Abstract
Objective
Inadequate adherence to highly active antiretroviral therapy (HAART) may lead to poor health outcomes and the development of HIV strains that are resistant to HAART. We developed a model to evaluate the cost effectiveness of counseling interventions to improve adherence to HAART among men who have sex with men (MSM).
Methods
We developed a dynamic compartmental model that incorporates HIV treatment, adherence to treatment, and infection transmission and progression. All data estimates were obtained from secondary sources. We evaluated a counseling intervention given prior to initiation of HAART and before all changes in drug regimens, combined with phone-in support while on HAART. We considered a moderate-prevalence and a high-prevalence population of MSM.
Results
If the impact of HIV transmission is ignored, the counseling intervention has a cost-effectiveness ratio of $25,500 per QALY gained. When HIV transmission is included, the cost-effectiveness ratio is much lower: $7,400 and $8,700 per QALY gained in the moderate- and high-prevalence populations, respectively. When the intervention is twice as costly per counseling session and half as effective as we estimated (in terms of the number of individuals who become highly adherent, and who remain highly adherent), then the intervention costs $17,100 and $19,600 per QALY gained in the two populations, respectively.
Conclusions
Counseling to improve adherence to HAART increased length of life, modestly reduced HIV transmission, and cost substantially less than $50,000 per QALY gained over a wide range of assumptions, but did not reduce the proportion of drug-resistant strains. Such counseling provides only modest benefit as a tool for HIV prevention, but can provide significant benefit for individual patients at an affordable cost.
Keywords: Cost Effectiveness, Adherence, HIV, Counseling, Computer Simulation
A number of strategies have been proposed to improve adherence to highly active antiretroviral therapy (HAART), including electronic reminders (1), easier-to-follow regimens (1–3), medication under supervised settings (4, 5), self-monitoring (6), counseling sessions (6), and other strategies (7, 8). Recent reviews have identified aspects of successful strategies to improve adherence (9–15). However, some strategies to improve HIV adherence “require considerable resources, and adherence is typically not sustained after the intervention is withdrawn” (15).
Since resistant HIV strains can be transmitted to others, improved adherence to HAART benefits not only those whose adherence is increased, but also those whom they may infect. Two recent papers examined the effectiveness (16) and cost effectiveness (17) of interventions to improve adherence to HAART, but did not account for changes in HIV transmission. To estimate the impact of improved adherence on the development and transmission of resistant strains of HIV, a model that incorporates mixing and infection transmission is needed. Recent papers have highlighted the importance of considering HIV transmission, resistance, and viral load when evaluating the effects of improved adherence (18–20) and have demonstrated the relationship between adherence and resistance (21) and viral load suppression (22, 23). An evaluation of the cost effectiveness of adherence interventions that does not include the benefits related to transmission may substantially underestimate cost effectiveness.
We evaluated the cost effectiveness of counseling to improve adherence to HAART. The analysis is based on a model of the HIV epidemic that incorporates infection transmission, disease progression, treatment and adherence to treatment. We included costs of HIV testing, viral load monitoring, resistance testing, counseling to improve adherence, HIV treatment, and non-HIV-related health care. We measured total quality-adjusted life years of survival (QALYs) experienced by the population, number of new HIV infections, and proportion of HIV cases in each resistance category.
METHODS
Model Overview
We constructed a dynamic compartmental model of HIV transmission and progression (Figure 1). We modeled an open population of men who have sex with men (MSM) aged 18–65. We constructed moderate- and high- prevalence (10% and 20%, respectively) HIV populations to reflect levels of HIV prevalence among MSM in different US cities (24–26). Key data and sources are shown in Table 1.
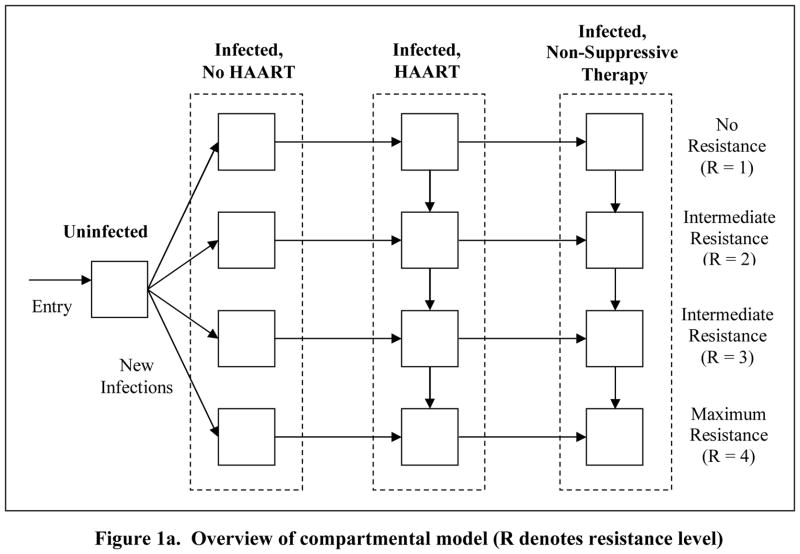
Figure 1. Schematic of model.
The model is divided into four sub-models: Uninfected; Infected, No HAART; Infected, HAART; and Infected, Non-suppressive Therapy (Figure 1a). Among infected individuals we considered four resistance levels. Infected individuals not receiving HAART are divided into three health states: asymptomatic and unaware of HIV status, asymptomatic and aware of HIV status, and symptomatic. Individuals receiving HAART (Figure 1b) are divided into three adherence levels high, intermediate, and low and six treatment states first, second, and third HAART regimens, and viral rebound states after each regimen (Figure 1b).
Table 1.
Parameter Estimates and Data Sources
| Parameter | Base Value [Range]* | Source |
|---|---|---|
| Demographic factors | ||
| Annual population growth rate | 4% [3–5%] | Derived§ |
| Annual maturation rate¶ | 2% [0–4%] | Calculated |
| Annual death rates | ||
| Uninfected individuals£ | 0.35% [.0–.5%] | (140) |
| Incremental mortality, asymptomatic HIV infection | 0% [0–1%] | (141, 142) |
| Incremental mortality, AIDS | 47% [35%–55%] | (130) |
| Baseline HIV prevalence | 10%, 20% | (24, 143) |
| Baseline HIV incidence | 1.6%, 3.2% [1.0%–2.1%] | (24) |
| Initial fraction of individuals with a resistant HIV strain¤ | 13% [0–25%] | (28–30) |
| Annual fraction of individuals with unknown HIV status who are screened each year | 75% [25%–99%] | (69–72) |
| HIV transmission | ||
| Number of new sexual partners per year | 3 [1–5]† | (45, 46) |
| Activity multiplier if aware of HIV infection | 1 [.5, 2] | (144–146) |
| Condom usage rate | .7 [.5–.9] | (35–37, 39–47, 147) |
| Condom effectiveness in preventing transmission | 87% [70%–99%] | (34) |
| Transmission probability from a partner with# | ||
| No AIDS, no viral suppression or viral load rebound | 6.6% [1.44%–7.35%] | (49–51, 53–56, 59, 60, 62, 63) |
| No AIDS, viral suppression due to HAART | 1.4% [0%–3.0%]‡ | (49–63) |
| AIDS | 14.7% [5%–20%] | (49–51, 53–56, 59, 60, 62, 63) |
| Proportion of patients on first HAART regimen who have not experienced viral load suppression | .10 [0–.14] | (48) |
| HIV progression (years) | ||
| Average time in Infected, No HAART sub-model | 7.3 [5.5–10] | (48) |
| Average time from AIDS to death in non-suppressive therapy sub-model | 2.1Ψ | |
| Average time from infection to death | 24 | Calculated |
| Adherence to HAART** | ||
| Average level of adherence | ||
| High-adherence group | .95 | Estimated based on (125, 148, 149) |
| Intermediate-adherence group | .80 | “ |
| Low-adherence group | .45 | “ |
| Fraction of individuals who are initially in | ||
| High-adherence group | .62 [0–.75] | (126, 127) |
| Intermediate-adherence group | .19 [0–.38]†† | Assumed |
| Low-adherence group | .19 §§ | “ |
| Annual rate of switching | ||
| From high- to intermediate-adherence group | .331 [.148–.5]¶¶ | (129) |
| Between all other groups | .01 [0–.10] | Assumed |
| Effect of counseling intervention | ||
| Fraction of individuals who are initially in: | ||
| High-adherence group | .697 [.50–.75]¥¥ | Estimated based on (126, 127) |
| Intermediate-adherence group | .1515¤¤ | “ |
| Low-adherence group | .1515¤¤ | “ |
| Annual rate of switching from high- to intermediate-adherence group | .148 [0–0.331] | (129) |
| Quality-of-life multipliers | ||
| Uninfected | 1.00 | Assumed |
| Asymptomatic, untreated | 0.87 [.79–.95] | (150–153) |
| Symptomatic, untreated | 0.79 [.70–.87] | (150–153) |
| HIV, treated | 0.83 [.79–.87] | (150–153) |
| AIDS | 0.73 [.50–.79] | (150–153) |
| Costs ($) | ||
| Annual non-HIV healthcare costs, all individuals## | 1,620 [1,200–1,750] | (140) |
| Ongoing annual HIV treatment costs | ||
| Pre-HAART, Asymptomatic | 1,976 [1,580–2,371] | (154) |
| Pre-HAART, Symptomatic | 14,944 [11,955–17,938] | (154) |
| HAART, Asymptomatic | 14,249 [11,399–17,099] | (154) |
| HAART, Symptomatic | 14,944 [11,955–17,938] | (154) |
| Non-suppressive therapy, CD4 >350 | 7,104 [5,683–8,525] | (154) |
| Non-suppressive therapy, CD4 200–350 | 12,353 [9,882–14,824] | (154) |
| Non-suppressive therapy, AIDS | 31,303 [25,042–37,567] | (154) |
| Viral load monitoringξξ | 456 [228–684] | (155) |
| One-time counseling and testing costs | ||
| HIV counseling and testing, positive test | 131.82 [105–158] | (156) |
| HIV counseling and testing, negative test | 42.41 [34–51] | (156) |
| Viral load test following treatment failure | 114 [220–660] | (155) |
| Resistance testing | 541 [433–649] | (157) |
| Enhanced counseling to improve adherence | 21.73 [21.73–200] | (131, 132) (See text) |
| Enhanced counseling provided with each viral load test | 21.73 [21.73–200] | (131, 132) (See text) |
| Monthly phone-in cost per patient for counseling intervention | 5.42 [0–100] | Estimatedψψ |
| Discount factor for QALYs and costs | 3% | (27) |
This column shows the base value assumed for each parameter, and the range of values considered in sensitivity analysis. When two base values are shown, they correspond to the moderate- and high-prevalence populations, respectively. Sensitivity analysis was performed for the moderate-prevalence population and ranges thus correspond to estimates for the moderate-prevalence population.
Derived to ensure approximately 1% growth per year in the MSM population.
