Abstract
Purpose
This study was designed to evaluate the association of metabolic syndrome and benign prostate enlargement in young Korean males. We analyzed the clinical data associated with metabolic syndrome and prostate volume in the study population.
Materials and Methods
We retrospectively analyzed the clinical data obtained from 1,506 young men under the age of 60 who visited the health promotion center in our institution for routine checkups. The patients were interviewed with a questionnaire including the International Prostate Symptom Score (IPSS) and were evaluated by medical history, blood chemistry, digital rectal examination, and prostate volume via transrectal ultrasonography. The presence of metabolic syndrome was determined according to the modified National Cholesterol Education Program Expert Panel on Detection, Evalution, And Treatment of High Blood Cholesterol in Adults criteria. We divided the subjects into two groups: those with metabolic syndrome and those without. Logistic regression analysis was carried out to determine which metabolic components were associated with an increased risk of benign prostate enlargement.
Results
Significant differences in prostate volume were noted between the groups. The prostate volumes were significantly larger in the metabolic syndrome group than in the non-metabolic syndrome group in all subgroups divided by age (in decades). However, no significant differences in IPSS or voiding or storage subscore were noted. In the multivariate regression analysis, only diabetes and obesity were identified as risk factors for benign prostate enlargement among the metabolic components.
Conclusions
Metabolic syndrome and prostate volume were significantly related, even in young males. Diabetes and obesity were identified as significant risk factors for benign prostate enlargement in young males under the age of 60.
Keywords: Metabolic syndrome X, Prostatic hyperplasia
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a prevalent disorder among older men and has received more attention as the average human lifespan has increased. In Korea, the prevalence of clinical prostatic hyperplasia was reported to range from 10.6% to 31% in men over 50 years of age, with an age-related increase [1,2].
The metabolic syndrome (MS) is a clinical constellation of metabolic abnormalities associated with an increased risk of cardiovascular disease. The major disorders in MS, which is characterized by insulin resistance and hyperinsulinemia, are localized in muscle, fat tissue, and the liver [3]. The components of this syndrome are type II diabetes mellitus, hypertension, obesity, and dyslipidemia. These components are proposed as risk factors for the development of prostatic hyperplasia [4,5].
In Korea, several studies have reported that men with MS had larger prostate volumes [6-8]. So far, however, there are insufficient data on the association of MS with prostate volume in young Korean men. We therefore investigated the possible association of MS with prostate volume in men under the age of 60.
MATERIALS AND METHODS
1. Study population and data collection
From January 2006 to September 2010, a total of 1,506 men aged between 30 and 60 years underwent prostate evaluation as a special option during routine health checkups in the Health Promotion Center in our institution. All men completed the International Prostate Symptom Score (IPSS) questionnaire, a digital rectal examination, transrectal ultrasonography (TRUSG), and anthropometric measurements, such as height, weight, and waist circumference. Body mass index was calculated by dividing weight (kg) by the surface area (m2) of the patients. The volume of the prostate was calculated by elliptical volume measurement (π/6 x transverse x anteroposterior x cephalocaudal diameter), and benign prostate enlargement (BPE) was defined as TRUSG-measured prostate volume over 20 ml. Blood samples were drawn from fasting patients to determine fasting blood sugar, high-density lipoprotein (HDL) cholesterol, and triglyceride. Self-reported information on medical history, major co-morbidities, lifestyle, and psychosocial factors as well as symptoms of urological conditions was also collected. Additionally, we collected information on prostate-related medical or surgical treatment history. We excluded 149 men who had a history of prostatitis, high prostate-specific antigen (PSA) (≥4 ng/ml), or abnormal findings on the digital rectal exam or TRUSG.
2. Assessment of metabolic syndrome
In this study, MS was defined by using a previously published modification of the National Cholesterol Education Program Expert Panel on Detection, Evalution, And Treatment of High Blood Cholesterol in Adults guidelines as the presence of 3 or more of the following 5 characteristics [9]: 1) waist circumference greater than 90 cm or body mass index (BMI) higher than 25 kg/m2; 2) systolic blood pressure 130 mmHg or greater or diastolic blood pressure 85 mmHg or greater, or antihypertensive medication use; 3) fasting blood sugar greater than 110 mg/dl or self-reported diabetes medication use; 4) triglyceride greater than 150 mg/dl; 5) HDL cholesterol less than 45 mg/dl or lipid medication use. As described above, the diagnosis of metabolic syndrome, including treated hypertension, diabetes mellitus, and hyperlipidemia, was provided by the patient's medical history. In the entry for obesity, BMI and waist circumference were applied in the Asia-Pacific perspective.
