Summary
Evidence reveals that L-tyrosine and L-DOPA, besides serving as substrates and intermediates of melanogenesis, are also bioregulatory agents acting not only as inducers and positive regulators of melanogenesis but also as regulators of other cellular functions. These can be mediated through action on specific receptors or through non-receptor mediated mechanisms. The substrate induced (L-tyrosine and/or L-DOPA) melanogenic pathway would autoregulate itself as well as it would regulate the melanocyte functions through activity of its structural or regulatory proteins and through intermediates of melanogenesis and melanin itself. Dissection of regulatory and autoregulatory elements of this process may elucidate how substrate induced autoregulatory pathways have evolved from prokaryotic or simple eukaryotic organisms to complex systems in vertebrates. This could substantiate older theory proposing that receptors for amino-acid derived hormones arose from the receptors for those amino acids, and that nuclear receptors evolved from primitive intracellular receptors binding nutritional factors or metabolic intermediates.
Keywords: L-tyrosine, L-DOPA, melanocytes, melanin, metabolic regulation, substrate induction, receptors
1. Introduction
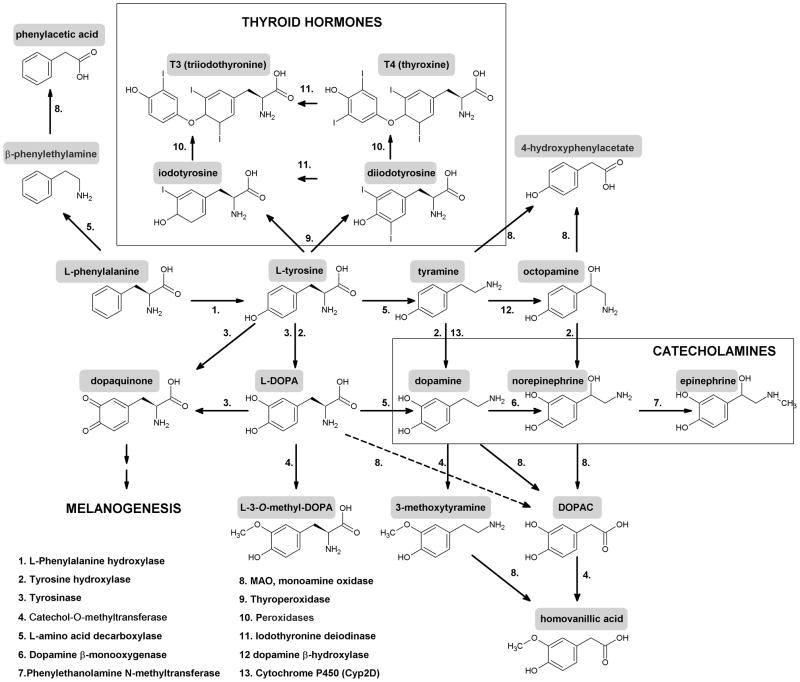
Non-essential aromatic amino acid L-tyrosine, in addition of being an element of protein synthesis, serves also as a precursor to melanin pigment, catecholamines, tyramine/octopamine and thyroid hormones through organification of iodine on tyrosine residue of thyroglobulin with monoiodo-and diiotyrosine as intermediates (Yen, 2001) (Fig. 1). In the body, L-tyrosine, to serve these diverse functions, is either delivered through the gastro-intestinal tract (GI) or is produced by L-phenylalanine hydroxylation, reaction mediated by L-phenylalanine hydroxylase (PH) with the liver being the main site of its systemic supply (Blau et al., 2010; Schallreuter et al., 2008). Depending on the cell type and enzymatic context, it can be hydroxylated to L-dihydroxyphenylalanine (L-DOPA) – reactions mediated either by tyrosine hydroxylase (TPH) or tyrosinase (Tyr)(Slominski et al., 2004); decarboxylated to tyramine; or through action of thyroperoxidases on thyroglobulin is transformed to monoiodo-and diiodotyrosine, with latter being transformed to thyroid hormones through sequential actions of thyroid peroxidase and deiodinases (Yen, 2001) (Fig. 1). L-DOPA is further decarboxylated to dopamine by L-amino acid decarboxylase (AAD) with further hydroxylation and methylation to produce norepinephrine or epinephrine or being oxidated by monoamine oxidase (MAO) to generate 3,4-dihydroxyphenylacetic acid (DOPAC) (Fig. 1).
Figure 1. Scheme showing metabolic transformation of L-tyrosine and its precursor L-phenylalanine to several bioregulatory molecules.
In addition of being transformed to catecholamines and melanins, L-tyrosine and L-DOPA can potentially be estrified as shown in the lower box, where R1 and R2 = phosphate, sulphate, glucuronide, acetyl or nitrate.
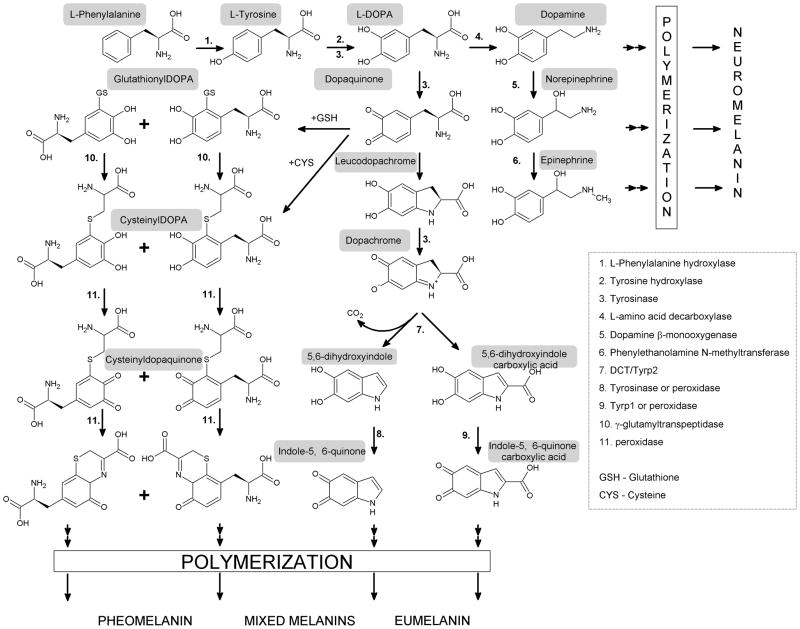
In the melanogenic pathway, L-DOPA is oxidized by tyrosinase to dopaquinone, an intermediate that is common to both eu- and pheomelanogenic pathways (Ito, 2003; Park et al., 2009; Simon et al., 2009) (Fig. 2). During eumelanogenesis dopaquinones transformed to leukodopachrome, followed by a series of oxido-reduction reactions with production of the intermediates dihydroxyindole (DHI) and DHI carboxylic acid (DHICA), that with final formation of eumelanin (Ito, 2003; Simon et al., 2009). The velocity of these reactions and the types of intermediates are regulated by tyrosinase related proteins type 1 (TRP1) and type 2 (TRP2) as well as by physicochemical milieu (metal ions, alkaline pH)(Hearing, 1999; Park et al., 2009; Schallreuter et al., 2008). Conjugation of dopaquinone to cysteine or glutathione to yield cysteinyldopa and glutathionyldopa initiate pheomelanogenesis with final production of pheomelanin (Ito, 2003; Simon et al., 2009).
Figure 2. Scheme show enzymatic steps of sequential transformation of L-tyrosine and L-DOPA to melanin pigments.
In vivo, initiation of the melanogenic pathway is dependent on either transport of L-tyrosine from the extracellular space into the melanosomal compartment of the melanocyte or intracellular generation through hydroxylation of L-phenylalanine by phenylalanine hydroxylase (PH)(Schallreuter and Wood, 1999; Slominski et al., 2004).
2. L-tyrosine and L-DOPA as a positive regulator of melanogenesis
Overview
Traditionally L-tyrosine and L-DOPA are recognized as the consecutive substrates and intermediates of melanogenesis, which in the past led to a prevalent opinion that these amino acids acted solely as substrates to melanin pigment without any modifying capability on their own. This view could find some justification, to certain degree, in observations that in many melanoma/melanocyte lines increased supply of L-tyrosine leads only to increased melanin pigmentation without apparent changes in an expression of proteins or genes of the melanin synthesis pathway. However, almost 20 years ago it has become clear that both L-tyrosine and L-DOPA also act as positive regulators of melanogenesis in a manner dependent on the species, cell genotype and its environment (Slominski and Paus, 1994; Slominski and Paus, 1990). The most comprehensive documentation on the positive regulation of the melanogenic apparatus of melanogenesis by L-tyrosine was provided in lines of rodent malignant melanocytes, see below.
