Abstract
Purpose
We performed a phase 2 study in children with recurrent or refractory leptomeningeal leukemia to determine the objective response rate after treatment with intrathecal topotecan.
Patients and Methods
Patients received age-adjusted intrathecal topotecan (0.4 mg/dose for patients > 3 years of age) administered twice weekly (every 3 to 4 days) for six weeks during induction, weekly for 4 weeks during consolidation, and twice monthly for 4 months and then monthly thereafter during maintenance.
Results
Twenty-two patients enrolled in the study, of whom 20 were eligible and assessable for toxicity and 16 were assessable for response. Of 16 patients, 6 (38%) had a complete response, 8 (50%) had stable disease, and 2 (13%) had progressive disease. The median EFS time (95% CI) was 3.1 (1.6 – 10.3) months and the median overall survival time (95% CI) was 18.0 (7.3 – 38.3) months. Eight patients (40%) experienced grade 3 or 4 adverse events. There were no grade 4 neurological events (Table III). Four patients experienced a total of 6 grade 3 neurological events including an olfactory seizure, a headache, transient grade 3 speech impairment, muscle weakness, motor neuropathy, and ataxia. Headache was the most common grade ≤ 2 neurologic event and two patients developed grade ≤ 2 arachnoiditis.
Conclusion
Intrathecal administration of topotecan was tolerable on this dose and schedule. The majority of adverse events were mild to moderate, reversible side effects. Complete central nervous system remissions were achieved in a subset of children with recurrent or refractory CNS leukemia.
Keywords: topotecan, intrathecal, CNS leukemia
INTRODUCTION
Refractory central nervous system (CNS) or leptomeningeal leukemia, though rare, presents a difficult clinical problem. Unlike the wide variety of anticancer drugs that show efficacy against systemic disease, relatively few agents have activity against CNS leukemia. Although systemic administration of some agents, such as high dose methotrexate, can produce clinically relevant cerebrospinal fluid (CSF) concentrations, the doses administered must be high, resulting in significant systemic toxicity. Furthermore, the blood-brain or blood-CSF barrier often prevents efficient penetration of many drugs into the CSF space. In contrast, regional delivery of drugs directly into the CSF is pharmacologically advantageous, with small doses producing high CSF concentrations with minimal systemic exposure.1 Unfortunately, only a limited number of drugs, primarily methotrexate and cytarabine, have been found to be both safe and efficacious when administered by the intrathecal (IT) route.
Topotecan is a topoisomerase I poison with anti-tumor activity against a variety of adult and childhood solid tumors. In preclinical and clinical studies, the CSF penetration of the active lactone form of topotecan was approximately 30% after systemic administration, and no significant neurologic toxicity was observed.2–4 In a phase 1 trial of IT topotecan, the maximum tolerated dose was 0.4 mg in children ≥ 3 years of age, with chemical arachnoiditis as the dose limiting toxicity.5 Preliminary evidence of activity was seen in the 3 children with CNS leukemia. We therefore conducted a phase 2 study to evaluate the response rate and safety of intrathecal topotecan in children with recurrent or refractory CNS leukemia. Event free survival is also reported. The preliminary results of a medulloblastoma stratum were previously reported.6
PATIENTS AND METHODS
Patient Eligibility
Informed consent was obtained in accordance with federal and institutional guidelines. Patients eligible for this study were at least 1 year of age and less than 22 years of age with leptomeningeal leukemia in second or greater relapse and refractory to conventional therapy, including radiation therapy. Patients were required to have a CSF white blood cell (WBC) count > 5/µL and evidence of blast cells on cytospin preparation or by cytology. Patients must not have received any systemic CNS-directed therapy within 3 weeks (6 weeks if a prior nitrosourea), or craniospinal irradiation within 8 weeks prior to starting treatment on this study. Patients must not have received IT chemotherapy within 1 week (2 weeks for liposomal cytarabine); patients who received IT chemotherapy in the 7 to 14 day period prior to study entry must have had evidence of subsequent increasing WBC and blasts percentage. Additional eligibility criteria included life expectancy > 8 weeks; Lansky or Karnofsky performance status ≥ 50%; recovery from the acute toxic effects of prior therapy; platelet count > 40,000/µL (transfusion supported); total bilirubin < 2.0 mg/dL and alanine aminotransferase (ALT) < 5 times normal; serum creatinine < 1.5 mg/dL; and normal serum electrolytes, calcium, and phosphorus. Patients with bone marrow relapse, obstructive hydrocephalus, compartmentalization of the CSF flow on radioisotope CSF flow study, ventriculoperitoneal or ventriculoatrial shunts, uncontrolled infection, CNS involvement requiring local XRT, or isolated bulky ventricular or leptomeningeal based lesions were not eligible.
