Abstract
The planar cell polarity (PCP) pathway plays a critical role in diverse developmental processes that require coordinated cellular movement, including neural tube closure and renal tubulogenesis. Recent studies have demonstrated that this pathway also has emerging relevance to the epidermis, as PCP signaling underpins many aspects of skin biology and pathology, including epidermal development, hair orientation, stem cell division and cancer. Coordinated cellular movement required for epidermal repair in mammals is also regulated by PCP signaling, and in this context, a new PCP gene encoding the developmental transcription factor Grainyhead-like 3 (Grhl3) is critical. This review focuses on the role that PCP signaling plays in the skin across a variety of epidermal functions and highlights perturbations that induce epidermal pathologies.
Key words: planar cell polarity, skin, wound repair, grainyhead-like, Drosophila, vertebrates, mammals, disease
Introduction
Epithelial cells within a planar “sheet,” such as the epidermis, display both apical-basolateral polarity at a single cell level and planar polarization extending broadly across the entire tissue. Whereas apical-basolateral polarity is often established simply through local extracellular cues, such as basal extracellular matrix or cell adhesion properties, planar cell polarity (PCP) establishment requires far-reaching, complex signal propagation to ensure that all cells in a tissue are precisely oriented. Diverse physiological processes, including many aspects of embryogenesis and patterning, chemotaxis, immune function and inflammatory response as well as wound healing, require polarized cell migration.1–3 Well-defined signaling pathways control these processes to polarize the cell within the plane of the epithelium. The identification of the frizzled (fz) serpentine receptor, its ligand wingless and the associated PCP signaling pathway in Drosophila highlighted the importance of the cell orientation and movement in many developmental events.4 Several ligands, receptors and cofactors have since been identified in vertebrates to regulate PCP, including the secreted glycoprotein cthrc1,5 secreted wnt5 6 and wnt11,7 the membrane-associated heparin sulfate proteoglycans knypek (kny) and glypican 4 (gpc4),8,9 the receptor for activated protein kinase C1 rack110 and the tyrosine kinase receptor ror2.11 PCP signaling requires activation of the cytoplasmic factors dishevelled (dvl1, dvl2, dvl3) 12,13 and prickle (pk1, pk2),14 the transmembrane protein Van Gogh/Strabismus (vangl1, vangl2),15,16 homologs of cadherin starry night/flamingo (celsr1, celsr2, celsr3)17 and the gene coding for cytoplasmic ankyrin repeat domain 6 diversin (Ankrd6),18 a homolog of Drosophila diego, which collectively are recognized as the core PCP genes.19
The list of ancillary factors which cooperate with these core genes to regulate PCP is continually expanding and includes inturned (in),20 fritz (frtz),21 fuzzy (fy/fuz),22 scrib1,15 lethal giant larvae (lgl) 23,24 and Grainyhead-like 3 (Grhl3).3,25–27 Although some of the instructive cues operating in parallel to the core PCP genes are known and broadly include fat, dachsous, four-jointed as well as other potential regulators, such as Hedgehog,28,29 this review will limit itself primarily to core PCP genes and downstream effectors of PCP signaling. The effector proteins in this pathway include Daam1, JNK, Profilin,30 the small GTPases of the Rho subfamily RhoA, Rac1 and cdc42 and the Rho-associated kinase (Rok), all of which are largely involved in re-modeling of the actin cytoskeleton.27,31,32 Disruption of any of the core PCP genes or their effector pathways results in polarity-related defects.