Maturation into and out of population. Individuals enter the population by turning 18, and leave the population through death and maturation. All individuals entering the population begin in the Uninfected state and progress through the remaining model compartments according to disease progression, treatment, and adherence. Assuming an equal age distribution among individuals age 18–65, approximately 1/(65–18) = 1/47 ~ 2.0% age out of the population every year. The maturation rate was set to zero for the scenarios that did not incorporate HIV transmission.
Average mortality rate among 18–65 year old men.
We assumed that initially 13% of all HIV-infected individuals were infected with a resistant strain of HIV (28). We derived the distribution of individuals among resistance categories by projecting forward with a model that initially has no resistant HIV until the prevalence of resistant strains was 13%. In sensitivity analysis we considered higher and lower prevalence of individuals with resistant strains (29, 30).
Wide ranges for annual number of sexual partners have been reported in studies of MSM (34–47). We selected an intermediate value.
A wide range of estimates of the probability of HIV transmission per unprotected sexual partnership have appeared (49–63) We assumed that the probability of sexual transmission was 14.7% per sexual partnership when the infected partner had AIDS (59) and that it would be reduced to 6.6% (a factor of 0.45) for infected individuals without AIDS whose viral load was not suppressed (64). Several studies have observed reduced risk sexual HIV transmission risk associated with reduced viral load (52, 53, 57, 58, 61). Studies of vertical HIV transmission estimate 25.5% chance of transmission when no antiretroviral treatment is received during pregnancy (65); less than 2.5% among those mothers whose viral load was less than 1730 (66); and 0% among mothers whose viral load was less than 1000 (67). We used a formula given elsewhere (48) to estimate the ratio of the probability of transmission when viral load is suppressed to the probability of transmission when viral load is not suppressed as being 0.21. When switching to subsequent regimens the transmission rate rises slightly when the individual is in a viral load rebound state. We assumed that the average transmission probability among individuals on their first HAART regimen would be slightly higher, reflecting the delay between HAART initiation and viral load suppression.
We estimated that the chance of transmission to an uninfected partner was 1.4% if the individual is on his first HAART regimen, and 1.0% if the individual is on his second or third HAART regimen; the chance of transmission is likely to be higher during the first HAART regimen than during later regimens due to the delay between HAART initiation and viral load suppression.
This is the inverse of the incremental mortality associated with AIDS and was not varied separately.
All estimates related to adherence to HAART were derived so that average adherence over the entire population and the rate of switching from high adherence to a lower adherence state closely approximated estimates in existing studies (125, 148, 149).
Varied as the proportion of those who did not enter the high-adherence state who entered the intermediate-adherence state, and assumed to be 50% in the base case.
This value automatically varies as the above two values vary.
The value ρ=.331 corresponds to e−.331 = 71.8% of individuals who initially enter the high-adherence state moving to the intermediate-adherence state per year. Thus, 28.2% of those who initially are non-adherent for 0–3 days per month become non-adherent for 6 days per month after one year.
In stochastic sensitivity analysis, the number entering the high-adherence state following the intervention was fixed at the maximum of this number and the pre-intervention proportion.
In stochastic sensitivity analysis, when the fraction of individuals in the high-adherence group changed, the distribution of the remaining individuals between the intermediate- and low-adherence groups was assumed to be the same as in the base case.
Average annual healthcare expenditure among 18–65 year old men.
We modeled three different types of transitions. First, we modeled the progression from uninfected to HIV-infected receiving treatment (active therapy and non-suppressive therapy), incorporating resistance (Figure 1a). Second, we modeled the transitions between adherence levels and therapeutic regimens during active therapy (Figure 2). Third, we modeled the progression from HIV to AIDS to death. Each of these is detailed below.
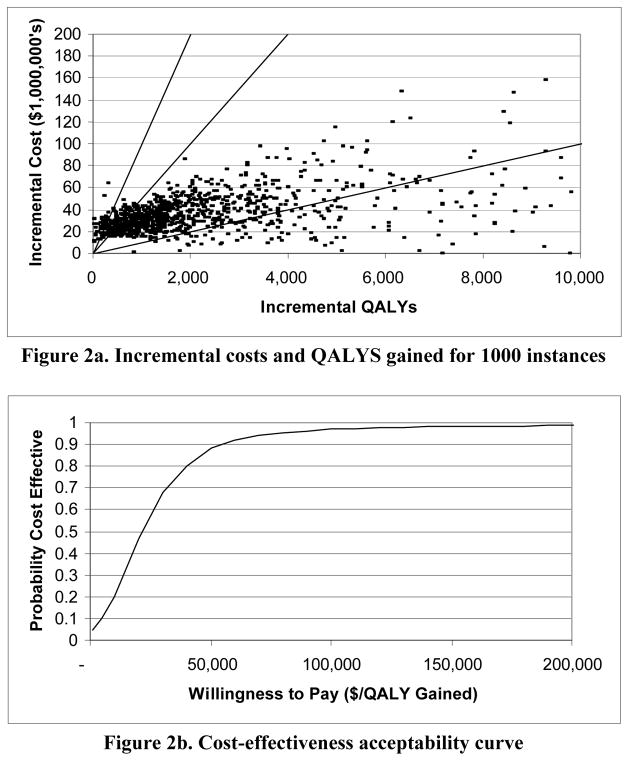
Figure 2. Results of stochastic sensitivity analysis for a moderate-prevalence population*.
* We modeled all rates and probabilities as beta distributions, all costs as normal distributions, and all other parameters as uniform distributions. For the uniform random variables, we used the upper and lower limits shown in Table 1. For the beta and normal variables, we assumed that the width of each range in Table 1 was equal to four times the standard deviation of the distribution. All failure and toxicity rates were varied within ± 20% of their base case values. Figure 2a shows the results of 1000 simulations. The x-axis shows incremental QALYs gained for each simulation instance and the y-axis shows the associated incremental cost. The three diagonal lines show thresholds of $10,000 per QALY gained (lower line), $50,000 per QALY gained (middle line), and $100,000 per QALY gained (upper line). Figure 2b shows the resulting cost-effectiveness acceptability curve.
Uninfected individuals become infected through sexual contact with infected individuals. Once infected, individuals remain unaware of their infection until either they develop HIV-related symptoms or opportunistic infections, or they are identified through routine screening. Once identified, individuals begin a HAART regimen.
While on HAART, individuals who achieve viral load suppression remain on that regimen until they either experience a treatment failure due to the development of a resistant strain or they develop a toxicity. Following three HAART regimens, individuals enter non-suppressive therapy.
Individuals whose viral load is suppressed are assumed to be asymptomatic, while those who experience a failure or toxicity while on HAART will be temporarily symptomatic until they switch regimens. Individuals can experience a declining CD4 count and a risk of both AIDS and AIDS-related death once they enter non-suppressive therapy.
The model was simulated for 20 years. For the first 20 years, costs and benefits were estimated directly through the model simulation. Costs and benefits beyond the first 20 years were estimated as the future expected costs and QALYs experienced by individuals in each model compartment assuming no more disease transmission or population growth. Costs are expressed in 2004 dollars. Costs and benefits were discounted at 3% (27).
Resistance
Clinically, HIV resistance is determined by specific mutations in the viral genome (which can confer resistance to single drugs or cross-resistance to other drugs in the same class), the number of mutations, and interactions between mutations. Rather than model specific drugs and resistance mutations, we classified individuals into four resistance groups reflecting their probability of sustaining virologic suppression with antiretroviral therapy. Resistance can be acquired at the time of infection or can develop during drug exposure (most frequently due to non-adherence). Accordingly, we assumed that resistance patterns are cumulative (and nondecreasing) and persist indefinitely.
The lowest resistance level (level 1) represents no resistant HIV strains. Individuals in this resistance class respond to all HAART regimens and derive the maximum benefit from HAART. Individuals with intermediate resistance levels (levels 2 and 3) have accumulated resistance mutations from past regimens or by being infected by an individual with a high resistance level, and therefore have restricted options for subsequent treatment. Because of cross-resistance between antiretroviral drugs, these individuals experience treatment failures at a higher rate than individuals with no resistant strains. Individuals with the highest resistance level (level 4) were assumed to have less than a 50% chance of achieving virologic suppression with HAART. We assumed that an individual’s resistance level changes only as a result of exposure to HAART (vertical arrows in Figure 1a).
In the base case, 14.3% of the population carried a resistant strain of HIV at the beginning of the time horizon; among these individuals, we estimated that 90.8% were in resistance level 1, 9.0% were in resistance level 2, and 0.2% were in resistance level 3. The distribution of individuals among compartments with resistant individuals was derived by starting with a model in which there were no resistant individuals and simulating forward until approximately 13% of the population was in resistance level 1 (comparable to (28)). The proportion initially resistant was varied in sensitivity analysis to reflect wide variations in the observed prevalence of resistant strains (29, 30).
HIV Transmission
Infection transmission occurs via sexual contacts. We assumed random mixing, which is a reasonable approximation for a homogenous population of MSM (31–33). We assumed that a newly infected individual has the same resistance level as the person who infected him. We calculated HIV incidence based on the average number of sexual partners (estimated from studies of MSM (34–47)), the condom usage rate, the reduction in transmission associated with condom use (34), and changes in infectivity associated with HAART (48–63).
A wide range of estimates of the probability of HIV transmission per unprotected sexual partnership have appeared (49–63). We estimated that the probability of sexual transmission was 14.7% per sexual partnership when the infected partner had AIDS (59) and that it would be reduced to 6.6% (a factor of 0.45) for infected individuals without AIDS whose viral load was not suppressed (64). Several studies have observed reduced sexual HIV transmission risk associated with reduced viral load (52, 53, 57, 58, 61). Studies of vertical HIV transmission have also observed reduced transmission risk associated with reduced viral load: a 25.5% chance of transmission when no antiretroviral treatment is received during pregnancy (65); less than 2.5% among those mothers whose viral load was less than 1730 (66); and 0% among mothers whose viral load was less than 1000 (67). We used a formula given elsewhere (48) to estimate the ratio of the probability of transmission when viral load is suppressed to the probability of transmission when viral load is not suppressed as being 0.21. When switching to subsequent regimens, the transmission rate rises slightly when the individual is in a viral load rebound state. We assumed that the average transmission probability among individuals on their first HAART regimen would be slightly higher than for individuals on later HAART regimens, reflecting the delay between HAART initiation and viral load suppression. Thus, we estimated that the chance of transmission to an uninfected partner was 1.4% for an individual on his first HAART regimen, and 1.0% for an individual on his second or third HAART regimen.
Treatment before HAART
We assumed that all newly infected individuals are untreated and unaware of their status. They may become aware of their HIV status through routine HIV screening or through the development of symptoms. CDC guidelines call for annual screening (68), although in practice not all at-risk individuals are screened. We estimated that 75% of individuals whose HIV status is unknown are screened each year (69–72). We assumed that individuals whose CD4 count is greater than 350 cells/mm3 are asymptomatic. Individuals initiate HAART if their CD4 count drops below 350 (73) or if they develop opportunistic infections. The expected time from infection until initiation of HAART was set to 7.3 years, consistent with another study (48).
