Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease of unclear etiology that affects mostly women of childbearing age. Profound abnormalities in both innate and adaptive immunity triggered by genetic and environmental factors are well documented to play an important part in the pathogenesis of SLE. Nonetheless, the role of neutrophils—the most abundant immune cell type—in the pathology of this disease has been unclear. Over the past decade, compelling evidence has emerged that implicates neutrophils in the initiation and perpetuation of SLE and also in the resultant organ damage frequently observed in patients with this disease. SLE-derived low-density granulocytes (LDGs) induce vascular damage and synthesize increased amounts of type I interferons and, as such, could play a prominent part in the pathogenesis of SLE. Furthermore, increased cell death and enhanced extracellular trap formation observed in SLE-derived neutrophils might have key roles in the induction of autoimmunity and the development of organ damage in patients with SLE. Together, these events could have significant deleterious effects and promote aberrant immune responses in this disease. This Review highlights the role of neutrophils in the pathogenesis of SLE, with a particular focus on the putative deleterious effects of LDGs and neutrophil extracellular trap formation.
Introduction
Systemic lupus erythematosus (SLE) is a multifactorial autoimmune disease of unclear etiology that affects multiple organs and afflicts mostly women of childbearing age. The development of SLE is attributed to disruptions in adaptive immunity, triggered by genetic predisposing factors and various environmental insults, which lead to the loss of tolerance of self-antigens. Indeed, the development and progression of SLE requires T lymphocytes and B lymphocytes, which highlights the key role of autoimmune reactivity in this disease.1,2
Evidence accrued over the past decade indicates that patients with SLE also have profound disruptions in innate immunity that could play a crucial part in the initiation and perpetuation of the disease, as well as in the development of organ damage to the kidneys, the vasculature, the skin and other tissues.3–6 Indeed, abnormalities in the phenotype and function of monocytes, macrophages, dendritic cells (DCs), and other cellular and humoral components of the innate immune system have been clearly identified in patients with SLE.3,7,8 These defects might be involved in key events in the pathogenesis of SLE, including regulation of cell death, presentation of putative autoantigens and synthesis of type I interferons (IFNs).9,10
Accumulating data support the concept that broad activation of the type I IFN pathway in SLE is associated with clinical manifestations and disease activity, and suggest that this pathway is important for disease pathogenesis.9,11,12 Although plasmacytoid DCs (pDCs) are the main source of IFN-α, they do not seem to account for all the increased type I IFN activity observed in individuals with SLE.7 As such, other cell subsets, primarily of myeloid lineage, have been proposed as important sources of these cytokines in humans with SLE and in mouse models of the disease.5,13 Over the past few years, type I IFNs have also been identified as important players in the development of accelerated atherosclerosis that occurs in patients with SLE.4,14
Although abnormalities in various immune cell subsets have been clearly described in SLE, the part played by neutrophils in this disease had not been well characterized. A potential role for neutrophils in the pathogenesis of SLE and the organ damage associated with this disease was described decades ago, including the description of the LE cell.15 Progress over the past decade indicates that neutrophils might indeed have been unfairly ignored and that they probably play an important part in the initiation and perpetuation of autoimmune responses in SLE. This Review discusses normal neutrophil biology and highlights the various abnormalities identified in granulocytes from patients with SLE, and how they could contribute to disease pathogenesis.
Overview of neutrophil biology
Normal neutrophil phenotype and function
Neutrophils are the most abundant leukocytes in the human body; however, they have a short lifespan and homeostasis is maintained by their continuous release from the bone marrow. As part of the innate immune system, neutrophils are a crucial component in the first line of defense against invading micro-organisms. The neutrophil-mediated inflammatory response is a multistep process, which involves the initial adhesion of circulating cells to the activated vascular endothelium, followed by their extravasation and migration towards inflammatory foci, and the ultimate in situ destruction of foreign micro-organisms.16 Elimination of microbes occurs through a number of processes that include phagocytosis, generation of reactive oxygen species (ROS) via the respiratory burst and the release of microbicidal substances from cytoplasmic granules (Table 1 lists the contents of cytoplasmic granules).17 In addition, a process characterized by the formation of neutrophil extracellular traps (NETs), termed `NETosis' (Box 1), is also involved in antimicrobial activity.18
Table 1.
Neutrophil antimicrobial peptides
| Peptide | Localization |
|---|---|
| Azurocidin | Primary granules Secretory vesicles |
| Bactericidal permeability increasing protein | Primary granules |
| Cathelicidin (LL-37) | Secondary granules NETs |
| Cathepsin G | Primary granules NETs |
| Elastase | Primary granules NETs |
| Histones | Nucleus NETs |
| HNPs | Primary granules NETs |
| Lactoferrin | Secondary granules |
| Lysozyme | Primary granules Secondary granules |
| Neutrophil gelatinase-associated lipocalin | Secondary granules |
| Peptidoglycan recognition proteins | Tertiary granules |
Abbreviations: HNPs, human neutrophil peptides; NETs, neutrophil extracellular traps.
Neutrophils contain various types of granules, enabling the sequential release of hundreds of constitutively expressed proteins into the extracellular environment, including proinflammatory mediators that have important effects on antigen-presenting cells (APCs) and induce DC maturation.19 Among the proteins included in primary (azurophilic) neutrophil granules are the alarmins, a class of molecules that activate APCs and trigger innate and adaptive immune responses.17 Neutrophil-derived alarmins include various antimicrobial peptides such as α-defensins, the cathelicidin human cationic antimicrobial protein 18 (hCAP18) and lactoferrin. Cathelicidin peptides such as LL-37, which is produced by proteolytic cleavage of the C-terminal antimicrobial domain of hCAP18, are chemotactic to various leukocytes. In conjunction with self-DNA release through NETosis, LL-37 also promotes activation of pDC, increasing their expression of co-stimulatory molecules and production of type I IFN.20 NETosis also results in the release of nuclear proteins which possess alarmin activity, such as high mobility group protein B1 (HMGB1). Other molecules released by neutrophils, including myeloperoxidase (MPO), neutrophil elastase and cathepsin G, also have important roles in the activation of innate immunity. Furthermore, neutrophils produce inflammatory cytokines and eicosanoids, regulate vascular permeability and can induce endothelial damage.21 In addition to microbial products, other stimuli (such as tissue deposition of immune complexes) can induce the respiratory burst, leading to enhanced inflammation and the recruitment of more neutrophils.22 Although not usually considered IFN-α-producing cells, mature neutrophils are capable of secreting this cytokine and other type I IFNs in response to certain stimuli, including granulocyte colony-stimulating factor (G-CSF),23 or via double-stranded RNA helicase signaling pathways.24 Moreover, neutrophils express Toll-like receptors (TLRs) 1–10, with the exception of TLR3, enabling them to initiate various potentially important immune responses upon recognition of pathogen-associated molecular patterns.