Dosage and Drug Administration
The Pharmaceutical Management Branch of the National Cancer Institute provided lyophilized topotecan in 4 mg vials. The contents of each vial were diluted in 4 ml of sterile water then further diluted with preservative-free, pyrogen-free saline to a final volume of 10 ml. Administration of the drug was performed at a constant rate of 2.0 ml/min (total 5 minutes) through an intraventricular reservoir or lumbar puncture. The volume of CSF equivalent to the volume of drug to be administered was removed prior to drug administration. Patients who received topotecan via intralumbar injection remained prone, flat, or in the Trendelenburg position for one hour following drug administration. After drug administration via an intraventricular reservoir, the reservoir was flushed slowly for 1 to 2 minutes with approximately 2 ml of either CSF or preservative-free normal saline, them pumped 4 to 6 times.
Patients > 3 years of age received a dose of 0.4 mg of topotecan. Patients > 2 and ≤ 3 years received 0.32 mg of drug, and patients > 1 and ≤ 2 years received 0.25 mg. During induction, topotecan was administered twice weekly (every 3 to 4 days) for a total of 6 weeks (12 doses). Patients with a complete response (CR) or stable disease (SD) after induction proceeded to consolidation therapy, which began 1 week after the last induction dose. Consolidation lasted four weeks, with one dose of topotecan administered each week (4 total doses). The maintenance phase began 2 weeks after the last consolidation dose. During maintenance, intrathecal topotecan was administered twice monthly for four months (8 doses), and monthly thereafter. The maximum duration of intrathecal topotecan treatment was one year.
Concomitant therapy
Patients could receive chemotherapy to control systemic disease provided the systemic chemotherapy was not a phase I agent, did not significantly penetrate the CSF, or was known to have serious unpredictable CNS side effects.
Pretreatment and Follow-Up Studies
Pretreatment evaluations, performed within 72 hours prior to enrollment, included a complete history, physical examination with detailed neurological examination, CBC with differential and platelet count, BUN, creatinine, SGPT, electrolytes, calcium, total bilirubin, and phosphorus. CSF studies performed included a cell count, differential, protein, glucose and cytologic examination for presence of blasts. In patients with Ommaya reservoirs, studies were performed on both lumber and ventricular CSF. All patients were required to undergo a head MRI with and without contrast within 2 weeks prior to protocol entry, as well as an MRI of the spine with and without contrast if clinically indicated.
During therapy, a history and physical exam with a detailed neurological exam were performed weekly during induction, every 2 weeks during consolidation, and monthly during maintenance. CSF was obtained and evaluated immediately prior to the first dose, weekly during induction, every 2 weeks during consolidation, monthly during maintenance, four weeks after the completion of maintenance therapy, and four weeks after the first negative CSF evaluation. CSF evaluation included total WBC and differential count, protein, glucose, and cytology. In patients with an Ommaya reservoir, lumbar and ventricular CSF were evaluated at the completion of induction, at the completion of consolidation, every 2 months during maintenance, four weeks after completion of maintenance therapy, and four weeks after the first negative CSF evaluation.
Follow-up observations, including a complete physical and neurological exams together with an assessment of change in the patient’s neurological status from baseline, were performed within 28 ± 7 days after the date of the last drug administration and subsequently every 28 ± 7 days for 3 months. For those patients still in disease remission after completing the study, additional clinical evaluation and CSF cytology were performed every 90 ± 30 days for up to 1 year or until relapse.
Criteria for Assessment of Toxicity, Response, and Survival
Adverse events were evaluated according to the NCI Common Toxicity Criteria (Version 2.0). Patients were withdrawn from the trial if they developed significant neurotoxicity or other organ dysfunction, grade 3 or higher that was considered to be primarily related to topotecan.