HAART
We defined HAART as a treatment regimen that includes three or more antiretroviral drugs taken in combination according to recent guidelines (73). Individuals on HAART (Figure 1b) continue on their first HAART regimen until they experience a viral load rebound or toxicity. If the regimen changes due to a viral load rebound, an individual progresses to the next regimen at a higher resistance level; if the regimen changes due to a toxicity, the individual progresses to the next regimen at the same resistance level. We assumed that individuals changing regimens receive genotypic resistance testing to identify an appropriate new drug regimen and undergo additional viral load testing to establish the new level prior to regimen change (after intolerance) or to confirm rebound (after failure). We assumed that viral load monitoring would occur every three months, so the average time in a treatment failure state is 1.5 months. We assumed that, while on HAART, viral load is suppressed except during temporary viral load rebounds; CD4 cell counts increase; and no one develops AIDS.
Viral Load Suppression and Treatment Change
Individuals who achieve viral load suppression can experience increased survival and quality-of-life gains from HAART. The probability of achieving viral load suppression on HAART and the probability of a HAART regimen failing within two years varied with the HAART regimen, the adherence level, and the resistance level (Table 2). We estimated that 80% of individuals on their first regimen, 65% of individuals on their second regimen, and 30% of individuals on their third regimen would achieve viral load suppression for both the high- and intermediate-adherence groups (74–122). These values are averages over all resistance levels. Mutations from early failures may persist, thus contributing to lower success rates for later regimens (123, 124). We estimated that 18% of individuals in the low-adherence group would achieve viral load suppression for all treatment regimens (125).
Table 2.
Chance of Viral Load Suppression and HAART Regimen Failure Within Two Years for Individuals on HAART
| Fraction of individuals on each HAART regimen and in each resistance level who experience initial viral load suppression* | |||||
|---|---|---|---|---|---|
| Average | R = 1† | R = 2 | R = 3 | R = 4 | |
| High or Intermediate | |||||
| Adherence | |||||
| First regimen | 0.80 | 0.90 | 0.80 | 0.55 | 0.45 |
| Second regimen | 0.65 | 0.79 | 0.71 | 0.48 | 0.40 |
| Third regimen | 0.30 | 0.46 | 0.41 | 0.28 | 0.23 |
| Low Adherence | |||||
| All regimens | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 |
| Fraction of individuals who remain on a HAART regimen two years after starting§ | ||||
|---|---|---|---|---|
| R = 1 | R = 2 | R = 3 | R = 4 | |
| High Adherence | ||||
| First regimen | 0.54 | 0.48 | 0.33 | 0.27 |
| Second regimen | 0.48 | 0.42 | 0.29 | 0.24 |
| Third regimen | 0.27 | 0.24 | 0.17 | 0.14 |
| Intermediate Adherence | ||||
| First regimen | 0.47 | 0.42 | 0.29 | 0.24 |
| Second regimen | 0.42 | 0.37 | 0.25 | 0.21 |
| Third regimen | 0.24 | 0.21 | 0.15 | 0.12 |
| Low Adherence | ||||
| All regimens | 0.09 | 0.09 | 0.09 | 0.09 |
Let αij denote the fraction of individuals in HAART regimen i and resistance level j who experience initial viral load suppression. We estimated the average values of αij for the first, second, and third regimens as 80%, 65% and 30%, respectively (48). The values of α11, α12, α13 and α14 were estimated elsewhere (48). We estimated all otherαij by assuming that the ratio αi,j+1:αi,j was the same for i = 2 and i = 3 as for i = 1.
R denotes resistance level. R = 1 is the lowest resistance level and R = 4 is the highest resistance level (see text).
Among those who achieve viral load suppression, let p1 be the proportion who experience a viral load rebound within two years of initiating HAART, and let p2 be the proportion of individuals who discontinue their HAART regimen within two years due to a toxicity. We assumed that p1 would be different for the high- and intermediate-adherence groups, consistent with numerous studies that have shown a relationship between adherence and viral load (22, 125, 128, 149, 158–162). The fraction of individuals who remain on a HAART regimen is calculated as αij×(1−p1–p2), where p1 = .15 for high-adherence individuals, p1 = .225 for intermediate- and low-adherence individuals, and p2 = .25 for all groups. To implement the compartmental model, we estimated continuous rates of failure and toxicity based on αij, p1, and p2.
Baseline Adherence
Adherence was defined as the percentage of prescribed doses of medicine taken. We defined three levels of adherence to HAART: high (> 90% adherence, averaged to be 95%), intermediate (70–90% adherence, averaged to be 80%), and low (< 70% adherence, averaged to be 45%).
The proportion of individuals in each adherence level at a given point in time is a function of the proportion who initiate HAART at each adherence level and the rate at which individuals change adherence levels. A review of studies to improve adherence found baseline rates ranging from 37% to 92%, with a median value of 62% (126, 127). We estimated the initial proportion of individuals in the high-adherence group to be 62%, and assumed that the remaining 38% would be split evenly between the intermediate- and low-adherence groups (19% in each group). Evidence suggests that adherence decreases over time (128). We estimated that, of 100 individuals with high adherence (with 95% average adherence), 28.2 would switch to intermediate adherence (with 80% average adherence) each year in the absence of any intervention (129), and 1 of every 100 persons would switch between other groups each year.
Adherence has an indirect impact on health outcomes in the model. Compared to individuals with high adherence, individuals with intermediate adherence are less likely to experience viral load suppression, and if they do, they experience treatment failure at a faster rate. Thus, they spend less time in asymptomatic states in which quality of life is high and they progress to non-suppressive therapy at a faster rate.
Toxicities and Treatment Failures
We assumed that, among individuals who achieve viral load suppression, 25% experience a toxicity within two years. Additionally, 15% of individuals with high adherence, 22.5% of individuals with intermediate adherence, and no individuals with low adherence would experience a treatment failure caused by the development of resistant strains within two years. The corresponding proportions of individuals who achieved viral load suppression and were still on their initial regimen are shown in Table 2. The values in Table 2 were used to calculate continuous rates of treatment failure and development of toxicities.
Non-suppressive Therapy
Individuals progress to non-suppressive therapy following three failed HAART regimens. We assumed that the rate of developing additional resistant strains in non-suppressive therapy was the same as on the third HAART regimen. We modeled three stages of HIV infection defined by CD4 cell counts (> 200, 50–200, < 50 cells/mm3). We estimated the amount of time on non-suppressive therapy based on a pre-HAART model of HIV progression (130).
Counseling to Improve Adherence
We assumed that the intervention to improve adherence to HAART would consist of individual counseling sessions provided by a registered nurse or similar skilled health-care professional and would be given to all individuals prior to HAART initiation and following all changes in HAART regimens. We assumed that patients would also have access to “phone-in” support services, provided by a registered nurse, throughout their treatment. Individuals not receiving counseling may still practice other techniques to improve adherence. We measured the improvement in adherence relative to the base case of no counseling. Two recent systematic reviews examined the effectiveness of interventions aimed at improving adherence to HAART (126, 127). The reviews differed with respect to inclusion criteria and analytical approach, but both found a wide range of intervention effectiveness. Amico et al. (126), including all comparison trials, found that interventions targeted to non-adherent patients were most effective. Rueda et al. (127), including only randomized trials, did not identify such a trend but did find that interventions targeted to individuals (rather than groups), interventions provided over a longer period of time (12 or more weeks), and interventions targeting practical skills (rather than psychological factors) seemed most successful. Amico et al. (126) estimated that the overall effect of interventions, measured by improvement in adherence, was modest.
In our analysis, we used the estimated odds ratio of 1.41 from Amico et al. (126) corresponding to untargeted interventions, but considered a 95% confidence interval (1.20, 1.63), recognizing the considerable heterogeneity in included studies. We modeled increases in adherence by estimating a baseline rate of adherence (with no intervention) and applying the odds ratios, converting between probabilities and odds as appropriate. Accordingly, we estimated that without a counseling intervention, 62% of participants would be highly adherent at baseline, and that with an intervention, this proportion would increase to 69.7% (range 66.2% to 72.3%). We estimated that the intervention would also reduce the annual rate of switching from high to intermediate adherence to 14.8 per 100 (129).
Neither of the systematic reviews reported the costs of (nor resources required for) the interventions. For our base case estimate, we assumed that the counseling would be provided by a registered nurse (average national wage rate of $26.87/hour in 2004 (131)). Schackman et al. (132) found that sessions provided by a registered nurse lasted 31.88 minutes on average, and that labor represented 65.7% of the total cost. Thus, we estimated the cost per counseling session to be (31.88/60)×($26.87)×(1/.657) = $21.73. Additionally, we assumed that patients used phone-in support costing an average of $5.42/month. We considered a wide range of cost estimates in sensitivity analysis.
Health Outcomes and Costs
We measured the number of new HIV infections, proportion of HIV cases in each resistance category, and QALYs experienced by the population. We included costs of HIV testing, viral load monitoring, resistance testing, counseling, HIV treatment, and non-HIV-related health care (Table 1). We estimated total costs (and health benefits) as the sum of the discounted costs (health benefits) experienced over the first 20 years in all model compartments plus the discounted value of the expected future lifetime healthcare costs (quality-adjusted life expectancy) for all individuals at the end of the initial 20 years.
We used the model to calculate lifetime future costs and quality-adjusted life expectancy for individuals in each model compartment. For example, for a newly infected individual with no resistant strains, we initialized the model with a cohort of 100,000 individuals in the compartment corresponding to “infected, unaware, no treatment, no resistant strains”. The populations of all other compartments were set to zero, as was the rate of migration into the population. We then projected the model forward for 100 years and calculated the total discounted cost and QALYs experienced. This results in a future discounted cost of $306,985 for a newly infected individual with no resistant strains, which is close to the estimate of $303,100 obtained by Schackman et al. (133). We repeated this process for all model compartments.
We present all results as total costs and health benefits accrued for a population initially consisting of 100,000 individuals. We present the results in this way because the adherence intervention affects HIV transmission and mortality, and thus affects population size.
Model Implementation
We implemented the model in an Excel spreadsheet. We calculated net present costs and QALYs for the base case (Table 1), with and without the effects of HIV transmission in the population. We performed one-way sensitivity analysis on all model parameters, a variety of two-way sensitivity analyses on important related variables, and a stochastic sensitivity analysis in which all parameters were varied simultaneously (details in Figure 2 legend).
RESULTS
Adherence
We first considered a cohort of 100,000 infected individuals. This analysis captures the benefits of the intervention only for those who receive the intervention: everyone in the population is infected, so no one avoids infection as a result of the intervention. In this case, the counseling intervention had an incremental cost-effectiveness ratio of $25,500 per QALY gained (13,847 QALYs gained at an incremental cost of $353.2 million). At the end of 20 years, 1,168 more people were alive and 65.9% of the population was infected with a resistant strain (an increase of less than 0.1% compared to no intervention).