Consequences of PCP Defects
In the fly, polarity has been mainly studied in the context of tissue organization.33,34 Disrupted PCP signaling in Drosophila leads to defects in wing hair polarity and disorganized ommatidia in the eye. Flies with disrupted expression of either the core PCP genes33 or downstream effectors26,35,36 present with misaligned wing hair orientation due to perturbed distribution of the actin pre-hair precursor.22 The polarized distribution of core PCP genes is also essential for development of the denticle belts of Drosophila larval cuticles37 as well as for dorsal closure,38 which is an invertebrate paradigm of neural tube closure. In vertebrates, PCP-related defects include aberrant convergence extension (CE)-mediated axial elongation resulting in defective neural tube closure (including exencephaly, spina bifida and cranioraschischisis),39 misaligned stereocilliary bundles of cochlear hair cells,15 disorganized body hair orientation,40 impaired ciliogenesis of primary and motile cilia41 and renal tubular disorganization.42,43
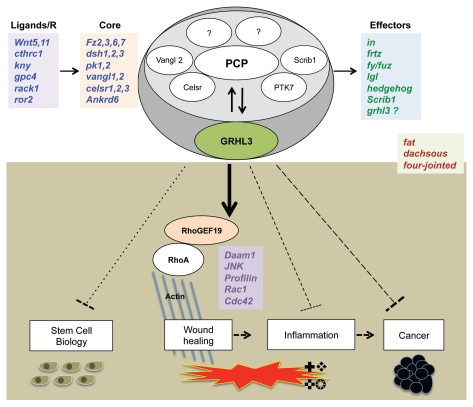
More recently, putative roles for individual members of the PCP signaling pathway have emerged in many aspects of epidermal development (Fig. 1). From the early establishment of a protective barrier during embryogenesis to stem cell biology, aging, hair development and newly discovered roles in epidermal cancers and wound repair, PCP signaling regulates many of the core elements of epidermal function. Here, we highlight the influences of PCP signaling in skin morphogenesis, where hair follicle orientation, eyelid closure and wound healing require this pathway,3,44 and review the growing body of literature implicating the grainyhead family as key players in skin homeostasis within many pathways related to epidermal PCP.
Figure 1.
Regulation of epidermal homeostasis by PCP signaling. As a part of this pathway, GRHL3 is known to positively induce wound healing via activation of RhoGEF/RhoA axis. Furthermore, we hypothesize its involvement as a negative regulator of skin biology and pathology, including stem cell proliferation, inflammation and cancer.
PCP in Epidermal Development during Embryogenesis
Much of our knowledge of PCP signaling in the epidermis comes from Drosophila. Alignment of the cells within the epidermal epithelium appears to require actomyosin-mediated contractility,45 and this feature also appears crucial for allowing at least some epidermal cells to withstand mechanical stress and preserve their shape and integrity.46 Furthermore, core PCP genes are distributed in specific cellular compartments to maintain the polarized status during epidermal development.38 Interestingly, although the gross defects in Drosophila cuticle formation are relatively mild following disruption of the core PCP genes, when one examines their function in higher vertebrates, severe ectodermal defects are seen. These include impaired skin barrier formation,40 defective wound healing3 and neural tube defects.47–49
Classically, the loss of vertebrate core PCP genes leads to neural tube defects, although it is important to note that not all PCP-mutants, such as mice deficient in Fzd6,40 display this particular phenotype. The role that the embryonic precursor of adult skin, the non-neural surface ectoderm (NNSE), plays in neural plate folding, elevation and eventual closure is a subject of intense speculation. Although PCP factors are expressed within the neuroepithelium of the neural plate, an intriguing hypothesis is that PCP signaling is crucial in polarizing the NNSE abutting the lateral edges of the neural plate. This NNSE would then provide instructive signaling cues for PCP-mediated neurulation to occur.50 It seems incongruous that the NNSE, which is both necessary and sufficient for neurulation to proceed (at least in the avian model),51,52 would not have an instructive role in neurulation, and future studies will focus on the role of PCP in this context.
A further PCP event hypothesized to occur in vertebrate development, which does not exist in the fly, is the formation of motile cilia. Cilia are found on a variety of epithelial cells, where their function ranges from ensuring smooth circulation of cerebro-spinal fluid in the brain53 to receiving and transmitting soluble signaling factors, such as Shh54,55 or Notch.56 PCP-mediated regulation of ciliogenesis in the epidermis has been suggested in Xenopus,57,58 although the relationship between PCP signaling and ciliogenesis remains controversial. Athough PCP proteins themselves are undoubtedly involved in ciliogenesis,20 the requirement for Wnt-mediated PCP signaling in ciliary polarity is less clear (elegantly reviewed by Wallingford and Mitchell, 2011).55 For example, ciliogenesis and planar cell polarity were disrupted in the cochlea of a mouse model lacking the cilia gene Polaris even though core components of the PCP pathway were correctly distributed intracellularly, suggesting that cilia themselves may contribute to epithelial polarity in this model59 or in other epithelial sheets, such as ependymal cells of the brain.60 Work by Ezratty and colleagues suggests that ciliogenesis during embryonic development occurs only after the epidermis has been polarized.56 Within the Xenopus epidermis, loss of cilia occurs through loss of Dvl, Inturned or fuzzy, but whether this is a PCP-mediated mechanism is unclear,13,29 particularly as defective Dvl signaling causes cell autonomous defects without disturbing planar polarity.58 Moreover, mice lacking PCP effector proteins, such as Fuzzy, display disturbed ciliogenesis but none of the disturbances in hair polarity (see below) associated with loss of core PCP genes.61 Lastly, although early polarized distribution of cilia may be influenced by PCP signaling,58 refinement of ciliary polarity occurs largely through mechanical sensing of fluid flow.62 Further work appears necessary to confirm that orientation of cilia in the epidermis is, indeed, a PCP-mediated event.
