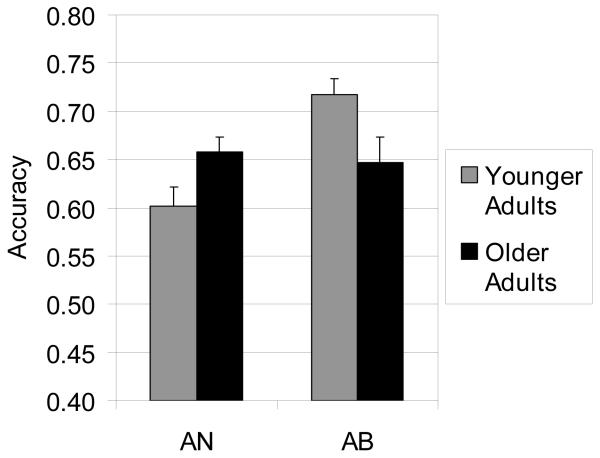
Figure 2 illustrates accuracy results from the test phase for younger and older adults for the AN and AB conditions. Accuracy in AN was 60.1% (SE = 2.0%) for younger adults and 65.7% (SE = 1.6%) for older adults. Accuracy in AB was 71.7% (SE = 1.7%) for younger adults and 64.7% (SE = 2.6%) for older adults. A 2 Group (Older vs. Younger) × 2 Task (AN vs. AB) between subjects ANOVA was conducted on test accuracy. The main effect of Group was not significant (p = 0.82) and the main effect of Task was significant, F(1, 108) = 7.5, p < 0.01, η2 = 0.07. However the main effect of Task was qualified by a significant Group by Task interaction, F(1, 108) = 10.9, p < 0.01, η2 = 0.09. The interaction suggested an age-related advantage in the AN task, t(56) = 2.18, p = 0.03, and an age-related deficit in the AB task, t(49) = 1.68, p = 0.03.
Figure 2.
Accuracy results for younger controls and older adults in the AN and AB tasks
Prototype Accuracy
We conducted a 2 Group (Older vs. Younger) × 2 Category (Prototype A vs. Prototype B) repeated measures ANOVA on test accuracy for the actual prototype exemplars which were both shown once per subject during testing. In AN, there was a significant main effect of Category such that accuracy was better for the A versus the B prototype (i.e., the anti-prototype), F(1, 55) = 7.6, p < 0.01, η2 = 0.12. There was also a significant Group by Category interaction, F(1, 55) = 5.6, p < 0.05, η2 = 0.09, such that older adults were more accurate for the anti-prototype than younger adults. In AB, there was no significant main effects nor a significant Group by Category interaction (ps > 0.2)
Computational Modeling
To examine the locus of the age-related AN advantage and AB deficit, we applied simple prototype models to the data from each individual. These models have been used extensively in the literature and provide important insights into underlying psychological processes that are unobservable with traditional performance measures (such as accuracy) (Ashby & Maddox, 1993; Posner & Keele, 1968; Smith & Minda, 1998). The model assumes that on each trial, a participant calculates an attention-weighted distance between the stimulus at hand (x) and the prototype of the categories (PA for category A, and PB for category B). The attention weights effectively stretch and shrink the perceptual space along each stimulus dimension with larger attention weights stretching the space (increasing the dimension-level discriminability) and smaller attention weights shrinking the space (decreasing the dimension-level discriminability). The (Euclidean) distance between x and P is calculated as:
| (1) |
where wi represents the attention-weight of dimension i. The attention weights are constrained to sum to 1, yielding 9 free wi parameters. The binary value of a dimension i is denoted by xi, and PAi denotes the binary feature value on dimension i for PA. PB is also calculated on each trial, and used with PA to calculate the predicted probability of responding A (or B) to a stimulus, P(A|x):
| (2) |
where ηiA = e−cd (where d is calculated by Equation 1) The c parameter represents the perceptual sensitivity of the system, and represents the 10th free parameter. Larger values of c effectively stretch the perceptual space uniformly leading to greater overall discriminabilty across stimuli, whereas smaller values of c effectively shrink the perceptual space uniformly leading to lesser overall discriminabilty across stimuli. For each participant, we fit the model to 42 test items using maximum likelihood procedures (Takani & Shibayama, 1992). The model provided a good account of the data yielded an average absolute deviation between predicted and observed accuracy of 0.04, 0.03, 0.04, and 0.05 for younger adults on AN and AB and older adults on AN and AB, respectively.
