Abstract
Planar cell polarity (PCP) describes the coordinated polarization of tissue cells in a direction that is orthogonal to their apical/basal axis. In the last several years, studies in flies and vertebrates have defined evolutionarily conserved pathways that establish and maintain PCP in various cellular contexts. Defective responses to the polarizing signal(s) have deleterious effects on the development and repair of a wide variety of organs/tissues. In this review, we cover the known and hypothesized roles for PCP in the metanephric kidney. We highlight the similarities and differences in PCP establishment in this organ compared with flies, especially the role of Wnt signaling in this process. Finally, we present a model whereby the signal(s) that organizes PCP in the kidney epithelium, at least in part, comes from the adjacent stromal fibroblasts.
Key words: planar cell polarity, kidney, cystic kidney disease, morphogenesis, non-canonical Wnt signaling
Kidney Development
The kidney is composed of epithelial and endothelial tubules that function together to maintain blood chemistry. The epithelial tubules of the metanephric kidney develop through two distinct mechanisms: branching morphogenesis and mesenchymal to epithelial transitions (MET). Briefly, an epithelial structure known as the ureteric bud (UB), branches within a population of pre-specified intermediate mesoderm known as the metanephric mesenchyme (MM) (Fig. 1). A signal(s) produced by the UB stimulates the nephron progenitors within the MM to survive and proliferate and induces a subpopulation to undergo MET forming the renal vesicle (RV). The nephron progenitors sit directly adjacent to the UB and give rise to all epithelial portions of the nephron.1,2 Reciprocally, signals from the mesenchyme cause the continued branching of the UB, which will eventually give rise to the collecting duct system and the ureter.
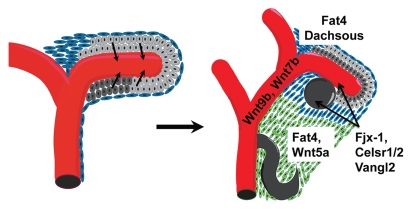
Figure 1.
Development of the metanephric kidney. The development of the kidney is initiated when the ureteric bud (shown here in red) invades the metanephric mesenchyme. The mesenchyme consists of two distinct cell populations: the nephron progenitors (light gray) and a layer of fibroblasts known as the stroma (blue). Signals from the bud induce differentiation in a progenitor-subset (dark gray) to form the renal vesicle (gray ball), while signals from the mesenchyme induce branching morphogenesis of the ureteric bud. At the same time, the stromal population is patterned and segregates itself as the cortical stroma (in blue) and medullary stroma (in green). Wnt5a and the PCP determinants Fat4 and Dachsous are primarity expressed in the stromal population, while Four-jointed, Vangl2 and Celsr1 are expressed primarily in the renal vesicles and ureteric buds. Wnt4 is also expressed in the renal vesicles. Wnt9b and Wnt7b are expressed in the ureteric bud epithelium.
After forming, the renal vesicles progress through a series of morphological steps known as the comma and S-shaped body, with distinct cellular populations giving rise to the various nephron segments, the most proximal being the renal corpuscle, progressing through the proximal tubule, loop of Henle, distal tubule and, finally, the connecting tubule that connects the distal tubule to the UB-derived collecting ducts. Likewise, the ureteric bud derivatives undergo extensive growth and morphogenesis to form the collecting ducts, renal pelvis and ureter. Interestingly, most, if not all, of the cell proliferation within both populations of epithelial tubules contributes to their lengthening, while the diameter remains largely constant. Proper length and diameter of the tubules is essential for their function.
In addition to the nephron progenitors (also known as the cap mesenchyme), the MM consists of a fibroblast layer with distinct molecular identity known as the stroma. Although for years the nephron progenitors and stromal progenitors were thought to be derived from the same population of MM, recent studies suggest that the two cell types either come from distinct lineages or take on their different fates very early in development.3,4 As nephron development continues, the stroma segregates itself into two unique populations: the cortical stroma that encapsulates the nephron progenitors and the medullary stoma that intercalates between the distal epithelial and endothelial tubules. The stroma will give rise to the majority of the interstitium of the kidney, including vascular smooth muscle cells, mesangial cells and pericytes but not the vasculature.5 The role of the stroma in nephron development is, however, not clear. Although it is widely speculated that the stroma plays a support role, it is possible that these fibroblasts play major roles in modulating signals emanating from the bud and the nephron progenitors.
In mice, metanephric kidney development initiates on embryonic day (E)10. The reciprocal nature of the signals between the UB and the MM lead to continuous, exponential growth of the UB and MM populations for the remainder of the fetal period and for a short time after birth, ultimately resulting in the formation of approximately 30,000 nephrons (1 million in a human). However, three days after birth, nephrogenesis ceases when the nephron progenitor population disappears.6
What is Planar Cell Polarity?
Most, if not all, epithelial cells show polarity along two distinct axes. The first, known as apical/basal polarity, defines the orientation of the cells relative to the underlying extracellular matrix. However, epithelial cells are also polarized within the plane of the tissue, orthogonal to the apical-basal axis. This type of polarity is known as planar cell polarity (PCP). The manifestation of planar polarity varies from tissue to tissue. In some cases, it is visible to the naked eye, such as in the coordinated, ordered alignment of bristles on the fly wing or the orientation of mammalian hair. In other tissues, it is visible only at the microscopic level as in the coordinated orientation of the stereociliary bundles in the mammalian cochlea, the orientation of cells and directed movements seen in gastrulating embryos or developing kidneys and the directional beating and tilt of cilia seen in the node of early stage mouse embryos. Over the last several years, mutation and overexpression studies in vertebrates and invertebrates have revealed a number of roles for PCP in normal embryonic development and tissue homeostasis. In this review, we will comment on the known and potential roles for PCP in the development and maintenance of the mammalian kidney.
Establishment of PCP
Before we can discuss the role of PCP in the kidney, it is necessary to review how PCP is established. We do not have space to cover this topic in detail in this review. Instead, we will attempt to briefly acquaint the reader with factors we will discuss later. For those interested in more thorough coverage, we recommend several excellent reviews, including one by Maung and Jenny in this issue of Organogenesis.7–9
The “Core” Group
PCP has been most extensively studied in the fly wing, eye and cuticle. Genetic studies in these systems have identified several distinct genetic pathways that are required to establish PCP. The first group consists of the “core” PCP proteins, comprising the membrane-bound proteins Frizzled (Fz), Strabismus/Van Gogh (vang) and Flamingo (fl), acting along with the cytoplasmic partners Disheveled (dsh), Diego (dg) and Prickle (pk). Recent studies have identified two additional proteins that may be members of the core group. The sodium/proton exchanger (nhe2) is required for the localization of Dsh protein and the regulation of PCP in the eye.10 Although determining its role in other tissues was prevented by pleiotrophic effects, the requirement for Nhe2 in Dsh localization in fly eye and mammalian cells suggests it may be a core determinant. Several groups also found that the prorenin receptor (prr), a subunit of the vacuolar ATPase (vATPase) was required for PCP in the fly eye and abdomen and for non-canonical Wnt signaling in vertebrates.11–13 Prr physically associates with the Frizzled receptor, affecting its subcellular localization and interaction with Dsh, once more indicating it may be a core determinant.
Many of the core proteins show distinct localization within the plane of the epithelium. For instance, in the fly wing imaginal disc, Vang and Pk are localized to the proximal side of epithelial cells while Fz, Dsh and Dg are localized to the distal side (Fl is not asymmetrically localized).14–20 Nhe2 and Prr subcellular localization has not been examined in vivo in great detail.
