Abstract
AIMS
The aim of this study was to define the underlying relative penetration of caffeine through hair follicles and through intact stratum corneum with time in vivo through pharmacokinetic modelling.
METHODS
Caffeine plasma concentration–time profiles after topical application into skin with or without hair follicle blocking were modelled using the Wagner–Nelson method or a compartmental model with first order absorption and elimination. Pharmacokinetic parameters describing absorption rate and extent of absorption through hair follicles or the stratum corneum were determined separately and compared with each other.
RESULTS
The obtained pharmacokinetic parameters from the two methods were similar. The absorption rate constant of caffeine for hair follicles was nearly 10 times higher than that for the stratum corneum and the percentage of absorption from hair follicles was more than half of that of the stratum corneum. In addition, the absorption from the stratum corneum showed an approximately 10 min delay while there was no delay for absorption from hair follicles. All caffeine absorbed by hair follicles occurs within 30 min of application and accounts for 10.5 to 33.8% of the total amount absorbed across the skin for all subjects, whereas absorption of caffeine through the stratum corneum can occur over several hours.
CONCLUSION
Hair follicles contribute significantly to percutaneous absorption of caffeine after topical application in man in vivo only at times soon after application.
Keywords: hair follicle, human skin penetration
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Depending on the body site and the substances, the hair follicles can contribute significantly to the penetration of topically applied substances. However, the quantitative and selective description of the follicular and intercellular penetration kinetics is difficult due to the lack of suitable models to differentiate between the different penetration pathways. Recently, for the first time, the follicular transport was measured directly in man in vivo indicating that the hair follicles are responsible for a fast delivery of topically applied substances.
WHAT THIS STUDY ADDS
In the present study, pharmacokinetic principles were applied to define the underlying relative penetration of caffeine through follicles and through intact stratum corneum with time in vivo. Absorption from hair follicles was found to contribute more than one third of the total percutaneous absorption of caffeine. Additionally, it was observed that the uptake by the hair follicles appeared at early times and was transient.
Introduction
The skin plays a major role in protecting us from our environment, in maintaining homeostasis and as a sensing organ. Much of the barrier properties of the skin have been ascribed to the outermost, ‘dead’ layer, the stratum corneum. However, the stratum corneum is pierced by a number of appendages and there has been a question as to what role they play in percutaneous absorption. One view has been that their role is insignificant, because hair follicles occupy only a small area of the total skin, usually less than 0.1% of the skin area [1]. Recently, this view has drastically changed. A number of studies have shown that hair follicles contribute significantly to the penetration of topically applied substances, depending on the body site and the substances applied [2].
The density of hair follicles varies between sites on the body, with the forehead being reported as having a very high density. Here, the surface area of the follicular stratum corneum contributes significantly to the total stratum corneum surface area [3]. In a comparison of the absorption of four compounds applied to different body sites, Rougier et al. found that the highest penetration occurred on the forehead and assumed that shunts played a significant role [4]. Our pharmacokinetic analysis of the likely penetration of steroids through the stratum corneum and through shunts suggested that less than 20% of the total penetration was due to shunts and that this mainly occurred at earlier times [5], as originally postulated by Scheuplein [6]. Others have used a comparison of normal and burn skin to show that liposomes can deposit drugs in the follicle regions [7] and that the follicles contribute to the absorption of hydrocortisone and testosterone into the skin [8]. Most recently, the novel sandwich system has been used to show that follicles can contribute 34 to 60% of the transport for low and intermediate polarity drugs but only 2 to 4% for lipophilic drugs [9].
A major limitation in the studies reported to date is that they have either involved animal skin or excised human skin. We are aware of only one study where a deliberate effort has been made to measure directly the role of follicular transport in man in vivo. Otberg et al. [10] applied caffeine in an ethanol : propylene glycol (30:70, v/v) solution to volunteers before and after blocking every hair follicle with a varnish solution. Caffeine was observed in blood samples at early times in normal skin but only after 20 min when the follicles were blocked. In a follow-up study using excised human skin, Trauer et al. [11] found that caffeine for both blocked and open follicles appeared in the receptor fluid in significant quantities after 120 min, a finding that paralleled their early in vivo data for unobstructed follicles [10].
In this study, we applied pharmacokinetic principles to define the underlying relative penetration of caffeine through follicles and intact stratum corneum with time in vivo. Both the pharmacokinetic model and a model free approach were employed. An important observation was that there was significant uptake by the follicles at early times but this was transient.
