Abstract
We report the creation of a new low-estrogen murine model of concurrent oral and vaginal C. albicans colonization that resembles human candidal carriage at both mucosal sites. Weekly estrogen administration of 5 μg intramuscular and subcutaneously was optimal for enhancement of oral colonization and was essential for vaginal colonization. In BALB/c mice, a number of C. albicans clinical isolates (n = 3) colonized both oral and/or vaginal sites, but only strain 529L colonized 100% of mice persistently for over 5 weeks. Laboratory strains SC5314 and NCPF 3153 did not colonize the model; however, NCPF 3156 showed vaginal colonization up to week 5. Prior passaging through mice enhanced subsequent colonization of SC5314. Intranasal immunization with a C. albicans virulence antigen (secreted aspartyl proteinase 2) significantly reduced or abolished the fungal burden orally and vaginally by week 2 and 7. Our concurrent model of mucosal colonization reduces the numbers of experimental mice by half, can be used to assess potential vaccine candidates, and permits the detailed analysis of host–fungal interactions during the natural state of Candida colonization.
Keywords: Candida albicans, Animal model, Oral, Vaginal
1. Introduction
Candida albicans remains the dominant human fungal pathogen, causing debilitating and often recurring diseases, and accounts for more than 50% of all fungal infections in man. Investigations into the complex Candida–host interaction often require the use of animals as an in vivo model of infection [1]. Over the last decade, a number of clinically relevant models of systemic and mucosal candidiasis have been established. While systemic models rely on intravenous injection of C. albicans, mucosal (oral) inoculation more closely mimics the predominant gastrointestinal portal of entry leading to systemic dissemination [2]. However, establishment of mucosal infection models generally requires the use of immunosuppressive agents, antibiotic or estrogen treatment, or the use of germ-free animals [3–5] (reviewed in [1,6]).
Murine vaginitis models are dependent upon prolonged pseudoestrus, usually induced with 17-β-estradiol, since rodents have a short 4-day estrus cycle [6–8]. In the absence of pseudoestrus vaginal infections are short-lived and usually cleared by the second week [6]. Estrogen levels appear to be the main hormonal factor affecting host susceptibility, with an estrogen range of 10–200 μg/mouse per week establishing infection for up to 5 weeks; usually a relatively high dose of 100 μg/mouse per week is used [7]. Susceptibility to vaginal candidiasis is independent of the major histocompatibility locus H-2 haplotype [8], since most mouse strains are susceptible to estrogen-induced C. albicans vaginitis [8,9]. One exception is the CD-1 mouse, which is innately less susceptible to candidal infection, probably due to its resistance to estradiol endocrine disruption [10].
A number of oral infection models have also been described, with persistent infections requiring immunosuppression [1]. In the absence of immunosuppression the fungal burden is variable and usually cleared by the second week [11]. However, Deslauriers and coworkers demonstrated in different mouse strains (BALB/c and DBA/2, and CD-1) that after an initial oral infection stage of 7–10 days fungal burdens reduced naturally to a carrier state for many weeks without the use of immunosuppressive agents [12–14]. To establish oral candidiasis, mice are usually treated with steroids [14], often with the addition of tetracycline [5,15]. Initially, fungal burdens can be high [5,14,15], but after cessation of drug administration fungal burdens diminish to very low levels [12,14].
One model that is currently lacking but which would be highly desirable is one that permits a relatively high level of C. albicans colonization of oral and vaginal sites concurrently in the same mouse over a 5–6 week period. This would allow detailed investigations into the delicate host–fungus interactions that exist at epithelial surfaces and also the analysis of fungal pathogenicity, host immunity, and the efficacy of anti-fungal therapies at multi-sites. Here we describe the establishment of such a model of concurrent oral and vaginal Candida colonization.
2. Materials and methods
2.1. Strains, media and growth conditions
Three laboratory strains of Candid albicans, SC5314, NCPF 3153 and NCPF 3156 (National Collection of Pathogenic Fungi) [16], and seven fresh clinical strains isolated from patients with oral (529L, GM2091, GM2093, GM808, GM1055) or vaginal (GV114, GV150) candidiasis regularly attending the Oral Medicine Clinic at Guy’s Hospital or the GenitoUrinary Medicine Clinic at St Thomas’s Hospital were used. Stock cultures were stored at −20 °C. For inoculations, a preculture from Sabouraud’s dextrose (SD) agar plates was grown to late-log phase for 18 h in SD liquid media at 25°C on an orbital incubator.
2.2. Establishment of murine model of concurrent oral and vaginal colonization
All animal work was performed under and complied with strict institutional guidelines. Female BALB/c, C57BL/6 and DBA/2 mice 6–8 weeks of age (20–22 g) were obtained from Harlan Olac UK and maintained on a normal mouse diet throughout the study. Estradiol valerate (Sigma) was dissolved in sesame oil (Sigma). To determine the optimal dose of estrogen to establish colonization, mice received weekly intramuscular (i.m.) or subcutaneous (s.c.) or both i.m. and s.c. injections of estradiol valerate ranging from 2.5–50 μg/mouse (equates to 0.125–2.5 mg/kg). Control mice were given only sesame seed oil or saline. Seven days later, mice were inoculated orally with 107 C. albicans cells in 100 μl phosphate buffered saline (PBS), applied topically and rubbed on to the teeth and gums with cotton buds, and vaginally with 2.5 × 106 C. albicans cells in 25 μl PBS by pipette. Ten different C. albicans strains were examined for their ability to colonize. Oral and vaginal swabs were collected weekly for up to six weeks. For oral swabbing, moist cotton buds were rubbed all over the oral cavity and suspended in 200 μl PBS before plating onto SD agar to determine colony forming units (CFU) per ml. Vaginal samples were obtained by washing the vagina twice with 50 μl PBS, flushing four times, and plating 100 μl to determine fungal burden. Groups of 5–6 mice were used.