Responses to the intrathecal topotecan therapy were assessed and classified as complete response (CR), stable disease (SD), or progressive disease (PD). For CR, patients had to have complete clearing of all malignant cells from lumbar CSF on two consecutive cytologic studies at least 4 weeks apart, with no worsening of physical or neurological findings clearly attributable to neoplastic meningitis. For patients with Ommaya reservoirs, complete clearing of ventricular CSF was required as well. PD was defined as the presence of blasts and an increase in the number of WBCs in the ventricular or lumbar CSF when compared to the lowest (best) CSF WBC count obtained on therapy. The minimum required CSF WBC count for PD was >10 cells/mm3. For asymptomatic patients with CSF WBC counts between 10 and 30/mm3, the increase in the CSF WBC count had to be at least 100% and not attributable to arachnoiditis or infection. In patients with CSF WBC counts > 30/mm3, the increase in the WBC count had to be at least 50% and not attributable to either arachnoiditis or infection. Patients with CSF blasts and worsening physical or neurologic findings clearly attributable to CNS leukemia were considered to have PD, regardless of the increase in the CSF WBC count.
Patients were considered to have stable disease (SD) if they did not meet the criteria for either a complete response or progressive disease. Patients with SD could not have worsening of physical findings clearly attributable to neoplastic meningitis.
The response rates for CR, SD and PD were estimated as the number of patients whose maximal response was in the noted category divided by the number of patients considered evaluable for response.7 Exact 95% confidence intervals for response were calculated.
Event-free survival (EFS) was taken to be the time from enrollment until disease progression, death, or last patient contact, whichever occurred first. Patients who experienced disease progression or death were considered to have experienced an EFS event; otherwise the patient was considered as censored at last contact. Survival (OS) was taken to be the time from enrollment to death or last patient contact, whichever occurred first. Patients who died were considered to have experienced a death event; otherwise the patient was considered as censored at last contact. EFS and OS as a functions of time since enrollment were estimated by the method of Kaplan and Meier.8 Confidence intervals were calculated using the complementary log-log transformation of the Kaplan-Meier estimate of the survival curve.9
All EFS events were either death or disease recurrence, including CNS recurrence. The cumulative incidence of CNS recurrence was estimated by the non-parametric estimator of the CNS-recurrence-cause-specific hazard.7
RESULTS
P9962 was opened for enrollment in April 2000 and closed in April 2006. Data current to December 2010 were used for this analysis. Twenty-two patients (Table I) enrolled in the study. Two patients were ineligible: 1 did not meet the eligibility requirements for serum phosphate and 1 did not have a diagnosis of CNS leukemia. The median number of doses administered to the twenty eligible patients was 15 (range, 4–25). Drug was administered through an Ommaya reservoir in 5 patients, lumbar puncture in 8 patients, and through both a combination of both routes (Ommaya reservoir or lumbar puncture) in 7 patients. In those who received the drug via both routes, dosing via Ommaya reservoir predominated.
Table I.
Patient demographics for eligible patients (n=20)
| Characteristic | No. of patients | |
|---|---|---|
| Gender | ||
| Male | 13 | |
| Female | 7 | |
| Age (years) | ||
| Median | 12.5 | |
| Range | 2–20 | |
| Race | ||
| White | 13 | |
| Black | 3 | |
| Asian | 1 | |
| Other | 1 | |
| Unknown | 2 | |
Eight patients experienced grade 3 or 4 adverse events. The predominant non-neurologic adverse events were hematologic, gastrointestinal, or metabolic (Table II); attribution of these to intrathecal topotecan is unlikely as patients were also receiving systemic anticancer drugs to maintain systemic remission. There were no grade 4 neurological events (Table III). Four patients experienced a total of 6 grade 3 neurological events: 1 patient each had an olfactory seizure, a headache, and transient grade 3 speech impairment that required removal from study and resolved within one month. In addition, 1 patient had grade 3 muscle weakness, motor neuropathy, and ataxia. This patient had evidence of cerebellar leukemic involvement on a pretreatment MRI. Headache was the most common grade ≤ 2 neurologic event. Two patients developed grade ≤ 2 arachnoiditis and received dexamethasone as per the study supportive care guidelines, with prompt resolution of symptoms. One patient had a grade 3 injection site reaction, described as tissue necrosis, at the site of a lumbar catheter and received all subsequent topotecan through an Ommaya reservoir.
Table II.