Melanoma cells
In cultured hamster melanoma lines supplementation of L-tyrosine from 10 to 600 μM stimulated not only melanin synthesis but also tyrosinase activity in a dose and time dependent manner with kinetics defined by the original melanogenic potential of the cell line (Slominski et al., 1988). In melanotic and hypomelanotic lines tyrosinase activity reached its peak at optimal media tyrosine concentration (200 or 400μM, respectively), and decreased at pharmacologic concentrations (400 or 600 μM, respectively), while in amelanotic cells, there was a continued increase, which however slowed (reaching plateau) when levels of melanization became high (Slominski et al., 1988). The most instructive results were obtained in lines of Bomirski hamster amelanotic melanoma cultured in Ham’s F10 media relatively low in tyrosine (10 μM). Increased L-tyrosine supplements produced concomitant induction and further increases melanin formation and stimulation of both tyrosine hydroxylase and DOPA oxidase activities of tyrosinase in a process dependent on new protein synthesis(Slominski et al., 1988). This was later confirmed by showing increased production of tyrosinase protein without a significant mRNA expression demonstrating translational regulation of the enzyme by its substrate in this model (Slominski and Costantino, 1991a). The effect was specific since under the same conditions the enantiomers (D-isoform), related (L-phenylalanine and L-tryptophan) or unrelated (L-valine) amino acids or N-acetyl-tyrosine had little or no effect (Slominski et al., 1988), and this process was independent from L-tyrosine transformation to catecholamines since both nor- and epinephrine as well as agonists of α and β adrenergic receptors had none or relatively lower effects on tyrosinase activity in comparison to their substrate (Howe et al., 1991). Importantly, induction of melanogenesis was preceded and accompanied by induction of melanosomes synthesis and translocation of tyrosinase enzyme from trans-Golgi- network (TGN) to premelanosomes with its further enzymatic activation (Slominski et al., 1989b; Slominski et al., 1988), and L-tyrosine stimulated MSH receptors expression and MSH receptor activity (Slominski et al., 1989a). L-tyrosine also induced translocation from TGN to melanosomes of other enzymes such as acid phosphatase indicating more general effect on the intracellular transport, however, without changing the enzymatic activity of selected lysosomal enzymes (Slominski et al., 1988).This indicated a pleiotropic effect of L-tyrosine on intracellular transport and processing, however, with a specificity for the stimulation of melanogenic proteins activity. The fundamental role of L-tyrosine in induction of melanosomes formation was confirmed by experiments with phenylthiourea (PTU), a non-toxic inhibitor of melanogenesis, which while inhibiting stimulation of tyrosinase by L-tyrosine, had not prevented L-tyrosine stimulation of melanosome synthesis (Slominski et al., 1989b). They presented as premelanosomes stage II with matrix presenting as multilamellar outer shell and paracrystalline core or as vesicular matrix (Slominski et al., 1989b). In this model MSH and agents that raise intracellular cyclic AMP induced dendrite formation, inhibited cell growth, and caused substantial increases in tyrosinase activity without inducing melanin synthesis; tyrosinase accumulated solely in the TGN and the mature melanosomes were absent (Slominski et al., 1989c). Therefore, it was concluded that L-tyrosine played a crucial role in the induction of the melanotic phenotype thought induction and enhancement of both melanosome synthesis/assembly and translocation of tyrosinase from the TGN to melanosomes leading to in vivo activation of melanogenesis (Slominski et al., 1989b; Slominski et al., 1988; Slominski and Paus, 1994). In parallel experiments performed with mouse Cloudman S91 melanoma cells L-tyrosine, while increasing melanin pigmentation, had no effect or even decreased tyrosinase activity (Slominski et al., 1988), indicating that in this model the activities of the melanogenic apparatus are largely independent from extracellular tyrosine. This was most likely due to an endogenous production of L-tyrosine from L-phenylalanine (Slominski et al., 2004), because of reported detection of PH activity in these cells (Breakefield et al., 1978).
The confirmation for L-tyrosine function as a positive regulator of melanogenesis was also provided in B16 melanoma (Price et al., 1988). In this cell line L-tyrosine and L-phenylanine not only stimulated melanogenesis but also increased dendrite formation and enhances metastatic capability of these cells (Prezioso et al., 1993) apparently through downregulation of protein kinase C (PKC)ζ (Sanz-Navares et al., 2001). Most recently Zmijewski et al. (unpublished) have also shown that L-tyrosine induced melanin pigmentation in the amelanotic subline of B16 and this induction was accompanied by the stimulation of tyrosinase mRNA, indicating transcriptional model of regulation that was absent in hamster melanoma. Similarly, other investigators demonstrated stimulation of tyrosinase by L-tyrosine in several human melanoma lines (Halaban et al., 2002a; Halaban et al., 2001; Ramirez-Bosca et al., 1992; Slominski et al., 1999; Winder and Harris, 1992) in a posttranslational (Halaban et al., 2001) and/or translational or transcriptional modes of action (Slominski et al., 1999).
The novel role for L-DOPA as a positive regulator of melanogenesis was for the first time shown in Cloudman S91 melanoma (McLane et al., 1987; Pawelek et al., 1988; Pawelek and Murray, 1986) and Bomirski hamster melanomas cells (Pawelek et al., 1988; Slominski et al., 1989b).Thus, the addition of phosphate at position C3 and/or C4 of L-DOPA, producing relatively stable phospho-DOPA (P-DOPA), resulted in the dose dependent stimulatory effects on tyrosinase activity and melanin pigmentation through amplification of the MSH receptor system (McLane et al., 1987; Pawelek et al., 1988; Pawelek and Osber, 1992). L-DOPA by itself did not affect melanogenesis being rapidly consumed by melanogenic pathway in Cloudman melanoma cells, however, at micromolar or lower concentrations it stimulated cell proliferation (McLane et al., 1987; Pawelek et al., 1988). In hamster amelanotic cells L-DOPA induced rapid and dose dependent increases of tyrosinase activity, being significantly more potent than its D- form, with a peak activity at 25 or 50 μM, which induced only moderate pigmentation and formation of predominantly immature melanosomes (Slominski et al., 1988). Tyrosinase accumulated predominantly in the TGN (Slominski et al., 1988) with increased concentration of the protein as documented by western blot that was accompanied by an initial increase in tyrosinase mRNA followed by a decrease below control levels (Slominski and Costantino, 1991b).
Normal melanocytes
Earlier studies performed with amphibian cells have shown that L-tyrosine can act as an inducer of melanogenesis in embryonic cells (Landstrom and Lovtrup, 1978; Lovtrup et al., 1984) and as a stimulator of differentiation program in cultured frog melanophores (Fukuzawa and Ide, 1988). These effects were strikingly similar to those described in hamster amelanotic melanomas (Slominski et al., 1989a; Slominski et al., 1988).
Follow-up studies performed on human normal melanocytes have clearly demonstrated that increasing concentration of L-tyrosine in culture medium stimulated tyrosinase activity in a dose dependent manner with optimal concentrations of 276–550 μM of the ligand (Ramirez-Bosca et al., 1992). Other investigators have shown that the pigmentary defect of the oculocutaneous albinism type 2 (OCA2) can be at least partially corrected by L-tyrosine (reviewed in (Orlow and Brilliant, 1999). OCA2 results from the mutations in pink-eyed dilution gene (P) leading to decreased pigmentation (Box et al., 1998; Oetting and King, 1999; Sturm et al., 2001) due to defective processing of tyrosinase and TRPs with intracellular misrouting, proteolysis and/or secretion to the extracellular environment (Halaban et al., 2002b; Manga et al., 2001; Toyofuku et al., 2002). Thus, in melanocytes from OCA-2 mice L-tyrosine partially restored pigmentary activity by properly targeting tyrosinase and TRP1 to melanosomes (Hirobe et al., 2002; Manga et al., 2001; Rosemblat et al., 1998), and increased the number of melanosomes at later stages of maturation as well as activity and concentration of tyrosinase (Hirobe et al., 2002; Manga et al., 2001; Rosemblat et al., 1998) without affecting tyrosinase mRNA levels (Potterf et al., 1998; Rosemblat et al., 1998). Similarly, L-tyrosine induced maturation and accumulation of tyrosinase in experimental models of OCA1B (Halaban et al., 2002a). Also studies on melanoblasts and melanocytes from C57BL/10 mice demonstrated that L-tyrosine also increases formation of premelanosomes and melanosomes in mutant (pp) melanocytes and increased intracellular concentration of c-kit, TyrP1 and TyrP2 protein in both non-mutant (PP) and pp cells with increased tyrosinase accumulation as demonstrated by DOPA cytochemistry (Hirobe et al., 2002). Interestingly, L-tyrosine, while stimulating proliferation of pp melanocytes inhibited growth of PP melanocytes (Hirobe et al., 2003). Furthermore, Hirobe et al,(Hirobe et al., 2007a) using melanocytes from mice with the a loss-of-function mutation in the MC1R (e/e), have shown that L-tyrosine stimulated tyrosinase activity and TRP1 and TRP2 expression, and maturation of melanosomes with an increased pigmentation as well as it increased proliferation of melanocytes. The same authors also showed that the inhibition of pheo- and eumelanin synthesis by the slaty mutation can be partially restored by the addition of excess L-tyrosine (Hirobe et al., 2007b). In mouse melanotic melanocyte cell line, TM10, 200 μM L-DOPA and P-DOPA but not cysteinyl-DOPA stimulated pheomelanosomes formation and pheomelanin synthesis without stimulating tyrosinase activity (Sato et al., 1987), indicating that even in cells fully expressing melanogenic pathway DOPA can have modulatory role.
The above findings substantiated the physiological significance of the mechanisms described originally in hamster and mouse melanoma models (Pawelek et al., 1988; Slominski et al., 1989a; Slominski et al., 1988). Notable, in vivo P-DOPA also stimulated skin melanin pigmentation (Agin et al., 1987; Pawelek and Osber, 1992), and synergized with the action of UVB (Pawelek et al., 1988). Similarly, topical application of L-tyrosine in Skh:HR2 mice amplified the cutaneous melanogenic response to UVB (Warren, 1986).