2.3. Histological examination of oral and vaginal tissues
Mice colonized with C. albicans from experimental and control groups were euthanized by CO2 inhalation at weeks 2, 3 and 5. The heads were removed and the vaginas dissected and fixed in 10% (v/v) formal-saline for 48 h. The heads were decalcified in 10% (v/v) EDTA (ethylenediaminetetraacetic acid) for 3–4 weeks and complete decalcification was confirmed radiographically. All experimental mice were sampled histologically (n = 6 per group). Coronal (medio-lateral) tissue blocks of the whole oral cavity from the lower incisors cranially to the tongue base caudally, and of the whole of the vaginal tract were taken. All tissue blocks were routinely processed trough paraffin wax, embedded and cut into 5-μm sections. Fifteen serial sections were prepared in the experimental group (5 anterior, 5 middle and 5 posterior oral cavity), whereas the entire paraffin blocks in the control group were ribboned out with every tenth section submitted for staining. All sections were stained with periodic acid Schiff (PAS) and visualized by light microscopy. Representative images are presented.
2.4. Passaging of C. albicans to enhance colonization
Estrogen-treated C57BL/6 mice (n = 6) were inoculated with C. albicans SC5314 both orally (107 cells) and vaginally (2.5 × 106 cells) as described. After 1 week, in those mice that were colonized, a single colony was taken from the oral swab and vaginal washing culture, regrown in SD medium, and reintroduced into the oral (107 cells) or vaginal (2.5 × 106 cells) cavities, respectively, of the same six mice at week 2. In a separate experiment, mice that failed to be colonized after the first inoculation were reinoclulated after one week with non-passaged SC5314. In both experiments, oral swabs and vaginal washings were collected as described for a further 3 weeks and the CFU/ml determined.
2.5. Intranasal immunization with a C. albicans Sap2
Two groups of Balb/c mice (n = 10) were given 5 μg estrogen i.m. and 5 μg estrogen s.c. at weekly intervals. On weeks 2 and 4 the experimental group was immunized intranasally on two consecutive days with 5 μg purified C. albicans 529L Sap2 (see below) and 1 μg cholera toxin (Sigma) suspended in 25 μl PBS. Control groups received PBS alone. On days 14 and 15 after the second Sap2 immunization, mice were inoculated orally and vaginally with C. albicans 529L as described and fungal burdens followed for an additional 5 weeks.
2.6. Purification of Sap2
C. albicans 529L were grown to stationary phase in 100 ml SD medium at 35 °C and 500 μl of packed cells were then transferred to 500 ml of yeast carbon base medium supplemented with 1% BSA (YCB/BSA) for 3 days at 27 °C to induce Sap2 production. After cell harvesting, the supernatant was adjusted to pH 6 and filter sterilized through 0.22-μm cell filtration systems (Corning Costar). Proteins were precipitated with ammonium sulfate to 75% saturation. The protein precipitate was dialyzed in sodium citrate buffer pH 6.5 and applied to a pre-equilibrated Affigel column (Pharmacia) and then eluted [17]. Fractions containing Sap2 were demonstrated by SDS–PAGE (Genomics Biolab) with a single band and confirmed by Western blotting using antisera raised against purified Sap2. Sap2 positive fractions were lyophilized, reconstituted in 5 ml sterile water and passed first through a PD-10 gel filtration column and then through Hitrap™ Q HP (Amersham Biosciences) to achieve maximum purity.
2.7. Statistical analyses
All data analyses were undertaken using the Mann–Whitney U-test on the Prism statistics package.
3. Results
3.1. Establishment of the concurrent oral and vaginal colonization murine model
Our concurrent mucosal model was based on the administration of estrogen since it is well recognized that persistent vaginal C. albicans colonization in mice is dependent upon prolonged pseudoestrus [6–8]. In initial experiments, when BALB/c mice were given between 2.5 and 50 μg estradiol valerate i.m., a clear dose–response curve was evident with both oral and vaginal colonization using the laboratory strain C. albicans 3153. The optimal dose to establish maximum oral colonization after 7 days was 5 μg of estrogen per mouse and for vaginal colonization it was 10 μg per mouse (not shown). At doses greater than 10 μg the mice often developed mild to severe bacterial vaginitis. Further optimization experiments revealed that s.c. estrogen administration enhanced vaginal colonization and i.m. administration enhanced oral colonization, and that a combined weekly estrogen dose of 5 μg (0.25 mg/kg) s.c. and 5 μg i.m. per mouse was more efficacious than either route alone to establish maximal colonization at both mucosal sites (not shown). Using these low levels of estrogen, no adverse effects such as swelling or redness were observed vaginally or orally.