3. Statistical analysis
All statistical analysis was performed by using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). We divided the study population into two groups: the MS group and the non-MS group. We compared the IPSS, voiding symptom subscore, storage symptom subscore, quality of life (QoL), and prostate- related parameters between the two groups. Statistical analysis including Student's t-test, Pearson's correlation coefficient, and logistic regression analysis was performed. Student's t-test was used to describe the difference in prostate volume and voiding-related symptom score. Pearson's correlation coefficient was used to test the linearity of the relationships between metabolic components and prostate volume. We used logistic regression analysis to determine the risk factors of BPE. In all comparisons of values, p-values of less than 0.05 were considered to be statistically significant.
RESULTS
1. Principal patient characteristics
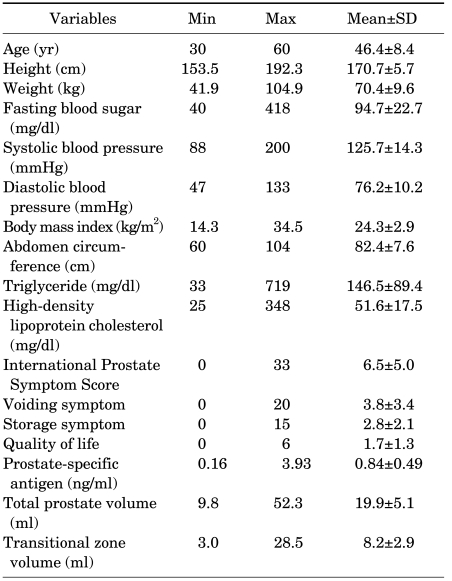
A total of 1,357 men aged 30 to 60 years were analyzed. Mean age at baseline in the entire cohort was 46.4±8.4 years. The age distribution of the patients was as follows: 389, 481, and 487 men were in their 30s (28.7%), 40s (35.4%), and 50s (35.9%), respectively. The median total prostate volume and transitional zone volume were 19.9±5.1 and 8.2±2.9, respectively. The descriptive data, including the voiding-related symptom score, QoL, PSA, and all anthropometric parameters in the men, are listed in Table 1.
TABLE 1.
Characteristics of the participants
2. Comparisons of variables between the metabolic syndrome group and the non-metabolic syndrome group
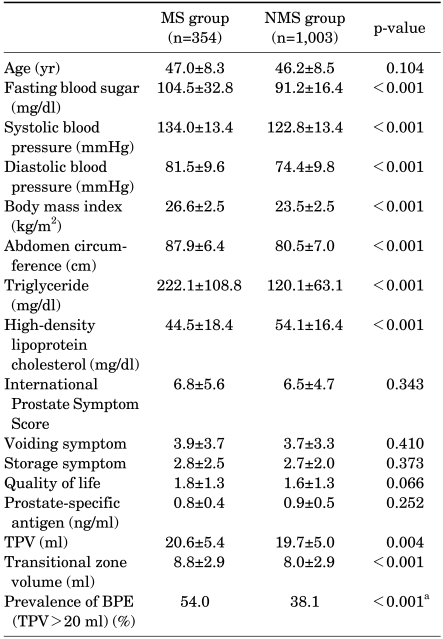
The prevalence of MS was 26.1%. Subjects with MS were older than subjects without MS but not significantly so. The mean total prostate volume and transitional zone volume of the MS group were larger than those of the non-MS group (20.6±5.4 vs. 19.7±5.0, p=0.004, and 8.8±2.9 vs. 8.0±2.9, p<0.001). There was also a significant difference in the prevalence of BPE (54.0% vs 38.1%, p<0.001) (Table 2). However, there were no statistically significant differences between the two groups regarding median age, IPSS, voiding and storage subscores, QoL, and PSA.
TABLE 2.
