Abstract
Exposure to repeated stress can lead to diverse and widespread behavioral consequences, including reduction in food and water intake and subsequent diminution in weight gain. Many reports have suggested that repeated stress substantially alters the neurochemistry, morphology and physiology of neurons within the bed nucleus of the stria terminalis (BNST). Here we investigate the role of the BNST in mediating the reduced weight gain observed during repeated stress. Rats exposed to a one-week variate stress paradigm exhibited a reduction in weight gain over the course of the 7 day paradigm. Excitotoxic lesions to a subregion of the anterolateral BNST containing the oval nucleus had no effects early in the 7 day paradigm, but significantly attenuated the effects of repeated stress on weight gain by the last day of stress. These data suggest that at least two mechanisms mediate the effects of stress on body weight gain, and that when stressor exposure becomes repeated, the BNST is recruited, worsening the symptoms of stressor exposure.
Keywords: extended amygdala, anorexia, anxiety, corticotropin-releasing hormone, oval nucleus, rat
Introduction
Repeated exposure to daily stressors produces a variety of behavioral changes that include anorexia and diminution in weight gain in rats (Krahn et al., 1990; Marti et al., 1994). Attenuated weight gain in rats is a commonly observed effect of stressor exposure that suggests that the central processing of stressful stimuli may influence feeding behavior and/or metabolic processes; hence, weight changes represent a useful mechanism to assess stress effects and may also reflect processes by which stressor exposure may decrease and/or increase feeding behavior and/or metabolism in other species, including humans. Many behavioral effects of repeated stress have been linked to the activity of corticotropin-releasing hormone (CRH), which is found in high concentrations in the paraventricular nucleus of the hypothalamus (PVN), central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST). CRH has been well-studied for its role in initiating peripheral stress-responses as well as coordinating behavioral responses to stressor exposure. Repeated central CRH injections mimic many stress-induced behavioral changes and can produce anorexia and weight loss (Buwalda et al., 1998; Hotta et al., 1991); by contrast, blockade of CRH receptors during repeated stress or CRH can attenuate these responses (Pelleymounter et al., 2000; Smagin et al., 1999). Hence, it is likely that the anorexigenic effects of repeated stress are mediated by CRH-expressing neurons in the PVN, CeA, and/or BNST.
BNST neurons respond to a variety of stressful stimuli, and have been implicated in mediating anxiety-like behavior (see (Walker et al., 2003; Walker et al., 2009) for review) and anorexia (Ciccocioppo et al., 2003). In particular, BNST CRH activity has been shown to be critical for the development of many anxiety-like behavioral phenotypes (for review see (Koob and Heinrichs, 1999; Owens and Nemeroff, 1993)), and activation of BNST CRH receptors produces an anxiogenic (Lee and Davis, 1997) and anorexic state (Ciccocioppo et al., 2003). The same regions of the BNST have been argued to mediate peripheral stress responding via projections to the PVN (Choi et al., 2006; Choi et al., 2007; Herman et al., 1994). Moreover, increases in BNST neuroplasticity have been shown to be altered following exposure to repeated stress paradigms (Pego et al., 2008; Vyas et al., 2003) or repeated administration of drugs of abuse (Dumont et al., 2008; Macey et al., 2003). While changes in BNST neuroplasticity have been argued to mediate stress-induced anxiety-like behavioral states, it is still not clear whether BNST activation is necessary for the observation of stress-induced anorexia.
Here we show that a 7-day repeated variate stress paradigm attenuates weight gain throughout the week of stress. Excitotoxic lesions of the BNST during repeated stress attenuated the effects of epeated stress on weight gain only towards the end of the 7 day paradigm. These data suggest that when stressor exposure becomes repeated, BNST activity is recruited, enhancing the effects of repeated stress on weight.
Method
Subjects
Male Sprague-Dawley rats (200–275 g) from Charles River Laboratories (Wilmington, MA) were single-housed with food and water available ad libitum, and kept on a 12 hr light/dark cycle (lights on at 7 AM). Rats were allowed one week of acclimation upon arrival to the facility prior to experimentation. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Vermont.
Stress procedure
Stressed rats were exposed to a 7-day repeated variate stress paradigm that has been previously described (Hammack et al., 2009). Briefly, rats were randomly assigned to either stress or control groups. Control rats were weighed daily, but received no other treatment during the 7-day stress period. Stressed rats were weighed daily prior to the administration of one of the following stressors:
Oscillation
Rats were placed inside a plastic chamber 28 × 17 × 13 cm (L × W × H), that was secured to a clinical rotator (Fisher Scientific, Morris Plains, NJ), and oscillated at low to medium speed for 30-min.