The asymmetric localization of the core determinants not only helps to define PCP but may also be required for it. For instance, the extracellular domains of Vang and Fz (expressed on opposing cells) interact and stabilize the localization of each other. Dsh, Fz and Dg form a complex on the distal side of cells that appears to block the distal localization and function of Pk. Reciprocally, Pk interacts with the cytoplasmic domain of Vang and blocks the recruitment of Dsh to the proximal side. Fl (possibly through the asymmetric expression and localization of distinct isoforms) is necessary for the localization of both Fz and Vang to their respective domains. Through their mutually antagonistic interactions, the two complexes appear to reinforce the localization of the other and maintain PCP.
Mutation of individual core components disrupts the asymmetric localization of other members of the complex resulting in randomization of planar polarity in both cell autonomous and non-autonomous fashion. Due to the interaction of the proximal and distal complexes in adjacent cells, mutation of a core component in one cell can affect the PCP of a neighboring wild-type cell in a phenomenon known as domineering non-autonomy.
A few studies have been done on the expression of PCP determinants in the developing kidney. For the most part, the core determinants appear to be expressed in the developing epithelia (ureteric bud, renal vesicles, s-shaped body21,22 and Fig. 2). However, little is known of their subcellular localization. Babayeva and colleagues suggest that Vangl2 is asymmetrically localized in the renal vesicles and s-shaped bodies.21 Although intriguing, more detailed studies with additional proteins will need to be performed to determine the extent to which this aspect of PCP is conserved within the kidney.
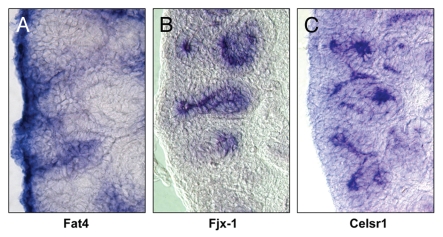
Figure 2.
Expression profile of genes involved in planar cell polarity in mouse E15.5 kidney. (A) Fat4 is expressed in the cortical stroma and in the interstitial cells. (B) Fjx-1 is expressed specifically in the ureteric bud tips and stalk, the renal vesicles. (C) Celsr1 is also expressed in the ureteric bud and renal vesicles, while Celsr2 shows predominant expression in the RV, C- and S-shaped bodies (data not shown).
The Fat/Ds Group
Another group of factors that regulates PCP will be referred to as the Fat/Dachsous group. This group includes the atypical cadherins Dachsous (ds, mammalian Dchs1 and 2) and Fat (fat, murine Fat-j or Fat4, Fat1, Fat2 and Fat3), the Golgi-associated kinase, Fourjointed (fj, murine Fjx-1), the transcription factor Atrophin (Atro, murine Atn1) and the Protein Phosphatase 2a subunit, Widerborst (Wdb, murine Ppp2r5e).
How these factors work is still not clear. Fat and Ds are proposed to physically interact in a heterotypic fashion to mediate PCP.23 These two proteins are expressed in a graded fashion across the plane of some tissues, for instance in the fly eye.24 Fj, which is also expressed in a gradient, is reported to regulate the phosphorylation state of the cadherin repeats of Fat and Ds and their ability to interact.23–26 Atro is a transcription factor that binds to the cytoplasmic domain of the Fat protein and may regulate the expression of specific effector molecules.27 Wdb functions in part to polarize the cytoskeleton, and this may regulate the localization of the core determinants.28
Initial studies suggested that the Fat/Ds group controlled the gradient of fz expression and, therefore, the direction of PCP, leading some to refer to them as the “upstream group”.29,30 However, this model has recently been challenged by the parallel hypothesis emphasizing the simultaneous, parallel activities of the fat-ds and the core complexes.31 In the Drosophila abdomen, it has been shown that overexpression of the upstream molecules can “repolarize effector cells” in the complete absence of core PCP proteins.32 Further mutation of either group of proteins exhibits a subtle effect compared with the complete randomized effect observed with the simultaneous depletion of both. These studies along with further evidence indicating the inability of the upstream molecules to affect core protein localization in all tissues indicated the possibility of the simultaneous existence of two or more pathways exhibiting independent influence on cell polarity.31
Interestingly, characterization of the expression of the mouse orthologs of Fat and Ds in the developing kidney suggested that they were predominantly expressed in the stroma, not the epithelium,33,34 while the Four-jointed homolog 1 (Fjx1) was expressed in the pre-tubular aggregates, renal vesicles and comma-shaped bodies.33 This is surprising, because if Fjx1 functions cell autonomously to modify Fat and Dachsous proteins as current models suggest, then it should be co-expressed in the stroma. Either the current models are incorrect, or the expression analysis is incorrect/incomplete (one cannot rule out low level expression of any of these factors in additional cell types or the possibility that there are additional paralogs of these genes that have not been characterized). There are several additional Fat paralogs, and at least one, Fat3, is expressed in the progenitor population.35
Mutation of either Fat4 or Dchs1 leads to defects in the kidney epithelium.34,36 The high expression levels of these genes in the stroma implicate this cell type in establishing PCP in the kidney. This is an intriguing yet sensible possibility given the close physical association of the stroma and epithelium and the building evidence of molecular crosstalk between these two cell types (see below). Indeed, kidney fibrosis frequently appears to lead to the development of cystic kidney tubules, and there is strong evidence that cystogenesis is caused by defects in PCP. However, stromal/epithelial interactions would seem better suited to playing a role in apical/basal polarity than planar polarity. The question of where the directional cues required for planar polarity of the kidney epithelium come from is still unresolved.
The Wnt Pathways
Several studies have shown that Wnt signaling plays a role in establishing PCP. However, the precise role of Wnt signaling in this process is complicated. Wnts are small, secreted glycoproteins with pleiotropic effects in cell fate specification and differentiation. Binding of a Wnt ligand to one of its receptors activates a signal transduction cascade that can affect cell proliferation, polarity, survival, differentiation and many other processes. Wnt signal transduction has been broadly categorized into canonical and non-canonical pathways based on whether the β-catenin protein is utilized (canonical) or not (non-canonical). In the canonical pathway, the Wnt ligand binds to a member of the Frizzled family of transmembrane receptors along with one of a number of co-receptors. Trafficking of Frizzled to the plasma membrane requires Prr/V-ATPase activity.13 Binding of the ligand to its receptor complex triggers a series of events that affect the subcellular localization of a cytoplasmic protein known as Disheveled (Dvl). Dvl activity inactivates a complex of proteins (collectively known as the β-catenin destruction complex) that cause the β-catenin protein to be degraded by the proteasome. Inactivation of the destruction complex allows β-catenin to be stabilized. The stabilized protein translocates to the nucleus and complexes with the T-cell factor/Lymphoid-enhancing factor (Tcf/Lef) family of transcription factors to form the β-catenin-Tcf/Lef DNA complex and regulate transcriptional targets.
