Abstract
ATP and adenosine are purines that play dual roles in cell metabolism and neuronal signaling. Acting at the A1 receptor (A1R) subtype, adenosine acts directly on neurons to inhibit excitability and is a powerful endogenous neuroprotective and anticonvulsant molecule. Previous research showed an increase in ATP and other cell energy parameters when an animal is administered a ketogenic diet, an established metabolic therapy to reduce epileptic seizures, but the relationship among purines, neuronal excitability and the ketogenic diet was unclear. Recent work in vivo and in vitro tested the specific hypothesis that adenosine acting at A1Rs is a key mechanism underlying the success of ketogenic diet therapy and yielded direct evidence linking A1Rs to the antiepileptic effects of a ketogenic diet. Specifically, an in vitro mimic of a ketogenic diet revealed an A1R-dependent metabolic autocrine hyperpolarization of hippocampal neurons. In parallel, applying the ketogenic diet in vivo to transgenic mouse models with spontaneous electrographic seizures revealed that intact A1Rs are necessary for the seizure-suppressing effects of the diet. This is the first direct in vivo evidence linking A1Rs to the antiepileptic effects of a ketogenic diet. Other predictions of the relationship between purines and the ketogenic diet are discussed. Taken together, recent research on the role of purines may offer new opportunities for metabolic therapy and insight into its underlying mechanisms.
Keywords: adenosine, ATP, epilepsy, hemichannel, hippocampus, long-term potentiation, metabolism, seizure, transgenic mouse
1.0 Purines: regulation of adenosine and adenosine A1 receptor activity
Purines are integral to many diverse cellular processes. The purine adenine is a nitrogenous base that likely played a role in prebiotic evolution (Oro and Kimball, 1961). Therefore, it is not surprising that it is both a building block of DNA and RNA, reflecting the metabolic activity of a cell, as well as a component of the main cell energy molecule, the adenine nucleotide adenosine triphosphate (ATP), reflecting the metabolic capacity of a cell. Thus ATP and its core molecule adenosine adapt cellular activity to energy homeostasis throughout the body. While the purines ATP and adenosine are evolutionarily ancient biochemical regulators, they also participate directly in cell signaling by binding to their own families of membrane-bound cell surface receptors.
Adenosine’s presence throughout the extracellular space exerts a tonic effect on central nervous system activity via G-protein coupled receptors, and - due to its interrelationship with adenine nucleotides, including ATP - adenosine is in a unique position to link metabolism and neuronal activity. Of the utmost relevance to epilepsy, increased activation of the adenosine A1 receptor subtype (A1R) profoundly inhibits neuronal activity. Due largely to these effects, adenosine is well known as a powerful seizure-reducing and neuroprotective molecule. The A1R exerts both presynaptic and postsynaptic inhibitory actions: presynaptically it reduces transmitter release by closing Ca2+ channels (Ribeiro et al., 1979), and postsynaptically it hyperpolarizes neurons by opening K+ channels (Haas and Greene, 1984).
Adenosine is released during cellular stress, including during hypoxia, ischemia, and seizures. Its neuroprotective role during conditions of high metabolic demand - where adenosine’s powerful inhibitory effect can reduce excitability and reduce excitotoxicity – has earned adenosine a reputation as a retaliatory metabolite (Newby et al., 1985). However, adenosine and ATP have also been shown to be regulated by a number of non-pathological stimuli, such as decreased pH and increased temperature (Gabriel et al., 1998; Masino and Dunwiddie, 1999; Dulla et al., 2005; Gourine et al., 2005; Dulla et al., 2009), and a main ongoing source of adenosine in the extracellular space is dephosphorylated ATP released from astrocytes (Pascual et al., 2005). Because of its prevalence throughout the brain, its connection to metabolism, and its dynamic and powerful actions, adenosine is a primary homeostatic bioenergetic network regulator (Boison et al., 2011).
2.0 Ketogenic diet – an established metabolic therapy
The ketogenic diet is a high-fat, low-carbohydrate diet which mimics fasting. It restricts glucose and forces metabolism of ketones (acetoacetate, acetone, β-hydroxybutyrate) to conversion into acetyl-CoA and thus generate ATP. Metabolic treatment with a ketogenic diet reduces seizures, and has been noted recently for neuroprotective benefits. Overall, ketogenic diet therapy is as effective as drugs, and is particularly effective in pediatric and medically refractory epilepsy (Hemingway et al., 2001; Freeman et al., 2007; Neal et al., 2008). There are some side effects – typically minor with good medical management – but the strict protocol can be unpalatable and difficult to maintain. The ketogenic diet was established in 1921 (Wilder, 1921), and although it has been in continuous use ever since – the key mechanism(s) are not known. An incomplete understanding of the translation between metabolic changes and reduced seizures has hampered efforts to develop better diets or pharmaceutical strategies.
3.0 Purines and the Ketogenic Diet
In 2008 we published an opinion paper hypothesizing that increased adenosine acting at A1Rs is critical to the therapeutic success of the ketogenic diet (Masino and Geiger, 2008). This concept was based initially on several parallel observations, including: (1) some of the stimuli thought to increase adenosine may be mobilized by the metabolic switch to ketolysis versus glycolysis (e.g. reduced glucose and/or pH), (2) the ketogenic diet increases ATP and mitochondrial biogenesis (thus providing additional substrate for adenosine formation), (3) both adenosine and the ketogenic diet are known to be effective anticonvulsants, even in drug-resistant epilepsy, and (4) the ketogenic diet has been found to be neuroprotective, thus matching predictions of the hypothesis that a ketogenic diet increases A1R activation.
