Abstract
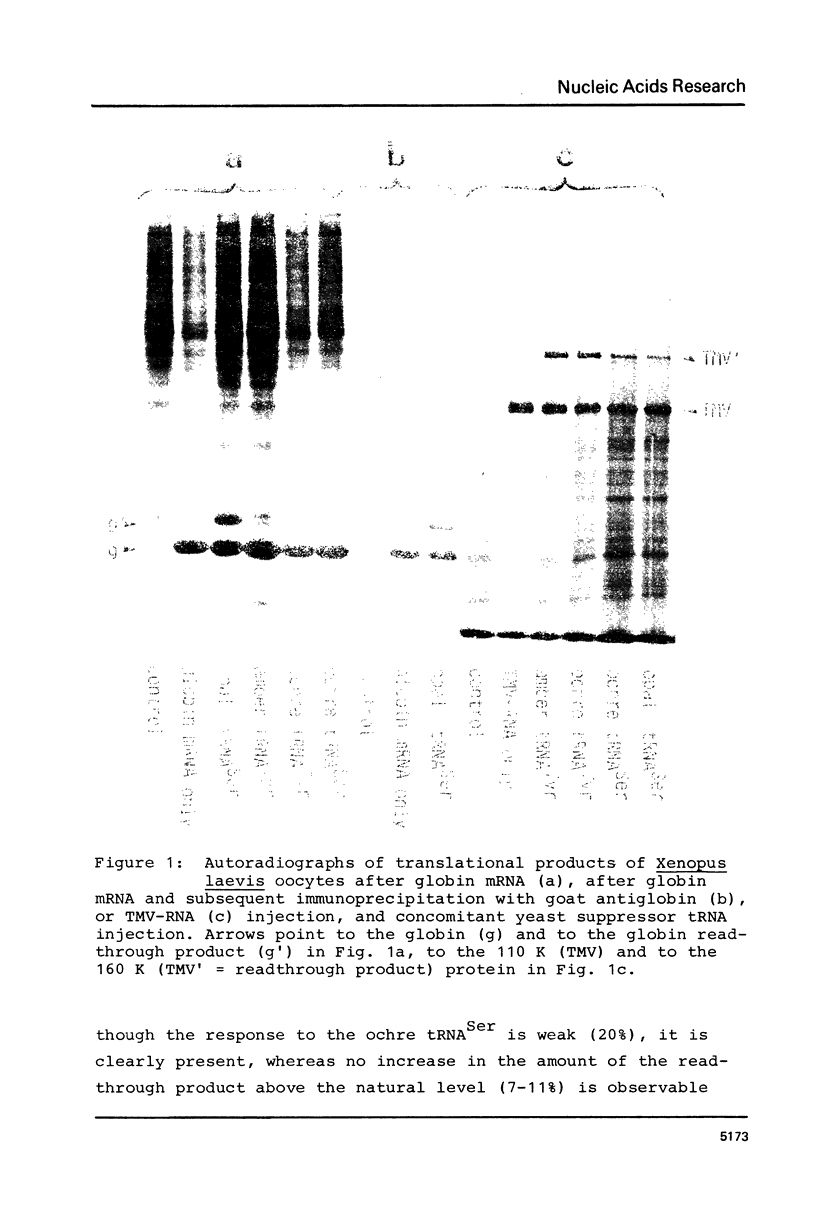
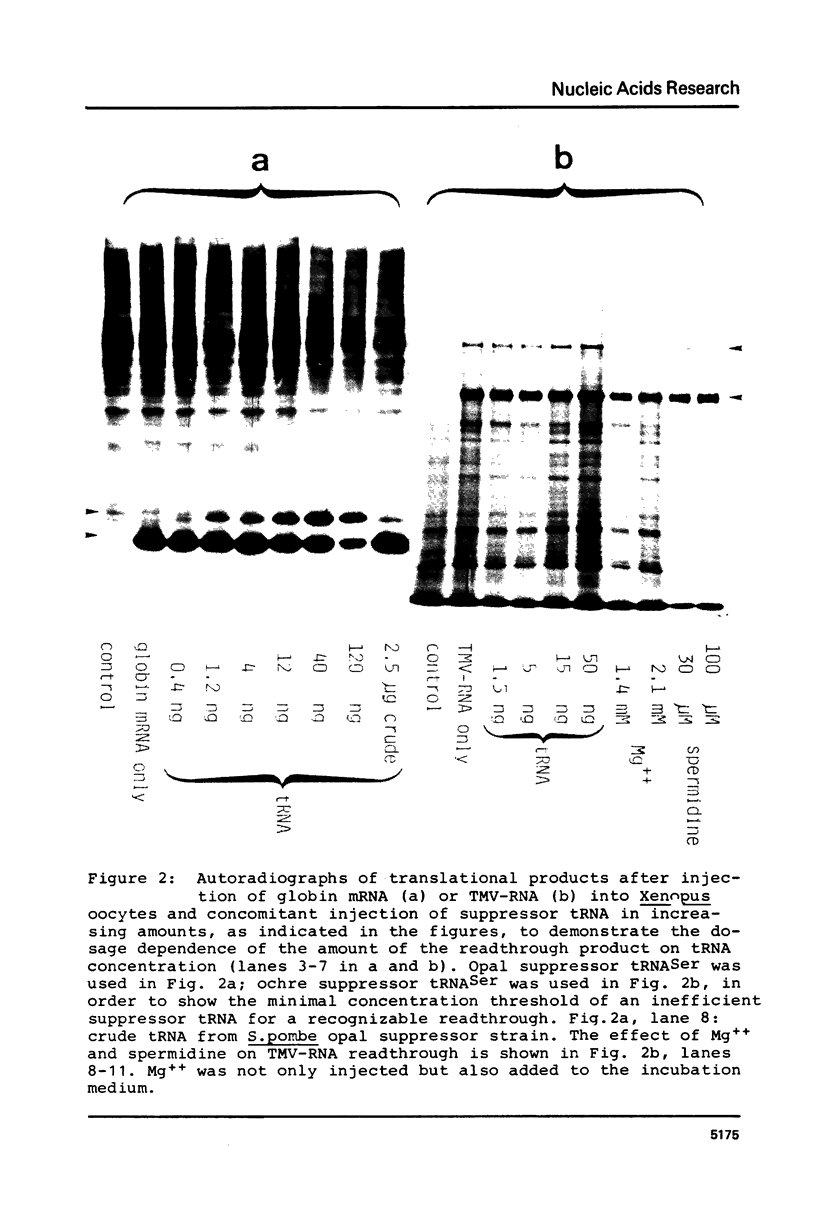
Amber, ochre, and opal nonsense suppressor tRNAs isolated from yeast were injected into Xenopus laevis oocytes together with purified mRNAs (globin mRNA from rabbit, tobacco mosaic virus-RNA). Yeast opal suppressor tRNA is able to read the UGA stop codon of the rabbit beta-globin mRNA, thus producing a readthrough protein. A large readthrough product is also obtained upon coinjection of yeast amber or ochre suppressor tRNA with TMV-RNA. The amount of readthrough product is dependent on the amount of injected suppressor tRNA. The suppression of the terminator codon of TMV-RNA is not susceptible to Mg++ concentration or polyamine addition. Therefore, the Xenopus laevis oocyte provides a simple, sensitive, and well buffered in vivo screening system for all three types of eukaryotic nonsense suppressor tRNAs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoi T., Kaesberg P. Read-through proteins of group 4 RNA bacteriophages TW19 and TW28. J Virol. 1976 Oct;20(1):330–333. doi: 10.1128/jvi.20.1.330-333.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R., Hughes S. H., Wahl G. M. Yeast super-suppressors are altered tRNAs capable of translating a nonsense codon in vitro. Cell. 1975 Nov;6(3):269–277. doi: 10.1016/0092-8674(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H., Dekel L., Israeli-Reches M., Belfort M. The requirement of nonsense suppression for the development of several phages. Mol Gen Genet. 1979 Feb 26;170(2):155–159. doi: 10.1007/BF00337791. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gatica M., Allende J. E. Aminoacyl transfer from phenylalanyl-tRNA microinjected into Xenopus laevis oocytes. Biochem Biophys Res Commun. 1977 Nov 21;79(2):352–356. doi: 10.1016/0006-291x(77)90164-4. [DOI] [PubMed] [Google Scholar]
