SUMMARY
Both H4K16 acetylation and H3K4 tri-methylation are required for gene activation. However, it is still largely unclear how these modifications are orchestrated by transcriptional factors. Here we analyzed the mechanism of the transcriptional activation by FOXP3, an X-linked suppressor of autoimmune diseases and cancers. FOXP3 binds near transcriptional start sites of its target genes. By recruiting MOF and displacing histone H3K4 demethylase PLU-1, FOXP3 increases both H4K16 acetylation and H3K4 tri-methylation at the FOXP3-associated chromatins of multiple FOXP3-activated genes. RNAi-mediated silencing of MOF reduced both gene activation and tumor suppression by FOXP3, while both somatic mutations in clinical cancer samples and targeted mutation of FOXP3 in mouse prostate epithelial disrupted nuclear localization of MOF. Our data demonstrate a pull-push model in which a single transcription factor orchestrates two epigenetic alterations necessary for gene activation and provide a mechanism for somatic inactivation of the FOXP3 protein function in cancer cells.
INTRODUCTION
FOXP3 was initially identified by severe autoimmune diseases associated with its mutations in mouse and human (Bennett et al., 2001; Brunkow et al., 2001; Chatila et al., 2000; Hori et al., 2003; Wildin et al., 2001), and has emerged as a key transcriptional regulator for the development and function of regulatory T cells (Treg) (Fontenot et al., 2003; Hori et al., 2003). Recently FOXP3 has emerged as an important X-linked tumor suppressor for breast and prostate cancers as it is somatically inactivated in both prostate and breast cancer samples (Liu et al., 2010; Wang et al., 2009a; Zuo et al., 2007b). A spontaneous germline mutation of Foxp3 in female mice resulted in significantly increased incidences of mammary carcinoma (Zuo et al., 2007b), while prostate-specific deletion of Foxp3 caused prostatic hyperplasia and prostatic intraepithelial neoplasm (Wang et al., 2009a). As a transcription factor, FOXP3 directly regulates transcription of important cancer-related genes such as ERBB2 (Her2/neu) (Zuo et al., 2007b), c-MYC (Wang et al., 2009a), SKP2 (Zuo et al., 2007a) and p21 (Liu et al., 2009). However, how FOXP3 regulates gene expression is largely unclear.
Dynamic histone modifications play a pivotal role in the regulation of gene transcription (Strahl and Allis, 2000). A subset of specific histone modification result in chromatin condensation (inactive state for transcription), while other subsets of histone modifications facilitate chromatin de-condensation (active state for transcription). Within the eukaryotic genome, actively transcribed euchromatin is marked with acetyl-H3 and -H4 and tri-methylation of H3K4 (H3K4me3), while transcriptionally inactive heterochromatin exhibits hypo-acetylation of H3 and H4 and tri-methylation of H3K27 (H3K27me3) and K9 (H3K9me3) and H4K20 (H4K20me3) (Lee and Workman, 2007; Martin and Zhang, 2005). Among lysine residues on histone H4, acetylation of H4K16 (H4K16ac) is thought to be a founder event of H4 acetylation and plays an important role in active transcription presumably by facilitating chromatin decondensation (Dion et al., 2005; Robinson et al., 2008; Shogren-Knaak et al., 2006). H3K4me3, occurring at transcription start sites (TSS), is also correlated with active transcription (Martin and Zhang, 2005; Wang et al., 2009b). These histone modifications are regulated by a variety of enzymes. MOF is a MYST family histone acetyltransferase and specifically acetylates histone H4K16 (Dou et al., 2005; Smith et al., 2005; Taipale et al., 2005). Likewise, methylation of H3K4 is positively regulated by Set-domain containing histone methyltransferases such as MLL/SET1 family members (MLL1-4 and hSET1) and negatively regulated by histone de-methylases such as JARID family members (e.g., PLU-1) and as yet unidentified enzymes (Martin and Zhang, 2005; Mosammaparast and Shi, 2010; Shi and Whetstine, 2007). MOF and MLL1 work in concert to activate HOX gene by facilitating both H4K16ac and H3K4me3 at the promoter (Dou et al., 2005). However, how these two enzymes are recruited to specific loci by transcription factors is largely unknown.
Dysregulations of histone modifications are important hall marks of cancer cells (Chi et al., 2010). Significant downregulations of H4K16ac and H4K20me3 in the global genome were observed in various cancers (Fraga et al., 2005). Multiple histone modification enzymes such as JARID1C, PLU-1, LSD1, SETD2, UTX, EZH2 and MOF are aberrantly expressed or somatically mutated in cancers (Dalgliesh et al., 2010; Duns et al., 2010; Kleer et al., 2003; Lu et al., 2010; Pfister et al., 2008; Yamane et al., 2007). Moreover, HDAC inhibitors have shown promising effects in cancer therapy (Minucci and Pelicci, 2006). These data suggest that improper histone modifications play an important role in the molecular pathogenesis of cancers.
Since FOXP3 changes various histone modifications including H3K27me3, H3K4me3 and acetylation of H3 and H4 at binding loci (Pan et al., 2009; Zheng et al., 2007), it is plausible that FOXP3 works in concert with histone modification enzymes to exert its function as a transcription factor. Supporting this hypothesis, recent studies revealed that FOXP3 interacts with histone acetyltransferase TIP60, HDACs and histone modifying complex EOS/CtBP1 on chromatin to repress its target genes (Li et al., 2007; Pan et al., 2009). While Foxp3 appears to increase H3K4me3 levels at its binding sites in T cells (Zheng et al., 2007), a general mechanism of FOXP3-mediated gene activation remains elusive. In breast cancer cells, FOXP3 increases H3 acetylation by removing HDAC2 and HDAC4 from its binding site at p21 locus (Liu et al., 2009).
We carried out chromatin-immunoprecipitation followed by next-generation sequencing (ChIP-seq) to identify FOXP3 binding sites in breast cancer cells. In combination with microarray analysis, we identified at least 845 direct targets. FOXP3-mediated gene activation correlated with both H4K16ac and H3K4me3. For multiple FOXP3 target genes, these crucial epigenetic modifications are simultaneously achieved by recruiting MOF and by displacing a H3K4 demethylase PLU-1. Our data suggest a pull-push model for gene activation by transcription factors.