3.2. Fresh clinical C. albicans isolates establish persistent colonization in different mouse strains
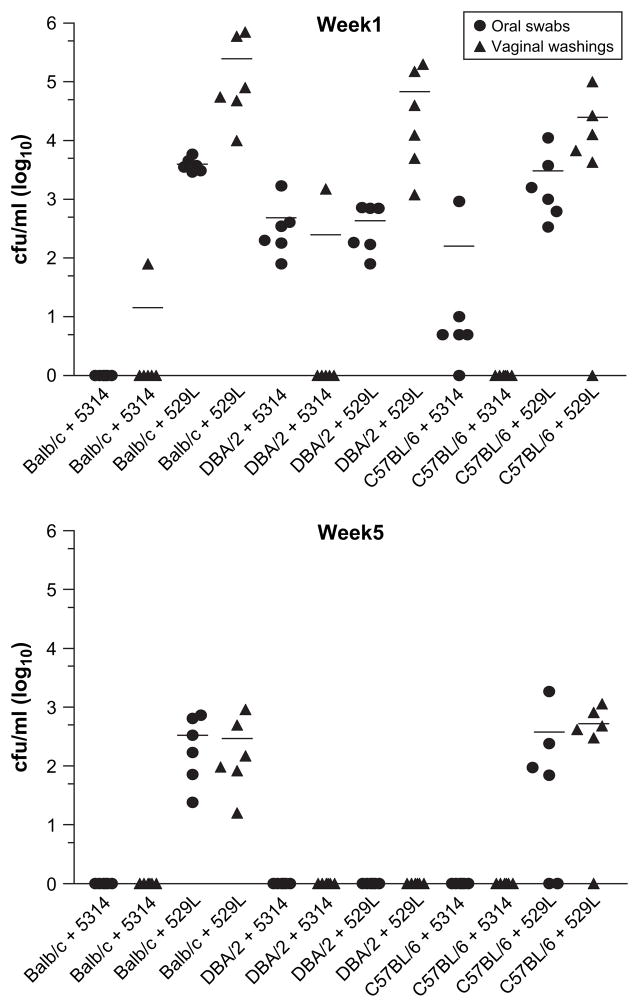
In initial experiments, in an attempt to establish persistent colonization over a 5–6 week period with fungal burdens similar to that found in human mucosal colonization, we assessed three mouse strains (BALB/c, C57BL/6 and DBA/2) and three C. albicans strains: two laboratory strains (SC5314 and NCPF 3153) and a freshly isolated strain obtained from a patient with oral candidiasis (529L)). C. albicans 3153 did not efficiently colonize BALB/c mice using the optimal estrogen concentrations and fungal burdens were highly variable, with concurrent oral and vaginal colonization rates between 35% and 50% (data not shown). Likewise, C. albicans SC5314 after 7 days was unable to reproducibly colonize both the oral and vaginal cavities of all three strains of mice examined (Fig. 1). In contrast, the freshly isolated patient strain 529L consistently colonized all three mouse strains both orally and vaginally past 7 days. By week 5, colonization with strain 529L was maintained at both mucosal sites in 100% of BALB/c mice and in the majority of C57BL/6 mice, but was cleared in DBA/2 mice (Fig. 1). Laboratory strains SC5314 and 3153 were totally cleared from both mucosal sites in the majority of mice by week 2 and from all mice by week 3 (data not shown). Fungal burdens were noticeably higher both orally and vaginally with the clinical strain 529L as compared with SC5314 or 3153 (Figs. 1 and 2) and persisted for up to 6 weeks (Fig. 2).
Fig. 1.
Persistent oral and vaginal colonization is dependent on both strain of mouse and strain of C. albicans. Three strains of mice (Balb/c, DBA/2 and C57BL/6) (n = 6) were induced into a state of pseudoestrus (see Section 2). Mice were inoculated orally and vaginally as described with two strains of C. albicans, the clinical human isolate 529L and the laboratory strain SC5314. Oral swabs and vaginal washings were collected at weekly intervals and C. albicans CFU/ml in individual mice are shown at 1 and 5 weeks after inoculation. C. albicans NCPF 3153 provided similar data to SC5314 (not shown). Experiments were repeated on at least two occasions with similar results.
Fig. 2.
Persistent vaginal colonization by C. albicans 529L requires estrogen. One week after administration of estrogen or saline (see Section 2), Balb/c mice (n = 6) were inoculated vaginally with C. albicans 529L (clinical human isolate) or C. albicans NCPF 3153 (laboratory strain). Weekly vaginal washings were collected and CFU/ml determined (mean ± S.E.M.). Similar data were observed using C57BL/6 mice (not shown). Experiments were repeated on at least two occasions with similar results.
