Abstract
Poisoning by paraquat herbicide is a major medical problem in parts of Asia while sporadic cases occur elsewhere. The very high case fatality of paraquat is due to inherent toxicity and lack of effective treatments. We conducted a systematic search for human studies that report toxicokinetics, mechanisms, clinical features, prognosis and treatment. Paraquat is rapidly but incompletely absorbed and then largely eliminated unchanged in urine within 12–24 h. Clinical features are largely due to intracellular effects. Paraquat generates reactive oxygen species which cause cellular damage via lipid peroxidation, activation of NF-κB, mitochondrial damage and apoptosis in many organs. Kinetics of distribution into these target tissues can be described by a two-compartment model. Paraquat is actively taken up against a concentration gradient into lung tissue leading to pneumonitis and lung fibrosis. Paraquat also causes renal and liver injury. Plasma paraquat concentrations, urine and plasma dithionite tests and clinical features provide a good guide to prognosis. Activated charcoal and Fuller's earth are routinely given to minimize further absorption. Gastric lavage should not be performed. Elimination methods such as haemodialysis and haemoperfusion are unlikely to change the clinical course. Immunosuppression with dexamethasone, cyclophosphamide and methylprednisolone is widely practised, but evidence for efficacy is very weak. Antioxidants such as acetylcysteine and salicylate might be beneficial through free radical scavenging, anti-inflammatory and NF-κB inhibitory actions. However, there are no published human trials. The case fatality is very high in all centres despite large variations in treatment.
Keywords: paraquat, self-poisoning, treatment
Introduction
Self-poisoning with pesticides is a major public health problem in developing countries with an estimated 300 000 deaths occurring in the Asia–Pacific region alone each year [1, 2]. For example, in Sri Lanka there are 3–400 self-poisonings with pesticides per 100 000 population each year [3, 4]. While the organophosphate class accounts for the majority of hospital admissions, the very high case fatality (>50%) of paraquat means that it is the leading single agent causing death from pesticide poisoning in many countries including Sri Lanka [5, 6]. Paraquat self-poisoning is not only a problem of the Asia–Pacific region. In 1986 to 1990, 63% of all suicide deaths in Trinidad and Tobago were due to paraquat [7]. A similar high contribution to total suicides was reported from south Trinidad (76% between 1996 and 1997) [7] and Samoa (70% between 1979 and 2000) [8]. The problem is not even confined to developing countries. For example, between 1945 and 1989, paraquat was responsible for 56% of all pesticide deaths in England and Wales [9, 10]. It was even responsible for more deaths in the American Association of Poison Control Centers' National Poison Data System in 2008 than any other pesticide [11]. In Sri Lanka, there have been trials of new formulations to reduce toxicity and recently a decrease in the maximum available concentration. These have had only very modest effects on case fatality [12, 13]. Very recently, paraquat has been banned in most European countries and also in Sri Lanka.
The very high case fatality of paraquat is due both to its inherent toxicity and the lack of any effective treatment. There are no widely accepted guidelines on treatment of patients with paraquat self-poisoning and the treatment varies from supportive care alone to various combinations of immune-modulation, anti-oxidant therapy, haemoperfusion and haemodialysis. However, the overall mortality remains >50% in centres routinely practising such intensive measures. Furthermore, these treatment options have been largely based on extrapolation of evidence from animal studies which often give the antidote before the poisoning, and small, mostly uncontrolled, and highly selected case series conducted in resource-poor settings with insufficient documentation of severity. Despite (or because of) the lack of a strong evidence base or consistent recommendations, any rational approach to treatment should consider both the mechanism of toxicity and toxicokinetics of paraquat poisoning.
We carried out a systematic search for relevant clinical studies searching PubMed, the UK National Research Register, the Injuries Group specialized Register, Clinicaltrials.gov and the Cochrane database by using the words ‘paraquat’ and ‘poisoning’. We also used unpublished information from our ongoing clinical trials and cohort studies of paraquat self-poisoning that have recruited close to 800 patients in Sri Lanka to date.
Mechanism of toxicity of paraquat self-poisoning
Generation of free radicals and oxidative stress
Paraquat-induced toxicity is a manifestation of its ability to undergo redox-cycling and subsequent generation of reactive oxygen species (ROS) [14–17]. Paraquat is metabolized by several enzyme systems (NADPH-cytochrome P450 reductase, xanthine oxidase, NADH-ubiquinone oxidoreductase and nitric oxide synthase) [18–22]. Its metabolism through these systems generates a paraquat mono-cation radical (PQ.+). Inside the cell, PQ.+ rapidly gets re-oxidized to PQ2+ and in the process it generates superoxide (O2.−). O2 acts as an electron acceptor and NADP as an electron donor in this reaction. This further gives rise to formation of the hydroxyl free radical (HO.) in the presence of iron via the Fenton reaction (Figure 1). NO. combines with O2.− to generate peroxinitrite (ONOO-) which is a very strong oxidant and a nitrating intermediate. NO. is enzymatically produced from L-arginine by NO synthase, and PQ also directly or indirectly induces NO synthase-mediated nitric oxide production [23]. Generation of highly reactive oxygen and nitrite species results in toxicity in most organs but the toxicity is particularly severe in the lungs as paraquat is taken up against a concentration gradient in to the lung [24].
Figure 1.
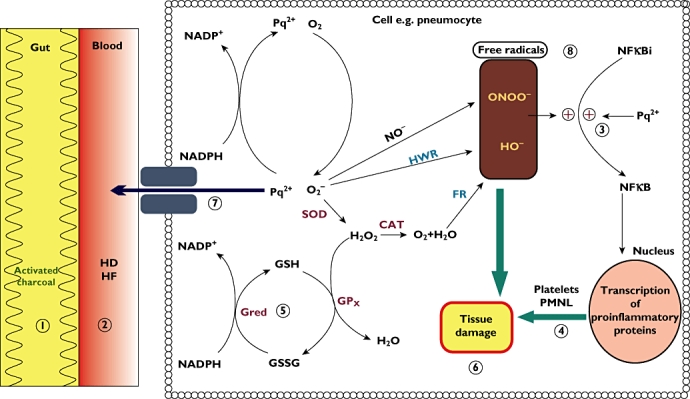
Graphical representation of paraquat toxicity inside a pneumocyte and potential sites of antidotal therapy. SOD, superoxide dismutase; CAT, catalase; Gred, glutathione reductase; Gpx, glutathione peroxidase; FR, Fenton reaction; HWR, Haber-Weiss reaction. 1–8: potential sites of action by available treatment options. 1: activated charcoal and Fuller's earth; 2: dialysis; 3, 4, 6 and 8: salicylates; 5 and 8: N-acetylcysteine; 7 (P-glycoprotein induction): dexamethasone; 4: immunosuppression
Secondary effects of oxidative stress
Lipid peroxidation
Electrophilic free radicals can extract hydrogen atoms from polyunsaturated fatty acids thus causing lipid peroxidation. In vitro, animal and human studies have demonstrated that paraquat can induce lipid peroxidation [25–27]. Widespread lipid peroxidation compromises cell membrane function and may trigger apoptosis. Lipid peroxidation is considered by some to be a key initial pathophysiological process in the cascade of events following paraquat poisoning, although the primary importance of this mechanism is not universally accepted, with others postulating these effects follow other pathological processes [28, 29].
Mitochondrial toxicity
Paraquat has been shown to cause mitochondrial damage in various cell lines. Paraquat appears to be principally reduced by complex I (NADH-ubiquinone oxidoreductase) in mitochondria [30]. Experiments with disrupted mitochondria showed that once in the matrix, paraquat reduced by complex I in mammals forms superoxide [31]. Paraquat induces a Ca2+-dependent permeability increase of the inner mitochondrial membrane (possibly due to lipid peroxidation) leading to membrane depolarization, uncoupling and matrix swelling [32]. There is a time dependency of paraquat effects such that mitochondrial complex I activities in rat brain as well as those in rat lung and liver decrease progressively within a few hours of exposure [33].
Oxidation of NADPH
Paraquat redox cycling rapidly oxidizes NADPH and this has been postulated to lead to secondary changes on cellular metabolism and impairs defenses against oxidative stress (e.g. decreased glutathione production) [34]. Fructose diphosphate worsens paraquat toxicity and this effect is attributed to negative effects on NADPH replenishment through inhibition of the pentose-phosphate shunt [35].
