Abstract
Purpose
In clinical practice, atypical small acinar proliferation (ASAP) and high-grade prostatic intraepithelial neoplasia (HGPIN) are two common findings on prostate biopsies. Knowing the frequency of a prostate cancer diagnosis on repeat biopsies would aid primary treating physicians regarding their decisions in suspicious cases.
Materials and Methods
One hundred forty-three patients in whom biopsies revealed ASAP or HGPIN or both were enrolled in the present study; prostate cancer was not reported in the biopsy specimens and at least one repeat biopsy was performed. Age, digital rectal examination findings, prostate volumes, and free and total prostate-specific antigen (PSA) levels and the biopsy results of the patients were recorded.
Results
Of the 97 patients with ASAP on the first set of biopsies, prostate cancer was diagnosed in the second and third biopsies of 32 and 6 patients, respectively. Prostate cancer was not detected in the second or third biopsies of the 40 patients with HGPIN in the first biopsy. Of the 6 patients with ASAP+HGPIN in the first biopsy, prostate cancer was detected in 3 patients in the second biopsy and in 1 patient in the third biopsy.
Conclusions
The diagnosis of ASAP is a strong risk factor for prostate cancer. A repeat biopsy should be performed for the entire prostate subsequent to the diagnosis of ASAP. In patients with HGPIN according to the biopsy result, the clinical decision should be based on other parameters, such as PSA values and rectal examination, and a repeat biopsy should be avoided if the initial biopsy was performed with multiple sampling.
Keywords: Biopsy, needle; Prostatic intraepithelial neoplasia; Prostatic neoplasms
INTRODUCTION
Prostate cancer is one of the most prevalent types of cancer in men, and transrectal ultrasound (TRUS)-guided prostate biopsy, a reliable and easy-to-perform method, is accepted as the gold standard in the diagnosis of this disease. The use of prostate-specific antigen (PSA) in clinical practice has led to an increase in the prevalence of prostate cancer [1]. Elevated serum PSA levels or suspected prostate cancer on the rectal examination require prostate biopsy. In patients with negative initial biopsy results, the cancer detection rate on repeat biopsy varies between 10% and 20% [2].
Some small foci of atypical glands with some features of adenocarcinoma are found in 1.5% to 9% of prostate biopsy specimens. This is referred to as atypical small acinar proliferation (ASAP). ASAP, which is defined as a "focus of small acinar structures formed by atypical epithelial cells," is a condition in which the pathologist has insufficient data to make a diagnosis and thus raises the suspicion of cancer. It has been reported that the cancer detection rate varies between 21% and 51% on the second biopsy in patients with ASAP [3,4].
Prostatic intraepithelial neoplasia (PIN) is defined as "premalignant proliferation of atypical ductal and acinar cells." High-grade prostatic intraepithelial neoplasia (HGPIN) is reported in 5% to 7% of prostate biopsies. The risk of subsequent cancer varies between 25% and 79% [3]. Because the sextant biopsy technique has been increasingly replaced by multiple sampling methods, cancer detection rates on repeat biopsies following HGPIN have declined.
In clinical practice, ASAP and HGPIN are two common findings observed on prostate biopsies. Knowing the rate of prostate cancer diagnosis on repeat biopsies would aid primary treating physicians in the decision-making process for suspicious cases. For this purpose, we examined the repeat biopsy results of patients with ASAP and HGPIN.
MATERIALS AND METHODS
We retrospectively reviewed the medical records of 2,433 patients who had undergone TRUS-guided prostate biopsy for the diagnosis of prostate cancer between 2003 and 2009 at our hospital. The biopsy results of the patients were classified into four categories: prostate cancer, normal prostates or benign prostatic hyperplasia (BPH), ASAP, and HGPIN.
In the present study, 143 patients with ASAP or HGPIN whose biopsy specimens had not revealed prostate cancer and who had undergone at least one repeat biopsy were enrolled. Age, digital rectal examination (DRE) findings, prostate volumes (PV), free and total PSA levels, and the biopsy results of the patients were recorded.
The tissue samples were stained with racemase (AMACR), p63, and high-molecular weight cytokeratin (clone 34βE12) in foci suspicious of cancer (ASAP) in an effort to make a definitive diagnosis of adenocarcinoma.
The patients in whom repeat biopsies had not been performed for any reason or those having <8 quadrant and extended initial biopsies were excluded from the study. Specimens in which transition zone sampling was performed were also excluded. Specimens from 8-12 quadrant (mean, 10.8±1.8) biopsies were studied.