3. L-tyrosine and L-DOPA modification
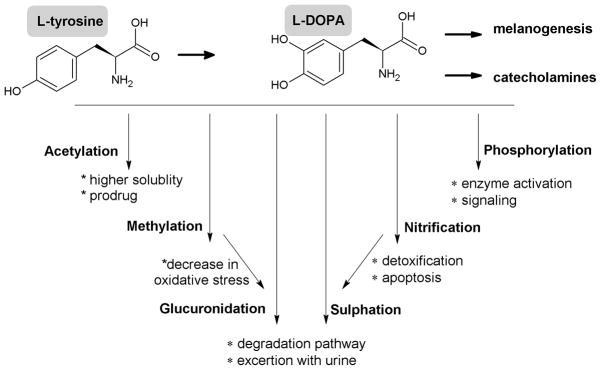
Since L-tyrosine and L-DOPA depending on genetic background, environmental factors and experimental models can act as an inducers/stimulators or modifiers of melanogenic apparatus or, only as a substrates for already existing constitutive melanogenic apparatus, it remains to be determined whether they can be modified in vivo and whether their derivatives are biologically active. Modification of tyrosine residue in protein by methylation, phosphorylation, sulphation or nitrification has been well studied and plays crucial role in protein activity but there is a shortage of information on regulation of free L-tyrosine and L-DOPA activity, although recent findings encourage further investigations on this subject (Liu et al., 2007; Rabbani and Thornalley, 2008; Yasuda et al., 2011). Current concepts concerning modifications of these free amino acids are summarized in Figure 3.
Figure 3. Potential modifications of L-tyrosine and L-DOPA and it oxidation products with predicted physiological significance.
O-methylation of L-DOPA and of other melanin precursors such as 5,6-dihydroxyindol or 5,6-dithydroxyindol-2-caboxylic acid by COMT plays a regulatory role in melanocyte activity by attenuating oxidative stress through removal of melanogenesis byproducts (Smit et al., 1994; Smit and Pavel, 1995). Similar mechanisms operate in Parkinson’s patients subjected to L-DOPA treatment (Muller, 2010)
Although phosphorylation of tyrosine residues plays important role in regulation of proteins activity, it has to be investigated whether phosphorylation of free tyrosine plays a significant role in physiology. However, study by Munoz and coworkers demonstrated that free phosphotyrosine induced platelet aggregation (Munoz et al., 1992). Importantly, phospho-L-tyrosine inhibited cellular growth in a number of non-melanocytic lines via inhibition of epidermal growth factor receptor tyrosine kinase and stimulation of protein tyrosine phosphatases (Mishra and Hamburger, 1993a; Mishra and Hamburger, 1993b). It also inhibited the insulin triggered insulin receptor tyrosine phosphorylation in the HEPG2 cell line and the tyrosine phosphorylation of a variety of cellular proteins in src-transformed NIH3T3 cells (Mishra and Hamburger, 1996). These findings support the concept that free phosphotyrosine or phosphodopa may play a role in regulation of melanocytes behavior (Pawelek et al., 1988; Slominski, 1991; Winder and Harris, 1992). Importantly, one should also consider P-DOPA as an attractive alternative for current therapy of Parkinson disease.
Presence of free 3-nitro-L-tyrosine as well as nitrification of L-tyrosine residue in proteins is a marker of oxidative/nitrosative stress (Rabbani and Thornalley, 2008). Thus, nitrification of free L-tyrosine (and probably L-DOPA), especially in melanin producing cells, could be a marker and a first line of defense against oxidative/nitrosative stress (Liu et al., 2007). On the other hand free 3-nitro-L-tyrosine is known to cause potential damage to cells including motor neuron apoptosis (Peluffo et al., 2004). It is possible that depending on condition, nitrification of L-tyrosine, and probably other byproducts of melanogenesis, may serve both as a protective mechanism for the cell survival and inducer of apoptosis. For example, such mechanism could play a role in neurodegenerative diseases (Beal, 2002; Pacher et al., 2007) including Parkinson disease (Tsang and Chung, 2009) or in development of vitiligo (Schallreuter et al., 2011). Importantly, L-DOPA stimulates nitric oxide synthetase (Pacher et al., 2007), and nitrate levels can serve as markers of nitrosative stress in vitiligo (Hazneci et al., 2005).
Sulfatation and glucuronidation of xenobiotics can increase their water solubility facilitating their excretion from the body. Both L-tyrosine and L-DOPA can be enzymatically sulfated (Liu et al., 2007; Suiko et al., 1996; Taskinen et al., 2003; Yasuda et al., 2011). Therefore, it is possible that sulfatation of L-DOPA and L-tyrosine may play a role in detoxification under oxidant stress related to melanogenesis as it has been shown in inflammatory responses (Liu et al., 2007; Yasuda et al., 2011). This is further supported by identification of ester glucuronide and sulfate conjugates of 5-hydroxy-6-methoxyindole-2-carboxylic acid and 6-hydroxy-5-methoxyindole-2-carboxylic acid in urine of melanoma patients (Wakamatsu and Ito, 1990) and rapid metabolism of L-DOPA to dopamine-4-O-glucuronide and 3-methoxytyramine-4-O-glucuronide in transplantable islet cell tumour of the golden hamster (Falck et al., 1977). Thus, modification of free L-tyrosine and L-DOPA or products of L-DOPA metabolism could modulate their functions with attendant pleiotropic phenotypic effects as summarized in Figure 3.
4. L-tyrosine and L-DOPA stimulate MSH receptor activities
L-tyrosine and P-DOPA both acted as positive regulators of the MSH receptor activity (McLane et al., 1987; Pawelek et al., 1988; Slominski et al., 1989a). Specifically, P-DOPA stimulated MSH receptors expression on S91 melanoma cells and amplified melanogenesis induced by MSH (McLane et al., 1987). In Bomirski hamster amelanotic melanoma cells, L-tyrosine stimulated MSH receptor expression with concomitant amplification of tyrosinase activity stimulated by MSH and reduced positive cooperatively among MSH receptors with a specificity indicated by lack of effect on unrelated insulin receptor (Slominski et al., 1989a). These findings have been confirmed in normal amphibian melanoblasts, where L-tyrosine acted synergistically with MSH in stimulation of melanogenesis (Fukuzawa and Bagnara, 1989), and in normal human epidermal melanocytes where increased tyrosine levels regulated the melanogenic response to α-MSH (Schwahn et al., 2001), as well as in human melanomas where L-tyrosine and L-DOPA stimulated MSH biding to cell surface in a dose restricted manner (Ghanem et al., 1989).
In mouse and hamster and melanoma cells stimulation of MSH receptors required prolonged exposure to P-DOPA or L-tyrosine (days) (McLane et al., 1987; Pawelek et al., 1988; Slominski et al., 1989a), with maximal biding capacity (in case of L-tyrosine) when full melanogenic potential was induced (Slominski et al., 1989a). Interestingly, in human melanomas increased MSH binding was seen within hours after L-tyrosine or L-DOPA exposure (Ghanem et al., 1989). In other melanoma models stimulation of melanogenesis increases expression of MSH receptors (Slominski et al., 1999). In hamster melanomas stimulation of melanogenesis did not change MC1-R mRNA concentrations (Slominski unpublished), however in human melanoma increased melanogenesis was accompanied by an increased MC1-R and tyrosinase mRNA concentrations (Slominski et al., 1999).
5. Mechanism of action for L-tyrosine and L-DOPA
Historical overview
These complex phenotypic effects of L-tyrosine and L-DOPA (including P-DOPA) in the pigmentary system would require diverse regulatory mechanism in which both of these compounds would act directly or indirectly to induce and further maintain or regulate the melanogenic system. For example, direct regulatory mechanism should involve interactions with regulatory proteins that specifically bind L-tyrosine or L-DOPA, including receptors for those amino acids of which existence in mammalian system was for the first time proposed in (Slominski and Paus, 1990; Slominski and Paus, 1994). The indirect mechanism would involve an action through intermediates of melanogenesis with L-DOPA acting as the initial and major “second messenger” and tyrosinase serving as a regulatory protein controlling production and inactivation of these non-standard second messengers, a concept originally proposed in (Slominski et al., 1989b). In this scenario melanocytes would regulate local and global homeostasis through control of L-tyrosine levels, production of L-DOPA (Slominski and Paus, 1990), and final formation of melanosomes that would serve as “regulatory packages/organelle second messengers” affecting not only the status of the melanocyte but also of the recipient cells to which they were transferred (Slominski et al., 1993). Remarkably, the premature age-related and noise-induced hearing loss or visual dysfunctions in albino mice can be corrected by L-DOPA either generated through the action of tyrosinase or tyrosine hydroxylase (Lavado and Montoliu, 2006; Murillo-Cuesta et al., 2010), supporting an original hypothesis on bioregulatory functions of L-DOPA and tyrosinase (Slominski et al., 1989b; Slominski and Paus, 1990). Below we discuss different mechanisms of action in different experimental models.
Role of tyrosinase
Tyrosinase and melanogenesis related proteins (MRP) are the classical proteins binding L-tyrosine and L-DOPA (Park et al., 2009; Schallreuter et al., 2008). Halaban et al. (Halaban et al., 2002a; Halaban et al., 2001), based on her observations in normal and malignant human melanocytes, has proposed that L-tyrosine and L-DOPA are necessary for the proper folding of tyrosinase in endoplasmic reticulum (ER), protecting it from entering the degradation pathway. In this model these amino acids enhanced tyrosinase exit from the ER, its carbohydrate modifications in the Golgi apparatus, and its transport into melanosomes with resulting increase in melanin pigmentation.