Extended predictions of this hypothesis are that the ketogenic diet should be therapeutic in multiple clinical conditions where adenosine is known to be effective. For example, the ketogenic diet, like adenosine, should offer neuroprotection, as noted above, and should also influence sleep and reduce pain. Indeed, multiple reports in recent years note neuroprotective effects of the ketogenic diet (Noh et al., 2003; Prins et al., 2005; Van der Auwera et al., 2005; Zhao et al., 2006; Puchowicz et al., 2008; Cheng et al., 2009; Hu et al., 2009). Furthermore, fasting – which similarly forces use of ketone bodies for fuel – has paradoxically been shown to improve recovery after injury (Davis et al., 2008). Metabolic manipulations such as mitochondrial uncouplers (Sullivan et al., 2004; Pandya et al., 2007) and glycolytic inhibition (Massieu et al., 2003; Garriga-Canut et al., 2006) are thought to mimic at least some aspects of a ketogenic diet; these manipulations can offer seizure protection (Garriga-Canut et al., 2006) and have been shown to increase adenosine (Tekkök et al., 1999). More limited reports link the ketogenic diet to improved sleep in children (Panico et al., 2000; Hallböök et al., 2007), and improved circadian patterns and rest-activity cycles in chronically epileptic Kcna1-null mice (Fenoglio-Simeone et al., 2009). In addition, we showed that a ketogenic diet reduced pain and inflammation in juvenile and adult rats (Ruskin et al., 2009).
Here we describe recent work testing the hypothesis that the ketogenic diet increases activation of purine receptors, particularly A1Rs, as a key mechanism underlying its therapeutic effects. We tested this hypothesis in vitro, using normal rat hippocampal slices, and in vivo, using several transgenic mouse models with chronic seizures due to altered adenosine signaling. In both cases we found evidence for increased A1R activation (Kawamura et al., 2010; Masino et al., 2011). Also in accord with increased adenosine, we found that a ketogenic diet reduces pain and inflammation in juvenile and adult rats (Ruskin et al., 2009).
3.1 In vitro model of metabolic effects of a ketogenic diet
We mimicked a ketogenic diet in vitro by focusing on two key metabolic endpoints – (1) increased intracellular ATP and (2) low extracellular glucose. It has been reported that a ketogenic diet increases brain concentrations of ATP in rodents (DeVivo et al., 1978; Nakazawa et al., 1983; Nylen et al., 2009). Clinically, Pan et al. (1999) showed that a ketogenic diet increased the phosphocreatine/ATP ratio (a measure of positive bioenergetic status) in cerebral cortical gray matter. In parallel, blood glucose levels are known to remain low during chronic treatment with the ketogenic diet (Huttenlocher, 1976; Bough et al., 2006) and mild hypoglycemia has been reported to control seizures in both animal models (Greene et al., 2001; Mantis et al., 2004) and clinical trials (Muzykewicz et al., 2009). Thus, we focused on these metabolic endpoints (increased ATP and reduced glucose) and developed a simple in vitro model of the ketogenic diet using whole-cell patch-clamp methods in hippocampal CA3 pyramidal cells, a cell type and region known to be involved in seizures.
While recording from individual CA3 neurons we simulated the effects of a ketogenic diet by varying the amount of ATP in the intracellular solution (above and below the standard ATP (2 mM) in aCSF) and reducing extracellular glucose from 11 mM (higher than physiological levels, but standard for brain slice recordings) to 3 mM (still physiologically relevant for brain tissue). We found that with high or sufficient intracellular ATP concentrations (1–5 mM), reducing glucose caused CA3 hippocampal pyramidal neurons to hyperpolarize - measured as an outward current in voltage-clamp mode. The current induced by reduced extracellular glucose was dose-dependent upon intracellular ATP between 0.5 mM and 2 mM, suggesting an autocrine modulation of the recorded neuron. The reduced glucose-induced outward current was abolished by an A1R antagonist and not observed in A1R knock-out mice.
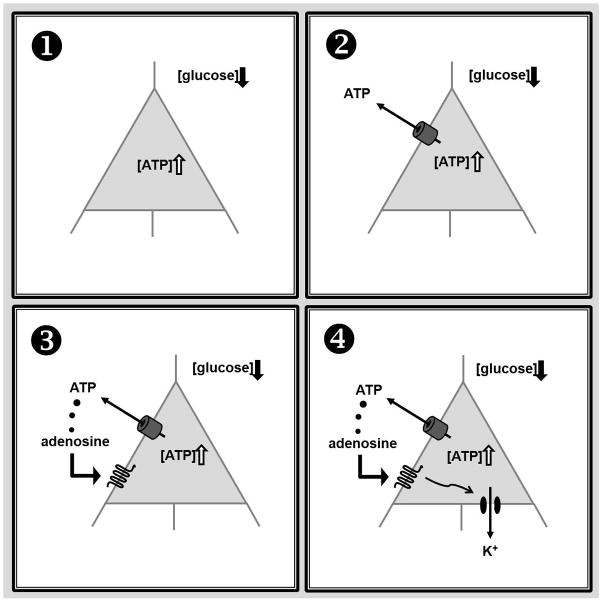
In contrast to the linear relationship between intracellular ATP and the membrane current observed for ATP concentrations between 0.5 mM and 2 mM, we found that the highest concentration of intracellular ATP - 5 mM – produced a lower amplitude of the reduced glucose-induced outward current, suggesting inhibition by high intracellular ATP. Furthermore, and in support of this observation, ATP-sensitive potassium channel blockers inhibited the outward currents. Gap-junction blockers and a peptide specific for blocking pannexin-1 channels also inhibited the outward current completely. Together, these results suggest that with high or sufficient intracellular ATP concentration and reduced extracellular glucose (a set of conditions present during ketogenic diet treatment), CA3 pyramidal neurons hyperpolarize themselves via direct ATP release through pannexin-1 channels with the subsequent activation of A1Rs and opening of ATP-sensitive potassium channels (Fig. 1 and Kawamura et al., 2010).
Figure 1.