Activation of nuclear factor kappa B (NF-κB)
Reactive oxygen species are well known to activate NF-κB from its dormant form [36]. In normal cells NF-κB is bound to an inhibitory protein (IκBα). IκBα is rapidly phosphorylated by inducers of NF-κB [37]. Once activated, NF-κB is translocated into the nucleus and binds to promoter regions and induces target genes involved in inflammation. As a result, NF-κB induces transcription of inflammatory enzymes, cytokines and chemokines. This leads to platelet aggregation, fibrogenesis and attraction of inflammatory cells [38].
Apoptosis
Paraquat can induce apoptosis by production of ROS and activation of NF-κB. This leads to nuclear condensation and DNA fragmentation [39–41]. Peroxinitrite also reacts with proteins, lipids and DNA altering cellular enzymatic and signalling pathways causing disruption of homeostasis and apoptosis [42].
Pathological processes in the major target organs
The above mechanisms are not exclusive; indeed, all may occur and are very likely to be synergistic. The multiplicity of pathways may be the underlying explanation for the observation that no agent aimed at any particular mechanism has been shown to alter toxicity substantially when given after poisoning. Indeed, the most widely used treatments are aimed at more non-specific secondary pathological processes such as inflammation.
Paraquat toxicity is most severe in the lungs and leads to an acute alveolitis. Further effects include diffuse alveolar collapse, vascular congestion and adherence of activated platelets and polymorphonuclear leucocytes to the vascular endothelium [43–45]. In the lung, as in most tissues, paraquat toxicity leads to apoptosis of affected cells [46–49].
The primary target of toxicity in the lung is the alveolar epithelium. During the acute ‘destructive phase’ both type I and type II pneumocytes demonstrate swelling, vacuolation and disruption of mitochondria and the endoplasmic reticulum. Sloughing of the alveoli is associated with pulmonary oedema. This initial phase is followed by a proliferative phase where the alveolar space is filled with mononuclear profibroblasts which mature into fibroblasts within days to weeks. This stage is followed by lung fibrosis [50–53]. Kidneys exposed to paraquat demonstrate development of large vacuolation in proximal convoluted tubules which leads to necrosis [54]. Congestion and hepatocellular injury associated with rough and smooth endoplasmic reticulum degranulation and mitochondrial damage occur in the liver. These changes can be observed within a few hours to days [55].
Toxicokinetics of paraquat
Upon ingestion, paraquat is rapidly but incompletely absorbed. It is rapidly distributed to lung, liver, kidney and muscle. Paraquat has a volume of distribution of 1.2–1.6 l kg−1[56]. Within 12–24 h after ingestion 90% of the absorbed paraquat is rapidly excreted unchanged in urine. Plasma paraquat concentrations can be well described by a two-compartment model with time-dependent elimination from the central compartment [57]. The kinetic parameters are non-linear largely due to progressive marked toxic effects on the organs that determine bioavailability and elimination. Bioavailability appears to increase substantially with increasing doses, perhaps due to gut and liver toxicity. After a few hours, renal clearance declines rapidly in severe poisoning. Thus, the small proportion of paraquat that distributes into the deeper compartments is only slowly eliminated by the kidneys over many days to weeks [56]. The initial elimination half-life is around 6 h but this is around 4 days after the first day [56]. Paraquat is actively taken up against a marked concentration gradient into the type II pneumocyte [58, 59] which could be considered a third ‘toxic effect’ compartment. Elimination from this compartment is even slower than from the other deep compartments.
Clinical course
The clinical manifestations that follow paraquat ingestion depend upon the quantity ingested [60, 61]. Ingestion of large amounts of liquid concentrate (>50–100 ml of 20% ion w/v) results in fulminant organ failure: pulmonary oedema, cardiac, renal and hepatic failure and convulsions due to CNS involvement. These patients generally have hypoxia, shock and a metabolic acidosis at presentation. Death results from multiple organ failure within several hours to a few days.
Ingestion of smaller quantities usually leads to toxicity in the two key target organs (kidneys and lungs) developing over the next 2–6 days. This is often referred to as ‘moderate to severe’ poisoning in the clinical literature. This grading only makes sense relative to the fulminant group as mortality in this group is still well over 50%. Renal failure develops quite rapidly, and creatinine and/or cystatin-C concentrations can be monitored over the first day to detect this group and these also predict long-term outcome [62, 63]. However, the major effect of this quantity of paraquat follows its accumulation in the lungs with lung cell damage producing decreased gas exchange and respiratory impairment. The pulmonary lesion has two phases: an acute alveolitis over 1–3 days followed by a secondary fibrosis. The patient typically develops increasing signs of respiratory involvement over 3–7 days and ultimately dies of severe anoxia due to rapidly progressive fibrosis up to 5 weeks later. Some liver toxicity (jaundice, transaminase rise) is also common in these patients. However, neither renal nor liver damage is the usual mode of death and in survivors no long-term effects on these organs have been reported.
Gastrointestinal toxicity is universal in those ingesting paraquat concentrate. Mucosal lesions of the mouth and the tongue (‘paraquat tongue’) begin to appear within the first few days and may become ulcerated with bleeding (Figure 2). These are of little prognostic significance as they occur even in those who spit paraquat out without swallowing (the products commonly contain stenching and bittering agents). Mucosal lesions in the pharynx, oesophagus and stomach are also very common and much more sinister. These may result in perforation, mediastinitis and/or pneumomediastinum. The contribution of this direct caustic effect to mortality is probably under-estimated.
Figure 2.
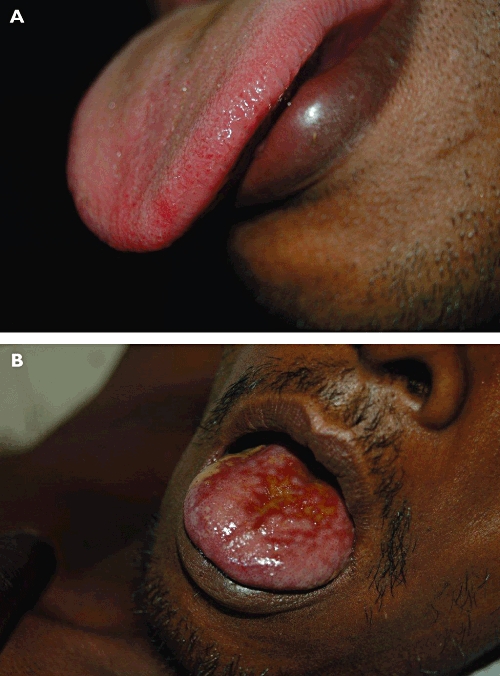
(A) ‘Paraquat tongue’ early lesion, within 24 h after ingestion. (B) ‘Paraquat tongue’ late lesion, 2 weeks after ingestion with extensive ulceration
Medical management of paraquat self-poisoning (Table 1)
Table 1.
Summary of treatment recommendations
| Treatment/investigation | Indications | Comment |
|---|---|---|
| Decontamination | If within 2–4 h | Use activated charcoal or Fuller's earth |
| Nasogastric tube | Pharyngeal/oesophageal burns or PQ in urine | Insert prophylactically as early as possible as swallowing becomes difficult later |
| Urine dithionite test | All patients. If negative, repeat within 24 h | Indicate prognosis. Survival expected if negative test – confirm with plasma paraquat |
| Plasma paraquat | All patients | Indicate prognosis |
| EUC, FBC, LFTs, ABG | Repeat at least daily and when clinically indicated | Look for reversible causes. Progressive changes indicate prognosis |
| Monitor fluid balance | All patients | Declining urine output- correct fluid balance and screen for acute renal failure |
| Intravenous fluids | Inability to swallow, hypotension | |
| Haemoperfusion/Haemodialysis | Presentation within 2 h. Acute renal failure WITHOUT pneumonitis | Most likely of use early and in cases with ‘borderline exposures’. Futile in very severe or late poisoning |
| Monitor respiratory rate and oxygen saturation | All patients. AVOID OXYGEN | Look for treatable causes (e.g. infection and pneumothorax). Acute pneumonitis (early) and fibrosis (late) indicate very poor prognosis |
| Monitor cardiovascular status | All patients | Hypotension not responsive to fluid indicates a very poor prognosis. |
| Monitor level of consciousness | All patients | If CNS toxicity secondary to hypoxia or acidosis, there is a very poor prognosis |
| Pain relief and sedation | All patients | Pain relief with opiates and sedation with benzodiazepines as required |
| Intubation and ventilation | Acute stage – as for any other medical condition | Avoid in acute pneumonitis due to large ingestions and lung fibrosis |
| Experimental therapy | Consenting patients and clinical trials | No evidence from human clinical trials. Dexamethasone, salicylates and NAC have most support in animal models |
Resuscitation
Patients in extremis have no realistic hope of recovery with current treatments. Treatment of such patients should be palliative once the diagnosis is established. Otherwise, the standard principles of resuscitation (assessment and management of airway, breathing and circulation) should generally be followed as per routine guidelines. The airway may be compromised due to mucosal toxicity or the presence of vomitus. Tachypnoea and/or hypoxia may be due to metabolic acidosis, aspiration and/or acute alveolitis and a blood gas and chest radiograph may help make the correct diagnosis. However, mild to moderate hypoxia should not be routinely treated with oxygen as it will worsen oxidative stress [64] and it greatly increases lethality in animal models [65].
