Abstract
Aims
The impact of a high-fat diet on the failing heart is unclear, and the differences between polyunsaturated fatty acids (PUFA) and saturated fat have not been assessed. Here, we compared a standard low-fat diet to high-fat diets enriched with either saturated fat (palmitate and stearate) or PUFA (linoleic and α-linolenic acids) in hamsters with genetic cardiomyopathy.
Methods and results
Male δ-sarcoglycan null Bio TO2 hamsters were fed a standard low-fat diet (12% energy from fat), or high-fat diets (45% fat) comprised of either saturated fat or PUFA. The median survival was increased by the high saturated fat diet (P< 0.01; 278 days with standard diet and 361 days with high saturated fat)), but not with high PUFA (260 days) (n = 30–35/group). Body mass was modestly elevated (∼10%) in both high fat groups. Subgroups evaluated after 24 weeks had similar left ventricular chamber size, function, and mass. Mitochondrial oxidative enzyme activity and the yield of interfibrillar mitochondria (IFM) were decreased to a similar extent in all TO2 groups compared with normal F1B hamsters. Ca2+-induced mitochondrial permeability transition pore opening was enhanced in IFM in all TO2 groups compared with F1B hamsters, but to a significantly greater extent in those fed the high PUFA diet compared with the standard or high saturated fat diet.
Conclusion
These results show that a high intake of saturated fat improves survival in heart failure compared with a high PUFA diet or low-fat diet, despite persistent mitochondrial defects.
Keywords: Cardiomyopathy, Low-carbohydrate diet, Metabolism, Obesity
1. Introduction
Little is known about the impact of dietary lipid on the development and progression of heart failure.1 Treatment guidelines for heart failure patients provide no specific recommendations regarding fat intake and suggest that heart failure patients follow the dietary guidelines provided for the prevention of coronary heart disease (CHD).2–4 Recent studies suggest that high-fat/low-carbohydrate diets in the absence of obesity exert favourable effects on cardiac function and survival in rodent models of heart failure induced by infarction,5–8 chronic hypertension,9–14 or genetic cardiomyopathy.15 Clinical studies have yet to address this issue; however, there is an extensive literature on dietary fat and CHD, with recent evidence showing no increase with greater intake of fat (including saturated fat) and reduced risk of CHD with high intake of polyunsaturated fatty acids (PUFA).16 This has prompted a paradigm shift for the prevention of coronary disease, with emphasis now on increased PUFA intake and decreased consumption of refined carbohydrate.17 At present, the dietary recommendation for heart failure patients in terms of fat intake is not clear.
Recent evidence suggests that there might be major differences between the intake of saturated fatty acids and PUFA in the development and progression of heart failure, although there has not been a direct comparison. In rodents with heart failure induced by aortic constriction or myocardial infarction, a high-fat diet rich in saturated fatty acids either improved or had no adverse effect on cardiac function and survival compared with a standard low-fat diet.5–12,18 On the other hand, a high intake of the n-3 PUFA α-linolenic acid (18:3n3) combined with a low intake of linoleic acid (18:2n6) prolonged survival in hamsters with dilated heart failure caused by δ-sarcoglycan deficiency.15 Feeding spontaneously hypertensive heart failure rats a high-fat diet comprised mainly of long-chain saturated fat shortens survival; however, treatment with a similar amount of fat that was mainly linoleic acid prolonged survival compared with a standard low-fat diet.14 This effect was associated with improved mitochondrial function and greater linoleic acid incorporation into mitochondrial membranes. Linoleic acid has been associated with pro-inflammatory effects via elongation and desaturation to form arachidonic acid, the precursor to prostanoids, while ω-3 PUFA such as α-linolenic acid inhibit this process. Dietary PUFA and saturated fatty acids also have divergent effects on gene expression, apoptosis, cell signalling, and mitochondrial function and could differentially affect the progression of heart failure.19 Feeding rats a high-fat diet comprised of saturated fatty acids (16:0 and 18:0) may be pro-apoptotic compared with linoleic acid.19 Thus, a diet rich in linoleic acid might improve or maintain mitochondrial function, prevent cardiomyocyte apoptosis, and improve cardiac function and survival in heart failure.
The goal of this study was to assess the effects of high-fat diets rich in PUFA or saturated fat on survival, mitochondrial function, and phospholipid fatty acid composition in a genetic model of heart failure. We hypothesized that a high PUFA diet (45% of energy from fat) enriched with both α-linolenic and linoleic acid would improve mitochondrial oxidative capacity, prevent Ca2+-induced mitochondrial permeability transition pore (MPTP) opening, and prolong survival compared with either a standard low-fat diet or a high-fat diet enriched with palmitate and stearate. Previous studies addressing the role of dietary fat in the development and progression of heart failure used rodent models with relatively short-term arterial hypertension or myocardial infarction in young animals.5–7,9–13,18 Here, we wished to assess the long-term effects of high-fat intake (6–15 months) on survival and biochemical mechanisms and we wanted to avoid the high variability and expense encountered with surgical models or high sodium intake. Thus, we used the δ-sarcoglycan null cardiomyopathic hamster, a well-established rodent model of dilated cardiomyopathy that has decreased mitochondrial oxidative capacity and has been shown to respond to nutritional and metabolic therapies.15,20,21
2. Methods
2.1. Experimental design
The animal protocol was approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee and conducted according to the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23). Investigators were blinded to treatment when measurements were performed. The animals were maintained on a 12 h light/dark cycle, and all procedures were performed between 2 and 5 h from the start of the light phase. Male δ-sarcoglycan null cardiomyopathic hamsters (Bio TO2 strain) were acquired at 5 weeks of age from Bio Breeder Inc. (Watertown, MA, USA) and 1 week later were assigned to either a standard high-carbohydrate/low-fat diet, a high PUFA/low-carbohydrate diet, or a high saturated fat/low-carbohydrate diet (Table 1). Healthy age-matched male F1B hamsters were used as a control group and were fed only the standard diet. Animals were housed six per cage and given food and water ad libitum.