The action of neutrophils usually results in the successful sequestration and resolution of inflammatory lesions; however, during unchecked inflammation their recruitment and activation can lead to the development of disease states with considerable subsequent tissue damage. To limit potentially excessive inflammatory responses, neutrophils are characteristically short-lived and die in circulation within 4–10 hours. However, neutrophil lifespan can increase several-fold—to 1–2 days—in response to cytokines or other proinflammatory agents typically present in infected and inflamed tissue.19
SLE neutrophil phenotype and function
Qualitative abnormalities in various neutrophil functions have been reported in SLE. Serum from patients with SLE induces increased neutrophil aggregation, compared with serum from healthy donors, and interferes with phagocytosis and lysosomal enzyme release by normal neutrophils in vitro.25 An impaired phagocytic capacity of SLE-derived neutrophils is well established,26 and aberrant clearance of apoptotic material by phagocytes, including neutrophils, has been proposed to play a part in the pathogenesis of SLE.27 In addition, decreased responsiveness to cytokines, including IL-8, has been described in SLE-derived neutrophils,28 as has premature telomere shortening, which is suggestive of enhanced senescence.29
Evidence suggests that neutrophils in patients with SLE are activated intravascularly, overexpress various adhesion molecules, and display a tendency to form aggregates.25,30 Several autoantibodies, including anti-β2-glycoprotein I, are implicated in the activation of neutrophils.31 Furthermore, nucleosomes—considered major SLE autoantigens—have been described as putative activators and recruiters of neutrophils in SLE, through as yet unidentified pathways.32 Moreover, the levels of various proteins synthesized and released by activated neutrophils and/or their precursors are increased in the serum of patients with SLE. These factors include defensins, lactoferrin and other bactericidal proteins, and the observed increases in their levels usually correlate with disease activity33–35 and the presence of autoantibodies directed against them.36 However, the exact roles that these abnormalities have in disease pathogenesis and organ damage remain unclear.
Neutropenia in SLE
Increased levels of neutrophil apoptosis and aberrant clearance of apoptotic bodies have been reported in patients with SLE,37,38 and are associated with disease activity. Furthermore, the in vitro rate of neutrophil secondary necrosis is increased in SLE-derived samples.37 In keeping with the increased loss of these cells, neutropenia (a condition characterized by a low number of circulating neutrophils) is a feature of the disease in a considerable proportion of patients with SLE.39 These observations have led to speculation that an increased number of circulating apoptotic neutrophils might be particularly relevant to the pathogenesis of SLE, given that these cells are numerically greater and have a shorter lifespan than other blood cell types. As such, apoptotic neutrophils could represent an abundant source of antigenic material and so contribute to excess production of SLE-related autoantibodies, including those against doublestranded DNA (dsDNA) and nucleosomes.40
The mechanisms that drive the development of neutropenia in patients with SLE include neutrophil-reactive autoantibody-driven cell removal; neutralizing autoantibodies against growth factors that act on neutrophils such as G-CSF; bone marrow suppression; increased neutrophil apoptosis and secondary necrosis; and, possibly, death by NETosis.41 Enhanced apoptosis and abnormal clearance of apoptotic neutrophils are considered to occur via diverse mechanisms, including decreased expression of CD44,42 increased FAS (TNF receptor superfamily member 6) expression and the production of autoantibodies against dsDNA27 and the ribonucleoprotein La.43 Anti-neutrophil cytoplasmic antibodies (ANCAs) develop in patients with SLE;44 however, although their antigen specificities have been described, their contribution to neutropenia and pathogenesis of disease is unclear.45 ANCAs detected in the serum of a series of pediatric patients with SLE were found to be directed toward a number of neutrophil proteins, including MPO, lactoferrin, cathepsin G and neutrophil elastase. Antigen specificities did not, however, correlate with disease activity or with target-organ involvement.36 Specific autoantibodies against the 60 kDa SS-A/Ro ribonucleoprotein can bind to a cross-reactive epitope presented on a neutrophil membrane protein.46 Furthermore, circulating SLE-derived neutrophils often exhibit increased membrane immunoglobulin, as a result both of immune complex deposition and of the binding of anti-neutrophil antibodies. These findings, therefore, suggest that autoantibodies contribute to neutropenia in patients with SLE, which is in line with well-documented evidence for antibody-mediated peripheral destruction in other autoimmune neutropenias. Autoantibodies to myeloid precursors have been identified in patients with autoimmune neutropenia;47 this observation might also be a factor in SLE, as suppression of hematopoiesis by autoantibodies of the IgG subtype from patients with this disease has been reported using in vitro colony forming assays [Au: Insertion OK].48
The observation of neutropenia and enhanced neutrophil-induced tissue damage in patients with SLE is difficult to reconcile. One possibility is that a proportion of the neutrophils that infiltrate organs are actively undergoing NETosis or apoptosis, which could contribute to promotion of neutropenia but also to organ damage and immune dysregulation. However, this possibility remains to be further investigated.