These findings confirmed earlier observations on Bomirski hamster amelanotic melanoma lines showing that L-tyrosine induced translocation of tyrosinase from TGN to melanosomes was a prerequisite for initiating the melanogenic pathway (Slominski et al., 1989c; Slominski et al., 1989b; Slominski et al., 1988), and stimulation of tyrosinase maturation in cells cultured in media containing high tyrosine levels. Importantly, stimulation of tyrosinase by MSH and factors raising intracellular cAMP levels that was accompanied by an increased formation of premelanosomes stage II failed to induce melanin production and tyrosinase accumulated in the cisternae, tubules and vesicles of the TGN (Slominski et al., 1989c), indicating the absence of an intrinsic recognition message signaling fusion between tyrosinase containing vesicles and premelanosomes. The crucial role of L-tyrosine in induction of melanogenic apparatus was also confirmed by observations that: 1) L-DOPA, while being more potent in stimulation of tyrosinase activity and protein accumulation was less efficient in induction of premelanosomes synthesis/assembly leading to relatively lower melanin production; 2) induction of melanin production was preceded by formation of premelanosomes and melanosomes; 3) L-tyrosine induced translocation of lysosomal enzymes from TGN to granular melanosomes 4) L-tyrosine by itself induced formation of premelanosomes; 5) L-tyrosine stimulated MSH receptors expression and activity (reviewed in (Slominski and Paus, 1994; Slominski et al., 2004)). Because of these pleiotropic effects we proposed that L-tyrosine must have wider regulatory effects on the melanogenic system than a simple protection of the enzyme from degradation. In models in which premelanosomes are already formed (Halaban et al., 2001; Potterf et al., 1998; Rosemblat et al., 1998) binding of L-tyrosine to tyrosinase, allowing its proper folding and protection from degradation, could induce chain of events affecting melanogenic activity. Here, because of the presence of cysteine rich domain tyrosinase could participate in protein-protein interactions (Slominski and Paus, 1994; Slominski and Paus, 1990; Slominski et al., 2004), a theory substantiated by formation of multimeric complexes with other MRP (Orlow et al., 1994). Thus, tyrosinase after binding L-tyrosine could form regulatory complexes with other proteins initiating an intracellular transport, processing and posttranslational modifications with further stimulation of organellogenesis, organelle maturation and melanin synthesis. In this context tyrosinase, a transmembrane protein, would act as a non-classical membrane bound receptor that after binding of the ligand (L-tyrosine) could initiate complex intracellular processes, as initially proposed (Slominski and Paus, 1990; Slominski and Paus, 1994) and discussed further (Slominski et al., 2004). In this context tyrosinase and other MRP, could also act as “receptors” for DOPA by initiating protein-protein interaction, leading to modified phenotypic responses, consistent with observation that both amino acids act through the overlapping but partially distinct mechanisms (Slominski et al., 1988). In this context, future studies on a possible role of alternatively spliced isoforms of tyrosinase or other MRPs in the regulation of a melanocyte behavior are warranted (Slominski and Paus, 1994; Slominski et al., 2004). In other systems (corticotropin releasing hormone receptors) it is already well documented that generation of alternative receptors forms can significantly affect interaction of the cell with its environment (Slominski et al., 2004).
One area that deserves further studies is the hypothesis on the bioregulatory role of tyrosinase based on its enzymatic activity that results in both generation and inactivation of biologically active L-DOPA and other intermediates of melanogenesis (Slominski et al., 1989b; Slominski and Paus, 1990) as well as it affects cellular metabolism through intermediates of melanin synthesis, melanogenesis induced changes in oxidative potential of the cell or intracellular oxygen consumption (reviewed in (Slominski et al., 2004), and see metabolic non-receptor mediated mechanism below). Strong support for this hypothesis has recently been provided by Schallreuter group showing that hair follicle pigmentation can be affected by changes in H2O2-redox homeostasis via tyrosinase, its substrate supply and signal transduction (Schallreuter et al., 2011). The general implications of the above model at the tissue and systemic levels cannot be underestimated taking into consideration that active melanogenesis can affect functions of other cell types, skin barrier and adnexal activity and immune function (discussed in (Gasparini et al., 2009; Slominski et al., 1993; Slominski et al., 2004).
Finally, general importance of tyrosinase aside of its role in melanogenesis is supported by findings that retinal network adaptation to bright light requires tyrosinase (Page-McCaw et al., 2004) and that it may provide TH-independent major pathway of peripheral dopamine synthesis in young, but not adult, mice (Eisenhofer et al., 2003).
Receptors for L-tyrosine and L-DOPA
The described hormone-like actions of L-tyrosine and L-DOPA in the pigmentary system strongly support a notion for the existence of specific receptors for these melanin precursors (see (Slominski and Paus, 1990; Slominski and Paus, 1994; Slominski et al., 2004). Indeed, OA1 was recently identified as the receptor for L-DOPA in retinal pigment epithelium (RPE) (Lopez et al., 2008) further substantiating previous work on defining receptors for this endogenously produced molecule (Slominski and Paus, 1994) and showing striking similarity to previously described properties of L-DOPA (Slominski and Pruski, 1992).
Such role was consistent with dose and time dependent L-tyrosine and L-DOPA actions with high degree of specificity, their stereo-selectivity for L-forms (Slominski and Costantino, 1991b; Slominski et al., 1988), presence of cell surface (L-tyrosine and L-DOPA) and nuclear (L-DOPA) binding sites, with binding being saturable, specific and reversible (Slominski, 1991; Slominski and Pruski, 1992). Crosslinking experiments of cells cultured in low in tyrosine media also identified cell surface proteins binding specifically L-tyrosine with molecular weight MW of approximately (+/−2 kD) of 55, 48, 45, 40, 30 and less than 20 kDa (those could be purified through tyrosine affinity chromatography) (Slominski, 1991) and L-DOPA with MW around of 55, 30, 25 and less than 20 kDa (Slominski and Pruski, 1992); these proteins were different from adrenergic or dopaminergic receptors or amino acids carriers (Slominski, 1991; Slominski and Pruski, 1992). Some of the crosslinked membrane bound proteins listed above (55, 48, 45 −/+ 2 kD) may indeed represented OA1, since the OA1 detected in melanocytes and melanoma cells presented as broad bands around 60 and 48 kD for glycosylated form and a band of 45–44 kD for unglycosylated form (Samaraweera et al., 2001; Schiaffino et al., 1996).In this context, they could represent the predicted membrane bound receptor(s) for L-DOPA and L-tyrosine as either non-radioactive L-DOPA or L-tyrosine competed mutually crosslinking of either of radiolabelled amino acid to cell surface proteins (Slominski, 1991; Slominski and Pruski, 1992). The requirement of culturing cells in low in tyrosine media for OA1 expression on cell surface (Lopez et al., 2008) further support the above hypothesis. It is interesting that L-tyrosine apparently induced redistribution of OA1 to intracellular compartments with no measurable second messenger activity, and L-DOPA induced PEDF production (Lopez et al., 2008). This further support the original hypothesis of L-DOPA and L-tyrosine acting through overlapping but distinct mechanism (Pawelek et al., 1988; Slominski et al., 1988) and L-DOPA acting as an endogenous ligand produced by tyrosinase regulating the melanogenic apparatus (Slominski et al., 1989b; Slominski and Pruski, 1992). Thus, it is possible that regulation of melanocytic functions by L-tyrosine and L-DOPA would also involve interaction with OA1 with L-DOPA acting as a high and L-tyrosine as a low affinity ligands inducing overlapping but distinct mechanisms (Slominski et al., 1988) in a process that is not coupled to the direct changes in production of either cAMP, cGMP or IP3 (Slominski et al., 1989c). One of the identified problems for future research is to clarify whether regulation of MSH receptor expression and activity by L-tyrosine, L-DOPA and P-DOPA (McLane et al., 1987; Pawelek et al., 1988; Slominski et al., 1989a) involves their interaction with OA1.
There is a strong evidence that L-DOPA itself acts as a neurotransmitter indicating a presence of membrane bound L-DOPA receptors in neuronal cells (Misu and Goshima, 2006; Misu et al., 1996; Misu et al., 2003). Accordingly, it remains to be tested whether there is a structural and functional overlap between such DOPA receptor and OA1. The receptor mediated functions of L-DOPA are further supported by L-tyrosine and nicotine induced synthesis of L-DOPA by human lymphocytes (Musso et al., 1997) and by reversible influx and efflux of L-DOPA in human epidermal Langerhans cells without its metabolism (Falck et al., 2004). There are also indications in mammalian systems that L-tyrosine and may act as a neurotransmitter (reviewed in (Slominski and Paus, 1990; Slominski and Paus, 1994). Thus further effort to define L-tyrosine and L-DOPA receptors in neural crest derived cells appears to be mandatory. Of broad interest should also be indications for existence of receptors for L-tyrosine and L-DOPA in lower organisms (uni- or multicellular) (reviewed in (Slominski and Paus, 1990),
Identification of nuclear binding sites for L-DOPA but not L-tyrosine in conjunction to its phenotypic activity described above suggests an existence of an additional nuclear receptor for this relatively short-lived molecule, allowing coordination of intracellular functions in an intracrine fashion but with a high level of specificity (Slominski and Paus, 1990; Slominski and Paus, 1994; Slominski and Pruski, 1992). This justifies further effort to test whether L-DOPA or its derivatives are interacting with already characterized receptor (s) from the nuclear receptors (NR) family or its isoform, with orphan NR for which the ligand has to be identified.