PCP in Hair Orientation
PCP signaling in the regulation of hair orientation may refer either to sensory hair cells on the cochlear epithelium of the inner ear15 or to the body hair covering (fur) appearing on the skin of terrestrial mammals. Although fur orientation in mammals is governed by many of the same PCP signals as the orientation of bristles in the Drosophila wing, it is important to note that the Drosophila wing bristles are simple, actin-based extensions (one bristle arising per cell), whereas fur in mammals results from the development and differentiation of multicellular complexes in the dermis.63 Although disruption of PCP signaling has been shown to regulate both sensory hair cell and fur polarity, this section will only focus on polarized orientation of body hair on the skin.
Most of our knowledge regarding mammalian body hair orientation comes from the mouse. Wild-type mice display an organized coat of hair, with individual hairs orientated caudally. Mice lacking core PCP proteins, including Frizzled6 40 or Celsr1,64 display abnormal whorl-like patterns centering around localized areas on the body, head and limbs.64 However, a certain degree of localized organization is apparent, as the hairs are still aligned in patterns and not randomly, as may be predicted from a loss of PCP-signaling. Localized interactions with adjacent cells presumably provide some instructive polarity signals, although these do not extend across the entire epithelial sheet. Importantly, the cellular structure of individual hairs or hair follicles is not disrupted, although the localization and distribution of hair follicles in the dermis is severely disorganized.64 Interestingly, mutants lacking Fzd6 are healthy and viable and, despite their hair polarity defects, display no defects in other PCP-related mechanisms, such as convergence extension movements or neural tube closure,40 suggesting localized tissue-specific roles for certain PCP factors in vertebrates.
PCP in Stem Cell Biology of the Skin
Polarized intracellular protein segregation is particularly relevant to the processes of asymmetric cell division and self-renewal in stem cells. The stem cell divides to produce two cells, one of which is an identical clone of itself, whereas the other is a more differentiated, committed progenitor. Just how external PCP signaling can regulate stem cell division in the epidermis is a key question of epidermogenesis. Wnt signaling through β-catenin is already a known major regulator of epidermal stem cell function. Mice expressing stabilized β-catenin in the skin show epidermal hyperplasia, hair follicle tumor-like outgrowth and precocious transition from telogen to anagen phase via activation of stem cells.65 It remains to be seen, however, whether Wnt signaling may also regulate PCP in this context independently of β-catenin.
Although not traditionally thought of as a PCP signal, asymmetric Notch signaling is involved in cell fate specification and pattern formation.66 Recent results suggest that asymmetric stem cell divisions promote Notch-dependent epidermal differentiation, as compromising asymmetric cell divisions induces defects in epidermal stratification, terminal differentiation and barrier formation.67 In the epidermis, recent work has shown that cilial defects lead to defective differentiation, putatively through altered Notch signaling,56 and Notch is also a known tumor suppressor in the skin of adult mice.68 Importantly, PCP signaling has been shown to directly regulate Notch pathway activation, for example, in regulating R3/R4 photoreceptors in the ommatidia of the Drosophila eye.66,69 Loss of Notch-mediated polarity regulation of unipotent hair cell progenitors in zebrafish also leads to defective planar polarization of the neuromast epithelium,70 raising the novel hypothesis that PCP-mediated regulation of the Notch pathway may be a crucial component in vertebrate epidermal stem cell biology.
In the mammalian epidermis, PCP proteins are asymmetrically localized in cells of the proliferative basal layer.71,72 Asymmetric division of stem cells in this layer gives rise to a mitotically active progenitor cell that retains polarized distribution of core PCP proteins.72 These cells are not only able to replenish the stratified, multi-layered epidermis, but can also regenerate dermal hair follicle precursors. A proposed mechanism by which epidermal stem cell and PCP fidelity is preserved is via “mitotic internalization,” whereby membrane-bound PCP proteins are endocytosed during division and are correctly redistributed in daughter progeny to maintain planar polarity.72 Mutants that were unable to internalize a core PCP gene, celsr1, exhibited misoriented hair follicles following mitosis, indicating that this internalization and resorting step is a key mechanism for maintaining planar polarity in the epidermis.
