Abstract
Aims
Myocardial infarction (MI) is associated with irreversible loss of viable cardiomyocytes. Cell therapy is a potential option to replace the lost cardiomyocytes and restore cardiac function. However, cell therapy is faced with a number of challenges, including survival of the transplanted cells in the infarct region, which is characterized by abundant levels of oxidants and lack of a pro-survival support mechanism. The goal of the present study was to evaluate the effect of supplemental oxygenation on cell engraftment and functional recovery in a rat model.
Methods and results
MI was induced in rats by a 60-min occlusion of the coronary artery, followed by restoration of flow. Mesenchymal stem cells (MSCs), isolated from adult rat bone marrow, were transplanted in the MI region. Rats with transplanted MSCs were exposed to hyperbaric oxygen (HBO: 100% O2, 2 atmospheres absolute) for 90 min, 5 days/week for 4 weeks. The experimental groups were: MI (control), Ox (MI + HBO), MSC (MI + MSC), and MSC + Ox (MI + MSC + HBO). HBO exposure (oxygenation) was started 3 days after induction of MI. MSCs were transplanted 1 week after induction of MI. Echocardiography showed a significant recovery of cardiac function in the MSC + Ox group, when compared with the MI or MSC group. Oxygenation increased the engraftment of MSCs and vascular density in the infarct region. Molecular analysis of infarct tissue showed a four-fold increase in NOS3 expression in the MSC + Ox group compared with the MI group.
Conclusions
The results showed that post-MI exposure of rats to daily cycles of hyperoxygenation (oxygen cycling) improved stem cell engraftment, cardiac function, and increased NOS3 expression.
Keywords: Mesenchymal stem cell, Hyperbaric oxygen, Myocardial infarction, Stem cell therapy, Endothelial nitric oxide synthase
1. Introduction
The most prevalent form of heart failure follows myocardial infarction (MI). Numerous studies have demonstrated that the damage to the heart following MI may be attenuated or repaired by cell-mediated myocardial regeneration (cell therapy).1 Cell therapy for MI is gaining interest as a viable means of replacing the lost cells. Several studies have reported the positive effects of cell transplantation on cardiac structure or function post-MI, irrespective of the cell type used.2–7 Experimental6,8 and clinical9 studies have clearly demonstrated that bone-marrow mononuclear cells may regenerate damaged myocardium following acute MI in humans. Adult stem cells have been proposed as a promising source for cardiac repair and regeneration, as well as restoration of cardiac function.6,7,10,11
A major limitation of cardiac stem cell therapy for MI heart is that the transplanted cells have low survival rates, negatively impacting their beneficial effects.12,13 To overcome this limitation, several strategies have been implemented to enhance the survival of cells using genetic engineering to overexpress prosurvival genes such as Akt.5 Transplantation of mesenchymal stem cells (MSCs) overexpressing Akt attenuated left ventricular remodelling and improved cardiac function. In an attempt to develop an optimal angiogenic strategy, other studies have overexpressed an adenoviral vector, a stable HIF-1α analogue (HIF-1α/VP16), in MSCs and showed a four-fold increase in VEGF release.14 Subsequently, in a murine model of hindlimb ischaemia, local injection of MSCs transduced with HIF-1α/VP16 significantly increased collateral perfusion compared with non-transduced cells.14 These strategies, although promising in principle and effectiveness, have practical limitations for clinical use.
The role of nitric oxide (NO) in myocardial ischaemia/preconditioning is one of the rapidly advancing areas in cardiovascular therapeutics and research.15 NO is a key mediator in attenuating the deleterious effects of superoxide generation, platelet aggregation, and post-ischaemic hyperpermeability.16–18 The administration of NO donors prior to ischaemia mitigates myocardial ischaemia-reperfusion (IR) injury, leading to decreased infarct size.19 Increased expression of endothelial nitric oxide synthase (eNOS, NOS3), one of the isoforms of NOS has been reported in endothelial cells subjected to IR injury and treated with hyperbaric oxygen (HBO) in vitro.20 In yet another study, preconditioning of the rat heart with hyperoxia/hyperbaria before the induction of IR injury led to improved cardiac function, decreased infarct size, and increased NOS3 expression.21
Our recent study has shown that HBO treatment enhances MSC survival and improves cardiac function and myocardial oxygenation in the infarcted heart during the short-term (2 weeks) post-transplantation period.22 The study utilized permanent left-anterior descending (LAD) coronary-artery ligation in rats. MSCs were transplanted after 30 min of ischaemia and the study was terminated 2 weeks after stem cell transplantation. The goal of the present study was to evaluate the effect of oxygenation along with stem cell therapy in a clinically relevant model of IR injury. Rats were subjected to LAD coronary-artery ligation for 1 h, after which cardiac perfusion was re-established (Figure 1). MSCs were transplanted at 1 week post-MI. HBO treatment began at 3 days post-MI and continued for 4 weeks after MSC transplantation. The results showed that combined treatment of MSC and oxygenation improved stem cell viability and engraftment in the infarcted heart leading to enhanced functional recovery and increased NOS3 expression.
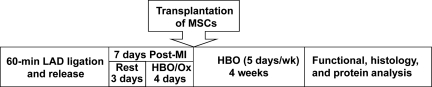
Figure 1.
Schematic representation of in vivo experimental studies.
2. Methods
Additional detailed methods are in Supplementary material online.