RESULTS
Characterization of FOXP3 Binding Sites in Breast Cancer Cells
As source of chromatin for ChIP-Seq, we induced FOXP3 in MCF7 human breast cancer cell with FOXP3-tet-off system (Zuo et al., 2007b). The DNA precipitated by control-IgG was used as a control. Remarkably, FOXP3 binding was highly focused to regions less than 100 bp of TSS (Fig. 1A). We combined the ChIP-seq result with our previous gene expression analysis of FOXP3-induced MCF7 cells (MIAExpress; E-MTAB-73) (Liu et al., 2009). As analyzed in Supplemental Fig. S1, FOXP3 binding sites of putative direct targets were most frequently located within 1 kb of TSS and, when the accumulating events were considered, most of the FOXP3 binding sites reside within 2 kb of TSS. Therefore, we defined direct target genes according to the following criteria; (1) genes with FOXP3 binding within 2kb of their TSS and (2) genes whose mRNA expressions in FOXP3-induced cells were >150% or <66% of those in control cells (Liu et al., 2009). Among in total 4,067 genes which have FOXP3 binding sites within 2 kb of its TSS (Fig. 1B), we identified 845 direct target genes, in which 270 and 575 genes were repressed and activated by FOXP3, respectively (Fig. 1B and Supplemental Table S1 and S2). Importantly, FOXP3-binding sites of these direct targets were also highly enriched around their TSS, although the distribution is slightly broader than the total pool of FOXP3 bound genes (Fig. 1C). A known forkhead DNA motif was significantly enriched among the FOXP3 binding sites (Fig. 1D and Supplemental Table S3), which indicated the robustness of our ChIP-seq analysis. The specific interactions of FOXP3 and the effects on gene expression were validated by ChIP-qPCR and RT-qPCR (Fig. 1E and 1F), respectively. Gene ontology analysis revealed that FOXP3’s direct targets were significantly enriched for genes related to cancer biology, cell cycles, cell death, and cellular development (Fig. 1G). Since normal epithelial cells expressed less FOXP3 than what was used for the study, we test whether, at levels found in normal epithelial cells, FOXP3 also induced similar spectrum of gene activation. As shown in Supplemental Fig. S2, inducing FOXP3 at levels found in normal breast/prostate epithelial cells also caused broad activation of most of the genes identified when higher levels of FOXP3 was induced, although the magnitude of gene activation is less pronounced. Moreover H4K16ac- and H3K4me3-ChIP on the LYPD1 promoter clearly showed that these histone modifications were also affected by the physiological expression of FOXP3 (Fig. S2D). Furthermore, we evaluated the effects of shRNA silencing of FOXP3 by profiling mRNA expression of putative FOXP3 targets in MCF10A, an immortalized but non-tumorigenic breast epithelial cell line. As shown in Fig. S2E, 41–46% of the activated FOXP3 targets in MCF7 cells were significantly down-regulated by the FOXP3-knockdown in MCF10A cells. This is significantly higher than either unaffected or up-regulated genes (P<0.05). Thus, high proportions of FOXP3 targets identified by our FOXP3-tet-off MCF7 system are physiologically relevant.
Fig. 1. FOXP3-ChIP-seq analysis identified direct target genes of FOXP3 in MCF7 cell.
(A) Distribution of FOXP3-binding sites revealed by ChIP-seq in relation to TSS of genes. X-axis represents the distance between ChIP-peaks and TSSs of genes, Y-axis indicate the number of binding sites. (B) ChIP-seq identified 4,067 genes which were directly bound by FOXP3 between −2kbp and +2kbp from their TSSs in the MCF7 cell, among which 270 were down-regulated while 575 were up-regulated by FOXP3 (genes are listed in Supplemental Table S1 and S2). (C) Distribution of FOXP3-binding sites among direct targets of FOXP3 in relation to TSS. The activated genes are depicted in red, while the repressed genes are shown in green. (D) A known Fork Head DNA binding motif that was enriched among FOXP3-binding sites with a statistical significance (overrepresentation=2.17, Z-score=5.38, see Supplemental Table S3). (E) ChIP-qPCR of target genes of FOXP3 using MCF7 cells with and without FOXP3 induction. Means of triplicate qPCR reactions are shown and error bars represent +1SD. Y-axis represents % of input DNA. HPRT gene locus and 5′-upstream of HIG2 gene were used as negative controls. (F) mRNA levels of target genes of FOXP3. qRT-PCR was performed to examine mRNA expression levels of FOXP3’s target genes before and after FOXP3 induction. Error bars in E and F represent +1SD of triplicate qPCR. *: p<0.05 (t-test). (G) Functional categories of the direct target genes of FOXP3. Enrichments of functional categories among FOXP3’s direct target genes (listed in Supplemental Table S1 and S2) were calculated by Ingenuity Pathway Analysis software (http://www.ingenuity.com) and by comparing with those among randomly pooled human genes in the software. Y-axis represents –log(p-value)s and categories ranked within top-15 smallest p-values are listed. Red line: p=0.05. Numbers of genes in each category are indicated as inserts in the bars.
Other groups have performed global FOXP3-ChIP analyses using human or mouse Treg cells (Birzele et al., 2011; Marson et al., 2007; Sadlon et al., 2010; Zheng et al., 2007). We compared our ChIP-seq results with those from others (Fig. S3). When compared to human FOXP3-ChIP databases, 58.5% (494/845) of the FOXP3 targets genes in MCF7 cells overlapped with either or both of previous databases (Fig. S3A). This overlap is considerably greater than other pairwise comparisons. When compared to mouse Foxp3-ChIP databases, only 13.4% (113/845 genes) of our genes were overlapped with previous databases (Fig. S3B). However, human Treg FOXP3-ChIP and mouse Treg Foxp3-ChIP also showed only small overlaps (Fig. S3C). Likewise, <10% overlaps were observed in two reports of mouse FOXP3 targets (Fig. S3C).
FOXP3 Induces H4K16ac on both Activated and Repressed Target Genes by Recruiting MOF
In Tregs, a subset of histone modifications such as H3K27me3 and acetyl-H3 are known to be correlated with the FOXP3-binding (Pan et al., 2009; Zheng et al., 2007). We confirmed that these histone modifications were also affected by FOXP3 in MCF7 cells (Supplemental Fig. S4). Acetyl-H4 is an important histone mark for gene activation, and a previous study reported that FOXP3 directly or indirectly mediates acetylation of pan-histone H4 in T cells (Pan et al., 2009). Histone H4 has various lysine residues which can be substrates for acetylation and, among them, H4K16ac is a founder event of the H4 acetylation (Dion et al., 2005). In order to investigate whether or not the H4K16ac is correlated with FOXP3-mediated gene activation in MCF7 cells, we compared H4K16ac levels at FOXP3 binding sites before and after FOXP3 induction. Surprisingly, ChIP-qPCR at FOXP3 binding sites of randomly chosen 4 activated and 3 repressed promoters demonstrated broad inductions of H4K16ac by FOXP3 regardless of whether the genes were activated or repressed (Fig. 2A). To substantiate the broad correlation between FOXP3 binding and H4K16ac, we performed a confocal imaging analysis. As shown in Fig. 2B and 2C, FOXP3 and H4K16ac exhibited an almost complete overlap throughout the nuclei of the MCF7 cells (Specificity controls are provided in Supplemental Fig. S5). The complete overlap was observed in all cells analyzed (10/10, data not shown).
Fig. 2. H4K16ac is induced by FOXP3-binding.
(A) H4K16ac levels at FOXP3 binding sites were examined by ChIP-qPCR before and after FOXP3 induction in the FOXP3-tet-off MCF7 cell. Y-axis represents enrichments of the H4K16ac (% of input DNA). Error bars represent +1SD of triplicate qPCR. *: p<0.05 (t-test). n.s.: not significant. (B) A representative confocal image of FOXP3 (red) and H4K16ac (green) in the MCF7 cell transfected with FOXP3. White bars represent 5μm. Similar pattern was observed in 10/10 cells analyzed. (C) A representative signal intensity profile in the confocal image (Fig. 2B) is shown. Red, green and blue graphs indicate signal intensities of FOXP3, H4K16ac and DAPI, respectively.