Methods
Experimental design
The details of the in vivo experimental designs have been published previously [10]. Briefly, 2 mg cm−2 of the 2.5% caffeine containing solution was applied to the 25 cm2 test area on the chest of six healthy Caucasian male volunteers. Blood samples were taken before and over 72 h after the topical caffeine application. After 3 days of a caffeine free diet, the experiment was repeated on exactly the same skin areas of the same volunteer under the same conditions, while each hair follicle orifice in the test area was blocked with a microdrop of the varnish-wax mixture prior to the caffeine solution being applied. Caffeine concentration in plasma was measured by a surface ionization mass spectrometry technique with a precision of ≤5% and with a detection limit of 1 ng ml−1. This technique has been developed and validated at the Arifov Institute of Electronics of the Uzbek Academy of Sciences, Taschkent, Utzbekistan. The experiments were approved by the Ethics Committee of the Charité (approval no. 272/03).
The details of the in vitro experimental design have likewise been described previously [11]. Briefly, 17.6 µl of the above mentioned formulation was applied to human full thickness breast skin mounted on pre-calibrated Franz diffusion cells with an area of 1.76 cm2 available for diffusion. The test samples previously underwent the follicular closing procedure, whereas the control samples remained untreated. Sampling from the receptor fluid was performed at time points 0, 1, 2, 5, 8 and 24 h. After 24 h, the compartments donor, epidermis, dermis and receptor fluid were analyzed separately. Caffeine concentrations were detected with HPLC with a detection limit for caffeine of 25 ng ml−1 at a wavelength of 262 nm. For this purpose, a WATERS liquid chromatograph equipped with a WATERS 510 high pressure pump, as well as a 712 WISP and a WATERS photo diode array detector were employed in combination with a reversed phase column TYPE WATERS RESOLVE™ C18 (5 µm, 3.9 mm × 150 mm). Every run was undertaken with a calibration curve, each having good linearity, i.e. r2≥ 0.99.
Pharmacokinetic analysis
Three methods were attempted in this analysis. The first of these, a constant rate of absorption model assuming a sink donor phase, was discontinued when it failed to describe the data obtained. Method A was the Wagner–Nelson method [12], which is a deconvolution method that allows one to estimate the amount absorbed (At) at various times (t) from apparent distribution volume for the compound (Vd), the plasma concentration at time t (Ct), and the elimination rate constant (ke) defined by the log-linear terminal plot of Ct vs. t. Here, the amount absorbed (At) at time t is given by:
where the cumulative area under the plasma concentration–time 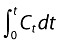 (AUC0–t) is estimated by the trapezoidal rule. The Wagner–Nelson method is most suitable when compounds have essentially mono-exponential disposition pharmacokinetics after intravenous administration, as observed for caffeine [13]. In order to simplify presentation of data, an apparent volume of distribution (Vd) of 0.35 l kg−1 for a low dose of caffeine intravenously administered [13] was assumed. The rate constant of absorption was obtained by fitting the At with the following equation:
(AUC0–t) is estimated by the trapezoidal rule. The Wagner–Nelson method is most suitable when compounds have essentially mono-exponential disposition pharmacokinetics after intravenous administration, as observed for caffeine [13]. In order to simplify presentation of data, an apparent volume of distribution (Vd) of 0.35 l kg−1 for a low dose of caffeine intravenously administered [13] was assumed. The rate constant of absorption was obtained by fitting the At with the following equation:
The plasma concentrations of caffeine data obtained from volunteers with closed and open hair follicles were fitted separately.
Method B was a compartmental model in which the plasma concentration of caffeine at various times after application to the skin occurred by two routes, the stratum corneum (SC) and hair follicles (HF), each assumed to be by a first order absorption process with absorption rate constants ka1 and ka2, respectively (a diagram of the model is illustrated in Figure 1). The elimination of caffeine from plasma was also assumed to be first order with an elimination rate constant ke. Accordingly, volunteer plasma concentrations are defined as either Cclose, caffeine plasma concentration for when the hair follicles were closed (caffeine was absorbed through the stratum corneum), or Copen, caffeine plasma concentration for subjects with the hair follicles open (caffeine was absorbed through both the stratum corneum and hair follicles):
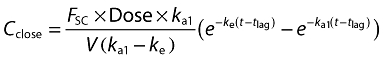 |
(1) |
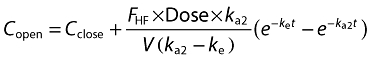 |
(2) |
where FSC and FHF are the percentage of the total amount of caffeine absorbed through the stratum corneum and hair follicles, respectively. tlag is the delay time of absorption from the stratum corneum. The data from the same subject with closed and open hair follicles were fitted simultaneously using the programme Scientist (MicroMath Scientific Software, Salt Lake City, UT).