In addition to the canonical pathway, several non-canonical Wnt pathways have been described in references 37–39. The only defining characteristic of these pathways is that none of them directly affect β-catenin stability. What determines which signal transduction pathway is activated by a Wnt is still not completely clear. Although some Wnts have been characterized as primarily signaling through the canonical or non-canonical pathways, indicating that the specificity may lie in the identity of the ligand, it is more accurate to state that the pathway utilized by a particular Wnt is highly dependent on the environment in which the ligand is received.37 In fact, a number of Wnts appear to be able to signal through both canonical and non-canonical pathways.40–44
Several recent studies suggest that rather than the identity of the ligand, pathway specificity is controlled by the presence or absence of various receptors/co-receptors in the receiving cells. Binding of a Wnt ligand to a Frizzled and Lrp5 or -6 promotes canonical signaling, while the Ror, Ptk7 and Ryk receptors appear to promote non-canonical signaling and may inhibit canonical signaling.40,45–51 There is still some question as to whether all of the non-canonical receptors utilize Fzds as co-receptors. Dvl, however, appears to be required for both branches of the pathway. There is considerable data suggesting that members of the Rho family of GTPases, protein kinase C (PKC) and the Jun kinases (Jnk) are activated downstream of Ror, Ptk7 and Ryk.37,39,47,52
There is data supporting roles for both canonical and noncanonical Wnt signaling in PCP. In flies, a Wnt ortholog, Wg, signaling through β-catenin is required for the graded expression of ds and fj in the eye.53 Thus, canonical Wnt signaling is required to set up the directional cues for PCP in this cell type.
Because Fz, Dvl and Prr are all core PCP determinants and components of the Wnt signal transduction cascade, it also seemed plausible that a Wnt gradient was directly involved in regulating the cellular “readout” of polarity. Several separate pieces of data based on the nature of polarity defects seen upon Wnt pathway misregulation and the cell type-specific requirements for canonical pathway components in PCP suggest that, in the fly, Wnts are not directly involved in PCP.53 Indeed, in the fly, there is no evidence that Wnt ligands (signaling through canonical or non-canonical pathways) play a direct role in PCP. Therefore, the model is that Fz, Dsh and Prr act in Wnt-dependent fashion to regulate expression of the factors that establish the direction of PCP and in a Wnt-independent fashion to coordinate the cellular readout of PCP.
In worms, localized Wnt production directly controls mitotic spindle orientation and the localization of Fz receptors in both embryonic and post-embryonic cells, and the role in spindle orientation does not require transcription, suggesting this is a non-canonical (β-catenin-independent) role.54–56 Further, during worm vulval development, PCP is regulated by non-canonical Wnt signaling through a Ror ortholog.50 There is additional data implicating non-canonical Wnt signaling (through both Ror and Ryk orthologs) in neurite migration, although it is not clear that the Wnts are directly regulating PCP in these systems.57 But these data would suggest that the mechanistic role of Wnts in PCP may differ between flies and worms.
In vertebrates, there is also significant data supporting a role for Wnts in PCP during various embryonic processes, many of which are reviewed in this issue of Organogenesis. In most of these cases, it is not clear whether the Wnts are affecting the readout or the upstream polarizing signal and which branch of the pathway is used. However, Witze and collegues showed that Wnt5a, most likely signaling in a non-canonical manner, was able to induce the planar polarized localization of actin, MCAM and Fz3 in cells.58 Unlike the situation in worms, the Wnt ligand appeared to play a permissive role with the instructive signal provided by the chemokine Cxcl12. In addition, Gao et al. recently found that Wnt5a, signaling through Ror2, directly regulated Vangl2 activity and PCP in the developing mouse limb.51 Although these two studies implicate non-canonical Wnt signaling in PCP in mammals, it is probable that the mechanisms are different in different tissues. Wnt signaling most likely plays multiple mechanistically distinct roles in PCP.
Cell Type-Specific Effectors
Cell type-specific effectors also regulate PCP. The identity of these molecules is specific to different tissues and the phenotypic readout of PCP. Multiple effectors of PCP have been identified in flies, and orthologs of these factors may play a role in PCP in the kidney. Alternatively, the kidney may have a unique set of effectors that regulate the various aspects of PCP in this organ. Many genes have been identified that affect (directly or indirectly) PCP within the kidney (see below), and most are not known PCP determinants. Genetic and molecular analysis will be required to determine if some (or all) of these factors are truly cell typespecific effectors of PCP.
What is the Role for PCP in Kidney Development?
Orthologs of most, if not all of the fly PCP determinants appear to be present in all metazoans that have been studied. Over the last several years, it has become quite apparent that their involvement in establishing PCP is also conserved, although the precise mechanisms may be altered relative to the fly.
Several recent studies have shown that disruption of PCP, including mutation of some PCP determinants, correlates with or directly results in kidney defects. In this last section, we will discuss various cellular processes that are controlled by PCP and how they might affect kidney structure.
Oriented Cell Division
One of the manifestations of PCP is oriented cell divisions (OCD) during tissue growth. Defects in this process can have significant impact on normal tissue development (Fig. 3). In 2006, Fisher and colleagues demonstrated that kidney tubule elongation is controlled, at least in part, by OCD.59 They further demonstrated that OCDs were disrupted in two distinct rodent models of CKD, including mice lacking the transcription factor Hnf1b. The defects in orientation were present prior to cyst formation, suggesting they played a causal role in disease progression. Since this initial discovery, several groups have shown a correlation between defects in oriented cell division and cystogenesis.36,42,60–62 The precise role these factors play in establishing or affecting PCP is not known. However, Lokmane and colleagues found that Hnf1b directly regulates the expression of Wnt9b, potentially explaining the PCP defects observed in these mice (see below).63
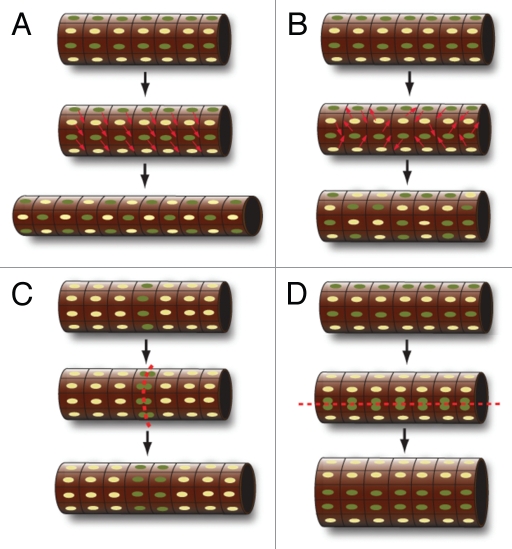
Figure 3.
Overview of convergent extension movements (A and B) and oriented cell division (C and D) in epithelial tubules. (A) During convergent extension movements, the cells intercalate medio-laterally or perpendicular to the proximo-distal axis of an epithelial tubule in the wild type. (B) In the absence of Wnt9b in kidney epithelial tubules, this movement is completely randomized, resulting in increased tubule diameter. (C) Oriented cell division in wild-type tubules causes the mitotic spindle to be within the plane (or parallel to the proximo-distal axis) of the epithelium, resulting in elongation without affecting the diameter. (D) In mutants with defects in oriented cell division, the plane of the mitotic spindle is randomized relative to the axis. In the absence of compensating forces, such as convergent extension, this will result in increased tubule diameter.
In 2008, Saburi et al. showed that mice lacking the ortholog of Drosophila fat, Fat4, developed cystic kidneys with defects in oriented cell division.36 They further showed that Fat4 genetically interacted with the orthologs of fj (Fjx-1) and vang (Vangl2), although mutations of neither of the latter two genes on their own were sufficient to give cysts. Nonetheless, this data suggested that the role for the Fat/Ds pathway (and perhaps the core pathway) in regulating PCP was conserved in the developing kidney. Additional support for this conclusion came from recent work by Mao and colleagues, who showed that mutation of the mouse ortholog of ds, Dchs1, also led to cyst formation.34 Genetic studies suggested that Fat4 and Dchs1 were most likely acting in the same pathway to regulate tubule diameter in the kidney, similar to the situation in the fly eye. An additional interesting observation made in this study was that Dchs1 and Fat4 mRNA and protein were primarily expressed in the stroma adjacent to the developing epithelia. This is quite distinct from the situation in the fly, where ds and ft are expressed in the epithelium itself. This is of interest, because several investigators have shown that multiple core components, including Fzds and Vangl2, are expressed in the epithelium.21,61,62 Indeed, the ultimate readout of PCP is on the epithelium. The data of Mao and colleagues suggest that the Fat/Ds pathways may be acting in completely distinct cell types from the core determinants in the kidney and may provide further support that these two pathways act in parallel.