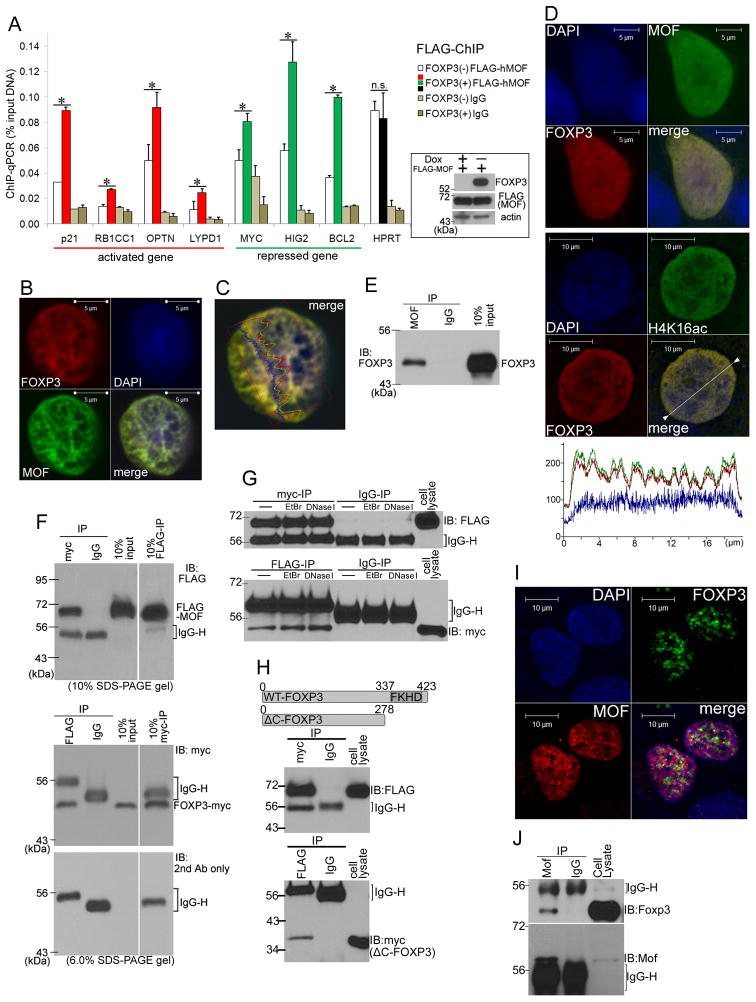
Since the histone acetyltransferase MOF is both necessary and sufficient for a great majority of H4K16ac in mammals (Li and Dou, 2010), the broad correlation between FOXP3-binding and H4K16ac suggested that FOXP3 may recruit MOF to its binding sites. To test this hypothesis, we performed anti-FLAG-ChIP before and after FOXP3 induction using FLAG-MOF transfected FOXP3-tet-off MCF7 cells. As shown in Fig. 3A, FOXP3 specifically recruited MOF onto FOXP3-binding sites. Confocal microscope analyses revealed virtual overlaps between FOXP3 and MOF throughout the nuclei in both MCF7 (Fig. 3B–C) and 293T cells (Data not shown). In order to confirm that the interaction between FOXP3 and MOF occurs on the chromatin, we performed immunofluorescent staining after in situ fractionation of the transfected cells. The procedure removed most of the cytoplasm, nuclear envelop and nucleoplasm, as judged by disappearance of γ-tubulin and Lamin B1, while retained chromatin based on the H3 staining (Supplemental Fig. S6). As shown in Fig. 3D, both MOF and H4K16ac overlapped with FOXP3, which confirmed the FOXP3-MOF interaction on the chromatin. We performed co-immunoprecipitation (co-IP) to determine if MOF and overexpressed FOXP3 associate with each other. As shown in Fig. 3E, an anti-MOF antibody targeting endogenous MOF brought down FOXP3 induced in the FOXP3-tet-off MCF7 cells. Furthermore, reciprocal co-IP, using 293T cells where FOXP3 and MOF were exogenously expressed, unequivocally demonstrated that Myc-tagged FOXP3 and Flag-tagged MOF physically interacted with each other (Fig. 3F).
Fig. 3. FOXP3 interacts with MOF at the chromatin of FOXP3 target genes.
(A) FLAG-MOF was expressed in FOXP3-tet-off MCF7 cells and ChIP-qPCR was performed using an anti-FLAG antibody. White and colored (red, green and black) bars represent [% of input DNA] before and after FOXP3 induction, respectively. Error bars represent +1SD of triplicate qPCR. *: p<0.05 (t-test). n.s.: not significant. Western blots of FOXP3 and MOF are shown as a right panel. DOX: doxycyline. (B) A representative confocal image of the MCF7 cell transfected with FOXP3 and MOF. FOXP3 (red) and MOF (green) were stained by an anti-FOXP3 and anti-MOF antibodies, respectively. Similar patterns were observed in all 10 cells analyzed. (C) A signal intensity profile of the FOXP3 and MOF in the MCF7 cell in Fig. 3B is shown. Red, green and blue graphs indicate signal intensities of FOXP3, MOF and DAPI, respectively. (D) Colocalization of MOF, H4K16ac and FOXP3 on chromatin as revealed by immunofluorescence after in situ subcellular fractionation. Controls are shown in Fig. S6. Signal intensity profile of the confocal image is shown as a bottom panel. Red, green and blue graphs indicate signal intensities of FOXP3, H4K16ac and DAPI, respectively. Similar patterns were observed in all 10 cells analyzed. (E) Co-IP targeting endogenous MOF and induced FOXP3 in the FOXP3-tet-off MCF7 cells. A co-IP was performed using an anti-MOF antibody. Precipitates were immunoblotted by an anti-FOXP3 antibody. Molecular weights are indicated to the left. (F) Upper Panel: FOXP3-myc/His and FLAG-MOF were expressed in 293T cells and co-IP with an anti-myc antibody was performed, and the precipitants were immunoblotted by an anti-FLAG antibody. IgG-H: heavy chains of IgG. Molecular weight is indicated at the left side. Lower Panels: Reciprocal co-IP with the anti-FLAG antibody was performed. Since the FOXP3-myc/His overlapped with IgG-H signals in a 10% SDS-PAGE gel, we used 6% SDS-PAGE gels in this experiment with a longer duration of electrophoresis. Immunoblots without primary myc-antibody was used to indicate the molecular sizes of IgG-H chains (lower panel). (G) Reciprocal co-IP was performed as in Fig. 3F with ethidium bromide (EtBr) or with DNase I treatment. EtBr: IP reactions were performed in an IP buffer containing EtBr (100μg/ml). DNase I: Before subjected to IP reaction, cell lysate was incubated with DNase I (10U/ml) for 30 minutes at room temperature. (H) FOXP3 lacking Forkhead domain (ΔC-FOXP3) and MOF were expressed in 293T cell and reciprocal co-IP was performed as in Fig. 3F. A diagram of ΔC-FOXP3 is shown in the upper panel. (I) A representative confocal image of U2OS sarcoma cells. Endogenous FOXP3 (green) and endogenous MOF (red) were stained by an anti-FOXP3 and anti-MOF antibodies, respectively. White bars indicate 10μm. Similar patterns were observed in at least 5 cells analyzed. (J) Endogenous interaction between Foxp3 and Mof in CD25-positive mouse T cell population as revealed by co-IP using anti-Foxp3 and anti-Mof antibodies.
We took two approaches to rule out the possibility that their interaction was due to their association with DNA. First, we tested if the interaction can be disrupted by either ethidium bromide or pretreatment with DNase I. As shown in Fig. 3G, neither treatment affected MOF-FOXP3 complex. Second, we evaluated if deletion of the forkhead DNA-binding domain prevented the FOXP3-MOF interaction. As shown in Fig. 3H, the deletion mutant co-precipitated with the MOF.