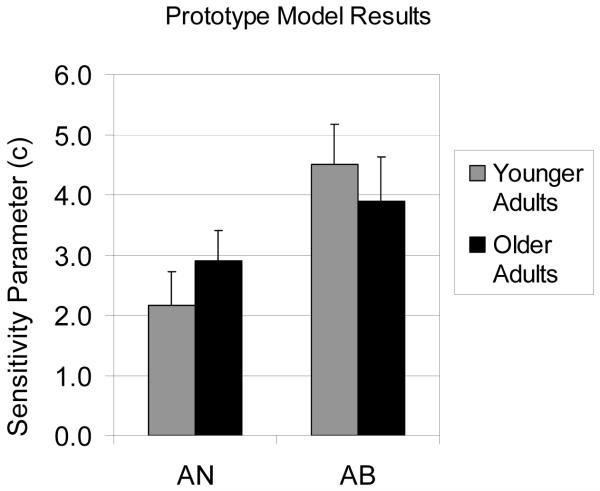
The perceptual sensitivity (c) values from the model are displayed in Figure 3. In AB, the perceptual sensitivity value was smaller for older adults than for younger adults, although the difference did not reach statistical significance[t(49) = 1.90, p = 0.06]. Interestingly, in AN the perceptual sensitivity value was larger for older adults than for younger adults, but again this difference did not reach statistical significance [t(56) = −0.98, p = 0.33].
Figure 3.
Perceptual sensitivity (c) value from computational modeling results for younger controls and older adults in the AN and AB tasks
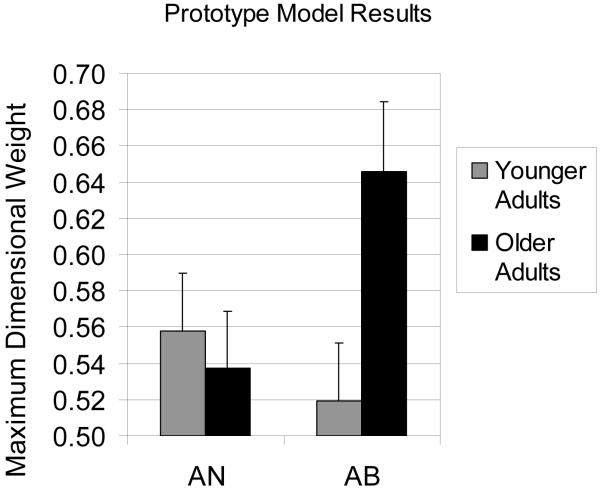
We examined attentional focus within the framework of the model in two ways. First, we examined the maximum dimensional attention weight parameter (out of the 10 wi parameters) for each subject. These values are displayed in Figure 4. The larger the maximum dimensional weight the greater the focus (usually interpreted as attentional focus) placed on a single feature dimension. An optimal classifier would evenly distribute attentional focus to all dimensions, resulting in a maximum dimensional weight of 0.1. In AB, the maximum attentional weight was significantly larger for older adults than younger adults [t(49) = 2.6, p < .05]. In AN, the maximum dimensional weight did not differ significantly between older and younger adults, but older adults were slightly less focused on a single dimension than younger adults.
Figure 4.
