Abstract
Dysregulation of autonomic nervous system dynamics is important in the pathophysiology of cardiovascular risk in obstructive sleep apnea (OSA). Heart rate variability (HRV) and impedance cardiography measures can estimate autonomic activity but have not gained traction clinically. We hypothesized that, even in a cohort of mild, asymptomatic OSA patients without overt cardiovascular disease, daytime HRV metrics and impedance cardiography measurements of pre-ejection period (PEP) would demonstrate increased sympathetic and decreased parasympathetic modulation compared with matched controls. Obese individuals (BMI ≥30 kg/m2) without any known cardiovascular or inflammatory comorbidities were recruited from the community. Subjects underwent standard in-laboratory polysomnograms (PSG), followed by simultaneous electrocardiography (ECG) and impedance cardiography recordings while supine, supine with paced breathing, and after standing. 74 subjects were studied, and 59% had OSA (apnea-hypopnea index (AHI) ≥10episodes/hr) with a median AHI of 25.8/hr. OSA subjects had significantly decreased daytime time- and frequency-domain HRV indices, but not significantly different PEP, when compared to controls. AHI was a significant independent predictor of time-domain HRV measures in all awake conditions, after controlling for age, gender, blood pressure, fasting cholesterol levels and hemoglobin A1C. In conclusion, our results demonstrate reductions in cardiac vagal modulation, as measured by multiple daytime time-domain markers of HRV, among asymptomatic OSA patients versus controls. Further prospective outcomes-based studies are needed to evaluate the applicability of these metrics for noninvasive screening of obese asymptomatic OSA patients, prior to the onset of overt cardiovascular disease.
Keywords: Sleep apnea, Sympathetic, Parasympathetic, Heart rate variability, Lung, Cardiac, Autonomic function, Cardiovascular risk factors
Introduction
In a cohort of community-based obese subjects with predominantly mild, asymptomatic obstructive sleep apnea (OSA), we sought to test prospectively the hypothesis that OSA per se is associated with increased sympathetic and decreased parasympathetic cardiac vagal modulation compared to obese controls. In addition, we sought to assess which autonomic metrics might be the most useful markers for OSA in subsequent studies, including pNNx measures, which are newer and readily computed heart rate variability metrics (HRV) based on inter-beat interval variability. By assessing a sample without cardiovascular comorbidities, we reasoned that any observed HRV or pre-ejection period (PEP) abnormalities would represent isolated effects of obesity and OSA and, therefore, might be useful as early markers prior to the onset of overt cardiovascular complications.
Methods
All subjects provided written informed consent. The study was approved by our Institutional Review Board. Nonsmoking individuals aged 18–70 years with a body mass index (BMI) ≥30 kg/m2, and without known cardiac, pulmonary, endocrine, or non-OSA sleep disorders, were recruited from the community by advertisements posted in local newspapers and in local medical clinics. Subjects were ineligible if taking any oral contraceptives, hormone replacement therapy, sedatives, steroids, non-steroidal anti-inflammatory agents, lipid-lowering medications, or anti-hypertensive medications. Potentially eligible subjects then underwent a screening examination by a licensed physician and were excluded from the study if blood counts, fasting glucose, thyroid stimulating hormone, lipid panel or blood pressure measurements were markedly abnormal. All subjects were also asked to keep a two-week sleep diary to ensure regular sleep patterns and to exclude sleep deprivation.
Subjects underwent a standard in-laboratory PSG; recorded signals included electroencephalogram (C4-A1, C3-A2, O2-A1, and O1-A2), left and right electro-oculogram, submental and bilateral tibial electromyogram, surface ECG, airflow, chest and abdominal excursion, oxyhemoglobin saturation, and body position. All data were collected on a Nihon Kohden (Foothill Ranch, CA) digital PSG system. PSGs were scored by a blinded, registered sleep technician according to standard criteria1. An apnea was scored if airflow was absent for 10 seconds, and a hypopnea was scored if there was a ≥ 50% reduction in airflow for 10 seconds or a discernable decrement in airflow for 10 seconds in association with either an oxyhemoglobin desaturation of ≥ 3% or an arousal.