Since the above studies involve FOXP3-transfected cells, we sought to confirmed that the endogenous FOXP3 interact with endogenous MOF by both confocal microscopy and co-IP. As shown in Fig. 3I, essentially all FOXP3 (green dots) co-localized with MOF (red dots) in U2OS sarcoma cells. Perhaps because of excess of MOF, not all MOF was found to be associated with endogenous FOXP3 in tumor cells. Given the important function of FOXP3 in Treg, we used co-IP to determine if endogenous FOXP3 interact with MOF in Treg. As shown in Fig. 3J, anti-MOF precipitated FOXP3. We have not been able to use anti-FOXP3 mAb to co-precipitate MOF. However, it is of note that even in the over-expression system, anti-FOXP3 mAb failed to co-precipitate MOF (data not shown). We suspect that the mAb we used blocked the FOXP3-MOF interaction as the anti-Myc mAb precipitated both Myc-tagged FOXP3 and Flag-tagged MOF from the same lysates (Fig. 3F and 3H).
A Major Role of MOF in the FOXP3-Mediated Global Gene Expression and Tumor Suppressive Function
In order to investigate whether MOF is required for FOXP3-dependent gene activation, we treated FOXP3-tet-off MCF7 cells with RNAi duplexes targeting endogenous MOF or control RNAi duplex (Fig. 4A). In this model, induced FOXP3 expression was identical between control RNAi and MOF-RNAi treated cells both in protein and mRNA levels (Fig. 4A and 4C). Global mRNA expression analysis revealed that endogenous MOF knockdown impaired FOXP3-dependent gene activation in most, if not all, of the direct target genes (Fig. 4B). Approximately 41.0% of target genes showed more than 30% impairment of FOXP3-mediated gene activation by MOF-RNAi as compared to control-RNAi. In contrast, in FOXP3-mediated gene repression, the affected gene numbers and magnitudes of impairments by the MOF knockdown seemed to be considerably smaller (Fig. 4C). Thus, at global level, MOF plays a more important role in FOXP3-mediated gene activation than in gene repression. Using real-time PCR, we confirmed the impacts of MOF knockdown on the FOXP3-dependent transcriptional activations of 6 randomly chosen activated target genes (Fig. 4D).
Fig. 4. MOF plays an important role in the FOXP3-mediated gene activation and in the FOXP3-dependent cell growth repression.
(A) RNAi-mediated knockdown of endogenous MOF was performed in the FOXP3-tet-off MCF7 cells together with FOXP3 induction. Protein expression levels of MOF and FOXP3 were examined by western blots with anti-MOF and anti-FOXP3 antibodies. (B–C) Global mRNA expression analysis of direct target genes of FOXP3 with and without MOF knockdown. RNAi #2 was used in this analysis as the knockdown is more efficient. B: The heat map represents ratios of mRNA expressions before and after FOXP3 induction in control-RNAi and MOF-RNAi treated MCF7 cells (expression values in cells without FOXP3 induction were normalized to 1.0). Color scale of the heat map is indicated at the bottom of the Fig.. C: Bar graph represents log-scaled ratio of the relative mRNA expression between control-RNAi and MOF-RNAi groups. Dashed lines (red and green) represent log (ratio) = +0.5 (ratio=1.41) and = −0.5 (ratio=0.71). The “direct target genes” were defined as (1) direct binding of FOXP3 between −2 kbp and +2 kbp from TSS of genes revealed by ChIP-seq, and (2) mRNA expression values were increased to more than 1.5 times or decreased to less than 2/3 after FOXP3 induction in the control-RNAi treated MCF7 cells. Ctrl: control. (D) qRT-PCR was performed to examine mRNA levels of FOXP3’s target genes with and without MOF knock down. (E) 1.0×105 FOXP3-tet-off MCF7 cells were plated into 6-well plates and treated with either control- or MOF-RNAi. After 6 days of FOXP3 induction, cell numbers were counted. Microscopic pictures of the cells on the 6th day are shown as a lower panel. White bars represent 20μm. (F) Cell growth repression rates (comparisons of cell numbers between FOXP3(−) and FOXP3(+) cells) in control- and MOF-RNAi treated groups were calculated from the data of Fig. 4e. Error bars in E–F represent +1SD. P-values were calculated by t-test. *: p<0.05, **: p<0.005, ***: p<0.0005. n.s.: not significant.
Next, we sought to investigate whether MOF is also important for FOXP3-mediated biological consequences in epithelial cells, as well as for the FOXP3-mediated gene regulation. Since FOXP3 in cancer cells showed significant cell growth suppression (Liu et al., 2009; Wang et al., 2009a; Zuo et al., 2007b), we tested if MOF knockdown would attenuate the growth suppressive function of FOXP3 in cancer cells. Interestingly, in this assay, only MOF knockdown without FOXP3 induction showed significant suppression of cell growths in MCF7 cells (Fig. 4E), which is consistent with a recent report showing that Mof knockout significantly suppressed cell growth in mouse cells (Li et al., 2010). Despite such a pro-growth function of MOF, the growth suppression by induced FOXP3 was significantly impaired by the MOF knockdown (Fig. 4E and 4F), showing an important role of MOF in the tumor suppressive function of FOXP3.
Somatic Mutations of FOXP3 Disrupt FOXP3-MOF Co-Localization in the Nuclei and Attenuate Acetylation of H4K16
In order to further investigate how FOXP3 and MOF interact with each other, we generated deletion and point mutants of FOXP3 (Fig. 5A). MCF7 cells co-transfected with FLAG-MOF and FOXP3-myc/His mutants were examined by confocal microscopy. As shown in Fig. 5B, full length (FL) FOXP3 and N-terminal deleted (ΔN) FOXP3 co-localized with MOF and, correspondingly, with H4K16ac in nucleus. Therefore, the N-terminal portion of FOXP3 does not regulate MOF-FOXP3 co-localization. Despite the subtle differences in the relative amounts of cytoplasmic-nuclear FOXP3, deletion mutants of either zinc finger (ZF) or leucine zipper (LZ) domains of FOXP3 (ΔZF or ΔLZ) accumulated in both cytoplasm and nuclei. In addition, these deletions had two significant effects. First, translocation of MOF to the central nuclear region was significantly reduced, with significant amounts of MOF protein accumulating in the peripheral nuclear region and the cytoplasm. Second, although substantial portions of ΔZF-FOXP3 and ΔLZ-FOXP3 did reach the central nuclear regions, much of them did not co-localize with MOF or H4K16ac. As expected, deletion of the C-terminal portion (ΔC) resulted in predominant cytoplasmic accumulation of FOXP3. Nevertheless, the cytoplasmic portion did not associate with MOF.
Fig. 5. FOXP3 mutations abrogate the proper formation and function of FOXP3/MOF complex.