Initially, hypotension is generally due to hypovolaemia and should be treated with boluses of fluids (15–20 ml kg−1 over 15–30 min) repeated as necessary. A high urine output is desirable. However, as renal failure commonly develops over the first 24 h, close monitoring of fluid balance is required to do this safely.
Patients generally maintain a normal level of consciousness. Any impairment usually indicates either co-ingestion of other agents (e.g. ethanol) or severe toxicity resulting in altered consciousness from hypoxia, hypotension and severe acidosis. In the latter cases, it is worth emphasizing that intubation and mechanical ventilation are futile in severe cases of paraquat poisoning.
Confirming the diagnosis and risk assessment
A semi-quantitative test using bicarbonate and sodium dithionite can be used as a bedside test to confirm systemic paraquat toxicity. In an alkaline medium, sodium dithionite reduces paraquat to a blue radical. If the urine paraquat concentration is more than 1 mg l−1, the urine will appear blue and this finding alone indicates a very poor prognosis [62]. Measurement of plasma paraquat concentration is also useful both to confirm poisoning and predict prognosis. There are five nomograms and formulae of plasma paraquat concentrations to predict outcome after self-poisoning [62, 66–69]. These offer prediction of outcome from 4 to 200 h after ingestion. All these nomograms and formulae have acceptable performance in predicting death within the range of their application [70]. The same very simple colorimetric methods used in urine can be used on plasma samples [71], although several more accurate and sensitive assays are available in specialized laboratories [70]. Plasma concentrations are useful for advising patients and making decisions on monitoring. As yet they do not have any role in guiding interventions and thus they are not urgent or essential.
Clinical and laboratory features also may provide an indication of the prognosis. Patients who present with overt systemic toxicity within the first day (e.g. hypotension, severe hypoxia, acidosis and low GCS) have no hope of survival. Other signs of toxicity have been used to differentiate those who are likely to succumb eventually over the next month from survivors. The development of renal failure, changes on chest radiograph and gastrointestinal lesions are all adverse prognostic signs [72]. We have recently observed that patients who complain of a ‘burning sensation’ in their skin also have a very poor prognosis [73].
Gastrointestinal decontamination
Gastric lavage followed by a dose of activated charcoal has been recommended for patients who present within 1 h of ingestion of paraquat [74, 75]. However, neither procedure has been proven to be of clinical benefit in pesticide self-poisoning [76, 77]. As paraquat is a life-threatening poison with no known antidote, a single dose of activated charcoal or Fuller's earth is recommended for consenting patients who have a protected airway. We do not recommend gastric lavage as its use is contraindicated in caustic injury and it is likely to add little to the amount removed by spontaneous vomiting and adsorbents.
Investigations
In addition to the paraquat concentrations discussed above, biochemistry (electrolytes and renal and liver function tests), and haematology (full blood count) should be done at least daily. A chest radiograph should be performed if pneumomediastinum, pneumothorax or lung fibrosis (Figure 3) is suspected. A CT scan of the chest may be useful in detecting early lung fibrosis (Figure 4) or assessing long-term damage in survivors. Amylase and lipase may diagnose acute pancreatitis. This should be suspected if patients develop abdominal pain and a raised blood sugar.
Figure 3.
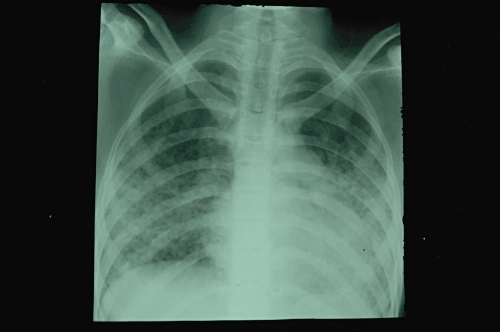
Chest radiograph demonstrating diffuse alveolar shadowing of a patient 7 days after ingestion of paraquat
Figure 4.
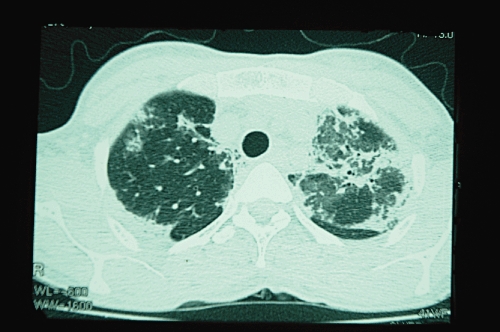
High resolution CT scan of chest demonstrating bilateral pulmonary fibrosis 11 days after paraquat poisoning
Clinical monitoring and ongoing care
Patients should be monitored for the development of:
Acute renal failure. Daily fluid balance should be maintained with the aim of ensuring a good urine output without overloading the patient.
Liver toxicity. Clinical examination will usually detect jaundice and right hypochondrial pain.
Respiratory failure: respiratory rate, auscultation of the lungs (for crepitations) and measurement of peripheral oxygen saturation should be performed on at least a twice-daily basis. However, supplementary oxygen should not be given except as a palliative measure in patients determined to be in terminal decline.
Mucosal injury: patients develop severe oral ulcers within a few days after ingestion of paraquat. This generally prevents patients from taking adequate food or oral fluids for up to 10 days. Early insertion of a nasogastric feeding tube will ensure adequate nutrition. This in turn may be important in ensuring innate anti-oxidant mechanisms are not compromised. In addition, pain relief with opiates is often required and these can then be given.
Elimination enhancement: haemodialysis (HD)/haemoperfusion (HP)
Haemodialysis and HP are part of the standard treatment in many centres [78–80]. However, the kinetics of paraquat suggest the benefits of this will be very limited for two reasons. Firstly, the endogenous clearance is high in the first 6 to 12 h and thus most paraquat is eliminated rapidly anyway and the additional amount eliminated will be relatively modest. Secondly, the timeframe in which the increased elimination will have an impact on the distribution into lungs is very short. In a dog model it was shown that HP was ineffective in reducing paraquat lung exposure unless it was started within 2 h post ingestion [57]. Using the data from this study it can be shown that commencing HD/HP after 2 to 4 h removes paraquat from the plasma compartment but reduces paraquat taken up by the lungs by negligible amounts and hence is unlikely to change overall outcome (Figure 5). The subsequent elimination of accumulated paraquat from the lung is minimally dependent on the plasma concentration. These conclusions are supported by the clinical studies that have been performed. Koo et al. in an uncontrolled study compared HP alone with HP followed by continued venovenous haemofiltration (CVVH) in a group of 80 patients with paraquat self-poisoning [78]. While survival was significantly longer with the additional intervention (5.0 ± 5.0 vs. 2.5 ± 2.1 days; P < 0.05), there was no difference in mortality between the two groups (66.7% vs. 63.6%; P = 0.82). Paraquat concentrations very similar to the blood concentration at the start of treatment were found in muscle and lung at post mortem 5 days after ingestion despite achieving clearances of 150 ml min−1 from combined HD/HP done for two half-lives from 3 to 8 h post ingestion achieving much lower blood concentrations [81]. The overall case fatality in centres routinely performing HP/HD is still over 50% [78, 79], comparable with that in our centres that never use the technique [12].
Figure 5.