Laboratory measurements were undertaken during the morning after the PSG, while patients were fasting. Blood samples were obtained in sterile fashion from the antecubital vein. Total serum cholesterol and triglycerides were measured using the Synchoron CX analyzer (Beckman Systems, Fullerton, CA). Serum high-density lipoprotein was measured directly (Sigma, St. Lois MO) and low-density lipoprotein was calculated. Whole blood glycated hemoglobin (HbA1C) was measured with ion-exchange high-performance liquid chromatography.
Each subject had 5 to 17 minutes of single-lead ECG recorded at 1000 Hz while (a) supine during basal breathing, (b) supine while breathing paced by an electronic tone at 12 breaths/minute (0.2 Hz), and (c) while standing during basal breathing. Continuous ECG data were recorded on Spike2 acquisition software (1401plus, Cambridge Electronic Design, UK), then extracted and converted to waveform database format. An automated QRS detector (ecgpuwave, PhysioNet, Cambridge, MA) was run on these rescaled ECG signals and every beat was annotated as normal or ectopic. The annotation file with the fewest number of ectopic beats was selected for further analysis, and the time series of normal-to-normal (NN) sinus intervals was extracted. The first 5 minutes of stable, stationary data were chosen for analysis from the three awake conditions. Outliers due to false or missed normal beat detections were removed using a sliding window average filter with a window of 41 data points and rejection of central points lying outside 20% of the window average. On average, the resulting annotation files contained 98.4% normal beats.
From the filtered NN interval files, time-domain statistics including AVNN (the average of all NN intervals), SDNN (the standard deviation of all NN intervals), RMSSD (root mean square of successive NN intervals), pNN50, pNN20, and pNN10 (pNNx is defined as the percentage of successive NN intervals differing by > × msec) were calculated2. Standard frequency-domain measures were also calculated, including LF power (spectral power of all NN intervals between 0.04 and 0.15 Hz), HF power (spectral power of all NN intervals between 0.15 and 0.4 Hz), LF/HF (ratio of low- to high- frequency power), and for paced breathing, power at the paced breathing frequency (PBF, or total spectral power of all NN intervals between 0.18 and 0.22 Hz). In order to eliminate the need for evenly sampled data that is required by the standard Fast Fourier Transform, frequency domain measures were calculated using the Lomb periodogram for unevenly sampled data3.
Approximately 45 minutes after awakening, simultaneous ECG and impedance cardiography recording was also performed. After skin preparation, 4 impedance electrode strips were placed in a standard circumference tetrapolar band configuration4, and 3 ECG leads were placed in the right and left upper chest and the left lower quadrant. ECG and dZ/dT signals were sampled at 512 Hz and ensemble-averaged over 1-minute epochs. PEPs were recorded as five 1-minute averages during each of the awake conditions described above. PEP was measured with the HIC 3000 Bioelectric Impedance Cardiograph System with Cop-Win/HRV version 6.0 data acquisition software (Bio-Impedance Technology, Inc, Chapel Hill, NC, USA).
Continuous data were inspected for normality of distribution and homogeneity of variance, and compared between groups using independent t-tests or Mann-Whitney tests as appropriate. Categorical data were compared with chi-square tests. Missing data were excluded pairwise for all analyses. To determine independent predictors of various time-domain HRV indices and PEP data, multiple linear regression forced-entry models were built. Multicollinearity between predictors was investigated using simple Pearson correlations, and inspection of tolerance and variance inflation factor values. Finally, standardized residuals were inspected to identify potential outliers. All reported p-values are 2-sided and p≤0.05 was considered significant.
Results
Of the 109 patients who were screened, 15 were excluded due to abnormal physical exams or laboratory values, 14 subjects withdrew consent prior to the PSG, and 2 subjects were withdrawn due to lightheadedness during testing. Data from 4 subjects were excluded entirely from HRV analysis due to poor waveform capture by signal acquisition software. Data from 8 subjects were excluded entirely from PEP analysis due to missing or corrupted raw files at the time of analysis.
OSA was defined as having an AHI ≥10/h. Of the 74 subjects, 44 subjects (59.5%) met this criterion with a median AHI of 25.8/h (IQR 25.7), indicative of mild to moderate severity. The median ESS of the OSA group (7.0; IQR 10.0) was within the normal range, suggesting that these patients were primarily asymptomatic. Baseline characteristics between OSA and control subjects were similar for many parameters (see Table 1).