(A) A schematic view of the FOXP3-mutants used in this study. ZF, LZ and FKHD represent zinc finger, leucine zipper and forkhead domains, respectively. a.a.: amino acid positions. A red and a blue dashed line represent deleted regions in ΔZF- and ΔLZ-FOXP3, respectively. P202L and V239I were found in human breast cancers and G203R was found in human prostate cancer. GST-fusion proteins used in this study are also indicated at the bottom. (B–C) Representative confocal images. Deletion (B) and somatic mutation (C) series of FOXP3-myc/His together with FLAG-MOF were expressed in MCF7 cells, and cells were stained using anti-myc, -FLAG and -acetyl-H4K16 antibodies as indicated. MCF7 cells transfected only with FLAG-MOF are also shown. White bars represent scales of the objects. Similar patterns were observed in all 5 cells analyzed. (D) Full length (FL) or deletion mutant FOXP3s were transfected into MCF7 cells. After 1 week of drug selection, ChIP-qPCR was performed using an anti-acetyl-H4K16 antibody. Enrichments of H4K16ac in the vector control cell were normalized to 1.0. (E) MCF7 cells were transfected with either vector, full-length FOXP3 or deletion/mutant-FOXP3 as indicated. After 2 weeks of blasticidin selection, cells were visualized by crystal violet dye and colony numbers were counted. (F) MCF7 cells were transfected with either vector, WT-FOXP3 or mutant-FOXP3 as indicated. After 1 week of drug selection, RT-PCRs were performed. Y-axis represents % of GAPDH expression. Error bars in D-F represent +1SD. P-values were calculated by t-test. *: p<0.05. (G) GST-pull down assay was performed using GST-FOXP3-domains and His-MOF proteins as indicated. (H) Left panel: Beads conjugated with either GST, LZ-GST or mutLZ(V239I)-GST were incubated with FLAG-MOF transfected 293T cell lysate. Precipitates were subjected to an immunoblot using anti-FLAG antibody. Right panel: GST pull down assay using LZ-GST conjugated beads was performed incubating with and without a blocking peptide corresponding to amino acids 232–246 of FOXP3 (CLLQREMVQSLEQQL). (I) Immunohistochemical staining using an anti-Mof antibody was performed using mouse prostate tissues with and without prostate-specific knockout of Foxp3 (16 weeks old Foxp3fl/y;PB-Cre+ and Foxp3wt/y;PB-Cre+ mice, respectively). Mof staining with a blocking peptide is shown as a negative control. Foxp3 staining is shown in the inserts. Black bars represent 50μm.
Since the ZF and LZ domains of FOXP3 are hot-spots of somatic mutation in breast and prostate cancers (Wang et al., 2009a; Zuo et al., 2007b), we tested whether the somatic mutations in ZF or LZ domains affect the MOF-FOXP3 association. As shown in Fig. 5C, mutations in the ZF domain, P202>L (breast cancer) and G203>R (prostate cancer), exhibited similar phenotypes with the ΔZF-FOXP3. Likewise, a mutation in the LZ domain, V239>I (breast cancer), partially phenocopied the ΔLZ-FOXP3.
To further confirm the confocal microscope analysis, we performed an anti-H4K16ac-ChIP-qPCR. As shown in Fig. 5D, in cells expressing ΔZF- or ΔLZ-FOXP3s, induction of H4K16ac at FOXP3-associated chromatin were significantly reduced as compared to those in cells expressing FL-FOXP3. Correspondingly, these FOXP3 mutants were largely inactive in both growth inhibition (Fig. 5E) and induction of p21 (Fig. 5F). Importantly, GST-pull down assay revealed that GST-LZ but not GST-ZF interacted with MOF in vitro (Fig. 5G), indicating a potential direct binding between MOF and LZ domain of FOXP3. The interaction between FOXP3 and MOF was abrogated by V239I mutation identified in human breast cancer tissue and blocked by synthetic peptide corresponding to amino acids 232–246 (CLLQREMVQSLEQQL) of FOXP3 (Fig. 5H).
In order to investigate whether FOXP3 is important for the nuclear localization of MOF in vivo, we performed immunohistochemical staining of Mof using Foxp−/y or Foxp3+/y prostates from the Foxp3fl/yPB-Cre+ and Foxp3+/y PB-Cre+ mice (Wang et al., 2009a). As shown in Fig. 5I, in the prostate epithelial cells, significant amounts of Mof were detected in cytoplasm of the Foxp3fl/y;PB-Cre+ cells, while majority of Mof was accumulated in nucleus in WT counterparts. Taken together, our data presented in this section demonstrated that FOXP3 directly interacts with MOF and regulates nuclear localization of MOF in both normal and cancer cells.
Both H4K16ac and H3K4me3 are Required for the FOXP3-Mediated Gene Activation
Since H4K16ac levels were significantly increased at FOXP3 binding sites regardless of whether the genes were activated or repressed, additional modifications should be required to determine the fate of FOXP3 targets. Apart from H4K16ac, H3K4me3 is an important mark of actively transcribed loci (Martin and Zhang, 2005). Moreover, it is known that MOF and MLL1 work in concert to simultaneously modify H4K16ac and H3K4me3 at promoters (Dou et al., 2005). Therefore we tested whether H3K4me3 correlated with FOXP3-dependent gene activation in MCF7 cells by an anti-H3K4me3-ChIP-qPCR. As shown in Fig. 6A, FOXP3 increased H3K4me3 levels at activated binding sites, while no such impacts were observed at repressed binding sites. Consistent with the ChIP-qPCR, a confocal image showed that FOXP3 only partially overlapped with H3K4me3 (Fig. 6B).
Fig. 6. H3K4me3 is also associated with the FOXP3-mediated gene activation.
(A) H3K4me3 levels at FOXP3 binding sites were examined by ChIP-qPCR before and after FOXP3 induction in the MCF7 cell. Error bars represent +1SD of triplicate qPCR. *: p<0.05 (t-test). n.s.: not significant. (B) A representative confocal image of FOXP3 (red) and H3K4me3 (green) in the MCF7 cell transfected with FOXP3. White bars represent 5μm. Signal intensity profile of the confocal image is shown as a bottom panel. Red, green and blue graphs indicate signal intensities of FOXP3, H3K4me3 and DAPI, respectively. Similar pattern was observed in 10/10 cells analyzed. (C) ChIP-qPCRs targeting endogenous MLL1, endogenous RbBP5 and exogenously expressed FLAG-WDR5 were performed using anti-MLL1, anti-RbBP5 and anti-FLAG antibodies, respectively. White and colored (red, green and black) bars represent [% of input DNA] before and after FOXP3 induction, respectively. GAPDH or 5′HIG2 were used as negative controls. Error bars represent +1SD of triplicate qPCR. n.s.: not significant (p>0.05, t-test).
MOF (H4K16ac) and MLL1 complex (H3K4me3) work in concert to activate transcription (Dou et al., 2005). Therefore we tested if FOXP3 also recruits the MLL1 complex (i.e. MLL1, RbBP5 and WDR5) to its activated binding sites. As shown in Fig. 6C, ChIP-qPCR indicated that the MLL1-complex proteins were detected at FOXP3-associated chromatin regardless of the FOXP3 induction. In order to explain how FOXP3 induces H3K4me3 at its activating binding sites, we carried out a motif scanning of the FOXP3-bound regions and searched for any enriched DNA motifs among activating and repressing binding sites. Apart from the FOXP3-binding motif (forkhead motif), the most enriched motif in the activated binding sites was a PLU-1-binding motif (Fig. 7A). Interestingly, PLU-1 is a H3K4me3 demethylase and a putative oncogene for breast cancer (Yamane et al., 2007). Importantly, the enrichment of the PLU-1 motif was specific in FOXP3’s activating binding sites (Fig. 7A).
Fig. 7. FOXP3 facilitates H3K4me3 presumably by replacing histone demethylase(s) from its binding sites: a hypothetical model.