Adherence and HIV Transmission
We next analyzed the effect of the intervention in a cohort of infected and uninfected individuals (Table 3). This analysis captures the impact of the intervention not only among those who receive the intervention, but also among those whom they might infect. In the moderate-prevalence population, the counseling intervention cost $7,400 per QALY gained (3,967 additional QALYs at a cost of $29.3 million). In the high-prevalence population, the intervention cost $8,700 per QALY gained (6,920 QALYs gained at a cost of $60.1 million). The contrast between these results and the individual-level results highlights the impact of considering mixing and population dynamics: the intervention to improve adherence appears more cost effective when its impact on disease transmission is considered. In the moderate-prevalence population, at the end of 20 years, approximately 57.7% of the population had a resistant strain of HIV without the intervention, compared to 57.8% with the intervention. In the high-prevalence population, the corresponding numbers are 58.5% and 58.6%, respectively.
Table 3.
Base Case Results
| Moderate-Prevalence Population | High-Prevalence Population | |||||
|---|---|---|---|---|---|---|
| Pre-Intervention | Post-Intervention | Incremental | Pre-Intervention | Post-Intervention | Incremental | |
| Health Outcomes | ||||||
| New infections | 11,162 | 10,854 | −309 | 18,930 | 18,432 | −498 |
| HIV infections after 20 years | ||||||
| Number resistant | 6,106 | 6,039 | −67 | 10,859 | 10,773 | −86 |
| Number not resistant | 4,473 | 4,408 | −66 | 7,707 | 7,616 | −91 |
| Total | 10,580 | 10,447 | −133 | 18,566 | 18,389 | −177 |
| HIV prevalence after 20 years | 8.0% | 7.9% | −0.1% | 14.8% | 14.6% | −0.2% |
| Proportion resistant | ||||||
| All HIV cases | 57.7% | 57.8% | 0.1% | 58.5% | 58.6% | 0.1% |
| New HIV cases | 57.2% | 57.6% | 0.4% | 58.2% | 58.6% | 0.4% |
| QALYs experienced | ||||||
| In 20 years | 1,692,000 | 1,692,000 | 566 | 1,624,000 | 1,625,000 | 1,045 |
| After 20 years | 1,531,000 | 1,534,000 | 3,400 | 1,395,000 | 1,401,000 | 5,875 |
| Total | 3,223,000 | 3,227,000 | 3,967 | 3,019,000 | 3,026,000 | 6,920 |
| Costs ($1,000) | ||||||
| Intervention | - | 15,461 | 15,461 | - | 29,714 | 29,714 |
| Total | 10,629,000 | 10,659,000 | 29,320 | 12,345,000 | 12,405,000 | 60,077 |
| Cost per QALY gained ($) | 7,392 | 8,682 | ||||
Although the cost-effectiveness ratios are favorable in both populations, the intervention is slightly more cost effective in the moderate-prevalence population. This is because relatively more infections are prevented in the moderate-prevalence population than in the high-prevalence population. In absolute terms, more infections are prevented in the high-prevalence population, but the impact of the intervention relative to the pre-intervention number of infections is larger in the moderate-prevalence population.
Improved adherence leads to more viral load suppression and thus reduced HIV transmission. In the moderate-prevalence population, the counseling intervention led to 309 fewer new HIV infections over a 20-year period and a 0.1% reduction in HIV prevalence at the end of 20 years. In the high-prevalence population, the intervention led to 498 fewer infections and a 0.2% reduction in prevalence. The total QALYs gained can be split into two groups: QALYs gained by the cohort during the 20 years of the simulation, and expected future QALYs among all members of the population who are alive at the end of 20 years. The latter increase in QALYs is due to fewer infections occurring during the simulation period, resulting in a healthier population at the end of 20 years. For both populations, this latter increase represents approximately 85% of the total QALYs gained.
In both populations, the counseling intervention reduced the total number of HIV infections that occurred over 20 years (by approximately 2.8% and 2.6% in the moderate-and high-prevalence populations, respectively) and reduced the total number of individuals infected with a resistant strain of HIV (by approximately 1.1% and 0.8% in the moderate- and high-prevalence populations, respectively), but slightly increased the proportion of infected individuals who were infected with a resistant HIV strain at the end of 20 years (by about .1% in both populations). The intervention can be thought of as shifting 3.9% of individuals on HAART from initial intermediate adherence to initial high adherence, and shifting an additional 3.9% from initial low adherence to initial high adherence. Shifting patients from initial intermediate adherence to high adherence causes the proportion of resistant strains to decrease because patients temporarily move from a state where resistant strains develop rapidly to one where they develop slowly. Shifting patients from initial low adherence to high adherence causes the proportion of resistant strains to increase because low-adherence patients, who would normally develop resistant strains very slowly, are moved to a state where they may eventually become intermediate-adherence patients who develop resistant strains rapidly. For the base case, these two effects approximately offset each other, leading to a slight increase in the proportion of resistant strains at the end of 20 years.
Sensitivity Analysis
In sensitivity analysis, for the case of no HIV transmission, we considered a cohort of newly infected individuals with no resistant strains. In this case, the intervention cost $24,900 per QALY gained (14,972 QALYs gained at an incremental cost of $373.0 million). At the end of 20 years, 63.5% of the population was infected with a resistant strain of HIV, an increase of less than 0.1% compared to the case of no intervention. The remaining sensitivity analyses were all conducted for the base model, moderate-prevalence scenario, which did include consideration of HIV transmission.
In one-way sensitivity analysis, the cost-effectiveness ratio remained below $50,000 per QALY gained over the entire range for all variables. The annual screening rate was varied from 25% to 99%; at 25% the intervention cost $7,500 per QALY gained, and at 99% the intervention cost $7,300 per QALY gained. In the base case we assumed no survival advantage associated with partial suppression in non-suppressive therapy. If there is a 50% survival advantage associated with non-suppressive therapy, then the cost-effectiveness ratio decreases to $6,400 per QALY gained. Base case incidence was approximately 1.6%. When we increased this to 2.1%, the cost-effectiveness ratio fell to $4,600 per QALY gained; when we decreased incidence to 1.0%, the ratio increased to $11,800 per QALY gained. In the base case, 62% of the population initially entered the high-adherence state when initiating treatment. We varied this number while holding the odds ratio for treatment constant. If only 50% enter the high-adherence state, then the cost-effectiveness ratio decreases to $6,700 per QALY gained; if the proportion initially adherent increases to 75%, then the ratio increases to $8,700 per QALY gained.
When we simultaneously varied the cost per counseling session and the ongoing cost of phone-in support (up to $200 per counseling session and $100 per patient per month), the intervention cost $54,300 per QALY gained. When the intervention is twice as costly per session and half as effective as we estimated (in terms of the number of individuals who move into the high-adherence state, and the annual rate of remaining in the high-adherence state), then the intervention cost $17,100 per QALY gained (and $19,600 per QALY gained in the high-prevalence population).
We conducted multi-way sensitivity analysis in which we varied the parameters related to the cost and effectiveness of the intervention (Table 4). In most instances considered, the incremental cost-effectiveness ratio was less than $50,000 per QALY gained, although for the most pessimistic combinations of parameter estimates, the incremental cost-effectiveness ratio exceeded $100,000 per QALY gained. The intervention would need to increase the proportion of individuals entering the high-adherence state to at least 65% (from the pre-intervention value of 62%, corresponding to shifting 1.5% of the population from intermediate to high adherence, and 1.5% from low to high adherence) in order for it to cost less than $50,000 per QALY gained.
Table 4.
Sensitivity Analysis on Cost and Effectiveness of the Enhanced Counseling Program in a Moderate-Prevalence Population: Cost per QALY Gained ($)*
| Proportion Initially in High-Adherence† (Post-Intervention) | Annual Rate of Switching from High to Intermediate Adherence (Post-Intervention) | Proportion of Individuals Not in High-Adherence State Who Are in Intermediate-Adherence State§ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25% | 50% | 100% | ||||||||
| Cost per Counseling Session | Cost per Counseling Session | Cost per Counseling Session | ||||||||
| $21.73 | $50 | $100 | $21.73 | $50 | $100 | $21.73 | $50 | $100 | ||
| 62.0% (OR = 1.00) | 0.083 | 13,100 | 16,300 | 22,000 | 13,100 | 16,300 | 21,900 | 13,000 | 16,200 | 21,900 |
| 0.148 | 15,900 | 21,400 | 31.100 | 15,900 | 21,400 | 31,100 | 15,800 | 21,400 | 31,100 | |
| 0.248 | 29,800 | 46,000 | 74,500 | 29,800 | 46,000 | 74,700 | 29,900 | 46,100 | 74,900 | |
| 0.331 | No Benefit | No Benefit | No Benefit | No Benefit | No Benefit | No Benefit | No Benefit | No Benefit | No Benefit | |
|
| ||||||||||
| 66.1% (OR = 1.20) | 0.083 | 8,600 | 10,900 | 14,900 | 9,800 | 12,200 | 16,600 | 12,700 | 15,600 | 20,700 |
| 0.148 | 8,300 | 11,600 | 17,500 | 10,0200 | 13,800 | 20,400 | 15,000 | 19,900 | 28,500 | |
| 0.248 | 7,900 | 13,700 | 24,000 | 11,200 | 18,300 | 31,000 | 25,100 | 37,800 | 60,100 | |
| 0.331 | 7,400 | 16,800 | 33,400 | 13,400 | 26,800 | 50,500 | 99,200 | 167,800 | 289,200 | |
|
| ||||||||||
| 69.7% (OR = 1.41) | 0.083 | 6,400 | 8,200 | 11,500 | 7,900 | 10,000 | 13,700 | 12,400 | 15,100 | 19,900 |
| 0.148 | 5,300 | 7,900 | 12,300 | 7,400¶ | 10,400 | 15,600 | 14,500 | 18,900 | 26,700 | |
| 0.248 | 3,500 | 7,300 | 14,100 | 6,500 | 11,300 | 20,000 | 22,600 | 33,200 | 52,000 | |
| 0.331 | 1,800 | 6,900 | 15,900 | 5,400 | 12,700 | 25,600 | 58,000 | 95,400 | 161,500 | |
|
| ||||||||||
| 72.3% (OR = 1.63) | 0.083 | 5,100 | 6,700 | 9,500 | 6,800 | 8,600 | 11,900 | 12,200 | 14,800 | 19,300 |
| 0.148 | 3,800 | 5,800 | 9,600 | 5,900 | 8,400 | 12,900 | 14,100 | 18,200 | 25,500 | |
| 0.248 | 1,700 | 4,600 | 9,800 | 4,300 | 8,200 | 15,000 | 21,000 | 30,300 | 46,900 | |
| 0.331 | CS | 3,600 | 10,100 | 2,700 | 8,000 | 17,300 | 44,400 | 71,300 | 119,100 | |
We varied the proportion of individuals initially entering the high-adherence state (post-intervention) from 62% to 72.3% (base case 69.7%); the annual rate of switching from the high-adherence state to the intermediate-adherence state from 0.083 to 0.331 (base case .148); the proportion of individuals initially not entering the high-adherence state who would enter the intermediate-adherence state from 25% to 100% (base case 50%); and the cost of each counseling session from $21.73–$100 (base case $21.73). All cost-effectiveness ratios are rounded to the nearest $100. OR = Odds Ratio, CS = Cost Saving (more effective and less costly than no program).