Low-density granulocytes
Discovery and association with SLE
Three studies have reported the presence of an abnormal subset of neutrophils isolated from the peripheral blood of patients with SLE.5,6,49 These low-density granulocytes (LDGs) are present in preparations of peripheral blood mononuclear cells (PBMCs) derived from both adult and pediatric patients with SLE. PBMCs are isolated by density-gradient fractionation and do not usually contain large numbers of granulocytes. The presence of LDGs in SLE-derived mononuclear cell fractions was established by immunohistochemistry and microscopy, as well as through the identification of a granulocyte-specific gene expression signature identified in PBMCs collected from pediatric patients with SLE.6,49 This granulocyte signature was coincident with a type I IFN gene expression profile.6 Further investigation revealed that the highly granular cells in the PBMC preparations covered all stages of granulocyte development, including promyelocytes, myelocytes and meta-myelocytes, band cells, and segmented neutrophils.6
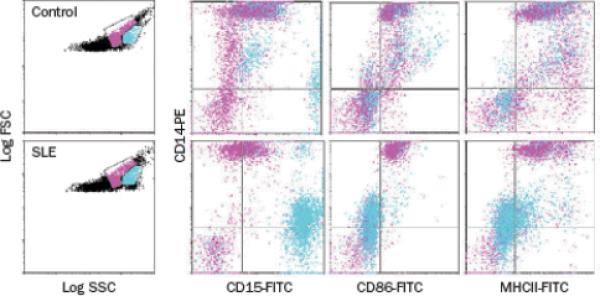
In 2010, my research group described the functional capacity and putative pathogenic role of LDGs isolated from adult patients with SLE, and explored their potential to contribute to clinical manifestations of this disease.5 The LDG population segregates directly adjacent to the monocyte pool by flow cytometric analysis using a dual-log scale of forward and side scatter intensities (Figure 1). LDGs are distinguished from monocytes on the basis of their high expression of the neutrophil marker CD15 and their low expression of CD14. Furthermore, SLE-derived LDGs express CD10 and CD16 but lack MHC class II and CD86 expression.5 All adult patients with SLE so far examined display LDGs in their PBMC fractions. Importantly, patients with high numbers of LDGs in peripheral blood have increased prevalence of skin involvement and vasculitis. By contrast, no appreciable associations have been found between age, disease duration, and/or use of immunosuppressive drugs and the presence of LDGs.5
Figure 1.
Identification of LDGs in SLE-derived PBMC fractions. PBMCs isolated from healthy controls (top panels) or patients with SLE (bottom panels) were stained for markers of the monocyte or granulocyte lineages and analyzed by flow cytometry. Gates that contained predominantly lymphocytes, monocytes, and granulocytes were established in dual-log scattergrams (left-hand panels). Granulocytes (blue) and monocytes (pink) are distinguished on the basis of CD14, CD15, CD86 and MHC class II expression. Monocytes express high levels of CD14 and are positive for CD86 and MHC class II, whereas CD15 is weak or absent. Granulocytes in the PBMC fraction express high levels of CD15, low levels of CD14 and are negative for CD86 and MHC class II. Abbreviations: FITC, fluorescein isothiocyanate; FSC, forward scatter; LDGs, low-density granulocytes; PBMCs, peripheral blood mononuclear cells; PE, phycoerythrin; SLE, systemic lupus erythematosus; SSC, side scatter. Permission to reproduce this figure was obtained from The American Association of Immunologists, Inc. © Denny, M. F. et al.J. Immunol. 184, 3284–3297 (2010).
Phenotype
LDGs express similar cell-surface markers to mature autologous or healthy control neutrophils; however, they differ from these cells in their nuclear morphology, which is consistent with an immature phenotype. LDGs present a proinflammatory phenotype characterized by augmented secretion of TNF as well as type I and type II IFNs upon stimulation, which could promote and increase tissue damage. In addition, LDGs have a considerably increased capacity to kill endothelial cells upon cell–cell contact,5 and display enhanced capacity to form NETs when compared with normal density SLE-derived neutrophils and control neutrophils.50 Given that LDGs are capable of synthesizing and secreting increased amounts of type I IFNs when compared with control neutrophils, they might account for the augmented type I IFN activity associated with SLE PBMC preparations.
Developmental origins
The developmental origin of LDGs and the cause of their production in patients with SLE remain to be characterized. On the basis of their phenotypic and functional properties, as well as their nuclear morphology, LDGs do not seem to represent a population of in vivo-activated and degranulated neutrophils. Indeed, in 2011 my group presented gene array and real-time PCR data that show LDGs express higher mRNA levels of various immunostimulatory bactericidal proteins and alarmins present in primary granules, relative to normal density SLE-derived and control neutrophils.50 These mRNAs include those encoding LL-37, neutrophil elastase, MPO and cathepsin G. Levels of mRNAs that encode neutrophil serine proteases are highest at the promyelocytic stage of differentiation in the bone marrow and are downregulated as these cells mature, which lends weight to the suggestion that LDGs possess a more immature phenotype than neutrophils. Interestingly, and in contrast to LDGs, gene profiles of adult SLE-derived normal density neutrophils did not differ from those of healthy controls.50 These results support the hypothesis that LDGs represent a phenotypically and functionally distinct subset of granulocytes.
Nakou et al.51 compared a gene array analysis of bone marrow from adult patients with SLE with samples obtained from healthy controls and found that granulopoiesis-related genes are predominantly upregulated in this disease. The genes shown to have increased expression include several of the `early granulopoiesis genes' that were also upregulated in LDG gene array studies.50 These observations are supported by a previous study, which showed that the PBMC granulocyte gene expression profiles observed in pediatric patients with SLE were consistent with those reported for the most immature granulocytes (myeloblast and promyelocytes) and coincided with the presence of immature neutrophils in peripheral blood samples.6 These findings further support that LDGs could represent an aberrant immature subset originating from the bone marrow that might persist or expand in the blood and/or other tissues in patients with SLE. Various cytokines abundant in SLE, including granulocyte macrophage colony-stimulating factor or type I IFNs, could enhance mobilization of neutrophil precursors from the bone marrow or hamper their differentiation into fully-matured granulocytes. However, the LDG microarray did not reveal evidence of increased expression of type I IFN-inducible genes,50 suggesting that LDGs do not represent a subset exposed to increased amounts of these cytokines. Flow cytometric and microscopic [Au: Insertion OK?] evidence suggests that LDGs are also present in the peripheral blood of patients with psoriasis.52 Consequently, future studies should assess whether LDGs are present in individuals with other autoimmune diseases and how they might contribute to disease pathogenesis and organ damage.
