Abstract
Prostate cancer is the most frequently diagnosed tumor in men and the second most common cause of cancer-related death for males in the United States. It has been shown that multiple signaling pathways are involved in the pathogenesis of prostate cancer, such as androgen receptor (AR), Akt, Wnt, Hedgehog (Hh) and Notch. Recently, burgeoning amounts of evidence have implicated that the F-box protein Skp2 (S-phase kinase associated protein 2), a well-characterized oncoprotein, also plays a critical role in the development and progression of prostate cancer. Therefore, this review discusses the recent literature regarding the function and regulation of Skp2 in the pathogenesis of prostate cancer. Furthermore, we highlight that Skp2 may represent an attractive therapeutic target, thus warrants further development of agents to target Skp2, which could have significant therapeutic impact on prostate cancer.
Keywords: Skp2, prostate cancer, p27, androgen receptor
Introduction
Prostate cancer is one of the most frequent non-cutaneous male malignancies, and the second leading cause of cancer-related death for men in the United States with an estimated 240,890 new cases and 33,720 deaths expected in 2011 [1]. The high mortality rate associated with patients diagnosed with prostate cancer could be partly due to the lack of effective therapies. Currently, prostate cancer is treated with surgery, chemotherapy, radiation therapy or hormonal ablation therapy [2]. Androgen deprivation is often used to shrink the cancer volume because prostate cancer is mostly characterized as an endocrine-related cancer that is driven by androgens [3]. However, most patients with advanced prostate cancer eventually develop resistance to androgen deprivation therapy which subsequently leads to the development of metastatic castrate-resistant prostate cancer (mCRPC), a clinical stage for which there is no curative therapy [4]. Due to the development of mCRPC, prostate cancer cause high morbidity and mortality with overall poor survival [1]. Taken together, although androgen ablation used in combination with other therapies are the leading treatment in clinical practice, there are many limitations associated with these types of treatment that retard the clinical expectation for curing prostate cancer. For example, many patients have poor response to androgen deprivation therapy, and patients develop tumor metastasis later on, leading to limited curative therapeutic options [5]. This disappointing outcome strongly suggests that understanding the molecular mechanism(s) by which prostate cancer arises is essential for the development of newer therapies to improve the treatment of prostate cancer.
In recent years, studies have shown that multiple cellular signaling cascades including those regulated by the AR (androgen receptor) [3], Akt [6], ER (estrogen receptor; ER) [7], EGFR (epidermal growth factor receptor) [8], PTEN (phosphatase and tensin homolog on chromosome 10) [9], mTOR (mammalian target of rapamycin) [10], NF-κB (nuclear factor-κB) [11], Wnt [12], Shh (sonic hedgehog) [13], PDGF (platelet-derived growth factor) [14], and Notch [15] have been investigated and these pathways are believed to play critical roles in the aggressive pathological progression of prostate cancer. The alterations in these pathways occur at different stages of prostate cancer from early stage such as high PIN (prostatic intraepithelial neoplasia) grades of malignancy to advanced disease [16]. It is important to note that the exact mechanisms by which prostate cancer develops and progresses still remain poorly understood. However, robust evidence has recently been accumulated to suggest that S-phase kinase associated protein 2 (Skp2) plays an important role in the development of prostate cancer [17–20]. Therefore, in this review article, we will focus our discussion on the role of Skp2 in the development and progression of prostate cancer and summarize approaches by which Skp2 could be inhibited.
2. SCFSkp2 is an E3 ubiquitin ligase
The Skp2 F-box protein is the substrate recruiting component of the SCF (Skp1-Cullin 1-F-box) type of E3 ubiqutin-ligase complexes. This family of E3 ligase belongs to the ubiquitin-proteasome system (UPS) that controls the stability of various key cell fate regulators [21]. There are three types of enzymes in the UPS: the ubiquitin-activating enzyme (E1), the ubiquitin-conjugating enzyme (E2), and ubiquitin ligase (E3). These three enzymes compose of a cascade of enzymatic reactions to exert UPS function [21]. The initial step is adenosine triphosphate (ATP)-dependent and involves the linkage of the ubiquitin to the E1. Ubiquitin, an evolutionarily conserved 76-amino acid protein, is then activated in an ATP-dependent manner by a thiol ester bond and subsequently transferred to the E2 enzyme [21]. The ubiquitin-charged E2 then interacts with a specific E3 partner and transfers the ubiquitin molecule onto the substrate by linking the COOH-terminus of ubiquitin to a lysine ε-amino residue on the protein substrate, leading to formation of a mono-ubiquitination or polyubiquitin chain due to the consecutive addition of ubiquitin moieties to target proteins [21]. In general, target protein specificity is defined by the E3, resulting in protein degradation in an ATP-dependent manner by the 26S proteasome [22]. This feature could be one of the reasons why deregulation of E3 ligase often leads to cancer development, while the involvement of E1 and E2 in cancer has not been widely reported except few published reports [23].
E3 ubiquitin ligases are classified into several groups based on the special domains they contain, such as RING (really interesting new gene)-domain, HECT (homologous to the E6-AP carboxyl terminus)-domain, U-box-domain, and PHD-domain [24]. Among them, RING-type E3 ligases contain the most members, which include the SCF type of E3 ligase complex. The SCF complex consists of four components: the invariable component Skp1, Rbx1 and Cullin1, and the interchangeable F-box protein that functions as a receptor for target proteins [24]. To date, there are more than 70 putative F-box proteins encoded in the human genome [24]. Among the many F-box proteins identified, Skp2 is one of the best characterized and has been shown to be involved in carcinogenesis by regulating the abundance of multiple tumor suppressor proteins associated with cancers [21].
3. Skp2 is a bona fide proto-oncoprotein
Skp2; a crucial component of the SCF complex, is the specificity factor of an E3 ligase involved in cell cycle progression through degradation of its ubiquitination targets [21]. It is worth mentioning that in most cases, Skp2 recognizes targeted substrates for ubiquitination after the phosphorylation of a consensus sequence within the target protein [21]. Recent research efforts have determined that the specific substrates of Skp2 include p21 [25], p27 [26], p57 [27], p130 [28], Tob1 (transducer of ERBB2) [29], FOXO1 [30], and others [31–33] (Figure 1). Without a doubt, Skp2 plays a critical role in regulating many cellular processes such as cell cycle regulation, cell proliferation, apoptosis, differentiation, and survival, all of which are closely related to cancer development through degradation of its substrates [21]. Since most of these substrates are tumor suppressor proteins, Skp2 is often believed to function as an oncogene.
Figure 1. Illustrated pathways of Skp2-mediated degradation of its major downstream targets.