Based on viral load monitoring every 3 months.
We assumed that the phone-in support line would need to provide adequate resources for each patient to call once every two months for 30 minutes per call, leading to a cost of $5.42 per patient per month (assuming an hourly wage rate of $21.68 for the phone counselor).
This column shows the proportion initially in high adherence and the corresponding odds ratio comparing post-intervention and pre-intervention proportions in high-adherence.
This number refers to the proportion of individuals with intermediate adherence among those whose adherence is not high, following the intervention. For example, if this proportion is 25% and the proportion in the high-adherence group is 70%, then the proportions of the population with intermediate and low adherence are 7.5% and 22.5%, respectively. In the absence of the intervention we assumed that this proportion would be 50%, and in the base case we assumed that the intervention would not change this proportion.
Base case.
We considered the possibility that 50% of individuals would inherit the same resistance level as the person who infected them, and 50% would inherit the next lower level. We also considered the possibility of no inherited resistance (i.e., all newly-infected individuals started at resistance level 1 regardless of the level of the person who infected them). In both cases, the incremental cost-effectiveness ratio remained at $7,400 per QALY gained, and the proportion of newly infected individuals with any level of resistance at the end of 20 years was reduced by 0.1%.
In stochastic sensitivity analysis (Figure 2), the intervention had a 20% chance of costing less than $10,000 per QALY gained, an 89% chance of costing less than $50,000 per QALY gained, and a 97% chance of costing less than $100,000 per QALY gained.
DISCUSSION
Counseling to improve adherence to HAART cost less than $10,000 per QALY gained in the base cases considered, and less than $50,000 per QALY gained over a wide range of sensitivity analyses. The cost-effectiveness ratio was most sensitive to the monthly cost of maintaining improved adherence. More than two-thirds of the QALYs gained were due to averted HIV infections.
A recent analysis of the cost effectiveness of improved adherence to HAART reported cost-effectiveness ratios as low as $22,400 per QALY gained in a cohort with late disease and as low as $27,100 per QALY gained in a cohort with early disease, but did not consider HIV transmission (17). When we ignored HIV transmission, we found that counseling to improve adherence had a cost-effectiveness ratio between $24,900 and $25,500 per QALY gained, depending on the prevalence of resistant HIV already in the population. When we included the impact of the intervention on HIV transmission, we found lower cost-effectiveness ratios: $7,400 and $8,700 per QALY gained in the moderate- and high-prevalence populations, respectively. Although the cost-effectiveness ratios are lower when we consider transmission, the counseling intervention appears cost effective when using common benchmarks regardless of whether transmission is considered.
We based our estimates of cost and effectiveness on one type of intervention for improving adherence. Numerous other interventions to improve adherence have been studied (e.g., (129, 134, 135)). Additionally, interventions that treat depression and alcohol abuse may improve adherence (136, 137). Evaluations of the costs and benefits of such programs that fail to include HIV transmission may significantly underestimate their cost effectiveness.
Our analysis suggests that modest gains can be expected from counseling interventions that improve adherence to HAART. The gains are modest because some of the benefits offset each other. For instance, individuals who receive the counseling intervention may live longer. They will spend more time in a state with reduced viral load, but the increased length of life leads to increased opportunities for infection transmission. As another example, the intervention prevents a small number of infections, thus reducing HIV treatment costs, but this reduction is offset by the increased survival and time in treatment among those who receive the intervention.
Our analysis suggests that improved adherence would lead to a very small increase in the proportion of resistant HIV strains after 20 years. The magnitude of the increase may depend on the adherence level of those receiving the counseling. Counseling interventions targeted to individuals expected to have only intermediate adherence may not produce this effect. Either with or without the counseling intervention we predict that approximately 58% of HIV infections would contain a resistant strain of HIV after 20 years. This compares with other models which suggest that the proportion resistant could be as high as 40% after 10 years (20); 60% after 10 years (138); or 100% after 30 years (139). This motivates continued drug development as well as research on drug dosing strategies that may reduce the rates at which resistant strains emerge.
In contrast to some existing research, our analysis suggests that improved adherence would lead only to a small reduction in HIV prevalence after 20 years. One model suggests reductions in prevalence of up to 10% after 30 years (139); another suggests that viral eradication may be possible if risky activity decreases (20). However, those analyses consider the impact of changing from limited to widespread use of HAART, whereas we considered the impact of changing adherence in a population of MSM who would have high access to HAART as part of usual HIV care.
Our model was restricted to MSM, a group that typically has higher adherence to HAART than other at-risk populations. Other populations would likely benefit from improved adherence to HAART. Further analysis is needed to evaluate the cost effectiveness of adherence interventions targeted to groups other than MSM.
Our analysis has several limitations. We assumed random mixing in the population. To the extent that individuals mix non-randomly, our analyses may overstate the benefits of improved adherence on HIV transmission (although, as we showed, counseling to improve adherence is cost effective even if there is no transmission benefit). We assumed that counseling is given to all individuals beginning a HAART regimen. Interventions to improve adherence may be most effective if targeted to those most likely to have low adherence. However, identifying such individuals may be difficult. While adherence interventions may become less important as new regimens are introduced that are easier to follow (including once-daily drug dosing), our model indicates that even at relatively high baseline levels of adherence, counseling to improve adherence remains cost effective. We modeled resistance categories, rather than focusing on single antiretrovirals or classes of drugs, recognizing that resistance-associated mutations frequently confer cross-resistance to other drugs in the same class, especially as mutations accumulate. With the development of new classes of drugs, individuals may have more treatment options than we modeled. However, effective interventions to support sustained adherence will still be needed. Existing research on adherence interventions shows mixed support for the hypothesis that adherence interventions improve adherence.
Our analyses show that counseling efforts to improve adherence to HAART among MSM are likely to be cost effective. Such counseling provides only modest benefit as a tool for HIV prevention, but can provide significant benefit for individual patients at an affordable cost.
Acknowledgments
Dr. Owens was supported by the Department of Veterans Affairs. Drs. Brandeau and Zaric were supported by a grant from the National Institute on Drug Abuse (DA-R01-15612). Drs. Zaric and Bayoumi were supported by a Research Operating Grant from the Ontario HIV Treatment Network. Dr. Bayoumi was supported by a Career Scientist Award from the Ontario HIV Treatment Network. Versions of this work have been presented at the SMDM, ISPOR, and INFORMS annual meetings.
References
- 1.Dunbar PJ, Madigan D, Grohskopf LA, et al. A two-way messaging system to enhance antiretroviral adherence. J Am Med Inform Assoc. 2003;10(1):11–5. doi: 10.1197/jamia.M1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggiolo F, Ripamonti D, Arici C, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3(5):371–8. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 3.Pollard RB. Can HIV infection be treated successfully with a once-daily regimen? AIDS Read. 2002;12(11):494–8. 500, 508. [PubMed] [Google Scholar]
- 4.Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden? AIDS Patient Care STDS. 2002;16(11):527–35. doi: 10.1089/108729102761041083. [DOI] [PubMed] [Google Scholar]
- 5.McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict. 2002;11(4):271–8. doi: 10.1080/10550490290088072. [DOI] [PubMed] [Google Scholar]
- 6.Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151–62. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 7.Remien R, Stirratt M, Dolezal C, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19(8):807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health. National Institutes of Health, ClinicalTrials.gov -Information on Clinical Trials and Human Research Studies: Trial List. 2005 [cited 2005 August 31]; Available from: http://www.clinicaltrials.gov/ct/search?term=hiv+adherence.