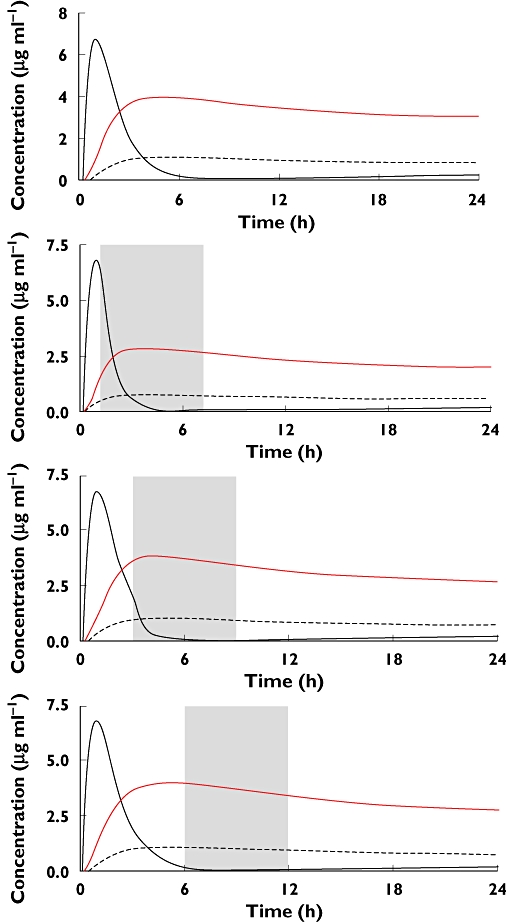
A model of the time-dependent effect of haemodialysis on plasma (black line), tissue (dashed line) and lung (red line) paraquat concentrations. It should be noted that there is minimal reduction in lung concentrations when instituted at 3 or 6 h post ingestion (parameters from model developed by Pond et al. [57])
The choice of method may be a moot point but has been studied. In an experimental model, HP provided moderately superior clearance to HD for 90 min after initiation of the procedure [79]. While, HD clearance remained static, clearance by HP then rapidly decreased. The plasma paraquat was reduced substantially by 4 h of HP in both survivors (80%) and non-survivors (76%). However, the most important consideration is starting the treatment within a few hours and the choice of method is secondary.
Haemodialysis could be considered in patients who have developed symptomatic acute renal failure. However, such patients have a very poor prognosis in terms of their lung injury, so this is unlikely to change outcome [63].
Other treatment options
There are no widely accepted treatment guidelines for paraquat self-poisoning or good-quality evidence to support the following treatments. However, given the dismal prognosis most physicians adopt one or more of the following into their practice.
Immunosuppression
‘Immunosuppression’ is widely practised as a treatment of paraquat self-poisoning. The theory is that as paraquat leads to an acute inflammatory response, interference with this may inhibit the processes that follow that then lead to lung fibrosis and death. The most widely studied regimen uses cyclophosphamide, MESNA, methylprednisolone and dexamethasone (Table 2). This includes agents with multiple other potential mechanisms (i.e. anti-inflammatory, induction of transporters, anti-oxidant) and thus the terminology is confusing. Dexamethasone in particular has been shown to increase the expression of P-glycoprotein in rats [82] (and may well increase expression of other transporters). There was a significant reduction of paraquat accumulation in the lung tissue and an increase of faecal excretion of paraquat in rats treated with lethal doses of paraquat. Dexamethasone has also been shown to possess the ability to ameliorate the histological and biochemical changes induced by paraquat and to reduce lipid peroxidation and survival rates in Wistar rats [83].
Table 2.
Summary of clinical studies
| Study | Description | Treatment | Mortality | Comments |
|---|---|---|---|---|
| Addo & Poon-King [84] | Uncontrolled 72 patients | CP, Dex, MP, vitamin B, vitamin C | 28% | No follow-up after discharge. Plasma paraquat measured in only 25 patients. Of the patients who had concentrations over 2 mg l−1, only 6/18 survived |
| Afzali & Gholyaf [87] | RCT 20 patients | CP, Dex, MP vs. conventional treatment | 33% vs. 81% | Plasma paraquat not measured. Sample size and power calculation not performed, no follow-up after discharge |
| Perriens et al. [89] | Uncontrolled 47 patients | CP, Dex vs. conventional treatment | 63% vs. 61% | Exposure confirmed |
| Lin et al. [86] | RCT 23 patients | CP, Dex, MP vs. conventional treatment | 31% vs. 86% | Power calculation not done. No follow-up after discharge |
| Lin et al. [80] | RCT 50 patients | CP, Dex, MP vs. conventional treatment | 68% vs. 82% | 71 patients were excluded from post hoc analysis. Plasma paraquat not measured. No follow-up after discharge |
| Lin et al. [85] | 16 patients 17 historic controls | CP, Dex, MP | 25% vs. 70% | Exposure unconfirmed. Used historic controls. No follow-up after discharge |
| Yasaka et al. [28] | Uncontrolled 9 patients | Vitamin E 100–4000 mg day−1 | 78% | |
| Hong et al. [99] | Uncontrolled 5 patients | Vitamin C escalating doses | 0% |
CP, cyclophosphamide; Dex, dexamethasone; MP, methylprednisolone; DFO, desferroxamine.
There are no animal studies that have demonstrated benefit of this ‘immunosuppression’ combination in experimental paraquat poisoning. The clinical evidence is also very limited. The regimen was promoted by Addo & Poon-King when they reported an uncontrolled study that used this combination along with supplementary K+ and Mg2+[84]. They claimed improved survival on the basis that six of the 18 patients who had plasma paraquat concentrations over 2 mg l−1 survived and that the mortality in this group should be 100%. The six survivors were all plotted close to the Proudfoot cut-off line [84] and their survival was not remarkable enough for this to be regarded as strong evidence. Following this initial report, a group from Taiwan carried out a series of further clinical studies using immunosuppression [80, 85, 86]. The first study was a cohort of 16 patients with moderate to severe paraquat poisoning treated with 1 g of cyclophosphamide daily for 2 days and 1 g of methylprednisolone daily for 3 days. Mortality was four out of 16 (25%) compared with 12 of 17 (71%) in a historical control group that had received only conventional therapy. This encouraged them to go on to perform a randomized controlled trial (RCT). On an intention to treat analysis, 12 of 65 control patients and 18 of 56 patients receiving immunosuppression survived (P = 0.09). The authors presented a post hoc analysis in which only patients who survived the first week after randomization were compared and claimed they had demonstrated benefit in this subgroup. This led to a third study of 23 patients randomized in a ratio of 1:2 ‘if their plasma paraquat concentrations were between the predictive mortality of >50% and <90% according to the formula of Hart et al. and Lin et al. [67, 86] All patients also underwent two sessions of charcoal haemoperfusion. The mortality rate (85.7%, six of seven) of the control group was higher than that of the study group (31.3%, five of 16). This study had no power calculation and indeed there is no power calculation that would feasibly have given rise to such a small study with mortality as the primary outcome. Another very small trial (n = 20) with an improbably large treatment effect (mortality 33.3% with immunosuppression vs. 81.8% in controls) has also been reported [87]. Even in the best-designed studies early termination of small RCTs ‘for benefit’ inevitably greatly exaggerates the effectiveness of the intervention [88]. An uncontrolled study showed no survival benefit of immunosuppression with cyclophosphamide and dexamethazone [89]. A 2003 systematic review of the effectiveness of immunosuppressive therapy in paraquat poisoning found no other RCT [90]. A much larger double-blind RCT of immunosuppression (ISRCTN85372848) should report in 2011. While no analysis has been performed, it is clear that the high overall mortality we have observed in the study is not consistent with the treatment more than halving the number of fatalities.
Antioxidants
Several antioxidants have been tested as potential antidotes for paraquat poisoning. As a general statement they have had impressive results in vitro, with mixed but modest results in animal studies. Human studies have either been absent or small and uncontrolled. As a further complication, none of these has established indications as anti-oxidants and the optimal dose to achieve this effect in humans is unknown. The following are those with therapeutic preparations and for which there is the most information.
Vitamin E
Vitamin E-deficient rats poisoned with paraquat have lower LD50 values and have shorter survival times [91]. Pre-treatment of rats with α-tocopherol liposomes [92] was shown to modify paraquat-induced lung toxicity. Furthermore, rat lungs, directly treated with vitamin E 24 h after exposure to paraquat, demonstrated less lipid peroxidation than controls [93]. Potential mechanisms may involve membrane stabilization of polyunsaturated fatty acids and ROS scavenging. Vitamin E also inhibits the activation of NF-κB [94].
However, in the only human study, only two of nine patients treated with vitamin E (200–4000 mg day−1) survived [28]. Other animal models have also failed to show either a survival benefit or an improvement in histology of lungs following paraquat toxicity [95].
Vitamin C
Vitamin C is an antioxidant based on its ability to donate an electron to free radicals thereby neutralizing them. When mutant rats unable to synthesize ascorbic acid were fed paraquat they developed signs and symptoms of paraquat toxicity at concentrations that do not produce features of toxicity in normal rats [96]. In mouse embryonic stem cells administration of ascorbic acid reduced the total ROS generated by exposure to paraquat [97]. However, while pre-treatment with intravenous vitamin C reduced oxidative stress from paraquat toxicity in rats, vitamin C given soon after paraquat ingestion worsened oxidative stress [98]. The latter effect was reduced by the iron chelator, desferoxamine and therefore attributed to the promotion of the Fenton reaction (see Figure 1) and increased redox-cycling of metals [98]. In the only published human study, 10 patients with paraquat poisoning with positive urine paraquat but ‘stable vital signs’, all survived after a combination of high-dose vitamin C and multiple other anti-oxidants. It was noted that the total anti-oxidant status increased progressively with increasing doses of vitamin C [99].