Maximum dimensional weights from computational modeling results for younger controls and older adults in the AN and AB tasks
Second, we calculated the number of dimensional weights needed to account for 95% of the weight allocation. This was done by sorting the weights in order of size, then summing cumulatively starting with the largest weight until a value equal to or larger than 0.95 was reachedb. These results converged with those for the maximum dimensional attention weight. Specifically, in AB, younger adults had on average 4.0 dimensions account for 95% of the weight allocation (SE = .29), while older adults had on average 3.1 (SE = .28), [t(49) = 2.1, p < 0.05]. In AN, there was no significant difference between older adults (3.7, SE = 0.26) and younger adults (3.6, SE = 0.27). Although one must always be careful not to over interpret the psychological meaning of model parameters, they can be informative and this application is no exception.
Neuropsychological Testing
Several neuropsychological tests were given to the participants before their participation in the present experiment. The testing battery included Trail Making Test Part A (visual attention), Trail Making Test Part B (task switching), Wisconsin Card Sorting Test (WCST; executive functioning and set shifting), WAIS-IV digit span (working memory), WMS-IV immediate visual reproduction recall (nonverbal memory), California Verbal Learning Test (CVLT) long delay free and cued recall (long-term memory), and WMS-III logical memory 30-minute recall test (long-term memory). All scores were normalized for age and converted to z-scores.
Importantly, the older adults were tested for memory impairment. All participants had positive Z-scores in the CVLT long delay free recall test (mean was 0.68 [SE=0.18] for the AN group and 0.73 [SE=0.16] for the AB group) and cued recall test (mean was 0.49 [SE=0.23] for the AN group and 0.54 [SE=0.19] for the AB group]), as well as positive Z-scores in the WMS-III logical memory 30-minute recall test (1.19 [SE=0.17] for the AN group and 1.32 [SE=0.17] for the AB group). Furthermore, these scores did not correlate with the task performance or model based analysis described below.
In the AN task, accuracy correlated negatively with Trail Making Test Part B (r2 = 0.16, p < 0.05, df = 31). Maximum attentional weight (associated with less evenly spread attention) was positively associated with WCST number of categories learned (r2 = 0.25, p < 0.01, df = 28) and years of education (r2 = 0.14, p < 0.05, df = 31). The sensitivity parameter correlated positively with WAIS-IV digit span total score (r2 = 0.13, p < 0.05, df = 31) and WMS-IV immediate visual reproduction recall (r2 = 0.14, p < 0.05, df = 31). Thus, executive functioning, working memory, nonverbal memory, and education level were indicators of good AN performance. Task switching ability was associated with worse accuracy.
In the AB task, accuracy correlated negatively with Trail Making Test Part A (r2 = 0.21, p < 0.05, df = 24). The number of dimensions comprising 95% of attentional weights (associated with more evenly spread attention) correlated positively with age (r2 = 0.29, p < 0.01, df = 25). Thus, visual attention was associated with worse AB performance, while higher age was associated with better AB performance.
Discussion
We examined the effects of normal aging on performance in two prototype learning tasks that, according to prior research, are likely subserved by distinct neural circuits. The AN task involves training on exemplars distorted from one prototype and testing on novel members from the same category as well as novel non-category members. AN prototype learning is thought to be mediated by a perceptual learning system involving the lateral occipital cortex and striatum (Zeithamova, et al., 2008). The AB task involves training on exemplars distorted from two prototypes and testing on novel members from each category. AB prototype learning is thought to be mediated by a rule-learning declarative memory system involving the parahippocampus, inferior parietal cortex, and orbitofrontal cortex (Zeithamova, et al., 2008).
Relative to young individuals, we observed an age-related deficit in AB performance and an age-related advantage in AN performance. The model-based analyses lends support to the idea that the AB deficit in older adults is due to increased attention to a few stimulus dimensions when a broader attentional focus would be optimal. The maximum dimensional weight was larger for older than for younger adults, and 95% of the (model defined) attentional resource was allocated to just over 3 dimensions for older adults but was spread across 4 dimensions for younger adults. Whereas the optimal strategy is to spread attention evenly across all 10 stimulus dimensions, both older and younger adults focus on a smaller subset of dimensions. One possibility is that older and younger adults are actively seeking a “rule”, with older adults seeking a simpler rule than younger adults (see also Maddox, et al., 2010). Another possibility is that changes in working memory may play a role with older adults finding it more difficult to maintain multiple dimensions of information across two categories, or maintaining two categories regardless of their number dimensions. However, we do not find that older adults who performed better in neuropsychology tests of memory functioning also did better on the AB tasks. Future work is needed to address this issue more fully.