Table 1.
Macronutrient content and fatty acid composition of the diets (expressed as a per cent of total energy in the chow)
| Standard (%) | High PUFA (%) | High saturated fat (%) | |
|---|---|---|---|
| Protein | 20 | 20 | 20 |
| Carbohydrate | 68 | 35 | 35 |
| Fat | 12 | 45 | 45 |
| Palmitate(16:0) | 2.8 | 3.6 | 10.9 |
| Palmitoleate (16:1n7) | 0.2 | 0.1 | 1.2 |
| Stearate (18:0) | 2.5 | 1.2 | 9.1 |
| Oleate (18:1n9) | 4.4 | 13.5 | 17.8 |
| Linoleate (18:2n6) | 1.8 | 20.7 | 3.1 |
| α-Linolenoate (18:3n3) | 0.2 | 6.0 | 0.3 |
Two series of experiments were performed: the ‘survival protocol’ assessed the effects of diet on mortality, and the ‘physiological evaluation protocol’ determined the impact of diet on cardiac and mitochondrial function prior to fully advanced cardiomyopathy and accelerated mortality (30 weeks of age). The survival protocol compared the standard low-fat diet with the high PUFA and high saturated fat diets. Since previous studies with Bio TO2 strain found a median survival of 37–46 weeks of age, with 100% death by 55–75 weeks,22–24 we set the maximum length of treatment at 78 weeks, after which any surviving animals were euthanized. Six-week-old TO2 hamsters were assigned to either the standard diet (n = 35), high PUFA diet (n = 30), or high saturated fat diet (n = 30). A control group of F1B hamsters were fed a standard diet (n = 17). There animals were weighed weekly and otherwise were not handled.
The physiological evaluation protocol assessed the effects of 24 weeks of dietary treatment (from 6 to 30 weeks of age). Animals were assigned to either the standard, high PUFA, or high saturated fat diet (n = 11 or 12/group) and after 23 weeks underwent an echocardiogram. The following week, they underwent a terminal study to assess cardiac biochemistry, histology, and mitochondria function.
2.2. Diets
The diets were custom-manufactured using purified ingredients (Research Diets, New Brunswick, NJ, USA). All diets contained 20% of energy from protein (casein + l-cystine) (Table 1). The carbohydrate sources were maltodextrin (12% of total energy) and corn starch (55% in standard diet and 22% in high-fat diets). The fat source for the standard diet was a mixture of lard, cocoa butter, and soybean oil totalling 12% of total energy. The total fat content was 45% of total energy in both the PUFA and saturated fat diets. The fat sources in the high PUFA diet were safflower, flaxseed, olive, and soybean oils, which generated a diet high in both linoleic acid (18:2n6) and α-linolenic acid, as both of these PUFA are frequently consumed at high levels in humans25,26 and are effective at prolonging survival in rodent models of heart failure.14,15 The high saturated fat diet consisted of lard, cocoa butter, and soybean oil.
2.3. Echocardiography
In the physiological evaluation protocol, cardiac function was assessed after 23 weeks of treatment using echocardiography (Vevo 770, Visual Sonics, Toronto, Canada) with a 15 MHz linear array transducer (model 716). Hamsters were anaesthetized with 2% isoflurane in oxygen and placed on a warming pad. Adequacy of anaesthesia was monitored by lack of reflex response to foot pinch. Two-dimensional cine loops and guided M-mode frames were recorded from the parasternal short axis. Absolute wall thickness was calculated as the sum of diastolic posterior and anterior wall thicknesses, and relative wall thickness as the ratio of absolute wall thickness to end-diastolic diameter. Fractional shorting was calculated as (end-diastolic diameter − end-systolic diameter)/end-diastolic diameter.
2.4. Terminal surgery
After 24 weeks of treatment, the hamsters in the physiological evaluation protocol were anaesthetized with 5.0% isoflurane between 3 and 6 h after initiation of the light phase while given free access to food. Adequacy of anaesthesia was monitored by lack of reflex response to foot pinch. The thorax was opened and blood was collected from the left ventricle (LV) and immediately placed on ice tubes containing EDTA, and centrifuged at 4°C to obtain plasma. The heart was removed, and sections of the LV free wall were taken for biochemical analysis and histology and stored at −80°C, and the remainder was used for mitochondrial isolation. Epididymal and retroperitoneal fat and lungs were removed.
2.5. Mitochondrial isolation
Mitochondria were isolated as described previously.27 Briefly LV tissue (∼250 mg) was minced and homogenized in 1:10 cold modified Chappel–Perry buffer (100 mM KCl, 50 mM MOPS, 5 mM MgSO4, 1 mM EGTA, 1 mM ATP, and 0.2 mg/mL BSA), and centrifuged at 500 g. Subsequent centrifugation separated and purified subsarcolemmal mitochondria (SSM). Separation of the interfibrillar mitochondria (IFM) was achieved by incubation on ice with trypsin (5 mg/g wet mass) for 10 min and subsequent centrifugations.
2.6. Mitochondrial respiration
Mitochondrial oxygen consumption was assessed in SSM and IFM.27,28 Isolated mitochondria (0.5 mg mitochondrial protein/mL) were respired in buffer containing 100 mM KCl, 50 mM MOPS, 5 mM KH2PO4, 1 mM EGTA, and 1 mg/mL BSA. State 3 and 4 respiration was measured with glutamate + malate (10 and 5 mM, respectively) and succinate + rotenone (10 mM and 7.5 µM, respectively). State 4 respiration was measured with oligomycin (4 µM). The mitochondrial diameter of IFM and SSM was measured by flow cytometry, as described previously.29
2.7. Mitochondrial Ca2+ retention
The ability of mitochondria to retain Ca2+, an index of MPTP opening, was assessed in isolated mitochondria as described previously in detail.27 Briefly, using a 96-well fluorescence plate reader, 25 µg of mitochondrial protein was suspended in 200 µL of respiration buffer without BSA but with 5 µM EGTA and 1 mM MgCl2. Glutamate and malate were added as substrates (10 and 5 mM, respectively), and extramitochondrial Ca2+ was monitored using 1 µM calcium green (5N) and fluorescence measured at 485 and 538 nm for excitation and emission wavelengths, respectively, at 37°C. Ca2+ was added in increments of 25 nmol/mg mitochondrial protein at 7 min intervals.