2.1. Isolation, culture, and characterization of MSCs
Fisher 344 rats (200–250 g) were euthanized by CO2 inhalation. After shaving the hind limbs and wiping with 70% ethanol, an incision was made around the perimeter of the hind legs using sterile scissors, and the skin was detached from the muscles and the tibia and the femur were removed. The ends of the bones were cut, using a sterile bone cutter, to expose the bone marrow. Bones were flushed with DMEM media using a sterile 22-G needle connected to a 10 mL syringe in a 100 mm culture dish. The cells were transferred into a 50 mL tube and uniformly suspended. The cells were centrifuged at 200 g for 5 min at room temperature. After aspirating and discarding the supernatant, the cell pellet was re-suspended in growth media and transferred to a T75 flask and incubated at 37°C, with a mixture of air and 5% CO2 in a humidified chamber. After 3 days of culture, 10 mL of pre-warmed fresh growth media was added to each flask. The culture media was changed every 2–3 days until scattered colonies of spindle-shaped cells were visualized by microscopy. Once the cells reached to passage 3, they were detached using accutase and the purity of the cells was determined by flow cytometry (see Supplementary material online, Figure S1) using CD29, CD44 as positive markers (≥85%), and CD14 and/or CD45 as negative markers (≤10%).
2.2. Characterization of MSC phenotype
MSCs of the second and third passages were analysed using flow cytometry to characterize the phenotype of the cells. The cells (1 × 106) were incubated with antibody or isotype control for 45 min on ice. Cell aliquots were incubated with fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated monoclonal antibody against CD44 (Chemicon), CD14 (Chemicon), CD29 (integrin β1; Biolegend), and CD45 (BD Pharmingen). Aliquots of cells were also stained with respective isotype controls, such as IgG conjugated to FITC or PE. Flow data were acquired using a FACS Calibur (BD) and analysed using CellQuest software (BD).
2.3. Labelling of MSCs with SPIO particles
MSCs (1 × 106 cells/15 mL medium) were incubated in a T75 flask with super paramagnetic iron oxide (SPIO) particles (0.9 µm diameter, 1.25 × 105/mL of medium; Bangs Laboratories, IN, USA) for 24 h. After incubation with SPIO, the cells were washed three times with PBS to remove un-internalized SPIOs and cells were re-suspended in fresh media as described previously.22
2.4. Induction of myocardial ischaemia/reflow injury in vivo
Male Fisher 344 rats (200–250 g) were used. Rats were randomly divided into four groups of eight animals each: (i) MI group (serum-free medium-treated); (ii) Ox group (MI with HBO treatment); (iii) MSC (MI with MSC transplantation); (iv) MSC + Ox (MI with MSC and HBO treatment). Rats were anaesthetized with ketamine (50 mg/kg, ip) and xylazine (5 mg/kg, ip) and maintained under anaesthesia using isofluorane (1.5–2.0%) mixed with air. During the surgical procedure, the absence of the pedal reflex was used as an indication that a surgical plane of anaesthesia has been maintained. MI was created by ligating the LAD coronary artery for 1 h. After 1 h of ischaemia, the ligation was released, flow was restored (reperfusion), and the chest cavity was closed. After reinstallation of spontaneous respiration, animals were extubated and allowed to recover from anaesthesia. No mortality was observed in any of the groups at the end of the study. All the procedures were performed with the approval of the Institutional Animal Care and Use Committee of The Ohio State University and conformed to the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86–23).
2.5. Echocardiography for cardiac functional analysis
Cardiac function was analysed using echocardiography prior to surgery (at baseline) 1 week after the induction of MI, and at 4 weeks after MSC transplantation. Rats were anaesthetized using 1.5–2.0% isofluorane and M-mode ultrasound images were acquired using a Vevo-2100 (Visualsonics; Toronto, Canada) high-resolution ultrasound imaging system.
2.6. Transplantation of MSCs in the ischaemic heart
MSCs were transplanted into the hearts at 1 week after the induction of MI. Three intramyocardial injections of MSCs labelled with SPIO were used to implant cells in the infarct (i) and peri-infarct (ii) regions of the hearts (total of 0.5 × 106 cells in 50 µL of serum-free medium).
2.7. Protocol for oxygen cycling
The rats were subjected to HBO treatment by placing them inside a custom-built small-animal hyperbaric chamber (Polyfab; Boston Plastics Manufacturing, Wilmington, MA, USA)22 connected to a compressed gas cylinder containing 100% oxygen. HBO was administered daily [100% O2, 2 atmospheres absolute (ATA), 90 min] for both HBO and HBO + MSC groups starting 4 days after MI and 3 days prior to cell transplantation. Following one day of post-transplantation rest, HBO was administered 5 days a week for 4 weeks. The animals were placed one per cage with up to three cages at a time in the chamber. After the chamber was closed, a fill-valve was slowly opened to allow the pressure within the chamber to reach 2 ATA over 5–10 min. The valve was then closed, sealing the chamber and the rats kept in the chamber for 90 min under constant observation. After this period, a safety pressure release valve was manually activated to slowly depressurize the unit to normal atmospheric pressure over 5–10 min.22 We did not observe any adverse effect like seizures or lung toxicity in rats after HBO (2 ATA) treatment in our study.
2.8. Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed using a 9.4 T horizontal-bore imaging system (Bruker BioSpin, Billerica, MA, USA). Rats were anaesthetized initially with 3.5% isofluorane mixed with carbogen (1 L/min) and maintained with 1.5–2% isofluorane during MR imaging. Physiologic parameters, such as the EKG and respiration, were monitored using an MRI-compatible system (Model 1025, Small Animal Instruments, Stonybrook, NY, USA). The EKG signal was obtained by placing electrode pads on the forepaw and leg of each animal. A pneumatic pillow was used to monitor the respiration of each animal. For gated cardiac imaging, the animal was secured to an animal bed and placed at the isocentre of the MRI scanner. Short-axis T1-weighted images were acquired so as to cover the entire left ventricle of each rat (FLASH sequence; parameters: TR = 16 ms, TE = 1.6 ms; matrix = 256 × 192; FOV = 5.1 × 5.1 cm; slice thickness = 2 mm, up to 20 frames per cardiac cycle).