N-acetylcysteine (NAC)
N-acetylcysteine replenishes cysteine which is rate-limiting in the synthesis of glutathione, a critical antioxidant defence. NAC reduced paraquat-induced apoptosis [48] and inflammatory response [100] in human lung cultures. In rats, NAC improved survival after paraquat poisoning, doubling the LD100 from 100 to 200 mg kg−1[100]. Beneficial effects of NAC appear to be mediated through reduction of ROS and inflammatory markers [101, 102]. NAC increased glutathione in alveolar type II pneumocytes in rats given paraquat [103].
Despite NAC having potentially beneficial effects through multiple mechanisms (scavenging of ROS, increasing glutathione and reducing inflammation, lipid peroxidation and apoptosis), strangely it has been minimally studied in human paraquat poisoning. S-carboxymethylcysteine 1500 mg day−1, a related cysteine/glutathione precursor, was used in 35 cases of paraquat poisoning with a case fatality of only 23% [104]. However, there is insufficient information to indicate how severely poisoned these patients were to contrast with the expected mortality.
Deferoxamine (DFO)
Iron is an important contributor to the generation of ROS through the Fenton reaction (Figure 1). Iron enhanced toxicity of paraquat in bacteria [105] and in mice [106]. Addition of DFO was protective in these experiments [105, 106]. However, DFO led to no survival benefit in rats poisoned with paraquat [107]. No human studies have looked at the efficacy of DFO in paraquat poisoning.
Salicylic acid (SA)
In addition to its established anti-inflammatory mechanism of inhibiting cyclo-oxygenase, SA has a variety of anti-oxidant effects. It can scavenge hydroxyl radicals and inhibit their production through the Fenton reaction [108]. SA can reduce oxidative stress [109, 110] and inhibit the activation of NF-κB [111]. The latter effect may be direct or mediated through inhibition of TNFα, a potent stimulator of NF-κB [112]. SA inhibits lipid peroxidation in both neuronal cells and mitochondria in vitro[113, 114]. Furthermore, SA reduces other inflammatory mediators such as IL4 [115] and activator protein-1 [116] independent of NF-κB inhibition.
A single dose of sodium salicylate (200 mg kg−1) to Wistar rats, 2 h after exposure to a toxic dose of paraquat (25 mg kg−1) resulted in no deaths compared with 100% mortality in the control group [43]. Treatment with salicylate significantly reduced oxidative stress, NF-κB activation, lipid peroxidation, platelet activation and histological lung damage. This was associated with reduced myeloperoxidase activity, reduced platelet activation, reduced mitochondrial dysfunction and apoptosis. There have been no published human studies.
Conclusions
Rational use of highly experimental treatment options
Based on animal studies and limited human data, it is our opinion that NAC, vitamin C, salicylate and dexamethasone appear to have the most promising mechanisms to counter the principle pathophysiological events following paraquat, and also have established safety profiles. However, more evidence is needed to guide the choice of doses, duration and combinations. Thus, these agents/combinations of agents should be tested first in small phase II studies utilizing biomarkers to select regimens to take into larger phase III clinical studies that can determine if any of these might significantly reduce the very high case fatality. We have recently completed two small phase II studies (n = 20–40) with combinations of the above. Laboratory results are pending but clinical results to date have been only slightly better than historical controls and we believe many further studies will be required to find an antioxidant regimen worth evaluating in large RCTs (full reports should be published in 2011).
Management conclusions
There are two competing philosophies that drive management decisions. The first recognizes that the outcome is dire and that no treatments are likely to be effective and aims to do minimal low-risk interventions (charcoal, i.v. fluids and maybe an anti-oxidant) and keep patients comfortable. The second recognizes that the outcome is dire and that no treatments are likely to be worse than the disease. This group does HP/HD, immunosuppression and often adds to this a cocktail of other treatments. We would encourage anyone seeing a substantial number of paraquat poisonings to adopt a consistent strategy for a number of patients, measure the paraquat concentration (the best prognostic predictor) and report their outcomes.
Competing Interests
Both authors have been involved as investigators on a study of immunosuppression and 2 studies on reformulation of paraquat funded by Syngenta Crop Protection AG, Basel, Switzerland (a manufacturer of paraquat). Both have been reimbursed for travel expenses from the study sponsor for attending investigator meetings.
Acknowledgments
Indika Gawarammana was supported by an Australian Leadership Award (ALA000379) from AusAid. South Asian Clinical Toxicology Research Collaboration has received extensive research grant support from the Wellcome Trust and NHMRC over the last 8 years.
REFERENCES
- 1.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. World Health Stat Q. 1990;43:139–44. [PubMed] [Google Scholar]
- 2.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–4. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. Where is the evidence for treatments used in pesticide poisoning? Is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol. 2004;4:113–6. doi: 10.1081/clt-120028756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manuel C, Gunnell DJ, van der Hoek W, Dawson A, Wijeratne IK, Konradsen F. Self-poisoning in rural Sri Lanka: small-area variations in incidence. BMC Public Health. 2008;8:26. doi: 10.1186/1471-2458-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. QJM. 2000;93:715–31. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 6.Dawson AH, Eddleston M, Senarathna L, Mohamed F, Gawarammana I, Bowe SJ, Manuweera G, Buckley NA. Acute human lethal toxicity of agricultural pesticides: a prospective cohort study. PLoS Med. 2010;7:e1000357. doi: 10.1371/journal.pmed.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hutchinson G, Daisley H, Simeon D, Simmonds V, Shetty M, Lynn D. High rates of paraquat-induced suicide in southern Trinidad. Suicide Life Threat Behav. 1999;29:186–91. [PubMed] [Google Scholar]
- 8.Bourke T. Suicide in Samoa. Pac Health Dialog. 2001;8:213–9. [PubMed] [Google Scholar]
- 9.Dargan P, Shiew C, Greene S, Gawarammana I, Jones A. Paraquat poisoning: caution in interpreting prognosis based on plasma paraquat concentration. Clin Toxicol. 2006;44:762. [Google Scholar]
- 10.Casey P, Vale JA. Deaths from pesticide poisoning in England and Wales: 1945–1989. Hum Exp Toxicol. 1994;13:95–101. doi: 10.1177/096032719401300206. [DOI] [PubMed] [Google Scholar]
- 11.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Giffin SL. 2008 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 26th annual report. Clin Toxicol (Phila) 2009;47:911–1084. doi: 10.3109/15563650903438566. [DOI] [PubMed] [Google Scholar]
- 12.Wilks MF, Fernando R, Ariyananda PL, Eddleston M, Berry DJ, Tomenson JA, Buckley NA, Jayamanne S, Gunnell D, Dawson A. Improvement in survival after paraquat ingestion following introduction of a new formulation in Sri Lanka. PLoS Med. 2008;5:e49. doi: 10.1371/journal.pmed.0050049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilks MF, Tomenson JA, Fernando R, Ariyananda PL, Berry DJ, Buckley NA, Gawarammana IB, Jayamanne S, Gunnell D, Dawson A. Formulation changes and time trends in outcome following paraquat ingestion in Sri Lanka. Clin Toxicol (Phila) 2011;49:21–8. doi: 10.3109/15563650.2010.544658. [DOI] [PubMed] [Google Scholar]