Interestingly, a very different pattern emerged in the AN model-based analyses. Acknowledging that one should not over interpret model parameters, the models suggest that when the task requires learning the structure of a single category, as opposed to learning two categories, older adults spread attention more broadly than younger adults, and older adults showed better global discrimination ability. In this case, it is possible that younger adults were less focused on the stimulus as a whole and, in addition, or perhaps as a result, they found the stimuli less discriminable.
Importantly, older adults outperformed younger adults on the AN task which was the task that yielded lower overall accuracy. This means that these findings cannot be attributed to a difference in difficulty, since younger adults would have outperformed older adults in both tasks. Of course other measures of difficulty are possible and should be explored in future research.
Correlating task performance with neuropsychological testing indicated that these two tasks tap very different underlying systems. Education level, executive functioning and both working memory and nonverbal memory were associated with better AN performance, while task switching ability was associated with worse AN performance. In the AB task, visual attention and lower age were associated with worse performance. The fact that nonverbal memory is important for the AN task but not the AB task could be seen to support the hypothesis that a perceptual learning system underlies AN prototype learning but not necessarily AB prototype learning. This differential pattern further supports the multiple systems view of prototype learning (Zeithamova, et al., 2008). Furthermore, enhanced executive functioning correlating with better AN performance could be seen to support a dual systems approach in that executive functioning could lead to a more efficient tradeoff from the declarative system to the perceptual learning system.
AB prototype learning is mediated by frontal and temporal lobe structures that are affected in normal aging, and as expected age-related AB deficits emerge. AN prototype learning is mediated by posterior visual systems and the striatum that are less affected in normal aging and AN deficits do not emerge. Instead an age-related advantage in AN learning emerged. One intriguing processing explanation for the age-related AN advantage is based on the growing literature suggesting that memory systems to do not operate independently, but rather tend to be highly interactive (Poldrack & Foerde, 2008; Poldrack & Packard, 2003). One finding from this literature is that declarative memory systems often tend to dominate early with control being passed to other systems only when performance demands dictate; for example, when better performance can be achieved by another system (Ashby, et al., 1998; Poldrack & Packard, 2003). If the declarative memory system show age-related declines, and the perceptual learning shows smaller declines or no decline at all, then an interactive memory system approach would predict an age-related deficit in AB performance but an age-related advantage in AN performance. The age-related deficit in AB would emerge because declarative memory systems should dominate but operate less efficiently with normal aging. The declarative memory system does not pass performance to the perceptual learning system perhaps because the risk of using perceptual learning to solve the task is known to be high. It is possible that more extensive training may reduce this uncertainty. The age-related advantage in AN would emerge because performance would be passed more quickly from the deficient declarative memory system to the more optimal perceptual learning system.
This prediction is not without precedence in the literature. Several studies in the literature have shown that information-integration category learning that is mediated by a procedural-based learning system can be enhanced by introducing experimental manipulations that impair frontal/declarative memory processing (Maddox, Love, Glass & Filoteo, 2008; Filoteo, Lauritzen, & Maddox, 2010). The “deficient” frontal/declarative processing speeds the transition to the procedural-based learning system effective enhancing learning and performance. The present study is the first to show this effect in normal aging, and to suggest that age-related declines in processing in the declarative memory system might encourage a shift to a more perceptual learning approach, and thus improve performance in an AN prototype learning task that is mediated by the perceptual learning system. While this explains early learning, it is likely that the discrepancy between younger- and older-adults would decrease over time.