Metabolic non-receptor mediated mechanisms
There are several experimental premises indicating non-receptor mechanisms triggered by L-DOPA and/or products of its metabolism. For example, L-DOPA dramatically inhibited an in vitro phosphorylation of glycoproteins purified from melanoma cells in a dose dependent manner, having no detectable effect on whole protein extracts (Slominski and Friedrich, 1992). The inhibitory effect was dependent on the presence of Mn+2 in the incubation mixture with significant inhibition seen only in glycoproteins purified from melanotic cells (Slominski and Friedrich, 1992). Since Mn+2 can readily stimulate L-DOPA oxidation in a dose dependent manner it is likely that the above effects are due to quinones production. Accordingly, in other model it has been found that L-DOPA and other catechols induce antioxidant response elements (ARE)-driven luciferase in a Cu2+-dependent manner indicating that their oxidation to quinones represents the rate limiting step in the activation of transcription factor NF-E2 p45-related factor 2 (Nrf2) to mediate cellular adaptation to oxidants and electrophiles (Wang et al., 2010). However, not all effects of L-DOPA on phosphorylation cascade could be explained by this mechanism, since in the absence of Mn+2 in the reaction mixture, 5 μM L-DOPA stimulated phosphorylation of glycoproteins with approximate MW of 118 and 68 kD (amelanotic cells) and of 150 and 50 kD (melanotic cells) (Slominski and Friedrich, 1992), a phenomenon requiring further studies.
Similarly, L-DOPA effects on cellular metabolism (Scislowski et al., 1984; Scislowski et al., 1985) are most likely mediated through intermediates of melanogenesis and its final product melanin (Slominski and Paus, 1994; Slominski et al., 2004). For example, L-DOPA induced switch of the energy metabolism from aerobic to anaerobic glycolysis was attenuated by L-phenylalanine (Scislowski et al., 1984). Also L-DOPA stimulation of the pentose phosphate pathway in isolated melanotic cells was consistent with generation of NADPH by isocitrate dehydrogenase and glucose-6-phosphate dehydrogenase stimulated the hydroxylation of L-tyrosine in the soluble fraction of melanomas (Scislowski et al., 1985). Thus, L-DOPA initiated/induced metabolic activity of melanosomes can regulate the catabolism of glucose and, in turn, the metabolic state of melanosomes can be influenced by the pathway of glucose metabolism (Scislowski et al., 1985; Slominski et al., 2004). These hypotheses have been further substantiated by observation of dramatic changes in cellular metabolism in hamster and human malignant melanocytes in which melanogenesis was stimulated/induced by riche in L-tyrosine culture medium (Li et al., 2009). Additional studies have shown that L-DOPA can reduce mitochondrial respiration in fibroblasts (Werner et al., 1994), can inhibit complex IV of the electron transport chain in human neuroblastoma (Pardo et al., 1995) and inhibit gluconeogenesis in rabbit kidney-cortex tubules (Drozak et al., 2005).
Furthermore, L-DOPA through oxidation products and active melanogenesis can affect functions of other cells of which the best documented is immunosuppressive activity (Slominski and Goodman-Snitkoff, 1992; Slominski et al., 2009); possible mechanisms were discussed previously (Slominski et al., 2004). Recent data also indicates that melanogenic activity inhibits vitamin D receptor expression in melanomas (Brozyna et al., 2011). Finally, a role for both free and protein-DOPA in cellular antioxidant defenses was suggested (Nelson et al., 2010), and inhibition of lipoxygenase-mediated arachidonic acid oxygenation by DHI and to lower degree by DHICA was demonstrated (Napolitano et al., 1993). Also, diffusible melanin-related metabolites are potent inhibitors of lipid peroxidation (Memoli et al., 1997), and 5-S-cysteinyldopa inhibits hydroxylation/oxidation reactions induced by the Fenton system (Napolitano et al., 1996). The potential cycling from indole to quinone form of L-DOPA and its derivatives may have direct effects on deactivation of reactive oxygen/nitrogen species or oxidation of intracellular proteins and lipids most probably in concentration depending manner.(Tsang and Chung, 2009). Finally, free radicals preferentially hydroxylate protein-bound tyrosine and its level can raise 5–10-fold during oxidative damage in vivo. Both free and protein-bound L-DOPA can trigger expression of several antioxidant enzymes including superoxide dismutase 1 (SOD) or NAD(P)H:Quinone oxidoreductase (NQO1) as part of ROS response (Nelson et al., 2007).
6. Concluding remarks
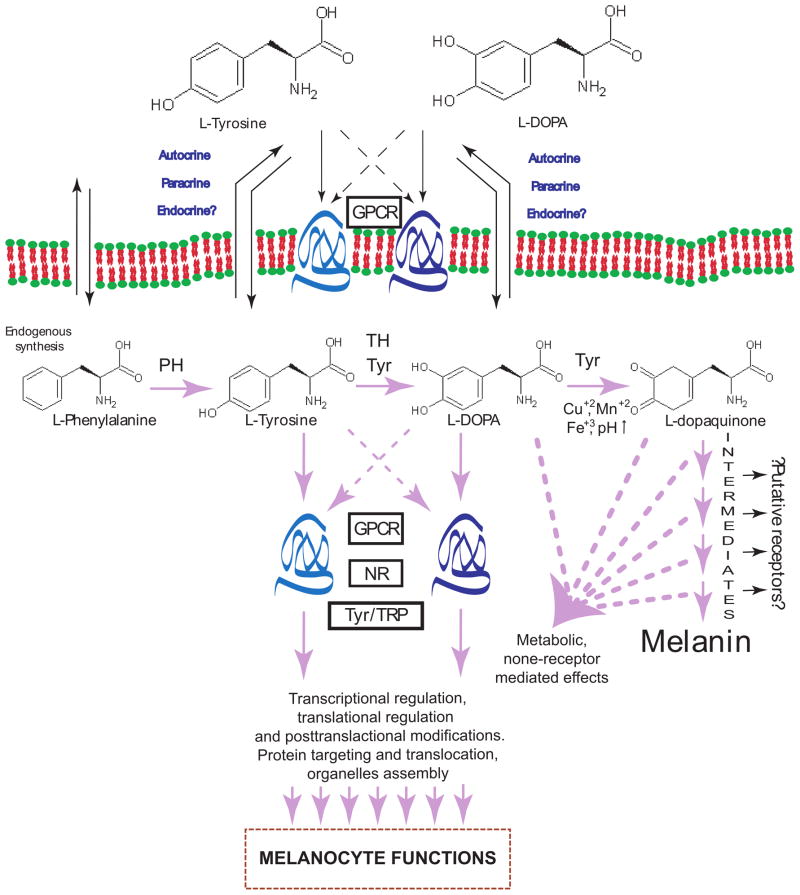
A cumulative evidence was provided that L-tyrosine and L-DOPA, besides serving as substrates and intermediates of melanogenesis, also act as inducers and positive regulators of melanogenic pathway as well as regulators of other cellular functions directly or indirectly through specific receptors or non-receptor mediated processes (Fig. 4).
Figure 4. Proposed mechanism of L-tyrosine and L-DOPA regulation of the melanocyte phenotype.
GPCR: membrane bound receptors including G-protein coupled receptor; NR: nuclear receptors; Tyr: tyrosinase; TRP: tyrosinase related proteins; PH: phenylalanine hydroxylase, TH: tyrosine hydroxylase,. Solid lines shows well established or most probable pathways, dashed line shows hypothetic interactions.
The major bullet points conclusions are listed below.
It is now well-substantiated that L-tyrosine is a modifier of melanogenesis and melanocytic phenotype, and the nature and magnitude of effects are dependent on the genetic background of the organism and environmental factors, and can vary depending on the experimental model used
Like L-tyrosine, L-DOPA or its more stable phosphoesters can regulate melanocyte function through overlapping but yet distinct mechanisms.
There is considerable evidence for an existence of L-tyrosine and L-DOPA receptors but their exact nature has yet to be determined.
Tyrosinase can both act as a regulatory protein interacting with other proteins or act as a regulator of intra-and extra-cellular concentrations of biologically active molecules (L-tyrosine and L-DOPA).
L-DOPA can also regulate cell functions and cellular metabolism through non-receptor mediated processes acting directly or through intermediates of melanogenesis generated by its non-enzymatic or enzymatic oxidation.
In the above model the substrate induced (L-tyrosine and/or L-DOPA) melanogenic pathway would autoregulate itself as well as it would regulate the melanocyte functions through activity of its structural or regulatory proteins and through intermediates of melanogenesis and melanin itself. Dissection of different regulatory and autoregulatory elements of this process may elucidate how similar processes (substrate induced autoregulatory pathways) have evolved from prokaryotic or simple eukaryotic organisms to complex systems in vertebrates. In some of them, nutritional regulation may have been conserved, while in others, hormonal regulation, have gained high level of complexity. In this context, it is worthy to revisit the two theories discussed previously of which one postulated that receptors for amino-acid derived hormones arose from the receptors for those amino acids, and the second postulating that nuclear receptors evolved from primitive intracellular receptors binding nutritional factors or metabolic intermediates(reviewed and discussed in (Slominski and Paus, 1990)). Thus, further studies on L-tyrosine and L-DOPA role in regulation of melanocyte functions will represent extremely fertile ground for investigation on a revised view on melanogenic pathway, its regulation and its role in the cellular and tissue ecosystem with possible systemic implications (Ducrest et al., 2008; Panzella et al., 2011; Roulin and Ducrest, 2011; Slominski et al., 2004).
Acknowledgments
We acknowledge partial NIH support (Grants R01-AR052190 and 1R01AR056666-01A2) during writing of this review.