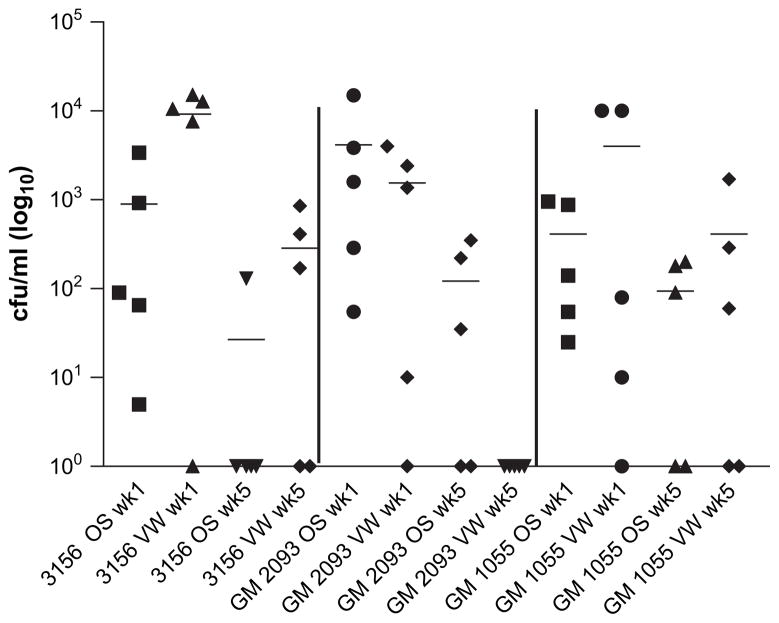
To determine the utility of the model, we tested whether an additional six fresh clinical C. albicans strains (see Section 2) and a laboratory strain (NCPF 3156) were also able to colonize the concurrent model. Although most strains colonized at week 1, by week 5 two of the six fresh clinical strains and NCPF 3156 were able to colonize at one or both mucosal sites, with similar fungal burdens to 529L, but only in up to 60% of BALB/c mice (Fig. 3). Only C. albicans 529L colonized 100% of BALB/c mice at both sites at week 5 (Fig. 1).
Fig. 3.
Fresh isolation of a C. albicans strain from an in vivo site is not a fundamental criterion in order to establish colonization. BALB/c mice (n = 5) were induced into a state of pseudoestrus and inoculated orally and vaginally as described with two clinical C. albicans isolates, GM2093 and GM1055, and the laboratory strain NCPF3156. Oral swabs and vaginal washings were collected at weekly intervals and C. albicans CFU/ml in individual mice are shown at 1 and 5 weeks after inoculation. All three strains colonized both sites at week 1, but at week 5 one or both mucosal sites only in up to 60% of mice and less persistently than 529L (compare with Fig. 1).
3.3. Histological analysis of oral and vaginal tissues
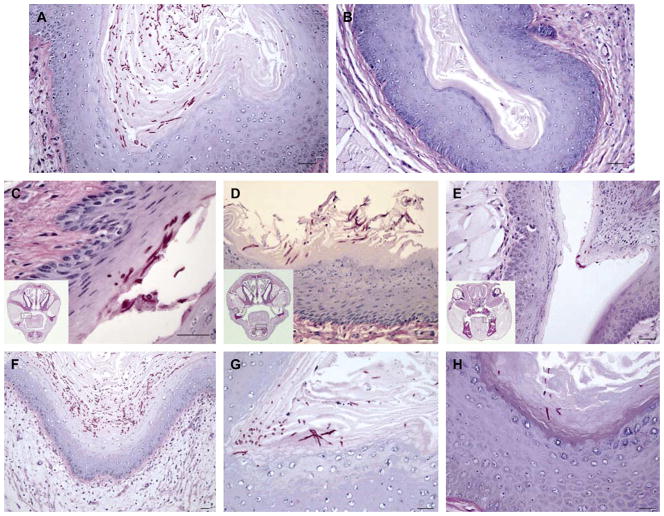
BALB/c mice were euthanized at 2, 3 and 5 weeks following inoculation with C. albicans 529L. In the oral cavity, fungal cells and hyphae were detected in the keratinized and upper prickle-cell layer of the epithelium at varying sites, namely, dorsum of tongue, buccal mucosa, floor of mouth, palate and lingual alveolar mucosa in both experimental and control groups at 2 and 3 weeks. By week 5, however, fungal cells and hyphae were still clearly present in oral epithelium of estrogenized animals but none were evident in the non-estrogenized animals. In the vagina, fungal cells and hyphae were detected in the keratinized layer of the epithelium and in the stacked keratin of the vaginal lumen of estrogenized at 2, 3 and 5 weeks. In stark contrast, no fungal cells were observed in the vaginal epithelium or lumen in non-estrogenized animals at any of the time points.
In spite of clear oral and vaginal colonization by C. albicans 529L at all time points examined, there were no clinically evident pseudomembranous or erythematous lesions, nor deep penetration by hyphae into the epithelial tissues, epithelial damage, or a polymorphonuclear (PMN)/lymphocyte infiltrate either within the epithelium or in the underlying lamina propria (Fig. 4). This strongly indicates colonization rather than a disease phenotype.
Fig. 4.
Representative photomicrographs of histological sections of test and control animals. (A, B) Coronal sections through the vagina of estrogen-treated (A) and untreated (B) animals at 3 weeks post-inoculation. (C–E) Histological sections through the oral cavity of estrogen treated animals at 2 weeks (C), 3 weeks (D) and 5 weeks (E) post-inoculation. The inset demonstrates the whole-mount coronal section and the boxed area represents the intra-oral site of colonization by C. albicans 529L (A, buccal mucosa; B, floor of mouth; C, Lingual alveolar mucosa). (F–H) Histological coronal sections through the vagina of estrogen-treated animals at 2 weeks (F), 3 weeks (G) and 5 weeks (H) post-inoculation. Scale bars = 100 μm.