Table 1. Baseline characteristics of study subjects.
Parametric data are presented as mean±SD; non-parametric data are represented as median (interquartile range). All p-values are 2-sided, and considered significant when ≤0.05 (shown in bold).
| Variable | Control (n=33) | OSA (n=41) | p-value |
|---|---|---|---|
| Men | 9 (27%) | 24 (58%) | <0.01 |
| Age (years) | 32.5±11.6 | 44.0±10.1 | <0.01 |
| Body Mass Index (kg/m2) | 37.8 (9.5) | 36.6 (12.2) | 0.77 |
| Epworth Sleepiness Scale score (/24) | 6 (7) | 7 (10) | 0.32 |
| Heart rate (beats/min) | 70±8 | 71±11 | 0.6 |
| Systolic Blood Pressure (mm Hg) | 127±12 | 130±10 | 0.31 |
| Diastolic Blood Pressure (mm Hg) | 73±7 | 78±10 | 0.02 |
| Total Cholesterol (mg/dL) | 185±32 | 193±35 | 0.37 |
| High-Density Lipoprotein (mg/dL) | 56±15 | 46±13 | 0.06 |
| Low-Density Lipoprotein (m/dL) | 99 (38) | 108 (50) | 0.47 |
| Triglycerides (mg/dL) | 87 (70) | 121 (94) | <0.01 |
| Hemoglobin A1C (%) | 5.5 (0.5%) | 5.6 (0.6%) | 0.01 |
| Apnea-Hypopnea Index (events/hr) | 2.8 (4.4) | 25.8 (25.7) | <0.01 |
| SpO2 minimum (%) | 87.0 (6.0%) | 76.0 (10.0%) | <0.01 |
| Time SpO2<90% | 0.9 (7.7%) | 12.7 (22.9%) | <0.01 |
| Arousal Index (events/hr) | 15.1 (10.2) | 30.3 (25.4) | <0.01 |
Time- and frequency-domain metrics for all subjects were graphically (see Figure 1) and statistically assessed (see Table 2). For frequency-domain metrics, both LF power and HF power were significantly decreased in OSA subjects compared to control subjects. Because HF power was decreased to a greater extent than LF power in OSA subjects, this finding resulted in significantly higher LF/HF ratios with OSA during supine basal breathing. During paced breathing, PBF power, a narrower HF band centered around the paced respiratory rate of 0.2 Hz, was also significantly decreased in OSA compared to control subjects.
Figure 1. Time series (RR in msec vs. time in sec) and Fourier transform (amplitude in msec vs time in sec) plots of representative control (panels A,C,E) and OSA subjects (panels B,D,E) during supine basal breathing (panels A and B), supine paced breathing (panels C and D), and standing (panels E and F).
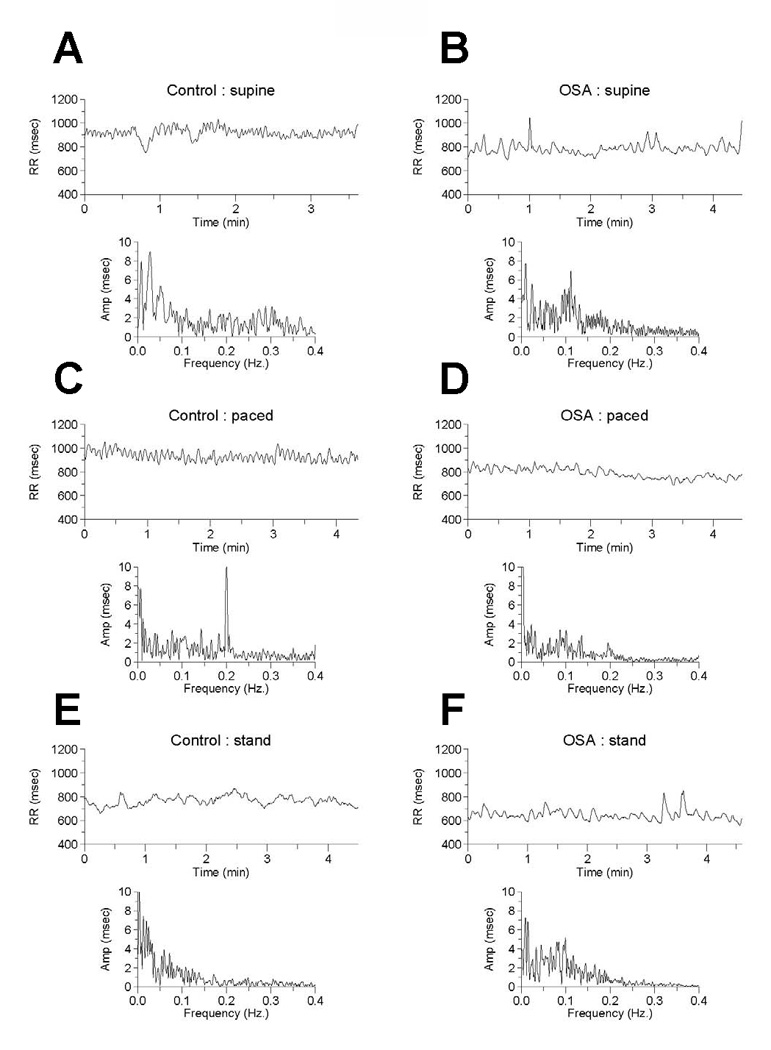
Panel A: The “fine-tooth” oscillations in the RR-time plot and the low, broad peak at about 0.3 Hz (18 cycles/min) of the Fourier transform represent cardiac vagal tone modulation related to respiratory sinus arrhythmia.
Panel B: Cardiac vagal tone modulation is reduced with OSA compared to control as noted by reduced high frequency fluctuations in the RR-time plot and absence of an apparent high frequency peak in the Fourier transform amplitude-frequency plot. Cardiac sympathetic tone may be increased, as noted by the relatively enhanced low frequency peak at 0.1 Hz.
Panel C: Paced breathing at 12 breaths/min leads is associated with a spectral spike at 0.2 Hz on the Fourier plot, related to accentuation of vagally-mediated respiratory sinus arrhythmia.