A schematic of purinergic autocrine regulation of CA3 pyramidal cell excitability. A: With abundant intracellular ATP and moderately reduced extracellular glucose - a scenario a ketogenic diet is thought to produce: (1) ATP is released directly via pannexin hemichannels, and (2) released ATP is dephosphorylated subsequently to adenosine (2) which activates adenosine A1Rs (3). This in turn opens KATP channels which hyperpolarizes the membrane, and decreases excitability (4). In addition to these autocrine postsynaptic effects, the elevated adenosine can function in a paracrine manner to reduce neurotransmitter release from afferent axon terminals. Adapted and modified from Kawamura et al., 2010.
The in vitro experiments described above demonstrate that relevant diet-induced metabolic shifts can produce an inhibitory purinergic autocrine/paracrine regulation. A combination of the synaptic inhibition and hyperpolarizing CA3 pyramidal neurons directly should confer a strong anticonvulsant effect, and this type of inhibition could be occurring in other brain regions; more work is needed to determine if this is observed in other brain regions and neuron subtypes. While this detailed mechanism has not been proven in vivo, a similar metabolic regulation of A1R actions might be an important mechanism underlying the clinical success of a ketogenic diet (Rho, 2010).
3.2 In vivo ketogenic diet administration in animals with spontaneous seizures and altered adenosine signaling
To test directly the relationship between a ketogenic diet and A1R activation, we tested adult wild-type (WT) and three types of transgenic mice that exhibit spontaneous hippocampal seizures and reduced A1R signaling (Li et al., 2007). The mice used were engineered genetically to have a complete absence of A1Rs (A1R−/−), a 50% reduction in A1Rs (A1R+/−) (Johansson et al., 2001), or an overexpression of adenosine kinase (Adk-tg) (Li et al., 2008b); adenosine kinase is an intracellular astrocyte-based enzyme (Studer et al., 2006) that catalyzes the metabolism of adenosine to 5′-AMP, and its overexpression is expected to decrease extracellular levels of adenosine resulting in increased susceptibility to neuronal injury (Pignataro et al., 2007) and spontaneous electrographic seizures (Li et al., 2008a). In comparison to the WT control mice, the A1R−/−, A1R−/+ and Adk-tg mice all exhibit spontaneous hippocampal seizures that are accompanied occasionally by behavioral arrest or staring episodes. The characterization of these adenosine-deficient mice is consistent with previously published results that spontaneous hippocampal seizures (Li et al., 2007; Li et al., 2008b) and enhanced vulnerability of brain to injury (Kochanek et al., 2006; Pignataro et al., 2007; Li et al., 2008b) are caused by deletion of A1Rs or decreased adenosine levels due to elevated expression levels of adenosine kinase.
If anti-seizure effects of a ketogenic diet are mediated through adenosine signaling systems, and particularly A1Rs, we predicted that the ketogenic diet (i) would suppress seizures in Adk-tg mice with their inherent deficiency in adenosine (but intact A1Rs), (ii) would suppress seizures somewhat in mice with reduced levels of adenosine receptors (A1R+/− mice), and (iii) would not exhibit anti-seizure effects in mice where A1Rs are absent (A1R−/− mice). Indeed, we found that when mice were fed the ketogenic diet for 3 weeks the incidence of spontaneous seizures was reduced by about 88% in Adk-tg mice, by about 53% in A1R+/− mice, and by only 4% in A1R−/− mice (Table 1 and Masino et al., 2011).
Table 1.
Predicted and observed effects of the ketogenic diet in mice.
| Mouse Model | A1R Expression | Predicted change in seizure frequency | Observed change in seizure frequency | Glucose-induced change in seizure frequency |
|---|---|---|---|---|
| Wild-type: (C57/BL6) | unaltered | n/a (no seizures) | n/a | no change |
| Transgenic: Adk-tg | unaltered | robust Suppression | 88% decrease (p<0.001) | reversed to 85% of baseline (p<0.001) |
| Transgenic: A1R+/− | 50% normal | partial suppression | 53% decrease (p<0.001) | reversed to 89% of baseline (p<0.001) |
| Transgenic: A1R−/− | no receptors | no Suppression | 4% decrease (n.s.) | no change (n.s.) |
Legend: Predicted and observed effects of a ketogenic diet in a complement of wild type and transgenic mice. All transgenic mice demonstrated adenosine-based recurrent electrographic seizures. Based on the level of A1R expression in each mouse strain we predicted the correlated effects of a ketogenic diet in reducing seizure frequency. The A1R receptor gene was not manipulated in the Adk-tg mice; thus, we predicted seizures would be reduced by a ketogenic diet. We observed a significant (88%) reduction in seizure frequency. Functional A1Rs are expressed partially (50%) in the A1R+/− mice, and thus we predicted partial seizure reduction. We observed that seizures decreased significantly but to a lesser extent than in the Adk-tg mice (53%). Finally, A1R−/− mice have a complete loss of functional A1Rs, we predicted no effects of the ketogenic diet on spontaneous seizures, and we observed no significant change (4%) in seizure frequency. The ketogenic diet-induced decrease in seizure frequency was reversed significantly by glucose injection in Adk-tg and A1R+/− mice, and electrographic activity was unaffected in wild-type and A1R−/− mice (adapted from Masino et al., 2011)
To confirm the metabolic specificity of the diet, we administered a single injection of glucose. In every case where seizure frequency was reduced significantly by the ketogenic diet (Adk-tg and A1R+/− mice) it was increased significantly within 30–90 minutes by acute glucose, confirming the metabolic antagonist (Masino et al., specificity of the effect. Similarly, seizure suppression was reversed by an A1R 2011). Together, these data suggest strongly that A1Rs are an important mechanism underlying the anti-seizure effects of the ketogenic diet.
4.0 Testing predictions
Adenosine is a pervasive neuromodulator, and so influences a wide range of neural and behavioral processes. Thus, in addition to the direct evidence above, supporting a link between the ketogenic diet and enhanced adenosine acting at A1Rs, correlative evidence supports the idea of a ketogenic diet promoting adenosine. Thus, predictions of this hypothesis can be “tested” by looking at published literature as well as conducting additional experiments.