The model based analyses provide important insights into the processing characteristics of this memory system interaction. The models suggest that the more efficient transition from the declarative to the perceptual learning system in older adults leads to a broader attentional focus, and to an increase in perceptual sensitivity to feature diagnostics, both of which increase performance. Although clearly more work is needed, this is an intriguing finding and is suggestive that deficits in one cognitive system can often lead to performance advantages in another, at least when those systems are potentially in competition with each other.
Although a dual system prospective provides a framework consistent with these results, a single system interpretation is also a viable possibility. This view would postulate that the deficits in declarative memory might underlie both the age-related AB deficit and the AN advantage. Assuming that all participants engaged in an explicit strategy to memorize training items, it is possible that older adults’ memory traces were less distinct or fuzzier than those of the younger adults. This might lead to greater generalization and support better abstraction of the prototype when only one category is present but would lead to worse generalization and worse abstraction of the prototype when two categories are present. Thus, this alternative would predict better performance in the AN task, but worse performance in the AB task as we observed in the experiment.
This single system hypothesis predicts that older adults should show better generalization in the AN condition as one moves away from the A prototype, and this prediction is supported by the data (accuracy for all test items other than the prototype: older adults = 66%; younger adults = 58%). However, this hypothesis would also predict that accuracy for the studied, A prototype in the AN condition should be higher for older adults than for younger adults, but this was not supported in the data (accuracy for the A prototype: older adults = 88%; younger adults = 77%). Thus the age-related AN performance advantage was likely not due to an enhanced representation of the A prototype, but rather was due to better generalization. Thus, declarative memory deficits in normal aging could account for our overall finding that older adults outperformed younger adults in the AN task but not in the AB task. Even so, adjudicating between these two possibilities will continue to require future research, perhaps some that include recognition memory tests.
As mentioned earlier, a single declarative system approach may predict increased accuracy for the category A prototype versus the anti-prototype in AN learning. However, the opposite turned out to be the case. Older adults were more accurate for the anti-prototype than younger adults, with no difference between the two groups for the category A prototype. Thus, this particular hypothesis that may follow from single system approaches does not seem viable.
While an interactive memory system approach is in line with the current results, it is possible that other underlying brain systems are leading to the results. A more definitive test would involve directly examining the neural correlates, perhaps using fMRI. Zeithamova et al. (2008) examined AN and AB prototype learning in young adults using brain imaging and an extension to older adults would be relatively straightforward and worthy of future research. In the only known study of classification learning in normal aging, Fera et al. (2005) examined the neural mechanisms associated with probabilistic category learning. Although they found no age-related performance differences, they did find a shift in the extent of activation in different regions as a function of age. Specifically, they found reduced caudate and prefrontal activation, but increased parietal activation in older adults. Although speculative, this increased parietal activation in older adults could be related to the differences in attentional focus found between the AN and AB tasks in the current study. This task is very different from the prototype learning tasks used in the current study, but does lend support to the claim that changes in activation patterns emerge with normal aging and can affect performance.
Acknowledgments
This research was supported by National Institutes of Health Grant R01 MH077708 to WTM. We would like to thank Bo Zhu, Devon Greer, Marissa Gorlick, Brittany Nix and other members of the Maddox Lab for their help with data collection.
Footnotes
Results were similar for 0.99, 0.95, and 0.90. Results were directional for 0.80, however lower values are less useful for assessing dimensional weight allocation.