Figure 1.
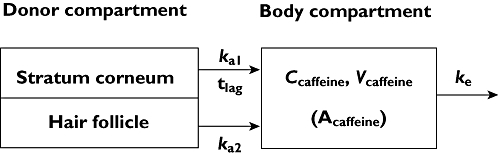
Schematic view of model for caffeine penetration through skin with open and closed hair follicles. Symbols: ka1, stratum corneum first order absorption rate constant with lag time, tlag; ka2, follicular first order absorption rate constant; Ccaffeine, plasma caffeine concentration; Vcaffeine, volume of distribution for caffeine; Acaffeine amount of caffeine in body (= Ccaffeine×Vcaffeine) and ke elimination rate constant for caffeine from body
As before, an apparent volume of distribution of 0.35 l kg−1 (V) was assumed for a low dose of caffeine [13]. The cumulative amount of absorption of caffeine from the stratum corneum (closed hair follicles) (ASC), hair follicles (AHF) and the whole skin (WS, open hair follicles) (AWS) was calculated as follows:
| (3) |
| (4) |
| (5) |
Statistical analysis
The pharmacokinetic parameters obtained from the first order absorption model and the Wagner–Nelson method were compared using Student's t-test. A P value less than 0.05 was considered as being significantly different.
Results
The pharmacokinetic parameters obtained using the Wagner–Nelson method (method A) and the first order absorption model (method B) are compared in Table 1. No statistically significant difference was found for all the parameters calculated from two methods (P > 0.05).
Table 1.
Summary of pharmacokinetic parameters for each subject obtained from the Wagner–Nelson method (method A) and the first order absorption model (method B)
| Parameter | Method | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | Subject 6 | Mean | SD |
|---|---|---|---|---|---|---|---|---|---|
| ka1 (h−1) | A | 0.62 | 1.51 | 3.15 | 4.02 | 1.13 | 0.61 | 1.84 | 1.42 |
| B | 1.25 | 1.59 | 2.38 | 4.42 | 2.76 | 1.88 | 2.38 | 1.14 | |
| ka2 (h−1) | A | 29.7 | 17.0 | 14.3 | 10.3 | 12.7 | 14.5 | 16.4 | 6.87 |
| B | 23.8 | 19.6 | 16.0 | 6.40 | 19.5 | 15.4 | 16.8 | 5.91 | |
| tlag (h) | A | 0.14 | 0.08 | 0.33 | 0.16 | 0.25 | 0.14 | 0.18 | 0.09 |
| B | 0.11 | 0.08 | 0.31 | 0.15 | 0.14 | 0.12 | 0.15 | 0.08 | |
| FWS (%) | A | 60.7 | 55.0 | 53.4 | 51.3 | 63.6 | 60.4 | 57.4 | 4.82 |
| B | 72.8 | 70.0 | 51.4 | 49.2 | 71.3 | 80 | 65.8 | 12.5 | |
| FSC (%) | A | 48.5 | 27.7 | 28.7 | 35.1 | 47.4 | 49.9 | 39.6 | 10.3 |
| B | 54.9 | 36.2 | 34.0 | 32.6 | 50.2 | 67.5 | 45.9 | 14.0 | |
| FHF (%) | A | 12.1 | 27.3 | 24.6 | 16.3 | 16.1 | 10.5 | 17.8 | 6.75 |
| B | 17.9 | 33.8 | 17.4 | 16.6 | 21.1 | 12.5 | 19.9 | 7.36 |
ka1, ka2 absorption rate constant from the stratum corneum and hair follicles, respectively; tlag, delay time for absorption from the stratum corneum; FWS, FSCand FHF the percentage of total amount of caffeine absorbed through whole skin, the stratum corneum and hair follicles, respectively.
The absorption rate constant of caffeine for hair follicles was nearly 10 times higher than that for the stratum corneum indicating the much faster absorption from hair follicles. There was no delay for absorption from hair follicles, whilst an approximate 10 min delay in absorption was shown for the stratum corneum. The percentage of absorption from hair follicles accounts for more than one-third of total absorption from the whole skin as shown in Table 1.