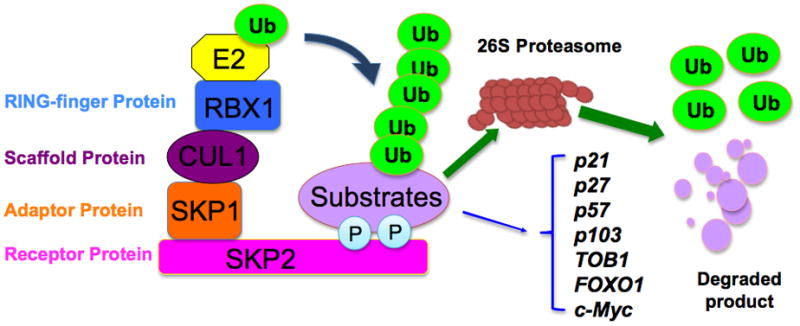
The SCF (Skp1-Cullin 1-F-box) complex consists of four components: the invariable component Skp1 (adaptor protein), Rbx1 (RING finger protein) and Cullin1 (Scaffold protein), and the interchangeable F-box protein that functions as a receptor for target proteins. Skp2 recognizes the targeted substrates, which are presented closely to the E2 enzyme to ensure consequent conjugation of ubiquitin. The addition of polyubiquitin targets proteins to the 26S-proteasome for degradation. The specific substrates of Skp2 include p21, p27, p57, p103, Tob1, FOXO1, and c-Myc.
Consistent with this notion, Skp2 over-expression has been detected in various types of cancers, including lymphomas [34], prostate cancer [20], melanoma [35], nasopharyngeal carcinoma [36], pancreatic cancer [37], and breast carcinomas [38, 39]. In addition, gene amplifications of the Skp2 have been reported in human gastric cancer [40]. Several mouse models also confirm the oncogenic role of Skp2 in tumorigenesis. For example, xenografts of breast cancer cell lines expressing high Skp2 grow much faster than xenografts expressing low levels of Skp2 [38]. In agreement with a critical role for Skp2 in tumor progression, Skp2 knock out mice are resistant to tumor development induced by loss of either p19ARF or the PTEN tumor suppressor protein [17]. Furthermore, a correlation between Skp2 over-expression and elevated Akt activity has also been reported in many carcinomas [41]. Further, consistent with this model, we and others have demonstrated that Akt1 phosphorylates Skp2 at Ser72, protecting Skp2 from Cdh1-mediated degradation and localizing a pool of Skp2 to the cytoplasm [42, 43]. Recently, it has been reported that Skp2 expression correlated significantly with histological grade and tumor size in human hepatocellular carcinoma [44]. Moreover, a correlation between elevated Skp2 protein expression and tumor metastasis has been noted in multiple tumors, including melanoma [35], oral squamous cell carcinomas [45], pancreatic cancer [46], and breast carcinoma [47]. Furthermore, Skp2 expression has been discovered to predict for poor prognosis in breast cancer [47], melanoma [35], and nasopharyngeal carcinoma [36]. Importantly, a recent study demonstrated that Skp2 confers upon a subset of pancreatic cancer cell lines resistance towards TRAIL (tumor factor-related apoptosis-inducing ligand) [37]. Taken together, these finding suggest that Skp2 serves as a proto-oncoprotein. In the next portion of this review, we will mainly focus on the role of Skp2 in prostate cancer progression.
4. The role of Skp2 in the development and progression of prostate cancer
Emerging evidence suggests that Skp2 plays important roles in prostate tumorigenesis. For example, prostate tissue-specific over-expression of Skp2 promoted marked over-proliferation, leading to hyperplasia, dysplasia, and low-grade carcinoma in the prostate gland [48]. A growing body of literature also strongly suggests that increased expression of Skp2 is detected in prostate cancer cells and tissues. Moreover, Skp2 protein expression was correlated with tumor stage, histological grade, and recurrence in prostate cancer [49–51], suggesting that Skp2 could be useful as a prognostic biomarker. Furthermore, Lin et al. reported that Skp2 deficiency restricts prostate cancer development by triggering cellular senescence through up-regulation of p21, p27 and ATF4 in vivo [17]. It is noteworthy that the molecular mechanism(s) by which Skp2 induces prostate tumor growth has not been fully elucidated. However, multiple signaling pathways, such as phosphatidylinositol 3-kinase (PI3K)/Akt [52], AR [53], PTEN [20], p27 [18], and BRCA2 [54] signaling have been reported to cross-talk with Skp2 in the prostate cancer, and thus it is believed that the cross-talk between Skp2 and these signaling pathways may play critical roles in prostate tumorigenesis. Here, we will discuss the recent advances in our understanding of the role of Skp2 in prostate tumor progression. Therefore, in the following paragraphs, we will summarize the results of emerging studies on Skp2, including the upstream regulators and downstream effectors of this protein, as well as its implication in human prostate cancer.
4.1 Upstream regulators of Skp2 in prostate cancer
In recent years, studies on Skp2 and its oncogenic roles have burst onto the scene; however, the upstream regulators of Skp2 in human cancer progression are largely unknown. Several groups have found that multiple genes can regulate Skp2 expression. For example, MYC can directly regulate Skp2 expression and MYC-mediated Skp2 induction leads to the reduction of p27 levels in human leukemia cells [55]. Skp2 was also revealed as a novel target for E2F regulation that is disrupted in several human tumor cell lines [56]. Additionally, over-expression of PPARγ (peroxisome proliferators activated receptor gamma) can down-regulate Skp2 expression in breast tumor cells [57]. BCR-ABL (breakpoint cluster region-abelson leukemia gene) controls Skp2 gene transcription via the PI3K/AKT/Sp1 pathway in leukemia cells [58]. Moreover, Tang et al. reported that WIF1 (Wnt inhibitory factor-1) induced cell cycle G1 arrest through down-regulation of Skp2, leading to p27 accumulation in bladder cancer cells [59]. Furthermore, Hu et al. found that thrombin, a trypsin-like serine protease, induces tumor cell cycle activation and promotes cell growth by the up-regulation of Skp2 as well as down-regulation of p27 in prostate cancer [60]. More recently, it has been found that PI3K/Akt, PTEN and the AR can regulate the expression of Skp2 through different mechanisms in prostate cancer [20, 52, 53]. The mechanisms by which these upstream genes regulate Skp2 are discussed in the following paragraphs.
4.1.1 PI3K/Akt regulates Skp2 in prostate cancer
The PI3Ks are enzymes that mediate cellular signal transduction. Both receptor tyrosine kinases (RTKs) and non-RTKs can activate PI3K, which subsequently converts membrane-bound phosphatidylinositol (4,5)-bisphosphoate (PIP2) to phosphatidylinositol (3,4,5)-triphosphoate (PIP3), leading to the activation of Akt by phosphorylation [61]. Akt, also namely protein kinase B (PKB), is one of the major regulators that control cell growth and apoptosis. It has been documented that there are three isoforms of Akt known as Akt 1, Akt 2 and Akt 3, which are encoded by PKBα, PKBβ and PKBγ in mammals, respectively. Akt is activated by 3-phosphoinositide-dependent protein kinase, which transmits signals through cytokines, growth factors, and oncoproteins to multiple targets [61]. Activated Akt could promote cell proliferation and survival by inhibiting apoptosis through regulation of multiple signaling pathways such as Bcl-xL/Bcl-2-Associated Death (BAD), IKK (Inhibitor of nuclear factor Kappa B Kinase), GSK3 (Glycogen synthase kinase 3), Forkhead-related transcription factor 1 (FKHR1), caspase-9 and mTOR [61]. The PI3K/Akt pathway has also been implicated in prostate carcinogenesis, although its precise function remains to be fully elucidated [6].