NETosis and SLE
NETs as a source of autoantigens
As mentioned above, one hallmark feature of SLE is the development of antibodies that recognize components of the cell nucleus.53,54 Implicit in the current model of SLE pathogenesis is that apoptotic debris is a predominant source of extracellular DNA, and that an underlying cause of the disease is related to an aberrant apoptotic process.55–57 However, some evidence indicates that extracellular DNA might frequently be present (and might even be necessary to aid clearance of micro-organisms) owing to the formation of NETs, which neutrophils produce in response to microbial infections.18
Considered a unique type of neutrophil cell death, NETosis is triggered by various stimuli and is characterized by active release of chromatin fibers containing antimicrobial peptides, which can trap and kill micro-organisms (Box 1).58 NETosis is achieved through translocation of neutrophil elastase into the nucleus from primary granules,59 where it partially degrades specific histones and—together with MPO,59 the autophagy machinery,60 and possibly ROS58,60—promotes chromatin decondensation and release of DNA from the cell. The antimicrobial effects of NETs are counteracted by the action of the DNA degrading enzyme, deoxyribonuclease 1 (DNase 1).61 Putative autoantigens are present within and attached to NET chromatin fibers, including citrullinated histones62 and various bactericidal molecules such as LL-37, neutrophil elastase and MPO, as well as dsDNA itself.59 In support of a role for this process in autoimmunity, neutrophils releasing NETs have been observed in kidney biopsies from patients with ANCA-positive vasculitis, and these NETs are enriched in MPO and LL-37.63 A link between NETosis and IL-17 release in skin and blood from patients with psoriasis has also been made, supporting their role in autoimmune disease.52 In addition, in vitro data indicate that NETs could directly harm endothelial cells and promote thrombosis,64,65 which might exacerbate organ damage.
Abnormal NETosis in the pathogenesis of SLE
New evidence from a number of sources suggests a potentially important role for aberrant NETosis or NET degradation in the pathogenesis of SLE. Impaired NET breakdown has been identified in a subset of patients with SLE and occurs owing to increased abundance of DNase 1 inhibitors, and production of anti-NET antibodies that prevent DNase 1 from accessing and degrading the NETs.66 In addition, increased NET formation has been documented in patients with SLE,50,67,68 and might contribute to development of autoimmunity. In keeping with this theory, Lande et al.68 reported that self-DNA in immune complexes present in sera from patients with SLE was associated with neutrophil antimicrobial peptides LL-37 and human neutrophil peptide. These peptides protected DNA from degradation by nucleases and, as such, stimulated self-DNA-induced triggering of TLR9 signaling in pDCs. Indeed, these DNA–antimicrobial peptide complexes led to enhanced IFN-α synthesis by pDCs. Furthermore, LL-37 autoantibodies were detected in sera from patients with SLE and promoted NETosis, suggesting that NETs might trigger B-cell activation and contribute to autoimmunity. Lande and colleagues68 proposed that increased release of antimicrobial peptides might be triggered by increased induction of NETosis upon priming with IFN-α and exposure to autoantibodies. As neutrophils were obtained from whole blood, these studies did not distinguish between LDGs and normal-density neutrophils.
Similar findings were reported by Garcia-Romo and colleagues67 in a cohort of pediatric patients with SLE, where mature SLE-derived neutrophils were primed in vivo by type I IFNs and died by NETosis upon exposure to SLE sera-derived anti-ribonucleoprotein antibodies. These NETs in turn activated pDCs to synthesize IFN-α in a DNA-dependent and TLR9-dependent manner.67
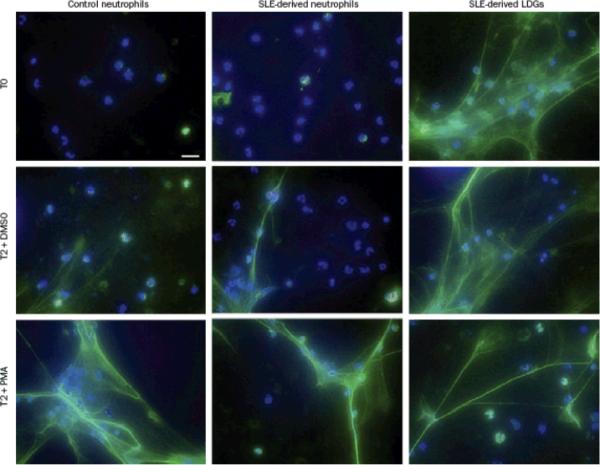
As mentioned above, my research group has reported that, compared with normal density neutrophils, SLE-derived LDGs have enhanced capacity to undergo NETosis (Figure 2). As such, the presence of these cells in patients with SLE might result in the exposure of more antigenic material, such as dsDNA and LL-37, as well as proinflammatory cytokines including IL-17. Furthermore, we reported that affected skin and kidneys from patients with SLE are infiltrated by neutrophils that are undergoing NETosis, which exposes these autoantigenic and proinflammatory factors at the tissue level.50 Indeed, NETosis in the skin and kidney of patients with SLE is associated with increased levels of serum anti-dsDNA antibodies, supporting the hypothesis that NETs could represent a source of nuclear material to which autoantibodies are elicited.50
Figure 2.
Circulating SLE-derived LDGs undergo increased NETosis. Representative images of control neutrophils, SLE-derived neutrophils and SLE-derived LDGs isolated from peripheral blood and analyzed at baseline (T0) or after 2 hours (T2) stimulation with DMSO or PMA. Panels show merged immunofluorescence images of NETs. Neutrophil elastase is shown in green. DNA was labeled with Hoechst 33342 and is shown in blue. Original magnification was ×40. Abbreviations: DMSO, dimethyl sulfoxide; LDGs, low-density granulocytes; NETs, neutrophil extracellular traps; PMA, phorbol 12-myristate 13-acetate; SLE, systemic lupus erythematosus. Permission to reproduce this figure was obtained from The American Association of Immunologists, Inc. © Villanueva, E. et al.J. Immunol. 187, 538–552 (2011).
The identification of neutrophils producing NETs in the skin in human cases of SLE is somewhat reminiscent to the findings reported by Guiducci et al.69 in a mouse model this disease, where skin injury led to leukocyte infiltration and activation, including production of IFN-α by pDCs and secretion of NETs by neutrophils.69 Therefore, these studies expand the potential pathogenic roles of aberrant neutrophils in SLE, and suggest that dysregulation of NET formation and/or degradation and subsequent responses have a prominent deleterious role in this disease (Figure 3).
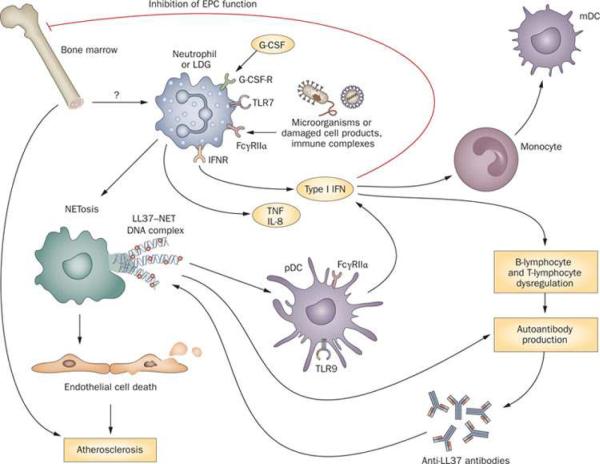
Figure 3.