Comparison of the metabolic components and symptom scores between the metabolic syndrome group and non-metabolic syndrome group
MS: metabolic syndrome, TPV: total prostate volume, BPE: benign prostate enlargement, a: χ2 analysis
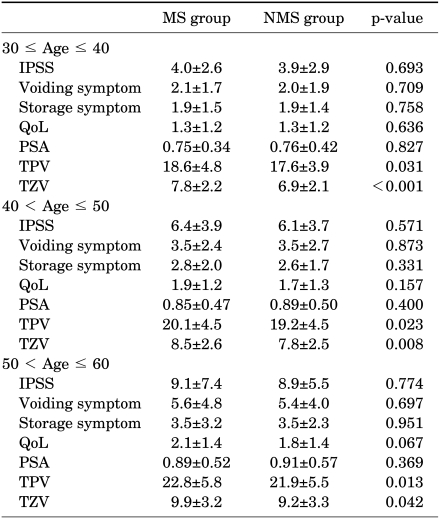
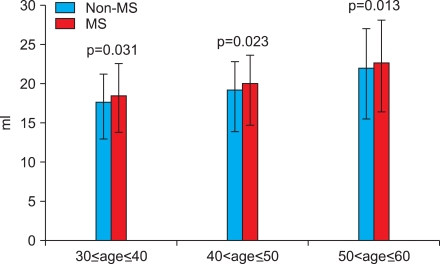
Because age is a common risk factor for MS and BPE, we performed subgroup analysis by dividing the patients into age decades. In all age decades from 30 to 60, prostate volumes were larger with statistical significance in the MS group than in the non-MS group (Table 3 and Fig. 1). However, no statistical significance was found in the IPSS, voiding and storage subscores, QoL, or PSA in the subgroup analysis (Table 3).
TABLE 3.
Comparison of the metabolic syndrome group and non-metabolic syndrome group according to age distribution
Satistical analysis by student's t-test, MS: metabolic syndrome, NMS: non-metabolic syndrome, IPSS: International Prostate Symptom Score, QoL: quality of life, PSA: prostate-specific antigen, TPV: total prostate volume, TZV: transitional zone volume, Statistical analysis by Student's t-test
FIG. 1.
Comparison of prostate volume between the metabolic syndrome group and the non-metabolic syndrome group. MS: metabolic syndrome group, Statistical analysis by Student's t-test.
3. Risk factors for benign prostate enlargement
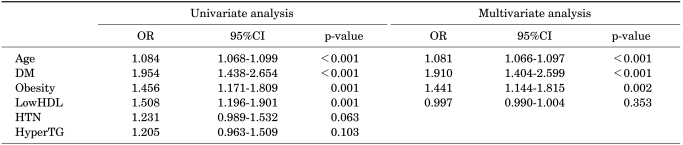
In the univariate logistic regression analysis, age, diabetes, obesity, and low HDL were significantly related to the presence of BPE. In the multivariate logistic regression analysis, only age, diabetes, and obesity were significant predictors of BPE (Table 4).
TABLE 4.
Logistic regression analysis to determine predictors of BPE (TPV>20 g)
BPE: benign prostate enlargement, TPV: total prostate volume, OR: odd ratio, CI: confidence interval, DM: diabetes mellitus, LowHDL: low high-density lipoprotein cholesterol, HTN: hypertension, HyperTG: hypertriglyceridemia
DISCUSSION
MS is a highly prevalent disorder and has been regarded as a risk factor for coronary disease, such as in type 2 diabetes mellitus, hypertension, obesity, and hyperlipidemia. The principal pathophysiology of MS is secondary hyperinsulinemia caused by tissue insulin resistance, which stimulates the autonomic nervous system, especially the sympathetic nerve system, thus resulting in bladder outlet obstruction [10,11]. Generally, metabolic components in adult males are highly prevalent, and the prevalence increases with increasing age.
Several studies have suggested that the components of metabolic syndrome are risk factors for prostatic hyperplasia [12-14]. As early as 1998, Hammarsten et al reported the relationship between MS and prostatic hyperplasia [12]. In their study, the annual prostate growth rate was significantly higher in prostatic hyperplasia patients with MS than in prostatic hyperplasia patients without MS. Ozden et al studied 78 men with prostatic hyperplasia and lower urinary tract symptoms and concluded that the presence of MS was associated with significantly higher total prostate volume and median annual transition zone growth rate [14]. Dahle et al reported that some metabolic components (such as abdominal obesity and hyperinsulinemia) are associated with a high risk of prostatic hyperplasia [13]. Several Korean studies have also reported that men with MS are more likely to have a large prostate volume [6-8].
Several studies have identified obesity and diabetes as risk factors for prostatic hyperplasia [13,15,16]. Parsons et al reported a positive relationship between prostate volume and obesity, elevated fasting plasma glucose, and diabetes [15]. Dahle et al also suggested that abdominal obesity and increasing serum insulin level are associated with a higher risk of prostatic hyperplasia [13]. The finding that men suffering from diabetes and obesity had larger prostate glands than did men without these disorders suggests that hyperinsulinemia may be related to the development of prostate enlargement, because all these conditions are associated with hyperinsulinemia.