- 14.Yang W, Tiffany-Castiglioni E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH-SY5Y cells. J Toxicol Environ Health A. 2007;70:1849–57. doi: 10.1080/15287390701459262. [DOI] [PubMed] [Google Scholar]
- 15.Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186–93. doi: 10.1074/jbc.M700827200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonneh-Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52–6. doi: 10.1016/j.molbrainres.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Adam A, Smith LL, Cohen GM. An assessment of the role of redox cycling in mediating the toxicity of paraquat and nitrofurantoin. Environ Health Perspect. 1990;85:113–7. doi: 10.1289/ehp.85-1568326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelner MJ, Bagnell R. Paraquat resistance associated with reduced NADPH reductase in an energy-dependent paraquat-accumulating cell line. Arch Biochem Biophys. 1989;274:366–74. doi: 10.1016/0003-9861(89)90450-5. [DOI] [PubMed] [Google Scholar]
- 19.Bus JS, Cagen SZ, Olgaard M, Gibson JE. A mechanism of paraquat toxicity in mice and rats. Toxicol Appl Pharmacol. 1976;35:501–13. doi: 10.1016/0041-008x(76)90073-9. [DOI] [PubMed] [Google Scholar]
- 20.Peng J, Stevenson FF, Oo ML, Andersen JK. Iron-enhanced paraquat-mediated dopaminergic cell death due to increased oxidative stress as a consequence of microglial activation. Free Radic Biol Med. 2009;46:312–20. doi: 10.1016/j.freeradbiomed.2008.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clejan L, Cederbaum AI. Synergistic interactions between NADPH-cytochrome P-450 reductase, paraquat, and iron in the generation of active oxygen radicals. Biochem Pharmacol. 1989;38:1779–86. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad I, Kumar A, Shukla S, Prasad Pandey H, Singh C. The involvement of nitric oxide in maneb- and paraquat-induced oxidative stress in rat polymorphonuclear leukocytes. Free Radic Res. 2008;42:849–62. doi: 10.1080/10715760802513733. [DOI] [PubMed] [Google Scholar]
- 24.Rannels DE, Kameji R, Pegg AE, Rannels SR. Spermidine uptake by type II pneumocytes: interactions of amine uptake pathways. Am J Physiol. 1989;257(Pt 1):L346–53. doi: 10.1152/ajplung.1989.257.6.L346. [DOI] [PubMed] [Google Scholar]
- 25.Bus JS, Aust SD, Gibson JE. Lipid peroxidation: a possible mechanism for paraquat toxicity. Res Commun Chem Pathol Pharmacol. 1975;11:31–8. [PubMed] [Google Scholar]
- 26.Bus JS, Aust SD, Gibson JE. Paraquat toxicity: proposed mechanism of action involving lipid peroxidation. Environ Health Perspect. 1976;16:139–46. doi: 10.1289/ehp.7616139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yasaka T, Ohya I, Matsumoto J, Shiramizu T, Sasaguri Y. Acceleration of lipid peroxidation in human paraquat poisoning. Arch Intern Med. 1981;141:1169–71. [PubMed] [Google Scholar]
- 28.Yasaka T, Okudaira K, Fujito H, Matsumoto J, Ohya I, Miyamoto Y. Further studies of lipid peroxidation in human paraquat poisoning. Arch Intern Med. 1986;146:681–5. [PubMed] [Google Scholar]
- 29.Kurisaki E. Lipid peroxidation in human paraquat poisoning. J Toxicol Sci. 1985;10:29–33. doi: 10.2131/jts.10.29. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Fukushima T. Mechanism of cytotoxicity of paraquat. II. Organ specificity of paraquat-stimulated lipid peroxidation in the inner membrane of mitochondria. Exp Toxicol Pathol. 1993;45:375–80. doi: 10.1016/S0940-2993(11)80433-1. [DOI] [PubMed] [Google Scholar]
- 31.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–98. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 32.Costantini P, Petronilli V, Colonna R, Bernardi P. On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitric oxide. Toxicology. 1995;99:77–88. doi: 10.1016/0300-483x(94)02997-9. [DOI] [PubMed] [Google Scholar]
- 33.Tawara T, Fukushima T, Hojo N, Isobe A, Shiwaku K, Setogawa T, Yamane Y. Effects of paraquat on mitochondrial electron transport system and catecholamine contents in rat brain. Arch Toxicol. 1996;70:585–9. doi: 10.1007/s002040050316. [DOI] [PubMed] [Google Scholar]
- 34.Keeling PL, Smith LL. Relevance of NADPH depletion and mixed disulphide formation in rat lung to the mechanism of cell damage following paraquat administration. Biochem Pharmacol. 1982;31:3243–9. doi: 10.1016/0006-2952(82)90557-3. [DOI] [PubMed] [Google Scholar]
- 35.Shibamoto T, Parker JC. Fructose 1,6-diphosphate augments paraquat injury in isolated dog lungs. J Appl Physiol. 1991;71:1830–5. doi: 10.1152/jappl.1991.71.5.1830. [DOI] [PubMed] [Google Scholar]
- 36.Kratsovnik E, Bromberg Y, Sperling O, Zoref-Shani E. Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J Mol Neurosci. 2005;26:27–32. doi: 10.1385/jmn:26:1:027. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside ST, Israel A. I kappa B proteins: structure, function and regulation. Semin Cancer Biol. 1997;8:75–82. doi: 10.1006/scbi.1997.0058. [DOI] [PubMed] [Google Scholar]
- 38.Schoonbroodt S, Piette J. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol. 2000;60:1075–83. doi: 10.1016/s0006-2952(00)00371-3. [DOI] [PubMed] [Google Scholar]
- 39.Rio MJ, Velez-Pardo C. Paraquat induces apoptosis in human lymphocytes: protective and rescue effects of glucose, cannabinoids and insulin-like growth factor-1. Growth Factors. 2008;26:49–60. doi: 10.1080/08977190801984205. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Tiffany-Castiglioni E. Paraquat-induced apoptosis in human neuroblastoma SH-SY5Y cells: involvement of p53 and mitochondria. J Toxicol Environ Health A. 2008;71:289–99. doi: 10.1080/15287390701738467. [DOI] [PubMed] [Google Scholar]
- 41.Fordel E, Thijs L, Martinet W, Lenjou M, Laufs T, Van Bockstaele D, Moens L, Dewilde S. Neuroglobin and cytoglobin overexpression protects human SH-SY5Y neuroblastoma cells against oxidative stress-induced cell death. Neurosci Lett. 2006;410:146–51. doi: 10.1016/j.neulet.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Denicola A, Radi R. Peroxynitrite and drug-dependent toxicity. Toxicology. 2005;208:273–88. doi: 10.1016/j.tox.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 43.Dinis-Oliveira RJ, Sousa C, Remiao F, Duarte JA, Navarro AS, Bastos ML, Carvalho F. Full survival of paraquat-exposed rats after treatment with sodium salicylate. Free Radic Biol Med. 2007;42:1017–28. doi: 10.1016/j.freeradbiomed.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 44.Dearden LC, Fairshter RD, McRae DM, Smith WR, Glauser FL, Wilson AF. Pulmonary ultrastructure of the late aspects of human paraquat poisoning. Am J Pathol. 1978;93:667–80. [PMC free article] [PubMed] [Google Scholar]
- 45.Dearden LC, Fairshter RD, Morrison JT, Wilson AF, Brundage M. Ultrastructural evidence of pulmonary capillary endothelial damage from paraquat. Toxicology. 1982;24:211–22. doi: 10.1016/0300-483x(82)90003-8. [DOI] [PubMed] [Google Scholar]
- 46.Melchiorri D, Del Duca C, Piccirilli S, Trombetta G, Bagetta G, Nistico G. Intrahippocampal injection of paraquat produces apoptotic cell death which is prevented by the lazaroid U74389G, in rats. Life Sci. 1998;62:1927–32. doi: 10.1016/s0024-3205(98)00161-1. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy S, Somayajulu M, Sikorska M, Borowy-Borowski H, Pandey S. Paraquat induces oxidative stress and neuronal cell death; neuroprotection by water-soluble Coenzyme Q10. Toxicol Appl Pharmacol. 2004;201:21–31. doi: 10.1016/j.taap.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 48.Cappelletti G, Maggioni MG, Maci R. Apoptosis in human lung epithelial cells: triggering by paraquat and modulation by antioxidants. Cell Biol Int. 1998;22:671–8. doi: 10.1006/cbir.1998.0305. [DOI] [PubMed] [Google Scholar]