- 9.Park-Wyllie L, Antoniou T, Bayoumi A, Glazier R. Effectiveness of patient support strategies for improving HAART adherence in HIV-infected individuals: a systematic review of controlled trials [abstract 236P]. Can J Infect Dis; 11th Annual Canadian Conference on HIV/AIDS Research; Winnipeg, MB. April 25 – 28, 2002; 2002. p. 34A. [Google Scholar]
- 10.Simoni JM, Frick PA, Pantalone DW, Turner BJ. Antiretroviral adherence interventions: a review of current literature and ongoing studies. Top HIV Med. 2003;11(6):185–98. [PubMed] [Google Scholar]
- 11.Stone VE. Strategies for optimizing adherence to highly active antiretroviral therapy: lessons from research and clinical practice. Clin Infect Dis. 2001;33(6):865–72. doi: 10.1086/322698. [DOI] [PubMed] [Google Scholar]
- 12.Tuldra A, Wu AW. Interventions to improve adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S154–7. doi: 10.1097/00126334-200212153-00014. [DOI] [PubMed] [Google Scholar]
- 13.Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. J Infect Dis. 2002;185(Suppl 2):143–51. doi: 10.1086/340197. [DOI] [PubMed] [Google Scholar]
- 14.Cote J, Godin G. Efficacy of interventions in improving adherence to antiretroviral therapy. Int J STD AIDS. 2005;16(5):335–43. doi: 10.1258/0956462053888934. [DOI] [PubMed] [Google Scholar]
- 15.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 16.Kagay CR, Porco TC, Liechty CA, et al. Modeling the impact of modified directly observed antiretroviral therapy on HIV suppression and resistance, disease progression, and death. Clin Infect Dis. 2004;38(Suppl 5):S414–20. doi: 10.1086/421406. [DOI] [PubMed] [Google Scholar]
- 17.Goldie SJ, Paltiel AD, Weinstein MC, et al. Projecting the cost-effectiveness of adherence interventions in persons with human immunodeficiency virus infection. Am J Med. 2003;115(8):632–41. doi: 10.1016/j.amjmed.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Blower SM, Aschenbach AN, Gershengorn HB, Kahn JO. Predicting the unpredictable: transmission of drug-resistant HIV. Nat Med. 2001;7(9):1016–20. doi: 10.1038/nm0901-1016. [DOI] [PubMed] [Google Scholar]
- 19.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000;287(5453):650–4. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 20.Velasco-Hernandez JX, Gershengorn HB, Blower SM. Could widespread use of combination antiretroviral therapy eradicate HIV epidemics? Lancet Infect Dis. 2002;2(8):487–93. doi: 10.1016/s1473-3099(02)00346-8. [DOI] [PubMed] [Google Scholar]
- 21.Paris D, Ledergerber B, Weber R, et al. Incidence and predictors of virologic failure of antiretroviral triple-drug therapy in a community-based cohort. AIDS Res Hum Retroviruses. 1999;15(18):1631–8. doi: 10.1089/088922299309676. [DOI] [PubMed] [Google Scholar]
- 22.Knobel H, Alonso J, Casado JL, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV-infected patients: the GEEMA Study. AIDS. 2002;16(4):605–13. doi: 10.1097/00002030-200203080-00012. [DOI] [PubMed] [Google Scholar]
- 23.Nachega JB, Hislop M, Dowdy DW, Chaisson RE, Regensberg L, Maartens G. Adherence to nonnucleoside reverse transcriptase inhibitor based HIV therapy and virologic outcomes. Ann Intern Med. 2007;146:564–573. doi: 10.7326/0003-4819-146-8-200704170-00007. [DOI] [PubMed] [Google Scholar]
- 24.Facer M, Ritieni A, Marino J, Grasso P Social Light Consulting Group. Consensus meeting on HIV/AIDS incidence and prevalence in California. Sacramento, CA: California Department of Health Services Office of AIDS; 2001. Dec, [Google Scholar]
- 25.Centers for Disease Control and Prevention. HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men--five U.S. cities, June 2004–April 2005. MMWR. 2005;52(24):597–601. [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. HIV Incidence Among Young Men Who Have Sex With Men --- Seven U.S. Cities, 1994–2000. MMWR. 2001;50(21):440–4. [PubMed] [Google Scholar]
- 27.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 28.Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. J Amer Med Assoc. 2002;288(2):181–8. doi: 10.1001/jama.288.2.181. [DOI] [PubMed] [Google Scholar]
- 29.Simon V, Vanderhoeven J, Hurley A, et al. Evolving patterns of HIV-1 resistance to antiretroviral agents in newly infected individuals. AIDS. 2002;16(11):1511–9. doi: 10.1097/00002030-200207260-00008. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan PS, Buskin SE, Turner JH, et al. Low prevalence of antiretroviral resistance among persons recently infected with human immunodeficiency virus in two US cities. Int J STD AIDS. 2002;13(8):554–8. doi: 10.1258/095646202760159684. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan EH. Asymptotic worst-case mixing in simple demographic models of HIV/AIDS. Math Biosci. 1992;108:141–156. doi: 10.1016/0025-5564(92)90009-l. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan EH, Crampton PC, Paltiel AD. Nonrandom mixing models of HIV transmission. In: Castillo-Chavez C, editor. Mathematical and Statistical Approaches to AIDS Epidemiology. Berlin: Springer-Verlag; 1989. p. 405. [Google Scholar]
- 33.Watts DJ. Small worlds: The dynamics of networks between order and randomness. Princeton, NJ: Princeton University Press; 1999. [Google Scholar]
- 34.Davis KR, Weller SC. The effectiveness of condoms in reducing heterosexual transmission of HIV. Fam Plann Perspect. 1999 Nov–Dec;31(6):272–9. [PubMed] [Google Scholar]
- 35.Diaz RM, Stall RD, Hoff C, Daigle D, Coates TJ. HIV risk among Latino gay men in the Southwestern United States. AIDS Educ Prev. 1996;8(5):415–29. [PubMed] [Google Scholar]
- 36.Dilley JW, McFarland W, Sullivan P, Discepola M. Psychosocial correlates of unprotected anal sex in a cohort of gay men attending an HIV-negative support group. AIDS Educ Prev. 1998;10(4):317–26. [PubMed] [Google Scholar]
- 37.Elford J, Bolding G, Maguire M, Sherr L. Sexual risk behaviour among gay men in a relationship. AIDS. 1999;13(11):1407–11. doi: 10.1097/00002030-199907300-00019. [DOI] [PubMed] [Google Scholar]
- 38.Ekstrand ML, Coates TJ. Maintenance of safer sexual behavior and predictors of risky sex: the San Francisco Men’s Health Study. Am J Public Health. 1990;80(8):973–7. doi: 10.2105/ajph.80.8.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hays RB, Kegeles SM, Coates TJ. High HIV risk-taking among young gay men. AIDS. 1990;4(9):901–7. doi: 10.1097/00002030-199009000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Hays RB, Kegeles SM, Coates TJ. Unprotected sex and HIV risk taking among young gay men within boyfriend relationships. AIDS Educ Prev. 1997;9(4):314–29. [PubMed] [Google Scholar]
- 41.Hays RB, Paul J, Ekstrand M, Kegeles SM, Stall R, Coates TJ. Actual versus perceived HIV status, sexual behaviors and predictors of unprotected sex among young gay and bisexual men who identify as HIV-negative, HIV-positive and untested. AIDS. 1997;11(12):1495–502. doi: 10.1097/00002030-199712000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Peterson JL, Coates TJ, Catania JA, Middleton L, Hilliard B, Hearst N. High-risk sexual behavior and condom use among gay and bisexual African-American men. Am J Public Health. 1992;82(11):1490–4. doi: 10.2105/ajph.82.11.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Posner SF, Marks G. Prevalence of high-risk sex among HIV-positive gay and bisexual men: a longitudinal analysis. Am J Prev Med. 1996;12(6):472–7. [PubMed] [Google Scholar]
- 44.Strathdee SA, Hogg RS, Martindale SL, et al. Determinants of sexual risk-taking among young HIV-negative gay and bisexual men. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(1):61–6. doi: 10.1097/00042560-199809010-00010. [DOI] [PubMed] [Google Scholar]
- 45.Strathdee SA, Martindale SL, Cornelisse PG, et al. HIV infection and risk behaviours among young gay and bisexual men in Vancouver. CMAJ. 2000;162(1):21–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Wold C, Seage GR, Lenderking WR, et al. Unsafe sex in men who have sex with both men and women. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(4):361–7. doi: 10.1097/00042560-199804010-00011. [DOI] [PubMed] [Google Scholar]
- 47.Waldo CR, McFarland W, Katz MH, MacKellar D, Valleroy LA. Very young gay and bisexual men are at risk for HIV infection: the San Francisco Bay Area Young Men’s Survey II. J Acquir Immune Defic Syndr Hum Retrovirol. 2000;24(2):168–74. doi: 10.1097/00126334-200006010-00012. [DOI] [PubMed] [Google Scholar]
- 48.Sanders GD, Bayoumi AM, Sundaram V, et al. Cost effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:32–47. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 49.Brandeau ML, Owens DK, Sox CH, Wachter RM. Screening women of childbearing age for human immunodeficiency virus: a cost-benefit analysis. Arch Intern Med. 1992;152(11):2229–37. [PubMed] [Google Scholar]
- 50.de Vincenzi I. A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. European Study Group on Heterosexual Transmission of HIV. N Engl J Med. 1994;331(6):341–6. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 51.Downs AM, de Vincenzi I. Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual acts. J Acquir Immun Defic Syndr Hum Retrovirol. 1996;11:388–395. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Hisada M, O’Brien TR, Rosenberg PS, Goedert JJ. Virus load and risk of heterosexual transmission of human immunodeficiency virus and hepatitis C virus by men with hemophilia. The Multicenter Hemophilia Cohort Study. J Infect Dis. 2000;181(4):1475–8. doi: 10.1086/315396. [DOI] [PubMed] [Google Scholar]
- 53.Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of HIV: variability of infectivity throughout the course of infection. Am J Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 54.Padian N, Marquis L, Francis DP, et al. Male-to-female transmission of human immunodeficiency virus. J Amer Med Assoc. 1987;258(6):788–90. [PubMed] [Google Scholar]
- 55.Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997;146(4):350–7. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- 56.Padian NS, Shiboski SC, Jewell NP. Female-to-male transmission of human immunodeficiency virus. J Amer Med Assoc. 1991;266(12):1664–7. [PubMed] [Google Scholar]
- 57.Pedraza MA, del Romero J, Roldan F, et al. Heterosexual transmission of HIV-1 is associated with high plasma viral load levels and a positive viral isolation in the infected partner. J Acquir Immune Defic Syndr. 1999;21(2):120–5. [PubMed] [Google Scholar]
- 58.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 59.Royce RA, Sena A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–8. doi: 10.1056/NEJM199704103361507. [DOI] [PubMed] [Google Scholar]
- 60.Samuel MC, Mohr MS, Speed TP, Winkelstein W. Infectivity of HIV by anal and oral intercourse among homosexual men: estimates from a prospective study in San Francisco. In: Kaplan EH, Brandeau ML, editors. Modeling the AIDS epidemic: Planning, policy, and prediction. New York: Raven Press; 1994. [Google Scholar]
- 61.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29(3):275–83. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 62.Vittinghoff E, Douglas J, Judson F, et al. Per-contact risk of HIV transmission between male sexual partners. Am J Epidemiol. 1999;150:307–311. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 63.Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost effectiveness of methadone maintenance. Am J Public Health. 2000;90:1100–1111. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brandeau ML, Owens DK, Sox CH, Wachter RM. Screening women of childbearing age for human immunodeficiency virus: a model-based policy analysis. Manage Sci. 1993;39(1):72–92. [Google Scholar]