3.4. Effect of in vivo passaging on the ability of C. albicans to colonize epithelium
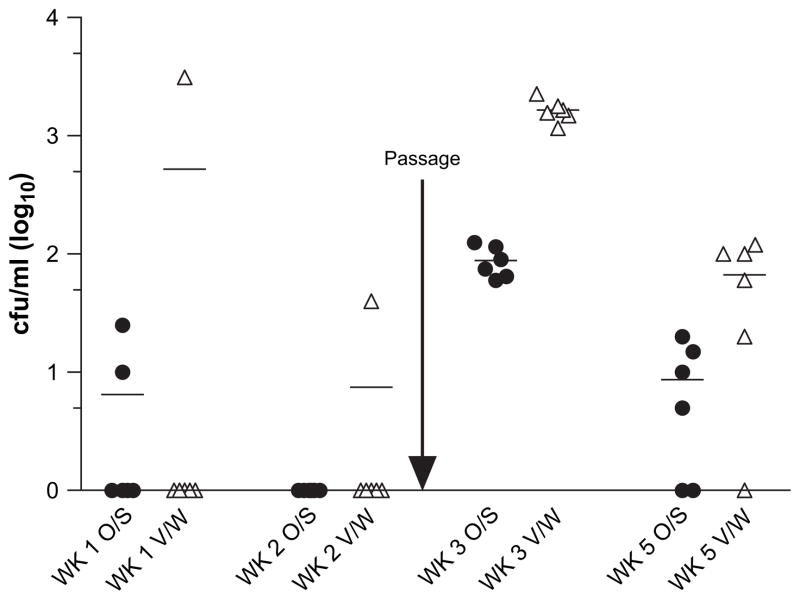
Since SC5314 was very poor at establishing mucosal colonization (Fig. 1), we examined whether in vivo passaging of this strain could enhance epithelial colonization. SC5314 cells isolated at week 1 from oral swabs or vaginal washes from an individual mouse were regrown and reinoculated into the oral or vaginal cavities, respectively of the same group of C57BL/6 mice (n = 6) at week 2. Colonization was significantly enhanced at both sites in all animals at weeks 3 and 4, and by week 5 the fungal burdens were still present, albeit at lower levels (Fig. 5). In comparison, non-passaged SC5314 did not colonize the model at weeks 3–5 weeks and when SC5314 was re-inoculated into mice that failed to be initially colonized, the fungus still did not colonize the model (data not shown).
Fig. 5.
Passaging of laboratory strain C. albicans SC5314 enhances colonization both orally and vaginally. Estrogen-treated C57BL/6 mice (n = 6) were inoculated with C. albicans SC5314 both orally and vaginally as described. After 1 week, only 2/6 mice were colonized orally and 1/6 vaginally. A single C. albicans colony (encircled) was taken from the oral and vaginal cultures, regrown, and reintroduced into the oral or vaginal cavities, respectively, of the same six mice at week 2. Oral swabs (O/S) and vaginal washings (V/W) were collected for a further 3 weeks. CFU/ml for weeks 1, 2, 3 and 5 are shown. Non-passaged SC5314 did not colonize the model at weeks 3–5 (data not shown).
3.5. Utilization of the model to study vaccine candidates
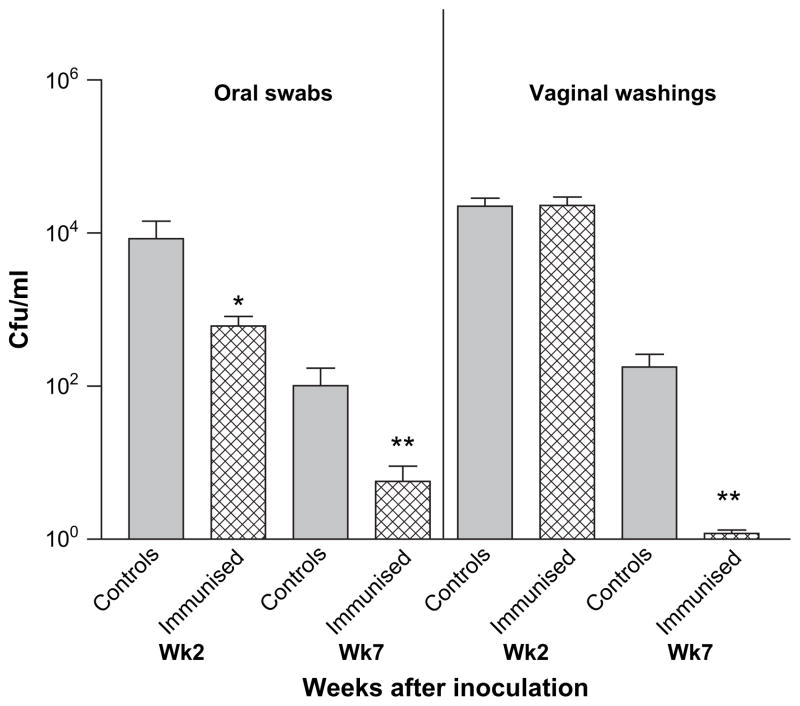
Mice (n = 10) were immunized intranasally with 5 μg purified Sap2 (a putative virulence factor of C. albicans thought to contribute to human mucosal infections reviewed in [1]) together with 1 μg cholera toxin, and subsequently challenged with C. albicans 529L orally and vaginally as described. Orally, at both weeks 2 and 7 Sap2 immunized mice had significantly reduced fungal burdens compared with the unimmunized control groups (*P < 0.05 and **P < 0.001, respectively) (Fig. 6). Vaginally, at week 2 there was no reduction in Candida loads, but by week 7 fungal burdens were abolished in the Sap2 immunized group (**P < 0.0001).