- 49.Dinis-Oliveira RJ, Sousa C, Remiao F, Duarte JA, Ferreira R, Sanchez Navarro A, Bastos ML, Carvalho F. Sodium salicylate prevents paraquat-induced apoptosis in the rat lung. Free Radic Biol Med. 2007;43:48–61. doi: 10.1016/j.freeradbiomed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 50.Smith P, Heath D, Kay JM. The pathogenesis and structure of paraquat-induced pulmonary fibrosis in rats. J Pathol. 1974;114:57–67. doi: 10.1002/path.1711140202. [DOI] [PubMed] [Google Scholar]
- 51.Smith P, Heath D. The pathology of the lung in paraquat poisoning. J Clin Pathol Suppl (R Coll Pathol) 1975;9:81–93. [PMC free article] [PubMed] [Google Scholar]
- 52.Smith P, Heath D. The ultrastructure and time sequence of the early stages of paraquat lung in rats. J Pathol. 1974;114:177–84. doi: 10.1002/path.1711140402. [DOI] [PubMed] [Google Scholar]
- 53.Vijeyaratnam GS, Corrin B. Experimental paraquat poisoning: a histological and electron-optical study of the changes in the lung. J Pathol. 1971;103:123–9. doi: 10.1002/path.1711030207. [DOI] [PubMed] [Google Scholar]
- 54.Fowler BA, Brooks RE. Effects of the herbicide paraquat on the ultrastructure of mouse kidney. Am J Pathol. 1971;63:505–20. [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumori H, Matsumoto T, Ishikawa H. Acute toxic effects of paraquat on ultrastructure of rat liver. Acta Pathol Jpn. 1984;34:507–18. doi: 10.1111/j.1440-1827.1984.tb07579.x. [DOI] [PubMed] [Google Scholar]
- 56.Houze P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM. Toxicokinetics of paraquat in humans. Hum Exp Toxicol. 1990;9:5–12. doi: 10.1177/096032719000900103. [DOI] [PubMed] [Google Scholar]
- 57.Pond SM, Rivory LP, Hampson EC, Roberts MS. Kinetics of toxic doses of paraquat and the effects of hemoperfusion in the dog. J Toxicol Clin Toxicol. 1993;31:229–46. doi: 10.3109/15563659309000391. [DOI] [PubMed] [Google Scholar]
- 58.Gaudreault P, Karl PI, Friedman PA. Paraquat and putrescine uptake by lung slices of fetal and newborn rats. Drug Metab Dispos. 1984;12:550–2. [PubMed] [Google Scholar]
- 59.Smith LL, Pratt I, Elliott C, Wyatt I. The accumulation of putrescine and paraquat into lung slices taken from BHT treated mice. Toxicology. 1983;27:1–13. doi: 10.1016/0300-483x(83)90071-9. [DOI] [PubMed] [Google Scholar]
- 60.Bismuth C, Hall AH. Paraquat Poisoning: Mechanisms, Prevention, Treatment. New York: Marcel Dekker Inc; 1995. [Google Scholar]
- 61.Proudfoot AT, Vale JA. Pesticides. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. Oxford Textbook of Medicine. 3rd edn. Oxford: Oxford University Press; 1996. pp. 1120–4. [Google Scholar]
- 62.Scherrmann JM, Houze P, Bismuth C, Bourdon R. Prognostic value of plasma and urine paraquat concentration. Hum Toxicol. 1987;6:91–3. doi: 10.1177/096032718700600116. [DOI] [PubMed] [Google Scholar]
- 63.Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202:69–74. doi: 10.1016/j.toxlet.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoet PH, Demedts M, Nemery B. Effects of oxygen pressure and medium volume on the toxicity of paraquat in rat and human type II pneumocytes. Hum Exp Toxicol. 1997;16:305–10. doi: 10.1177/096032719701600602. [DOI] [PubMed] [Google Scholar]
- 65.Pratt IS, Keeling PL, Smith LL. The effect of high concentrations of oxygen on paraquat and diquat toxicity in rats. Arch Toxicol Suppl. 1980;4:415–8. doi: 10.1007/978-3-642-67729-8_95. [DOI] [PubMed] [Google Scholar]
- 66.Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma paraquat concentrations. Lancet. 1979;2:330–2. doi: 10.1016/s0140-6736(79)90345-3. [DOI] [PubMed] [Google Scholar]
- 67.Hart TB, Nevitt A, Whitehead A. A new statistical approach to the prognostic significance of plasma paraquat concentrations. Lancet. 1984;2:1222–3. doi: 10.1016/s0140-6736(84)92784-3. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto I, Saito T, Harunari N, Sato Y, Kato H, Nakagawa Y, Inokuchi S, Sawada Y, Makuuchi H. Correlating the severity of paraquat poisoning with specific hemodynamic and oxygen metabolism variables. Crit Care Med. 2000;28:1877–83. doi: 10.1097/00003246-200006000-00032. [DOI] [PubMed] [Google Scholar]
- 69.Jones AL, Elton R, Flanagan R. Multiple logistic regression analysis of plasma paraquat concentrations as a predictor of outcome in 375 cases of paraquat poisoning. QJM. 1999;92:573–8. doi: 10.1093/qjmed/92.10.573. [DOI] [PubMed] [Google Scholar]
- 70.Senarathna L, Eddleston M, Wilks MF, Woollen BH, Tomenson JA, Roberts DM, Buckley NA. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251–9. doi: 10.1093/qjmed/hcp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo JR, Yoon JW, Han SJ, Choi MJ, Park, Lee YK, Kim SG, Oh JE, Seo JW, Kim HJ, Noh JW. Rapid analysis of plasma paraquat using sodium dithionite as a predictor of outcome in acute paraquat poisoning. Am J Med Sci. 2009;338:373–7. doi: 10.1097/MAJ.0b013e3181b4deee. [DOI] [PubMed] [Google Scholar]
- 72.Bismuth C, Dally S, Schermann JM, Gaultier M, Fournier PE. [Paraquat poisoning. Prognostic and therapeutic evaluation in 28 cases] Nouv Presse Med. 1982;11:3239–44. [PubMed] [Google Scholar]
- 73.Gawarammana I, Dawson A. Peripheral burning sensation: a novel clinical marker of poor prognosis and higher plasma paraquat levels in paraquat poisoning. Clin Toxicol. 2010;48:347–9. doi: 10.3109/15563651003641794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vale JA. Position statement: gastric lavage. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:711–9. doi: 10.3109/15563659709162568. [DOI] [PubMed] [Google Scholar]
- 75.Vale JA, Kulig K. Position paper: gastric lavage. J Toxicol Clin Toxicol. 2004;42:933–43. doi: 10.1081/clt-200045006. [DOI] [PubMed] [Google Scholar]
- 76.Eddleston M, Juszczak E, Buckley NA, Senarathna L, Mohamed F, Dissanayake W, Hittarage A, Azher S, Jeganathan K, Jayamanne S, Sheriff MR, Warrell DA. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371:579–87. doi: 10.1016/S0140-6736(08)60270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Tse ML, Gawarammana I, Buckley N, Eddleston M. Systematic review of controlled clinical trials of gastric lavage in acute organophosphorus pesticide poisoning. Clin Toxicol (Phila) 2009;47:179–92. doi: 10.1080/15563650701846262. [DOI] [PubMed] [Google Scholar]
- 78.Koo JR, Kim JC, Yoon JW, Kim GH, Jeon RW, Kim HJ, Chae DW, Noh JW. Failure of continuous venovenous hemofiltration to prevent death in paraquat poisoning. Am J Kidney Dis. 2002;39:55–9. doi: 10.1053/ajkd.2002.29880. [DOI] [PubMed] [Google Scholar]
- 79.Hong SY, Yang JO, Lee EY, Kim SH. Effect of haemoperfusion on plasma paraquat concentration in vitro and in vivo. Toxicol Ind Health. 2003;19:17–23. doi: 10.1191/0748233703th171oa. [DOI] [PubMed] [Google Scholar]
- 80.Lin JL, Leu ML, Liu YC, Chen GH. A prospective clinical trial of pulse therapy with glucocorticoid and cyclophosphamide in moderate to severe paraquat-poisoned patients. Am J Respir Crit Care Med. 1999;159:357–60. doi: 10.1164/ajrccm.159.2.9803089. [DOI] [PubMed] [Google Scholar]
- 81.Van de Vyver FL, Giuliano RA, Paulus GJ, Verpooten GA, Franke JP, De Zeeuw RA, Van Gaal LF, De Broe ME. Hemoperfusion-hemodialysis ineffective for paraquat removal in life-threatening poisoning? J Toxicol Clin Toxicol. 1985;23:117–31. doi: 10.3109/15563658508990622. [DOI] [PubMed] [Google Scholar]