The characteristics of patients with ASAP and HGPIN on the initial biopsy were compared. The levels of PSA and free PSA, PVs, and DRE findings of the patients with ASAP/HGPIN on the initial biopsy were compared with respect to the presence of cancer. The Pearson's chi-square test was used to analyze differences in the cancer development rate for ASAP and HGPIN. The significance level was considered as p<0.05.
RESULTS
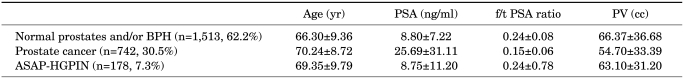
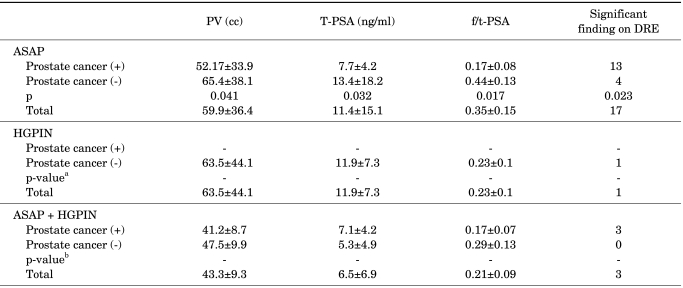
After the initial biopsy, prostate cancer was diagnosed in 742 of 2,433 patients (30.5%) and normal prostates or BPH was reported in 1,513 patients (62.2%). ASAP was found in 114 patients (4.7%), HGPIN in 58 patients (2.4%), and ASAP+HGPIN in 6 patients (0.2%) (Table 1). One hundred forty-three patients in whom repeat biopsy results were available were included in the study. The mean age of the 143 patients was 68.05±8.79 years. Of these patients, the mean PSA was 11.33±10.9 ng/ml, the mean free/total PSA ratio was 0.31±0.73, and the mean prostate volume was 60.2±32.4 cc. In 21 patients, DRE findings were found to be significant regarding the presence of cancer (Table 2). Age, PV, and PSA values did not show any statistically significant differences when patients reported as having ASAP and those reported as having HGPIN were compared (p>0.05), whereas abnormal DRE findings were significantly higher in the ASAP group.
TABLE 1.
Characteristics of the 2,433 patients
PSA: prostate-specific antigen, f/t PSA: free/total prostate-specific antigen, PV: prostate volume, BPH: benign prostatic hyperplasia, ASAP: atypical small acinar proliferation, HGPIN: high-grade prostatic intraepithelial neoplasia
TABLE 2.
Comparison of the patients with ASAP, HGPIN, and ASAP+HGPIN regarding the presence of prostate cancer
ASAP: atypical small acinar proliferation, DRE: digital rectal examination, f/t-PSA: free/total prostate-specific antigen, HGPIN: high-grade prostatic intraepithelial neoplasia, PV: prostate volume, T-PSA: total prostate-specific antigen, a: Statistical analysis was not performed in the patients with HGPIN in the initial biopsy because prostate cancer was not detected in any of the patients, b: Statistical analysis was not performed in the patients with ASAP+HGPIN because of the small number of patients
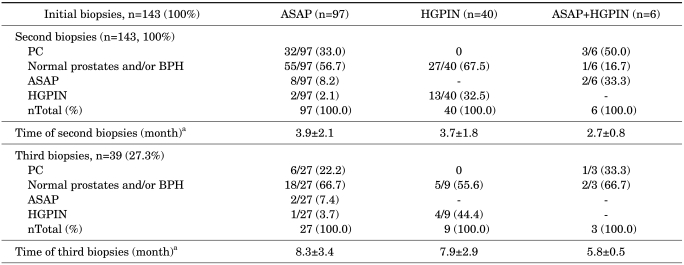
Of the 143 patients, ASAP, HGPIN, and ASAP+HGPIN were detected in 97, 40, and 6 patients, respectively, in the initial biopsies. Although second biopsies were performed in all patients, third biopsies were performed in only 27 patients with ASAP and 9 patients with HGPIN.
Of the 97 patients with ASAP, prostate cancer was detected in the second and third biopsies of 32 and 6 patients, respectively. The Gleason score was found to be 6 in 26 patients and 7 in 6 patients who were diagnosed as having prostate cancer after the second biopsy. All the patients who were diagnosed as having prostate cancer after the third biopsy had a Gleason score of 6. The total number of patients diagnosed as having prostate cancer was 38, and 22 of them had T1c tumors, whereas 16 had T2a. None of the 40 patients with HGPIN had evidence of prostate cancer in the second or third biopsy. Of the 6 patients with ASAP+HGPIN, prostate cancer was detected in 3 patients in the second biopsy and in 1 patient in the third biopsy (Table 3). Furthermore, on repeat biopsies, ASAP and HGPIN were detected in 12 and 5 patients, respectively.