Forced Swim
Rats were placed in a cylindrical container 29 × 37 cm (D × H) filled with room temperature water to a depth that prevented the rat tail from touching the bottom. After 5-min of monitored swimming, rats were placed in a holding chamber for 30 min prior to being returned to their home cage.
Footshock
Rats were placed inside a Plexiglas conditioning chamber (Med Associates, St. Albans, VT) 30 × 25 ×35 cm (L × W × H). After a 5-min acclimation period, two 1.0 mA 5-sec scrambled footshocks were delivered through the grid floor with a 1-min inter-trial interval.
Restraint
Rats were placed in a cylindrical restraining device 9 × 15 cm (D × H) for 60-min.
Pedestal
Rats were placed on an elevated platform 20 × 20 cm (L × W) that was 60 cm from the floor. Rats remained on the platform for 30-min before being returned their home cages.
Stressed rats received one stressor a day in the following order: oscillation, forced swim, footshock, restraint, pedestal, forced swim, footshock. Rats were returned to their home cages immediately after each stressor.
Drugs
N-methyl-D-aspartate (NMDA) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in saline 0.9%.
Statistics
A 2 × 2 × 8 (stress treatment × lesion treatment × time) repeated measures Analysis of Variance (ANOVA) was used to analyze weight data over the entire 7 day stress period using IBM SPSS Statistics Version 19 (International Business Machines, Armonk, NY). Because data did not meet assumptions of sphericity, Greenhouse-Geisser corrections were used. In order to assess effects in the early and late phase of the stress paradigm, one-way ANOVAs and post-hoc tests were conducted on the percent weight change from Days 1 to 4, and also on weight change from Days 4 to 8. Because we predicted that the Stress-Sham group should differ from the other three groups, we followed the one-way ANOVA analyses with a Dunnett’s Multiple Comparison Test comparing all groups against the Stress-Sham rats.
Procedure
Rats were anesthetized with isoflurane vapor (1.5 – 3.5%), and secured in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). After incision, the skull was exposed and two drill holes were made at the following coordinates: AP = −.26, ML = +/− 3.82. For each rat, a 10 µl syringe attached to an infusion pump was stereotaxically placed into the BNST (from bregma in mm, AP = −0.26, DV = −6.8, and ML ± 3.82 at a 20 degree angle). NMDA was infused (4 µg in 200 nl) into each BNST over 4 min followed by a delay period of 4 minutes before retracting the syringe to prevent spreading. For sham-operated control animals, an equivolume of saline was infused. After infusion, the wound was closed with wound clips. Once awake, rats were returned to their home cages for 7 day post-surgery recovery, during which all rats were monitored and weighed daily.
After surgery recovery, rats were administered the 7 - day repeated variate stress paradigm described above. Twenty-four h after last stressor exposure (day 8), rats were weighed and perfused transcardially with saline followed by 4% paraformaldehyde.
Neu-N immunohistochemistry
Excitotoxic lesions were verified using Neu-N immunohistochemistry (see Figure 1). After perfusion, brains were removed, post-fixed for 48 hours at 4°C in 4% paraformaldehyde, and equilibrated in 30% sucrose and rapidly frozen in a dry-ice slurry for cryosectioning (30 µm). Sections were mounted onto gelatin-coated slides and washed in 0.1 M sodium phosphate buffer. Sections were then permeabilized and blocked in 0.3% triton and 1% bovine serum albumin in phosphate buffered saline for 15 minutes each, and incubated with mouse monoclonal anti-NeuN antibody overnight at 4°C. After subsequent washes in buffer, sections were incubated in a Cy3-conjugated donkey anti-mouse secondary antibody for 2 hours at room temperature. Lesions were then verified using a fluorescence microscope.