2.8. Metabolic and biochemical parameters
Plasma glucose and free fatty acids were measured by spectrophotometric enzymatic assays and insulin by ELISA.11 Maximal activities of medium-chain acyl-CoA dehydrogenase and citrate synthase were measured spectophotometrically.11
2.9. Histology
Histological analysis was performed on LV tissue from the lateral free wall for myocyte cross-sectional area, interstitial fibrosis, and cardiomyocyte apoptosis as described previously.11
2.10. Fatty acid analysis
Myocardial phospholipid fatty acid composition was assessed in homogenates of the right ventricle by gas chromatography coupled with mass spectrometry according to a modification of the transesterification method as described previously.30 The same method was used to analyse the diets. The double bond index (DBI) was calculated as the sum of the concentration of each fatty acid times the number of double bonds.
2.11. Statistical analysis
Data are presented as mean ± the standard error. Differences among groups in the measurements from the physiological evaluation protocol were determined using a non-parametric one-way ANOVA with a Dunn's post hoc test for multiple comparisons. Data for extramitochondrial Ca2+ from the retention assay and body mass were evaluated using a two-way ANOVA with a Holm–Sidak post hoc test appropriate. Survival was assessed with a Kaplan–Meier analysis. A value of P < 0.05 was considered significant.
3. Results
3.1. Survival
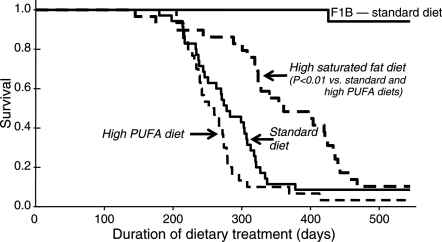
The median survival on treatment increased from 278 days with the standard diet to 361 days with high saturated fat (P < 0.01) (Figure 1). The high PUFA group was not different from the standard diet (260 days), but was shorter than the high saturated fat group (P < 0.01).
Figure 1.
Animal survival plotted as a function of duration of dietary treatment. Hamsters initiated dietary treatment at 6 weeks of age.
3.2. Body and organ mass
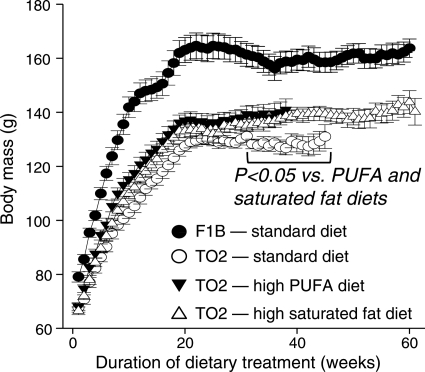
After 24 weeks of treatment in the physiological evaluation protocol, there were no significant differences among the three diets in the mass of the whole body or LV (Table 2) or in wet or dry lung mass or liver mass (data not shown). Fat pad mass was higher with high PUFA diet compared with the standard or high saturated fat diets. The lung wet/dry mass ratio was not different between the healthy F1B hamsters compared with the TO2 hamsters (data not shown), consistent with well-compensated cardiac function at this time point. More prolonged treatment resulted in significant and equivalent increases in body mass of ∼10% after ∼30 weeks in the high PUFA and saturated fat groups (Figure 2).
Table 2.
Body and organ mass, and echocardiographic and histological data after 24 weeks of dietary treatment after 24 weeks of treatment from the physiological evaluation protocol
| Normal F1B | Cardiomyopathic TO2 hamsters |
|||
|---|---|---|---|---|
| Standard diet | Standard diet | High PUFA | High SAT | |
| Body mass (g)—6 weeks old | 77.8 ± 1.5 | 69.4 ± 2.1* | 68.8 ± 3.7* | 72.7 ± 1.8 |
| Body mass (g)—30 weeks old | 166.4 ± 4.5 | 131.3 ± 3.3§ | 136.9 ± 4.5§ | 134.2 ± 4.9§ |
| Retroperitoneal fat pad mass (g) | 1.23 ± 0.06 | 0.52 ± 0.03§,† | 0.89 ± 0.07§ | 0.52 ± 0.06§,† |
| Epididymal fat pad mass (g) | 3.1 ± 0.17 | 1.4 ± 0.07* | 2.2 ± 0.15‡ | 1.6 ± 0.2* |
| LV mass (mg) | 395 ± 9 | 304 ± 8§ | 317 ± 12§ | 321 ± 7§ |
| LV mass/body mass (mg/g) | 2.38 ± 0.05 | 2.32 ± 0.04 | 2.32 ± 0.05 | 2.41 ± 0.09 |
| LV mass/tibia length (mg/mm) | 15.1 ± 0.3 | 12.3 ± 0.2§ | 13.1 ± 0.4§ | 12.8 ± 0.3§ |
| LV end-diastolic diameter (cm) | 0.47 ± 0.02 | 0.50 ± 0.02 | 0.45 ± 0.05 | 0.50 ± 0.09 |
| LV end-systolic diameter (cm) | 0.29 ± 0.03 | 0.38 ± 0.02 | 0.32 ± 0.02 | 0.37 ± 0.03 |
| LV fractional shortening | 0.39 ± 0.02 | 0.26 ± 0.03* | 0.29 ± 0.02* | 0.28 ± 0.02* |
| Absolute wall thickness (cm) | 0.37 ± 0.01 | 0.29 ± 0.01* | 0.31 ± 0.01* | 0.28 ± 0.01* |
| Relative wall thickness | 0.80 ± 0.04 | 0.59 ± 0.03* | 0.69 ± 0.04 | 0.57 ± 0.03* |
| Myocyte cross-sectional area (μm2) | 603 ± 25 | 665 ± 26 | 622 ± 27 | 635 ± 18 |
| Interstitial fibrosis (% area) | 9.8 ± 1.0 | 10.6 ± 0.5 | 9.7 ± 0.75 | 10.4 ± 0.7 |
| Cardiomyocyte apoptosis (DNAfr/10 000 myocytes) | 2.3 ± 0.8 | 5.2 ± 1.3 | 3.1 ± 0.6 | 3.6 ± 0.5 |
*P < 0.05 vs. F1B.