- 65.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 66.Sperling RS, Shapiro DE, Coombs RW, et al. Maternal viral load, zidovudine treatment, and the risk of transmission of human immunodeficiency virus type 1 from mother to infant. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1996;335(22):1621–9. doi: 10.1056/NEJM199611283352201. [DOI] [PubMed] [Google Scholar]
- 67.Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma human immunodeficiency virus type 1 RNA and the risk of perinatal transmission. Women and Infants Transmission Study Group. N Engl J Med. 1999;341(6):394–402. doi: 10.1056/NEJM199908053410602. [DOI] [PubMed] [Google Scholar]
- 68.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE1–4. [PubMed] [Google Scholar]
- 69.Binson D, Woods WJ, Pollack L, Sheon N. Bringing HIV/STI testing programmes to high-risk men. Int J STD AIDS. 2005;16(9):600–4. doi: 10.1258/0956462054944462. [DOI] [PubMed] [Google Scholar]
- 70.Manning SE, Thorpe LE, Ramaswamy C, et al. Estimation of HIV Prevalence, Risk Factors, and Testing Frequency among Sexually Active Men Who Have Sex with Men, Aged 18–64 Years-New York City, 2002. J Urban Health. 2007;84(2):212–25. doi: 10.1007/s11524-006-9135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roark RA, Webster RD, Darrow WW, Stempel RR. HIV testing among men who have sex with men: how often should one test? J Public Health Manag Pract. 2005;11(1):18–24. doi: 10.1097/00124784-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Ryder N, Bourne C, Rohrsheim R. Clinical audit: adherence to sexually transmitted infection screening guidelines for men who have sex with men. Int J STD AIDS. 2005;16(6):446–9. doi: 10.1258/0956462054093980. [DOI] [PubMed] [Google Scholar]
- 73.Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK Panel on Clinical Practices for Treatment of HIV. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137(5 Pt 2):381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 74.AVANTI Steering Committee. Analysis of HIV-1 clinical trials: statistical magic? Lancet. 1999;353(9169):2061–4. [PubMed] [Google Scholar]
- 75.Baxter JD, Mayers DL, Wentworth DN, et al. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. CPCRA 046 Study Team for the Terry Beirn Community Programs for Clinical Research on AIDS. AIDS. 2000;14(9):F83–93. doi: 10.1097/00002030-200006160-00001. [DOI] [PubMed] [Google Scholar]
- 76.Bonfanti P, Valsecchi L, Parazzini F, et al. Incidence of adverse reactions in HIV patients treated with protease inhibitors: a cohort study. Coordinamento Italiano Studio Allergia e Infezione da HIV (CISAI) Group. J Acquir Immune Defic Syndr. 2000;23(3):236–45. doi: 10.1097/00126334-200003010-00004. [DOI] [PubMed] [Google Scholar]
- 77.Butcher D, Greene J, Duong P, Markson L. Virologic response associated with a change in protease inhibitor therapy [letter] Arch Intern Med. 2000;160(3):394–5. doi: 10.1001/archinte.160.3.394-a. [DOI] [PubMed] [Google Scholar]
- 78.Carpenter CC, Cooper DA, Fischl MA, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. J Amer Med Assoc. 2000;283(3):381–90. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 79.Casado JL, Perez-Elias MJ, Antela A, et al. Predictors of long-term response to protease inhibitor therapy in a cohort of HIV-infected patients. AIDS. 1998;12(11):F131–5. doi: 10.1097/00002030-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 80.Cohen C, Hunt S, Sension M, et al. Phenotypic resistance testing significantly improves response to therapy (Tx): a randomized trial (VIRA 3001). 7th Conference on Retroviruses and Opportunistic Infections; 2000 January 30-February 2; San Francisco, CA. 2000. [Google Scholar]
- 81.Cohen Stuart JW, Schuurman R, Burger DM, et al. Randomized trial comparing saquinavir soft gelatin capsules versus indinavir as part of triple therapy (CHEESE study) AIDS. 1999;13(7):F53–8. doi: 10.1097/00002030-199905070-00001. [DOI] [PubMed] [Google Scholar]
- 82.d’Arminio Monforte A, Testa L, Adorni F, et al. Clinical outcome and predictive factors of failure of highly active antiretroviral therapy in antiretroviral-experienced patients in advanced stages of HIV-1 infection. AIDS. 1998;12(13):1631–7. doi: 10.1097/00002030-199813000-00010. [DOI] [PubMed] [Google Scholar]
- 83.Deeks SG, Hecht FM, Swanson M, et al. HIV RNA and CD4 cell count response to protease inhibitor therapy in an urban AIDS clinic: response to both initial and salvage therapy. AIDS. 1999;13(6):F35–43. doi: 10.1097/00002030-199904160-00001. [DOI] [PubMed] [Google Scholar]
- 84.Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet. 1999;353(9171):2195–9. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 85.Erb P, Battegay M, Zimmerli W, Rickenbach M, Egger M. Effect of antiretroviral therapy on viral load, CD4 cell count, and progression to acquired immunodeficiency syndrome in a community human immunodeficiency virus-infected cohort. Swiss HIV Cohort Study. Arch Intern Med. 2000;160(8):1134–40. doi: 10.1001/archinte.160.8.1134. [DOI] [PubMed] [Google Scholar]
- 86.Guardiola JM, Domingo P, Vazquez G. Switching HIV-1 protease inhibitor therapy: which? When? And why? [letter] Arch Intern Med. 1999;159(2):194–5. doi: 10.1001/archinte.159.2.194. [DOI] [PubMed] [Google Scholar]
- 87.Gulick RM, Mellors JW, Havlir D, et al. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection: 100-week follow-up. J Amer Med Assoc. 1998;280(1):35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 88.Hogg RS, Rhone SA, Yip B, et al. Antiviral effect of double and triple drug combinations amongst HIV- infected adults: lessons from the implementation of viral load-driven antiretroviral therapy. AIDS. 1998;12(3):279–84. doi: 10.1097/00002030-199803000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Kirk O, Katzenstein TL, Gerstoft J, et al. Combination therapy containing ritonavir plus saquinavir has superior short-term antiretroviral efficacy: a randomized trial. AIDS. 1999;13(1):F9–16. doi: 10.1097/00002030-199901140-00002. [DOI] [PubMed] [Google Scholar]
- 90.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131(2):81–7. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 91.Maher K, Klimas N, Fletcher MA, et al. Disease progression, adherence, and response to protease inhibitor therapy for HIV infection in an urban Veterans Affairs Medical Center. J Acquir Immune Defic Syndr. 1999;22(4):358–63. doi: 10.1097/00126334-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 92.Mocroft A, Gill MJ, Davidson W, Phillips AN. Predictors of a viral response and subsequent virological treatment failure in patients with HIV starting a protease inhibitor. AIDS. 1998;12(16):2161–7. doi: 10.1097/00002030-199816000-00011. [DOI] [PubMed] [Google Scholar]
- 93.Montaner JS, Reiss P, Cooper D, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study. J Amer Med Assoc. 1998;279(12):930–7. doi: 10.1001/jama.279.12.930. [DOI] [PubMed] [Google Scholar]
- 94.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents, January 28, 2000. HIV Clin Trials. 2001;1(1):60–110. doi: 10.1310/hct.2000.1.1.008. [DOI] [PubMed] [Google Scholar]
- 95.Raboud JM, Montaner JS, Conway B, et al. Suppression of plasma viral load below 20 copies/ml is required to achieve a long-term response to therapy. AIDS. 1998;12(13):1619–24. doi: 10.1097/00002030-199813000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Rhone SA, Hogg RS, Yip B, et al. The antiviral effect of ritonavir and saquinavir in combination amongst HIV-infected adults: results from a community-based study. AIDS. 1998;12(6):619–24. doi: 10.1097/00002030-199806000-00011. [DOI] [PubMed] [Google Scholar]
- 97.Shulman NS, Zolopa AR, Passaro DJ, et al. Efavirenz- and adefovir dipivoxil-based salvage therapy in highly treatment-experienced patients: clinical and genotypic predictors of virologic response. J Acquir Immune Defic Syndr. 2000;23(3):221–6. doi: 10.1097/00126334-200003010-00002. [DOI] [PubMed] [Google Scholar]
- 98.Valdez H, Lederman MM, Woolley I, et al. Human immunodeficiency virus 1 protease inhibitors in clinical practice: predictors of virological outcome. Arch Intern Med. 1999;159(15):1771–6. doi: 10.1001/archinte.159.15.1771. [DOI] [PubMed] [Google Scholar]
- 99.Zolopa AR, Shafer RW, Warford A, et al. HIV-1 genotypic resistance patterns predict response to saquinavir-ritonavir therapy in patients in whom previous protease inhibitor therapy had failed. Ann Intern Med. 1999;131(11):813–21. doi: 10.7326/0003-4819-131-11-199912070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellman PC. Clinical experience with adding delavirdine to combination therapy in patients in whom multiple antiretroviral treatment including protease inhibitors has failed. AIDS. 1998;12(11):1333–40. doi: 10.1097/00002030-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 101.Bernasconi E, Magenta L, Piffaretti JC, Carota A, Moccetti T. Are nelfinavir-containing regimens effective as second-line triple therapy? [letter] AIDS. 2000;14(1):95–6. doi: 10.1097/00002030-200001070-00015. [DOI] [PubMed] [Google Scholar]
- 102.Bonfanti P, Capetti A, Di Mattei P, Niero F, Rizzardini G. Virological treatment failure of highly active antiretroviral therapy in an unselected cohort of HIV-infected patients [letter] AIDS. 1998;12(9):1111. [PubMed] [Google Scholar]
- 103.Cameron DW, Japour AJ, Xu Y, et al. Ritonavir and saquinavir combination therapy for the treatment of HIV infection. AIDS. 1999;13(2):213–24. doi: 10.1097/00002030-199902040-00009. [DOI] [PubMed] [Google Scholar]
- 104.Clough LA, D’Agata E, Raffanti S, Haas DW. Factors that predict incomplete virological response to protease inhibitor-based antiretroviral therapy. Clin Infect Dis. 1999;29(1):75–81. doi: 10.1086/520185. discussion 82–4. [DOI] [PubMed] [Google Scholar]
- 105.De Wit S, Cassano P, Hermans P, et al. Salvage therapy with ritonavir-saquinavir plus two nucleoside reverse transcriptase inhibitors in patients failing with amprenavir-zidovudine- lamivudine [letter] AIDS. 1999;13(7):864–5. doi: 10.1097/00002030-199905070-00020. [DOI] [PubMed] [Google Scholar]
- 106.Gulick RM, Mellors JW, Havlir D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy [see comments] N Engl J Med. 1997;337(11):734–9. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 107.Hall CS, Raines CP, Barnett SH, Moore RD, Gallant JE. Efficacy of salvage therapy containing ritonavir and saquinavir after failure of single protease inhibitor-containing regimens. AIDS. 1999;13(10):1207–12. doi: 10.1097/00002030-199907090-00009. [DOI] [PubMed] [Google Scholar]
- 108.Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study [letter] Lancet. 1998;351(9104):723–4. doi: 10.1016/s0140-6736(98)24010-4. [DOI] [PubMed] [Google Scholar]
- 109.Kaufmann GR, Duncombe C, Cunningham P, et al. Treatment response and durability of a double protease inhibitor therapy with saquinavir and ritonavir in an observational cohort of HIV- 1-infected individuals. AIDS. 1998;12(13):1625–30. doi: 10.1097/00002030-199813000-00009. [DOI] [PubMed] [Google Scholar]
- 110.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active antiretroviral therapy in HIV-1 patients: a prospective cohort study. Swiss HIV Cohort Study. Lancet. 1999;353(9156):863–8. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 111.Moyle G, Pozniak A, Opravil M, et al. The SPICE study: 48-week activity of combinations of saquinavir soft gelatin and nelfinavir with and without nucleoside analogues. Study of Protease Inhibitor Combinations in Europe. J Acquir Immune Defic Syndr. 2000;23(2):128–37. doi: 10.1097/00126334-200002010-00004. [DOI] [PubMed] [Google Scholar]