TABLE 3.
Second and third biopsies results of 143 patients in the study
Values are presented as number (%), ASAP: atypical small acinar proliferation, BPH: benign prostatic hyperplasia, HGPIN: high-grade prostatic intraepithelial neoplasia, PC: prostate cancer, a: Time elapsed since the first biopsies
DISCUSSION
The incidence of prostate cancer increases with age, and it ranks second to lung cancer in terms of incidence and mortality risk [5]. Significant advances have been achieved in the diagnosis and treatment of prostate cancer with the introduction of PSA and prostate biopsy techniques. However, the histological diagnosis of prostate cancer in biopsy specimens remains a challenge for pathologists. A lack of availability of immunohistochemical staining, which may differentiate benign from malignant acini, and the poor diagnostic significance of the absence of basal cells in some cases complicate the diagnosis of prostate cancer.
TRUS-guided prostate biopsy, which is a reliable and easy-to-perform method, is accepted as the gold standard in the diagnosis of prostate cancer [6]. The purpose of the biopsy is to determine prostate cancer foci by obtaining a sufficient amount of samples at the true location without increasing morbidity. In the prostate biopsies performed for suspected prostate cancer, positive results confirm the diagnosis; however, the suspicion of cancer remains with negative results. Moreover, the suspicion of cancer becomes more prominent if the results of the biopsy reveal ASAP or HGPIN. Cancer detection rates decline after the first two biopsies. Keetch et al reported cancer detection rates in 4 consecutive biopsies to be 34%, 19%, 8%, and 7%, respectively in a group of 1,136 patients with PSA values higher than 4 ng/ml [7]. In accordance with the literature, in the present study, prostate cancer was detected in 30.5% of the biopsies performed after the first biopsy.
PIN is defined as "premalignant proliferation of atypical ductal and acinar cells." Four main patterns of HGPIN have been identified as follows: flat, micropapillary, cribriform, and tufting. However, other unusual histologic types of HGPIN, such as foamy gland, signet-ring cells, and mucinous have also been reported [8]. Borboroglu et al showed a 4% HPIN rate in extensive repeat biopsies in a series of 57 cases [3]. Because the sextant biopsy technique has been increasingly replaced by multiple sampling methods, cancer detection rates on repeat biopsies following HGPIN have declined. Thus, repeat biopsy should be performed if HGPIN is detected by a sextant biopsy technique, and if the biopsy has been performed with multiple sampling, other parameters, such as PSA or rectal examination status, should be considered [1,9,10]. In the present study, prostate cancer was not detected in any of the 40 patients with HGPIN. Recently, multiple sampling with laterally directed systematic sextant biopsies has been shown to increase the cancer detection rate by providing sufficient sampling in the first biopsy. There are direct and indirect findings indicating that such lesions are precancerous lesions. Thus, HGPIN appears to be a precancerous lesion of prostate cancer. As with prostate cancer, the prevalence of HGPIN also increases with age and represents a similar geographic/ethnic distribution. It is important to note that the detection of prostate cancer on repeat biopsies in patients with HGPIN detected in their first biopsies is not a missed diagnosis in the initial biopsy, but in fact might reflect the development of a new cancer arising from HGPIN [11]. The rate of prostate cancer diagnosis in a 3 year follow- up among patients who were initially diagnosed as having HPIN was 25.8% in a small group studied by Lefkowitz et al [12].
It is known that needle biopsy does not provide clear findings for the diagnosis of prostate cancer. Although the structural and cytological features of a small number of glands in a small focus may have atypical appearances on needle biopsy, such findings may be insufficient for the diagnosis of adenocarcinoma. The terms ASAP, focal glandular atypia, atypical biopsy suspicious for malignancy, and borderline lesions are used in such conditions [13]. The term ASAP was coined by Iczkowski et al in 1997 [14]; however, it is not recommended by all pathologists. In a consensus meeting in 2004 sponsored by the World Health Organization [15], the committee members recommended designating ASAP as either suspicious or highly suspicious for cancer. The reasons for this include the equation by some urologists of the term ASAP with HGPIN, and because all of the atypical foci are not always "small" acinar but may include glands with a larger diameter (such as a pseudohyperplastic pattern of cancer or adenocarcinoma with ductal features). Indeed, ASAP is a condition in which the pathologist has insufficient data to make diagnosis, and thus raises the suspicion of cancer. Therefore, a repeat biopsy should be performed in such cases. The cancer detection rate on repeat biopsy is 21% to 51%, and a subsequent biopsy should be directed to the locations in which ASAP was found in the initial biopsy, because a significant portion of the cancers are located in these regions [1,16]. In the present study, 38.2% of the patients shown to have ASAP in the initial biopsy were diagnosed with prostate cancer. Therefore, the high percentage of prostate cancer in patients with ASAP in the initial biopsies makes the need for aggressive follow-up reasonable.