Insulin resistance and secondary hyperinsulinemia constitute important etiologic links between MS and increased prostate enlargement. As has been demonstrated in many studies, diabetes and hyperinsulinemia are closely related [17,18]. Hammarsten et al suggested that prostatic hyperplasia may be an insulin resistance condition with secondary hyperinsulinemia as a possible etiological factor for prostate enlargement [12]. High insulin levels may induce prostatic growth via a variety of mechanisms. One pathway that explains the increased risk for prostate enlargement in hyperinsulinemia is the insulin-like growth factor (IGF) axis. IGF-1 is a strong mitogen and increases cell proliferation and induces apoptosis in many tissues, including prostatic stroma and epithelium [4]. Similarly, increased glucose levels are likely to be accompanied by hyperinsulinemia, which results in an increase in insulin-like growth factor, a known prostatic mitogen, and induces a reduction in proapoptotic cascades within the prostate [5]. These changes should culminate in increased prostate growth. Furthermore, hyperinsulinemia increases catecholamine levels in plasma and tissues [19], which may have a trophic effect on the growth of prostatic cells by down-regulating the apoptotic progress, thereby suggesting a link between hyperinsulinemia and the development of prostate enlargement. In a previous clinical study, doxazosin, an alpha-blocker used for symptomatic prostatic hyperplasia treatment, was shown to increase insulin sensitivity and reduce insulin levels [20].
Obesity has been implicated in the etiology of BPE due to its influence on metabolic and endocrine changes. Adipose tissue, which accumulates with age, aromatizes circulating testosterone into testosterone into estrogen, and it has been hypothesized that alterations in the balance between testosterone and estrogen levels in prostate tissue with age may contribute to prostate enlargement [21]. Abdominal obesity increases the estrogen/androgen ratio and may induce sympathetic nervous activity, both of which are known to affect the development of prostate enlargement and the severity of urinary obstructive symptoms [22].
The results of previous studies have suggested a possible relationship between obesity and prostate enlargement. Hammarsten and Hogstedt examined 250 men with lower urinary tract symptoms with or without manifestations of MS [23]. BMI, waist circumference, hip measurement, and the waist/hip ratio were all significantly associated with an increased rate of growth of prostatic hyperplasia. Kristal et al examined several modifiable lifestyle factors associated with the development of symptomatic prostatic hyperplasia [24]. They discovered significant increases in symptomatic prostatic hyperplasia with abdominal obesity as measured by the waist/hip ratio. Moreover, they also suggested that clinical perspective weight loss may prove useful for the management of the prostatic hyperplasia symptoms. Additionally, men with greater abdominal adiposity were reported to be more likely to undergo surgery for prostatic hyperplasia and also to be more likely to exhibit frequent obstructive urinary symptoms [22]. The results of some Korean studies have suggested that obesity is associated with large prostate volume. Kim et al reported that patients with an elevated BMI tended to have larger prostate volumes [25]. Additionally, Kim et al also reported that prostate volume was correlated positively with all obesity- related parameters: BMI, waist circumference, and waist-to-hip ratio [26]. In another study, prostate volume was greater in the obese groups than in the normal group; the results of this study suggested that central obesity (>90 cm) is an important risk factor for prostatic hyperplasia (>20 ml) [27].
In our study, total prostate volume and transitional zone volume in the MS group were significantly larger than in the non-MS group. Also, in the subgroup analysis by age decades, significant differences in the prevalence of BPE were noted. Prostate volumes in the MS groups were significantly larger than in the non-MS groups in all age subgroups. With increasing age, this trend tends to become more evident. The presence of diabetes and obesity were also significant risk factors for BPE as measured by TRUSG. Although the clinical significance was unclear, statistical significance in prostate volume was found even in men with metabolic syndrome from as early an age as their thirties.
However, our study has its own limitations. This study was conducted in a single institution and may have been subject to selection bias. Further large-scale studies in the general population will be necessary to confirm our results.
CONCLUSIONS
Metabolic syndrome and prostate volume were significantly related even in young males. Diabetes and obesity were identified as significant risk factors for benign prostate enlargement in young males under the age of 60.
Footnotes
The authors have nothing to disclose.