Non-neurologic adverse events that were possibly, probably or definitely attributed to IT topotecan in 20 eligible patients*
| Event | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Fatigue | 1 | - | - | - |
| Fever | 1 | 2 | - | - |
| Weight Loss | - | 1 | - | - |
| Injection Site Reaction | - | 1 | 1 | - |
| Infection/Febrile Neutropenia | - | - | 1 | 1 |
| Pain | 1 | 1 | 1 | - |
| Dermatologic | 1 | - | - | - |
| Gastrointestinal | 5 | 4 | - | 1 |
| Hepatic | 1 | - | 3 | - |
| Metabolic | 2 | 1 | - | - |
| Hematologic | - | 2 | 1 | 3 |
More than 1 event may have occurred in the same patient
Table III.
Neurologic adverse events that were possibly, probably or definitely attributed to IT topotecan in 20 eligible patients*
| Event | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Arachnoiditis | 1 | 1 | - | - |
| Ataxia | 1 | - | 1 | - |
| Depression | - | 1 | - | - |
| Diplopia / blurred vision | - | 2 | - | |
| Dizziness/lightheadedness | 1 | - | - | - |
| Involuntary movement / restlessness | 1 | - | - | - |
| Facial weakness/droop | 1 | - | ||
| Headache | 2 | 6 | 1 | - |
| Insomnia | - | 1 | - | - |
| Leukoencephalopathy | 1 | - | - | - |
| Motor neuropathy | 1 | - | 1 | - |
| Muscle weakness | 1 | - | 1 | |
| Seizures (olfactory) | - | - | 1 | - |
| Sensory neuropathy | - | 1 | - | - |
| Speech impairment | - | 1 | 1 | - |
More than 1 event may have occurred in the same patient
Antitumor Activity
Of the twenty eligible patients, 4 were inevaluable for response: 2 because of incomplete lumbar CSF sampling to confirm response, 1 who was removed from protocol therapy by the treating physician to use an alternate dosing schedule, and 1 patient who was removed from study early due to toxicity (grade 3 speech impairment). The CR and SD rates for the 16 evaluable patients were estimated as 37.5% (95% CI: 15.2%–64.6%) and 50.0% (95% CI 24.7%–75.3%), respectively (Table IV). The patient with incomplete lumbar sampling had a CR in the ventricular CSF that was maintained for the entire duration of protocol therapy; however, a lumbar puncture was not performed due to previous skin necrosis associated with a lumbar catheter, thus the patient was considered inevaluable for response.
Table IV.
Responses in evaluable patients (n=16)
| Response | No. | Percentage |
|---|---|---|
| Complete response | 6 | (37.5%) |
| Stable disease | 8 | (50%) |
| Progressive disease | 2 | (12.5%) |
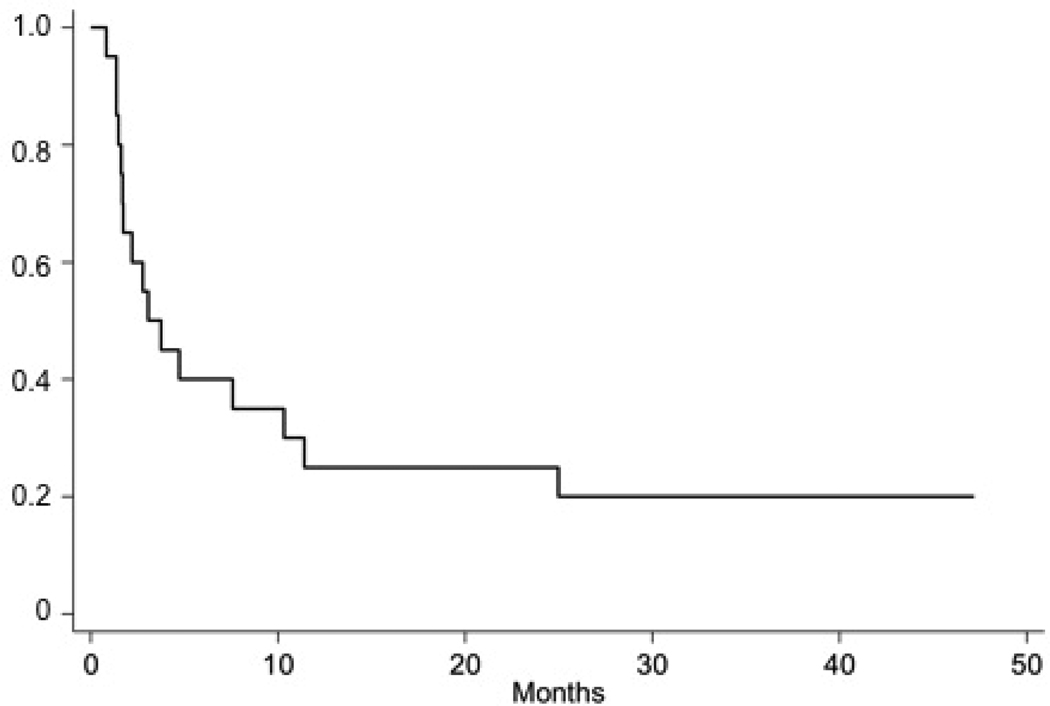
All eligible patients on the ALL stratum were included in EFS and OS analyses. The median follow up time was 47.2 months (range 34.9 – 83.5 months) in patients who were alive at last contace. The median EFS time (95% CI) was 3.1 (1.6 – 10.3) months and the median overall survival time (95% CI) was 18.0 (7.3 – 38.3) (Figure 1). The estimated cumulative incidence of CNS relapse at 30 months was 80.0% (Figure 2).