Role of neutrophils and LDGs in the pathogenesis of SLE and associated organ damage. Upon exposure to micro-organisms, damaged cell products, immune complexes and other as yet unidentified stimuli, neutrophils and LDGs undergo NETosis. NETs externalize bactericidal immunostimulatory peptides such as LL-37, autoantigens including dsDNA, and inflammatory cytokines such as IL-17. LL-37-DNA complexes stimulate pDCs to synthesize IFN-α and might also promote B-cell stimulation and the development of antibodies against antimicrobial peptides. In addition, upon stimulation with G-CSF and/or via induction of dsRNA helicase signaling, neutrophils and LDGs display augmented synthesis of type I IFNs. Increased levels of type I IFNs promote differentiation of mDCs as well as dysregulation of B lymphocytes and T lymphocytes and synthesis of autoantibodies. LDGs also promote endothelial cell death through a NET-mediated effect. Furthermore, increased levels of type I IFNs have a detrimental effect on bone marrow EPCs and on circulating angiogenic cells, leading to aberrant vascular repair. As such, LDGs might have a dual effect on the vasculature by enhancing damage and inhibiting repair. Abbreviations: dsDNA, double stranded DNA; dsRNA, double stranded RNA; EPCs, endothelial progenitor cells; Fcγ RIIα, Low affinity immunoglobulin-γ Fc region receptor II-α, G-CSF, granulocyte-colony stimulating factor; G-CSF-R, G-CSF receptor; IFNR, interferon receptor; LDGs, low-density granulocytes; mDC, myeloid dendritic cell; NET, neutrophil extracellular trap; pDC, plasmacytoid dendritic cell; SLE, systemic lupus erythematosus; TLR, Toll-like receptor.
Future studies should assess the exact part that abnormal NET formation plays as an inducer or a contributor to autoimmunity. Furthermore, as enhanced NETosis occurs in other chronic inflammatory conditions such as ANCA-positive vasculitides and psoriasis,50,52,63 it will be important to examine whether this phenomenon is also seen in other systemic autoimmune diseases that are characterized by enhanced type I IFN synthesis.
Neutrophils and organ damage in SLE
Vascular damage
Patients with SLE have a strikingly higher risk of developing cardiovascular atherosclerotic complications compared with age-matched and gender-matched control individuals.70 We have proposed that accelerated vascular disease in SLE occurs owing to a strong imbalance between endothelial damage and vascular repair.4,71 Indeed, we and others have reported that type I IFNs have a crucial role in the aberrant vascular repair that occurs in SLE, through deleterious effects on endothelial progenitor cells (EPCs) and circulating myeloid angiogenic cells (CACs).4,72 LDGs seem to account for the increased type I IFN production that leads to abnormal EPC and/or CAC function in vitro and, potentially, in vivo in SLE. As such, depleting LDGs (but not pDCs) from SLE proangiogenic cultures restores the capacity of EPCs and/or CACs to differentiate into a mature endothelium.5 Furthermore, our findings suggest that LDGs induce marked endothelial cytotoxicity, at least in part, through a NET-mediated effect.5,50 Therefore, the possibility exists that this granulocyte subset has an important dual role in the induction of premature cardiovascular damage in patients with SLE, by promoting endothelial damage and inflammation while inhibiting vascular repair. This theory is supported by the finding that high numbers of LDGs correlate with vascular inflammation in patients with SLE.5
Lupus nephritis
Neutrophils are likely contributors to the pathogenesis of antibody-mediated lupus nephritis, particularly to acute flares of this condition, as confirmed by depletion studies and adoptive transfer experiments, in which this cell type is selectively removed from wild-type animals or reintroduced into deficient animals, respectively.73 Neutrophils are predominantly localized within the glomerular tuft in several types of glomerulonephritis,74 and various enzymes released by them, including neutrophil elastase, MPO and various cathepsins, can destroy glomerular structures.16,75,76 In addition, proinflammatory mediators elicit secretion of B-lymphocyte stimulator, which can be stored in activated neutrophils.77 The activity of neutrophils in lupus nephritis is highlighted by the suggested adoption of neutrophil gelatinase-associated lipocalin as a biomarker of this condition.78 Aberrant clearance of NETs in SLE is associated with the development of glomerulonephritis,66 and neutrophils producing NETs were identified in SLE-affected kidneys and correlated with levels of anti-dsDNA antibodies in these patients.50 Additional evidence for a putative role of neutrophils in SLE-associated glomerulonephritis comes from studies assessing the association of polymorphisms in neutrophil-related genes, such as the MPO G463A polymorphism and IL8 polymorphisms, with renal disease in African Americans with SLE.79,80
Skin disease
The exact part that neutrophils play in the development and severity of cutaneous involvement in SLE as well as in isolated cutaneous lupus erythematosus remains to be determined; however, various types of skin involvement in SLE are associated with neutrophil infiltration. Infiltration of neutrophils and neutrophilic debris beneath the basement membrane zone occurs in acute cutaneous lupus erythematosus.81 The neutrophilic component is most prominent in bullous lupus erythematosus (an autoantibody-mediated subepidermal blistering disease that occurs in patients with SLE); nonetheless, non-bullous neutrophilic lesions can develop as the presenting manifestation of cutaneous lupus erythematosus, although such lesions are rare. These non-bullous neutrophilic lesions include uncommon conditions such as neutrophilic urticarial dermatosis, palisaded neutrophilic granulomatous dermatitis and acute febrile neutrophilic dermatosis.82–85 Neutrophils have been proposed to act as key players in an autoinflammatory process triggered by the innate immune system in cutaneous lupus erythematosus.86 As described above, affected skin from patients with SLE is infiltrated by neutrophils that subsequently die by NETosis, which exposes LL-37, dsDNA and IL-17 at the tissue level. Furthermore, skin NETosis is associated with increased levels of serum anti-dsDNA.50 Similar findings have been reported in skin from lupus-prone mice.69 In addition, high numbers of LDGs in peripheral blood are associated with cutaneous manifestations in patients with SLE.5
Conclusions
The observations summarized in this Review indicate that patients with SLE display marked abnormalities in neutrophil phenotype and function, and enhanced neutrophil death through apoptosis and NETosis. Increased NETosis in SLE could be a key factor in the induction of autoimmunity, at least in part, through augmentation of type I IFN synthesis. Furthermore, NETosis could have pivotal roles in accelerated vascular disease in SLE: a hypothesis that requires additional investigation. Longitudinal studies will be necessary to assess whether the prevalence of NETosis, antibacterial protein autoantibodies and/or LDGs can be useful as biomarkers for or predictors of tissue damage in patients with SLE. In addition, the role of IL-17 externalization during NETosis in the pathogenesis of SLE requires further investigation. This cytokine might be important in disease pathogenesis and organ damage in SLE,87 and has a negative effect on vascular function in other diseases. The role of neutrophils and aberrant NETosis in the damage of organs other than the vasculature, skin and kidneys in SLE (for example, brain, lungs and joints) has not been systematically determined and certainly warrants further investigation. Future studies should also examine whether inhibition of aberrant NETosis or neutrophilic proteins implicated in the pathogenesis of SLE will lead to amelioration of this disease. Finally, research should be conducted to explore the possibility that LDGs are also present in individuals with other systemic autoimmune diseases where type I IFNs and endothelial damage might have an important pathogenic role in disease progression.