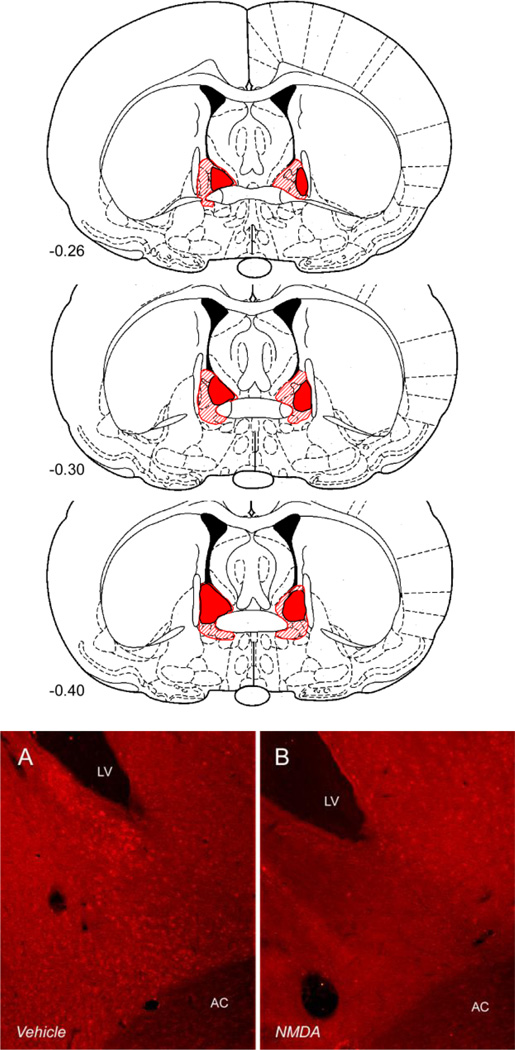
Figure 1.
Above: extent of the smallest (solid) and largest (hatched) anterolataral NMDA excitotoxic BNST lesions accepted for analysis. Below: a representative excitotoxic BNST lesion visualized using anti-NeuN immunohistochemistry. BNST tissue from a vehicle treated (sham) rat is shown on the left; the same region is shown from an NMDA (lesion) rat on the right.
Results
The extent of the largest and smallest NMDA BNST lesions acceptable for analysis is shown in Figure 1 and in all cases, the oval nucleus was targeted; only small areas of the ventral BNST were affected. Notably, the extent of these lesions was similar to those we have previously observed one-week after BNST NMDA infusion using the same procedures (Hammack et al., 2004); hence, BNST lesions created using these procedures do not increase in size between one and two weeks following NMDA infusion, and such an increase in the extent of BNST lesions cannot explain the time-dependency of the effects reported below. As shown in Figure 2, weight increased over the course of the experiment, except in stressed rats, and BNST lesions reduced stress-induced attenuations in weight gain. Weight data were analyzed as a percent change from each rat’s weight immediately prior to the first stressor exposure using a 2 × 2 × 8 (stress treatment × lesion treatment × time) repeated measures ANOVA. As noted above, because data did not meet assumptions of sphericity, Greenhouse-Geisser corrections were used. There was a main effect of day, such that overall weight reliably increased over the 7 day span F(2.873/66.070) = 101.765, p < 0.001, and an interaction between day and stress treatment F(2.873/66.070) = 28.599, p < 0.001. There was also an interaction between day and BNST lesion treatment F(2.873/66.070) = 3.856, p = 0.014. We did not observe a three-way interaction between day, stress treatment and lesion treatment F(2.873/66.070) = 0.905, p = 0.440.