- 82.Dinis-Oliveira RJ, Remiao F, Duarte JA, Ferreira R, Sanchez Navarro A, Bastos ML, Carvalho F. P-glycoprotein induction: an antidotal pathway for paraquat-induced lung toxicity. Free Radic Biol Med. 2006;41:1213–24. doi: 10.1016/j.freeradbiomed.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 83.Dinis-Oliveira RJ, Duarte JA, Remiao F, Sanchez-Navarro A, Bastos ML, Carvalho F. Single high dose dexamethasone treatment decreases the pathological score and increases the survival rate of paraquat-intoxicated rats. Toxicology. 2006;227:73–85. doi: 10.1016/j.tox.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 84.Addo E, Poon-King T. Leucocyte suppression in treatment of 72 patients with paraquat poisoning. Lancet. 1986;1:1117–20. doi: 10.1016/s0140-6736(86)91836-2. [DOI] [PubMed] [Google Scholar]
- 85.Lin JL, Wei MC, Liu YC. Pulse therapy with cyclophosphamide and methylprednisolone in patients with moderate to severe paraquat poisoning: a preliminary report. Thorax. 1996;51:661–3. doi: 10.1136/thx.51.7.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34:368–73. doi: 10.1097/01.ccm.0000195013.47004.a8. [DOI] [PubMed] [Google Scholar]
- 87.Afzali S, Gholyaf M. The effectiveness of combined treatment with methylprednisolone and cyclophosphamide in oral paraquat poisoning. Arch Iran Med. 2008;11:387–91. [PubMed] [Google Scholar]
- 88.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schunemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NK, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KE, Vandvik PO, Coto-Yglesias F, Chrispim PP, Ramsay T. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–7. doi: 10.1001/jama.2010.310. [DOI] [PubMed] [Google Scholar]
- 89.Perriens JH, Benimadho S, Kiauw IL, Wisse J, Chee H. High-dose cyclophosphamide and dexamethasone in paraquat poisoning: a prospective study. Hum Exp Toxicol. 1992;11:129–34. doi: 10.1177/096032719201100212. [DOI] [PubMed] [Google Scholar]
- 90.Eddleston M, Wilks MF, Buckley NA. Prospects for treatment of paraquat-induced lung fibrosis with immunosuppressive drugs and the need for better prediction of outcome: a systematic review. QJM. 2003;96:809–24. doi: 10.1093/qjmed/hcg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Block ER. Potentiation of acute paraquat toxicity by vitamin E deficiency. Lung. 1979;156:195–203. doi: 10.1007/BF02714010. [DOI] [PubMed] [Google Scholar]
- 92.Suntres ZE, Shek PN. Intratracheally administered liposomal alpha-tocopherol protects the lung against long-term toxic effects of paraquat. Biomed Environ Sci. 1995;8:289–300. [PubMed] [Google Scholar]
- 93.Suntres ZE, Shek PN. Liposomal alpha-tocopherol alleviates the progression of paraquat-induced lung damage. J Drug Target. 1995;2:493–500. doi: 10.3109/10611869509015919. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki YJ, Inhibition PL, NF-kappa B. DNA binding activity by alpha-tocopheryl succinate. Biochem Mol Biol Int. 1993;31:693–700. [PubMed] [Google Scholar]
- 95.Redetzki HM, Wood CD, Grafton WD. Vitamin E and paraquat poisoning. Vet Hum Toxicol. 1980;22:395–7. [PubMed] [Google Scholar]
- 96.Minakata K, Suzuki O, Saito S, Harada N. Effect of dietary paraquat on a rat mutant unable to synthesize ascorbic acid. Arch Toxicol. 1996;70:256–8. doi: 10.1007/s002040050270. [DOI] [PubMed] [Google Scholar]
- 97.Perla V, Perrin NA, Greenlee AR. Paraquat toxicity in a mouse embryonic stem cell model. Toxicol In Vitro. 2008;22:515–24. doi: 10.1016/j.tiv.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 98.Kang SA, Jang YJ, Park H. In vivo dual effects of vitamin C on paraquat-induced lung damage: dependence on released metals from the damaged tissue. Free Radic Res. 1998;28:93–107. doi: 10.3109/10715769809097880. [DOI] [PubMed] [Google Scholar]
- 99.Hong SY, Hwang KY, Lee EY, Eun SW, Cho SR, Han CS, Park YH, Chang SK. Effect of vitamin C on plasma total antioxidant status in patients with paraquat intoxication. Toxicol Lett. 2002;126:51–9. doi: 10.1016/s0378-4274(01)00431-3. [DOI] [PubMed] [Google Scholar]
- 100.Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, Chang MC, Wang YJ. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–90. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Hoffer E, Avidor I, Benjaminov O, Shenker L, Tabak A, Tamir A, Merzbach D, Taitelman U. N-acetylcysteine delays the infiltration of inflammatory cells into the lungs of paraquat-intoxicated rats. Toxicol Appl Pharmacol. 1993;120:8–12. doi: 10.1006/taap.1993.1080. [DOI] [PubMed] [Google Scholar]
- 102.Hoffer E, Shenker L, Baum Y, Tabak A. Paraquat-induced formation of leukotriene B4 in rat lungs: modulation by N-acetylcysteine. Free Radic Biol Med. 1997;22:567–72. doi: 10.1016/s0891-5849(96)00385-1. [DOI] [PubMed] [Google Scholar]
- 103.Hoffer E, Baum Y, Tabak A, Taitelman U. N-acetylcysteine increases the glutathione content and protects rat alveolar type II cells against paraquat-induced cytotoxicity. Toxicol Lett. 1996;84:7–12. doi: 10.1016/0378-4274(95)03446-3. [DOI] [PubMed] [Google Scholar]
- 104.Lugo-Vallin N, Maradei-Irastorza I, Pascuzzo-Lima C, Ramirez-Sanchez M, Montesinos C. Thirty-five cases of S-carboxymethylcysteine use in paraquat poisoning. Vet Hum Toxicol. 2003;45:45–6. [PubMed] [Google Scholar]
- 105.Korbashi P, Kohen R, Katzhendler J, Chevion M. Iron mediates paraquat toxicity in Escherichia coli. J Biol Chem. 1986;261:12472–6. [PubMed] [Google Scholar]
- 106.Kohen R, Chevion M. Paraquat toxicity is enhanced by iron and reduced by desferrioxamine in laboratory mice. Biochem Pharmacol. 1985;34:1841–3. doi: 10.1016/0006-2952(85)90659-8. [DOI] [PubMed] [Google Scholar]
- 107.Hoffer E, Zonis Z, Tabak A, Taitelman U. The administration of desferrioxamine to paraquat-intoxicated rats. Vet Hum Toxicol. 1992;34:300–3. [PubMed] [Google Scholar]
- 108.Arouma O, Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988;18:459–70. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- 109.Silverman FP, Petracek PD, Fledderman CM, Ju Z, Heiman DF, Warrior P. Salicylate activity. 1. Protection of plants from paraquat injury. J Agric Food Chem. 2005;53:9764–8. doi: 10.1021/jf0513819. [DOI] [PubMed] [Google Scholar]
- 110.Drew JE, Padidar S, Horgan G, Duthie GG, Russell WR, Reid M, Duncan G, Rucklidge GJ. Salicylate modulates oxidative stress in the rat colon: a proteomic approach. Biochem Pharmacol. 2006;72:204–16. doi: 10.1016/j.bcp.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 111.Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 112.Katerinaki E, Haycock JW, Lalla R, Carlson KE, Yang Y, Hill RP, Lorigan PC, MacNeil S. Sodium salicylate inhibits TNF-alpha-induced NF-kappaB activation, cell migration, invasion and ICAM-1 expression in human melanoma cells. Melanoma Res. 2006;16:11–22. doi: 10.1097/01.cmr.0000195698.58013.53. [DOI] [PubMed] [Google Scholar]
- 113.Maharaj DS, Saravanan KS, Maharaj H, Mohanakumar KP, Daya S. Acetaminophen and aspirin inhibit superoxide anion generation and lipid peroxidation, and protect against 1-methyl-4-phenyl pyridinium-induced dopaminergic neurotoxicity in rats. Neurochem Int. 2004;44:355–60. doi: 10.1016/s0197-0186(03)00170-0. [DOI] [PubMed] [Google Scholar]
- 114.Manjula TS, Devi CS. Effect of aspirin on mitochondrial lipids in experimental myocardial infarction in rats. Biochem Mol Biol Int. 1993;29:921–8. [PubMed] [Google Scholar]
- 115.Cianferoni A, Schroeder JT, Kim J, Schmidt JW, Lichtenstein LM, Georas SN, Casolaro V. Selective inhibition of interleukin-4 gene expression in human T cells by aspirin. Blood. 2001;97:1742–9. doi: 10.1182/blood.v97.6.1742. [DOI] [PubMed] [Google Scholar]
- 116.Furst R, Blumenthal SB, Kiemer AK, Zahler S, Vollmar AM. Nuclear factor-kappa B-independent anti-inflammatory action of salicylate in human endothelial cells: induction of heme oxygenase-1 by the c-jun N-terminal kinase/activator protein-1 pathway. J Pharmacol Exp Ther. 2006;318:389–94. doi: 10.1124/jpet.106.102251. [DOI] [PubMed] [Google Scholar]