Work from our laboratory has identified a novel vertebrate mediator of PCP, Grainyhead-like 3 (Grhl3), in the context of several processes, including neural tube closure, skin barrier formation, orientation of cochlear hair cells and epidermal wound healing. Grhl3 is a vertebrate ortholog of the Drosophila PCP factor grainyhead, which, among other functions, regulates wing hair polarity,26 cuticle formation,25 expression of epidermis-specific genes,73 wound healing74,75 and the mitotic activity of neuroblasts.76,77 In the context of epidermogenesis in mice, Grhl3 regulates differentiation,78 wound healing3 and proliferation,78,100 suggesting that Grhl3 is a key determinant in the fine-tuning of cellular turnover in skin homeostasis.
PCP and Grhl3 in wound healing.
Wound healing requires both cell number regulation and cell migration. During development of the Drosophila ectoderm, PCP components contribute to maintain morphology through regulation of oriented cell division, as dividing cells develop tissues and organs.79 In wound healing, replacement of damaged cells necessitates cell rearrangement to reconstitute organ shape in addition to cell number regulation, which occurs by mitotic reorientation. Recently, PCP genes have been shown to preserve tissue polarity during proliferation of the mammalian epidermal basal cells. Celsr1, Vangl2 and Fzd6 were inherited equally by progeny following mitosis and were able to properly re-localize and reorient themselves to keep a collective cell polarization across the epidermis.72 In addition, the healing process represents an ideal model to study polarized cell migration, a fundamental element in this process. Despite this, the links between cellular migration in wound repair and PCP signaling have only recently been established.3 Much like embryonic closure events mediated by PCP-CE signaling, spatial restriction of PCP components at the membrane of leading edge cells with receptor tyrosine kinase (RTK) activation transforms the extracellular gradient into a robust intracellular polarity, leading to polarized intracellular distribution of members of the Rho family of GTPases.3,27,80
Work from our laboratory has implicated many of the core and effector PCP genes (Vangl2, PTK7, Scrib1 and Celsr1) in epidermal repair and has led to the identification of Grhl3 as an important contributor to the PCP pathway.3 Grhl3 interacts genetically with these core PCP genes during epidermal healing and also in other PCP-mediated developmental events, including neural tube closure and stereociliary orientation in the cochlea. Like mutants harboring deletions in the other PCP genes, Grhl3-/- mice also present with severe neural tube defects, suggesting that Grhl3 is a key novel regulator of the PCP pathway in mammalian epidermis.
The identification of Grhl3 as a mammalian PCP determinant closely mirrors the function of the antecedent member of the family, Drosophila grainyhead (grh). grh genetically interacts with the core PCP genes in maintaining wing hair polarity,26 and grh is also directly involved in cuticle repair.74 Stitcher (Stit) induces grh expression in wound closure,81 and extracellularregulated kinase (ERK) signaling has been shown to be essential for grh activation in this context.75 This pathway directly leads to the expression of grh target genes involved in wound healing, including Dopa decarboxylase (Ddc) and pale (ple). Independent of its role in wound healing, grh also regulates several genes involved in epidermal development, namely ultrabithorax, engrailed and fushi tarazu.73 Thus, grh in the fly, as per Grhl3 in the mouse, clearly has a significant role in both homeostatic epidermal development and the polarity-mediated processes of wound healing.
In mammals, both PCP core and effector genes contribute to epidermal development, particularly in regulating hair orientation and maintaining skin homeostasis.3,61 In response to wounding, Grhl3 expression is required for cells at the wound margin to establish directional polarity of the leading edge. This is achieved through direct transcriptional activation of the PCP effector molecule, RhoGEF19 by Grhl3.3 RhoGEF19, a member of the small GTPase family, functions as a direct activator of RhoA, leading to actin polymerization, cytoskeletal arrangement and directed migration in wound healing.3 The Xenopus ortholog of this factor, WGEF, is critical for CE and neural tube closure.82 WGEF has been shown to bind Dishevelled and Daam-1 and to connect the PCP pathway to Rho activation in the frog, and preliminary studies from our laboratory indicate that it also plays a role in these processes in zebrafish (Dworkin, unpublished). Loss of RhoGEF19 function induced by shRNA in a human epidermal cell line, HaCaT, leads to defective cellular migration across a scratch wound. This is accompanied by disruption of Golgi structure and Golgi pericentrosomal positioning, which impairs polarized secretion and directional cell migration.83 Cells lacking RhoGEF19 also fail to activate RhoA, resulting in loss of the coordinated actin polymerization necessary for oriented cell migration. Whether the mechanistic basis of epidermal wound-healing defects caused by loss of the core PCP genes relates to altered RhoGEF19 expression remains to be determined.