Recently, we have found that the function of Akt at regulating Skp2 levels is primarily through the regulation of Skp2 protein stability by Cdh1 [41]. It has also been reported that activation of Akt promotes the binding of E2F-1 to the proximal Skp2 promoter in pancreatic cancer [62]. Therefore, Skp2 up-regulation in most human cancers might be due to a synergistic action of up-regulated Skp2 mRNA levels with a concomitant evasion of Cdh1-mediated degradation. Similarly, one study showed that PI3K/Akt signaling regulates Skp2 expression as blocking PI3K/Akt signaling in prostate cancer cells resulted in decreased Skp2 protein expression and increased p27 abundance [52]. However, further research toward exploration of the molecular mechanisms by which PI3K/Akt regulates Skp2 requires in-depth investigations.
4.1.2 PTEN regulates Skp2 in prostate cancer
It has been well documented that PTEN functions as a tumor suppressor by negatively regulating the PI3K/Akt signaling pathway. PTEN is among the most frequently inactivated or deleted tumor suppressor genes in many cancers including prostate tumorigenesis. Interestingly, mutation of the PTEN gene is rare in primary prostate cancer [63]. It has been shown that re-expression of PTEN in prostate-cancer cell lines causes morphological changes associated with apoptosis [63]. Similarly, deletion of PTEN in tentative prostate stem cells causes development of prostate cancer in mice, which recapitulates the disease progression seen in humans [64]. Restoration of functional PTEN activity can inhibit the growth of PTEN−/−prostate cancer xenografts in mice and restore sensitivity to chemotherapy [65]. Moreover, PI3K/Akt signaling is up-regulated in 30–50% of prostate cancer cases, often due to the loss of PTEN function [10]. Together, it is well characterized that PTEN serves as a tumor suppressor in prostate cancer without a doubt.
Recently, PTEN has been found to regulate the oncogenic functions of Skp2 in prostate cancer. Down-regulation of PTEN in prostate cancer DU145 cells showed PTEN/Akt-dependent regulation of Skp2 and p27 [52]. Moreover, an inverse correlation between the PTEN tumor suppressor protein and expression of Skp2 as well as its target p27 in the prostate cancer has been observed, implicating that PTEN may regulate Skp2 expression in vivo [20]. These data indicate that the PI3K/PTEN/Akt could modulate p27 through Skp2 in prostate cancer. This regulatory role for PTEN towards Skp2 might be partly attributed to the ability of PTEN to activate the E3 ligase activity of APC/Cdh1 through a direct physical interaction [66]. Thus, in PTEN−/−cells, impaired APC/Cdh1 E3 ligase activity might cause elevated Skp2 abundance, allowing Skp2 to exert its oncogenic functions in facilitating prostate cancer development. Nonetheless, further research towards exploration of the molecular mechanisms by which PTEN regulates Skp2 should require immediate attention.
4.1.3 Androgen and AR regulate Skp2 in prostate cancer
AR, a ligand-activated transcription factor, has been well documented to play a critical role in the pathological progression of prostate cancer. AR without binding to its ligand androgen is sequestered in the cytoplasm and bound to HSPs (heat shock proteins) [67]. After binding to androgen, AR undergoes a dramatic conformational change, resulting in its dissociation from HSPs and its subsequent phosphorylation. These change allow AR to localize to the nuclear and form homodimer. facilitating its interaction with DNA [67]. The activated AR then initiates gene transcription by binding to specific androgen response elements in the promoter regions of its target genes such as PSA (prostate specific antigen), leading to promoting cell growth. It has been reported that androgens and AR are involved in all stages of prostate carcinogenesis including initiation, progression, and treatment resistance, suggesting that AR signaling could be a critical target for prostate cancer prevention and treatment. Importantly, it has been found that Akt is an important activator of the AR, which is required for androgen-independent survival and growth of prostate cancer cells [68].
Emerging evidence has shown that androgen and the AR are involved in the regulation of Skp2. Pernicova and his co-workers found that androgen depletion decreased prostate cancer cell proliferation partly through down-regulation of Skp2 [69]. Recent studies have also shown that the AR is a robust upstream regulator of Skp2 through blocking its D-box-dependent degradation in prostate cancer cells [19]. Interestingly, Skp2, in turn, serves as an essential downstream effector of the AR in promoting proliferation independent of the differentiation-promoting function of the AR [19]. Very interestingly, it has been demonstrated that expression of Skp2 is inhibited by androgen in an AR-dependent manner [70]. Specifically, androgen represses Skp2 expression via both p107-dependent and p107-independent pathways in prostate cancer cells [71]. Similarly, one study led by Chuu et al. reported that androgen suppresses cell growth in prostate cancer by inducing G1 cell cycle arrest via reduction of Skp2 and c-Myc as well as induction of p27 [53]. This evidence suggests that there is a direct link between Skp2 and AR-mediated cell signaling; however, the molecular mechanism of this feedback signaling loop is still not yet clear. Thus, a better understanding of the precise role of Skp2 and its interrelationship with AR requires further in-depth investigation.
4.2 Downstream effectors of Skp2 in prostate cancer
Recent studies have clearly demonstrated that behaving as a proto-oncoprotein, Skp2 regulates a variety of cellular processes including cell cycle progression, cell proliferation, apoptosis, differentiation, migration, invasion, and survival, all of which are related to cancer development and progression. This is mainly achieved through directly promoting the degradation of Skp2 downstream substrates. However, it has also been shown that Skp2 affects the expression of proteins other than its substrates. For example, Skp2 over-expression increases the expression of MMP-2 as well as MMP-9 and invasion of lung cancer cells [72], which might be indirectly through regulating Skp2 substrates such as FOXO1 [30] or c-Myc [32, 33]. Although the exact molecular mechanisms still remain elusive, it expands the understanding of the oncogenic role of Skp2 and articulates the significant contribution of Skp2 during the tumorigenesis process via affecting a wide spectrum of signaling cascades. Here, we mainly focus on discussing the recent advances in the understanding of the role of Skp2 in prostate tumor progression.