§P < 0.001 vs. F1B.
†P < 0.001 vs. TO2 high PUFA.
‡P < 0.05 vs. TO2 standard diet.
Figure 2.
Body mass from the survival protocol assessed weekly from initiation of the diet until 67% mortality was achieved in a treatment group.
3.3. Cardiac function and histology
Dietary fat intake did not affect LV systolic or diastolic diameters, fractional shortening, or absolute or relative wall thickness (Table 2). Cardiomyocyte apoptosis was low in both the F1B and TO2 hamsters on the standard diet and was unaffected by the high-fat diets (Table 2). Interstitial fibrosis and cardiomyocyte cross-sectional area were similar among groups (Table 2).
3.4. Metabolic measures
Plasma glucose was higher in the cardiomyopathic hamster on the standard and high PUFA diets compared with the normal F1B hamsters on the standard diet, but there were no differences in glucose concentration among the three diet groups in cardiomyopathic hamsters (Table 3). Plasma insulin concentrations were not affected by the high PUFA or saturated fat diets (Table 3). The plasma free fatty acid concentration was elevated by the high PUFA diet, with no other difference among groups.
Table 3.
Metabolic data after 24 weeks of dietary treatment
| Normal F1B | Cardiomyopathic TO2 hamsters |
|||
|---|---|---|---|---|
| Standard diet | Standard diet | High PUFA | High SAT | |
| Plasma glucose (mg/dL) | 110 ± 4 | 137 ± 6* | 166 ± 17* | 135 ± 9 |
| Plasma insulin (ng/mL) | 1.2 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 0.7 ± 0.3 |
| Plasma free fatty acids (mM) | 0.50 ± 0.07 | 0.67 ± 0.07 | 1.23 ± 0.2* | 0.73 ± 0.09 |
| Urine thromboxane B2 (pg/µmol creatine) | 22.8 ± 5.9 | 25.6 ± 6.6 | 33.2 ± 6.4 | 25.1 ± 3.2 |
Samples were taken in unfasted animals given free access to food and water.
*P < 0.05 vs. F1B.
3.5. Mitochondrial enzymes and function
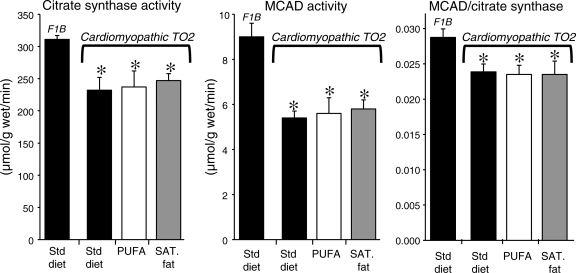
The activity of the mitochondrial marker enzymes citrate synthase (citric acid cycle) and medium-chain acyl-CoA dehydrogenase (fatty acid β-oxidation) was significantly reduced to a similar extent in all three TO2 groups compared with the normal F1B animals. Consistent with this was a significant decrease in mitochondrial yield in the TO2 hamsters compared with the F1B that was primarily in the IFM subpopulation (Table 4). There were no differences in mitochondrial respiratory parameters, or the size of SSM or IFM as assessed by flow cytometry, among any of the groups (Table 4). The cardiomyopathic animals had a lower medium-chain acyl-CoA dehydrogenase/citrate synthase activity ratio, consistent with a greater down-regulation of the capacity for fatty oxidation capacity relative to citric acid cycle flux (Figure 3). Activation of peroxisome proliferator-activated receptor α (PPARα) by a high-fat diet or a pharmacological agonist increases the oxidative capacity for fat relative to the citric acid cycle or electron transport chain.5,31 The lack of effect of the high-fat diets on the ratio of medium-chain acyl-CoA dehydrogenase to citrate synthase activity suggests that the high PUFA and high saturated fat diets did not activate PPARα sufficiently to elevate the capacity for fatty acid oxidation.
Table 4.