4.1 Ketogenic Diet, Adenosine and Neuroprotection
Adenosine acting at A1Rs is well-established as a neuroprotective mechanism, and reduces brain injury from mechanical, ischemic, and hypoglycemic insults (Evans et al., 1987; Goldberg et al., 1988; Varma et al., 2002). There is now abundant evidence from a number of laboratories that the ketogenic diet can also reduce brain injury from mechanical damage (Prins et al., 2005; Appelberg et al., 2009; Hu et al., 2009) and ischemic/hypoglycemic damage (Yamada et al., 2005; Puchowicz et al., 2008; Tai et al., 2008). Furthermore, treatment with ketones themselves is also effective against ischemic/hypoglycemic damage to neural tissue (Suzuki et al., 2001; Massieu et al., 2003; Samoilova et al., 2010). At this time, in addition to epilepsy, neuroprotection is an ideal target to test directly the relationship between ketogenic diet and adenosine.
4.2 Ketogenic Diet, Adenosine and Pain
Adenosine agonists are well-known to reduce pain by acting at both central and peripheral receptors (Yarbrough and McGuffin-Clineschmidt, 1981; Karlsten et al., 1992), primarily via A1Rs (Jarvis et al., 2000; Johansson et al., 2001; Schmidt et al., 2009), and effective against pain induced by thermal, chemical, and mechanical stimuli. Antinociceptive actions of adenosine are also present clinically (Belfrage et al., 1995) and animal research suggests that adenosine may underlie the antinociceptive effects of acupuncture (Goldman et al., 2010). Despite the common use of antiepileptic drugs to reduce pain (Vanotti et al., 2007), very little work has been done with the ketogenic diet and pain.
We tested the ketogenic diet for effects on pain using thermal stimulation (paw withdrawal from a hotplate) in rats, and found significant diet-related hypoalgesia at several temperatures (Ruskin et al., 2009). This effect was observed consistently in juvenile and adult rats (pooled data shown in Fig. 2, left). Intriguingly, hypoalgesia evolved gradually over many days after diet onset, with a similar time-course to the delayed appearance of the ketogenic diet’s anticonvulsant effect, and the thermal hypoalgesia did not depend directly on reduced glucose or increased ketones (Ruskin et al., 2010). These results provide initial evidence that the diet can reduce pain sensation, consistent with a hypothesis linking a ketogenic diet to increased actions of adenosine.
Figure 2.
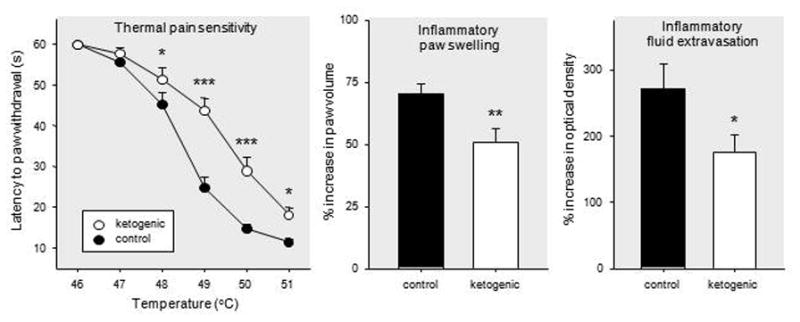
Effects of a ketogenic diet on nociception and inflammation in juvenile and adult rats. Left: Latency to nocifensive hindpaw response across a range of hotplate temperatures. Ketogenic diet feeding significantly lengthened latencies at a majority of tested temperatures, suggesting that the diet reduced sensitivity to thermal pain. Middle: Hindpaw swelling after experimentally-induced inflammation. Ketogenic diet reduced inflammatory swelling, expressed as percent increase in paw volume compared to pretreatment paw volume. Right: Movement of fluid out of blood vessels (extravasation) after experimentally-induced inflammation. Ketogenic diet feeding reduced this aspect of inflammation, expressed as percent increase in treated paw compared to contralateral untreated paw. For clarity, all panels show combined data from adolescent and adult rats; dietary effects were significant at both ages (Ruskin et al., 2009). Number of subjects = 26–28 (pain, left panel), 15–19 (swelling and extravasation, middle and right panels). *p<0.05, **p<0.01, ***p<.001, compared to control diet. Modified from Ruskin et al., 2009.
4.3 Ketogenic Diet, Adenosine, and Inflammation
Adenosine can attenuate peripheral and central inflammation by acting locally in the periphery as well as by reducing peripheral inflammation indirectly through central effects (Akimitsu et al., 1995; Poon and Sawynok, 1999; Sorkin et al., 2003; Tsutsui et al., 2004). Both A1Rs and A2ARs appear to be involved (Tsutsui et al., 2004; Sitkovsky and Ohta, 2005). In the study described above we also tested the ketogenic diet for anti-inflammatory effects by chemically-inducing paw inflammation in juvenile and adult rats after three weeks of control or ketogenic diet feeding (Ruskin et al., 2009). When we tested inflammatory parameters (two days after paw injection with complete Freund’s adjuvant) we found significantly less tissue swelling in animals fed the ketogenic diet (Fig. 2, middle). In addition, fluid movement out of the local blood vessels (fluid extravasation, a measure of vascular permeability), measured by tracking movement of the dye Evans Blue, was significantly reduced by ketogenic diet feeding (Fig. 2, right). Similar to the effects of the ketogenic diet on thermal nociception, the reduced inflammation was found consistently in juvenile and adult rats (pooled data shown in Fig. 2). Other researchers are also beginning to characterize the anti-inflammatory effects of the ketogenic diet, including in clinical settings (Tendler et al., 2007; Yang and Cheng, 2010).