4.2.1 Skp2 regulates p27 in prostate cancer
Cell proliferation is tightly regulated by expression and activation of cell cycle-dependent cyclins, cyclin-dependent kinases (Cdks) and cyclin-dependent kinase inhibitors (CdkIs) [73]. There are two classes of CdkIs that regulate different Cdks, namely the INK4 family and the KIP/CIP family. Members of the INK4 family include p15INK4A, p16INK4B, p18INK4C, and p19INK4D, while the KIP (kinase inhibitor protein)/CIP family contains p21CIP, p27KIP1, and p57KIP2 [73]. It is well established that p27, an inhibitor of cyclin-dependent kinases, is a negative cell cycle regulator that functions as a tumor suppressor [74]. Low or absent p27 expression has been frequently observed in many human cancers including prostate cancer [74]. Furthermore, down-regulation of p27 correlates with aggressive tumor grade and poor prognosis in prostate cancer [75, 76]. However, the mechanism of p27 down-regulation in prostate cancer is not fully understood.
It has been reported that Skp2, which targets p27 for degradation, is highly expressed in prostate cancer [20]. This raises the question of whether the low levels of p27 in prostate cancer may be due to increased expression of Skp2. Indeed, several studies by different groups have shown that Skp2 acts as an oncoprotein probably through its degradation of p27. First of all, Skp2 expression was inversely correlated with p27 expression in prostate cancer [49]. Consistent with this notion, over-expression of Skp2 in transgenic mice caused significant down-regulation of p27 in prostate glands [48]. Moreover, up-regulation of Skp2 led to ectopic down-regulation of p27 in prostate cancer cells [18]. However, it is recognized that more thorough studies are required to fully understand how Skp2 regulates p27 in human prostate cancer.
4.2.2 Skp2 regulates BRCA2 in prostate cancer
BRCA2 (Breast cancer 2), originally identified in familial breast cancer patients, was found to be involved in prostate cancer as a tumor suppressor [77]. Studies have shown that men with mutations in BRCA2 will be more likely to develop prostate cancer, indicating that there is a genetic link between BRCA2 mutations and the overall risk for prostate cancer [78]. Moreover, BRCA2 mutation carriers are typically diganosed with more aggressive prostate cancer phenotype, such as shorter survival [78], suggesting that BRCA2 mutation is a prognostic factor for prostate cancer outcome.
Recently, it has been demonstrated that Skp2 controls the proteolytic degradation of BRCA2 in a PI3K-dependent manner in prostate cancer cells [79], indicating that the interaction between BRCA2 and Skp2 may play a critical role in prostate tumorigenesis. Specifically, inhibition of Skp2 expression prevented BRCA2 depletion and inhibited prostate cancer cell growth [79]. More recently, Arbini et al. reported that decreased BRCA2 was inversely correlated with Skp2 expression in prostate cancer [54]. This group also found that Skp2 over-expression reduced BRCA2 protein and promoted prostate cell growth and migration, suggesting that the loss of BRCA2 expression is correlated with prostate cancer growth and migratory behavior due to over-expression of Skp2 [54].
4.2.3 Skp2 regulates the expression of other targets in prostate cancer
Recently, Lin et al. reported that Skp2 depletion markedly reduced tumor weight and invasive prostate cancer after complete PTEN inactivation [17]. Moreover, this group showed that Skp2 deficiency restricts prostate cancer development by triggering cellular senescence through p27, p21 and ATF4 induction [17], suggesting that Skp2 could regulate these gene expressions in prostate cancer. It is important to note that Skp2 deficiency did not elicit cellular senescence on its own, but it triggers a senescence response after oncogenic stress [17]. Therefore, Skp2 maybe regulating other gene expressions that have not yet been discovered which might contribute to the development of prostate cancer. Additionally, it has been found that c-Myc is overexpressed in prostate cancer and been linked to prostate cancer tumorigenesis [80, 81], while Skp2 participates in c-Myc proteosomal degradation and also induces c-Myc-responsive genes, [32, 33], suggesting that Skp2 may promote prostate carcinogenesis partly through regulation of c-Myc. More recently, Chan et al. demonstrated that Skp2 cooperates with Myc to induce RhoA transcription by recruiting Miz1 and p300 to the RhoA promoter independently of the SCFSkp2 E3 ligase activity [82]. In addition, the Myc–Skp2–Miz1 complex is overexpressed and correlated with the elevated RhoA expression in metastatic prostate cancer [82], indicating a novel SCFSkp2 E3-ligase-independent function accounting for the oncogenic activity of Skp2 in prostate cancer. Clearly, further in-depth studies are still needed to ascertain more downstream targets that could be regulated by Skp2, either in an E3-ligase-dependent or an E3-ligase-independent fashion, which will help to elucidate the underlying molecular mechanisms by which Skp2 governs the tumorigenesis in the prostate cancer setting.
5 Skp2 is a potential target for prostate cancer therapy
Given the importance of Skp2 in tumor cell cycle regulation, cell growth, apoptosis, migration, and metastasis and its cross-talk with many signaling pathways in human cancers including prostate cancer (Figure 2), Skp2 has emerged as an attractive pharmacological target for the development of novel cancer therapy in the past decade. While proteasome inhibitors have been developed such as bortezomib (Velcade TM Millennium Pharmaceuticals, Inc), the first proteasome inhibitor approved by the FDA for treating multiple myeloma [83], agents inhibiting Skp2 may represent more directly targeted drugs with the promise of enhanced efficacy and reduced toxicity. For example, MG-132, a proteasome inhibitor, suppresses cell proliferation and induces apoptosis by down-regulation of Skp2 and accumulation of p27 in lymphoma cell lines [84]. However, patients receiving proteasome inhibition therapy have numerous undesirable side effects such as nausea, diarrhea, fever, anemia, neutropenia, and neuropathy [85]. These adverse effects could be due to inhibition of the wide-ranging functions of the UPS, indicating that targeting specific components of the UPS, such as Skp2 might overcome these problems. Therefore, the development of small molecule inhibitors against Skp2 with a higher level of specificity and selectivity without unwanted side effects would be more likely to have a significant impact on cancer therapy.
Figure 2. Diagram of Skp2 cross-talks with other pathways in prostate cancer.