PCP in Epidermal Cancer
In Drosophila, several components that interact with the PCP pathway in determining both planar and apico-basal polarity, including disc large (dlg),84 lethal giant larvae (lgl) 85,86 and scribble (scrib),87,88 function as tumor suppressor genes.89 Loss of these genes causes overgrowth of imaginal disc epithelia, a hyperproliferation accompanied by loss of apical-basal polarity and abnormal cell shapes.90 In mammals, several studies suggest a link between PCP genes and cancer,91,92 potentially via involvement of the ERK/MAPK93 or Rac1-JNK30 pathways. These studies show that the PCP pathway can interfere with other signaling pathways to influence proliferation and, as such, represent suitable targets for cancer prevention and therapy. The PCP activator Wnt is implicated in vertebrate tumor progression via non-canonical (including PCP) signaling,30,94,95 and loss of the core PCP-component Vangl2 promotes cellular migration and ECM invasion of fibrosarcoma tumor cells.96 Aberrant distribution of core PCP proteins could lead to subsequent localized deregulation of PCP signaling and would represent a critical step in tumorigenesis by deregulating control of not only proliferation, but also metastasis, angiogenesis and tumor invasion.30
The concept of cancer as an aberrant developmental process is not new, and putative links between the core PCP genes and tumor suppressor function are emerging. While less is known about PCP and skin cancer, two interesting yet contradictory theories exist, namely, that of skin cancer arising from a wound that doesn't heal, vs. cancer arising from an “overhealing” wound.97 However, it remains crucial to uncover the molecular determinants that affect PCP signaling in cancer under variable transforming conditions, including exposure to UV irradiation, carcinogens, mechanical or oxidative stress and inflammation as well as wound healing.
Consistent with a role for the PCP pathway in suppression of proliferation and armed with the knowledge that wounding, and by extension aberrant wound healing, can promote both tumor initiation98 and metastasis,99 we show that Grhl3 is implicated in the prevention of skin cancer (Darido, Cancer Cell 2011, In press). Additionally, we and others have shown that loss of Grhl3 in embryonic epidermis results in both impaired epidermal terminal differentiation100 and increased cellular proliferation.78,100 Given the suggested antiproliferative role of this gene, further investigations into the PCP-mediated functions of Grhl3 and GRHL3 target genes in maintaining the balance between proliferation and differentiation are warranted and could provide further insights into the link between PCP signaling and regulation of skin homeostasis.
Conclusion
Although many aspects of the PCP signaling pathway are well-characterized, continuing studies in the field reveal new roles attributable to increasing numbers of PCP regulators, effectors and partner proteins. The epidermis provides a unique system to study the roles of the PCP pathway in both Drosophila and vertebrates through its myriad of developmental, homeostatic, protective and pathological states. Future directions will ultimately bring regulators of PCP signaling into the clinic, whereby treatment of skin-related disorders will greatly benefit.