Figure 1.
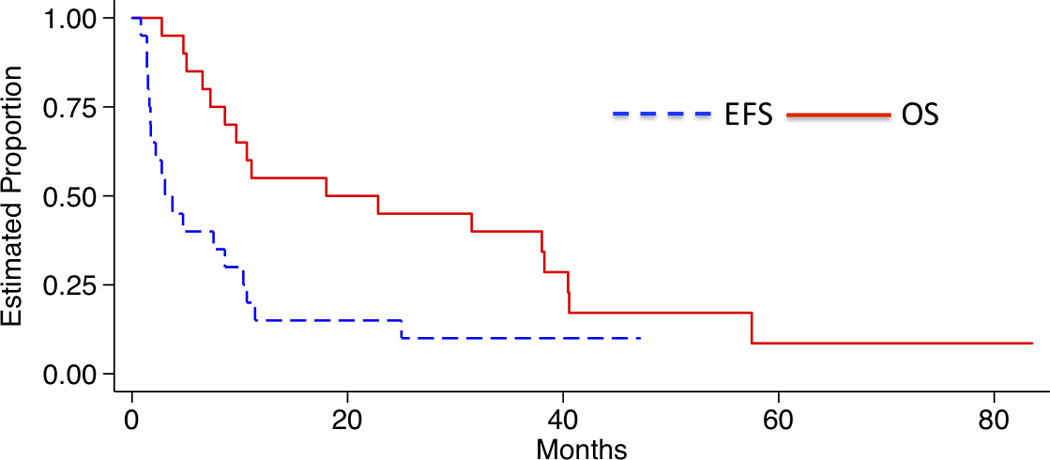
The median EFS time (95% CI) was 3.1 (1.6 – 10.3) months and the median OS time (95% CI) was 18.0 (7.3 – 38.3) months.
Figure 2.
Estimated proportion of patients who will not experience a CNS relapse following intrathecal topotecan administration according to time since starting treatment.
DISCUSSION
Although leukemia and lymphoma are generally considered chemosensitive tumors, leptomeningeal relapse of acute leukemia or lymphoma remains difficult to treat. Standard therapy includes intrathecal methotrexate, cytarabine, or “triple” intrathecal therapy (methotrexate, cytarabine, and hydrocortisone). The “concentration times time” (C × T) approach of giving small doses of methotrexate and cytarabine frequently into the ventricular CSF is pharmacologically advantageous and produces remission in up to 90% of leukemia patients, with a median CNS remission duration of 15 months.10,11 In addition, half the patients who relapsed on the maintenance phase of C × T therapy, when doses are given less frequently, experience a second remission when the induction or frequent administration schedule of C × T therapy was reinstated.11 The primary disadvantage of the C × T approach is that it requires placement of an intraventricular reservoir.