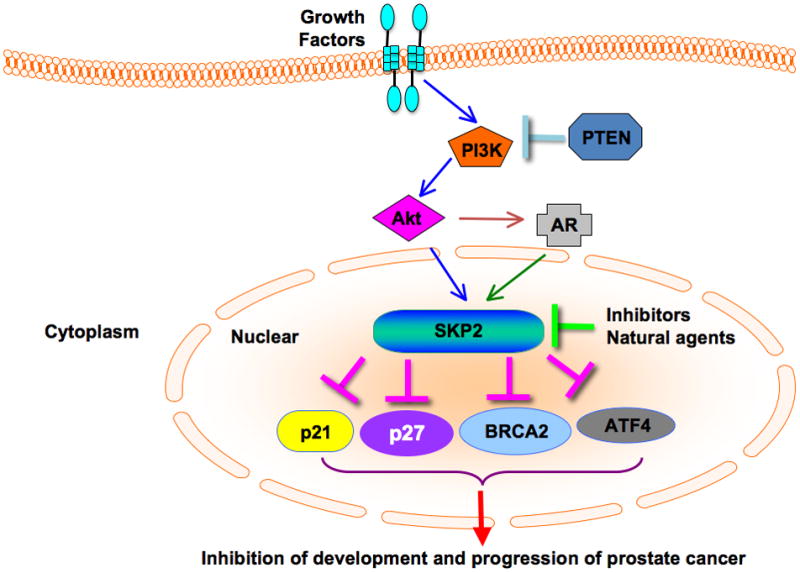
PI3K/Akt, PTEN and AR regulate the expression of Skp2 in prostate cancer. Skp2 regulates the abundance of its substrates p27, p21 as well as the expression of ATF4 and BRCA2. Skp2 inhibitors including natural agents such as silibinin could induce cell cycle arrest through downregulation of Skp2 expression in prostate cancer. Skp2: S-phase kinase associated protein 2; AR: androgen receptor; BRCA2: breast cancer 2; PI3K: phosphatidylinositol 3-kinase; PTEN: phosphatase and tensin homolog on chromosome 10.
Several small molecule inhibitors that block the Skp2 have been developed through a high-throughput screening [86, 87]. Recently, a small molecule inhibitor CpdA (Compound A), which blocks the recruitment of Skp2 to the SCF ligase complex, inhibited cell growth via cell cycle arrest and induced apoptosis in multiple myeloma cells [86]. Moreover, CpdA overcame resistance to chemotherapeutic agents such as dexamethasone, doxorubicin, and melphalan, as well as to proteasome inhibitor bortezomib in multiple myeloma [86]. Furthermore, one chemical compound known as SMIP0004 was reported to down-regulate Skp2 in prostate cancer cells, leading to p27 stabilization [87]. Interestingly, the PPARγ agonist troglitazone and its analogs mediated the proteasomal degradation of β-catenin by up-regulating β-TRCP (beta transducin repeat containing protein) as well as by down-regulation of Skp2 in prostate cancer cells [88].
Using chemical compounds to inhibit Skp2 has limitations for the treatment of human cancer because of the lack of appropriate in vivo delivery systems. To overcome such limitations, researchers recently considered “natural agents” to target Skp2. Indeed, it has been shown that natural agents including curcumin, quercetin, lycopene, silibinin, epigallocatechin-3-gallate, Vitamin D3, and others could induce cell cycle arrest through down-regulation of Skp2 expression in human cancers [89–92]. For example, Roy et al. found that silibinin inhibited Skp2 expression and reduced its binding with p27, leading to accumulation of p27, subsequently causing cell cycle arrest in prostate cancer cells [89]. Yang et al. observed that 1,25-(OH)2 vitamin D3 exerts anti-proliferative effects via cell cycle regulation by reduction of Skp2 and accumulation of p27 in prostate cancer cells [90]. Considering the relatively non-toxic nature of “natural agents”, targeting Skp2 by these agents combined with conventional chemotherapeutics could be a novel and safer approach for achieving better treatment outcome. However, further in-depth preclinical and clinical studies are warranted in order to appreciate the value of “natural agents” for the prevention and treatment of prostate cancer.
6. Conclusions and perspectives
In conclusion, Skp2 plays an important role in the development and progression of human cancers including prostate cancer mainly through regulation of its substrates such as p27, p21 and FOXO1. Therefore, development of inhibitors that target Skp2 could be a novel strategy for the treatment of prostate cancer. One alternative strategy may be to target several signaling pathways that control Skp2 expression, such as PI3K, Akt, and the AR. Furthermore, we summarize that several chemical compounds as Skp2 inhibitors caused cell cycle arrest in prostate cancer, suggesting that targeting Skp2 could represent a novel therapeutic strategy. Interestingly, natural agents were also found to target Skp2 expression in prostate cancer. Due to their non-toxic features, targeting Skp2 by natural agents combined with conventional chemotherapeutics could be an attractive and safer approach for anti-prostate cancer therapy. We believe that this review article would draw significant attention and ignite further work to investigate the molecular mechanism(s) by which Skp2 could be targeted, and will aid in further design of novel approaches by single agent or by using a combination to achieve more effective treatment outcome of prostate cancer patients especially those who are diagnosed with mCRPC.
Acknowledgments
In this review article, we sincerely apologize to those authors whose work was not cited because of the succinct nature of this review article and due to space limitation. The authors’ work cited in this review was funded by grants from the National Institute of General Medicines, NIH (GM089763) to W.W., and Massachusetts Life Science Center New Investigator award (W.W.), and Department of Defense Prostate New Investigator award to W.W.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Thoms J, Goda JS, Zlotta AR, Fleshner NE, van der Kwast TH, Supiot S, Warde P, Bristow RG. Neoadjuvant radiotherapy for locally advanced and high-risk prostate cancer. Nat Rev Clin Oncol. 2011;8:107–113. doi: 10.1038/nrclinonc.2010.207. [DOI] [PubMed] [Google Scholar]
- 3.Attard G, Richards J, de Bono JS. New strategies in metastatic prostate cancer: targeting the androgen receptor signaling pathway. Clin Cancer Res. 2011;17:1649–1657. doi: 10.1158/1078-0432.CCR-10-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadar MD. Small molecule inhibitors targeting the “achilles' heel” of androgen receptor activity. Cancer Res. 2011;71:1208–1213. doi: 10.1158/0008-5472.CAN_10-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karavitakis M, Ahmed HU, Abel PD, Hazell S, Winkler MH. Tumor focality in prostate cancer: implications for focal therapy. Nat Rev Clin Oncol. 2011;8:48–55. doi: 10.1038/nrclinonc.2010.190. [DOI] [PubMed] [Google Scholar]
- 6.Sarker D, Reid AH, Yap TA, de Bono JS. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro DJ, Mao C, Cherian MT. Small molecule inhibitors as probes for estrogen and androgen receptor action. J Biol Chem. 2011;286:4043–4048. doi: 10.1074/jbc.R110.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kambhampati S, Ray G, Sengupta K, Reddy VP, Banerjee SK, Van Veldhuizen PJ. Growth factors involved in prostate carcinogenesis. Front Biosci. 2005;10:1355–1367. doi: 10.2741/1625. [DOI] [PubMed] [Google Scholar]