In addition to Fat and Ds, at least two Wnts, Wnt9b and Wnt7b, are required for OCD in the kidney. Wnt7b is expressed in the distal collecting ducts of the embryonic and adult kidney. Ablation of Wnt7b from embryonic tissue results in a shortened renal medulla (collecting ducts and loop of Henle).64 Although mutants do not form cysts, dilation of the collecting ducts is apparent in mutants. Yu and colleagues found significant deficits in OCD within the collecting ducts. Although this observation indicated a defect in planar cell polarity, it was discovered that Wnt7b was not signaling to the epithelium, but instead to the medullary stroma through β-catenin.64 It was speculated that the PCP defect might be an indirect consequence of a failure to activate Wnt5a and Wnt11 expression in the stroma adjacent to the area of Wnt7b activity. Both Wnt5a and -11 have been implicated in regulating PCP by acting through a non-canonical pathway that utilizes the Rho GTPases and Jnk.40,52,65–69 Further, recent studies have shown that Wnt5a can regulate the subcellular localization of Vangl2 by interacting with the co-receptor Ror2.51 Interestingly, Vangl2 mutants show defects in the renal pelvis and mild tubular dilation that appears similar to the Wnt7b phenotype.70
The findings with Wnt7b, Fat4 and Ds are intriguing, because they suggest a novel mechanism for establishing PCP in the kidney, whereby the epithelium produces a Wnt ligand (like Wnt7b) that is received and, signaling through β-catenin, is necessary for the proper differentiation of the adjacent stroma. Reciprocally, the stromal microenvironment produces a non-canonical Wnt (like Wnt5a), Fat and Ds that act directly or indirectly on the adjacent epithelia to regulate its planar polarity. It is possible that, similar to the findings in fly, canonical Wnt signaling also regulates the expression of fat4 and ds, which, in turn, act as the directional cues for PCP by interacting with other molecules within the epithelium (such as other Fat or Ds orthologs). A final possibility is that Fat4 and Ds are directly regulating planar polarity of the stroma itself, which secondarily affects the PCP of the adjacent epithelium, perhaps by regulating the orientation or tension of the extracellular matrix.71–73
Interestingly, no individual role for core determinants in OCD has been discovered. As mentioned, Vangl2 and Fjx mutation enhances the Fat4 phenotype,36 but its mutation alone does not result in obvious cysts or other indicators of perturbed PCP. Vangl2 mutant kidneys branch less and have glomerular defects, but the precise cellular cause of these phenotypes is unknown.70 Several other orthologs of PCP determinants have been mutated in mice, but, to date, none have been reported to have defects in OCD. However, mutations in the Inversin gene (Nphp2) lead to a type of cystic disease in humans known as nephronopthisis.74 Inversin has some sequence and functional similarity to the fly core PCP protein Dg.75 Mutation of Inversin in the mouse is hypothesized to lead to cystic kidneys, at least in part by causing inappropriate stabilization of the Wnt/β-catenin pathway75 (although also see ref. 76). Recent studies have shown that Inv mutant kidneys have defects in oriented cell division that occur prior to cystogenesis.76 If Inversin is functionally redundant with the Diego ortholog Diversin, then this data would implicate core determinants in OCD during kidney development.
An obvious question is whether defects in PCP play a role in human cystic kidney diseases. The phenotypes resulting from Wnt7b, Fat4 and Dchs mutation are defects in kidney tubule formation/development. However, in most types of human cystic disease, including autosomal dominant polycystic kidney disease (ADPKD), cysts do not arise until relatively late in life. In other words, CKDs represent defects in tubule diameter maintenance, not establishment. What is the evidence that PCP is needed to maintain epithelial diameter? Current models suggest that adult onset of CKD may be the result of a failure to adequately repair damaged organs.77–80 Cysts may form when cells in an injured kidney divide in order to replace dead cells. Interestingly, studies in Drosophila have shown that the ability to properly repair damaged tissue requires the Ft/Ds pathway.81 In the absence of Ft, Atro or Ds, cells do not orient their spindles correctly to replace dead cells. Several groups have now shown that in the mouse kidney, cell division is tightly oriented in response to injury, and that this process is perturbed in several models of PKD prior to signs of tubule dilation.77–80 A reasonable hypothesis is that injury response requires proper communication between the epithelium and the surrounding stroma, and that interruption of this process disrupts normal repair. This is particularly interesting given the observation that cysts seem to form as a secondary consequence of several types of fibrotic kidney disease. Unfortunately, there is currently no evidence that the PCP determinants are required for proper response to injury. This type of study will require the use of various types of injury models in kidneys that have had PCP determinants mutated at adult stages.
Directed Cell Movements
Although the mitotic spindle is oriented along the long axis of the tubule after birth, Karner et al. demonstrated that prior to birth, spindle orientation is random.42 According to the oriented cell division hypothesis, the random orientation of spindles during the embryonic period should result in tubules whose diameter continues to increase until the spindle becomes oriented (which does not occur until shortly after birth). However, this was not the case. The number of cells comprising the circumference of the collecting duct actually decreased during development.
How does a tubule with randomly oriented cell divisions increase in length while decreasing in diameter? Karner et al. suggested that directed cell movements, similar to the processes that occur during gastrulation and neurulation, must be occurring during tubule morphogenesis (Fig. 3). This process, frequently referred to as convergent extension, is regulated by PCP and non-canonical Wnt signaling.82–84 Interestingly, Karner and colleagues found that during embryogenesis, kidney epithelial cells had planar polarity. Specifically, they found that the epithelial cells were elongated, and that the axis of elongation was vertical to the proximal-distal axis of the tubule, precisely what one would expect of cells intercalating between each other. Further, they found Wnt9b was required for this orientation (in the absence of Wnt9b, orientation was randomized) and for establishment of tubule diameter. This was independent of changes in the rates of cell proliferation or death. The cause and effect relationship between cell orientation and convergent extension movements is still not clear. Although it has been suggested that cell orientation is required for normal convergent extension movements during gastrulation in the frog, recent studies in the fly wing imaginal disc have shown that directed cell movements (referred to as cell flow) can actually re-orient the axis of PCP.85
Biochemical data suggested that the mode of action for Wnt9b during tubule diameter establishment was β-catenin-independent or non-canonical, while earlier studies had suggested its role in tubule induction was canonical.41,42 Non-canonical Wnt signaling has been proposed to activate the GTPases Rho, Rac and Cdc42 and the c-Jun N-terminal kinase (Jnk1), and activated Rho and Jnk2 were reduced in Wnt9b mutants. Interestingly, previous studies using Rho kinase (Rock) inhibitors found that tubule branching was reduced and that the diameter of the epithelia appeared to be increased,86,87 similar to the Wnt9b mutant phenotype, suggesting Rho signaling may lie downstream of Wnt9b. It will be of great interest to determine the relationship between cell movement and orientation in the kidney epithelium, but this type of analysis will require live imaging of individual cells within a growing tubule.
Interestingly, Karner et al. also showed that Wnt9b signaling was required for the oriented cell divisions that took place after birth. This finding raises the possibility that convergent extension and oriented cell division both rely on uniform cellular orientation. Alternatively, cell polarity and OCD could be controlled by distinct Wnt-dependent events.