4.4 Ketogenic Diet, Adenosine and Synaptic Plasticity
Adenosine interacts with synaptic plasticity, including long-term potentiation at hippocampal synapses. Typically, prior application of adenosine or receptor agonists has been shown to block induction of long term potentiation (Arai et al., 1990; Mitchell et al., 1993), including evidence in vivo (Dolphin, 1983), and there is mixed evidence that endogenous extracellular adenosine acts as a gate for synaptic plasticity (Moore et al., 2003; Kukley et al., 2005). Despite the clear effect of a ketogenic diet on seizures, and its use in children who are in a period of rapid growth and plasticity, there has been little research on the effects of a ketogenic diet on synaptic plasticity.
Using chronic recordings in awake, behaving rats, we found baseline parameters of synaptic transmission at entorhinal cortex-to-dentate gyrus synapses (input/output relationship, paired-pulse plasticity) were not changed significantly by 3 weeks of ketogenic diet feeding. After a theta-burst stimulus was applied to induce long-term potentiation, all animals showed significant synaptic potentiation in the dentate gyrus. However, a significantly reduced magnitude of potentiation was found in ketogenic diet-fed animals at both short-term (1 – 15 minutes) and long-term (30 minutes +) time points after induction (Koranda et al., 2011). The amplitude of the long-term potentiation remained diminished significantly in the ketogenic diet group out to the longest recorded time, 48 hours. Non-significant effects of the ketogenic diet on long-term potentiation in vivo in a prior electrophysiology study (Thio et al., 2010) might have been due to the use of general anesthesia, which could overwhelm the mild inhibition produced by a ketogenic diet. Overall, the effects of a ketogenic diet on long-term potentiation observed so far are minor – it was diminished, not abolished - and in the expected direction based on a relationship among a ketogenic diet, adenosine and long-term potentiation.
4.5 Emerging Evidence and Additional Mechanisms
Together, these findings suggest multiple commonalities in adenosine-based and ketogenic diet-induced effects. Relatively few studies have explored other implications of this relationship. For example, adenosine is well-known as a sleep-promoting agent, particularly in the basal forebrain (Porkka-Heiskanen, 1999; Huang et al., 2007). Thus far there is only one published report (in children with epilepsy) linking a ketogenic diet to changes in sleep behavior; however it found an overall increase in sleep quality (Hallböök et al., 2007). Notably, epilepsy is associated with sleep disruption, and chronically epileptic Kcna-1null mice with impaired circadian rhythms showed both decreased seizures and improved rest-activity cycles after treatment with a ketogenic diet (Fenoglio-Simeone et al., 2009). Decreased adenosine increases anxiety, and the regulation of the influence of adenosine by caffeine has been implicated in a number of disorders (Lara, 2010; Ribeiro and Sebastiao, 2010). There is ample evidence for adenosine/dopamine receptors forming functional heteromers (Fuxe et al., 2007), and thus additional predictions arise from this adenosine/dopamine interaction. Recently we found that a ketogenic diet delays weight loss and does not negatively affect locomotor activity in a mouse model of Huntington’s Disease, suggesting some metabolic promise in aspects of this progressive genetic disorder (Ruskin et al., 2011). Further experiments with pharmacological and transgenic methods will be needed to test strictly the involvement of adenosine in these and other phenomena and disorders (Masino et al., 2009; Gomes et al., 2011).
Certainly the metabolic effects of a ketogenic diet are many, and its benefits may rely on a key set of mechanisms for a given clinical condition or even for a particular individual. Non-adenosine actions are likely to be involved in some of its therapeutic benefits: for instance, a ketone-based metabolism modulates mitochondrial energy production (Sato et al., 1995) to produce fewer free radicals (Noh et al., 2006) which may alleviate inflammation, and polyunsaturated fatty acids (elevated by ketogenic diets) might reduce pain through activation of potassium channels in nociceptive circuitry (Lauritzen et al., 2000; Xu et al., 2008). More research is needed into these and other mechanisms.
5.0 Conclusions
Data is accumulating that purines play a key role in the anticonvulsant effects of a ketogenic diet, particularly recent in vivo work showing that the ketogenic diet can suppress seizures via A1Rs in vivo (Masino et al., 2011). In general, a cohort of recent work on purines incorporates and comports with previous evidence outlining a connection between increased mitochondria and energy molecules with a ketogenic diet, and previous research linking purines to epilepsy in general – e.g. the relationship between ATP and seizures, and anticonvulsant effects of adenosine - even in pharmacoresistant epilepsy.
An in vitro mimic of a ketogenic diet revealed new cellular mechanisms and a metabolic autocrine hyperpolarization of neurons via A1Rs linked to KATP channels (Kawamura et al., 2010). Previous work linked direct application of two ketones – β-hydroxybyturate and acetoacetate – to hyperpolarization and reduced firing rate via KATP channels (Ma et al., 2007), so metabolic or pharmaceutical enhancement of adenosine itself, A1R activation, or direct activation of neuronal post-synaptic KATP channels to hyperpolarize neuron membrane potential would be key targets. Recent work has found ketone bodies reduce the activity of vesicular glutamate transporters (Juge et al., 2010). The role of these mechanisms in the clinical effects of the ketogenic diet is unknown, but the consequences of such a global diet-induced change in metabolism are diverse and multiple targets could offer anticonvulsant potential.
Many questions remain regarding the relationship between purines and a ketogenic diet: Does a ketogenic diet in vivo open pannexins, reduce adenosine kinase, and/or increase the number of A1Rs? Are there changes in the metabolic profile of all purines, and are there differential effects across brain regions and cell types? Is there a key combination of diet-mobilized mechanisms and effects for optimal clinical benefits? Based on initial evidence, the adenosine-enhancing effects have the potential to be strongest exactly where more adenosine is needed – regions of gliosis and high adenosine kinase activity. More mechanistic details are needed to elulcidate the effects on purines in vivo.