- 112.Notermans DW, Jurriaans S, de Wolf F, et al. Decrease of HIV-1 RNA levels in lymphoid tissue and peripheral blood during treatment with ritonavir, lamivudine and zidovudine. Ritonavir/3TC/ZDV Study Group. AIDS. 1998;12(2):167–73. doi: 10.1097/00002030-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Paredes R, Mocroft A, Kirk O, et al. Predictors of virological success and ensuing failure in HIV-positive patients starting highly active antiretroviral therapy in Europe: results from the EuroSIDA study. Arch Intern Med. 2000;160(8):1123–32. doi: 10.1001/archinte.160.8.1123. [DOI] [PubMed] [Google Scholar]
- 114.Paredes R, Puig T, Arno A, et al. High-dose saquinavir plus ritonavir: long-term efficacy in HIV-positive protease inhibitor-experienced patients and predictors of virologic response. J Acquir Immune Defic Syndr. 1999;22(2):132–8. doi: 10.1097/00126334-199910010-00004. [DOI] [PubMed] [Google Scholar]
- 115.Powderly WG, Saag MS, Chapman S, Yu G, Quart B, Clendeninn NJ. Predictors of optimal virological response to potent antiretroviral therapy. AIDS. 1999;13(14):1873–80. doi: 10.1097/00002030-199910010-00009. [DOI] [PubMed] [Google Scholar]
- 116.Roca B, Gomez CJ, Arnedo A. Stavudine, lamivudine and indinavir in drug abusing and non-drug abusing HIV-infected patients: adherence, side effects and efficacy. J Infect. 1999;39(2):141–5. doi: 10.1016/s0163-4453(99)90006-3. [DOI] [PubMed] [Google Scholar]
- 117.Roca B, Gomez CJ, Arnedo A. A randomized, comparative study of lamivudine plus stavudine, with indinavir or nelfinavir, in treatment-experienced HIV-infected patients. AIDS. 2000;14(2):157–61. doi: 10.1097/00002030-200001280-00011. [DOI] [PubMed] [Google Scholar]
- 118.Salzberger B, Rockstroh J, Wieland U, et al. Clinical efficacy of protease inhibitor based antiretroviral combination therapy--a prospective cohort study. Eur J Med Res. 1999;4(11):449–55. [PubMed] [Google Scholar]
- 119.Staszewski S, Morales-Ramirez J, Tashima KT, et al. Efavirenz plus zidovudine and lamivudine, efavirenz plus indinavir, and indinavir plus zidovudine and lamivudine in the treatment of HIV-1 infection in adults. Study 006 Team. N Engl J Med. 1999;341(25):1865–73. doi: 10.1056/NEJM199912163412501. [DOI] [PubMed] [Google Scholar]
- 120.van Roon EN, Verzijl JM, Juttmann JR, Lenderink AW, Blans MJ, Egberts AC. Incidence of discontinuation of highly active antiretroviral combination therapy (HAART) and its determinants. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(3):290–4. doi: 10.1097/00042560-199903010-00012. [DOI] [PubMed] [Google Scholar]
- 121.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20(16):2051–64. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 122.Fatkenheuer G, Theisen A, Rockstroh J, et al. Virological treatment failure of protease inhibitor therapy in an unselected cohort of HIV-infected patients. AIDS. 1997;11(14):F113–6. doi: 10.1097/00002030-199714000-00001. [DOI] [PubMed] [Google Scholar]
- 123.Casado JL, Perez-Elias MJ, Antela A, et al. Risk factors for ritonavir intolerance and outcome after change to indinavir [letter] AIDS. 1998;12(3):335–6. [PubMed] [Google Scholar]
- 124.Svedhem V, Lindkvist A, Lidman K, Sonnerborg A. Persistence of earlier HIV-1 drug resistance mutations at new treatment failure. J Med Virol. 2002 Dec;68(4):473–8. doi: 10.1002/jmv.10246. [DOI] [PubMed] [Google Scholar]
- 125.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 126.Amico KR, Harman JJ, Johnson BT. Efficacy of antiretroviral therapy adherence interventions: a research synthesis of trials, 1996 to 2004. J Acquir Immune Defic Syndr. 2006;41(3):285–97. doi: 10.1097/01.qai.0000197870.99196.ea. [DOI] [PubMed] [Google Scholar]
- 127.Rueda S, Park-Wyllie LY, Bayoumi AM, et al. Patient support and education for promoting adherence to highly active antiretroviral therapy for HIV/AIDS. Cochrane Database of Systematic Reviews. 2006:3. doi: 10.1002/14651858.CD001442.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gross R, Bilker WB, Friedman HM, Strom BL. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–17. doi: 10.1097/00002030-200111090-00006. [DOI] [PubMed] [Google Scholar]
- 129.Weber R, Christen L, Christen S, et al. Effect of individual cognitive behaviour intervention on adherence to antiretroviral therapy: prospective randomized trial. Antivir Ther. 2004;9(1):85–95. [PubMed] [Google Scholar]
- 130.Longini IM, Clark WS, Byers RH, et al. Statistical analysis of the stages of HIV infection using a Markov model. Stat Med. 1989;8(7):831–43. doi: 10.1002/sim.4780080708. [DOI] [PubMed] [Google Scholar]
- 131.Bureau of Labor Statistics. National Compensation Survey. 2007 April 5;2007 [cited; Available from: http://data.bls.gov/PDQ/outside.jsp?survey=nc.
- 132.Schackman BR, Finkelstein R, Neukermans CP, Lewis L, Eldred L. The cost of HIV medication adherence support interventions: results of a cross-site evaluation. AIDS Care. 2005;17(8):927–37. doi: 10.1080/09540120500100635. [DOI] [PubMed] [Google Scholar]
- 133.Schackman BR, Gebo KA, Walensky RP, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44(11):990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 134.Berrien VM, Salazar JC, Reynolds E, McKay K HIV Medication Adherence Intervention Group. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18(6):355–63. doi: 10.1089/1087291041444078. [DOI] [PubMed] [Google Scholar]
- 135.Parsons JT, Rosof E, Punzalan JC, Di Maria L. Integration of motivational interviewing and cognitive behavioral therapy to improve HIV medication adherence and reduce substance use among HIV-positive men and women: results of a pilot project. AIDS Patient Care STDS. 2005;19(1):31–9. doi: 10.1089/apc.2005.19.31. [DOI] [PubMed] [Google Scholar]
- 136.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38(4):432–438. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 137.Mohammed H, Kieltyka L, Richardson-Alston G, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care STDS. 2004;18(5):289–96. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- 138.Zaric GS, Brandeau ML, Bayoumi AM, Owens DK. The effects of protease inhibitors on the spread of HIV and the development of drug-resistant HIV strains: a simulation study. Simulation. 1998;71:262–275. [Google Scholar]
- 139.Nagelkerke NJ, Jha P, de Vlas SJ, et al. Modelling HIV/AIDS epidemics in Botswana and India: impact of interventions to prevent transmission. Bull World Health Organ. 2002;80(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 140.United States Census Bureau. Statistical abstract of the United States. Washington, DC: US Bureau of the Census; 2002. [Google Scholar]
- 141.Phillips A. Short-term risk of AIDS according to current CD4 cell count and viral load in antiretroviral drug-naive individuals and those treated in the monotherapy era. AIDS. 2004;18(1):51–8. doi: 10.1097/00002030-200401020-00006. [DOI] [PubMed] [Google Scholar]
- 142.Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. J Amer Med Assoc. 2002;288(2):199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 143.Centers for Disease Control and Prevention. HIV incidence among young men who have sex with men--seven U.S. cities, 1994–2000. MMWR. 2001;50(21):440–4. [PubMed] [Google Scholar]
- 144.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. J Amer Med Assoc. 2004;292(2):224–36. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 145.Van de Ven P, Kippax S, Crawford J, et al. In a minority of gay men, sexual risk practice indicates strategic positioning for perceived risk reduction rather than unbridled sex. AIDS Care. 2002;14(4):471–80. doi: 10.1080/09540120208629666. [DOI] [PubMed] [Google Scholar]
- 146.Wolitski RJ, Rietmeijer CA, Goldbaum GM, Wilson RM. HIV serostatus disclosure among gay and bisexual men in four American cities: general patterns and relation to sexual practices. AIDS Care. 1998;10(5):599–610. doi: 10.1080/09540129848451. [DOI] [PubMed] [Google Scholar]
- 147.Ekstrand ML, Stall RD, Paul JP, Osmond DH, Coates TJ. Gay men report high rates of unprotected anal sex with partners of unknown or discordant HIV status. AIDS. 1999;13(12):1525–33. doi: 10.1097/00002030-199908200-00013. [DOI] [PubMed] [Google Scholar]
- 148.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16(16):2175–82. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 149.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–77. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 150.Bayoumi AM, Redelmeier DA. Economic methods for measuring the quality of life associated with HIV infection. Qual Life Res. 1999;8(6):471–80. doi: 10.1023/a:1008969512182. [DOI] [PubMed] [Google Scholar]
- 151.Revicki DA, Wu AW, Murray MI. Change in clinical status, health status, and health utility outcomes in HIV-infected patients. Medical Care. 1995;33(4 Suppl):AS173–82. [PubMed] [Google Scholar]
- 152.Tsevat J, Sherman SN, McElwee JA, et al. The will to live among HIV-infected patients. Ann Intern Med. 1999;131(3):194–8. doi: 10.7326/0003-4819-131-3-199908030-00006. [DOI] [PubMed] [Google Scholar]
- 153.Tsevat J, Solzan JG, Kuntz KM, et al. Health values of patients infected with human immunodeficiency virus. Relationship to mental health and physical functioning. Medical Care. 1996;34(1):44–57. doi: 10.1097/00005650-199601000-00004. [DOI] [PubMed] [Google Scholar]
- 154.Bozzette SA, Joyce G, McCaffrey DF, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344(11):817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 155.Elbeik T, Charlebois E, Nassos P, et al. Quantitative and cost comparison of ultrasensitive human immunodeficiency virus type 1 RNA viral load assays: Bayer bDNA quantiplex versions 3.0 and 2.0 and Roche PCR Amplicor monitor version 1.5. J Clin Microbiol. 2000;38(3):1113–20. doi: 10.1128/jcm.38.3.1113-1120.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holtgrave DR, Pinkerton SD, Merson M. Estimating the cost of unmet HIV-prevention needs in the United States. Am J Prev Med. 2002;23(1):7–12. doi: 10.1016/s0749-3797(02)00447-6. [DOI] [PubMed] [Google Scholar]
- 157.Chaix C, Grenier-Sennelier C, Clevenbergh P, et al. Economic evaluation of drug resistance genotyping for the adaptation of treatment in HIV-infected patients in the VIRADAPT study. J Acquir Immune Defic Syndr. 2000;24(3):227–31. doi: 10.1097/00126334-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 158.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 159.Bernard L, Vuagnat A, Peytavin G, et al. Relationship between levels of indinavir in hair and virologic response to highly active antiretroviral therapy. Ann Intern Med. 2002;137(8):656–9. doi: 10.7326/0003-4819-137-8-200210150-00009. [DOI] [PubMed] [Google Scholar]
- 160.Duong M, Piroth L, Peytavin G, et al. Value of patient self-report and plasma human immunodeficiency virus protease inhibitor level as markers of adherence to antiretroviral therapy: relationship to virologic response. Clin Infect Dis. 2001;33(3):386–92. doi: 10.1086/321876. [DOI] [PubMed] [Google Scholar]
- 161.Giron-Gonzalez JA, Lopez-Sanchez A, Elvira J, Perez E, Fernandez-Gutierrez C. Effect of patient adherence to antiretroviral therapy on CD4+ cell count, HIV-1 RNA, and serum concentrations of tumor necrosis factor and its soluble receptors. Eur J Clin Microbiol Infect Dis. 2000;19(11):852–8. doi: 10.1007/s100960000379. [DOI] [PubMed] [Google Scholar]
- 162.Moreno A, Perez-Elias MJ, Casado JL, et al. Effectiveness and pitfalls of initial highly active antiretroviral therapy in HIV-infected patients in routine clinical practice. Antivir Ther. 2000;5(4):243–8. [PubMed] [Google Scholar]