Fig. 6.
Fungal burdens are significantly reduced after mucosal immunization with a putative C. albicans virulence factor. Balb/c mice (n = 10) induced into a state of pseudoestrus (see Section 2) were immunized intranasally with 5 μg of C. albicans secreted aspartyl protease (Sap2) and 1 μg cholera toxin or PBS (control). Two weeks after immunization, mice were challenged orally and vaginally with C. albicans 529L as described. Weekly oral swabs and vaginal washings were collected and fungal burdens followed for an additional 5 weeks (mean ± S.E.M.). Significant reduction in fungal burdens was observed orally at week 2 (*P < 0.05) and at week 7 both orally (**P < 0.001) and vaginally (**P < 0.0001). The data are representative of two independent experiments.
4. Discussion
Oral and vaginal candidiasis are two of the most common forms of human fungal infections and detailed investigations into these complex diseases are greatly facilitated by the use of animal models. Here, we report the creation of a new low-estrogen murine model of concurrent oral and vaginal C. albicans colonization that resembles human candidal carriage, which reduces the numbers of experimental mice by half, allows the study of delicate host–pathogen interactions during the natural state of C. albicans colonization, and facilitates the assessment of potential mucosal vaccine candidates.
One of the unique features of our model is that Candida ‘colonization’ rather than ‘disease’ can be established at two mucosal sites simultaneously in the same mouse. The concept of ‘colonization’ versus ‘infection’ (or ‘disease’) is an important issue. Some regard ‘infection’ as similar to ‘colonization’ in that the simple presence of an organism signifies that someone is ‘infected’, irrespective of any symptomatic evidence. However, most regard ‘infection’ as similar to ‘disease’, where clinical symptomatic or histological evidence (signs, symptoms, invasion, cell damage, host immune cell infiltrates) are present in addition to colonization. In this paper, ‘colonization’ is defined as the presence of fungal cells demonstrated histologically and by culture but without (a) pseudomembranous or erythematous lesions, (b) signs of epithelial damage, (c) deep epithelial penetration by hyphae, or (d) PMN/lymphocyte infiltration. Nearly all current oral or vaginal murine Candida models establish a ‘disease’ state at one or other site and are based on immunosuppressive therapies or high estrogen doses [1,7]. In our model there is no evidence of these four criteria (Fig. 4), strongly indicating a ‘colonization’ rather than a ‘disease’ phenotype.
In respect of our vaginal data, the low levels of estrogen used (10 μg weekly/mouse) are in contrast to most current murine models of vaginal candidiasis that typically use between 100 and 500 μg estrogen weekly/mouse [7,8]. Although these high estrogen levels permit the establishment of persistent vaginal infections with relatively high fungal burdens, they are also likely to have undesirable host effects and significantly influence the defense response [19]. In support of this, when we administered pilocarpine (to stimulate saliva flow for antibody studies) to mice given 100 μg of estrogen, we often observed severe vaginitis and secondary bacterial infections, and occasionally death. In our low estrogen model, no adverse health effects to pilocarpine were observed and the mice remained healthy. This indicates a less profound effect of estrogen in our model and, therefore, probably a more relevant host physiological and immunological response to the fungus when colonized.
In respect of our oral data, low-dose estrogen appeared to enhance oral colonization, although for C. albicans 529L the oral fungal burdens were less affected by estrogen than vaginal burdens, which were estrogen-dependent (Fig. 2). This was demonstrated by the ability of C. albicans 529L to orally colonize estrogenized mice for at least 5 weeks (Fig. 1) but not non-estrogenized mice (data not shown). Low estrogen doses may subtly alter the finely balanced host–fungus equilibrium at oral sites to promote fungal adherence and colonization by acting upon the host or the pathogen [19]. For example, the action of estrogens may affect estrogen receptor (ER)-β expression, the predominant ER subtype present in human oral tissues, and/or saliva secretion and composition [20]. In addition, C. albicans produces an estrogen binding protein (EBP) [21] and estrogens may thus enhance fungal colonization directly [22]. Recently, it has been suggested that the mechanism for estrogen-sensitive vaginal colonization by C. albicans includes a functional ligand-EBP interaction within the yeast [23].