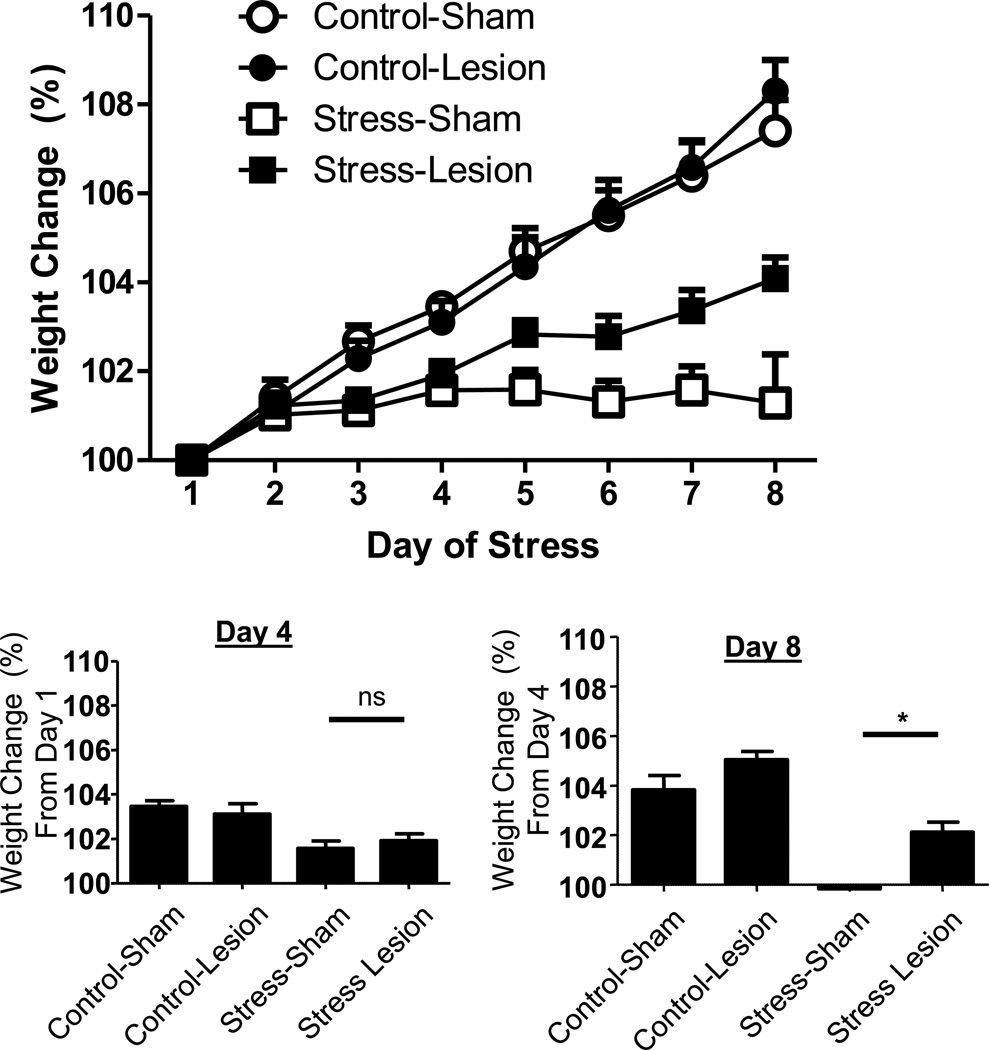
Figure 2.
Above: weight change in rats that received excitotoxic lesions to the BNST 1-week before exposure to the 7-day chronic variate stress procedure. Repeated variate stress significantly reduced weight over the course of the 7 day paradigm. BNST lesions attenuated this effect, but only toward the end of the 7 day period. Below: BNST lesions had no effect on stress-induced weight change from Day 1 to Day 4, but significantly attenuated the effects of stress on weight from Day 4 to Day 8. Ns = non-significant, * = p < 0.05.
The analysis described above suggested that both stress and BNST lesion effects on weight gain depended on the duration of the chronic stress paradigm. In order to determine whether the effects of BNST lesion were more pronounced towards the end of the 7 day paradigm, we conducted one-way ANOVA on the percent weight change between Day 1 and Day 4, and also between Day 4 at Day 8. As noted above, because we predicted that the Stress-Sham group should differ from the other three groups, we followed these analyses with a Dunnett’s Multiple Comparison Test comparing all groups against the Stress-Sham rats. From Day 1 to Day 4, a one-way ANOVA revealed a significant difference between the groups F(3,26) = 6.468, p < 0.01; post hoc analyses revealed that the Stress-Sham group differed from both Control groups, but not the Stress-Lesion group (Figure 2, bottom left). From Day 4 to Day 8, a one-way ANOVA revealed a significant difference between the groups F(3,26) = 17.40, p < 0.001; post hoc analyses revealed that the Stress-Sham group differed from all of the other groups (Figure 2, bottom right). Hence, BNST lesions attenuated the stress-induced reductions on weight during the second half, but not first half of the 7 day stress paradigm.
Together, these data suggest that weight gain was attenuated by repeated variate stress treatment. Excitotoxic lesions of the BNST had no effect on weight during the early phase of repeated variate stress; however, during the late phase of repeated variate stress, BNST lesion significantly attenuated the effects of repeated variate stress on weight gain. These data imply that as stress becomes repeated, BNST activity is recruited, and mediates the effects of repeated stress on weight gain.