References
- Ashby FG, Alfonso-Reese LA, Turken AU, Waldron EM. A neuropsychological theory of multiple systems in category learning. Psychological Review. 1998;105:442–481. doi: 10.1037/0033-295x.105.3.442. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Maddox WT. Relations between prototype, exemplar, and decision bound models of categorization. Journal of Mathematical Psychology. 1993;37:372–400. [Google Scholar]
- Ashby FG, Maddox WT. Human category learning. Annual Review of Psychology. 2005;56:149–178. doi: 10.1146/annurev.psych.56.091103.070217. [DOI] [PubMed] [Google Scholar]
- Bozoki A, Grossman M, Smith EE. Can patients with Alzheimer’s disease learn a category implicitly? Neuropsychologia. 2006;44:816–827. doi: 10.1016/j.neuropsychologia.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26:809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Reed BR. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 2001;130:746–763. [PubMed] [Google Scholar]
- Corrigan JD, Hinkeldey MS. Relationships between parts A and B of the Trail Making Test. Journal of Clinical Psychology. 1987;43:402–409. doi: 10.1002/1097-4679(198707)43:4<402::aid-jclp2270430411>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera F, et al. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience. 2005;25:11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV, Maddox WT. A quantitative model-based approach to examining aging effects on information-integration category learning. Psychology & Aging. 2004;19:171–182. doi: 10.1037/0882-7974.19.1.171. [DOI] [PubMed] [Google Scholar]
- Filoteo JV, Lauritzen JS, Maddox WT. Removing the Frontal Lobes: The Effects of Engaging Executive Functions on Perceptual Category Learning. Psychological Science. 2010;21:415–423. doi: 10.1177/0956797610362646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopie N, Craik FIM, Hasher L. A double association of implicit and explicit memory in younger and older adults. Psychological Science. 2011 doi: 10.1177/0956797611403321. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cerebral Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Heaton RK. A manual for the Wisconsin Card Sorting Test. Psychological Assessment Resources, Inc.; Odessa, FL: 1980. [Google Scholar]
- Hess TM. Visual abstraction processes in young and older adults. Developmental Psychology. 1982;18:473–484. [Google Scholar]
- Hess TM, Slaughter SJ. Aging effects on prototype abstraction and concept identification. Journal of Gerontology. 1986a;41:214–221. doi: 10.1093/geronj/41.2.214. [DOI] [PubMed] [Google Scholar]
- Hess TM, Slaughter SJ. Specific exemplar retention and prototype abstraction in young and old adults. Psychology and Aging. 1986b;1:202–207. doi: 10.1037//0882-7974.1.3.202. [DOI] [PubMed] [Google Scholar]
- Homa D, Sterling S, Trepel L. Limitation of expemplar-based generalization and the abstraction of categorical information. Journal of Experimental Psychology: Human Learning and Memory. 1981;7:418–439. [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Benedek G, Janka Z. Intact prototype learning in schizophrenia. Schizophrenia Research. 2001;52:261–264. doi: 10.1016/s0920-9964(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Koenig P, Smith EE, Glosser G, DeVita C, Moore P, McMillan C, Gee J, Grossman G. The neural basis for novel semantic categorization. Neuroimage. 2005;24:369–383. doi: 10.1016/j.neuroimage.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Maddox WT, Pacheco J, Reeves M, Zhu B, Schnyer DM. Rule-based and information-integration category learning in normal aging. Neuropsychologia. 2010;48:2998–3008. doi: 10.1016/j.neuropsychologia.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox WT, Love BC, Glass BD, Filoteo JV. When More is Less: Feedback Effects in Perceptual Category Learning. Cognition. 2008;108:578–589. doi: 10.1016/j.cognition.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Palmer MR, Patlak CS, Calne DB. Nigrostriatal function in humans studied with positron emission tomography. Annual Neurology. 1989;26:535–542. doi: 10.1002/ana.410260407. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MG, Holroyd CB, Kok A, van der Molen MW. A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cognitive Affect Behavioral Neuroscience. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM. Selective attention and the formation of linear decision boundaries: Reply to Maddox and Ashby (1998) Journal of Experimental Psychology: Human Perception & Performance. 1998;24:322–339. doi: 10.1037//0096-1523.22.2.294. [DOI] [PubMed] [Google Scholar]
- Nosofsky RM, Zaki SR. Dissociations between categorization and recognition in amnesic and normal individuals: An exemplar-based interpretation. Psychological Science. 1998;9:247–255. [Google Scholar]