Fungal burdens were noticeably higher both orally and vaginally with the fresh clinical strains 529L as compared with laboratory strains SC5314 or 3153 (Figs. 1 and 2) and persisted for up to 6 weeks (Fig. 2). Oral colonization burdens were generally 1–2 log higher (102–103 CFU per ml saliva or per oral or vaginal swab) than previously described oral models without immunosuppression [12–14], which are compatible to C. albicans carriage levels found in humans (although there is more variability vaginally) [24,25]. Given that C. albicans virulence might be tissue specific and dependent upon a strains origin [26], one reason why 529L exhibited enhanced colonization and persistence over the laboratory strains SC5314 or 3153 might be that 529L is a fresh human oral isolate. Thus, 529L might be innately adapted to the in vivo environment and may naturally express more relevant adherence/virulence moieties that enhance mucosal colonization. This hypothesis was partially supported by additional experiments, whereby although fresh clinical isolates consistently colonized initially, by week 5 only 2/6 colonized one or both mucosal sites at similar levels to 529L (Fig. 3). However, another laboratory strain (NCPF 3516) was as effective as the two additional clinical strains at establishing colonization. Nonetheless, colonization for 5 weeks or longer by fresh human isolates was greater (n = 3/7, with higher CFU counts) than by laboratory strains (n = 1/3, with lower CFU counts). Further evidence for in vivo adaptation is supported by the passaging experiments, whereby passaged SC5314 colonized both mucosal sites in all animals for up to three weeks (Fig. 5). An alternative explanation that primary infection makes the mice more susceptible to secondary challenge seems less likely since reinoculation by non-passaged strains did not lead to enhanced colonization. Together, the data suggest that although fresh isolation of a strain from an in vivo site may enhance mucosal colonization, it is not a fundamental criterion in order to establish colonization.
Since SC5314 is the wild-type strain used in the majority of gene mutation studies to assess virulence phenotypes, our model using passaged SC5314 provides an option of using this strain to investigate the effect of gene disruption on mucosal colonization at both anatomical sites over a 3-week period. However, detailed analysis of persistent colonization (up to 6 weeks) will be limited with SC5314. On the other hand, our model could be potentially very useful in studying host–pathogen colonization interactions of fresh clinical isolates, which may be more relevant than studying laboratory isolates. Moreover, advances in gene disruption techniques now allow the relatively quick generation of null mutants in clinical wild-type strains; therefore, our model could be widely utilized to study C. albicans colonization in clinically relevant strains if desired.
One of the primary objectives of this study was to establish a concurrent mucosal colonization model of sufficient longevity that could be utilized for immunization studies to study the efficacy of potential vaccine candidates. We found that immunization with Sap2, a known virulence factor of C. albicans associated with mucosal infection [18,24,25,27] significantly reduced fungal burdens both orally and vaginally (P < 0.001 and P < 0.0001, respectively) (Fig. 6). This finding corroborates previous studies [28,29] demonstrating that Sap2 is an immunogenic antigen capable of inducing protective responses against C. albicans colonization and infection, and tentatively supports its targeting as a potential vaccine candidate. The data demonstrate proof-of-principle that our concurrent mucosal model can be used for both immunological and potential vaccine studies and permits the detailed analysis of host–fungal interactions during the natural state of Candida colonization [30].
Acknowledgments
The authors would like to thank Alex Woodman for the preparation of histological sections, Katherine Rodgers for collection of the clinical vaginal Candida samples, and Paul Fidel for helpful discussions. This work was supported in part by institutional funding, by NIH grant R21 DE015528-01 (to J.R.N.) and a Wellcome Value in People (VIP) personal award (to J.R.N.).
Footnotes
The authors have no conflicting financial interests.
References
- 1.Samaranayake YH, Samaranayake LP. Experimental oral candidiasis in animal models. Clin Microbiol Rev. 2001;14:398–429. doi: 10.1128/CMR.14.2.398-429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Repentigny L. Animal models in the analysis of Candida host–pathogen interactions. Curr Opin Microbiol. 2004;7:324–329. doi: 10.1016/j.mib.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Rahman D, Challacombe SJ. Oral immunization against mucosal candidiasis in a mouse model. Adv Exp Med Biol. 1995;371B:1663–1666. [PubMed] [Google Scholar]
- 4.Rahman D, Mistry M, Challacombe SJ. Mucosal antibodies to Candida antigens in saliva and vaginal secretions after intranasal immunisation. J Dent Res. 1997;76:328. [Google Scholar]
- 5.Kamai Y, Kubota M, Kamai Y, Hosokawa T, Fukuoka T, Filler SG. New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother. 2001;45:3195–3197. doi: 10.1128/AAC.45.11.3195-3197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fidel PLJ, Sobel JD. Murine models of Candida vaginal infections. In: Sande M, Zak O, editors. Handbook of Animal Models of Infection. Academic Press; 1999. pp. 741–748. [Google Scholar]