Function of isolated cardiac mitochondria after 24 weeks of dietary treatment
| Normal F1B | Cardiomyopathic TO2 hamsters |
|||
|---|---|---|---|---|
| Standard diet | Standard diet | High PUFA | High SAT | |
| Subsarcolemmal mitochondrial | ||||
| Yield (mg mito prot./g wet mass) | 6.0 ± 0.6 | 4.1 ± 0.3* | 5.9 ± 0.5‡ | 5.2 ± 0.4 |
| Mitochondria diameter (nm) | 619 ± 22 | 558 ± 11 | 555 ± 20 | 565 ± 20 |
| Glutamate + malate: State III | 148 ± 10 | 148 ± 17 | 143 ± 9 | 124 ± 18 |
| Glutamate + malate: State IV | 42.2 ± 2.6 | 41 ± 4.5 | 38 ± 2.7 | 38 ± 4.8 |
| Glutamate + malate: RCR | 7.4 ± 1.5 | 10.3 ± 2.8 | 8.6 ± 2.2 | 10.6 ± 4.1 |
| Succinate + rotenone: State III | 296 ± 16 | 258 ± 15 | 260 ± 24 | 244 ± 9.5 |
| Succinate + rotenone: State IV | 108 ± 8.4 | 119 ± 13 | 93 ± 6 | 102 ± 6.6 |
| Succinate + rotenone: RCR | 4.1 ± 0.4 | 5.1 ± 0.5 | 4.4 ± 0.7 | 3.9 ± 0.5 |
| Interfibrillar mitochondria | ||||
| Yield (mg mito prot./g wet mass) | 9.0 ± 0.6 | 4.1 ± 0.6* | 4.6 ± 0.5* | 4.0 ± 0.5* |
| Mitochondria diameter (nm) | 572 ± 19 | 560 ± 14 | 555 ± 15 | 584 ± 17 |
| Glutamate + malate: State III | 201 ± 26 | 154 ± 23 | 190 ± 28 | 175.1 ± 16 |
| Glutamate + malate: State IV | 60 ± 6 | 53 ± 8.4 | 63 ± 9 | 60 ± 8 |
| Glutamate + malate: RCR | 10 ± 4.4 | 5 ± 0.7 | 5 ± 1 | 6 ± 0.8 |
| Succinate + rotenone: State III | 357 ± 28 | 281 ± 20 | 329 ± 22 | 339 ± 28 |
| Succinate + rotenone: State IV | 122 ± 11 | 132 ± 11 | 137 ± 12 | 150 ± 11 |
| Succinate + rotenone: RCR | 3.8 ± 0.4 | 4.0 ± 0.3 | 3.6 ± 0.3 | 4.8 ± 0.9 |
*P < 0.05 vs. F1B.
‡P < 0.05 vs. TO2 standard diet.
Figure 3.
Myocardial activity of the mitochondrial oxidative enzymes citrate synthase and medium-chain acyl-CoA dehydrogenase, and the ratio of medium-chain acyl-CoA dehydrogenase to citrate synthase. *P < 0.05 vs. F1B.
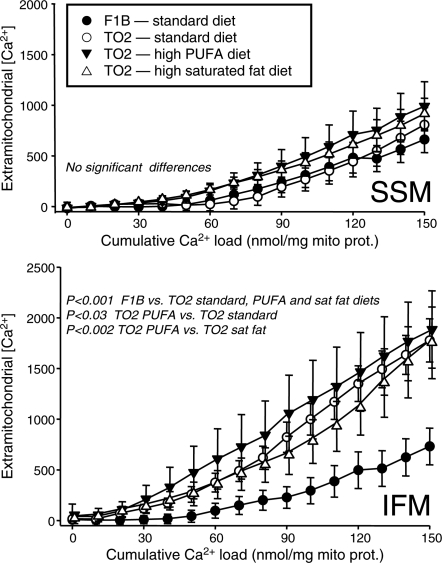
Heart failure induced by severe myocardial injury alters mitochondria in a manner that causes greater susceptible to stress-induced MPTP opening.32,33 Here, we observed a decrease in the capacity of isolated mitochondria to take up exogenous Ca2+, an index of enhanced MPTP opening. As shown in Figure 4, IFM, but not SSM, showed a higher extramitochondrial [Ca2+] for a given cumulative Ca2+ load in all three TO2 groups compared with the F1B group. The PUFA diet further increased the sensitivity of IFM to Ca2+-induced MPTP opening compared with the TO2 hamsters fed the standard or high saturated fat diets. There were no effects of diet or strain in SSM (Figure 4).
Figure 4.
Extramitochondrial [Ca2+] plotted as a function of the cumulative Ca2+ added to SSM (top panel) and IFM (lower panel).
3.6. Cardiac phospholipid fatty acid composition
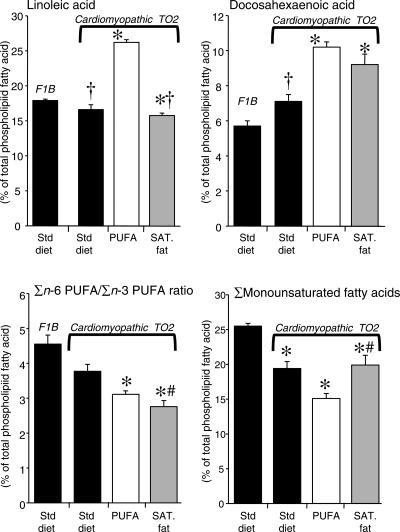
Compared with the F1B strain, the cardiomyopathic TO2 animals had a significant increase in stearic acid, eicosapentaenoic acid, docosahexaenoic acid, and the sum of n-3 PUFA, and a decrease in oleic acid (Figure 5 and Table 5).
Figure 5.
Myocardial phospholipid fatty acid composition expressed as a per cent of total fatty acids (top), and the ratio of the sum of n-6 PUFA/n-3 PUFA (bottom left), and the sum of monounsaturated fatty acids (right) from the right ventricular myocardium after 24 weeks of dietary treatment. *P < 0.05 vs. F1B; §P < 0.001 vs. F1B; †P < 0.05 vs. TO2 on the high PUFA diet; #P < 0.05 vs. TO2 of the standard diet.
Table 5.