There is tantalizing data that other established PCP determinants are required for directed cell movements in the kidney as well. As mentioned above, Inversin has some similarity to Diego, and its loss causes CKD. Although the cellular mechanism is not well-understood, recent studies suggest that the Inversin ortholog in frogs is required for directed cell movements during pronephric kidney tubule morphogenesis.88 It is possible that it controls similar processes in the developing mammalian kidney as well. Also, the increased tubular diameter found in the embryonic kidneys of Vangl2, Fat4 and Dchs1 mutants (especially the increased number of cells found in the circumference of Dchs1 mutants) are strong indicators that cell movements are perturbed. Given that cell division is random until after birth, we would suggest that any mutants showing diameter defects prior to birth (without accompanying changes in cell number) must have defects in directed cell movements.
Recent studies indicate that directed cell movements not only regulate tubule development, but also maintenance and/or recovery from injury. Although several studies have demonstrated that loss of oriented cell division precedes tubular dilation in several distinct mouse models of cystic kidney disease, Nishio and colleagues found it was not sufficient to cause cysts.89 Mice carrying a partial loss-of-function mutant in the fibrocystin gene (Pkhd1) showed randomized orientation of division but did not form cysts. The authors suggested that the randomization of the division plane was compensated for by oriented re-intercalation of the cells. This suggests that dividing cells within the adult tubule maintain the capability to undergo directed cell movements, and that this process is required to maintain tubule diameter. However, at the same time, although randomized orientation of cell division is not sufficient to cause cysts, we would predict that defects in convergent extension alone also would not be. To form a cyst, we suggest that there needs to be randomization of both cell divisions and cell movements.
Finally, it is tempting to speculate that in addition to playing a role in tubule diameter, directional cell movement will play additional roles in kidney development, such as branching morphogenesis, transition of the mesenchymal progenitors into a renal vesicle and the 3D anatomy of the glomeruli. However, providing evidence of this may have to wait for the development of techniques that allow high-resolution live cell imaging or additional genetic studies.
Differential Cell Adhesion
Differential cell adhesion describes differences in the relative adhesiveness of cells within a tissue. The process has been implicated in ureteric bud branching morphogenesis, patterning of the nephron and formation of cysts in CKD.90–96 Further, it is likely to be involved in MET and potentially glomerulus development and differentiation.
In several systems, there is direct evidence that PCP regulates cell adhesion.97–104 For example, loss of non-canonical Wnt signaling leads to a reduction in the levels of E-cadherin at the membrane and is accompanied by a reduction in cell-cell adhesiveness during zebrafish gastrulation.97 Loss of polycomb group genes in the fly ovary leads to upregulation of both canonical and non-canonical Wnt pathway components and the formation of tumor-like masses that are extruded from the basal side of the epithelium (somewhat reminiscent of cysts). Knockdown of the non-canonical pathway does not rescue the hyper-proliferative qualities of these tumors, but it does prevent the basal extrusion, suggesting that the PCP pathway regulates cell adhesion.105 Finally, knockdown of the orthologs of Prickle1 and Wnt11 leads to defects in the zebrafish laterality organ known as the Kuppfers vesicle.106 The resultant epithelium is misshapen and frequently displays cyst-like vesicles that have separated from the main body of the organ. The authors show decreased adhesiveness of Kuppfers vesicle progenitors that prevent the cells from organizing into a normal epithelium.
Although there has been no study demonstrating defects in cell adhesion in kidneys lacking any PCP determinant, there may be a connection. Mosaic ablation of β-catenin from the Wolffian ducts resulted in ectopic ureteric buds, while mosaic deletion in the collecting ducts resulted in cysts.107 In both cases, the mutant cells appeared to be more adhesive to each other than to the wild-type cells. However, whether this phenotype was related to defects in non-canonical signaling is unclear. Interestingly, mice lacking both Fat4 and Fjx1 formed ectopic ureteric buds and duplexed kidneys.36 It is possible that the ectopic buds are caused by a failure of the UB progenitor cells within the Wolffian duct to properly migrate toward their budding site or to adhere to each other during bud invasion, as proposed by Chi and colleagues.108
Cell Shape/Morphology
Previous studies have demonstrated a clear connection between the shape of cells and epithelial morphogenesis.109,110 In the kidney, it has been shown that cells within the ureteric bud undergo cell shape changes, including apical constriction, during branching morphogenesis.111 Although it has not been described in detail, similar processes are most certainly occurring during MET and later stages of nephrogenesis and morphogenesis.
Numerous factors contribute to the size and shape of individual cells, and many of these also affect PCP, such as the Par3 and -6, Crumbs, Scribble, Discs large (Dlg) and lethal giant larvae (Lgl orthologs).29 So far, direct roles for Par3 in glomerulogenesis and Dlg complex in branching and tubule diameter establishment have been demonstrated.91,112–116 It will be interesting to analyze cell shape changes during tubule formation and morphogenesis in greater detail and to determine the relationship between cell shape and kidney tubule defects.
The Cilium, PCP and Cystic Kidney Disease
CKDs represent some of the most common lethal genetic disorders known to man. They are a frequent cause of renal failure and compromise the quality of life of millions of people worldwide. In mice, mutations in over one dozen different genes result in CKD, and many of these appear to affect PCP. However, the question of why these lesions lead to defects in PCP is still open.
It is possible that some of the cystogenesis genes may represent cell type-specific effectors of PCP. These proteins would act downstream of the classical PCP determinants to regulate the various PCP-dependent processes within the epithelium. Based on the various readouts of PCP in the kidney and during cyst formation, it is likely that various cell type-specific effectors will play multiple, distinct roles in PCP in the kidney. However, some mechanistic insight may be gained by the observation that several cystogenesis proteins are localized to and/or are necessary for the formation/function of the apical monocilium, an evolutionarily conserved organelle that is essential for the development and maintenance of multiple vertebrate tissues.117
The cilium is composed of nine doublets of microtubules that are anchored at their base within a centriole and surrounding pericentriolar molecules (motile cilia have an additional two microtubules that lie within the ring of nine doublets. Non-motile cilia lack the center microtubules). The ciliary cytoskeleton is covered by a specialized plasma membrane that is molecularly distinct from the apical plasma membrane of the cell.118–122
Several pieces of data suggest that one of the roles of the cilium is to regulate Wnt signaling.123 In this context, the cilium has been proposed to act as a switch between the canonical/β-catenin pathway and the non-canonical pathway with an “active” cilium repressing the canonical pathway and promoting the non-canonical pathway. Given that gain of canonical Wnt signaling or loss of non-canonical signaling can lead to cysts,107,124–126 this model seems quite reasonable. Loss of the cilia would result in inappropriate activation of the canonical pathway, potentially leading to changes in cell adhesiveness and differentiation along with increased rates of proliferation. Further, as it appears that activation of the canonical pathway represses the non-canonical pathway (and vice versa), one would also expect to see defects associated with loss of the non-canonical pathway, such as randomized cell division and migration when the canonical pathway is activated. These combined defects would explain most if not all of the defects seen in CKD.
How the cilium regulates Wnt signaling is still not clear. Although in some tissues, such as the embryonic node, the cilium is motile and directs the movement of extracellular molecules setting up morphogen gradients, in the kidney, the cilium is not motile. Some data suggest that the centrioles of non-motile cilia act as scaffolds to tether and regulate the activity of various intracellular signaling molecules. In this model, the cilia can actually bring signaling molecules together to activate them or prevent their activation by separating them.127
A second model suggests that the separation of the plasma and ciliary membrane acts to compartmentalize signaling molecules. Once again, there is precedence for this type of role. Rohatgi et al. have shown that the Sonic hedgehog (Shh) receptor patched is localized to the cilia, while its co-factor smoothened is in the plasma membrane.118,119 In the presence of the Shh ligand, smoothened is transported to the cilia, and the hedgehog signal transduction pathway is activated. This is in the absence of any known fluid flow. Loss of the cilia presumably allows the intermingling of Smo and Ptc proteins inappropriately activating the pathway.