- 9.Uzoh CC, Perks CM, Bahl A, Holly JM, Sugiono M, Persad RA. PTEN-mediated pathways and their association with treatment-resistant prostate cancer. BJU Int. 2009;104:556–561. doi: 10.1111/j.1464-410X.2009.08411.x. [DOI] [PubMed] [Google Scholar]
- 10.Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9:237–249. doi: 10.2174/156800909787580999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson DR, Zylstra CR, Williams BO. Wnt signaling and prostate cancer. Curr Drug Targets. 2008;9:571–580. doi: 10.2174/138945008784911831. [DOI] [PubMed] [Google Scholar]
- 13.Datta S, Datta MW. Sonic Hedgehog signaling in advanced prostate cancer. Cell Mol Life Sci. 2006;63:435–448. doi: 10.1007/s00018-005-5389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Ahmad A, Li Y, Kong D, Azmi AS, Banerjee S, Sarkar FH. Emerging roles of PDGF-D signaling pathway in tumor development and progression. Biochim Biophys Acta. 2010;1806:122–130. doi: 10.1016/j.bbcan.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villaronga MA, Bevan CL, Belandia B. Notch signaling: a potential therapeutic target in prostate cancer. Curr Cancer Drug Targets. 2008;8:566–580. doi: 10.2174/156800908786241096. [DOI] [PubMed] [Google Scholar]
- 16.Shen MM, Abate-Shen C. Molecular genetics of prostate cancer: new prospects for old challenges. Genes Dev. 2010;24:1967–2000. doi: 10.1101/gad.1965810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH, Yang WL, Wang J, Egia A, Nakayama KI, Cordon-Cardo C, Teruya-Feldstein J, Pandolfi PP. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature. 2010;464:374–379. doi: 10.1038/nature08815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Schulz H, Wolf DA. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 2002;3:22. doi: 10.1186/1471-2121-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Sun D, Ji P, Mohler J, Zhu L. An AR-Skp2 pathway for proliferation of androgen-dependent prostate-cancer cells. J Cell Sci. 2008;121:2578–2587. doi: 10.1242/jcs.030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang G, Ayala G, De Marzo A, Tian W, Frolov A, Wheeler TM, Thompson TC, Harper JW. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res. 2002;8:3419–3426. [PubMed] [Google Scholar]
- 21.Frescas D, Pagano M. Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer. 2008;8:438–449. doi: 10.1038/nrc2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedford L, Lowe J, Dick LR, Mayer RJ, Brownell JE. Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets. Nat Rev Drug Discov. 2011;10:29–46. doi: 10.1038/nrd3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto Y, Ozaki T, Miyazaki K, Aoyama M, Miyazaki M, Nakagawara A. UbcH10 is the cancer-related E2 ubiquitin-conjugating enzyme. Cancer Res. 2003;63:4167–4173. [PubMed] [Google Scholar]
- 24.Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. doi: 10.1038/nrc1881. [DOI] [PubMed] [Google Scholar]
- 25.Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21(CIP1/WAF1) and cyclin D proteins. Proc Natl Acad Sci U S A. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 27.Kamura T, Hara T, Kotoshiba S, Yada M, Ishida N, Imaki H, Hatakeyama S, Nakayama K, Nakayama KI. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci U S A. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2) Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hiramatsu Y, Kitagawa K, Suzuki T, Uchida C, Hattori T, Kikuchi H, Oda T, Hatakeyama S, Nakayama KI, Yamamoto T, Konno H, Kitagawa M. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 2006;66:8477–8483. doi: 10.1158/0008-5472.CAN-06-1603. [DOI] [PubMed] [Google Scholar]
- 30.Huang H, Regan KM, Wang F, Wang D, Smith DI, van Deursen JM, Tindall DJ. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc Natl Acad Sci U S A. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song MS, Song SJ, Kim SJ, Nakayama K, Nakayama KI, Lim DS. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G1-S transition. Oncogene. 2008;27:3176–3185. doi: 10.1038/sj.onc.1210971. [DOI] [PubMed] [Google Scholar]
- 32.von der Lehr N, Johansson S, Wu S, Bahram F, Castell A, Cetinkaya C, Hydbring P, Weidung I, Nakayama K, Nakayama KI, Soderberg O, Kerppola TK, Larsson LG. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003;11:1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 33.Kim SY, Herbst A, Tworkowski KA, Salghetti SE, Tansey WP. Skp2 regulates Myc protein stability and activity. Mol Cell. 2003;11:1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- 34.Lim MS, Adamson A, Lin Z, Perez-Ordonez B, Jordan RC, Tripp S, Perkins SL, Elenitoba-Johnson KS. Expression of Skp2, a p27(Kip1) ubiquitin ligase, in malignant lymphoma: correlation with p27(Kip1) and proliferation index. Blood. 2002;100:2950–2956. doi: 10.1182/blood.V100.8.2950. [DOI] [PubMed] [Google Scholar]
- 35.Rose AE, Wang G, Hanniford D, Monni S, Tu T, Shapiro RL, Berman RS, Pavlick AC, Pagano M, Darvishian F, Mazumdar M, Hernando E, Osman I. Clinical relevance of SKP2 alterations in metastatic melanoma. Pigment Cell Melanoma Res. 2011;24:197–206. doi: 10.1111/j.1755-148X.2010.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HM, Liang Y, Chen Q, Wu QN, Guo YM, Shen GP, Zhang RH, He ZW, Zeng YX, Xie FY, Kang TB. Correlation of Skp2 overexpression to prognosis of patients with nasopharyngeal carcinoma from South China. Chin J Cancer. 2011;30:204–212. doi: 10.5732/cjc.010.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuler S, Diersch S, Hamacher R, Schmid RM, Saur D, Schneider G. SKP2 confers resistance of pancreatic cancer cells towards TRAIL-induced apoptosis. Int J Oncol. 2011;38:219–225. [PubMed] [Google Scholar]
- 38.Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448–3458. doi: 10.1038/sj.onc.1208328. [DOI] [PubMed] [Google Scholar]
- 39.Zheng WQ, Zheng JM, Ma R, Meng FF, Ni CR. Relationship between levels of Skp2 and P27 in breast carcinomas and possible role of Skp2 as targeted therapy. Steroids. 2005;70:770–774. doi: 10.1016/j.steroids.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Masuda TA, Inoue H, Sonoda H, Mine S, Yoshikawa Y, Nakayama K, Mori M. Clinical and biological significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002;62:3819–3825. [PubMed] [Google Scholar]