2.9. Assessment of fibrosis in cardiac tissue
The rats were euthanized by injecting pentobarbital sodium (50 mg/kg body weight, ip) and the hearts were quickly excised, washed with cold PBS and fixed in formalin. After 12 h, the heart sections were embedded in paraffin and left ventricular (LV) cross-sections from apex, mid-LV, and base were stained with Masson-Trichrome. Images of the left ventricular area of each slide were taken using a Nikon Model C-PS (objective×20) dissecting microscope equipped with a Spot Insight camera (Diagnostic).
2.10. Prussian blue staining of cells and cardiac tissue
Transplanted cells, labelled with SPIO particles, were identified using Prussian blue staining as described previously.22
2.11. Immunohistochemical staining of cardiac tissue for CD68/Macrophage, α-SMA, vWF, connexin-43, and NOS3 expression
Hearts were fixed in formalin and embedded in paraffin. Five micron sections were cut and Immunohistochemical/immunofluorescence staining for CD68/Macrophage, alpha-smooth muscle actin (α-SMA), von Willebrand Factor VIII (vWF), connexin-43, and NOS3 expression was performed and quantified as described previously.22
2.12. Western blot analysis
Western blots for NOS3 and HIF-1α signalling were performed in the cardiac tissue homogenates prepared from the anterior wall of the left ventricles from rats of all four groups. At 4 weeks after treatment rats in MI, Ox, MSC, and MSC + Ox were anaesthetized and sacrificed. Control rats (non-I/R/Sham, n= 4) were also used. Heart tissues were homogenized for protein extraction (Supplementary material online) and probed with primary antibodies for HIF-1α (Novus Littleton, CO, USA), NOS3, phospho-NOS3 (ser-495/1177, Cell Signaling; Beverly, MA, USA), and HIF-1α (Novus Biologicals, Littleton, CO, USA).23–25 Data were expressed as per cent of the expression in the control group.
2.13. Reverse transcriptase PCR
Snap-frozen cardiac tissue was homogenized using mortar and pestle (cooled to temperature in a liquid nitrogen bath). TRIZOL (1 mL/50 mg) was mixed with the sample and the tissue was transferred to the pestle for grinding (Supplementary material online). Single strand c-DNA was synthesized for incubation using sense and anti-sense primers and a revert aid TM h minus first strand cDNA synthesis kit (Fermentas, MD, USA). The resulting cDNA was diluted 1:10 before proceeding with the PCR reaction. PCR was conducted in a Mastercycler gradient (Brinkmann Instruments). Each PCR reaction used 50 µL cDNA, 2.5 U Taq polymerase (Eppendorf Scientific), 0.2 mM dNTPs, and 0.5 µM primer. PCR products were resolved on 2% agarose gel containing 0.01% (v/v) ethidium bromide and visualized using an UV illuminator.
2.14. RT–PCR primers
Name Sequence
β-actin forward 5′-CACCCGCGAGTACAACCTTC-3′
β-actin reverse 5′-CCCATACCCACCATCACACC-3′
NOS3 forward 5′-ACGTGGAGATCACCGAGCTC-3′
NOS3 reverse 5′-GTGCTCATGTACCAGCCACTG-3′
2.15. Immunoprecipitation
Proteins were isolated from cardiac tissue samples using a Total Protein Extraction kit from Millipore according to the manufacturer's protocol. After cell lysis, the extracted DNA was fragmented by passing the lysed suspension through a needle attached to a 1 mL syringe a total of 5–10 times. The lysates were pre-cleared to help reduce non-specific binding of proteins to agarose or sepharose beads. A 50 μL of anti-rabbit GAPDH antibody was added for 3 h to pre-clear the lysate. NOS3 antibody was then added to the pre-cleared lysate. The lysate was incubated on ice with the antibodies overnight and 100 μL of bead slurry was added to the solution. The bead and lysate mix was incubated for 4 h at 4°C with gentle agitation. The mixture was spun at 14 000 g for 10 min at 4°C. The bead pellet was discarded and the supernatant was saved for immunoprecipitation. In order to increase the yield, the beads washed 1 or 2 more times in lysis buffer and the supernatants were collected.
2.16. Data analysis
The statistical significance of the results was evaluated using one-way ANOVA (Tukey's post hoc test) and Student's t-test. All pairwise comparisons between groups were made only after the ANOVA showed an overall difference among all experimental groups and there was no adjustment for multiple comparison analysis. The values were expressed as mean ± SD. A P-value of <0.05 was considered significant.