Discussion
Here we showed that exposure to one week of repeated variate stress attenuated weight gain, and excitotoxic BNST lesions had no effect on stress-attenuation of weight gain during the early phase of repeated variate stress, but significantly attenuated the late phase effects of repeated variate stress on weight gain. These results suggest that stressor exposure activates at least two mechanisms that lead to reduced body weight gain, a BNST-independent mechanism that is activated early and a later BNST-dependent mechanism that is recruited with increasing stressor frequency. These data are consistent with several prior reports suggesting that repeated stress enhances BNST neuroplasticity and function (see below), and is also consistent with prior studies suggesting a role for the BNST in mediating stress-induced anorexia (Ciccocioppo et al., 2003).
Choi et al. (2007, 2008a, 2008b) have shown that lesions to the posteromedial or anteroventral BNST differentially regulate stress responding such that hypothalamic-pituitary-adrenal axis activity can be increased or decreased, respectively. Moreover, the role of the anteroventral BNST in mediating the stress response varies depending on whether the stressor exposure is acute or repeated (Choi et al., 2007; Choi et al., 2008a; Choi et al., 2008b). Notably, lesions of neither of these two BNST subregions affected the reduction in body weight gain observed during repeated variate stress (Choi et al., 2007; Choi et al., 2008a; Choi et al., 2008b). Unlike the posteromedial or anteroventral BNST, we show that lesions to the anterolateral BNST, a region dorsal to the anterior commissure encompassing the oval nucleus, do attenuate the stress-induced reductions in weight gain. Interestingly, the BNST oval nucleus sends robust projections to the BNST fusiform nucleus in the anteroventral BNST as well as the BNST rhomboid nucleus (Dong et al., 2001) which project heavily to hypothalamic regions controlling food intake. However, BNST fusiform lesions do not reduce repeated stress-induced anorexia (Choi et al., 2008a). Dorsal anterolateral BNST lesions do attenuate repeated stressinduced anorexia (present study) suggesting that the effects of BNST oval nucleus activation may be mediated by projections to other areas, such as the BNST rhomboid nucleus.
Consistent with a role for the anterolateral BNST in mediating the consequences of repeated stress, several repeated stress paradigms have been shown to induce plasticity in this BNST subregion. For example, BNST CRH expression is increased following prolonged social stress, repeated exposure to mild stressors, and repeated corticosterone injection (Makino et al., 1994; Schulkin et al., 1998; Stout et al., 2000; Watts and Sanchez-Watts, 1995). Increased expression of norepinheprhine transporters have also been observed in the BNST in rhesus monkeys after repeated administration of drugs of abuse (Macey et al., 2003). Moreover, similar treatments cause morphological changes in BNST neurons, such that that exposure to a repeated unpredictable stress paradigm increased total BNST volume and dendritic length of BNST neurons (Pego et al., 2008), repeated immobilization increased the number of branch points observed in the dendritic arborization of BNST neurons (Vyas et al., 2003), and we have shown that the dendritic length of BNST oval nucleus neurons is enhanced after the same 7-day repeated stress paradigm used in the present study (Hammack and May, unpublished observation). In addition to morphological changes, repeated stressor exposure can enhance physiological correlates of BNST neuroplasticity, such as the enhancement in excitatory postsynaptic currents observed in ventral-tegmental area (VTA)-projecting BNST neurons after repeated infusions of drugs of abuse (Dumont et al., 2008). Hence, several reports suggest that BNST activity may be enhanced following several different stress paradigms, and one salient characteristic common to all of these paradigms is that stressor presentation was repeated or chronic, similar to the current study. The results of the current study corroborate the anatomical evidence above, and suggest at a behavioral level that BNST function is enhanced after repeated, but not acute stressor exposure.
Ciccocioppo et al., (2003) reported that the activation of BNST CRH receptors produces anorexia in rats, and that activation of the BNST nociceptin/orphanin FQ system completely reverses the anorexia induced by ventricular CRH infusion (Ciccocioppo et al., 2003). In addition to suggesting that the BNST is the site of action for CRH-induced anorexia, these results suggest that the activation of BNST neuropeptides tightly modulate feeding behavior and anorexia. We have found that repeated variate stress substantially enhances the expression of pituitary adenylate cyclase activating polypeptides (PACAP) in the dorsal aspect of the anterolateral BNST ((Hammack et al.), the same region lesioned in the present report). Similar to the nociceptin/orphanin FQ system, BNST PACAP is closely associated with BNST CRH (Kozicz et al., 1997), although the relationship is likely excitatory. Hence, we have argued that BNST PACAP expression mediates behavioral consequences of repeated variate stress, such as increased anxiety and anorexia (Hammack et al.). For the present study, it is unknown whether the effects of repeated stress on weight gain were mediated by stress-induced anorexia and/or stress-induced changes in metabolism. An analysis of food intake in a few of the animals was suggestive of an anorexic state (data not shown), but effects on metabolism were also possible.