Abbreviations
- PCP
planar cell polarity
- grh
grainyhead
- grhl
grainyhead-like
- fz
frizzled
- cthrc1
collagen triple helix repeat containing 1
- wnt
wg and int hybrid (wingless-type MMTV integration site)
- kny
knypek
- gpc
glypican
- rack
receptor of activated protein kinase C1
- ror2
receptor tyrosine kinase-like orphan receptor 2
- dvl
disheveled
- pk
prickle
- vangl
Van gogh-like
- celsr
cadherin EGF LAG seven pass G-type receptor
- ankrd6
ankyrin repeat domain 6 diversin
- in
inturned
- frtz
fritz
- fy/fuz
fuzzy
- lgl
lethal giant larvae
- JNK
c-jun N-terminal kinase
- RhoGEF
Rho guanine nucleotide exchange factor
- WGEF
weak-similarity GEF
- dlg
disc large
- PTK7
protein tyrosine kinase 7
- Stit
Stitcher
- RTK
receptor tyrosine kinase
- CE
convergence-extension
- NNSE
non-neural surface ectoderm
- Scrib
scribble
- Rok
Rho-associated kinase
- Ddc
Dopa decarboxylase
- Ple
pale
- shRNA
small hairpin ribonucleic acid
- ERK
extracellular regulated kinase
- MAPK
mitogen-activated protein kinase
- UV
ultraviolet
- ECM
extracellular matrix
- cdc42
cell division control protein 42
- Shh
sonic hedgehog
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cell-bio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 3.Caddy J, Wilanowski T, Darido C, Dworkin S, Ting SB, Zhao Q, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19:138–147. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler PN, Krasnow RE, Liu J. Tissue polarity points from cells that have higher Frizzled levels towards cells that have lower Frizzled levels. Curr Biol. 1997;7:940–949. doi: 10.1016/S0960-9822(06)00413-1. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, et al. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 8.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, et al. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/S1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 9.Ohkawara B, Yamamoto TS, Tada M, Ueno N. Role of glypican 4 in the regulation of convergent extension movements during gastrulation in Xenopus laevis. Development. 2003;130:2129–2138. doi: 10.1242/dev.00435. [DOI] [PubMed] [Google Scholar]
- 10.Wehner P, Shnitsar I, Urlaub H, Borchers A. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development. 2011;138:1321–1327. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- 11.Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, et al. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell. 2011;20:163–176. doi: 10.1016/j.devcel.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 13.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gubb D, Green C, Huen D, Coulson D, Johnson G, Tree D, et al. The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev. 1999;13:2315–2327. doi: 10.1101/gad.13.17.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 16.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, et al. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tissir F, Bar I, Goffinet AM. Lambert De Rouvroit C. Expression of the ankyrin repeat domain 6 gene (Ankrd6) during mouse brain development. Dev Dyn. 2002;224:465–469. doi: 10.1002/dvdy.10126. [DOI] [PubMed] [Google Scholar]
- 19.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 20.Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J Cell Biol. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan NA, Tolwinski NS. Spatially defined Dsh-Lgl interaction contributes to directional tissue morphogenesis. J Cell Sci. 2010;123:3157–3165. doi: 10.1242/jcs.069898. [DOI] [PubMed] [Google Scholar]
- 24.Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–617. doi: 10.1016/S0092-8674(01)00246-X. [DOI] [PubMed] [Google Scholar]
- 25.Bray SJ, Kafatos FC. Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev. 1991;5:1672–1683. doi: 10.1101/gad.5.9.1672. [DOI] [PubMed] [Google Scholar]
- 26.Lee H, Adler PN. The grainyhead transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Darido C, Jane SM. Grhl3 and GEF19 in the front rho. Small GTPases. 2010;1:104–107. doi: 10.4161/sgtp.1.2.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colosimo PF, Tolwinski NS. Wnt, Hedgehog and junctional Armadillo/beta-catenin establish planar polarity in the Drosophila embryo. PLoS ONE. 2006;1:9. doi: 10.1371/journal.pone.0000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- 31.Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/S0960-9822(00)00645-X. [DOI] [PubMed] [Google Scholar]
- 32.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 33.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/S1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 34.Stein JA, Broihier HT, Moore LA, Lehmann R. Slow as molasses is required for polarized membrane growth and germ cell migration in Drosophila. Development. 2002;129:3925–3934. doi: 10.1242/dev.129.16.3925. [DOI] [PubMed] [Google Scholar]