References
- Agin PP, Sayre RM, Pawelek JM. Phosphorylated mixed isomers of L-dopa increase melanin content in skins of Skh-2 pigmented hairless mice. Pigment Cell Research. 1987;1:137–42. doi: 10.1111/j.1600-0749.1987.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Beal MF. Oxidatively modified proteins in aging and disease. Free radical biology & medicine. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- Blau N, Van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–27. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- Box NF, Wyeth JR, Mayne CJ, O’gorman LE, Martin NG, Sturm RA. Complete sequence and polymorphism study of the human TYRP1 gene encoding tyrosinase-related protein 1. Mamm Genome. 1998;9:50–3. doi: 10.1007/s003359900678. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Castiglione CM, Halaban R, Pawelek J, Shiman R. Phenylalanine hydroxylase in melanoma cells. J Cell Physiol. 1978;94:307–14. doi: 10.1002/jcp.1040940308. [DOI] [PubMed] [Google Scholar]
- Brozyna AA, Jozwicki W, Janjetovic Z, Slominski AT. Expression of vitamin D receptor decreases during progression of pigmented skin lesions. Hum Pathol. 2011;42:618–31. doi: 10.1016/j.humpath.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozak J, Doroszewska R, Chodnicka K, Winiarska K, Bryla J. Contribution of L-3,4-dihydroxyphenylalanine metabolism to the inhibition of gluconeogenesis in rabbit kidney-cortex tubules. Int J Biochem Cell Biol. 2005;37:1269–80. doi: 10.1016/j.biocel.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Ducrest AL, Keller L, Roulin A. Pleiotropy in the melanocortin system, coloration and behavioural syndromes. Trends Ecol Evol. 2008;23:502–10. doi: 10.1016/j.tree.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Tian H, Holmes C, Matsunaga J, Roffler-Tarlov S, Hearing VJ. Tyrosinase: a developmentally specific major determinant of peripheral dopamine. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17:1248–55. doi: 10.1096/fj.02-0736com. [DOI] [PubMed] [Google Scholar]
- Falck B, Bendsoe N, Ronquist G. Mediated exodus of L-dopa from human epidermal Langerhans cells. Amino Acids. 2004;26:133–8. doi: 10.1007/s00726-003-0052-6. [DOI] [PubMed] [Google Scholar]
- Falck B, Hansson C, Kennedy BM, Rosengren E. Conjugated catechol derivatives in a transplantable islet cell tumour of the golden hamster. Acta physiologica Scandinavica. 1977;99:217–24. doi: 10.1111/j.1748-1716.1977.tb10372.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa T, Bagnara JT. Control of melanoblast differentiation in amphibia by alpha-melanocyte stimulating hormone, a serum melanization factor, and a melanization inhibiting factor. Pigment Cell Research. 1989;2:171–81. doi: 10.1111/j.1600-0749.1989.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Fukuzawa T, Ide H. A ventrally localized inhibitor of melanization in Xenopus laevis skin. Dev Biol. 1988;129:25–36. doi: 10.1016/0012-1606(88)90158-3. [DOI] [PubMed] [Google Scholar]
- Gasparini J, Bize P, Piault R, Wakamatsu K, Blount JD, Ducrest AL, Roulin A. Strength and cost of an induced immune response are associated with a heritable melanin-based colour trait in female tawny owls. J Anim Ecol. 2009;78:608–16. doi: 10.1111/j.1365-2656.2008.01521.x. [DOI] [PubMed] [Google Scholar]
- Ghanem G, Verstegen J, De Rijcke S, Hanson P, Van Onderbergen A, Libert A, Del Marmol V, Arnould R, Vercammen-Grandjean A, Lejeune F. Studies on factors influencing human plasma alpha-MSH. Anticancer Res. 1989;9:1691–6. [PubMed] [Google Scholar]
- Halaban R, Cheng E, Hebert DN. Coexpression of wild-type tyrosinase enhances maturation of temperature-sensitive tyrosinase mutants. J Invest Dermatol. 2002a;119:481–8. doi: 10.1046/j.1523-1747.2002.01824.x. [DOI] [PubMed] [Google Scholar]
- Halaban R, Cheng E, Svedine S, Aron R, Hebert DN. Proper folding and endoplasmic reticulum to golgi transport of tyrosinase are induced by its substrates, DOPA and tyrosine. J Biol Chem. 2001;276:11933–8. doi: 10.1074/jbc.M008703200. [DOI] [PubMed] [Google Scholar]
- Halaban R, Patton RS, Cheng E, Svedine S, Trombetta ES, Wahl ML, Ariyan S, Hebert DN. Abnormal acidification of melanoma cells induces tyrosinase retention in the early secretory pathway. J Biol Chem. 2002b;277:14821–8. doi: 10.1074/jbc.M111497200. [DOI] [PubMed] [Google Scholar]
- Hazneci E, Karabulut AB, Ozturk C, Batcioglu K, Dogan G, Karaca S, Esrefoglu M. A comparative study of superoxide dismutase, catalase, and glutathione peroxidase activities and nitrate levels in vitiligo patients. International journal of dermatology. 2005;44:636–40. doi: 10.1111/j.1365-4632.2004.02027.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4:24–8. doi: 10.1038/sj.jidsp.5640176. [DOI] [PubMed] [Google Scholar]
- Hirobe T, Abe H, Wakamatsu K, Ito S, Kawa Y, Soma Y, Mizoguchi M. Excess tyrosine rescues the reduced activity of proliferation and differentiation of cultured recessive yellow melanocytes derived from neonatal mouse epidermis. Eur J Cell Biol. 2007a;86:315–30. doi: 10.1016/j.ejcb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hirobe T, Wakamatsu K, Ito S. Changes in the proliferation and differentiation of neonatal mouse pink-eyed dilution melanocytes in the presence of excess tyrosine. Pigment Cell Research. 2003;16:619–28. doi: 10.1046/j.1600-0749.2003.00091.x. [DOI] [PubMed] [Google Scholar]
- Hirobe T, Wakamatsu K, Ito S. Excess tyrosine stimulates eumelanin and pheomelanin synthesis in cultured slaty melanocytes from neonatal mouse epidermis. Zoolog Sci. 2007b;24:209–17. doi: 10.2108/zsj.24.209. [DOI] [PubMed] [Google Scholar]
- Hirobe T, Wakamatsu K, Ito S, Abe H, Kawa Y, Mizoguchi M. Stimulation of the proliferation and differentiation of mouse pink-eyed dilution epidermal melanocytes by excess tyrosine in serum-free primary culture. J Cell Physiol. 2002;191:162–72. doi: 10.1002/jcp.10085. [DOI] [PubMed] [Google Scholar]
- Howe J, Costantino R, Slominski A. On the putative mechanism of induction and regulation of melanogenesis by L-tyrosine. Acta Derm Venereol. 1991;71:150–2. [PubMed] [Google Scholar]
- Ito S. The IFPCS presidential lecture: a chemist’s view of melanogenesis. Pigment Cell Research. 2003;16:230–6. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- Landstrom U, Lovtrup S. Tyrosine and the Synthesis of Melanin in Embryonic Cells from Ambystoma mexicanum. Roux’s Archives of Developmental Biology. 1978;185:201–207. doi: 10.1007/BF00848351. [DOI] [PubMed] [Google Scholar]
- Lavado A, Montoliu L. New animal models to study the role of tyrosinase in normal retinal development. Front Biosci. 2006;11:743–52. doi: 10.2741/1832. [DOI] [PubMed] [Google Scholar]
- Li W, Slominski R, Slominski AT. High-resolution magic angle spinning nuclear magnetic resonance analysis of metabolic changes in melanoma cells after induction of melanogenesis. Anal Biochem. 2009;386:282–4. doi: 10.1016/j.ab.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MC, Yasuda S, Idell S. Sulfation of nitrotyrosine: biochemistry and functional implications. IUBMB life. 2007;59:622–7. doi: 10.1080/15216540701589320. [DOI] [PubMed] [Google Scholar]
- Lopez VM, Decatur CL, Stamer WD, Lynch RM, Mckay BS. L-DOPA is an endogenous ligand for OA1. PLoS Biol. 2008;6:e236. doi: 10.1371/journal.pbio.0060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovtrup, Rehnholm A, Perris R. Induction of the synthesis of melanin and pteridinein celss isolated from the otolotl embryo. Development, Growth & Differentiation. 1984;26:445–450. doi: 10.1111/j.1440-169X.1984.00445.x. [DOI] [PubMed] [Google Scholar]
- Manga P, Boissy RE, Pifko-Hirst S, Zhou BK, Orlow SJ. Mislocalization of melanosomal proteins in melanocytes from mice with oculocutaneous albinism type 2. Exp Eye Res. 2001;72:695–710. doi: 10.1006/exer.2001.1006. [DOI] [PubMed] [Google Scholar]
- Mclane J, Osber M, Pawelek JM. Phosphorylated isomers of L-dopa stimulate MSH binding capacity and responsiveness to MSH in cultured melanoma cells. Biochem Biophys Res Commun. 1987;145:719–25. doi: 10.1016/0006-291x(87)91024-2. [DOI] [PubMed] [Google Scholar]
- Memoli S, Napolitano A, D’ischia M, Misuraca G, Palumbo A, Prota G. Diffusible melanin-related metabolites are potent inhibitors of lipid peroxidation. Biochimica et biophysica acta. 1997;1346:61–8. doi: 10.1016/s0005-2760(97)00018-0. [DOI] [PubMed] [Google Scholar]