Key points.
Patients with systemic lupus erythematosus (SLE) display marked abnormalities in neutrophil phenotype and function, and enhanced neutrophil death through apoptosis and `NETosis'
A distinct subset of proinflammatory low-density granulocytes isolated from patients with SLE induces vascular damage, displays enhanced bactericidal gene signatures and synthesizes increased amounts of type I IFNs
Enhanced NETosis observed in SLE-derived neutrophils might have key roles in the induction of autoimmunity and the development of organ damage in SLE
Neutrophil dysfunction and increased NETosis might contribute to SLE pathology and disease manifestations, such as vascular complications, lupus nephritis and cutaneous lupus erythematosus
Box 1 | Neutrophil NETosis.
Upon encountering various stimuli—including micro-organisms, proinflammatory cytokines, and activated platelets or endothelial cells—neutrophils can undergo a specialized form of cell death termed NETosis. NETosis is driven by reactive oxygen species generation, as well as by the action of neutrophil granule components after their translocation to the cytoplasm and nucleus, promoting decondensation of chromatin and breakdown of the nuclear membrane. Expansion of the usually tightly packaged chromatin fibers eventually results in rupture of the cellular membranes and expulsion of a mesh-like structure termed a neutrophil extracellular trap (NET). These web-like NETs contain a backbone of DNA and nuclear histones as well as many granular antimicrobial peptides normally contained in the neutrophil granules and some cytoplasmic proteins, which can trap and promote the clearance of micro-organisms.
Acknowledgments
The writing of this manuscript was supported by the NIH through Public Health Service Grant HL-088,419.
Footnotes
Competing interests The author declares no competing interests.
References
- 1.Crispin JC, Kyttaris VC, Terhorst C, Tsokos GC. T cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 2010;6:317–325. doi: 10.1038/nrrheum.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorner T, Jacobi AM, Lee J, Lipsky PE. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. Immunol. Methods. 2011;363:187–197. doi: 10.1016/j.jim.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Denny MF, et al. Accelerated macrophage apoptosis induces autoantibody formation and organ damage in systemic lupus erythematosus. J. Immunol. 2006;176:2095–2104. doi: 10.4049/jimmunol.176.4.2095. [DOI] [PubMed] [Google Scholar]
- 4.Denny MF, et al. Interferon-α promotes abnormal vasculogenesis in lupus: a potential pathway for premature atherosclerosis. Blood. 2007;110:2907–2915. doi: 10.1182/blood-2007-05-089086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denny MF, et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J. Immunol. 2006;177:5878–5889. doi: 10.4049/jimmunol.177.9.5878. [DOI] [PubMed] [Google Scholar]
- 9.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MJ. Apoptosis in systemic lupus erythematosus. Clin. Immunol. 2004;112:210–218. doi: 10.1016/j.clim.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Hua J, Kirou K, Lee C, Crow MK. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006;54:1906–1916. doi: 10.1002/art.21890. [DOI] [PubMed] [Google Scholar]
- 12.Kariuki S, et al. Cutting edge: autoimmune disease risk variant of STAT4 confers increased sensitivity to IFN-α in lupus patients in vivo. J. Immunol. 2009;182:34–38. doi: 10.4049/jimmunol.182.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PY, et al. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan MJ, Salmon JE. How does interferon-α insult the vasculature? Let me count the ways. Arthritis Rheum. 2011;63:334–336. doi: 10.1002/art.30161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holman HR. The L. E. cell phenomenon. Annu. Rev. Med. 1960;11:231–242. doi: 10.1146/annurev.me.11.020160.001311. [DOI] [PubMed] [Google Scholar]
- 16.Henson PM. Pathologic mechanisms in neutrophil-mediated injury. Am. J. Pathol. 1972;68:593–612. [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi SD, DeLeo FR. Role of neutrophils in innate immunity: a systems biology-level approach. Wiley Interdiscip. Rev. Syst. Biol. Med. 2009;1:309–333. doi: 10.1002/wsbm.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 19.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 21.Murphy HS, Bakopoulos N, Dame MK, Varani J, Ward PA. Heterogeneity of vascular endothelial cells: differences in susceptibility to neutrophil-mediated injury. Microvasc. Res. 1998;56:203–211. doi: 10.1006/mvre.1998.2110. [DOI] [PubMed] [Google Scholar]
- 22.Marzocchi-Machado CM, et al. Fcgamma and complement receptors: expression, role and co-operation in mediating the oxidative burst and degranulation of neutrophils of Brazilian systemic lupus erythematosus patients. Lupus. 2002;11:240–248. doi: 10.1191/0961203302lu172oa. [DOI] [PubMed] [Google Scholar]
- 23.Shirafuji N, et al. Granulocyte colony-stimulating factor stimulates human mature neutrophilic granulocytes to produce interferon-α. Blood. 1990;75:17–19. [PubMed] [Google Scholar]
- 24.Tamassia N, et al. Activation of an immunoregulatory and antiviral gene expression program in poly(I:C)-transfected human neutrophils. J. Immunol. 2008;181:6563–6573. doi: 10.4049/jimmunol.181.9.6563. [DOI] [PubMed] [Google Scholar]
- 25.Abramson SB, Given WP, Edelson HS, Weissmann G. Neutrophil aggregation induced by sera from patients with active systemic lupus erythematosus. Arthritis Rheum. 1983;26:630–636. doi: 10.1002/art.1780260509. [DOI] [PubMed] [Google Scholar]
- 26.Brandt L, Hedberg H. Impaired phagocytosis by peripheral blood granulocytes in systemic lupus erythematosus. Scand. J. Haematol. 1969;6:348–353. doi: 10.1111/j.1600-0609.1969.tb02420.x. [DOI] [PubMed] [Google Scholar]