3. Results
3.1. Oxygen cycling improved the recovery of cardiac function in cell-treated hearts
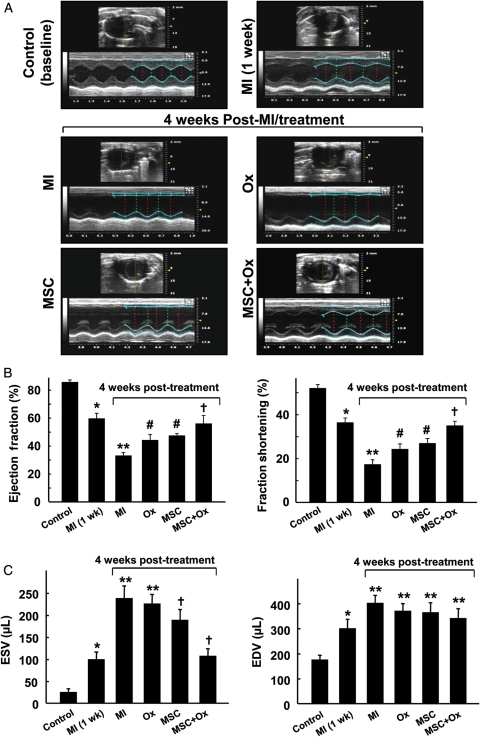
Cardiac function was measured at baseline (before surgery), 1 week, and 4 weeks after MI using transthoracic M-mode echocardiography. A significant reduction in the ejection fraction and fraction shortening was observed at 1 week after induction of MI (Figure 2A). Significant improvements in cardiac function were observed at 4 weeks in hearts treated with MSCs and oxygen when compared with untreated MI hearts. In particular, the hearts treated with both MSC and oxygen (MSC + Ox group) showed a significantly higher recovery of cardiac function when compared with MSC- and oxygen- treated groups (Figure 2B). Similarly, the left ventricular end-systolic volumes were significantly decreased in MSC and MSC + Ox groups when compared with the MI group (Figure 2C). However, we did not observe any significant difference in left ventricular end-diastolic volumes compared with the MI group (Figure 2C). These results clearly indicated that oxygen cycling, in conjunction with MSC therapy, had a beneficial effect on the recovery of cardiac function in MI hearts.
Figure 2.
Recovery of cardiac function following MSC transplantation and oxygen cycling. Cardiac function was measured in stem cell/oxygen cycled MI hearts using transthoracic M-mode echocardiography. The measurements were performed in rats prior to induction of MI (control/baseline), 1-week post-MI (prior to cell therapy), and 4 weeks after cell therapy. The four groups included untreated (MI), oxygen cycled (Ox), stem cell transplanted (MSC), and stem cell transplanted and oxygen cycled (MSC + Ox) (A) Representative echocardiograms. (B) Left ventricular ejection fraction and fractional shortening. (C) Left ventricular end-systolic and end-diastolic volumes. Data represent mean ± SD; n= 6; *P< 0.05 vs. Control; **P< 0.05 vs. Control; #P< 0.05 vs. MI; †P< 0.05 vs. MSC. The results show that oxygenation resulted in significantly higher cardiac functional recovery when compared with normoxic groups (MI and MSC).
3.2. Oxygen cycling enhanced MSC engraftment and integration with resident cardiomyocytes
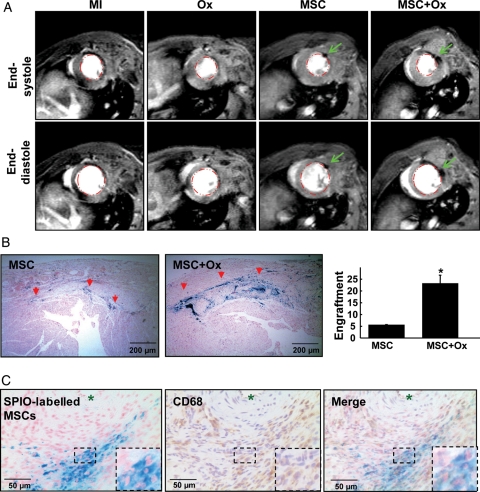
MSCs, labelled with SPIO markers, were identified in hearts 4 weeks after transplantation using MRI and Prussian blue staining. MRI showed hypointense regions in the left ventricular tissue suggesting the presence of transplanted stem cells (Figure 3A). Prussian blue staining showed higher intensity of iron oxide staining in the MSC + Ox group when compared with the MSC group (Figure 3B), indicating an enhancement of MSC engraftment in the hearts of the MSC + Ox group. In both the MSC and MSC + Ox groups, the transplanted MSCs were aligned in the same orientation as the resident myocytes. In order to determine whether the SPIO particles were localized in the engrafted MSCs or engulfed by macrophages, we stained for CD68, which is specifically expressed in tissue macrophages. The results, as shown in Figure 3C, showed very few of the SPIO-labelled MSCs in the heart was positive for CD68 suggesting that the Prussian blue staining was mostly from the engrafted MSCs.
Figure 3.
Tracking of MSC engraftment in the infarct heart. MSCs, pre-labelled with SPIO, were tracked 4 weeks after transplantation in the infarcted hearts using MRI in vivo and Prussian blue staining in vitro. The measurements were performed on MI (medium treated), Ox, MSC, and MSC + Ox hearts. (A) Representative short-axis MR images of hearts corresponding to end-systolic and end-diastolic cardiac time-points. The images show hypointense (black) regions (pointed by arrows) indicating the distribution of the SPIO particles in the LV anterior wall. (B) Prussian blue staining showing the engraftment of SPIO-labelled cells (red arrows) in the infarcted heart. The bar graph indicates a quantification of engrafted MSCs. There was a substantial increase in MSC engraftment in the MSC + Ox group when compared with the MSC group (*P< 0.05 vs. MSC group, n= 4). (C) Prussian blue staining showing the engraftment of SPIO-labelled MSCs (left panel) in the infarcted heart; CD68/macrophage staining showing the co-localization of macrophages in same tissue section (middle panel) and the merge image showing co-localization of both SPIO-labelled MSCs and CD68 staining (right panel). Results show that majority of engrafted cells are negative for CD68 staining.