(A) Transcription factor binding motifs which were significantly enriched among FOXP3-binding sites at either activated or repressed gene promoters are listed. TOP-10 ranked motifs as sorted by overrepresentation scores were included. Statistical significances were evaluated by Z-score according to the database www.genomatix.de. (B) A known DNA binding motif of PLU-1. (C) Genomic regions around the FOXP3-ChIP-seq peaks were partitioned into 150 bp windows, and enrichments of the Fork Head (FOXP3) and PLU-1 motifs among each of these 150 bp partitions are evaluated by the overrepresentation scores as in Fig. 7A. The YY1 motif was used as an unrelated negative control. *: Fork Head motifs were not identified in these regions. (D) ChIP-qPCR was performed using an anti-PLU-1 antibody targeting endogenous PLU-1 before and after FOXP3 induction. Y-axis represents qPCR signals (% of input DNA). Error bars represent +1SD of triplicate qPCR. *: p<0.05 (t-test). n.s.: not significant. (*1) P-value was calculated by chi-square test. With Yates modification: p = 0.0058. Fisher exact test: p = 0.0024. (E) A proposed model by which FOXP3 activates multiple gene expression by pulling MOF and causing displacement of H3K4me demethylase(s).
PLU-1 has a known DNA-binding motif (Fig. 7B) (Scibetta et al., 2007). Strikingly, DNA binding motifs of FOXP3 and PLU-1 were enriched at essentially overlapping regions (Fig. 7C). This close proximity suggested a model in which FOXP3 binding may competitively displace PLU-1 from FOXP3-associated chromatin. To test this model, we evaluated whether FOXP3 binding reduced enrichments of PLU-1 at FOXP3 binding sites. Fifteen activated and 11 repressed genes were chosen according to the following criteria: (1) genes whose expression were strongly affected by FOXP3 (more than twice or less than half compared to FOXP3(−) cells) and (2) both of the FOXP3 and PLU-1 motifs were identified around ChIP-seq peaks (within 500 bp of the ChIP-seq peaks). As shown in Fig. 7D, induction of FOXP3 significantly reduced the binding of PLU-1 at 9 out of 15 activated promoters. FOXP3 and PLU-1 motifs were essentially overlapped at p21, YPEL2 and ITGB8 promoters and located closely at other promoters (Supplemental Fig. S7). Interestingly, the distance between FOXP3 and PLU-1 motifs is likely a major contributor for the displacements of PLU-1 caused by FOXP3, as all 9 genes that showed significant displacements of PLU-1 had FOXP3 motifs within 100 bp from the PLU-1 motif (Supplemental Fig. S8). However, proximity of the binding sites is not sufficient since displacements were not observed in 2 genes with the distance less than 100 bp (Supplemental Fig. S8). Importantly, no displacements of PLU-1 were observed among repressed genes (Fig. 7D). Since JARID1 family members may not always bind to chromatin in a DNA sequence-specific fashion, it is possible that, at the repressed promoters, PLU-1 binds to other chromatin-associated proteins and is not displaced when FOXP3 bound to the loci.
DISCUSSION
Our integrated analysis of FOXP3-binding sites and gene regulation presented herein identified 845 direct targets in a cancer cell. The most remarkable feature of the FOXP3 binding sites is their proximities to TSS. Such proximity strongly suggests a direct involvement of FOXP3 in the regulation of transcription. The precise identification of FOXP3 binding sites also allow us to define how FOXP3 coordinates local histone modifications (Jenuwein and Allis, 2001; Lee et al., 2010). We have demonstrated a broad requirement of MOF in FOXP3-mediated gene activation, while the impact of MOF knockdown on FOXP3-dependent gene repression was negligible. Surprisingly, FOXP3 induces local H4K16ac regardless of whether the genes are activated or repressed. The strong association between FOXP3 and H4K16ac is due to a direct interaction between FOXP3 and MOF. These data raise two intriguing issues. First, how can the H4K16ac be recognized as a part of repressive histone codes in FOXP3-mediated gene repression? While a definitive answer remains to be elucidated, it is of note that in embryonic stem cells, H3K4me3 and H3K27me3 can frequently be observed at the same loci in transcriptionally silenced genes (Mikkelsen et al., 2007; Pan et al., 2007; Vastenhouw et al., 2010). The authors proposed a bivalent histone code in which a mixture of activating (H3K4me3) and repressive (H3K27me3) histone codes could be read as a repressive code. Since repressed targets of FOXP3 exhibit significantly increased H3K27me3 levels, it is likely that H3K27me3 (repressive code) somehow overrides the H4K16ac (activating code) to cause gene silencing at FOXP3-associated chromatin. Second, what other histone modifications co-operate with H4K16ac to constitute activating histone codes for the activated target genes? Considering a previous report demonstrating that MLL1 complex co-operates with MOF in Hox gene activation (Dou et al., 2005), we tested whether FOXP3 also recruits MLL1 complex to activate target genes. In this case, although MLL1 complexes were found at FOXP3 target sites, their recruitments are FOXP3-independent. Of note, since FOXP3 recruited neither RbBP5 nor WDR5 to its binding sites, it is unlikely that other MLL/SET1 family methyltransferases are recruited by FOXP3. Steady state levels of H3K4me3 can be maintained by both H3K4 methyltransferases and H3K4 de-methylases (Shi et al., 2004; Shi and Whetstine, 2007; Yamane et al., 2007). Since our ChIP-qPCR revealed that FOXP3 binding sites are basically decorated with MLL1 even before FOXP3 inductions, it is intriguing that displacements of H3K4 demethylase(s) may explain how the H3K4me3 is induced by FOXP3. We identified closely located enrichments of FOXP3 and PLU-1 motifs specifically among activated targets. This selectivity prompted us to test and confirmed a hypothesis that FOXP3 cause displacement of PLU-1 from at least a major subset of activated FOXP3 binding regions. We demonstrated that FOXP3 significantly displaced PLU-1 at 9 out of 15 (60.0%) activated targets tested. It would be of interest to determine whether displacement of other H3K4me3 demethylases is responsible for activation of FOXP3 targets.
H3K4me3 recruits various downstream effectors to activate transcription of the loci (Chi et al., 2010; Levy and Gozani, 2010; Ruthenburg et al., 2007). For example, chromatin remodeling NURF complex, PHD finger containing ING family and human SAGA complexes can specifically recognize H3K4me3 (Chi et al., 2010; Levy and Gozani, 2010; Vermeulen et al., 2010). Among them, BPTF in NURF complex is of particular interest since BPTF can recognize both H3K4me3 and H4K16ac by its PHD and bromo domains, respectively (Kwon et al., 2009; Wysocka et al., 2006). A more recent study demonstrated that H3K4me3 and H4K16ac combination is selectively recognized by BPTF if they are present in the same mononucleosome (Ruthenburg et al., 2011). It is plausible that BPTF may be selectively recruited to the FOXP3-bound chromatins to initiate chromatin remodeling necessary for transcriptional activation.
Based on these considerations, we hereby propose a model by which FOXP3 activates multiple, although not necessarily all target genes (Fig. 7E). In essence, FOXP3 pulls MOF to its binding sites where the MOF induces H4K16ac. Perhaps by competitive DNA binding or other unknown mechanisms, FOXP3 caused displacement of H3K4 demethylase(s) from FOXP3 binding sites, and thereby facilitates H3K4me3 by MLL complex. FOXP3 may thus create a histone code (H4K16ac/H3K4me3) locally, perhaps within a nucleosome, which leads to active transcription, as recently reported by Ruthenburg et al. (Ruthenburg et al., 2011).