- Mishra S, Hamburger AW. Exogenous phosphotyrosine modulates epidermal growth factor receptor tyrosine phosphorylation. Carcinogenesis. 1993a;14:269–73. doi: 10.1093/carcin/14.2.269. [DOI] [PubMed] [Google Scholar]
- Mishra S, Hamburger AW. O-phospho-L-tyrosine inhibits cellular growth by activating protein tyrosine phosphatases. Cancer Research. 1993b;53:557–63. [PubMed] [Google Scholar]
- Mishra S, Hamburger AW. Association of inhibition of cell growth by O-phospho-L-tyrosine with decreased tyrosine phosphorylation. Cancer Lett. 1996;102:65–71. doi: 10.1016/0304-3835(96)04164-x. [DOI] [PubMed] [Google Scholar]
- Misu Y, Goshima Y. Nerobiology of DOPA as a Neurotransmitter. Boca Raton: CRC Press/Taylor & Francis Group; 2006. [Google Scholar]
- Misu Y, Goshima Y, Ueda H, Okamura H. Neurobiology of L-DOPAergic systems. Prog Neurobiol. 1996;49:415–54. doi: 10.1016/0301-0082(96)00025-1. [DOI] [PubMed] [Google Scholar]
- Misu Y, Kitahama K, Goshima Y. L-3,4-Dihydroxyphenylalanine as a neurotransmitter candidate in the central nervous system. Pharmacol Ther. 2003;97:117–37. doi: 10.1016/s0163-7258(02)00325-x. [DOI] [PubMed] [Google Scholar]
- Muller T. Catechol-O-methyltransferase enzyme: cofactor S-adenosyl-L-methionine and related mechanisms. International review of neurobiology. 2010;95:49–71. doi: 10.1016/B978-0-12-381326-8.00004-1. [DOI] [PubMed] [Google Scholar]
- Munoz GE, Arenas-Diaz G, Marshall SH. Exogenously added free phosphotyrosine induces aggregation of circulating platelets in rabbits. Cellular and molecular biology (Noisy-le-Grand, France) 1992;38:719–22. [PubMed] [Google Scholar]
- Murillo-Cuesta S, Contreras J, Zurita E, Cediel R, Cantero M, Varela-Nieto I, Montoliu L. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment cell & melanoma research. 2010;23:72–83. doi: 10.1111/j.1755-148X.2009.00646.x. [DOI] [PubMed] [Google Scholar]
- Musso NR, Brenci S, Indiveri F, Lotti G. L-tyrosine and nicotine induce synthesis of L-Dopa and norepinephrine in human lymphocytes. J Neuroimmunol. 1997;74:117–20. doi: 10.1016/s0165-5728(96)00212-3. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Memoli S, Nappi AJ, D’ischia M, Prota G. 5-S-cysteinyldopa, a diffusible product of melanocyte activity, is an efficient inhibitor of hydroxylation/oxidation reactions induced by the Fenton system. Biochimica et biophysica acta. 1996;1291:75–82. doi: 10.1016/0304-4165(96)00047-5. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Palumbo A, Misuraca G, Prota G. Inhibitory effect of melanin precursors on arachidonic acid peroxidation. Biochimica et biophysica acta. 1993;1168:175–80. doi: 10.1016/0005-2760(93)90122-p. [DOI] [PubMed] [Google Scholar]
- Nelson M, Foxwell AR, Tyrer P, Dean RT. Protein-bound 3,4-dihydroxy-phenylanine (DOPA), a redox-active product of protein oxidation, as a trigger for antioxidant defences. Int J Biochem Cell Biol. 2007;39:879–89. doi: 10.1016/j.biocel.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Nelson M, Foxwell AR, Tyrer P, Dean RT. Radical sequestration by protein-bound 3,4-dihydroxyphenylalanine. Int J Biochem Cell Biol. 2010;42:755–61. doi: 10.1016/j.biocel.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Oetting WS, King RA. Molecular basis of albinism: mutations and polymorphisms of pigmentation genes associated with albinism. Hum Mutat. 1999;13:99–115. doi: 10.1002/(SICI)1098-1004(1999)13:2<99::AID-HUMU2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Brilliant MH. The pink-eyed dilution locus controls the biogenesis of melanosomes and levels of melanosomal proteins in the eye. Exp Eye Res. 1999;68:147–54. doi: 10.1006/exer.1998.0599. [DOI] [PubMed] [Google Scholar]
- Orlow SJ, Zhou BK, Chakraborty AK, Drucker M, Pifko-Hirst S, Pawelek JM. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994;103:196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiological reviews. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-Mccaw PS, Chung SC, Muto A, Roeser T, Staub W, Finger-Baier KC, Korenbrot JI, Baier H. Retinal network adaptation to bright light requires tyrosinase. Nat Neurosci. 2004;7:1329–36. doi: 10.1038/nn1344. [DOI] [PubMed] [Google Scholar]
- Panzella L, Wakamatsu K, Monfrecola G, Ito S, Ayala F, Napolitano A. Increased cysteinyldopa plasma levels hint to melanocyte as stress sensor in psoriasis. Experimental dermatology. 2011;20:288–90. doi: 10.1111/j.1600-0625.2010.01211.x. [DOI] [PubMed] [Google Scholar]
- Pardo B, Mena MA, De Yebenes JG. L-dopa inhibits complex IV of the electron transport chain in catecholamine-rich human neuroblastoma NB69 cells. Journal of neurochemistry. 1995;64:576–82. doi: 10.1046/j.1471-4159.1995.64020576.x. [DOI] [PubMed] [Google Scholar]
- Park HY, Kosmadaki M, Yaar M, Gilchrest BA. Cellular mechanisms regulating human melanogenesis. Cell Mol Life Sci. 2009;66:1493–506. doi: 10.1007/s00018-009-8703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek J, Bolognia J, Mclane J, Murray M, Osber M, Slominski A. A possible role for melanin precursors in regulating both pigmentation and proliferation of melanocytes. Prog Clin Biol Res. 1988;256:143–54. [PubMed] [Google Scholar]
- Pawelek JM, Murray M. Increase in melanin formation and promotion of cytotoxicity in cultured melanoma cells caused by phosphorylated isomers of L-dopa. Cancer Research. 1986;46:493–7. [PubMed] [Google Scholar]
- Pawelek JM, Osber MP. Phosphorylated derivatives of L-DOPA and composition and methods for increasing the melanin contents in mammalian skin and hair. USA: Yale University (New Haven, CT); 1992. [Google Scholar]
- Peluffo H, Shacka JJ, Ricart K, Bisig CG, Martinez-Palma L, Pritsch O, Kamaid A, Eiserich JP, Crow JP, Barbeito L, et al. Induction of motor neuron apoptosis by free 3-nitro-L-tyrosine. Journal of neurochemistry. 2004;89:602–12. doi: 10.1046/j.1471-4159.2004.02363.x. [DOI] [PubMed] [Google Scholar]
- Potterf SB, Furumura M, Sviderskaya EV, Santis C, Bennett DC, Hearing VJ. Normal tyrosine transport and abnormal tyrosinase routing in pink-eyed dilution melanocytes. Experimental Cell Research. 1998;244:319–26. doi: 10.1006/excr.1998.4173. [DOI] [PubMed] [Google Scholar]
- Prezioso JA, Wang N, Duty L, Bloomer WD, Gorelik E. Enhancement of pulmonary metastasis formation and gamma-glutamyltranspeptidase activity in B16 melanoma induced by differentiation in vitro. Clin Exp Metastasis. 1993;11:263–74. doi: 10.1007/BF00121169. [DOI] [PubMed] [Google Scholar]
- Price JE, Tarin D, Fidler IJ. Influence of organ microenvironment on pigmentation of a metastatic murine melanoma. Cancer Res. 1988;48:2258–64. [PubMed] [Google Scholar]
- Rabbani N, Thornalley PJ. Assay of 3-nitrotyrosine in tissues and body fluids by liquid chromatography with tandem mass spectrometric detection. Methods in enzymology. 2008;440:337–59. doi: 10.1016/S0076-6879(07)00822-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Bosca A, Bernd A, Theilig C, Werner R, Kippenberger S, Dold K, Holzmann H. Effect of L-dopa and L-tyrosine on the tyrosinase activity in human melanocytes and melanoma cells taken from adult skin. European Journal of Dermatology. 1992;2:179–184. [Google Scholar]
- Rosemblat S, Sviderskaya EV, Easty DJ, Wilson A, Kwon BS, Bennett DC, Orlow SJ. Melanosomal defects in melanocytes from mice lacking expression of the pink-eyed dilution gene: correction by culture in the presence of excess tyrosine. Exp Cell Res. 1998;239:344–52. doi: 10.1006/excr.1997.3901. [DOI] [PubMed] [Google Scholar]
- Roulin A, Ducrest AL. Association between melanism, physiology and behaviour: a role for the melanocortin system. Eur J Pharmacol. 2011;660:226–33. doi: 10.1016/j.ejphar.2011.01.036. [DOI] [PubMed] [Google Scholar]
- Samaraweera P, Shen B, Newton JM, Barsh GS, Orlow SJ. The mouse ocular albinism 1 gene product is an endolysosomal protein. Experimental Eye Research. 2001;72:319–29. doi: 10.1006/exer.2000.0962. [DOI] [PubMed] [Google Scholar]
- Sanz-Navares E, Fernandez N, Kazanietz MG, Rotenberg SA. Atypical protein kinase Czeta suppresses migration of mouse melanoma cells. Cell Growth Differ. 2001;12:517–24. [PubMed] [Google Scholar]
- Sato C, Ito S, Takeuchi T. Enhancement of pheomelanogenesis by L-dopa in the mouse melanocyte cell line, TM10, in vitro. Journal of Cell Science. 1987;87(Pt 4):507–12. doi: 10.1242/jcs.87.4.507. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Kothari S, Chavan B, Spencer JD. Regulation of melanogenesis--controversies and new concepts. Experimental dermatology. 2008;17:395–404. doi: 10.1111/j.1600-0625.2007.00675.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Salem MM, Hasse S, Rokos H. The redox--biochemistry of human hair pigmentation. Pigment cell & melanoma research. 2011;24:51–62. doi: 10.1111/j.1755-148X.2010.00794.x. [DOI] [PubMed] [Google Scholar]