References
- 1.Park YH, Chung MK. The prevalence of clinical benign prostatic hyperplasia and lower urinary tract symptoms in South-East Korea: a community-based study. J Pusan Natl Univ Hosp. 2001;9:141–157. [Google Scholar]
- 2.Lee MW, Lee KS. The prevalence of benign prostatic hyperglasia in self-referral populations over age 50. Korean J Urol. 1996;37:263–267. [Google Scholar]
- 3.Krotkiewski M. Role of muscle morphology in the development of insulin resistance and metabolic syndrome. Presse Med. 1994;23:1393–1399. [PubMed] [Google Scholar]
- 4.Peehl DM, Cohen P, Rosenfeld RG. The role of insulin-like growth factors in prostate biology. J Androl. 1996;17:2–4. [PubMed] [Google Scholar]
- 5.Kasturi S, Russell S, Mcvary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006;7:288–292. doi: 10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 6.Sohn JC, Chang HS, Kim CI. The correlation between metabolic syndrome and the prostate volume. Korean J Urol. 2007;48:603–607. [Google Scholar]
- 7.Koo KC, Cho KS, Kang EM, Kwon SW, Hong SJ. The relationship between metabolic syndrome and prostate volume in men over sixties who underwent prostate health check-up. Korean J Urol. 2008;49:813–817. [Google Scholar]
- 8.Park JS, Park JK. The meaning of metabolic syndrome X in patients suffering with benign prostatic hyperplasia. Korean J Urol. 2007;48:696–700. [Google Scholar]
- 9.Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 11.Rett K, Wicklmayr M, Mehnert H. New aspects of insulin resistance in hypertension. Eur Heart J. 1994;15(Suppl C):78–81. doi: 10.1093/eurheartj/15.suppl_c.78. [DOI] [PubMed] [Google Scholar]
- 12.Hammarsten J, Hogstedt B, Holthuis N, Mellstrom D. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 1998;1:157–162. doi: 10.1038/sj.pcan.4500221. [DOI] [PubMed] [Google Scholar]
- 13.Dahle SE, Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Hsing AW. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002;168:599–604. [PubMed] [Google Scholar]
- 14.Ozden C, Ozdal OL, Urgancioglu G, Koyuncu H, Gokkaya S, Memis A. The correlation between metabolic syndrome and prostatic growth in patients with benign prostatic hyperplasia. Eur Urol. 2007;51:199–203. doi: 10.1016/j.eururo.2006.05.040. [DOI] [PubMed] [Google Scholar]
- 15.Parsons JK, Carter HB, Partin AW, Windham BG, Metter EJ, Ferrucci L, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab. 2006;91:2562–2568. doi: 10.1210/jc.2005-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yim SJ, Cho YS, Joo KJ. Relationship between metabolic syndrome and prostate volume in Korean men under 50 years of age. Korean J Urol. 2011;52:390–395. doi: 10.4111/kju.2011.52.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med. 1990;113:909–915. doi: 10.7326/0003-4819-113-12-909. [DOI] [PubMed] [Google Scholar]
- 18.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 19.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–1090. [PubMed] [Google Scholar]
- 20.Shieh SM, Sheu WH, Shen DC, Fuh MM, Chen YD, Reaven GM. Glucose, insulin, and lipid metabolism in doxazosin-treated patients with hypertension. Am J Hypertens. 1992;5:827–831. doi: 10.1093/ajh/5.11.827. [DOI] [PubMed] [Google Scholar]
- 21.Shibata Y, Ito K, Suzuki K, Nakano K, Fukabori Y, Suzuki R, et al. Changes in the endocrine environment of the human prostate transition zone with aging: simultaneous quantitative analysis of prostatic sex steroids and comparison with human prostatic histological composition. Prostate. 2000;42:45–55. doi: 10.1002/(sici)1097-0045(20000101)42:1<45::aid-pros6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Rimm EB, Chute CG, Kawachi I, Colditz GA, Stampfer MJ, et al. Obesity and benign prostatic hyperplasia. Am J Epidemiol. 1994;140:989–1002. doi: 10.1093/oxfordjournals.aje.a117206. [DOI] [PubMed] [Google Scholar]
- 23.Hammarsten J, Högstedt B. Clinical, anthropometric, metabolic and insulin profile of men with fast annual growth rates of benign prostatic hyperplasia. Blood press. 1999;8:29–36. doi: 10.1080/080370599438365. [DOI] [PubMed] [Google Scholar]
- 24.Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Weiss N, Goodman P, et al. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395–1400. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Song PH, Kim HT, Moon KH. Effect of obesity on prostate-specific antigen, prostate volume, and International Prostate Symptom Score in patients with benign prostatic hyperplasia. Korean J Urol. 2011;52:401–405. doi: 10.4111/kju.2011.52.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim GW, Doo SW, Yang WJ, Song YS. Effects of obesity on prostate volume and lower urinary tract symptoms in Korean men. Korean J Urol. 2010;51:344–347. doi: 10.4111/kju.2010.51.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Min HG, Choi SH, Kim YJ, Oh SW, Kim YJ, et al. Central obesity as a risk factor for prostatic hyperplasia. Obesity (Silver Spring) 2006;14:172–179. doi: 10.1038/oby.2006.21. [DOI] [PubMed] [Google Scholar]