What triggers ciliary activation is still not clear. It could be the binding of a ligand or other molecule. Alternatively, in vitro studies have shown that the cilium in kidney cell lines can function as a mechanosensor and is activated by fluid flow.128–130 Indeed, fluid flow has been shown to orient the basal bodies and the cilia of the ependymal cells in the ventricles of the embryonic mouse brain.131 Thus, some models suggest that the kidney cilium is activated and PCP is established by urine flow. Further, it was shown that mechanical stimuli resulted in the cleavage of Polycystin-1 protein releasing a C-terminal fragment that then was able to move to the nucleus.132–134 This fragment interacted with nuclear β-catenin and blocked the ability of β-catenin to turn on target genes. These data provided a model for how the cilia and polycystins inhibited canonical (and perhaps promoted non-canonical) Wnt signaling.
Although this model is quite attractive, recent findings have cast some doubt as to whether it is correct. First, the studies of Karner et al. suggest that PCP is established prior to the timepoint where urine is thought to begin flowing.42 Second, Patel and colleagues found that if they ablated the cilium during embryonic development or early post-natal stages, the mice developed an aggressive type of CKD.80 However, ablation of the monocilia from one month old mice had a relatively mild effect on tubule morphology. If the function of the cilium was as a urine mechanosensor, one would expect a much more severe defect after its removal from a functioning kidney. Severe cysts and defects in OCD did become more prominent when the kif3a mutant kidneys were injured. Similar findings have been reported for other mutants, including Pkd1, leading to the hypothesis that high rates of cell proliferation and/or dedifferentiation of the epithelial cells are required for cyst formation.61 It is important to note that cells within the mature epithelium are already planar polarized, and the cilia (and PCP determinants) are most likely not necessary to maintain PCP in mitotically inactive cells. It may only be in the situation where PCP needs to be re-established in dividing cells (especially after injury) that these signals are required.
Finally, given the recent data implicating calcium and non-canonical Wnt signaling in tubule differentiation,135,136 one cannot ignore the role of the polycystin complex as ion channels. It is possible that the polycystins regulate calcium levels that are required for proper Wnt pathway determination.
In summary, it is already abundantly clear that proper establishment and maintenance of planar cell polarity is required for the proper development and function of the kidney, and this fundamental biological process most likely plays a crucial role in various kidney pathologies. However, there are still many more questions that need to be answered. It will be of great interest to determine the precise role of the cystogenesis genes (and the cilia) in PCP and how this affects normal development. However, some caution should be taken in interpretation of these data, as we know that every gene that affects PCP is not a direct determinant of this process. Defects in multiple subcellular processes, such as apical/basal polarity and cytoskeletal organization, will also affect PCP as a secondary consequence. Over the next several years, we will gain more insights into how PCP is established, what it does and how it might affect therapies for various kidney diseases.
Acknowledgments
We thank Moushumi Dey for reading and contacting on this manuscript and Courtney Karner for providing unpublished data. Work in the Carroll lab is supported by grants from the NIH (R01DK080004), American Heart Association and March of Dimes. A.D. is supported by a post-doctoral fellowship from the AHA.
References
- 1.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyle S, Shioda T, Perantoni AO, de Caestecker M. Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev Dyn. 2007;236:2321–2330. doi: 10.1002/dvdy.21242. [DOI] [PubMed] [Google Scholar]
- 3.Guillaume R, Bressan M, Herzlinger D. Paraxial mesoderm contributes stromal cells to the developing kidney. Dev Biol. 2009;329:169–175. doi: 10.1016/j.ydbio.2009.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mugford JW, Yu J, Kobayashi A, McMahon AP. High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol. 2009;333:312–323. doi: 10.1016/j.ydbio.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strutt D. Gradients and the specification of planar polarity in the insect cuticle. Cold Spring Harb Perspect. Biol 2009;1:489. doi: 10.1101/cshperspect.a000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buechling T, Bartscherer K, Ohkawara B, Chaudhary V, Spirohn K, Niehrs C, et al. Wnt/Frizzled signaling requires dPRR, the Drosophila homolog of the prorenin receptor. Curr Biol. 2010;20:1263–1268. doi: 10.1016/j.cub.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–1276. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 13.Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, Ingelfinger D, et al. Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science. 2010;327:459–463. doi: 10.1126/science.1179802. [DOI] [PubMed] [Google Scholar]
- 14.Feiguin F, Hannus M, Mlodzik M, Eaton S. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev Cell. 2001;1:93–101. doi: 10.1016/S1534-5807(01)00010-7. [DOI] [PubMed] [Google Scholar]
- 15.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, Schwarz TL, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/S0092-8674(00)80046-X. [DOI] [PubMed] [Google Scholar]
- 16.Das G, Jenny A, Klein TJ, Eaton S, Mlodzik M. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development. 2004;131:4467–4476. doi: 10.1242/dev.01317. [DOI] [PubMed] [Google Scholar]
- 17.Tree DR, Shulman JM, Rousset R, Scott MP, Gubb D, Axelrod JD. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell. 2002;109:371–381. doi: 10.1016/S0092-8674(02)00715-8. [DOI] [PubMed] [Google Scholar]
- 18.Axelrod JD. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 2001;15:1182–1187. doi: 10.1101/gad.890501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastock R, Strutt H, Strutt D. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development. 2003;130:3007–3014. doi: 10.1242/dev.00526. [DOI] [PubMed] [Google Scholar]
- 20.Strutt DI. Asymmetric localization of frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell. 2001;7:367–375. doi: 10.1016/S1097-2765(01)00184-8. [DOI] [PubMed] [Google Scholar]
- 21.Babayeva S, Zilber Y, Torban E. Planar cell polarity pathway regulates actin rearrangement, cell shape, motility and nephrin distribution in podocytes. Am J Physiol Renal Physiol. 2011;300:549–560. doi: 10.1152/ajprenal.00566.2009. [DOI] [PubMed] [Google Scholar]
- 22.Shima Y, Copeland NG, Gilbert DJ, Jenkins NA, Chisaka O, Takeichi M, et al. Differential expression of the seven-pass transmembrane cadherin genes Celsr1-3 and distribution of the Celsr2 protein during mouse development. Dev Dyn. 2002;223:321–332. doi: 10.1002/dvdy.10054. [DOI] [PubMed] [Google Scholar]
- 23.Matakatsu H, Blair SS. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 24.Simon MA. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 25.Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 26.Zeidler MP, Perrimon N, Strutt DI. Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol. 2000;228:181–196. doi: 10.1006/dbio.2000.9940. [DOI] [PubMed] [Google Scholar]
- 27.Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, et al. The tumor-suppressor and cell adhesion molecule Fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. [DOI] [PubMed] [Google Scholar]
- 28.Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B' regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- 29.Karner C, Wharton KA, Jr, Carroll TJ. Planar cell polarity and vertebrate organogenesis. Semin Cell Dev Biol. 2006;17:194–203. doi: 10.1016/j.semcdb.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/S0092-8674(02)00658-X. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock R, Schrauth S, Gessler M. Expression of mouse dchs1, fjx1 and fat-j suggests conservation of the planar cell polarity pathway identified in Drosophila. Dev Dyn. 2005;234:747–755. doi: 10.1002/dvdy.20515. [DOI] [PubMed] [Google Scholar]