Understanding the metabolic control of central nervous system adenosine offers opens many powerful clinical opportunities beyond epilepsy. If a key role for purines in ketogenic diet therapy - and specifically adenosine acting at the A1R - is borne out through additional research, this lends new insight into metabolic therapy and new targets for drug development. In addition, diet-based approaches are cost-effective across cultures (Kossoff et al., 2009). Ultimately, and for the first time, the relationship between a ketogenic diet and adenosine may provide insight into a global regulation of network activity – increased adenosine can enhance the signal-to-noise ratio, and prevent inappropriate hyperexcitability, regardless of the underlying cause(s). This emerging work that the ketogenic diet enhances the actions of a homeostatic bioenergetic network regulator such as adenosine (Boison et al., 2011), is an exciting development in ketogenic diet research and may yield insight into other neurological disorders.
Acknowledgments
We acknowledge the support of National Institutes of Health NINDS R01NS065957, R15NS066392, R15065446 and 2P20RR017699 from the NCRR (a component of the NIH), CHDI, and National Science Foundation IOS-0843585.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akimitsu T, White JA, Carden DL, Gute DC, Korthius RJ. Fructose-1,6-diphosphate or adenosine attenuate leukocyte adherence in postischemic skeletal muscle. Am J Physiol Heart Circ Physiol. 1995;269:H1743–H1751. doi: 10.1152/ajpheart.1995.269.5.H1743. [DOI] [PubMed] [Google Scholar]
- Appelberg KS, Hovda DA, Prins ML. The effects of a ketogenic diet on behavioral outcome after controlled cortical impact injury in the juvenile and adult rat. J Neurotrauma. 2009;26:497–506. doi: 10.1089/neu.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai A, Kessler M, Lynch GS. The effects of adenosine on the development of long-term potentiation. Neurosci Lett. 1990;119:41–44. doi: 10.1016/0304-3940(90)90750-4. [DOI] [PubMed] [Google Scholar]
- Belfrage M, Sollevi A, Segerdahl M, Sjölund KF, Hansson P. Systemic adenosine infusion alleviates spontaneous and stimulus evoked pain in patients with peripheral neuropathic pain. Anesth Analg. 1995;81:713–717. doi: 10.1097/00000539-199510000-00010. [DOI] [PubMed] [Google Scholar]
- Boison D, Masino SA, Geiger JD. Homeostatic bioenergetic network regulation: a novel concept to avoid pharmacoresistance in epilepsy. Expert Opin Drug Discov. 2011;6:713–724. doi: 10.1517/17460441.2011.575777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine RJ. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Cheng B, Yang X, An L, Gao B, Liu X, Liu S. Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease. Brain Res. 2009;1286:25–31. doi: 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J Neurosci Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. The adenosine agonist 2-chloroadenosine inhibits the induction of long-term potentiation of the perforant path. Neurosci Lett. 1983;39:83–89. doi: 10.1016/0304-3940(83)90169-6. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Frenguelli BG, Staley KJ, Masino SA. Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. J Neurophysiol. 2009;102:1984–1993. doi: 10.1152/jn.90695.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MC, Swan JH, Meldrum BS. An adenosine analogue, 2-chloroadenosine, protects against long term development of ischaemic cell loss in the rat hippocampus. Neurosci Lett. 1987;83:287–292. doi: 10.1016/0304-3940(87)90101-7. [DOI] [PubMed] [Google Scholar]
- Fenoglio-Simeone KA, Wilke JC, Milligan HL, Allen CN, Rho JM, Maganti RK. Ketogenic diet treatment abolishes seizure periodicity and improves diurnal rhythmicity in epileptic Kcna1-null mice. Epilepsia. 2009;50:2027–2034. doi: 10.1111/j.1528-1167.2009.02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JM, Kossoff EH, Hartman AL. The ketogenic diet: one decade later. Pediatrics. 2007;119:535–543. doi: 10.1542/peds.2006-2447. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Genedani S, Franco F, Agnati LF. Adenosine receptor–dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Klussmann FW, Igelmund P. Rapid temperature changes induce adenosine-mediated depression of synaptic transmission in hippocampal slices from rats (non-hibernators) but not in slices from golden hamsters (hibernators) Neuroscience. 1998;86:67–77. doi: 10.1016/s0306-4522(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Goldberg MP, Monyer H, Weiss JH, Choi DW. Adenosine reduces cortical neuronal injury induced by oxygen or glucose deprivation in vitro. Neurosci Lett. 1988;89:323–327. doi: 10.1016/0304-3940(88)90547-2. [DOI] [PubMed] [Google Scholar]
- Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2011;1808:1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–11. doi: 10.1038/nature03690. [DOI] [PubMed] [Google Scholar]
- Greene AE, Todorova MT, McGowan R, Seyfried TN. Caloric restriction inhibits seizure susceptibility in epileptic EL mice by reducing blood glucose. Epilepsia. 2001;42:1371–1378. doi: 10.1046/j.1528-1157.2001.17601.x. [DOI] [PubMed] [Google Scholar]
- Haas HL, Greene RW. Adenosine enhances afterhyperpolarization and accommodation in hippocampal pyramidal cells. Pflugers Archiv. 1984;402:244–247. doi: 10.1007/BF00585506. [DOI] [PubMed] [Google Scholar]
- Hallböök T, Lundgren J, Rosén I. Ketogenic diet improves sleep quality in children with therapy-resistant epilepsy. Epilepsia. 2007;48:59–65. doi: 10.1111/j.1528-1167.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- Hemingway C, Freeman JM, Pillas DJ, Pyzik PL. The ketogenic diet: a 3- to 6-year follow-up of 150 children enrolled prospectively. Pediatrics. 2001;108:898–905. doi: 10.1542/peds.108.4.898. [DOI] [PubMed] [Google Scholar]