- 27.Courtney PA, et al. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann. Rheum. Dis. 1999;58:309–314. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsieh SC, et al. Abnormal in vitro CXCR2 modulation and defective cationic ion transporter expression on polymorphonuclear neutrophils responsible for hyporesponsiveness to IL-8 stimulation in patients with active systemic lupus erythematosus. Rheumatology (Oxford) 2008;47:150–157. doi: 10.1093/rheumatology/kem320. [DOI] [PubMed] [Google Scholar]
- 29.Wu CH, Hsieh SC, Li KJ, Lu MC, Yu CL. Premature telomere shortening in polymorphonuclear neutrophils from patients with systemic lupus erythematosus is related to the lupus disease activity. Lupus. 2007;16:265–272. doi: 10.1177/0961203307077155. [DOI] [PubMed] [Google Scholar]
- 30.Molad Y, Buyon J, Anderson DC, Abramson SB, Cronstein BN. Intravascular neutrophil activation in systemic lupus erythematosus (SLE): dissociation between increased expression of CD11b/CD18 and diminished expression of L-selectin on neutrophils from patients with active SLE. Clin. Immunol. Immunopathol. 1994;71:281–286. doi: 10.1006/clin.1994.1087. [DOI] [PubMed] [Google Scholar]
- 31.Arvieux J, Jacob MC, Roussel B, Bensa JC, Colomb MG. Neutrophil activation by anti-β2 glycoprotein I monoclonal antibodies via Fcγ receptor II. J. Leukoc. Biol. 1995;57:387–394. doi: 10.1002/jlb.57.3.387. [DOI] [PubMed] [Google Scholar]
- 32.Ronnefarth VM, et al. TLR2/TLR4-independent neutrophil activation and recruitment upon endocytosis of nucleosomes reveals a new pathway of innate immunity in systemic lupus erythematosus. J. Immunol. 2006;177:7740–7749. doi: 10.4049/jimmunol.177.11.7740. [DOI] [PubMed] [Google Scholar]
- 33.Sthoeger ZM, Bezalel S, Chapnik N, Asher I, Froy O. High α-defensin levels in patients with systemic lupus erythematosus. Immunology. 2009;127:116–122. doi: 10.1111/j.1365-2567.2008.02997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vordenbaumen S, et al. Elevated levels of human β-defensin 2 and human neutrophil peptides in systemic lupus erythematosus. Lupus. 2010;19:1648–1653. doi: 10.1177/0961203310377089. [DOI] [PubMed] [Google Scholar]
- 35.Ma CY, et al. Elevated plasma level of HMGB1 is associated with disease activity and combined alterations with IFN-α and TNF-α in systemic lupus erythematosus. Rheumatol. Int. doi: 10.1007/s00296-010-1636-6. http://dx.doi.org/10.1007/s00296-010-1636-6. [DOI] [PubMed]
- 36.Bakkaloglu A, et al. Antineutrophil cytoplasmic antibodies in childhood systemic lupus erythematosus. Clin. Rheumatol. 1998;17:265–267. doi: 10.1007/BF01451065. [DOI] [PubMed] [Google Scholar]
- 37.Ren Y, et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2888–2897. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 38.Donnelly S, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum. 2006;54:1543–1556. doi: 10.1002/art.21783. [DOI] [PubMed] [Google Scholar]
- 39.Budman DR, Steinberg AD. Hematologic aspects of systemic lupus erythematosus. Current concepts. Ann. Intern. Med. 1977;86:220–229. doi: 10.7326/0003-4819-86-2-220. [DOI] [PubMed] [Google Scholar]
- 40.Kramers C, et al. Anti-nucleosome antibodies complexed to nucleosomal antigens show anti-DNA reactivity and bind to rat glomerular basement membrane in vivo. J. Clin. Invest. 1994;94:568–577. doi: 10.1172/JCI117371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arenas M, Abad A, Valverde V, Ferriz P, Pascual R. Selective inhibition of granulopoiesis with severe neutropenia in systemic lupus erythematosus. Arthritis Rheum. 1992;35:979–980. doi: 10.1002/art.1780350821. [DOI] [PubMed] [Google Scholar]
- 42.Cairns AP, Crockard AD, McConnell JR, Courtney PA, Bell AL. Reduced expression of CD44 on monocytes and neutrophils in systemic lupus erythematosus: relations with apoptotic neutrophils and disease activity. Ann. Rheum. Dis. 2001;60:950–955. doi: 10.1136/ard.60.10.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh SC, et al. Anti-SSB/La is one of the antineutrophil autoantibodies responsible for neutropenia and functional impairment of polymorphonuclear neutrophils in patients with systemic lupus erythematosus. Clin. Exp. Immunol. 2003;131:506–516. doi: 10.1046/j.1365-2249.2003.02084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nassberger L, Sjoholm AG, Jonsson H, Sturfelt G, Akesson A. Autoantibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clin. Exp. Immunol. 1990;81:380–383. doi: 10.1111/j.1365-2249.1990.tb05342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galeazzi M, et al. Anti-neutrophil cytoplasmic antibodies in 566 European patients with systemic lupus erythematosus: prevalence, clinical associations and correlation with other autoantibodies. European Concerted Action on the Immunogenetics of SLE. Clin. Exp. Rheumatol. 1998;16:541–546. [PubMed] [Google Scholar]
- 46.Kurien BT, Newland J, Paczkowski C, Moore KL, Scofield RH. Association of neutropenia in systemic lupus erythematosus (SLE) with anti-Ro and binding of an immunologically cross-reactive neutrophil membrane antigen. Clin. Exp. Immunol. 2000;120:209–217. doi: 10.1046/j.1365-2249.2000.01195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartman KR, et al. Antibodies to myeloid precursor cells in autoimmune neutropenia. Blood. 1994;84:625–631. [PubMed] [Google Scholar]
- 48.Liu H, et al. Suppression of haematopoiesis by IgG autoantibodies from patients with systemic lupus erythematosus (SLE) Clin. Exp. Immunol. 1995;100:480–485. doi: 10.1111/j.1365-2249.1995.tb03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hacbarth E, Kajdacsy-Balla A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986;29:1334–1342. doi: 10.1002/art.1780291105. [DOI] [PubMed] [Google Scholar]