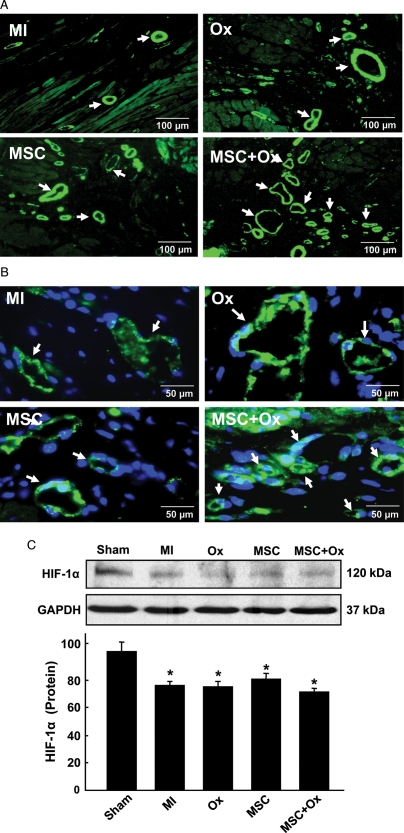
3.3. Oxygen cycling increased angiogenesis in the cell-transplanted heart
Immunohistochemical staining showed an increase in vasculogenesis in the cell-treated heart upon oxygen cycling. Immunostaining for α-SMA showed a marked increase in vessel density in the MSC + Ox group compared with the MSC group (Figure 4A). Similarly, immunostaining with vWF showed increased capillary density in the MSC + Ox-treated group when compared with the MSC-treated group (Figure 4B). Hypoxia-inducible factor-1α (HIF1α) is a transcriptional regulatory factor that plays a key role in tissue adaptation to hypoxia and angiogenesis. Western blot analysis of the heart tissue showed a significant decrease in HIF-1α expression in all the treatment groups at 4 weeks after MI compared with untreated control heart (Figure 4C).
Figure 4.
Blood vessel formation, capillary density and HIF-1α expression in the infarcted heart 4 weeks after transplantation of MSCs. (A) α-smooth-muscle actin (α-SMA) staining for blood vessels. The images show an increased number of blood vessels (indicated by arrows) in the peri-infarct region of MSC + Ox group when compared with the MSC group. (B) von Willebrand Factor VIII (vWF) staining shows increased capillary density (indicated by arrows) in the MSC + Ox group. (C) HIF-1α expression was significantly decreased in all the groups compared with untreated control (Sham). Data represent mean ± SD; *P< 0.05 vs. Sham. No significant difference was observed in MI, Ox, MSC, and MSC + Ox groups.
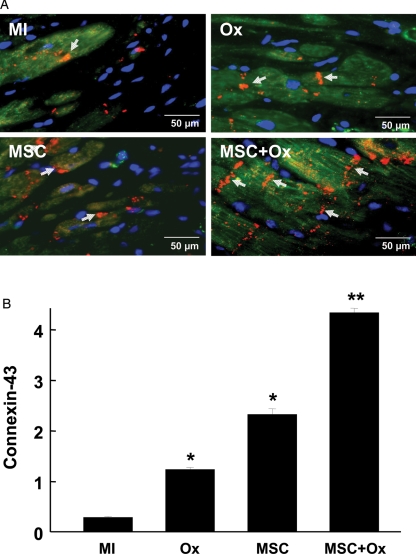
3.4. Oxygen cycling induced the expression of connexin-43 in MSCs transplanted in the heart
To assess whether oxygen cycling can induce differentiation of transplanted MSCs into a cardiomyocyte-like cell type, we performed immunostaining for connexin-43, a gap-junction protein responsible for cardiac conduction. MSCs were labelled with fluorescent Dragon-Green iron-oxide probes and anti-connexin-43 antibodies. An image was then produced by overlaying the dragon-green and anti-connexin-43 images (Figure 5A). A significant increase in connexin-43 expression was observed in the region of MSCs, which appeared to fuse with resident myocytes in the peri-infarct region (Figure 5B).
Figure 5.
Connexin-43 expression in the infarct tissue 4 weeks after transplantation of MSCs. Immunostaining for the connexin-43 expression. (A) Overlay images of Dragon green fluorescence (green), connexin-43 immunofluorescence (red), and DAPI staining (blue) in the peri-infarct region of hearts are shown. Arrows (yellow) indicate positive staining for connexin-43 expression in engrafted MSCs. (B) Connexin-43 expression was significantly increased in MSC and MSC + Ox groups compared with MI. Data represent as mean ± SD; *P< 0.05 vs. MI; **P< 0.05 vs. Ox and MSC.
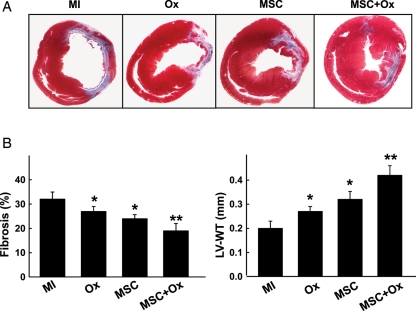
3.5. Oxygen cycling attenuated fibrosis in the cell-transplanted heart
The extent of fibrosis was lower in Ox-, MSC-, and MSC + Ox-treated groups when compared with the MI group (Figure 6). The MSC + Ox group showed a significant decrease in cardiac fibrosis when compared with the MSC or Ox group. Similarly, there was a significant improvement in wall thickness in the MSC + Ox group when compared with the MSC or Ox group.
Figure 6.
Cardiac fibrosis and remodelling. Masson-trichrome staining was used to measure fibrosis and remodelling in the infarct regions. (A) Representative images of heart sections from each group. (B) Fibrosis and left ventricular wall thickness (LV-WT) determined by computer planimetry. Data represent mean ± SD; *P< 0.05 vs. MI; **P< 0.05 vs. MSC. The MSC + Ox group show a significant reduction in the fibrosis and increase in LV-WT compared with the MSC group.