- 35.Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee H, Adler PN. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics. 2002;160:1535–1547. doi: 10.1093/genetics/160.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirano M, Neff D, Collier S. Cell shape and epithelial patterning in the Drosophila embryonic epidermis. Fly (Austin) 2009;3:185–191. doi: 10.4161/fly.3.3.9138. [DOI] [PubMed] [Google Scholar]
- 38.Morel V, Arias AM. Armadillo/beta-catenin-dependent Wnt signalling is required for the polarisation of epidermal cells during dorsal closure in Drosophila. Development. 2004;131:3273–3283. doi: 10.1242/dev.01217. [DOI] [PubMed] [Google Scholar]
- 39.Ueno N, Greene ND. Planar cell polarity genes and neural tube closure. Birth Defects Res C Embryo Today. 2003;69:318–324. doi: 10.1002/bdrc.10029. [DOI] [PubMed] [Google Scholar]
- 40.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proc Natl Acad Sci USA. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 43.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 45.Walters JW, Dilks SA, DiNardo S. Planar polarization of the denticle field in the Drosophila embryo: roles for Myosin II (zipper) and fringe. Dev Biol. 2006;297:323–339. doi: 10.1016/j.ydbio.2006.04.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olguin P, Glavic A, Mlodzik M. Intertissue mechanical stress affects Frizzled-mediated planar cell polarity in the Drosophila notum epidermis. Curr Biol. 2011;21:236–242. doi: 10.1016/j.cub.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- 48.Doudney K, Stanier P. Epithelial cell polarity genes are required for neural tube closure. Am J Med Genet C Semin Med Genet. 2005;135:42–47. doi: 10.1002/ajmg.c.30052. [DOI] [PubMed] [Google Scholar]
- 49.Paudyal A, Damrau C, Patterson VL, Ermakov A, Formstone C, Lalanne Z, et al. The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. BMC Dev Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- 51.Hackett DA, Smith JL, Schoenwolf GC. Epidermal ectoderm is required for full elevation and for convergence during bending of the avian neural plate. Dev Dyn. 1997;210:397–406. doi: 10.1002/(SICI)1097-0177(199712)210:4<397::AID-AJA4>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 52.Moury JD, Schoenwolf GC. Cooperative model of epithelial shaping and bending during avian neurulation: autonomous movements of the neural plate, autonomous movements of the epidermis and interactions in the neural plate/epidermis transition zone. Dev Dyn. 1995;204:323–337. doi: 10.1002/aja.1002040310. [DOI] [PubMed] [Google Scholar]
- 53.Malaterre J, Mantamadiotis T, Dworkin S, Lightowler S, Yang Q, Ransome MI, et al. c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells. 2008;26:173–181. doi: 10.1634/stemcells.2007-0293. [DOI] [PubMed] [Google Scholar]
- 54.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity and cilia. Genes Dev. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ezratty EJ, Stokes N, Chai S, Shah AS, Williams SE, Fuchs E. A role for the primary cilium in Notch signaling and epidermal differentiation during skin development. Cell. 2011;145:1129–1141. doi: 10.1016/j.cell.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konig G, Hausen P. Planar polarity in the ciliated epidermis of Xenopus embryos. Dev Biol. 1993;160:355–368. doi: 10.1006/dbio.1993.1312. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Curr Biol. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, et al. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 60.Mirzadeh Z, Han YG, Soriano-Navarro M, Garcia-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dai D, Zhu H, Wlodarczyk B, Zhang L, Li L, Li AG, et al. Fuz controls the morphogenesis and differentiation of hair follicles through the formation of primary cilia. J Invest Dermatol. 2011;131:302–310. doi: 10.1038/jid.2010.306. [DOI] [PubMed] [Google Scholar]
- 62.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 63.McNeill H. Planar cell polarity: keeping hairs straight is not so simple. Cold Spring Harb Perspect Biol. 2010;2:3376. doi: 10.1101/cshperspect.a003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravni A, Qu Y, Goffinet AM, Tissir F. Planar cell polarity cadherin Celsr1 regulates skin hair patterning in the mouse. J Invest Dermatol. 2009;129:2507–2509. doi: 10.1038/jid.2009.84. [DOI] [PubMed] [Google Scholar]
- 65.Murayama K, Kimura T, Tarutani M, Tomooka M, Hayashi R, Okabe M, et al. Akt activation induces epidermal hyperplasia and proliferation of epidermal progenitors. Oncogene. 2007;26:4882–4888. doi: 10.1038/sj.onc.1210274. [DOI] [PubMed] [Google Scholar]
- 66.Cho B, Fischer JA. Ral GTPase promotes asymmetric Notch activation in the Drosophila eye in response to Frizzled/PCP signaling by repressing ligand-independent receptor activation. Development. 2011;138:1349–1359. doi: 10.1242/dev.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 69.del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Dev Cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 70.Wibowo I, Pinto-Teixeira F, Satou C, Higashijima S, Lopez-Schier H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development. 2011;138:1143–1152. doi: 10.1242/dev.060566. [DOI] [PubMed] [Google Scholar]