Our data provided here also explain a mechanism for functional inactivation of FOXP3 protein in cancer cell. We presented direct evidence that MOF is required not only for the FOXP3-mediated gene activation but also for the FOXP3-dependent cell growth suppression in cancer cells. Therefore, it is not surprising that this interaction is targeted during tumorigenesis, as demonstrated herein. Our data is consistent with previous reports that MOF expression is frequently down-regulated in tumors (Pfister et al., 2008) and that global H4K16ac levels are significantly reduced in breast cancer cells (Fraga et al., 2005). Paradoxically, it was recently reported that MOF promote the survival of mouse embryonic fibroblasts (Li et al., 2010). Likewise, our data also showed that, in the absence of FOXP3, silencing of MOF retarded the growth of MCF7 cells. Therefore, function of MOF in growth likely depends on activity of other tumor suppressors. Since our data showed that aberrant expression of FOXP3 affects nuclear localization of MOF, it is of interest to determine whether the documented defects of FOXP3 nuclear localization (Katoh et al., 2010; Ladoire et al., 2011; Wang et al., 2010) explains defective H4K16ac in clinical breast cancer samples (Fraga et al., 2005).
EXPERIMENTAL PROCEDURES
Cell Lines, Plasmids, and Bacterial Expression Vectors, Oligonucleotides
An MCF7 human breast cancer cell line with a FOXP3-tet-off system was described previously (Zuo et al., 2007b). 293T and MCF7 cells were cultured with DMEM supplemented with 10% FBS and P/S. FOXP3-myc/His, 3xFLAG -MOF and -WDR5 were cloned into pcDNA6 (Invitrogen, CA) and p3xFLAG-CMV-7.1 (Sigma Aldrich, MO) vectors, respectively. Deletion constructs of FOXP3 and mutant FOXP3s were cloned using GeneTailor Site-Directed Mutagenesis System (Invitrogen) using pcDNA6-FOXP3-myc/His as a template according to a manufacture’s protocol. Luecine Zipper and Zinc Finger domains of FOXP3 were cloned into pGEX-KG vector (ATCC, VA). DNA and RNAi Oligonucleotides used in this study are listed in Supplemental Table S4.
Antibodies
Commercial antibodies used in this study were as follows; mouse anti-FOXP3 (eBio7979, eBioscience, CA), rabbit anti-MOF (A300-992A, Bethyl Laboratories, TX), mouse anti-actin (ab3280, ABcam, MA), rabbit anti-acetyl-H4 at K16 (#07-329, Millipore, MA), rabbit anti-tri-methyl-H3 at K4 (#9727, Cell Signaling, MA), rabbit anti-acetyl-H3 (#9677, Cell Signaling), rabbit anti-tri-methyl-H3 at K27 (#07-449, Millipore), rabbit anti-RbBP5 (A300-109A, Bethyl Laboratories), rabbit anti-PLU-1 (ab50958, ABcam), mouse anti-FLAG (clone M2, Sigma Aldrich), mouse anti-myc (9E10, Convance, NJ), mouse anti-6xHis (34660, Qiagen, MA), goat anti-mouse IgG Alexa Fluor 568-conjugated (A11031, Invitrogen) and goat anti-rabbit IgG Alexa Fluor 488-conjugated (A11034, Invitrogen). For an immunofluorescent staining of U2OS cells, rabbit anti-FOXP3 (ab10563, ABcam) and mouse anti-PLU-1 (ab54276, ABcam) were used. Rabbit anti-MLL1c antibody was raised as described previously (Dou et al., 2005). Blocking peptide for the MOF antibody (BP300-992) was used for immunohistochemistry.
ChIP-sequence and data analysis
ChIP DNA libraries were modified for sequencing using ChIP-Seq Sample Prep Kit (Illumina, CA) according to the manufacturer’s protocol. ChIP-seq was performed using Illumina Genome Analyzer (Illumina) as described previously (Yu et al., 2010). Briefly, raw sequence image data were processed by Illumina analysis pipeline, aligned onto unmasked human genome (NCBI v36, hg18) using ELAND software (Illumina). Hpeak, a Hidden Markov Model (HMM)-based peak identifying algorithm (Qin et al., 2010), was used. Assuming the average size of ChIP-DNA fragments was 200 bp (range: 175–225 bp), we extended each of the 36 bp sequencing read to make a 200 bp hypothetical genomic DNA fragment (HDF). Under the null hypothesis of no enrichment in the FOXP3-ChIP treated sample versus IgG-ChIP control sample, the numbers of HDFs from the two libraries were assumed to follow the same distribution. The entire genome was partitioned into 25 bp windows and HMM was applied to define where FOXP3-enriched regions start and end. Assignments of ChIP-seq peaks with coding genes were defined as follows; (1) if a binding site locates in introns or exons of a gene, it was assigned to this gene, (2) if a site is between genes, it was assigned to the nearest downstream gene. Searching for transcription factor binding motifs was performed using MatInspector (Cartharius et al., 2005) as part of the Genomatix software suite (Genomatix Software GmbH, Munich, Germany) (www.genomatix.de). Overrepresentations of motifs in the FOXP3 ChIP-enriched peaks were evaluated against length-matched control sequences that were randomly selected from Human promoter genomic sequences.
Immunofluorescence after in situ subcellular fractionation
FOXP3-myc/His and FLAG-MOF were transfected into MCF7 cells. Before proceeding to fixations for immunofruorescent stainings, in situ subcellular fractionation was performed as described (Sawasdichai et al., 2010) with some modifications. Briefly, (1) Cytoplasmic fraction of the cells was removed by incubating culture slides on ice for 1min in a buffer containing 10mM PIPES, 300mM Sucrose, 100mM NaCl, 3mM MgCl2, 1mM EGTA, 0.1% Triton-X. (2) The culture slides were washed twice by PBS. (3) Nuclear fraction of the cells was removed by incubating the culture slides on ice for 20 min in a buffer containing 10mM PIPES, 300mM Sucrose, 100mM NaCl, 3mM MgCl2, 1mM EGTA, 0.5% Triton-X. (4) The culture slides were washed 3 times by PBS. (4) Immunofruorescent stainings were then performed.
Supplementary Material
Acknowledgments
H.K. is a recipient of a Postdoctoral Fellowship Award from Breast Cancer Research Program, Department of Defense. This work has been supported by National Institute of Health and Department of Defense, the United States of America. We are grateful to Dr. Jindan Yu for critical reading of the manuscript and Ms. Chun-Shu Wong for editorial assistance.