The cancer detection rate declines with increasing prostate volume. Levine et al reported that the cancer diagnosis rate was 43%, 27%, and 24% in men with prostate volumes less than 30 cc, between 30 cc and 50 cc, and greater than 50 cc, respectively [17]. In second biopsies, two-fold higher positive results were obtained in patients with larger prostate volumes. It is now accepted that the standard sextant biopsy technique does not provide adequate sampling. Hence, multiple sampling must be performed in patients with larger prostate volumes either in the initial or on repeat biopsies.
Although PSA is specific to prostate tissue, it is not specific to prostate cancer. The positive predictive value of serum PSA >4.0 ng/ml is reported to be 25% for prostate cancer [18]. When the cut off value is accepted to be 2.5 ng/ml, prostate cancer detection rates increase, but the number of unnecessary biopsies also increases [19]. Although the percent free PSA maintains sensitivity for the early detection of prostate cancer, it also significantly improves the specificity of PSA. A low percent free PSA indicates an increased risk of cancer; however, cancer is detected on biopsies of 30% to 50% of men with percent free PSA levels <15% [20]. In the present study, we observed that the total PSA and the percent free PSA levels significantly increased the cancer detection rates in patients with ASAP in the initial biopsy. This condition is predictable for ASAP, which is associated with higher cancer detection rates on repeat biopsies.
A limitation of the present study was that the biopsy samples were not examined by the same pathologist, although all biopsies were examined in the same pathology laboratory. Furthermore, the retrospective design of the study and changes in PSA threshold level over time might have influenced the homogeneous distribution of the study population. In the present study, the PSA threshold value was 4.0 ng/ml in approximately one-half of the patients, whereas it was 2.5 ng/ml for the rest of the patients.
CONCLUSIONS
The diagnosis of ASAP is a strong risk factor for prostate cancer. A repeat biopsy should be performed for the entire prostate subsequent to the diagnosis of ASAP and the patients should be closely followed for the risk of cancer. If the initial biopsies are performed with multiple sampling, the final decision should be based on other parameters, such as PSA values and rectal examinations in patients with a biopsy result of HGPIN. We need to carefully follow-up such patients for their progression towards cancer in the future.
Footnotes
The authors have nothing to disclose.
References
- 1.Moore CK, Karikehalli S, Nazeer T, Fisher HA, Kaufman RP, Jr, Mian BM. Prognostic significance of high grade prostatic intraepithelial neoplasia and atypical small acinar proliferation in the contemporary era. J Urol. 2005;173:70–72. doi: 10.1097/01.ju.0000148260.69779.c5. [DOI] [PubMed] [Google Scholar]
- 2.Djavan B, Zlotta AR, Ekane S, Remzi M, Kramer G, Roumeguère T, et al. Is one set of sextant biopsies enough to rule out prostate Cancer? Influence of transition and total prostate volumes on prostate cancer yield. Eur Urol. 2000;38:218–224. doi: 10.1159/000020282. [DOI] [PubMed] [Google Scholar]
- 3.Borboroglu PG, Comer SW, Riffenburgh RH, Amling CL. Extensive repeat transrectal ultrasound guided prostate biopsy in patients with previous benign sextant biopsies. J Urol. 2000;163:158–162. [PubMed] [Google Scholar]
- 4.Leite KR, Srougi M, Dall'Oglio MF, Sanudo A, Camara-Lopes LH. Histopathological findings in extended prostate biopsy with PSA < or = 4 ng/mL. Int Braz J Urol. 2008;34:283–290. doi: 10.1590/s1677-55382008000300005. [DOI] [PubMed] [Google Scholar]
- 5.Polat K, Tüzel E, Aktepe F, Akdoğan B, Güler C, Uzun İ. Investigation of the incidence of latent prostate cancer and high-grade prostatic intraepithelial neoplasia in an autopsy series of Turkish males. Turkish Journal of Urology. 2009;35:96–100. [Google Scholar]