Figure 2 shows a typical compartmental model fit of whole skin (open follicles) and skin with follicles closed. It clearly shows that the applied model could adequately describe the plasma concentration–time profile obtained from volunteers, both with open and closed hair follicles.
Figure 2.
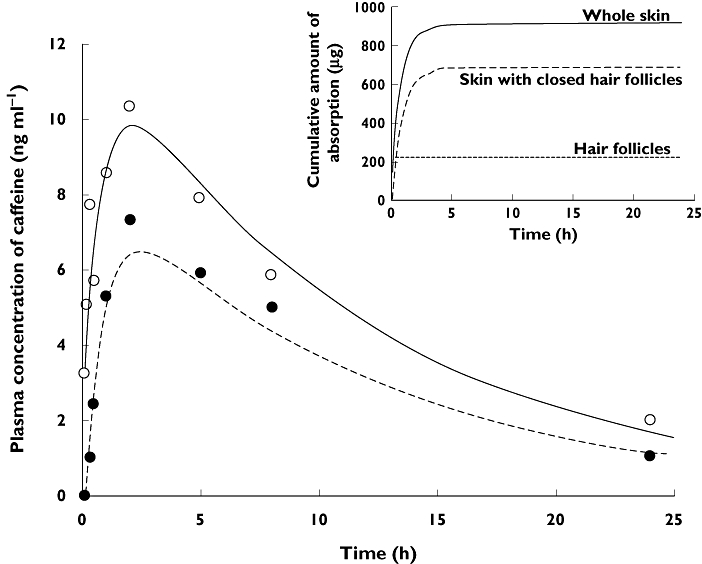
Representative plasma concentration-time profile of caffeine and the model fitting curve (Subject 1) from the first order absorption model. (○) open hair follicles, (•) closed hair follicles. Lines represent the fitting curve. Insert is cumulative absorption profile for this subject
Figure 3 shows the cumulative amount of absorption from the whole skin, hair follicles and the stratum corneum. It is obvious from this figure that the absorption of caffeine from hair follicles reaches its maximum value within 0.5 h while the absorption from the stratum corneum takes nearly 5 h to reach its maximum absorption.
Figure 3.
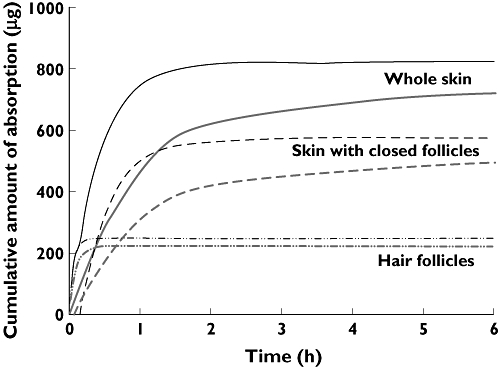
Mean cumulative absorption profile obtained from the Wagner–Nelson method (thick grey line) and first order absorption model (thin black line) (n = 6)
As shown in Figure 4, the in vivo absorption of caffeine from the whole skin, hair follicles or the stratum corneum alone was much faster and higher than the in vitro absorption.
Figure 4.
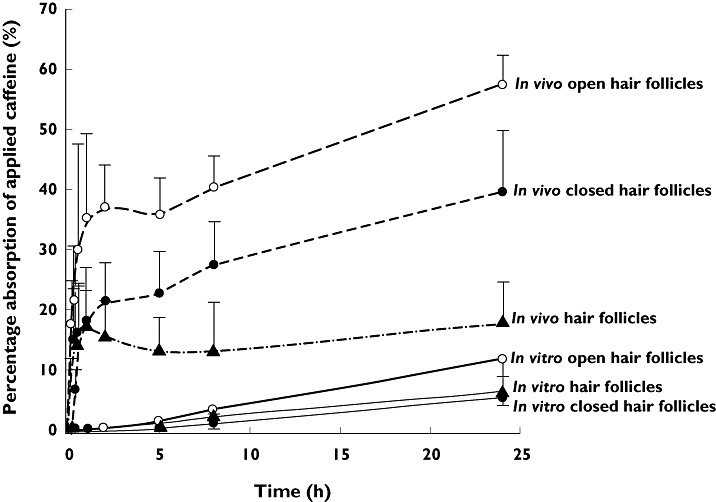
Comparison of mean absorption of applied caffeine in vivo (dashed lines) and in vitro (solid lines) for open and closed follicles and estimated hair follicle only penetration. (○) open hair follicles, (•) closed hair follicles, (Δ) hair follicles. Error bar represents SD (n = 6)
Discussion
Drug penetration through a follicular pathway has been studied for more than 20 years and demonstrated to be an efficient pathway through or into the skin [14]. Moreover, the hair follicles offer interesting therapeutic target sites as they represent complex and dynamic three dimensional structures [15]. Recently, it has been observed that the utilization of particulate substances allows selective targeting of specific structures within the hair follicles. These include specific cell populations in and around the hair follicle, such as immune cells, stem cells and melanocytes, sebaceous glands and perifollicular blood vessels [16–19]. Whereas transfollicular penetration for particulate substances is not realistic due to their size, for smaller molecules, such as caffeine, the hair follicle can be regarded as a transport route into the living tissue. Especially, the lower infundibulum region provides, on the one hand, reduced barrier properties, and, on the other hand, is surrounded by a dense network of blood capillaries ensuring a rapid uptake of penetrated substances [2].