- 34.Mao Y, Mulvaney J, Zakaria S, Yu T, Morgan KM, Allen S, et al. Characterization of a Dchs1 mutant mouse reveals requirements for Dchs1-Fat4 signaling during mammalian development. Development. 2011;138:947–957. doi: 10.1242/dev.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunskill EW, Lai HL, Jamison DC, Potter SS, Patterson LT. Microarrays and RNA-Seq identify molecular mechanisms driving the end of nephron production. BMC Dev Biol. 2011;11:15. doi: 10.1186/1471-213X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, et al. Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet. 2008;40:1010–1055. doi: 10.1038/ng.179. [DOI] [PubMed] [Google Scholar]
- 37.Cadigan KM, Liu YI. Wnt signaling: complexity at the surface. J Cell Sci. 2006;119:395–402. doi: 10.1242/jcs.02826. [DOI] [PubMed] [Google Scholar]
- 38.O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camilli TC, Weeraratna AT. Striking the target in Wnt-y conditions: intervening in Wnt signaling during cancer progression. Biochem Pharmacol. 2010;80:702–711. doi: 10.1016/j.bcp.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, et al. Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development. 2011;138:1247–1257. doi: 10.1242/dev.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 44.Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, et al. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 45.Vincent JP, Beckett K. Off-track takes Frizzled off the canonical path. EMBO J. 2011;30:3665–3666. doi: 10.1038/emboj.2011.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 47.Peradziryi H, Kaplan NA, Podleschny M, Liu X, Wehner P, Borchers A, et al. PTK7/Otk interacts with Wnts and inhibits canonical Wnt signalling. EMBO J. 2011;30:3729–3740. doi: 10.1038/emboj.2011.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Inoue T, Oz HS, Wiland D, Gharib S, Deshpande R, Hill RJ, et al. C. elegans LIN-18 is a Ryk ortholog and functions in parallel to LIN-17/Frizzled in Wnt signaling. Cell. 2004;118:795–806. doi: 10.1016/j.cell.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 50.Green JL, Inoue T, Sternberg PW. Opposing Wnt pathways orient cell polarity during organogenesis. Cell. 2008;134:646–656. doi: 10.1016/j.cell.2008.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, et al. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- 53.Wehrli M, Tomlinson A. Independent regulation of anterior/posterior and equatorial/polar polarity in the Drosophila eye; evidence for the involvement of Wnt signaling in the equatorial/polar axis. Development. 1998;125:1421–1432. doi: 10.1242/dev.125.8.1421. [DOI] [PubMed] [Google Scholar]
- 54.Schlesinger A, Shelton CA, Maloof JN, Meneghini M, Bowerman B. Wnt pathway components orient a mitotic spindle in the early Caenorhabditis elegans embryo without requiring gene transcription in the responding cell. Genes Dev. 1999;13:2028–2038. doi: 10.1101/gad.13.15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorpe CJ, Schlesinger A, Carter JC, Bowerman B. Wnt signaling polarizes an early C, elegans blastomere to distinguish endoderm from mesoderm. Cell. 1997;90:695–705. doi: 10.1016/S0092-8674(00)80530-9. [DOI] [PubMed] [Google Scholar]
- 56.Goldstein B, Takeshita H, Mizumoto K, Sawa H. Wnt signals can function as positional cues in establishing cell polarity. Dev Cell. 2006;10:391–396. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song S, Zhang B, Sun H, Li X, Xiang Y, Liu Z, et al. A Wnt-Frz/Ror-Dsh pathway regulates neurite outgrowth in Caenorhabditis elegans. PLoS Gene. 2010;6:1001056. doi: 10.1371/journal.pgen.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 60.Bonnet CS, Aldred M, von Ruhland C, Harris R, Sandford R, Cheadle JP. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum Mol Genet. 2009;18:2166–2176. doi: 10.1093/hmg/ddp149. [DOI] [PubMed] [Google Scholar]
- 61.Luyten A, Su X, Gondela S, Chen Y, Rompani S, Takakura A, et al. Aberrant regulation of planar cell polarity in polycystic kidney disease. J Am Soc Nephrol. 2010;21:1521–1532. doi: 10.1681/ASN.2010010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li L, Zepeda-Orozco D, Patel V, Truong P, Karner CM, Carroll TJ, et al. Aberrant planar cell polarity induced by urinary tract obstruction. Am J Physiol Renal Physiol. 2009;297:1526–1533. doi: 10.1152/ajprenal.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S. vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development. 2010;137:347–357. doi: 10.1242/dev.042226. [DOI] [PubMed] [Google Scholar]
- 64.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon APA. Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development. 2009;136:161–171. doi: 10.1242/dev.022087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wallingford JB, Vogeli KM, Harland RM. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int J Dev Biol. 2001;45:225–227. [PubMed] [Google Scholar]
- 66.Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/S0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 68.Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, Rodriguez-Esteban C, et al. Non-canonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–175. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Habas R, He X. Activation of Rho and Rac by Wnt/frizzled signaling. Methods Enzymol. 2006;406:500–511. doi: 10.1016/S0076-6879(06)06038-1. [DOI] [PubMed] [Google Scholar]
- 70.Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Hum Mol Genet. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goto T, Davidson L, Asashima M, Keller R. Planar cell polarity genes regulate polarized extracellular matrix deposition during frog gastrulation. Curr Biol. 2005;15:787–793. doi: 10.1016/j.cub.2005.03.040. [DOI] [PubMed] [Google Scholar]
- 72.Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Curr Opin Cell Biol. 2010;22:589–596. doi: 10.1016/j.ceb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wallingford JB. Vertebrate gastrulation: polarity genes control the matrix. Curr Biol. 2005;15:414–416. doi: 10.1016/j.cub.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 74.Otto EA, Schermer B, Obara T, O'Toole JF, Hiller KS, Mueller AM, et al. Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and leftright axis determination. Nat Genet. 2003;34:413–420. doi: 10.1038/ng1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, et al. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet. 2005;37:537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T. The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int. 2011;79:957–965. doi: 10.1038/ki.2010.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, et al. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet. 2009;18:2532–2542. doi: 10.1093/hmg/ddp190. [DOI] [PubMed] [Google Scholar]
- 78.Bastos AP, Piontek K, Silva AM, Martini D, Menezes LF, Fonseca JM, et al. Pkd1 haploinsufficiency increases renal damage and induces microcyst formation following ischemia/reperfusion. J Am Soc Nephrol. 2009;20:2389–2402. doi: 10.1681/ASN.2008040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, et al. Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet. 2009;18:2523–2531. doi: 10.1093/hmg/ddp147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li W, Kale A, Baker NE. Oriented cell division as a response to cell death and cell competition. Curr Biol. 2009;19:1821–1826. doi: 10.1016/j.cub.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 83.Djiane A, Riou J, Umbhauer M, Boucaut J, Shi D. Role of frizzled 7 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2000;127:3091–3100. doi: 10.1242/dev.127.14.3091. [DOI] [PubMed] [Google Scholar]