- Schallreuter KU, Wood JM. The importance of L-phenylalanine transport and its autocrine turnover to L-tyrosine for melanogenesis in human epidermal melanocytes. Biochem Biophys Res Commun. 1999;262:423–428. doi: 10.1006/bbrc.1999.1241. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M, Ballabio A. The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci U S A. 1996;93:9055–60. doi: 10.1073/pnas.93.17.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn DJ, Xu W, Herrin AB, Bales ES, Medrano EE. Tyrosine levels regulate the melanogenic response to alpha-melanocyte-stimulating hormone in human melanocytes: implications for pigmentation and proliferation. Pigment Cell Research. 2001;14:32–9. doi: 10.1034/j.1600-0749.2001.140106.x. [DOI] [PubMed] [Google Scholar]
- Scislowski PW, Slominski A, Bomirski A. Biochemical characterization of three hamster melanoma variants--II. Glycolysis and oxygen consumption. Int J Biochem. 1984;16:327–31. doi: 10.1016/0020-711x(84)90107-1. [DOI] [PubMed] [Google Scholar]
- Scislowski PW, Slominski A, Bomirski A, Zydowo M. Metabolic characterization of three hamster melanoma variants. Neoplasma. 1985;32:593–8. [PubMed] [Google Scholar]
- Simon JD, Peles D, Wakamatsu K, Ito S. Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment cell & melanoma research. 2009;22:563–79. doi: 10.1111/j.1755-148X.2009.00610.x. [DOI] [PubMed] [Google Scholar]
- Slominski A. L-tyrosine-binding proteins on melanoma cells. In Vitro Cell Dev Biol. 1991;27A:735–8. doi: 10.1007/BF02633219. [DOI] [PubMed] [Google Scholar]
- Slominski A, Costantino R. L-tyrosine induces tyrosinase expression via a posttranscriptional mechanism. Experientia. 1991a;47:721–4. doi: 10.1007/BF01958826. [DOI] [PubMed] [Google Scholar]
- Slominski A, Costantino R. Molecular mechanism of tyrosinase regulation by L-dopa in hamster melanoma cells. Life Sci. 1991b;48:2075–9. doi: 10.1016/0024-3205(91)90164-7. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Wortsman J. Modification of melanogenesis in cultured human melanoma cells. In Vitro Cell Dev Biol Anim. 1999;35:564–5. doi: 10.1007/s11626-999-0093-6. [DOI] [PubMed] [Google Scholar]
- Slominski A, Friedrich T. L-DOPA inhibits in vitro phosphorylation of melanoma glycoproteins. Pigment Cell Res. 1992;5:396–400. doi: 10.1111/j.1600-0749.1992.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Goodman-Snitkoff GG. Dopa inhibits induced proliferative activity of murine and human lymphocytes. Anticancer Res. 1992;12:753–6. [PubMed] [Google Scholar]
- Slominski A, Jastreboff P, Pawelek J. L-tyrosine stimulates induction of tyrosinase activity by MSH and reduces cooperative interactions between MSH receptors in hamster melanoma cells. Biosci Rep. 1989a;9:579–86. doi: 10.1007/BF01119801. [DOI] [PubMed] [Google Scholar]
- Slominski A, Moellmann G, Kuklinska E. L-tyrosine, L-dopa, and tyrosinase as positive regulators of the subcellular apparatus of melanogenesis in Bomirski Ab amelanotic melanoma cells. Pigment Cell Research. 1989b;2:109–16. doi: 10.1111/j.1600-0749.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Moellmann G, Kuklinska E. MSH inhibits growth in a line of amelanotic hamster melanoma cells and induces increases in cyclic AMP levels and tyrosinase activity without inducing melanogenesis. J Cell Sci. 1989c;92(Pt 4):551–9. doi: 10.1242/jcs.92.4.551. [DOI] [PubMed] [Google Scholar]
- Slominski A, Moellmann G, Kuklinska E, Bomirski A, Pawelek J. Positive regulation of melanin pigmentation by two key substrates of the melanogenic pathway, L-tyrosine and L-dopa. J Cell Sci. 1988;89(Pt 3):287–96. doi: 10.1242/jcs.89.3.287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Are L-tyrosine and L-dopa hormone-like bioregulators. J Theor Biol. 1990;143:123–138. doi: 10.1016/s0022-5193(05)80292-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R. Towards defining receptors for L-tyrosine and L-dopa. Mol Cell Endocrinol. 1994;99:C7–11. doi: 10.1016/0303-7207(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Slominski A, Paus R, Schadendorf D. Melanocytes as “sensory” and regulatory cells in the epidermis. J Theor Biol. 1993;164:103–20. doi: 10.1006/jtbi.1993.1142. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pruski D. L-dopa binding sites in rodent melanoma cells. Biochimica et biophysica acta. 1992;1139:324–8. doi: 10.1016/0925-4439(92)90109-z. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological reviews. 2004;84:1155–228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–7. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit N, Tilgmann C, Karhunen T, Slingerland R, Ulmanen I, Westerhof W, Pavel S. O-methylation of L-dopa in melanin metabolism and the presence of catechol-O-methyltransferase in melanocytes. Pigment Cell Research. 1994;7:403–8. doi: 10.1111/j.1600-0749.1994.tb00069.x. [DOI] [PubMed] [Google Scholar]
- Smit NP, Pavel S. Induction of cytotoxicity in melanoma cells through inhibition of catechol-O-methyltransferase. Biochemical pharmacology. 1995;50:1955–62. doi: 10.1016/0006-2952(95)00243-x. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Teasdale RD, Box NF. Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene. 2001;277:49–62. doi: 10.1016/s0378-1119(01)00694-1. [DOI] [PubMed] [Google Scholar]
- Suiko M, Sakakibara Y, Nakajima H, Sakaida H, Liu MC. Enzymic sulphation of dopa and tyrosine isomers by HepG2 human hepatoma cells: stereoselectivity and stimulation by Mn2+ The Biochemical journal. 1996;314(Pt 1):151–8. doi: 10.1042/bj3140151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taskinen J, Ethell BT, Pihlavisto P, Hood AM, Burchell B, Coughtrie MW. Conjugation of catechols by recombinant human sulfotransferases, UDP-glucuronosyltransferases, and soluble catechol O-methyltransferase: structure-conjugation relationships and predictive models. Drug metabolism and disposition: the biological fate of chemicals. 2003;31:1187–97. doi: 10.1124/dmd.31.9.1187. [DOI] [PubMed] [Google Scholar]
- Toyofuku K, Valencia JC, Kushimoto T, Costin GE, Virador VM, Vieira WD, Ferrans VJ, Hearing VJ. The etiology of oculocutaneous albinism (OCA) type II: the pink protein modulates the processing and transport of tyrosinase. Pigment Cell Research. 2002;15:217–24. doi: 10.1034/j.1600-0749.2002.02007.x. [DOI] [PubMed] [Google Scholar]
- Tsang AH, Chung KK. Oxidative and nitrosative stress in Parkinson’s disease. Biochimica et biophysica acta. 2009;1792:643–50. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Wakamatsu K, Ito S. Identification of ester glucuronide and sulfate conjugates of 5-hydroxy-6-methoxyindole-2-carboxylic acid and 6-hydroxy-5-methoxyindole-2-carboxylic acid in melanoma urine. J Dermatol Sci. 1990;1:253–9. doi: 10.1016/0923-1811(90)90117-v. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Hayes JD, Higgins LG, Wolf CR, Dinkova-Kostova AT. Activation of the NRF2 signaling pathway by copper-mediated redox cycling of para-and ortho-hydroquinones. Chem Biol. 2010;17:75–85. doi: 10.1016/j.chembiol.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Warren R. Sensitivity of mouse Skh-HR-2 to ultraviolet light: mouse pigmentation model. Photochem Photobiol. 1986;43:41–7. doi: 10.1111/j.1751-1097.1986.tb05589.x. [DOI] [PubMed] [Google Scholar]
- Werner P, Mytilineou C, Cohen G, Yahr MD. Impaired oxidation of pyruvate in human embryonic fibroblasts after exposure to L-dopa. Eur J Pharmacol. 1994;263:157–62. doi: 10.1016/0014-2999(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Winder AJ, Harris H. Induction of tyrosinase in human melanoma cells by L-tyrosine phosphate and cytochalasin D. Exp Cell Res. 1992;199:248–54. doi: 10.1016/0014-4827(92)90431-7. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Yasuda T, Liu MY, Shetty S, Idell S, Boggaram V, Suiko M, Sakakibara Y, Fu J, Liu MC. Sulfation of chlorotyrosine and nitrotyrosine by human lung endothelial and epithelial cells: role of the human SULT1A3. Toxicology and applied pharmacology. 2011;251:104–9. doi: 10.1016/j.taap.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Yen PM. Physiological and molecular basis of thyroid hormone action. Physiological reviews. 2001;81:1097–142. doi: 10.1152/physrev.2001.81.3.1097. [DOI] [PubMed] [Google Scholar]