However, the quantitative and selective description of the follicular and the intercellular penetration kinetic process has not been reported due to the lack of suitable models to differentiate between the different penetration pathways. In the present study, the percutaneous absorption of caffeine in vivo with and without the influence of hair follicles was estimated using the Wagner–Nelson method and a first order absorption model based on the in vivo data of caffeine absorption obtained by Otberg et al. [8]. The caffeine plasma concentration–time profile of subjects with closed and open hair follicles was well described by the first order absorption model (Figure 2). The pharmacokinetic parameters obtained from both methods are compared in Table 1 and were shown to be quite similar. Absorption from hair follicles was found to contribute more than one-third of the total percutaneous absorption of caffeine. In addition, the absorption of caffeine through hair follicles was much faster compared with the absorption through the stratum corneum and reached the maximum absorption within 1 h after topical application of caffeine, while it took more than 5 h for absorption of caffeine to reach its maximum through the stratum corneum. This effect is understandable when considering that exclusive absorption via the stratum corneum means that caffeine has to diffuse through all cell layers of the complete epidermis before systemic uptake via the blood capillaries of the dermis becomes possible, whereas follicular penetration allows a rapid and direct transport into the infundibulum region where penetration into the living tissue is facilitated by reduced barrier properties.
In Figure 4, the in vivo absorption of caffeine was compared with the in vitro absorption based on the data obtained by Trauer et al. [11]. The comparison revealed that the in vivo absorption of caffeine from the whole skin, hair follicle or stratum corneum alone was much faster and higher than the in vitro absorption. A possible explanation for the faster appearance of caffeine in the blood in comparison with the receptor medium might represent the blood flow in vivo allowing a fast evacuation of the permeated substance. In vitro, this mechanism is absent. After permeating the hair follicle, caffeine reaches the living tissue. Whereas, in vivo, caffeine would have been taken up by the blood system, in vitro caffeine has to penetrate through all skin layers of the dermis to reach the receptor medium, which is more time-consuming. Additionally, differences in the in vivo and in vitro results may derive from differences in the investigated skin. For the in vivo study, breast skin areas of male volunteers with terminal hair follicles were investigated, whereas, for the in vitro study, excised breast skin of female volunteers was used providing significantly smaller vellus hair follicles on this body site. In addition, hairy regions of excised skin show a great contraction. Previous investigations have shown that the follicular reservoir can be reduced up to 90% in excised skin [20]. These aspects have to be considered when comparing in vivo and in vitro data.
Nevertheless, the results of the present investigation clearly indicate that hair follicles contribute significantly to percutaneous absorption of caffeine only at early times after topical application in man in vivo.