- 71.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Devenport D, Oristian D, Heller E, Fuchs E. Mitotic internalization of planar cell polarity proteins preserves tissue polarity. Nat Cell Biol. 2011 doi: 10.1038/ncb2284. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dynlacht BD, Hoey T, Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991;66:563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- 74.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainyhead. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 75.Kim M, McGinnis W. Phosphorylation of Grainyhead by ERK is essential for wound-dependent regeneration but not for development of an epidermal barrier. Proc Natl Acad Sci USA. 2011;108:650–655. doi: 10.1073/pnas.1016386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cenci C, Gould AP. Drosophila Grainyhead specifies late programmes of neural proliferation by regulating the mitotic activity and Hox-dependent apoptosis of neuroblasts. Development. 2005;132:3835–3845. doi: 10.1242/dev.01932. [DOI] [PubMed] [Google Scholar]
- 77.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 78.Ting SB, Caddy J, Wilanowski T, Auden A, Cunningham JM, Elias PM, et al. The epidermis of grhl3-null mice displays altered lipid processing and cellular hyperproliferation. Organogenesis. 2005;2:33–35. doi: 10.4161/org.2.2.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W, Kale A, Baker NE. Oriented cell division as a response to cell death and cell competition. Curr Biol. 2009;19:1821–1826. doi: 10.1016/j.cub.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang S, Tsarouhas V, Xylourgidis N, Sabri N, Tiklova K, Nautiyal N, et al. The tyrosine kinase Stitcher activates Grainyhead and epidermal wound healing in Drosophila. Nat Cell Biol. 2009;11:890–895. doi: 10.1038/ncb1898. [DOI] [PubMed] [Google Scholar]
- 82.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27:606–617. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yadav S, Puri S, Linstedt AD. A primary role for Golgi positioning in directed secretion, cell polarity and wound healing. Mol Biol Cell. 2009;20:1728–1736. doi: 10.1091/mbc.E08-10-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-X. [DOI] [PubMed] [Google Scholar]
- 85.Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- 86.Gateff E. The genetics and epigenetics of neoplasms in Drosophila. Biol Rev Camb Philos Soc. 1978;53:123–168. doi: 10.1111/j.1469-185X.1978.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 87.Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- 88.Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature. 2000;403:676–680. doi: 10.1038/35001108. [DOI] [PubMed] [Google Scholar]
- 89.Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- 90.Wodarz A. Cell polarity: no need to reinvent the wheel. Curr Biol. 2001;11:975–978. doi: 10.1016/S0960-9822(01)00578-4. [DOI] [PubMed] [Google Scholar]
- 91.Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- 92.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 93.Dow LE, Elsum IA, King CL, Kinross KM, Richardson HE, Humbert PO. Loss of human Scribble cooperates with H-Ras to promote cell invasion through deregulation of MAPK signalling. Oncogene. 2008;27:5988–6001. doi: 10.1038/onc.2008.219. [DOI] [PubMed] [Google Scholar]
- 94.Katoh M. WNT/PCP signaling pathway and human cancer. Oncol Rep. 2005;14:1583–1588. [PubMed] [Google Scholar]
- 95.Darido C, Buchert M, Pannequin J, Bastide P, Zalzali H, Mantamadiotis T, et al. Defective claudin-7 regulation by Tcf-4 and Sox-9 disrupts the polarity and increases the tumorigenicity of colorectal cancer cells. Cancer Res. 2008;68:4258–4268. doi: 10.1158/0008-5472.CAN-07-5805. [DOI] [PubMed] [Google Scholar]
- 96.Cantrell VA, Jessen JR. The planar cell polarity protein Van Gogh-Like 2 regulates tumor cell migration and matrix metalloproteinase-dependent invasion. Cancer Lett. 2010;287:54–61. doi: 10.1016/j.canlet.2009.05.041. [DOI] [PubMed] [Google Scholar]
- 97.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 98.Schuh AC, Keating SJ, Monteclaro FS, Vogt PK, Breitman ML. Obligatory wounding requirement for tumorigenesis in v-jun transgenic mice. Nature. 1990;346:756–760. doi: 10.1038/346756a0. [DOI] [PubMed] [Google Scholar]
- 99.Walter ND, Rice PL, Redente EF, Kauvar EF, Lemond L, Aly T, et al. Wound healing after trauma may predispose to lung cancer metastasis: review of potential mechanisms. Am J Respir Cell Mol Biol. 2011;44:591–596. doi: 10.1165/rcmb.2010-0187RT. [DOI] [PubMed] [Google Scholar]
- 100.Yu Z, Lin KK, Bhandari A, Spencer JA, Xu X, Wang N, et al. The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev Biol. 2006;299:122–136. doi: 10.1016/j.ydbio.2006.07.015. [DOI] [PubMed] [Google Scholar]