- Hu ZG, Wang HD, Jin W, Yin HX. Ketogenic diet reduces cytochrome c release and cellular apoptosis following traumatic brain injury in juvenile rats. Ann Clin Lab Sci. 2009;39:76–83. [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Curr Opin Pharmacol. 2007;7:33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Ketonemia and seizures: metabolic and anticonvulsant effects of two ketogenic diets in childhood epilepsy. Pediatr Res. 1976;10:536–540. doi: 10.1203/00006450-197605000-00006. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Yu H, Kohlhass K, Alexander K, Lee CH, Jiang M, Bhagwat SS, Williams M, Kowaluk EA. ABT-702 (4-Amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2,3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther. 2000;295:1156–1164. [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela LM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hårdemark A, Betscholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsten R, Gordh T, Post C. Local antinociceptive and hyperalgesic effects in the formalin test after peripheral administration of adenosine analogues in mice. Pharmacol Toxicol. 1992;70:434–438. doi: 10.1111/j.1600-0773.1992.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Kawamura M, Jr, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors and KATP channels. J Neurosci. 2010;30:3886–3895. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RSB, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- Koranda JL, Ruskin DN, Masino SA, Blaise JH. A ketogenic diet reduces long-term potentiation in the dentate gyrus of freely-behaving rats. J Neurophysiol. 2011 May 25; doi: 10.1152/jn.00001.2011. (online before print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossoff EH, Zupec-Kania BA, Rho JM. Ketogenic diets: an update for child neurologists. J Child Neurol. 2009;24:979–988. doi: 10.1177/0883073809337162. [DOI] [PubMed] [Google Scholar]
- Kukley M, Schwan M, Fredholm BB, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara DR. Caffeine, mental health, and psychiatric disorders. J Alzheimers Dis. 2010;20 (Suppl 1):S239–S248. doi: 10.3233/JAD-2010-1378. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J. 2000;19:1784–1793. doi: 10.1093/emboj/19.8.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biol. 2008a;4:91–99. doi: 10.1017/S1740925X09990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biol. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Invest. 2008b;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis JG, Centeno NA, Todorova MT, McGowan R, Seyfried TN. Management of multifactorial idiopathic epilepsy in EL mice with caloric restriction and the ketogenic diet: role of glucose and ketone bodies. Nutr Metab. 2004;1:11. doi: 10.1186/1743-7075-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Dunwiddie TV. Temperature-dependent modulation of excitatory transmission in hippocampal slices is mediated by extracellular adenosine. J Neurosci. 1999;19:1932–1939. doi: 10.1523/JNEUROSCI.19-06-01932.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Geiger JD. Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci. 2008;31:273–278. doi: 10.1016/j.tins.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Kawamura M, Jr, Wasser CD, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7:257–268. doi: 10.2174/157015909789152164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massieu L, Haces ML, Montiel T, Hernández-Fonseca K. Acetoacetate protects hippocampal neurons against glutamate-mediated neuronal damage during glycolysis inhibition. Neuroscience. 2003;120:365–378. doi: 10.1016/s0306-4522(03)00266-5. [DOI] [PubMed] [Google Scholar]
- Mitchell JB, Miller K, Dunwiddie TV. Adenosine-induced suppression of synaptic responses and the initiation and expression of long-term potentiation in the CA1 region of the hippocampus. Hippocampus. 1993;3:77–86. doi: 10.1002/hipo.450030108. [DOI] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc Natl Acad Sci USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykewicz DA, Lyczkowski DA, Memon N, Conant KD, Pfeifer HH, Thiele EA. Efficacy, safety, and tolerability of the low glycemic index treatment in pediatric epilepsy. Epilepsia. 2009;50:1118–1126. doi: 10.1111/j.1528-1167.2008.01959.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Kodama S, Matsuo T. Effects of ketogenic diet on electroconvulsive threshold and brain contents of adenosine nucleotides. Brain Dev. 1983;5:375–380. doi: 10.1016/s0387-7604(83)80042-4. [DOI] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- Newby AC, Worku Y, Holmquist CA. Adenosine formation: Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol. 1985;6:273–284. [PubMed] [Google Scholar]
- Noh HS, Hah YS, Nilufar R, Han J, Bong JH, Kang SS, Cho GJ, Choi WS. Acetoacetate protects neuronal cells from oxidative glutamate toxicity. J Neurosci Res. 2006;83:702–709. doi: 10.1002/jnr.20736. [DOI] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Nylen K, Velazquez JLP, Sayed V, Gibson KM, Burnham WM, Snead OC., III The effects of a ketogenic diet on ATP concentrations and the number of hippocampal mitochondria in Aldh5a1−/− mice. Biochim Biophys Acta. 2009;1790:208–212. doi: 10.1016/j.bbagen.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oro J, Kimball AP. Synthesis of purines under possible primitive earth conditions. I Adenine from hydrogen cyanide. Arch Biochem Biophys. 1961;94:217–227. doi: 10.1016/0003-9861(61)90033-9. [DOI] [PubMed] [Google Scholar]
- Pan JW, Bebin EM, Chu WJ, Hetherington HP. Ketosis and epilepsy: 31P spectroscopic imaging at 4.1 T. Epilepsia. 1999;40:703–707. doi: 10.1111/j.1528-1157.1999.tb00766.x. [DOI] [PubMed] [Google Scholar]
- Pandya JD, Pauly JR, Nukala VN, Sebastian AH, Day KM, Korde AS, Maragos WF, Hall ED, Sullivan PG. Post-injury administration of mitochondrial uncouplers increases tissue sparing and improves behavioral outcome following traumatic brain injury in rodents. J Neurotrauma. 2007;24:798–811. doi: 10.1089/neu.2006.3673. [DOI] [PubMed] [Google Scholar]