- 6.Taş M, Kaygısız O, Inal G, Uğurlu Ö, Adsan Ö, Öztürk B. Comparison of patient comfort and complications of transrectal ultrasonography guided prostate biopsies using 16 and 18 gauge needles. Turkish Journal of Urology. 2005;31:119–122. [Google Scholar]
- 7.Keetch DW, Catalona WJ, Smith DS. Serial prostatic biopsies in men with persistently elevated serum prostate specific antigen values. J Urol. 1994;151:1571–1574. doi: 10.1016/s0022-5347(17)35304-1. [DOI] [PubMed] [Google Scholar]
- 8.Bostwick DG, Amin MB, Dundore P, Marsh W, Schultz DS. Architectural patterns of high-grade prostatic intraepithelial neoplasia. Hum Pathol. 1993;24:298–310. doi: 10.1016/0046-8177(93)90041-e. [DOI] [PubMed] [Google Scholar]
- 9.Eskicorapci SY, Guliyev F, Islamoglu E, Ergen A, Ozen H. The effect of prior biopsy scheme on prostate cancer detection for repeat biopsy population: results of the 14-core prostate biopsy technique. Int Urol Nephrol. 2007;39:189–195. doi: 10.1007/s11255-006-9009-5. [DOI] [PubMed] [Google Scholar]
- 10.Epstein JI, Herawi M. Prostate needle biopsies containing prostatic intraepithelial neoplasia or atypical foci suspicious for carcinoma: implications for patient care. J Urol. 2006;175:820–834. doi: 10.1016/S0022-5347(05)00337-X. [DOI] [PubMed] [Google Scholar]
- 11.Meng MV, Shinohara K, Grossfeld GD. Significance of high-grade prostatic intraepithelial neoplasia on prostate biopsy. Urol Oncol. 2003;21:145–151. doi: 10.1016/s1078-1439(03)00009-7. [DOI] [PubMed] [Google Scholar]
- 12.Lefkowitz GK, Taneja SS, Brown J, Melamed J, Lepor H. Followup interval prostate biopsy 3 years after diagnosis of high grade prostatic intraepithelial neoplasia is associated with high likelihood of prostate cancer, independent of change in prostate specific antigen levels. J Urol. 2002;168:1415–1418. doi: 10.1016/S0022-5347(05)64463-1. [DOI] [PubMed] [Google Scholar]
- 13.Humperey P. The prostate gland. In: Silverbeng SG, Delellis RA, Frable WJ, editors. Silverberg's principles and practice of surgical pathology and chytopathology. 4th ed. New York: Churchill Livingstone; 2006. pp. 1791–1830. [Google Scholar]
- 14.Iczkowski KA, MacLennan GT, Bostwick DG. Atypical small acinar proliferation suspicious for malignancy in prostate needle biopsies: clinical significance in 33 cases. Am J Surg Pathol. 1997;21:1489–1495. doi: 10.1097/00000478-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Amin M, Boccon-Gibod L, Egevad L, Epstein JI, Humphrey PA, Mikuz G, et al. Prognostic and predictive factors and reporting of prostate carcinoma in prostate needle biopsy specimens. Scand J Urol Nephrol Suppl. 2005;216:20–33. doi: 10.1080/03008880510030923. [DOI] [PubMed] [Google Scholar]
- 16.Hong YM, Lai FC, Chon CH, McNeal JE, Presti JC., Jr Impact of prior biopsy scheme on pathologic features of cancers detected on repeat biopsies. Urol Oncol. 2004;22:7–10. doi: 10.1016/S1078-1439(03)00147-9. [DOI] [PubMed] [Google Scholar]
- 17.Levine MA, Ittman M, Melamed J, Lepor H. Two consecutive sets of transrectal ultrasound guided sextant biopsies of the prostate for the detection of prostate cancer. J Urol. 1998;159:471–475. doi: 10.1016/s0022-5347(01)63951-x. [DOI] [PubMed] [Google Scholar]
- 18.Mistry K, Cable G. Meta-analysis of prostate-specific antigen and digital rectal examination as screening tests for prostate carcinoma. J Am Board Fam Pract. 2003;16:95–101. doi: 10.3122/jabfm.16.2.95. [DOI] [PubMed] [Google Scholar]
- 19.Wright JL, Lange PH. Newer potential biomarkers in prostate cancer. Rev Urol. 2007;9:207–213. [PMC free article] [PubMed] [Google Scholar]
- 20.Khan MA, Sokoll LJ, Chan DW, Mangold LA, Mohr P, Mikolajczyk SD, et al. Clinical utility of proPSA and "benign" PSA when percent free PSA is less than 15% Urology. 2004;64:1160–1164. doi: 10.1016/j.urology.2004.06.033. [DOI] [PubMed] [Google Scholar]