In conclusion, the results of this study suggest that during the late phase of a 7-day repeated variate stress paradigm, BNST activity is recruited, which mediates the effects of stressor exposure on weight gain in rats when stress is repeated. These data are consistent with a burgeoning literature suggesting enhanced BNST function after repeated stress, and also further implicate a role for the BNST in mediating the behavioral consequences of repeated stress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buwalda B, Van Kalkeren AA, de Boer SF, Koolhaas JM. Behavioral and physiological consequences of repeated daily intracerebroventricular injection of corticotropin-releasing factor in the rat. Psychoneuroendocrinology. 1998;23:205–218. doi: 10.1016/s0306-4530(97)00096-6. [DOI] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiology & Behavior. 2006;89:301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. Journal of Neuroscience. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Evanson NK, Furay AR, Ulrich-Lai YM, Ostrander MM, Herman JP. The anteroventral bed nucleus of the stria terminalis differentially regulates hypothalamic-pituitary-adrenocortical axis responses to acute and chronic stress. Endocrinology. 2008a;149:818–826. doi: 10.1210/en.2007-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008b;33:659–669. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. Journal of Comparative Neurology. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dumont EC, Rycroft BK, Maiz J, Williams JT. Morphine produces circuit-specific neuroplasticity in the bed nucleus of the stria terminalis. Neuroscience. 2008;153:232–239. doi: 10.1016/j.neuroscience.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V. Roles for Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Expression and Signaling in the Bed Nucleus of the Stria Terminalis (BNST) in Mediating the Behavioral Consequences of Chronic Stress. J Mol Neurosci. doi: 10.1007/s12031-010-9364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Richey KJ, Watkins LR, Maier SF. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behavioral Neuroscience. 2004;118:443–448. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- Hammack SE, Cheung J, Rhodes KM, Schutz KC, Falls WA, Braas KM, May V. Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34:833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Watson SJ. Involvement of the bed nucleus of the stria terminalis in tonic regulation of paraventricular hypothalamic CRH and AVP mRNA expression. Journal of Neuroendocrinology. 1994;6:433–442. doi: 10.1111/j.1365-2826.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Yamauchi N, Ohno H, Benoit R, Ling N, Demura H. The effects of chronic central administration of corticotropin-releasing factor on food intake, body weight, and hypothalamic-pituitary-adrenocortical hormones. Life Sciences. 1991;48:1483–1491. doi: 10.1016/0024-3205(91)90186-f. [DOI] [PubMed] [Google Scholar]
- Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Research. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Vigh S, Arimura A. Axon terminals containing PACAP- and VIP-immunoreactivity form synapses with CRF-immunoreactive neurons in the dorsolateral division of the bed nucleus of the stria terminalis in the rat. Brain Research. 1997;767:109–119. doi: 10.1016/s0006-8993(97)00737-3. [DOI] [PubMed] [Google Scholar]
- Krahn DD, Gosnell BA, Majchrzak MJ. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Smith HR, Nader MA, Porrino LJ. Chronic cocaine self-administration upregulates the norepinephrine transporter and alters functional activity in the bed nucleus of the stria terminalis of the rhesus monkey. Journal of Neuroscience. 2003;23:12–16. doi: 10.1523/JNEUROSCI.23-01-00012.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Research. 1994;657:141–149. doi: 10.1016/0006-8993(94)90961-x. [DOI] [PubMed] [Google Scholar]
- Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–753. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Foundation Symposium. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308-16. [DOI] [PubMed] [Google Scholar]
- Pego JM, Morgado P, Pinto LG, Cerqueira JJ, Almeida OFX, Sousa N. Dissociation of the morphological correlates of stress-induced anxiety and fear. European Journal of Neuroscience. 2008;27:1503–1516. doi: 10.1111/j.1460-9568.2008.06112.x. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. American Journal of Physiology. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. European Journal of Pharmacology. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Research. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G. Region-specific regulation of neuropeptide mRNAs in rat limbic forebrain neurones by aldosterone and corticosterone. Journal of Physiology. 1995;484:721–736. doi: 10.1113/jphysiol.1995.sp020698. [DOI] [PMC free article] [PubMed] [Google Scholar]