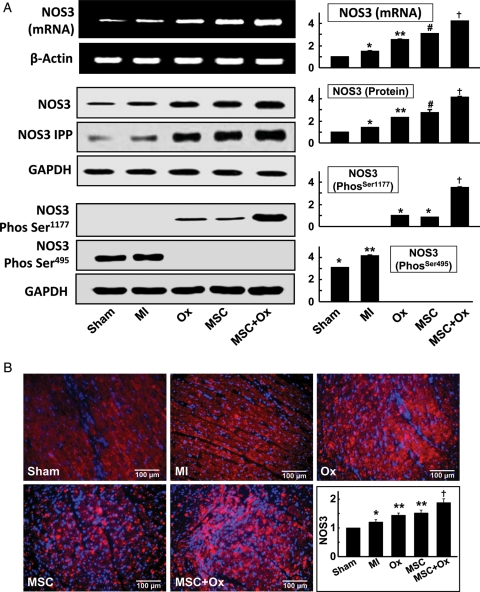
3.6. Oxygen cycling along with stem cell treatment increased NOS3 expression in the infarcted heart
NOS3 has been implicated as an important mediator in cardiovascular disease. In the present study, we have investigated the effect of oxygenation, stem cell transplantation, and the combination of both, on NOS3 gene expression in infarcted heart tissue. The expression of NOS3 mRNA, observed using RT–PCR, showed a basal level of NOS3 mRNA expression in the untreated control (MI) and infarcted hearts (MSC and Ox; Figure 7A). The expression of NOS3 mRNA was found to increase four-fold vs. the basal level in rat heart tissues recovered from the MSC + Ox group. The results suggested that stem cell engraftment with hyperoxygenation induced NOS3 gene transcription, thus increasing NOS3 mRNA levels. Similar results were observed in NOS3 protein expression measured by western blotting, where hyper oxygenation along with stem cell therapy was able to induce a four-fold increase in NOS3 protein expression when compared with other groups. Oxygenation or stem cell therapy alone was not as effective in increasing NOS3 protein expression.
Figure 7.
NOS3 expression. The expressions of NOS3 gene and protein in the infarcted hearts were measured. (A) NOS3 mRNA (by RT–PCR), NOS3 protein (by western blot and immunoprecipitation), NOS3 phosphorylation at Ser-1177 and NOS3 de-phosphorylation at Ser-495 (by western blot). NOS3 was not phosphorylated at Ser-1177 in sham and MI hearts; however, NOS3 was phosphorylated in the treated groups. NOS3 was phosphorylated on the Ser-495 residue in sham and MI hearts, indicative of an inactive state of NOS3 in the tissue. *P< 0.05 vs. Sham; **P< 0.05 vs. MI; #P< 0.05 vs. Ox; †P< 0.05 vs. MSC). (B) Representative NOS3 immunostaining images and quantitative results expressed as a per cent (mean ± SD; n= 6) of Sham hearts. *P< 0.05 vs. Sham; **P< 0.05 vs. MI; †P< 0.05 vs. MSC). The results show a significant increase in NOS3 expression in the treated groups when compared with the MI group.
Immunoprecipitation of NOS3 protein from the heart tissue samples showed the combination of oxygenation and stem cell grafting was able to induce significant increases in NOS3 protein expression in the infarcted heart. The expression of NOS3 enzyme is not the sole factor which determines NOS3 activation. NOS3 activity requires phosphorylation at Ser-1177, which is downstream of p53-dependent kinase signalling, and concomitant de-phosphorylation of Thr-495.26–29 The effect of oxygenation- and stem cell therapy-based treatments on NOS3 phosphorylation at Ser-1177 and NOS3 de-phosphorylation at Ser-495 was analysed by immunoblotting. It was found that NOS3 was not phosphorylated at Ser-1177 in MI heart tissue. Oxygenation or stem cell therapy alone induced an increase in the phosphorylation of Ser-1177. The combined therapy of oxygenation and stem cell transplantation led to a 3.5-fold increase in the phosphorylation of the Ser-1177 residue of NOS3. In contrast, NOS3 was also found to be phosphorylated on the Ser-495 residue in infarcted hearts, indicative of an inactive state of NOS3 in the tissue. Oxygenation, stem cells, and the combination of both reversed NOS3 phosphorylation at the Ser-495 residue. Immunostaining for NOS3 in cardiac tissues treated with stem cells and oxygen cycling showed a marked increase in NOS3 levels in MSC + Ox hearts (Figure 7B), when compared with the MSC-alone treated group. Overall, the results established that MI reduced NOS3 mRNA and gene expression in untreated infarcted heart tissue. Furthermore, the combination of oxygenation and stem cells led to a substantial increase in NOS3 gene and protein expression through regulation of NOS post-translational modifications.
4. Discussion
The key finding from this study is that oxygenation led to improved engraftment of MSCs in the infarcted heart possibly through increased NOS3 expression. The combinatorial therapy of oxygenation and MSCs engraftment showed a cumulative effect on cardiac regeneration and function. We showed that the oxygenation- and MSC-mediated effects on cardiac function were possibly associated with increased expression of NOS3. We further established that oxygenation and MSCs were able to induce NOS3 expression at the gene level, as well as at the protein level. In addition, this combinatorial therapy led to alterations in the post-translational modification patterns of NOS3 protein. NOS3 protein was rendered activated through increases in phosphorylation of NOS3 at the Ser-1177 residue and by de-phosphorylation of the Thr-495 residue.