- 50.Villanueva E, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakou M, et al. Gene expression in systemic lupus erythematosus: bone marrow analysis differentiates active from inactive disease and reveals apoptosis and granulopoiesis signatures. Arthritis Rheum. 2008;58:3541–3549. doi: 10.1002/art.23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin AM, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J. Exp. Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruns A, Blass S, Hausdorf G, Burmester GR, Hiepe F. Nucleosomes are major T and B cell autoantigens in systemic lupus erythematosus. Arthritis Rheum. 2000;43:2307–2315. doi: 10.1002/1529-0131(200010)43:10<2307::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 55.Amoura Z, et al. Nucleosome-restricted antibodies are detected before anti-dsDNA and/or antihistone antibodies in serum of MRL-Mp lpr/lpr and +/+ mice, and are present in kidney eluates of lupus mice with proteinuria. Arthritis Rheum. 1994;37:1684–1688. doi: 10.1002/art.1780371118. [DOI] [PubMed] [Google Scholar]
- 56.Licht R, van Bruggen MC, Oppers-Walgreen B, Rijke TP, Berden JH. Plasma levels of nucleosomes and nucleosome-autoantibody complexes in murine lupus: effects of disease progression and lipopolyssacharide administration. Arthritis Rheum. 2001;44:1320–1330. doi: 10.1002/1529-0131(200106)44:6<1320::AID-ART224>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 57.McHugh NJ. Systemic lupus erythematosus and dysregulated apoptosis-what is the evidence? Rheumatology (Oxford) 2002;41:242–245. doi: 10.1093/rheumatology/41.3.242. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs TA, et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Remijsen Q, et al. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 2011;21:290–304. doi: 10.1038/cr.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buchanan JT, et al. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 62.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 63.Kessenbrock K, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuchs TA, et al. Extracellular DNA traps promote thrombosis. Proc. Natl Acad. Sci. USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta AK, et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 66.Hakkim A, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl Acad. Sci. USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–346. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 71.Rajagopalan S, et al. Endothelial cell apoptosis in systemic lupus erythematosus: a common pathway for abnormal vascular function and thrombosis propensity. Blood. 2004;103:3677–3683. doi: 10.1182/blood-2003-09-3198. [DOI] [PubMed] [Google Scholar]
- 72.Lee P, et al. Type I interferon as a novel risk factor for endothelial progenitor cell depletion and endothelial dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3759–3769. doi: 10.1002/art.23035. [DOI] [PubMed] [Google Scholar]
- 73.Cochrane CG, Unanue ER, Dixon FJ. A role of polymorphonuclear leukocytes and complement in nephrotoxic nephritis. J. Exp. Med. 1965;122:99–116. doi: 10.1084/jem.122.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hotta O, et al. Role of neutrophil elastase in the development of renal necrotizing vasculitis. Clin. Nephrol. 1996;45:211–216. [PubMed] [Google Scholar]
- 75.Camussi G, et al. The polymorphonuclear neutrophil (PMN) immunohistological technique: detection of immune complexes bound to the PMN membrane in acute poststreptococcal and lupus nephritis. Clin. Nephrol. 1980;14:280–287. [PubMed] [Google Scholar]
- 76.Johnson RJ, et al. The human neutrophil serine proteinases, elastase and cathepsin G, can mediate glomerular injury in vivo. J. Exp. Med. 1988;168:1169–1174. doi: 10.1084/jem.168.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scapini P, et al. Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood. 2005;105:830–837. doi: 10.1182/blood-2004-02-0564. [DOI] [PubMed] [Google Scholar]
- 78.Hinze CH, et al. Neutrophil gelatinase-associated lipocalin is a predictor of the course of global and renal childhood-onset systemic lupus erythematosus disease activity. Arthritis Rheum. 2009;60:2772–2781. doi: 10.1002/art.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouali H, et al. Association of the G-463A myeloperoxidase gene polymorphism with renal disease in African Americans with systemic lupus erythematosus. J. Rheumatol. 2007;34:2028–2034. [PMC free article] [PubMed] [Google Scholar]
- 80.Rovin BH, Lu L, Zhang X. A novel interleukin-8 polymorphism is associated with severe systemic lupus erythematosus nephritis. Kidney Int. 2002;62:261–265. doi: 10.1046/j.1523-1755.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- 81.Obermoser G, Sontheimer RD, Zelger B. Overview of common, rare and atypical manifestations of cutaneous lupus erythematosus and histopathological correlates. Lupus. 2010;19:1050–1070. doi: 10.1177/0961203310370048. [DOI] [PubMed] [Google Scholar]
- 82.Kieffer C, Cribier B, Lipsker D. Neutrophilic urticarial dermatosis: a variant of neutrophilic urticaria strongly associated with systemic disease. Report of 9 new cases and review of the literature. Medicine (Baltimore) 2009;88:23–31. doi: 10.1097/MD.0b013e3181943f5e. [DOI] [PubMed] [Google Scholar]
- 83.Gulati A, et al. Palisaded neutrophilic granulomatous dermatitis associated with systemic lupus erythematosus presenting with the burning rope sign. J. Am. Acad. Dermatol. 2009;61:711–714. doi: 10.1016/j.jaad.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Misago N, Inoue H, Inoue T, Nagasawa K, Narisawa Y. Palisaded neutrophilic granulomatous dermatitis in systemic lupus erythematosus with a butterfly rash-like lesion. Eur. J. Dermatol. 2010;20:128–129. doi: 10.1684/ejd.2010.0822. [DOI] [PubMed] [Google Scholar]
- 85.Hospach T, von den Driesch P, Dannecker GE. Acute febrile neutrophilic dermatosis (Sweet's syndrome) in childhood and adolescence: two new patients and review of the literature on associated diseases. Eur. J. Pediatr. 2009;168:1–9. doi: 10.1007/s00431-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 86.Lipsker D, Saurat JH. Neutrophilic cutaneous lupus erythematosus. At the edge between innate and acquired immunity? Dermatology. 2008;216:283–286. doi: 10.1159/000113940. [DOI] [PubMed] [Google Scholar]
- 87.Yang J, et al. TH17 and natural TREG cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]