- Gatica M., Tarragó A., Allende C. C., Allende J. E. Aminoacylation of transfer RNA microinjected into Xenopus laevis oocytes. Nature. 1975 Aug 21;256(5519):675–678. doi: 10.1038/256675a0. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Wolfner M., Grisafi P., Fink G., Botstein D., Roth J. R. Yeast suppressors of UAA and UAG nonsense codons work efficiently in vitro via tRNA. Cell. 1976 Mar;7(3):381–390. doi: 10.1016/0092-8674(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Giglioni B., Gianni A. M., Comi P., Ottolenghi S., Rungger D. Translational control of globin synthesis by haemin in Xenopus oocytes. Nat New Biol. 1973 Nov 28;246(152):99–102. doi: 10.1038/newbio246099a0. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Cornelis P., Giveon D., Littauer U. Z. Functionally impaired tRNA from ethionine treated rats as detected in injected Xenopus oocytes. Nucleic Acids Res. 1979 Feb;6(2):657–672. doi: 10.1093/nar/6.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Changes in somatic cell nuclei inserted into growing and maturing amphibian oocytes. J Embryol Exp Morphol. 1968 Nov;20(3):401–414. [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Molecular biology in a living cell. Nature. 1974 Apr 26;248(5451):772–776. doi: 10.1038/248772a0. [DOI] [PubMed] [Google Scholar]
- Hawthorne D. C., Leupold U. Suppressors in yeast. Curr Top Microbiol Immunol. 1974;64(0):1–47. doi: 10.1007/978-3-642-65848-8_1. [DOI] [PubMed] [Google Scholar]
- Horiuchi K., Webster R. E., Matsuhashi S. Gene products of bacteriophage Q beta. Virology. 1971 Aug;45(2):429–439. doi: 10.1016/0042-6822(71)90343-6. [DOI] [PubMed] [Google Scholar]
- Knowland J. Protein synthesis directed by the RNA from a plant virus in a normal animal cell. Genetics. 1974 Sep;78(1):383–394. doi: 10.1093/genetics/78.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J., Kwong T., Altruda F., Söll D., Wahl G. Characterization of a UGA-suppressing serine tRNA from Schizosaccharomyces pombe with the help of a new in vitro assay system for eukaryotic suppressor tRNAs. J Biol Chem. 1979 Mar 10;254(5):1546–1551. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Gesteland R. F. Suppression of amber mutants in vitro induced by low temperature. J Mol Biol. 1978 Nov 15;125(4):433–447. doi: 10.1016/0022-2836(78)90309-1. [DOI] [PubMed] [Google Scholar]
- Marbaix G., Huez G., Nokin P., Cleuter Y. Free cytoplasmic alpha-globin messenger RNA appears during the maturation of rabbit reticulocytes. FEBS Lett. 1976 Jul 15;66(2):269–273. doi: 10.1016/0014-5793(76)80520-0. [DOI] [PubMed] [Google Scholar]
- Marcus A., Seal S. N., Weeks D. P. Protein chain initiation in wheat embryo. Methods Enzymol. 1974;30:94–101. doi: 10.1016/0076-6879(74)30013-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch M. D., Benicourt C. Polyamines stimulate suppression of amber termination codons in vitro by normal tRNAs. Eur J Biochem. 1980 Apr;105(3):445–451. doi: 10.1111/j.1432-1033.1980.tb04519.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Piper P. W., Wasserstein M., Engbaek F., Kaltoft K., Celis J. E., Zeuthen J., Liebman S., Sherman F. Nonsense suppressors of Saccharomyces cerevisiae can be generated by mutation of the tyrosine tRNA anticodon. Nature. 1976 Aug 26;262(5571):757–761. doi: 10.1038/262757a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Complete 3' noncoding region sequences of rabbit and human beta-globin messenger RNAs. Cell. 1977 Apr;10(4):559–570. doi: 10.1016/0092-8674(77)90089-7. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T., Gesteland R. F., Spahr P. F. Translation of bacteriophage R17 and Qbeta RNA in a mammalian cell-free system. J Mol Biol. 1973 Apr 15;75(3):575–578. doi: 10.1016/0022-2836(73)90462-2. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. A single UGA codon functions as a natural termination signal in the coliphage q beta coat protein cistron. J Mol Biol. 1973 Nov 15;80(4):837–855. doi: 10.1016/0022-2836(73)90213-1. [DOI] [PubMed] [Google Scholar]