Over the last decade hypoxia-inducible transcription factor (a.k.a., HIF) is gaining importance in cardiovascular disease as a key regulator of gene expression in response to hypoxia.30 The two hetrodimers of HIF, α and β subunits, are expressed extensively in response hypoxia.31,32 A recent study showed a significant increase in HIF-1α mRNA at 1 week after MI and this increased expression of HIF lasted for up to 4 weeks in both infarct and peri-infarct regions of the heart.33 A similar study by Kido et al.34 demonstrated that overexpression of HIF-1α in the myocardium attenuated the progression of cardiac dysfunction and decreased infarction. On the other hand, in our present study we did not observe any significant difference in HIF-1α expression in MSC or HBO-treated groups when compared with MI at 5 weeks after MI suggesting that HIF-1α may not be playing a role in the hyperoxygenation-mediated recovery of cardiac function.
The role of oxygenation in combating post-surgery IR injury is gradually gaining acceptance as an important factor in the progression and treatment of acute MI. It has been reported that hyperbaric preconditioning of the heart prior to the induction of IR injury limits myocardial damage and decreases infarct size.35 There is a growing body of evidence that pharmacological/ischaemic preconditioning stimulates the production of endogenous NO, which is one possible mechanism whereby IR injury can be attenuated.15,36 One possible mechanism for the production of oxygenation-induced NO and its role in mediating myocardial protection might involve the synthesis of heat shock protein.21 In their study, rats treated with hyperoxia for 1 h (100% O2 at 1 ATA), showed a large recovery of cardiac function and simultaneously a four-fold increase in NOS3 expression. It has been reported that the first site where phosphorylation was found to significantly influence NOS3 function is the Ser-1179/1177 (bovine/human and mouse).37,38 NOS3 Ser-1179 was initially reported to be phosphorylated by Akt, thus enhancing NOS3 function. The phosphorylation of Ser-1179 and various extracellular stimuli and kinases also influence NOS3 activity by modulating Ser-1179 phosphorylation.39
In our study, we have shown that the combined treatment of oxygenation and MSCs led to a synergistic increase in NOS3 mRNA and protein expression in infarcted cardiac tissue, thus suggesting the role towards NOS3-dependent cardiac recovery. We further established that oxygenation and MSCs not only induce NOS3 expression, but also alter the post-translational modification status of the NOS3 in hearts subjected to hyperbaric oxygenation. NOS3 enzyme consists of specific serine and threonine residues which interact with cellular kinases and phosphatases.40 It is well established that phosphorylation of NOS3 on the Ser1177 residue and de-phosphorylation of NOS3 on the Thr495 residue are required for NOS3 enzyme activity. Our results indicate that NOS3 was both phosphorylated at the Ser1177 residue and de-phosphorylated at the Ser495 residue in rat hearts treated with oxygenation and MSCs.
Recent studies have indicated that ROS may be involved in the activation of NOS41 and thus the production of NO.42 It has also been shown that oxygenation results in oxidative stress and generates ROS.43 Oxygenation-induced ROS has been shown to activate NOS.44 Transcription factors like p53 are also known to induce ROS45 and may induce NOS3 expression through another mechanistic pathway.46 However, the mechanism of NOS3 gene regulation in the infarcted heart and its positive regulation in hearts subjected to stem cell therapy and oxygen cycling is yet to be fully investigated. NOS family genes are regulated by a variety of transcription factors, of which p53 is a prime contributor and it would be interesting to explore the relationship between NOS3 transcriptional regulations during oxygenation and MSC treatment in our future studies.
5. Conclusions
The present study demonstrates the first evidence that MSC therapy and oxygenation, either independently or in combination, induced expression and activation of NOS3. We also found that NOS3 was altered post-translationally during treatment with both oxygen and MSCs. Additionally, we observed that the increase in phosphorylation of Ser1177 and subsequent activation of NOS3 led to an improvement in cardiac function, increased angiogenesis and decreased fibrosis in the MSC and Ox-treated group. These data provide a novel insight in understanding the mechanisms involved with the combined therapeutic approaches of oxygenation and stem cell therapy and the role of these mechanisms in myocardial protection against IR injury.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by grants from AHA (SDG 0930181N to M.K.) and NIH (R01 EB006153 to P.K.).