Panel D: The reduction in cardiac vagal tone with OSA (compare with panel C) during paced breathing is visually evidence. Note that the fine oscillations in the RR-time series plot are absent and the 0.2 Hz spike on the Fourier plot is markedly diminished.
Panels E and F: Standing results in a higher 0.1 Hz peak with OSA compared to controls, suggestive of higher cardiac sympathetic tone.
Table 2. Heart rate variability and impedance cardiography metrics during awake conditions.
Parametric data are presented as mean±SD; non-parametric data are represented as median (interquartile range). All p-values are 2-sided, and considered significant when ≤0.05 (shown in bold).
| Control | OSA | p-value | |
|---|---|---|---|
| 5-minute supine basal breathing | |||
| AVNN (msec) | 920 (96) | 887 (215) | NS |
| SDNN (msec) | 51.2 (42.6) | 35.9 (21.2) | <0.01 |
| RMSSD (msec) | 41.1 (39.1) | 22.1 (17.1) | <0.01 |
| pNN10 (%) | 80.8 (15.2) | 58.3 (26.8) | <0.01 |
| pNN20 (%) | 61.8 (30.1) | 26.52(28.9) | <0.01 |
| pNN50 (%) | 17.5 (38.6) | 2.72 (7.73) | <0.01 |
| LF power (msec2) | 698 (1254) | 356 (526) | <0.01 |
| HF power (msec2) | 564 (1572) | 165 (322) | <0.01 |
| LF/HF ratio | 1.08 (0.94) | 2.40 (2.25) | <0.01 |
| PEP (msec) | 112±10 | 11±18 | NS |
| 5-minute supine paced breathing | |||
| AVNN (msec) | 931 (119) | 896 (219) | NS |
| SDNN (msec) | 45.9 (35.5) | 37.9 (19.9) | <0.05 |
| RMSSD (msec) | 37.3 (45.4) | 22.3 (18.1) | <0.01 |
| pNN10 (%) | 76.2±15.2 | 57.9±15.9 | <0.01 |
| pNN20 (%) | 57.1±22.0 | 31.1±18.0 | <0.01 |
| pNN50 (%) | 13.8 (42.1) | 3.26 (7.68) | <0.01 |
| PBF power (msec2) | 524 (1495) | 123 (245) | <0.01 |
| PEP (msec) | 111±11 | 111±8 | NS |
| 5-minute standing basal breathing | |||
| AVNN (msec) | 739±90 | 729±121 | NS |
| SDNN (msec) | 44.0 (24.6) | 36.5 (14.5) | <0.01 |
| RMSSD (msec) | 17.2 (15.6) | 12.5 (6.9) | <0.01 |
| pNN10 (%) | 47.9±19.6 | 34.0±18.5 | <0.01 |
| pNN20 (%) | 16.7 (27.1) | 8.56 (11.2) | <0.01 |
| pNN50 (%) | 1.45 (5.97) | 0.32 (0.99) | <0.01 |
| LF (msec2) | 644 (807) | 412 (515) | <0.01 |
| HF (msec2) | 131 (235) | 70.6 (85.4) | <0.01 |
| LF/HF | 5.08 (6.04) | 7.05 (6.10) | NS |
| PEP (msec) | 139±10.5 | 139±11.0 | NS |
(AVNN = average of normal interbeat intervals; SDNN = standard deviation of normal interbeat intervals; RMSSD = root mean square of successive normal interbeat intervals; pNNx = percent of normal interbeat intervals >×msec; PEP = pre-ejection period; PBF = paced breathing frequency; NS = non-significant)
For all time-domain measures except AVNN (SDNN, RMSSD, pNN10, pNN20, pNN50), OSA subjects had decreased HRV when compared to controls in all awake conditions (see Table 2).
As shown in Table 1, there were minor differences between control and OSA subjects with regard to gender, age, diastolic blood pressure (DBP), triglyceride level (Tg), and hemoglobin A1C (HbA1C) level. These variables, along with AHI, were added to nine multivariate linear regression models assessing the independent contribution of these predictors to pNN10, pNN20 and pNN50 in the three awake conditions. In all awake conditions, AHI was a significant independent predictor of pNN10 and pNN20 (see Table 3).