Myocardial phospholipid fatty acid composition after 24 weeks of dietary treatment
| Normal F1B | Cardiomyopathic TO2 hamsters |
|||
|---|---|---|---|---|
| Standard diet | Standard diet | High PUFA | High SAT | |
| Palmitic acid (16:0) | 18.7 ± 0.2 | 18.9 ± 0.4† | 14.2 ± 0.3*,‡ | 16.6 ± 0.4* |
| Palmitoleic acid (16:1n7) | 1.03 ± 0.06 | 0.91 ± 0.03 | 0.46 ± 0.09* | 0.54 ± 0.04 |
| Stearic acid (18:0) | 20.3 ± 0.4 | 23.9 ± 0.7* | 23.8 ± 0.4* | 26.2 ± 1.0* |
| Oleic acid (18:1n9) | 22.4 ± 0.4 | 16.6 ± 0.9* | 13.6 ± 0.7* | 18.3 ± 1.3† |
| Vaccenic acid (18:1n7) | 2.01 ± 0.12† | 1.88 ± 0.06† | 1.06 ± 0.02 | 1.12 ± 0.01 |
| Linoleic acid (18:2n6) | 17.9 ± 0.2 | 16.6 ± 0.7† | 26.2 ± 0.4* | 15.8 ± 0.3*,† |
| α-Linolenic acid (18:3n3) | 0.12 ± .01† | 0.16 ± .0.1† | 0.31 ± 0.01 | 0.14 ± 0.01† |
| Arachidonic acid (20:4n6) | 10.0 ± 0.2 | 12.2 ± 1.0† | 8.3 ± 0.2* | 10.3 ± 0.5† |
| Eicosapentaenoic acid (20:5n3) | 0.29 ± 0.01 | 0.42 ± 0.02* | 0.41 ± 0.02* | 0.35 ± 0.02‡ |
| Docosapentaenoic acid (22:5n3) | 0.40 ± 0.01 | 0.50 ± 0.02 | 0.57 ± 0.02 | 0.53 ± 0.03 |
| Docosahexaenoic acid (22:6n3) | 5.7 ± 0.3 | 7.1 ± 0.4† | 10.2 ± 0.3* | 9.2 ± 0.6* |
| ∑Saturated fatty acids | 39.2 ± 0.3 | 42.3 ± 0.6* | 38.1 ± 0.4‡ | 42.9 ± 1.1*,# |
| ∑Monounsaturated fatty acids | 25.4 ± 0.4 | 19.4 ± 1.0* | 15.1 ± 0.7* | 19.9 ± 1.4*,# |
| ∑n-3 PUFA | 6.5 ± 0.3 | 8.2 ± 0.4 | 11.4 ± 0.4‡,* | 10.2 ± 0.6* |
| ∑n-6 PUFA | 28.9 ± 0.2 | 30.0 ± 0.6 | 35.4 ± 0.3* | 27.0 ± 0.4‡,# |
| Double bond index | 142.0 ± 1.7 | 153.0 ± 4.0 | 170.0 ± 2.0*,‡ | 155.2 ± 4.5* |
*P < 0.05 vs. F1B.
‡P < 0.05 vs. TO2 standard diet.
†P < 0.001 vs. TO2 high PUFA.
#P < 0.05 vs. TO2 high PUFA.
Among the TO2 hamsters, there were only modest differences between the standard diet and the high saturated fat group, as the only significant differences were a small decrease in eicosapentaenoic acid and the sum of n-6 PUFA in the high saturated fat group (Figure 5 and Table 5). On the other hand, the high PUFA diet had a profound effect on cardiac phospholipid fatty acid composition, as seen in a ∼60% increase in linoleic acid and a doubling in α-linolenic acid (Figure 5 and Table 5), both of which were highly enriched in the diet (Table 1). The increase in PUFA in cardiac phospholipids resulted in a significant increase in the DBI compared with all other groups (Table 5).
The high PUFA diet resulted in a significant reduction in oleic acid and the sum of monounsaturated fatty acids (oleic acid + palmitoleic acid) compared with the standard diet or high saturated fat group (P < 0.05). This occurred despite relatively similar oleic acid content in the two high-fat diets (Table 1). On the other hand, there were no differences between the two high-fat diets in docosahexaenoic acid, the main n-3 PUFA in cardiac phospholipids, or the sum of n-3 PUFA.
Treatment with the high PUFA diet decreased arachidonic acid compared with the TO2 animals on the standard and high saturated fat diets (Figure 5 and Table 5). Since the PUFA diet was high in both linoleic acid and α-linolenic acid (18:3n3), the fall in arachidonic acid was presumably due to inhibition of the elongation of linoleic acid by elevated n-3 PUFA. There were no differences in the thromboxane B2/creatinine ratio in urine, an index of systemic inflammatory prostaglandin production, which suggests there were no major alterations in prostanoid production.
4. Discussion
While we hypothesized that a high PUFA diet enriched with both α-linolenic and linoleic acid would improve mitochondrial oxidative capacity, prevent Ca2+-induced MPTP opening, and prolong survival compared with a standard low-fat diet or a high saturated fat diet, our results were very much to the contrary. Surprisingly, consumption of the diet high in long-chain saturated fatty acids prolonged life compared with a standard low-fat diet. This clear beneficial effect was not due to correction of myocardial mitochondrial oxidative capacity or greater susceptibility to MPTP opening, nor to improved cardiac pump function. In contrast to our hypothesis, a high PUFA fat diet enriched with α-linolenic acid and linoleic acid from vegetable sources did not improve survival, cardiac mechanical function, or mitochondrial energetics or resistance to MPTP opening. In contrast to previous studies in rodents that found a beneficial effect on survival when diets high in α-linolenic acid and linoleic acid were given separately to rodents with heart failure,14,15 our findings suggest that high intake of both α-linolenic acid and linoleic acid has a negative effect.
The lack of improvement in survival with the high PUFA diet compared with the high saturated fat diet was associated with a 75% increase in plasma free fatty acids with the PUFA diet compared with the standard and high saturated fat groups. Clinical studies in the 1960s found an association between elevated free fatty acids and ventricular arrhythmias,34 and it was later shown that plasma fatty acid concentration is a strong predictor of sudden cardiac death.35 Experimental evidence showed that elevated free fatty acids can prevent normal sarcolemmal ion handling and could potentially trigger fatal arrhythmias.36 Thus, it is possible that elevated free fatty acids triggered arrhythmias and sudden death in the PUFA group.
The two high-fat diets were low in carbohydrate compared with the standard diet (Table 1), as protein content was constant across diets. We were careful to use a standard diet free of sugars, as we previously observed that high sugar intake can accelerated the development of heart failure in pressure overload models.1 This does not seem to be the case in cardiomyopathic hamsters, as we recently observed in a parallel study that high sugar intake did not adversely affect cardiac function or survival compared with the standard diet (P.A.H., W.C.S. et al., unpublished observation). Thus, the beneficial effect of the high saturated fat diet does not appear to be due to the reduction in ‘glycaemic load’ that accompanied reduced carbohydrate intake.