- 7.Fidel PLJ, Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun. 2000;68:651–657. doi: 10.1128/iai.68.2.651-657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon L, Williams R, Martinez M, Clemons KV, Stevens DA. Genetic susceptibility to vaginal candidiasis. Med Mycol. 2003;41:143–147. doi: 10.1080/mmy.41.2.143.147. [DOI] [PubMed] [Google Scholar]
- 9.Fidel PLJ, Cutright JL, Sobel JD. Effects of systemic cell-mediated immunity on vaginal candidiasis in mice resistant and susceptible to Candida albicans infections. Infect Immun. 1995;63:4191–4194. doi: 10.1128/iai.63.10.4191-4194.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science. 1999;285:1259–1261. doi: 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- 11.Elahi S, Pang G, Clancy R, Ashman RB. Cellular and cytokine correlates of mucosal protection in murine model of oral candidiasis. Infect Immun. 2000;68:5771–5777. doi: 10.1128/iai.68.10.5771-5777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakir J, Cote L, Coulombe C, Deslauriers N. Differential pattern of infection and immune response during experimental oral candidiasis in BALB/c and DBA/2 (H-2d) mice. Oral Microbiol Immunol. 1994;9:88–94. doi: 10.1111/j.1399-302x.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 13.Lacasse M, Fortier C, Chakir J, Cote L, Deslauriers N. Acquired resistance and persistence of Candida albicans following oral candidiasis in the mouse: a model of the carrier state in humans. Oral Microbiol Immunol. 1993;8:313–318. doi: 10.1111/j.1399-302x.1993.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 14.Deslauriers N, Coulombe C, Carre B, Goulet JP. Topical application of a corticosteroid destabilizes the host–parasite relationship in an experimental model of the oral carrier state of Candida albicans. FEMS Immunol Med Microbiol. 1995;11:45–55. doi: 10.1111/j.1574-695X.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 15.Takakura N, Sato Y, Ishibashi H, Oshima H, Uchida K, Yamaguchi H, Abe S. A novel murine model of oral candidiasis with local symptoms characteristic of oral thrush. Microbiol Immunol. 2003;47:321–326. doi: 10.1111/j.1348-0421.2003.tb03403.x. [DOI] [PubMed] [Google Scholar]
- 16.Gillum AM, Tsay EY, Kirsch DR. Isolation of the Candida albicans gene for orotidine-5′-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198:179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- 17.Ray TL, Payne CD, Ruchel R, Ritter B, Schaffrinski M. Comparative production and rapid purification of Candida acid proteinase from protein-supplemented cultures. Infect Immun. 1990;273:391–403. doi: 10.1128/iai.58.2.508-514.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naglik JR, Challacombe SJ, Hube B. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol Mol Biol Rev. 2003;67:400–428. doi: 10.1128/MMBR.67.3.400-428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Styrt B, Sugarman B. Estrogens and infection. Rev Infect Dis. 1991;13:1139–1150. doi: 10.1093/clinids/13.6.1139. [DOI] [PubMed] [Google Scholar]
- 20.Valimaa H, Savolainen S, Soukka T, Silvoniemi P, Makela S, Kujari H, Gustafsson JA, Laine M. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J Endocrinol. 2004;180:55–62. doi: 10.1677/joe.0.1800055. [DOI] [PubMed] [Google Scholar]
- 21.Madani ND, Malloy PJ, Rodriguez-Pombo P, Krishnan AV, Feldman D. Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol. Proc Natl Acad Sci USA. 1994;91:922–926. doi: 10.1073/pnas.91.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Essmann M, Burt ET, Larsen B. Estrogen effects on Candida albicans: a potential virulence-regulating mechanism. J Infect Dis. 2000;181:1441–1446. doi: 10.1086/315406. [DOI] [PubMed] [Google Scholar]
- 23.Tarry W, Fisher M, Shen S, Mawhinney M. Candida albicans The Estrogen Target for Vaginal Colonization. J Surg Res. 2005;129:278–282. doi: 10.1016/j.jss.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Naglik JR, Newport G, White TC, Fernandes-Naglik LL, Greenspan JS, Greenspan D, Sweet SP, Challacombe SJ, Agabian N. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect Immun. 1999;67:2482–2490. doi: 10.1128/iai.67.5.2482-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis. 2003;188:469–479. doi: 10.1086/376536. [DOI] [PubMed] [Google Scholar]
- 26.Taylor BN, Fichtenbaum C, Saavedra M, Slavinsky III, Swoboda R, Wozniak K, Arribas A, Powderly W, Fidel JP. In vivo virulence of Candida albicans isolates causing mucosal infections in people infected with the human immunodeficiency virus. J Infect Dis. 2000;182:955–959. doi: 10.1086/315768. [DOI] [PubMed] [Google Scholar]
- 27.Naglik J, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–926. doi: 10.1111/j.1462-5822.2004.00439.x. [DOI] [PubMed] [Google Scholar]
- 28.Suenobu N, Kweon MN, Kiyono H. Nasal vaccination induces the ability to eliminate Candida colonization without influencing the pre-existing antigen-specific IgE Abs: a possibility for the control of Candida-related atopic dermatitis. Vaccine. 2002;20:2972–2980. doi: 10.1016/s0264-410x(02)00218-9. [DOI] [PubMed] [Google Scholar]
- 29.De Bernardis F, Boccanera M, Adriani D, Girolamo A, Cassone A. Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect Immun. 2002;70:2725–2729. doi: 10.1128/IAI.70.5.2725-2729.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Challacombe SJ, Naglik JR. The effects of HIV infection on oral mucosal immunity. Adv Dent Res. 2006;19:29–35. doi: 10.1177/154407370601900107. [DOI] [PubMed] [Google Scholar]