Acknowledgments
We would like to thank the Foundation ‘Skin Physiology’ of the Donor Association for German Science and Humanities for financial support and the Australian National Health & Medical Research Council for support.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Redelmeier TE, Schaefer H. Skin Barrier: Principles of Percutaneous Absorption. Basel: Karger; 1996. [Google Scholar]
- 2.Knorr F, Lademann J, Patzelt A, Sterry W, Blume-Peytavi U, Vogt A. Follicular transport route – research progress and future perspectives. Eur J Pharm Biopharm. 2009;71:173–80. doi: 10.1016/j.ejpb.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol. 2004;122:14–9. doi: 10.1046/j.0022-202X.2003.22110.x. [DOI] [PubMed] [Google Scholar]
- 4.Rougier A, Lotte C, Maibach HI. In vivo percutaneous penetration of some organic compounds related to anatomic site in humans: predictive assessment by the stripping method. J Pharm Sci. 1987;76:451–4. doi: 10.1002/jps.2600760608. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui O, Roberts MS, Polack AE. Percutaneous absorption of steroids: relative contributions of epidermal penetration and dermal clearance. J Pharmacokinet Biopharm. 1989;17:405–24. doi: 10.1007/BF01061455. [DOI] [PubMed] [Google Scholar]
- 6.Scheuplein RJ. Mechanism of percutaneous absorption. II. Transient diffusion and the relative importance of various routes of skin penetration. J Invest Dermatol. 1967;48:79–88. [PubMed] [Google Scholar]
- 7.Bernard E, Dubois JL, Wepierre J. Importance of sebaceous glands in cutaneous penetration of an antiandrogen: target effect of liposomes. J Pharm Sci. 1997;86:573–8. doi: 10.1021/js960394l. [DOI] [PubMed] [Google Scholar]
- 8.Hueber F, Wepierre J, Schaefer H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: in vivo study in the hairless rat. Skin Pharmacol. 1992;5:99–107. doi: 10.1159/000211026. [DOI] [PubMed] [Google Scholar]
- 9.Frum Y, Bonner MC, Eccleston GM, Meidan VM. The influence of drug partition coefficient on follicular penetration: in vitro human skin studies. Eur J Pharm Sci. 2007;30:280–7. doi: 10.1016/j.ejps.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Otberg N, Patzelt A, Rasulev U, Hagemeister T, Linscheid M, Sinkgraven R, Sterry W, Lademann J. The role of hair follicles in the percutaneous absorption of caffeine. Br J Clin Pharmacol. 2008;65:488–92. doi: 10.1111/j.1365-2125.2007.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trauer S, Patzelt A, Otberg N, Knorr F, Rozycki C, Balizs G, Buttermeyer R, Linscheid M, Liebsch M, Lademann J. Permeation of topically applied caffeine through human skin – a comparison of in vivo and in vitro data. Br J Clin Pharmacol. 2009;68:181–6. doi: 10.1111/j.1365-2125.2009.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner JG, Nelson E. Per cent absorbed time plots derived from blood level and/or urinary excretion data. J Pharm Sci. 1963;52:610–1. doi: 10.1002/jps.2600520629. [DOI] [PubMed] [Google Scholar]
- 13.Newton R, Broughton LJ, Lind MJ, Morrison PJ, Rogers HJ, Bradbrook ID. Plasma and salivary pharmacokinetics of caffeine in man. Eur J Clin Pharmacol. 1981;21:45–52. doi: 10.1007/BF00609587. [DOI] [PubMed] [Google Scholar]
- 14.Lademann J, Knorr F, Richter H, Blume-Peytavi U, Vogt A, Antoniou C, Sterry W, Patzelt A. Hair follicles – an efficient storage and penetration pathway for topically applied substances. Summary of recent results obtained at the Center of Experimental and Applied Cutaneous Physiology, Charite-Universitatsmedizin Berlin, Germany. Skin Pharmacol Physiol. 2008;21:150–5. doi: 10.1159/000131079. [DOI] [PubMed] [Google Scholar]
- 15.Rogers GE. Hair follicle differentiation and regulation. Int J Dev Biol. 2004;48:163–70. [PubMed] [Google Scholar]
- 16.Gupta S, Domashenko A, Cotsarelis G. The hair follicle as a target for gene therapy. European Journal of. Dermatology. 2001;11:353–6. [PubMed] [Google Scholar]
- 17.Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–60. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- 18.Patzelt A, Knorr F, Blume-Peytavi U, Sterry W, Lademann J. Hair follicles, their disorders and their opportunities. Drug Discovery Today. 2008;5:173–81. [Google Scholar]
- 19.Vogt A, Combadiere B, Hadam S, Stieler KM, Lademann J, Schaefer H, Autran B, Sterry W, Blume-Peytavi U. 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. Journal of Investigative. Dermatology. 2006;126:1316–22. doi: 10.1038/sj.jid.5700226. [DOI] [PubMed] [Google Scholar]
- 20.Patzelt A, Richter H, Buettemeyer R, Huber HJ, Blume-Peytavi U, Sterry W, Lademann J. Differential stripping demonstrates a significant reduction of the hair follicle reservoir in vitro compared to in vivo. Eur J Pharm Biopharm. 2008;70:234–8. doi: 10.1016/j.ejpb.2008.02.024. [DOI] [PubMed] [Google Scholar]