Administration of liposomal cytarabine is an alternate approach that permits intralumbar injections at intervals of approximately 2 weeks. In a phase 1 study, all 7 patients with leukemia had objective responses (4 CR, 3 PR) to intralumbar liposomal cytarabine, and cytotoxic drug concentrations were maintained in the CSF for more than a week after a single dose.12
The present study shows that intraventricular administration of topotecan is feasible and produces responses in patients with refractory leptomeningeal leukemia, with a CR rate of nearly 40%. Caution should be exercised when comparing this response rate to other small studies, as the individual patient characteristics, especially prior therapies, may be quite different. Similarly, adverse events in this study cannot be directly compared to other studies, but appeared typical of those experienced by patients with refractory CNS disease receiving intrathecal and systemic therapy.
In contrast to the experience in leukemia, there were no objective responses for children with leptomenigeal dissemination of medulloblastoma or other solid tumors who received intrathecal topotecan; the median 6 month progression-free survivals for these patients were 19% ± 12% and 13% ± 12%, respectively.13
In summary, IT topotecan is tolerable and active against recurrent or refractory leptomeningeal leukemia. Future studies are necessary to determine its role in prevention and treatment of CNS leukemia. However, it may have provided benefit for many months in a subset of patients with CNS leukemia who are refractory to traditional anti-metabolite therapy.
Acknowledgments
Research support: Supported by National Cancer Institute Grants U10 CA98543 and U10CA98413 and NCRR M01 RR00188.
References
- 1.Blaney SM, Poplack DG. Neoplastic meningitis: diagnosis and treatment considerations. Med Oncol. 2000;17:151–162. doi: 10.1007/BF02780522. [DOI] [PubMed] [Google Scholar]
- 2.Blaney S, Cole D, Godwin K, et al. Intrathecal administration of topotecan in nonhuman primates. Cancer Chemother Pharmacol. 1995;36:121–124. doi: 10.1007/BF00689195. [DOI] [PubMed] [Google Scholar]
- 3.Sung C, Blaney S, Cole D, et al. A pharmacokinetic model of topotecan clearance from plasma and cerebrospinal fluid. Cancer Res. 1994;54:5118–5112. [PubMed] [Google Scholar]
- 4.Baker S, Heideman R, Crom W, et al. Cerebrospinal fluid pharmacokinetics and penetration of continuous infusion topotecan in children with central nervous system tumors. Cancer Chemother Pharmacol. 1996:195–202. doi: 10.1007/BF00688317. [DOI] [PubMed] [Google Scholar]
- 5.Blaney SM, Heideman R, Berg S, et al. Phase I clinical trial of intrathecal topotecan in patients with neoplastic meningitis. J Clin Oncol. 2003;21:143–147. doi: 10.1200/JCO.2003.04.053. [DOI] [PubMed] [Google Scholar]
- 6.Blaney SM, Berg SL, Krailo M, et al. Phase II clinical trial of intrathecal topotecan in children with leptomeningeal dissemination from medulloblastoma or an underlying solid or CNS tumor: a Children’s Oncology Group Study. International Society of Pediatric Neuro-Oncology (2006) Neuro-Oncology. 2007;9:188. Abstract #128. [Google Scholar]
- 7.Kalbfleisch J. Probability and Statistical Inference. New York: Springer-Verlag; 1979. [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 9.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley and Sons; 2002. [Google Scholar]
- 10.Bleyer W, Poplack D, Simon R. 'Concentration-time' methotrexate via a subcutaneous reservoir: a less toxic regmine for intraventricular chemotherapy of central nervous system neoplasms. Blood. 1978;51:835–842. [PubMed] [Google Scholar]
- 11.Moser A, Adamson PC, Gillespie A, et al. Intraventricular concentration times time (C x T) methotrexate and cytarabine for patients with recurrent meningeal leukemia and lymphoma. Cancer. 1999;85:511–516. doi: 10.1002/(sici)1097-0142(19990115)85:2<511::aid-cncr33>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Bomgaars L, Geyer J, Franklin J, et al. A phase I trial or intrathecal liposomal cytarabine in children with neoplastic meningitis. J Clin Oncol. 2004;22:3916–3921. doi: 10.1200/JCO.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Blaney SM, Berg, Krailo M, et al. Phase II clinical trial of intrathecal topotecan in children with leptomeningeal dissemination from medulloblastoma or an underlying solid or CNS tumor: a Children’s Oncology Group Study. International Society of Pediatric Neuro-Oncology (2006) Neuro-Oncology. 2007;9:188. Abstract #128. [Google Scholar]