Table 3. Multiple linear regression analyses of predictors of pNNx metrics.
For each of the nine models, data are represented as the standardized beta in univariate regression (left column) and standardized beta in multivariate regression (right column), along with the statistical significance where * = p≤0.05 and ** = p≤0.01.
| pNN10 | pNN20 | pNN50 | |||||
|---|---|---|---|---|---|---|---|
| 5-minute supine basal breathing |
Univariate Standardized Beta |
Multivariate Standardized Beta |
Univariate Standardized Beta |
Multivariate Standardized Beta |
Univariate Standardized Beta |
Multivariate Standardized Beta |
|
| Age | −0.250* | −0.154 | −0.335** | −0.245* | −0.373** | −0.299* | |
| Gender | 0.071 | −0.068 | 0.069 | −0.043 | 0.063 | −0.019 | |
| AHI | −0.526** | −0.507** | −0.452** | −0.401** | −0.349** | −0.274* | |
| Diastolic Pressure | −0.110 | −0.036 | −0.162 | −0.078 | −0.159 | −0.071 | |
| Triglyceride Level | −0.191 | −0.028 | −0.221 | −0.053 | −0.237* | −0.087 | |
| Hemoglobin A1C | −0.044 | −0.050 | −0.054 | −0.072 | −0.066 | −0.094 | |
| Multivariate Model | R2=0.312 F(6,67)=5.06** |
R2=0.288 F(6,67)=4.52** |
R2=0.245 F(6,67)=3.61** |
||||
|
5-minute supine paced breathing |
|||||||
| Age | −0.352** | −0.287* | −0.404** | −0.338** | −0.375** | −0.323** | |
| Gender | 0.115 | 0.016 | 0.100 | 0.011 | 0.019 | −0.044 | |
| AHI | −0.469** | −0.427** | −0.448** | −0.392** | −0.306** | −0.247 | |
| Diastolic Pressure | −0.140 | −0.067 | −0.179 | −0.098 | −0.129 | −0.045 | |
| Triglyceride Level | −0.133 | 0.065 | −0.153 | 0.051 | −0.182 | −0.044 | |
| Hemoglobin A1C | −0.027 | −0.048 | −0.038 | −0.062 | −0.089 | −0.112* | |
| Multivariate Model | R2=0.305 F(6,67)=4.89** |
R2=0.325 F(6,67)=5.38** |
R2=0.219 F(6,67)=3.14** |
||||
|
5-minute standing basal breathing |
|||||||
| Age | −0.157 | −0.089 | −0.142 | −0.087 | −0.139 | −0.100 | |
| Gender | 0.051 | −0.039 | 0.012 | −0.065 | −0.018 | −0.080 | |
| AHI | −0.407** | −0.411** | −0.345** | −0.354** | −0.231* | −0.227 | |
| Diastolic Pressure | −0.146 | −0.106 | −0.111 | −0.071 | −0.021 | 0.024 | |
| Triglyceride Level | −0.074 | −0.063 | −0.064 | 0.044 | −0.101 | −0.042 | |
| Hemoglobin A1C | −0.052 | −0.048 | −0.077 | −0.070 | −0.071 | −0.072 | |
| Multivariate Model | R2=0.120 F(6,67)=2.66* |
R2=0.144 F(6,67)=1.88 |
R2=0.077 F(6,67)=0.926 |
||||
A standardized beta is the number of standard deviations the outcome will change, in the appropriate direction, as a result of one standard deviation change in the predictor. The R2 for each multivariate model represents the proportion of overall variance explained by the model; this is tested on an F-distribution indicating the statistical significance of the model where * = p≤0.05 and ** = p≤0.01. (pNNx = percent of normal interbeat intervals > × msec; AHI = Apnea- Hypopnea Index)