Footnotes
Supplemental Data including 8 Supplemental Fig.s and 4 Supplemental Tables are available online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- Birzele F, Fauti T, Stahl H, Lenter MC, Simon E, Knebel D, Weith A, Hildebrandt T, Mennerich D. Next-generation insights into regulatory T cells: expression profiling and FoxP3 occupancy in Human. Nucleic acids research. 2011 doi: 10.1093/nar/gkr444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics (Oxford, England) 2005;21:2933–2942. doi: 10.1093/bioinformatics/bti473. [DOI] [PubMed] [Google Scholar]
- Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. The Journal of clinical investigation. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Allis CD, Wang GG. Covalent histone modifications--miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010;10:457–469. doi: 10.1038/nrc2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgliesh GL, Furge K, Greenman C, Chen L, Bignell G, Butler A, Davies H, Edkins S, Hardy C, Latimer C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463:360–363. doi: 10.1038/nature08672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion MF, Altschuler SJ, Wu LF, Rando OJ. Genomic characterization reveals a simple histone H4 acetylation code. Proc Natl Acad Sci U S A. 2005;102:5501–5506. doi: 10.1073/pnas.0500136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y, Milne TA, Tackett AJ, Smith ER, Fukuda A, Wysocka J, Allis CD, Chait BT, Hess JL, Roeder RG. Physical association and coordinate function of the H3 K4 methyltransferase MLL1 and the H4 K16 acetyltransferase MOF. Cell. 2005;121:873–885. doi: 10.1016/j.cell.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H, Hofstra RM, Kok K. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer research. 2010;70:4287–4291. doi: 10.1158/0008-5472.CAN-10-0120. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nature genetics. 2005;37:391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Katoh H, Zheng P, Liu Y. Signalling through FOXP3 as an X-linked Tumor Suppressor. Int J Biochem Cell Biol. 2010;42:1784–1787. doi: 10.1016/j.biocel.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SY, Xiao H, Wu C, Badenhorst P. Alternative splicing of NURF301 generates distinct NURF chromatin remodeling complexes with altered modified histone binding specificities. PLoS Genet. 2009;5:e1000574. doi: 10.1371/journal.pgen.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- Lee JS, Smith E, Shilatifard A. The language of histone crosstalk. Cell. 2010;142:682–685. doi: 10.1016/j.cell.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8:284–295. doi: 10.1038/nrm2145. [DOI] [PubMed] [Google Scholar]
- Levy D, Gozani O. Decoding chromatin goes high tech. Cell. 2010;142:844–846. doi: 10.1016/j.cell.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, Basu S, Riley JL, Hancock WW, Shen Y, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci U S A. 2007;104:4571–4576. doi: 10.1073/pnas.0700298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Corsa CA, Pan PW, Wu L, Ferguson D, Yu X, Min J, Dou Y. MOF and H4 K16 acetylation play important roles in DNA damage repair by modulating recruitment of DNA damage repair protein Mdc1. Molecular and cellular biology. 2010;30:5335–5347. doi: 10.1128/MCB.00350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Dou Y. New perspectives for the regulation of acetyltransferase MOF. Epigenetics. 2010;5:185– 188. doi: 10.4161/epi.5.3.11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang L, Chen G, Katoh H, Chen C, Liu Y, Zheng P. FOXP3 up-regulates p21 expression by site-specific inhibition of histone deacetylase 2/histone deacetylase 4 association to the locus. Cancer research. 2009;69:2252–2259. doi: 10.1158/0008-5472.CAN-08-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang L, Zheng P. X-linked tumor suppressors: perplexing inheritance, a unique therapeutic opportunity. Trends Genet. 2010;26:260–265. doi: 10.1016/j.tig.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, Armaiz-Pena GN, Hu W, Stone RL, Munkarah A, et al. Regulation of tumor angiogenesis by EZH2. Cancer cell. 2010;18:185–197. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Zhang Y. The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol. 2005;6:838–849. doi: 10.1038/nrm1761. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, et al. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, Thomson JA. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister S, Rea S, Taipale M, Mendrzyk F, Straub B, Ittrich C, Thuerigen O, Sinn HP, Akhtar A, Lichter P. The histone acetyltransferase hMOF is frequently downregulated in primary breast carcinoma and medulloblastoma and constitutes a biomarker for clinical outcome in medulloblastoma. Int J Cancer. 2008;122:1207–1213. doi: 10.1002/ijc.23283. [DOI] [PubMed] [Google Scholar]
- Qin ZS, Yu J, Shen J, Maher CA, Hu M, Kalyana-Sundaram S, Yu J, Chinnaiyan AM. HPeak: an HMM-based algorithm for defining read-enriched regions in ChIP-Seq data. BMC bioinformatics. 2010;11:369. doi: 10.1186/1471-2105-11-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, An W, Routh A, Martino F, Chapman L, Roeder RG, Rhodes D. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. J Mol Biol. 2008;381:816–825. doi: 10.1016/j.jmb.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25:15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Ruthenburg AJ, Li H, Milne TA, Dewell S, McGinty RK, Yuen M, Ueberheide B, Dou Y, Muir TW, Patel DJ, et al. Recognition of a Mononucleosomal Histone Modification Pattern by BPTF via Multivalent Interactions. Cell. 2011;145:692–706. doi: 10.1016/j.cell.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlon TJ, Wilkinson BG, Pederson S, Brown CY, Bresatz S, Gargett T, Melville EL, Peng K, D’Andrea RJ, Glonek GG, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J Immunol. 2010;185:1071–1081. doi: 10.4049/jimmunol.1000082. [DOI] [PubMed] [Google Scholar]
- Sawasdichai A, Chen HT, Abdul Hamid N, Jayaraman PS, Gaston K. In situ subcellular fractionation of adherent and non-adherent mammalian cells. J Vis Exp. 2010:23. doi: 10.3791/1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scibetta AG, Santangelo S, Coleman J, Hall D, Chaplin T, Copier J, Catchpole S, Burchell J, Taylor-Papadimitriou J. Functional analysis of the transcription repressor PLU-1/JARID1B. Mol Cell Biol. 2007;27:7220–7235. doi: 10.1128/MCB.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311:844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- Smith CM, Gafken PR, Zhang Z, Gottschling DE, Smith JB, Smith DL. Mass spectrometric quantification of acetylation at specific lysines within the amino-terminal tail of histone H4. Anal Biochem. 2003;316:23–33. doi: 10.1016/s0003-2697(03)00032-0. [DOI] [PubMed] [Google Scholar]
- Smith ER, Cayrou C, Huang R, Lane WS, Cote J, Lucchesi JC. A human protein complex homologous to the Drosophila MSL complex is responsible for the majority of histone H4 acetylation at lysine 16. Molecular and cellular biology. 2005;25:9175–9188. doi: 10.1128/MCB.25.21.9175-9188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- Taipale M, Rea S, Richter K, Vilar A, Lichter P, Imhof A, Akhtar A. hMOF histone acetyltransferase is required for histone H4 lysine 16 acetylation in mammalian cells. Molecular and cellular biology. 2005;25:6798–6810. doi: 10.1128/MCB.25.15.6798-6810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastenhouw NL, Zhang Y, Woods IG, Imam F, Regev A, Liu XS, Rinn J, Schier AF. Chromatin signature of embryonic pluripotency is established during genome activation. Nature. 2010;464:922–926. doi: 10.1038/nature08866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M, Eberl HC, Matarese F, Marks H, Denissov S, Butter F, Lee KK, Olsen JV, Hyman AA, Stunnenberg HG, et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell. 2010;142:967–980. doi: 10.1016/j.cell.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, McNally B, Lin L, Zhou P, Zuo T, et al. Somatic Single Hits Inactivate the X-Linked Tumor Suppressor FOXP3 in the Prostate. Cancer Cell. 2009a;16:336–346. doi: 10.1016/j.ccr.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu R, Ribick M, Zheng P, Liu Y. FOXP3 as an X-linked tumor suppressor. Discov Med. 2010;10:322–328. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Zang C, Cui K, Schones DE, Barski A, Peng W, Zhao K. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009b;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, et al. A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling. Nature. 2006;442:86–90. doi: 10.1038/nature04815. [DOI] [PubMed] [Google Scholar]
- Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25:801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, Wang X, Wu L, Li J, Hu M, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443–454. doi: 10.1016/j.ccr.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, Zheng P. FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest. 2007a;117:3765–3773. doi: 10.1172/JCI32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, Liu Y, Wang Y, Liu X, Chan MW, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007b;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.