Examination of the fatty acid profile of cardiac phospholipids revealed only subtle differences between the high saturated fat diet and the standard diet groups, but a dramatic difference in survival. These findings suggest that the difference in survival between these two groups is not due to changes in membrane fatty acid composition. On the other hand, when one compares the high PUFA and high saturated fat groups, there is a striking elevation in linoleic acid and depletion of oleic acid and total monounsaturated fatty acids (Figure 5 and Table 5), suggesting that these alterations may have contributed to the failure of the high PUFA diet to improve survival. A similar relationship between the cardiac oleic acid content and life span was observed in normal mice, although mice have a very low level of monounsaturated fatty acid (∼5–7%) in the myocardium compared with hamsters (∼20%) or humans (∼14%).37 It is important to note that our analysis of total tissue phospholipid fatty acid composition provides a gross profile of fatty acyl moieties in pooled myocardial membrane. Future studies should assess the effects of dietary lipid in heart failure on isolated myocardial membrane fractions (i.e. sarcolemmal, sarcoplasmic reticulum, mitochondria) and the fatty acyl composition of distinct lipid classes (phosphotidylethanolamine, phosphotidylcholine, cardiolipin, etc.).
The elevation in linoleic acid in myocardial phospholipids with the PUFA diet compared with both the standard and high saturated fat diets could accelerate inflammation if it increased arachidonic acid (20:4n6), the precursor to inflammatory prostaglandins. Arachidonic acid is highly abundant in cardiac phospholipids, and it has been suggested that diets high in linoleic acid will accelerate conversion to arachidonic acid and production of inflammatory prostanoids. This was not observed, presumably because conversion of linoleic acid to arachidonic acid was inhibited by high intake of α-linolenic acid. Together, these finding suggest that the improved survival in the high saturated fat group compared with the PUFA diet was not mediated via changes in inflammatory prostaglandins.
IFM from the cardiomyopathic hamster showed a clear increase in sensitivity to Ca2+-induced MPTP opening compared with normal animals; however, this defect was not observed in SSM (Figure 4). Thirty years ago, Hoppel et al.21 showed a defect in oxidative phosphorylation in IFM but not SSM in cardiomyopathic hamsters. Here, we extend this finding, showing that greater sensitivity to Ca2+-induced MPTP opening is also isolated to IFM. While diet had no effect in SSM, in IFM, the high PUFA diet further increased susceptibility to permeability transition. While we observed a trend for a lower state 3 respiration rate IFM in cardiomyopathic animals, it was not statistically significant, but our mitochondrial protein yield was decreased by ∼50%. This is in contrast to Hoppel et al., who observed similar mitochondrial yields in normal and cardiomyopathic hamsters, but decreased state 3 respiration. This difference could perhaps be due to strain, as they used the Bio 14.6 strain while we used the Bio TO2, though we both used the same vendor. In any case, available evidence suggests a clear defect in mitochondrial function in this model that is specific to IFM.
Limitations to the present investigation need to be addressed. First, we did not assess the effects of the high PUFA or high saturated fat diets in normal hamsters. There is a clear possibility that what is good for the failing heart might be bad for the normal healthy myocardium. We previously assessed the effects of various high PUFA and high saturated fat diets in normal rats and mice and observed no major differences in cardiac function or structure,9–12,18 though we did observe elevated myocardial palmitoylceramide content and cardiomyocyte apoptosis in normal rats fed a high saturated fat diet compared with a high n-6 PUFA diet.19 A similar increase in apoptosis was not observed in cardiomyopathic hamsters in the present study, suggesting that the differences might be due to species or to the underlying effects of heart failure. A second limitation is that we measured the fatty acid profile in total cardiac phospholipids, which does not reflect the complexity and biological activities of the various pools of individual phospholipid classes in specific organelles. Future studies need to isolate subcellular membrane fractions and identify the composition of the various phospholipid subclasses. Another limitation is the lack of assessment of cardiac function and myocardial pathology at a later time point (e.g. 40–50 weeks) when mitochondrial pathology and cardiomyocyte apoptosis are further advanced.21 Lastly, we did not assess the electrocardiogram or cause of death in the present study. The improved survival with high saturated fat compared with high PUFA may be due to greater ventricular arrhythmias and cardiac sudden death. In addition, the precise cause of death with the standard diet is unclear in the model. Thus, future studies should employ telemetry to evaluate possible diet-induced changes in cardiac electrophysiology and to determine whether death is due to ventricular arrhythmias.
In summary, our results show that consuming a diet high in saturated fat prolongs life compared with a standard low-fat diet in an established genetic model of heart failure. This appeared to be independent of mitochondrial function. In contrast, consuming a high PUFA fat diet rich in α-linolenic acid and linoleic acid did not improve survival nor LV or mitochondrial function. The failure of the high PUFA diet to improve survival in this model was associated with an increase in linoleic acid and a decrease in oleic acid in cardiac phospholipids, suggesting that future studies should pursue a thorough analysis of the phospholipid composition in various cardiac membrane compartments in an effort to unmask the underlying causes for the differences in survival.
Funding
This work was supported by the National Institutes of Health, grant numbers HL074237, HL101434, and HL072751.
Acknowledgements
The authors wish to thank Ramzi Khairallah for assistance with the mitochondrial assays.
Conflict of interest: none declared.