There were no significant differences in PEP between OSA and control subjects during any of the awake conditions. The same 6 predictor variables as above (age, gender, AHI, DBP, Tg, HbA1c) were added to a multivariate linear regression model with the change in PEP between supine and standing basal breathing as the outcome variable; this overall model was not significant (R2=0.066; F(6,56)=1.735, p=0.13).
Discussion
The main finding of our study is that multiple daytime time- and frequency-domain markers of HRV are reduced in OSA compared to control subjects in multiple awake conditions. This finding suggests that the effects of asymptomatic, mild-to-moderate OSA are not limited to sleep, and that daytime autonomic modulation of heart rate is altered, even in patients without symptoms or comorbidities.
Our findings confirm a reduction in cardiac vagal modulation (reduced HF power, reduced time-domain markers) during wakefulness in OSA patients, as previously noted by prior investigators5,6,7. Furthermore, we found a reduction in LF power in OSA patients compared to controls. LF power is a mixed HRV estimate that includes both sympathetic and parasympathetic components2. This finding demonstrates how an overall increase in LF/HF ratio can occur even in the setting of a reduction in LF power, and adds to other known limitations of using frequency domain measurements to assess ‘sympatho-vagal balance’8,9. With severe OSA and its resultant marked sympathetic modulation, frequency-domain markers of HRV may be more readily evident; this pattern has been noted by prior studies10,11. However, with mild disease as in our study population, effects of vagal modulation may lead to variable LF power and LF/HF estimates.
With regard to sympathetic activity, PEP has been previously noted to correlate with cardiac sympathetic tone12,13. In our study, the presence of OSA was not associated with a significant change in PEP, perhaps due to the mild severity of disease in our study population compared to prior studies or the reduced sensitivity of this measure.
Our findings add support to the concept of using time-domain markers of HRV for assessing cardiac vagal modulation. As noted previously, pNNx thresholds with x< 50 msec may be more useful than the standard pNN50 in distinguishing patients with cardiac dysfunction from normal subjects14,15. Our study extends these findings by demonstrating that the pNN10 and pNN20, in addition to the standard PNN50 marker, were also significantly lower in the OSA group during various awake conditions. Furthermore, increasing AHI was a significant predictor of a reduction in several pNNx metrics, based on multivariate analyses controlling for common confounders such as gender, age, blood pressure, triglyceride level, and hemoglobin A1C level. These markers have previously been noted to be sensitive indicators of vagal function2.