- Panico LR, Ríos VG, Demartini MG, Carniello MA. The electroencephalographic evolution of a group of patients on a ketonic diet. Rev Neurol. 2000;30:8–15. [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27:1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- Poon A, Sawynok J. Antinociceptive and anti-inflammatory properties of an adenosine kinase inhibitor and an adenosine deaminase inhibitor. Eur J Pharmacol. 1999;1999:123–138. doi: 10.1016/s0014-2999(99)00626-3. [DOI] [PubMed] [Google Scholar]
- Porkka-Heiskanen T. Adenosine in sleep and wakefulness. Ann Med. 1999;31:125–129. doi: 10.3109/07853899908998788. [DOI] [PubMed] [Google Scholar]
- Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413–420. doi: 10.1002/jnr.20633. [DOI] [PubMed] [Google Scholar]
- Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JM. How does altered metabolism lead to seizure control? Partially filling the knowledge gap. Epilepsy Curr. 2010;10:159–161. doi: 10.1111/j.1535-7511.2010.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro JA, Sá-Almeida AM, Namorado JM. Adenosine and adenosine triphosphate decrease 45Ca uptake by synaptosomes stimulated by potassium. Biochem Pharmacol. 1979;28:1297–1300. doi: 10.1016/0006-2952(79)90428-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro JA, Sebastião AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20 (Suppl 1):S3–S15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Kawamura M, Jr, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Ross JL, Kawamura M, Jr, Ruiz TL, Geiger JD, Masino SA. A ketogenic diet delays weight loss and does not impair working memory or motor function in the R6/2 1J mouse model of Huntington’s disease. Physiol Behav. 2011;103:501–507. doi: 10.1016/j.physbeh.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin DN, Suter TACS, Masino SA. Ketogenic diet-induced thermal hypoalgesia follows a delayed time course in comparison to ketosis in juvenile rats. Soc Neurosci Abstr. 2010:375.4. [Google Scholar]
- Samoilova M, Weisspapir M, Abdelmalik P, Velumian AA, Carlen PL. Chronic in vitro ketosis is neuroprotective but not anticonvulsant. J Neurochem. 2010;113:826–835. doi: 10.1111/j.1471-4159.2010.06645.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies, and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- Schmidt AP, Böhmer AE, Antunes C, Schallenberger C, Porciúncula LO, Elisabetsky E, Lara DR, Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br J Pharmacol. 2009;156:163–172. doi: 10.1111/j.1476-5381.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Moore J, Boyle DL, Yang L, Firestein GS. Regulation of peripheral inflammation by spinal adenosine: role of somatic afferent fibers. Exp Neurol. 2003;184:162–168. doi: 10.1016/s0014-4886(03)00102-x. [DOI] [PubMed] [Google Scholar]
- Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Rippy NA, Dorenbos K, Concepcion RC, Agarwal AK, Rho JM. The ketogenic diet increases mitochondrial uncoupling protein levels and activity. Ann Neurol. 2004;55:576–580. doi: 10.1002/ana.20062. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Suzuki M, Sato K, Dohi S, Sato T, Matsuura A, Hiraide A. Effect of β-hydroxybutyrate, a cerebral function improving agent, on cerebral hypoxia, anoxia and ischemia in mice and rats. Jpn J Pharmacol. 2001;87:143–150. doi: 10.1254/jjp.87.143. [DOI] [PubMed] [Google Scholar]
- Tai KK, Nguyen N, Pham L, Truong DD. Ketogenic diet prevents cardiac arrest-induced cerebral ischemic neurodegeneration. J Neural Transm. 2008;115:1011–1017. doi: 10.1007/s00702-008-0050-7. [DOI] [PubMed] [Google Scholar]
- Tekkök S, Medina I, Krnjevid K. Intraneuronal [Ca2+] changes induced by 2-deoxy-D-glucose in rat hippocampal slices. J Neurophysiol. 1999;81:174–183. doi: 10.1152/jn.1999.81.1.174. [DOI] [PubMed] [Google Scholar]
- Tendler D, Lin S, Yancy WS, Jr, Mavropoulos J, Sylvestre P, Rockey DC, Westman EC. The effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study. Dig Dis Sci. 2007;52:589–593. doi: 10.1007/s10620-006-9433-5. [DOI] [PubMed] [Google Scholar]
- Thio LL, Rensing N, Maloney S, Wozniak DF, Xiong C, Yamada KA. A ketogenic diet does not impair rat behavior or long-term potentiation. Epilepsia. 2010;51:1619–1623. doi: 10.1111/j.1528-1167.2009.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Wera S, Van Leuven F, Henderson ST. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr Metab. 2005;2:28. doi: 10.1186/1743-7075-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanotti A, Osio M, Mailland E, Nascimbene C, Capiluppi E, Mariani C. Overview of pathophysiology and newer approaches to treatment of peripheral neuropathies. CNS Drugs. 2007;21 (Suppl 1):3–12. doi: 10.2165/00023210-200721001-00002. [DOI] [PubMed] [Google Scholar]
- Varma MR, Dixon CE, Jackson EK, Peters GW, Melick JA, Griffith RP, Vagni VA, Clark RSB, Jenkins LW, Kochanek PM. Administration of adenosine receptor agonists or antagonists after controlled cortical impact in mice: effects on function and histopathology. Brain Res. 2002;951:191–201. doi: 10.1016/s0006-8993(02)03161-x. [DOI] [PubMed] [Google Scholar]
- Wilder RM. The effects of ketonemia on the course of epilepsy. Mayo Clin Proc. 1921;2:307–308. [Google Scholar]
- Xu X-p, Erichsen D, Börjesson SI, Dahlin M, Åmark P, Elinder F. Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: A putative mechanism of antiseizure action. Epilepsy Res. 2008;80:57–66. doi: 10.1016/j.eplepsyres.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Rensing N, Thio LL. Ketogenic diet reduces hypoglycemia-induced neuronal death in young rats. Neurosci Lett. 2005;385:210–214. doi: 10.1016/j.neulet.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Yang X, Cheng B. Neuroprotective and anti-inflammatory activities of ketogenic diet on MPTP-induced neurotoxicity. J Mol Neurosci. 2010;42:145–153. doi: 10.1007/s12031-010-9336-y. [DOI] [PubMed] [Google Scholar]
- Yarbrough GG, McGuffin-Clineschmidt JC. In vivo behavioral assessment of central nervous system purinergic receptors. Eur J Pharmacol. 1981;76:137–144. doi: 10.1016/0014-2999(81)90495-7. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]