References
- 1.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 2.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 4.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 5.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, et al. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–H1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 6.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, et al. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–H1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 7.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, et al. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res. 2008;79:331–340. doi: 10.1093/cvr/cvn066. [DOI] [PubMed] [Google Scholar]
- 8.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, et al. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail. 2008;14:82–88. doi: 10.1016/j.cardfail.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chess DJ, Khairallah RJ, O'Shea KM, Xu W, Stanley WC. A high-fat diet increases adiposity but maintains mitochondrial oxidative enzymes without affecting development of heart failure with pressure overload. Am J Physiol Heart Circ Physiol. 2009;297:H1585–H1593. doi: 10.1152/ajpheart.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007;20:403–409. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Sharma N, Okere IC, Barrows BR, Lei B, Duda MK, Yuan CL, et al. High-sugar diets increase cardiac dysfunction and mortality in hypertension compared to low-carbohydrate or high-starch diets. J Hypertens. 2008;26:1402–1410. doi: 10.1097/HJH.0b013e3283007dda. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chicco AJ, Sparagna GC, McCune SA, Johnson CA, Murphy RC, Bolden DA, et al. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 2008;52:549–555. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiaccavento R, Carotenuto F, Minieri M, Masuelli L, Vecchini A, Bei R, et al. Alpha-linolenic acid-enriched diet prevents myocardial damage and expands longevity in cardiomyopathic hamsters. Am J Pathol. 2006;169:1913–1924. doi: 10.2353/ajpath.2006.051320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 17.Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. J Am Coll Cardiol. 2007;50:22–24. doi: 10.1016/j.jacc.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, et al. Low-carbohydrate/high-fat diet attenuates pressure overload-induced ventricular remodeling and dysfunction. J Card Fail. 2008;14:327–335. doi: 10.1016/j.cardfail.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 20.D'Hahan N, Taouil K, Dassouli A, Morel JE. Long-term therapy with trimetazidine in cardiomyopathic Syrian hamster BIO 14:6. Eur J Pharmacol. 1997;328:163–174. doi: 10.1016/s0014-2999(97)83042-7. [DOI] [PubMed] [Google Scholar]
- 21.Hoppel CL, Tandler B, Parland W, Turkaly JS, Albers LD. Hamster cardiomyopathy. A defect in oxidative phosphorylation in the cardiac interfibrillar mitochondria. J Biol Chem. 1982;257:1540–1548. [PubMed] [Google Scholar]
- 22.Shimizu T, Okamoto H, Chiba S, Matsui Y, Sugawara T, Akino M, et al. VEGF-mediated angiogenesis is impaired by angiotensin type 1 receptor blockade in cardiomyopathic hamster hearts. Cardiovasc Res. 2003;58:203–212. doi: 10.1016/s0008-6363(02)00843-x. [DOI] [PubMed] [Google Scholar]
- 23.Mattera GG, Lo GP, Loi FM, Vanoli E, Gagnol JP, Borsini F, et al. Istaroxime: a new luso-inotropic agent for heart failure. Am J Cardiol. 2007;99:33A–40A. doi: 10.1016/j.amjcard.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, et al. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005;112:2650–2659. doi: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]
- 25.Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJ, et al. Omega-6 fatty acids and risk for cardiovascular disease. A science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119:902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 26.Harris WS. Alpha-linolenic acid: a gift from the land? Circulation. 2005;111:2872–2874. doi: 10.1161/CIRCULATIONAHA.105.545640. [DOI] [PubMed] [Google Scholar]
- 27.Khairallah RJ, Sparagna GC, Khanna N, O'Shea KM, Hecker PA, Kristian T, et al. Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochim Biophys Acta. 2010;1797:1555–1562. doi: 10.1016/j.bbabio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Shea KM, Khairallah RJ, Sparagna GC, Xu W, Hecker PA, Robillard-Frayne I, et al. Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. J Mol Cell Cardiol. 2009;47:819–827. doi: 10.1016/j.yjmcc.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabkowski ER, Williamson CL, Hollander JM. Mitochondria-specific transgenic overexpression of phospholipid hydroperoxide glutathione peroxidase (GPx4) attenuates ischemia/reperfusion-associated cardiac dysfunction. Free Radic Biol Med. 2008;45:855–865. doi: 10.1016/j.freeradbiomed.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Gelinas R, Thompson-Legault J, Bouchard B, Daneault C, Mansour A, Gillis MA, et al. Prolonged QT interval and lipid alterations beyond {beta}-oxidation in very long-chain acyl-CoA dehydrogenase null mouse hearts. Am J Physiol Heart Circ Physiol. 2011;301:H813–H823. doi: 10.1152/ajpheart.01275.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lionetti V, Stanley WC, Recchia FA. Modulating fatty acid oxidation in heart failure. Cardiovasc Res. 2011;90:202–209. doi: 10.1093/cvr/cvr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharov VG, Todor AV, Imai M, Sabbah HN. Inhibition of mitochondrial permeability transition pores by cyclosporine A improves cytochrome C oxidase function and increases rate of ATP synthesis in failing cardiomyocytes. Heart Fail Rev. 2005;10:305–310. doi: 10.1007/s10741-005-7545-1. [DOI] [PubMed] [Google Scholar]
- 33.Javadov S, Huang C, Kirshenbaum L, Karmazyn M. NHE-1 inhibition improves impaired mitochondrial permeability transition and respiratory function during postinfarction remodelling in the rat. J Mol Cell Cardiol. 2005;38:135–143. doi: 10.1016/j.yjmcc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Oliver MF, Kurien VA, Greenwood TW. Relation between serum-free-fatty acids and arrhythmias and death after acute myocardial infarction. Lancet. 1968;1:710–714. doi: 10.1016/s0140-6736(68)92163-6. [DOI] [PubMed] [Google Scholar]
- 35.Jouven X, Charles MA, Desnos M, Ducimetiere P. Circulating nonesterified fatty acid level as a predictive risk factor for sudden death in the population. Circulation. 2001;104:756–761. doi: 10.1161/hc3201.094151. [DOI] [PubMed] [Google Scholar]
- 36.Corr PB, Yamada KA. Selected metabolic alterations in the ischemic heart and their contributions to arrhythmogenesis. Herz. 1995;20:156–168. [PubMed] [Google Scholar]
- 37.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, et al. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]