Our study is limited by inexact age and gender matching between groups, which necessitated multivariate analyses. In spite of our adjusted analysis, the relatively young age of our cohort and the fact that control subjects in our study had a higher BMI than the national average might limit the applicability of our results to the general population. In addition, though our study demonstrates reductions in daytime cardiac vagal tone among mild, asymptomatic OSA patients and suggests that readily-computed HRV measures such as the pNNx metrics may be useful in detecting these changes, further prospective outcomes-based studies are warranted.
Acknowledgments
Funding: Dr. Malhotra is PI of National Institutes of Health 1 P01 HL 095491, K24 HL 093218, R01 HL 090897, AHA 0840159N and R01 HL 085188, and Harvard Catalyst UL1 RR 025758-01. Dr. Goldberger is PI of National Institutes of Health U01 EB 008577 and is supported by the G. Harold and Leila Y. Mathers Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Association of Sleep Disorders Centres and the Association for the Psychophysiological Study of Sleep. Diagnostic classification of sleep and arousal disorders 1979 first edition. Sleep. 1979;2:1–154. [PubMed] [Google Scholar]
- 2.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 3.Clifford GD, Tarassenko L. Quantifying errors in spectral estimates of HRV due to beat replacement and resampling. IEEE Trans Biomed Eng. 2005;52:630–638. doi: 10.1109/TBME.2005.844028. [DOI] [PubMed] [Google Scholar]
- 4.Woltjer HH, Bogaard HJ, de Vries PMJM. The technique of impedance cardiography. Eur Heart J. 1997;18:1396–1403. doi: 10.1093/oxfordjournals.eurheartj.a015464. [DOI] [PubMed] [Google Scholar]
- 5.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. mechanisms and usefulness of 24 h electrocardiography as a screening technique. Lancet. 1984;8369:126–131. doi: 10.1016/s0140-6736(84)90062-x. [DOI] [PubMed] [Google Scholar]
- 6.Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, Bonsignore G, Mancia G. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens. 1997;15:1621–1626. doi: 10.1097/00004872-199715120-00063. [DOI] [PubMed] [Google Scholar]
- 7.Aydin M, Altin R, Ozeren A, Kart L, Bilge M, Unalacak M. Cardiac autonomic activity in obstructive sleep apnea: time-dependent and spectral analysis of heart rate variability using 24-hour holter electrocardiograms. Tex Heart Inst J. 2004;31:132–136. [PMC free article] [PubMed] [Google Scholar]
- 8.Brown TE, Beightol LA, Koh L, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J App Phys. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 9.Zollei E, Csillik A, Rabi S, Gingl Z, Rudas L. Respiratory effect on the reproducibility of cardiovascular autonomic parameters. Clin Physiol Funct Imaging. 2007;27:205–210. doi: 10.1111/j.1475-097X.2007.00736.x. [DOI] [PubMed] [Google Scholar]
- 10.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation. 1998;98:1071–1077. doi: 10.1161/01.cir.98.11.1071. [DOI] [PubMed] [Google Scholar]
- 11.Wiklund U, Olofsson BO, Franklin K, Blom H, Bjerle P, Niklasson U. Autonomic cardiovascular regulation in patients with obstructive sleep apnoea: a study based on spectral analysis of heart rate variability. Clin Physiol. 2000;20:234–241. doi: 10.1046/j.1365-2281.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- 12.Berntson GG, Cacioppo JT, Quigley KS. Autonomic cardiac control. i. estimation and validation from pharmacological blockades. Psychophysiology. 1994;31:572–585. doi: 10.1111/j.1469-8986.1994.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 13.Schachinger H, Weinbacher M, Kiss A, Ritz R, Langewitz W. Cardiovascular indices of peripheral and central sympathetic activation. Psychosom Med. 2001;63:788–796. doi: 10.1097/00006842-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Mietus JE, Peng CK, Henry I, Goldsmith RL, Goldberger AL. The pNNx files: re-examining a widely used heart rate variability measure. Heart. 2002;88:378–380. doi: 10.1136/heart.88.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Yi SH, Ahn YM. The pNNx heart rate variability statistics: an application to neuroautonomic dysfunction of clozapine-treated subjects. Psychiatry Investig. 2009;6:294–298. doi: 10.4306/pi.2009.6.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]