- 41.Gao D, Inuzuka H, Tseng A, Wei W. Akt finds its new path to regulate cell cycle through modulating Skp2 activity and its destruction by APC/Cdh1. Cell Div. 2009;4:11. doi: 10.1186/1747-1028-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, Yang WL, Erdjument-Bromage H, Nakayama KI, Nimer S, Tempst P, Pandolfi PP. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu M, Ma J, Xue W, Cheng C, Wang Y, Zhao Y, Ke Q, Liu H, Liu Y, Li P, Cui X, He S, Shen A. The expression and prognosis of FOXO3a and Skp2 in human hepatocellular carcinoma. Pathol Oncol Res. 2009;15:679–687. doi: 10.1007/s12253-009-9171-z. [DOI] [PubMed] [Google Scholar]
- 45.Tosco P, La Terra Maggiore GM, Forni P, Berrone S, Chiusa L, Garzino-Demo P. Correlation between Skp2 expression and nodal metastasis in stage I and II oral squamous cell carcinomas. Oral Dis. 2011;17:102–108. doi: 10.1111/j.1601-0825.2010.01713.x. [DOI] [PubMed] [Google Scholar]
- 46.Einama T, Kagata Y, Tsuda H, Morita D, Ogata S, Ueda S, Takigawa T, Kawarabayashi N, Fukatsu K, Sugiura Y, Matsubara O, Hatsuse K. High-level Skp2 expression in pancreatic ductal adenocarcinoma: correlation with the extent of lymph node metastasis, higher histological grade, and poorer patient outcome. Pancreas. 2006;32:376–381. doi: 10.1097/01.mpa.0000220862.78248.c4. [DOI] [PubMed] [Google Scholar]
- 47.Voduc D, Nielsen TO, Cheang MC, Foulkes WD. The combination of high cyclin E and Skp2 expression in breast cancer is associated with a poor prognosis and the basal phenotype. Hum Pathol. 2008;39:1431–1437. doi: 10.1016/j.humpath.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Shim EH, Johnson L, Noh HL, Kim YJ, Sun H, Zeiss C, Zhang H. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- 49.Zheng XY, Ding W, Xie LP, Chen ZD. Correlation of Skp2 and P27kip1 protein expression and clinicopathological features of prostate cancer. Ai Zheng. 2004;23:215–218. [PubMed] [Google Scholar]
- 50.Nguyen PL, Lin DI, Lei J, Fiorentino M, Mueller E, Weinstein MH, Pagano M, Loda M. The impact of Skp2 overexpression on recurrence-free survival following radical prostatectomy. Urol Oncol. 2011;29:302–308. doi: 10.1016/j.urolonc.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Izhak O, Lahav-Baratz S, Meretyk S, Ben-Eliezer S, Sabo E, Dirnfeld M, Cohen S, Ciechanover A. Inverse relationship between Skp2 ubiquitin ligase and the cyclin dependent kinase inhibitor p27Kip1 in prostate cancer. J Urol. 2003;170:241–245. doi: 10.1097/01.ju.0000072113.34524.a7. [DOI] [PubMed] [Google Scholar]
- 52.van Duijn PW, Trapman J. PI3K/Akt signaling regulates p27(kip1) expression via Skp2 in PC3 and DU145 prostate cancer cells, but is not a major factor in p27(kip1) regulation in LNCaP and PC346 cells. Prostate. 2006;66:749–760. doi: 10.1002/pros.20398. [DOI] [PubMed] [Google Scholar]
- 53.Chuu CP, Kokontis JM, Hiipakka RA, Fukuchi J, Lin HP, Lin CY, Huo C, Su LC, Liao S. Androgen Suppresses Proliferation of Castration-Resistant LNCaP 104-R2 Prostate Cancer Cells via Androgen Receptor, Skp2, and c-Myc. Cancer Sci. 2011 doi: 10.1111/j.1349-7006.2011.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arbini AA, Greco M, Yao JL, Bourne P, Marra E, Hsieh JT, di Sant'agnese PA, Moro L. Skp2 overexpression is associated with loss of BRCA2 protein in human prostate cancer. Am J Pathol. 2011;178:2367–2376. doi: 10.1016/j.ajpath.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bretones G, Acosta JC, Caraballo JM, Ferrandiz N, Gomez-Casares MT, Albajar M, Blanco R, Ruiz P, Hung WC, Albero MP, Perez-Roger I, Leon J. SKP2 oncogene is a direct MYC target gene and MYC down-regulates p27(KIP1) through SKP2 in human leukemia cells. J Biol Chem. 2011;286:9815–9825. doi: 10.1074/jbc.M110.165977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L, Wang C. F-box protein Skp2: a novel transcriptional target of E2F. Oncogene. 2006;25:2615–2627. doi: 10.1038/sj.onc.1209286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng J, Ding Y, Shen A, Yan M, He F, Ji H, Zou L, Liu Y, Wang Y, Lu X, Wang H. Overexpression of PPARgamma can down-regulate Skp2 expression in MDA-MB-231 breast tumor cells. Mol Cell Biochem. 2010;345:171–180. doi: 10.1007/s11010-010-0570-y. [DOI] [PubMed] [Google Scholar]
- 58.Chen JY, Wang MC, Hung WC. Transcriptional activation of Skp2 by BCR-ABL in K562 chronic myeloid leukemia cells. Leuk Res. 2009;33:1520–1524. doi: 10.1016/j.leukres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Tang Y, Simoneau AR, Liao WX, Yi G, Hope C, Liu F, Li S, Xie J, Holcombe RF, Jurnak FA, Mercola D, Hoang BH, Zi X. WIF1, a Wnt pathway inhibitor, regulates SKP2 and c-myc expression leading to G1 arrest and growth inhibition of human invasive urinary bladder cancer cells. Mol Cancer Ther. 2009;8:458–468. doi: 10.1158/1535-7163.MCT-08-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu L, Ibrahim S, Liu C, Skaar J, Pagano M, Karpatkin S. Thrombin induces tumor cell cycle activation and spontaneous growth by down-regulation of p27Kip1, in association with the up-regulation of Skp2 and MiR-222. Cancer Res. 2009;69:3374–3381. doi: 10.1158/0008-5472.CAN-08-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 62.Reichert M, Saur D, Hamacher R, Schmid RM, Schneider G. Phosphoinositide-3-kinase signaling controls S-phase kinase-associated protein 2 transcription via E2F1 in pancreatic ductal adenocarcinoma cells. Cancer Res. 2007;67:4149–4156. doi: 10.1158/0008-5472.CAN-06-4484. [DOI] [PubMed] [Google Scholar]
- 63.Assinder SJ, Dong Q, Kovacevic Z, Richardson DR. The TGF-beta, PI3K/Akt and PTEN pathways: established and proposed biochemical integration in prostate cancer. Biochem J. 2009;417:411–421. doi: 10.1042/BJ20081610. [DOI] [PubMed] [Google Scholar]