Supplementary Material
References
- 1.van Laake LW, Passier R, Monshouwer-Kloots J, Nederhoff MG, Ward-van Oostwaard D, Field LJ, et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat Protoc. 2007;2:2551–2567. doi: 10.1038/nprot.2007.371. [DOI] [PubMed] [Google Scholar]
- 2.Afzal MR, Haider H, Idris NM, Jiang S, Ahmed RP, Ashraf M. Preconditioning promotes survival and angiomyogenic potential of mesenchymal stem cells in the infarcted heart via NF-kappaB signaling. Antioxid Redox Signal. 2010;12:693–702. doi: 10.1089/ars.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed RP, Haider KH, Shujia J, Afzal MR, Ashraf M. Sonic Hedgehog gene delivery to the rodent heart promotes angiogenesis via iNOS/netrin-1/PKC pathway. PLoS One. 2011;5:e8576. doi: 10.1371/journal.pone.0008576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 6.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 7.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem cell transplantation for myocardial regeneration. Lancet. 2003;361:45–46. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–1119. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 9.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, et al. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 10.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 11.Rosenzweig A. Cardiac cell therapy—mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 12.Gussoni E, Blau HM, Kunkel LM. The fate of individual myoblasts after transplantation into muscles of DMD patients. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 13.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent KA, Shyu KG, Luo Y, Magner M, Tio RA, Jiang C, et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1alpha/VP16 hybrid transcription factor. Circulation. 2000;102:2255–2261. doi: 10.1161/01.cir.102.18.2255. [DOI] [PubMed] [Google Scholar]
- 15.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 16.Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol. 1992;262:H611–H615. doi: 10.1152/ajpheart.1992.262.2.H611. [DOI] [PubMed] [Google Scholar]
- 17.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharm. 1987;92:181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangialardi G, Monopoli A, Ongini E, Spinetti G, Fortunato O, Emanueli C, et al. Nitric oxide-donating statin improves multiple functions of circulating angiogenic cells. Br J Pharm. 2011;164:570–583. doi: 10.1111/j.1476-5381.2011.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buras JA, Stahl GL, Svoboda KK, Reenstra WR. Hyperbaric oxygen downregulates ICAM-1 expression induced by hypoxia and hypoglycemia: the role of NOS. Am J Physiol Cell Physiol. 2000;278:C292–C302. doi: 10.1152/ajpcell.2000.278.2.C292. [DOI] [PubMed] [Google Scholar]
- 21.Cabigas BP, Su J, Hutchins W, Shi Y, Schaefer RB, Recinos RF, et al. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72:143–151. doi: 10.1016/j.cardiores.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 22.Khan M, Meduru S, Mohan IK, Kuppusamy ML, Wisel S, Kulkarni A, et al. Hyperbaric oxygenation enhances transplanted cell graft and functional recovery in the infarct heart. J Mol Cell Cardiol. 2009;47:275–287. doi: 10.1016/j.yjmcc.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan M, Meduru S, Mostafa M, Khan S, Hideg K, Kuppusamy P. Trimetazidine, administered at the onset of reperfusion, ameliorates myocardial dysfunction and injury by activation of p38 mitogen-activated protein kinase and Akt signaling. J Pharmacol Exp Ther. 2010;333:421–429. doi: 10.1124/jpet.109.165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M, Mohan IK, Kutala VK, Kotha SR, Parinandi NL, Hamlin RL, et al. Sulfaphenazole protects heart against ischemia-reperfusion injury and cardiac dysfunction by overexpression of iNOS, leading to enhancement of nitric oxide bioavailability and tissue oxygenation. Antioxid Redox Signal. 2009;11:725–738. doi: 10.1089/ars.2008.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, et al. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2,3,4-trimethoxybenzyl]piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharmacol Exp Ther. 2009;329:543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr. Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 27.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T, Nakamura H, Yodoi J, Bloom ET. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic Biol Med. 2005;38:1231–1242. doi: 10.1016/j.freeradbiomed.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SR, Chen K, Keaney JF., Jr. Hydrogen peroxide activates endothelial nitric-oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3-kinase-dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. doi: 10.1074/jbc.M109107200. [DOI] [PubMed] [Google Scholar]
- 30.Mazure NM, Brahimi-Horn MC, Berta MA, Benizri E, Bilton RL, Dayan F, et al. HIF-1: master and commander of the hypoxic world. A pharmacological approach to its regulation by siRNAs. Biochem Pharmacol. 2004;68:971–980. doi: 10.1016/j.bcp.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 31.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993;268:21513–21518. [PubMed] [Google Scholar]
- 33.Jurgensen JS, Rosenberger C, Wiesener MS, Warnecke C, Horstrup JH, Grafe M, et al. Persistent induction of HIF-1alpha and -2alpha in cardiomyocytes and stromal cells of ischemic myocardium. Faseb J. 2004;18:1415–1417. doi: 10.1096/fj.04-1605fje. [DOI] [PubMed] [Google Scholar]
- 34.Kido M, Du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, et al. Hypoxia-inducible factor 1-alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005;46:2116–2124. doi: 10.1016/j.jacc.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 35.Yogaratnam JZ, Laden G, Guvendik L, Cowen M, Cale A, Griffin S. Pharmacological preconditioning with hyperbaric oxygen: can this therapy attenuate myocardial ischemic reperfusion injury and induce myocardial protection via nitric oxide? J Surg Res. 2008;149:155–164. doi: 10.1016/j.jss.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Rakhit RD, Marber MS. Nitric oxide: an emerging role in cardioprotection? Heart (British Cardiac Society) 2001;86:368–372. doi: 10.1136/heart.86.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 38.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi Y, Baker JE, Zhang C, Tweddell JS, Su J, Pritchard KA., Jr Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ Res. 2002;91:300–306. doi: 10.1161/01.res.0000031799.12850.1e. [DOI] [PubMed] [Google Scholar]
- 41.Drummond GR, Cai H, Davis ME, Ramasamy S, Harrison DG. Transcriptional and posttranscriptional regulation of endothelial nitric oxide synthase expression by hydrogen peroxide. Circ Res. 2000;86:347–354. doi: 10.1161/01.res.86.3.347. [DOI] [PubMed] [Google Scholar]
- 42.Mathy-Hartert M, Deby-Dupont GP, Reginster JY, Ayache N, Pujol JP, Henrotin YE. Regulation by reactive oxygen species of interleukin-1beta, nitric oxide and prostaglandin E(2) production by human chondrocytes. Osteoarthr Cartil. 2002;10:547–555. doi: 10.1053/joca.2002.0789. [DOI] [PubMed] [Google Scholar]
- 43.Benedetti S, Lamorgese A, Piersantelli M, Pagliarani S, Benvenuti F, Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin Biochem. 2004;37:312–317. doi: 10.1016/j.clinbiochem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 44.Elayan IM, Axley MJ, Prasad PV, Ahlers ST, Auker CR. Effect of hyperbaric oxygen treatment on nitric oxide and oxygen free radicals in rat brain. J Neurophysiol. 2000;83:2022–2029. doi: 10.1152/jn.2000.83.4.2022. [DOI] [PubMed] [Google Scholar]
- 45.Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010;2:471–474. doi: 10.18632/aging.100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.