- 84.Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/S1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 85.Aigouy B, Farhadifar R, Staple DB, Sagner A, Roper JC, Julicher F, et al. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell. 2010;142:773–786. doi: 10.1016/j.cell.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 86.Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, et al. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638–647. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 87.Michael L, Sweeney DE, Davies JA. A role for microfilament-based contraction in branching morphogenesis of the ureteric bud. Kidney Int. 2005;68:2010–2018. doi: 10.1111/j.1523-755.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 88.Lienkamp S, Ganner A, Boehlke C, Schmidt T, Arnold SJ, Schafer T, et al. Inversin relays Frizzled-8 signals to promote proximal pronephros development. Proc Natl Acad Sci USA. 2010;107:20388–20393. doi: 10.1073/pnas.1013070107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishio S, Tian X, Gallagher AR, Yu Z, Patel V, Igarashi P, et al. Loss of Oriented Cell Division Does not Initiate Cyst Formation. J Am Soc Nephrol. 2010;21:295–300. doi: 10.1681/ASN.2009060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ibraghimov-Beskrovnaya O, Bukanov N. Polycystic kidney diseases: from molecular discoveries to targeted therapeutic strategies. Cell Mol Life Sci. 2008;65:605–619. doi: 10.1007/s00018-007-7362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Olsen O, Funke L, Long JF, Fukata M, Kazuta T, Trinidad JC, et al. Renal defects associated with improper polarization of the CRB and DLG polarity complexes in MALS-3 knockout mice. J Cell Biol. 2007;179:151–164. doi: 10.1083/jcb.200702054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson PD. Polycystic kidney disease: new understanding in the pathogenesis. Int J Biochem Cell Biol. 2004;36:1868–1873. doi: 10.1016/j.biocel.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 93.Debiec H, Kutsche M, Schachner M, Ronco P. Abnormal renal phenotype in L1 knockout mice: a novel cause of CAKUT. Nephrol Dial Transplant. 2002;17:42–44. doi: 10.1093/ndt/17.suppl_9.42. [DOI] [PubMed] [Google Scholar]
- 94.Muller U, Brandli AW. Cell adhesion molecules and extracellular-matrix constituents in kidney development and disease. J Cell Sci. 1999;112:3855–3867. doi: 10.1242/jcs.112.22.3855. [DOI] [PubMed] [Google Scholar]
- 95.Wilson PD, Burrow CR. Cystic diseases of the kidney: role of adhesion molecules in normal and abnormal tubulogenesis. Exp Nephrol. 1999;7:114–124. doi: 10.1159/000020592. [DOI] [PubMed] [Google Scholar]
- 96.Muller U, Brandli AW. Cell adhesion molecules and extracellular-matrix constituents in kidney development and disease. J Cell Sci. 1999;112:3855–3867. doi: 10.1242/jcs.112.22.3855. [DOI] [PubMed] [Google Scholar]
- 97.Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Saburi S, McNeill H. Organising cells into tissues: new roles for cell adhesion molecules in planar cell polarity. Curr Opin Cell Biol. 2005;17:482–488. doi: 10.1016/j.ceb.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 99.Carreira-Barbosa F, Kajita M, Morel V, Wada H, Okamoto H, Martinez Arias A, et al. Flamingo regulates epiboly and convergence/extension movements through cell cohesive and signalling functions during zebrafish gastrulation. Development. 2009;136:383–392. doi: 10.1242/dev.026542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pope KL, Harris TJ. Control of cell flattening and junctional remodeling during squamous epithelial morphogenesis in Drosophila. Development. 2008;135:2227–2238. doi: 10.1242/dev.019802. [DOI] [PubMed] [Google Scholar]
- 101.Zallen JA, Blankenship JT. Multicellular dynamics during epithelial elongation. Semin Cell Dev Biol. 2008;19:263–270. doi: 10.1016/j.semcdb.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harrington MJ, Hong E, Fasanmi O, Brewster R. Cadherin-mediated adhesion regulates posterior body formation. BMC Dev Biol. 2007;7:130. doi: 10.1186/1471-213X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Medina A, Swain RK, Kuerner KM, Steinbeisser H. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. EMBO J. 2004;23:3249–3258. doi: 10.1038/sj.emboj.7600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 105.Li X, Han Y, Xi R. Polycomb group genes Psc and Su(z)2 restrict follicle stem cell self-renewal and extrusion by controlling canonical and non-canonical Wnt signaling. Genes Dev. 2010;24:933–946. doi: 10.1101/gad.1901510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oteiza P, Koppen M, Krieg M, Pulgar E, Farias C, Melo C, et al. Planar cell polarity signalling regulates cell adhesion properties in progenitors of the zebrafish laterality organ. Development. 2010;137:3459–3468. doi: 10.1242/dev.049981. [DOI] [PubMed] [Google Scholar]
- 107.Marose TD, Merkel CE, McMahon AP, Carroll TJ. B-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state. Dev Biol. 2008;314:112–126. doi: 10.1016/j.ydbio.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell. 2009;17:199–209. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ma D, Amonlirdviman K, Raffard RL, Abate A, Tomlin CJ, Axelrod JD. Cell packing influences planar cell polarity signaling. Proc Natl Acad Sci USA. 2008;105:18800–18805. doi: 10.1073/pnas.0808868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Classen AK, Anderson KI, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 111.Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, et al. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev Biol. 2004;275:44–67. doi: 10.1016/j.ydbio.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 112.Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development. 2007;134:1799–1807. doi: 10.1242/dev.02830. [DOI] [PubMed] [Google Scholar]
- 113.Naim E, Bernstein A, Bertram JF, Caruana G. Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney. Kidney Int. 2005;68:955–965. doi: 10.1111/j.1523-755.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- 114.Simons M, Hartleben B, Huber TB. Podocyte polarity signalling. Curr Opin Nephrol Hypertens. 2009;18:324–330. doi: 10.1097/MNH.0b013e32832e316d. [DOI] [PubMed] [Google Scholar]
- 115.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, et al. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J Biol Chem. 2008;283:23033–23038. doi: 10.1074/jbc.M803143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cai Y, Stafford LJ, Bryan BA, Mitchell D, Liu M. G-protein-activated phospholipase C-beta, new partners for cell polarity proteins Par3 and Par6. Oncogene. 2005;24:4293–4300. doi: 10.1038/sj.onc.1208593. [DOI] [PubMed] [Google Scholar]
- 117.Deane JA, Ricardo SD. Polycystic kidney disease and the renal cilium. Nephrology (Carlton) 2007;12:559–564. doi: 10.1111/j.1440-797.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 118.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 120.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity and cilia. Genes Dev. 2010;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, et al. Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem. 2005;280:3938–3945. doi: 10.1074/jbc.M410697200. [DOI] [PubMed] [Google Scholar]
- 125.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, et al. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the β-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 126.Romagnolo B, Berrebi D, Saadi-Keddoucci S, Porteu A, Pichard AL, Peuchmaur M, et al. Intestinal dysplasia and adenoma in transgenic mice after overexpression of an activated β-catenin. Cancer Res. 1999;59:3875–3879. [PubMed] [Google Scholar]
- 127.Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, et al. Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc Natl Acad Sci USA. 2011;108:10679–10684. doi: 10.1073/pnas.1016214108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 129.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 130.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 131.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 132.Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, et al. Polycystin-1 C-terminal tail associates with β-catenin and inhibits canonical Wnt signaling. Hum Mol Genet. 2008;17:3105–3117. doi: 10.1093/hmg/ddn208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 134.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Burn SF, Webb A, Berry RL, Davies JA, Ferrer-Vaquer A, Hadjantonakis AK, et al. Calcium/NFAT signalling promotes early nephrogenesis. Dev Biol. 2011;352:288–298. doi: 10.1016/j.ydbio.2011.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tanigawa S, Wang H, Yang Y, Sharma N, Tarasova N, Ajima R, et al. Wnt4 induces nephronic tubules in metanephric mesenchyme by a non-canonical mechanism. Dev Biol. 2011;352:58–69. doi: 10.1016/j.ydbio.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]