- 64.Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. Proc Natl Acad Sci U S A. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci U S A. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song MS, Carracedo A, Salmena L, Song SJ, Egia A, Malumbres M, Pandolfi PP. Nuclear PTEN regulates the APC-CDH1 tumor-suppressive complex in a phosphatase-independent manner. Cell. 2011;144:187–199. doi: 10.1016/j.cell.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Niu Y, Chang TM, Yeh S, Ma WL, Wang YZ, Chang C. Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene. 2010;29:3593–3604. doi: 10.1038/onc.2010.121. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Curr Cancer Drug Targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 69.Pernicova Z, Slabakova E, Kharaishvili G, Bouchal J, Kral M, Kunicka Z, Machala M, Kozubik A, Souccek K. Androgen Depletion Induces Senescence in Prostate Cancer Cells through Down-regulation of Skp2. Neoplasia. 2011;13:526–536. doi: 10.1593/neo.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang H, Zegarra-Moro OL, Benson D, Tindall DJ. Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene. 2004;23:2161–2176. doi: 10.1038/sj.onc.1207326. [DOI] [PubMed] [Google Scholar]
- 71.Jiang J, Pan Y, Regan KM, Wu C, Zhang X, Tindall DJ, Huang H. Androgens repress expression of the f-box protein Skp2 via p107 dependent and independent mechanisms in LNCaP prostate cancer cells. Prostate. 2011 doi: 10.1002/pros.21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hung WC, Tseng WL, Shiea J, Chang HC. Skp2 overexpression increases the expression of MMP-2 and MMP-9 and invasion of lung cancer cells. Cancer Lett. 2010;288:156–161. doi: 10.1016/j.canlet.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 73.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 74.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8:253–267. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 75.Guo Y, Sklar GN, Borkowski A, Kyprianou N. Loss of the cyclin-dependent kinase inhibitor p27(Kip1) protein in human prostate cancer correlates with tumor grade. Clin Cancer Res. 1997;3:2269–2274. [PubMed] [Google Scholar]
- 76.Tsihlias J, Kapusta LR, DeBoer G, Morava-Protzner I, Zbieranowski I, Bhattacharya N, Catzavelos GC, Klotz LH, Slingerland JM. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–548. [PubMed] [Google Scholar]
- 77.Narod SA, Foulkes WD. BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer. 2004;4:665–676. doi: 10.1038/nrc1431. [DOI] [PubMed] [Google Scholar]
- 78.Mitra A, Fisher C, Foster CS, Jameson C, Barbachanno Y, Bartlett J, Bancroft E, Doherty R, Kote-Jarai Z, Peock S, Easton D, Eeles R. Prostate cancer in male BRCA1 and BRCA2 mutation carriers has a more aggressive phenotype. Br J Cancer. 2008;98:502–507. doi: 10.1038/sj.bjc.6604132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moro L, Arbini AA, Marra E, Greco M. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J Biol Chem. 2006;281:22100–22107. doi: 10.1074/jbc.M604636200. [DOI] [PubMed] [Google Scholar]
- 80.Fleming WH, Hamel A, MacDonald R, Ramsey E, Pettigrew NM, Johnston B, Dodd JG, Matusik RJ. Expression of the c-myc protooncogene in human prostatic carcinoma and benign prostatic hyperplasia. Cancer Res. 1986;46:1535–1538. [PubMed] [Google Scholar]
- 81.Buttyan R, Sawczuk IS, Benson MC, Siegal JD, Olsson CA. Enhanced expression of the c-myc protooncogene in high-grade human prostate cancers. Prostate. 1987;11:327–337. doi: 10.1002/pros.2990110405. [DOI] [PubMed] [Google Scholar]
- 82.Chan CH, Lee SW, Li CF, Wang J, Yang WL, Wu CY, Wu J, Nakayama KI, Kang HY, Huang HY, Hung MC, Pandolfi PP, Lin HK. Deciphering the transcriptional complex critical for RhoA gene expression and cancer metastasis. Nat Cell Biol. 2010;12:457–467. doi: 10.1038/ncb2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adams J. The proteasome: a suitable antineoplastic target. Nat Rev Cancer. 2004;4:349–360. doi: 10.1038/nrc1361. [DOI] [PubMed] [Google Scholar]
- 84.Hussain AR, Ahmed M, Ahmed SO, Al-Thari S, Khan AS, Razack S, Platanias LC, Al-Kuraya KS, Uddin S. Proteasome inhibitor MG-132 mediated expression of p27Kip1 via S-phase kinase protein 2 degradation induces cell cycle coupled apoptosis in primary effusion lymphoma cells. Leuk Lymphoma. 2009;50:1204–1213. doi: 10.1080/10428190902951799. [DOI] [PubMed] [Google Scholar]
- 85.Palumbo A, Gay F, Bringhen S, Falcone A, Pescosta N, Callea V, Caravita T, Morabito F, Magarotto V, Ruggeri M, Avonto I, Musto P, Cascavilla N, Bruno B, Boccadoro M. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19:1160–1165. doi: 10.1093/annonc/mdn018. [DOI] [PubMed] [Google Scholar]
- 86.Chen Q, Xie W, Kuhn DJ, Voorhees PM, Lopez-Girona A, Mendy D, Corral LG, Krenitsky VP, Xu W, Parseval L Moutouh-de, Webb DR, Mercurio F, Nakayama KI, Nakayama K, Orlowski RZ. Targeting the p27 E3 ligase SCF(Skp2) results in p27- and Skp2-mediated cell-cycle arrest and activation of autophagy. Blood. 2008;111:4690–4699. doi: 10.1182/blood-2007-09-112904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rico-Bautista E, Yang CC, Lu L, Roth GP, Wolf DA. Chemical genetics approach to restoring p27Kip1 reveals novel compounds with antiproliferative activity in prostate cancer cells. BMC Biol. 2010;8:153. doi: 10.1186/1741-7007-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei S, Lin LF, Yang CC, Wang YC, Chang GD, Chen H, Chen CS. Thiazolidinediones modulate the expression of beta-catenin and other cell-cycle regulatory proteins by targeting the F-box proteins of Skp1-Cul1-F-box protein E3 ubiquitin ligase independently of peroxisome proliferator-activated receptor gamma. Mol Pharmacol. 2007;72:725–733. doi: 10.1124/mol.107.035287. [DOI] [PubMed] [Google Scholar]
- 89.Roy S, Kaur M, Agarwal C, Tecklenburg M, Sclafani RA, Agarwal R. p21 and p27 induction by silibinin is essential for its cell cycle arrest effect in prostate carcinoma cells. Mol Cancer Ther. 2007;6:2696–2707. doi: 10.1158/1535-7163.MCT-07-0104. [DOI] [PubMed] [Google Scholar]
- 90.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 91.Huang HC, Lin CL, Lin JK. 1,2,3,4,6-penta-O-galloyl-beta-D-glucose, quercetin, curcumin and lycopene induce cell-cycle arrest in MDA-MB-231 and BT474 cells through downregulation of Skp2 protein. J Agric Food Chem. 2011;59:6765–6775. doi: 10.1021/jf201096v. [DOI] [PubMed] [Google Scholar]
- 92.Huang HC, Way TD, Lin CL, Lin JK. EGCG stabilizes p27kip1 in E2-stimulated MCF-7 cells through down-regulation of the Skp2 protein. Endocrinology. 2008;149:5972–5983. doi: 10.1210/en.2008-0408. [DOI] [PubMed] [Google Scholar]