- Palmeri TJ, Flannery MA. Learning about categories in the absence of training: profound amnesia and the relationship between perceptual categorization and recognition memory. Psychological Science. 1999;10:526–530. [Google Scholar]
- Palmeri TJ, Flanery MA. Memory systems and perceptual categorization. In: Ross BH, editor. The Psychology of Learning and Motivation. Vol. 41. Academic Press; 2002. [Google Scholar]
- Palmeri TJ, Nosofsky RM. Central tendencies, extreme points, and prototype enhancement effects in ill-defined perceptual categorization. Quarterly Journal of Experimental Psychology: Human Experimental Psychology. 2001;54:197–235. doi: 10.1080/02724980042000084. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Foerde K. Category learning and the memory systems debate. Neuroscience & Biobehavioral Reviews. 2008;32:197–205. doi: 10.1016/j.neubiorev.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Posner MI, Keele SW. On the genesis of abstract ideas. Journal of Experimental Psychology. 1968;77:304–63. doi: 10.1037/h0025953. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Dupuis J, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: general trends, individual differences, and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Haacke EM. Brain aging and its modifiers: insights from in vivo neuromorphometry and susceptibility weighted imaging. Annals of the New York Academy of Sciences. 2007;1097:84–93. doi: 10.1196/annals.1379.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Dahle C, Head D, Acker JD. Differential age-related changes in the regional metencephalic volumes in humans: A five-year follow-up. Neuroscience Letters. 2003;349:163–166. doi: 10.1016/s0304-3940(03)00820-6. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR. Intact learning of artificial grammars and intact category learning by patients with Parkinson’s disease. Behavioral Neuroscience. 1999;113:235–242. doi: 10.1037//0735-7044.113.2.235. [DOI] [PubMed] [Google Scholar]
- Rosch E. On the internal structure of perceptual and semanic categories. Academic Press; New York: 1973. [Google Scholar]
- Rosch E, Mervis CB. Family resemblances: Studies in the internal structure of categories. Cognitive Psychology. 1975;7:573–605. [Google Scholar]
- Reed SK. Structural descriptions and the limitations of visual images. Memory & Cognition. 1974;2:319–336. doi: 10.3758/BF03209004. [DOI] [PubMed] [Google Scholar]
- Seger CA. How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback. Neuroscience & Biobehavioral Reviews. 2008;32:265–278. doi: 10.1016/j.neubiorev.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD, Minda JP. Prototypes in the mist: The early epochs of category learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1411–36. [Google Scholar]
- Smith EE, Patalano AL, Jonides J. Alternate strategies of categorization. Cognition. 1998;65:167–196. doi: 10.1016/s0010-0277(97)00043-7. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;28:643–662. [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiology of Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Takane Y, Shibayama T. Structure in stimulus identification data. Erlbaum; Hillsdale: 1992. San Antonio, TX: Pearson. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale: Technical and interpretive manual. 4th ed. Pearson; San Antonio, TX: 2008. [Google Scholar]
- van Dyck CH, Seibyl JP, Malison RT, Laruelle M, Zoghbi SS, Baldwin RM, Innis RB. Age-related decline in dopamine transporters: Analysis of striatal subregions, nonlinear effects, and hemispheric asymmetries. American Journal of Geriatric Psychiatry. 2002;10:36–43. [PubMed] [Google Scholar]
- Volkow ND, Logan J, Fowler JS, Wang GJ, Gur RC, Wong C, et al. Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. American Journal of Psychiatry. 2000;157:75–80. doi: 10.1176/ajp.157.1.75. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS, Gur RC, Gatley J, et al. Parallel loss of presynaptic and postsynaptic dopamine markers in normal aging. Annual Neurology. 1998;44:143–147. doi: 10.1002/ana.410440125. [DOI] [PubMed] [Google Scholar]
- Zaki SR, Nosofsky RM, Jessup NM, Unverzagt FW. Categorization and recognition performance of a memory-impaired group: evidence for single-system models. Journal of the International Neuropsychological Society. 2003;9:394–406. doi: 10.1017/S1355617703930050. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Maddox WT, Schnyer DM. Dissociable prototype learning systems: Evidence from brain imaging and behavior. The Journal of Neuroscience. 2008;28:13194–13201. doi: 10.1523/JNEUROSCI.2915-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]