Abstract
A naturally-occurring H275Y oseltamivir resistant variant of influenza A (H1N1) virus emerged in 2007, subsequently becoming prevalent worldwide, via an undetermined mechanism. To understand the antigenic properties of the H275Y variant, oseltamivir resistant and susceptible strains of H1N1 viruses were analyzed by hemagglutination inhibition (HI) and microneutralization assays. HI analysis with H1-positive sera obtained from seasonal flu vaccine immunized and non-immunized individuals, and H1-specific monoclonal antibodies, revealed that resistant strains exhibited a reduced reactivity to these antisera and antibodies in the HI assay, as compared to susceptible strains. Neutralization assay testing demonstrated that oseltamivir resistant H1N1 strains are also less susceptible to antibody inhibition during infection. Mice inoculated with a resistant clinical isolate exhibit 4-fold lower virus-specific antibody titers than mice infected with a susceptible strain under the same conditions. Resistant and sensitive variants of 2009 pandemic H1N1 virus did not exhibit such differences. While HA1 and NA phylogenetic trees show that both oseltamivir resistant and susceptible strains belong to clade 2B, NA D354G and HA A189T substitutions were found exclusively, and universally, in oseltamivir resistant variants. Our results suggest that the reduced susceptibility to antibody inhibition and lesser in vivo immunogenicity of the oseltamivir resistant 2008–2009 H1N1 influenza A virus is conferred by coupled NA and HA mutations, and may contribute to the prevalence of this H1N1 variant.
Keywords: influenza, H1N1, oseltamivir, resistance, neuraminidase, H275Y
1. Introduction
Resistance to rimantadine and amantadine, viral M2 ion channel blockers, has been commonly observed in H3N2 influenza viruses since 2003 and also in the 2009 pandemic H1N1 virus (Bright et al., 2005; Bright et al., 2006; Garten et al., 2009); as such, treatment of influenza virus infection has relied mainly on neuraminidase (NA) inhibitors, which inhibit virus release from infected cells by targeting viral NA. The NA inhibitors oseltamivir and zanamivir have been available for treatment of influenza since 1999, while another NA inhibitor, peramivir, was recently registered for use in Japan (Kohno et al., 2011; Sugaya, 2011; Zambon and Hayden, 2001). Surveillance studies facilitated via the global neuraminidase inhibitor susceptibility network (NISN) found that resistance to each neuraminidase inhibitor was rare during the first few years of clinical use (Hurt et al., 2009b; Kiso et al., 2004; McKimm-Breschkin et al., 2003; McKimm-Breschkin, 2000; Monto et al., 2006). It was also shown that resistant mutants obtained through selection in culture or isolated from patients treated with neuraminidase inhibitors are subtype specific, with E119V and R292K NA mutations occurring mainly in H3N2 subtype viruses exposed to either oseltamivir or zanamivir, and the H275Y mutation in the NA protein found almost exclusively among H1N1 subtype viruses in response to oseltamivir treatment (McKimm-Breschkin et al., 2003; McKimm-Breschkin, 2000; Sheu et al., 2008; Yen et al., 2006; Zambon and Hayden, 2001). Further characterization revealed that those non-naturally occurring resistant mutants selected by exposure to oseltamivir or zanamivir have compromised infectivity and replication abilities (Bouvier et al., 2008; Carr et al., 2002; Herlocher et al., 2002; Herlocher et al., 2004; Ives et al., 2002; Yen et al., 2005).
Resistance to oseltamivir (Tamiflu) has been monitored extensively as it is used more commonly than zanamivir and peramivir due to its oral availability, and is one of the most stockpiled antiviral drugs for the preparedness for a potential pandemic, as recommended by the World Health Organization (WHO) (Oxford, 2005; Reddy, 2010). Prior to 2007, only a very small number of H275Y mutant oseltamivir resistant viruses were recognized, mainly clinical isolates following oseltamivir treatment (Hurt et al., 2009b). However, the emergence of a H275Y resistant H1N1 variant in patients without pre-exposure to oseltamivir was identified in Norway in 2007 (Hauge et al., 2009). Similar resistant isolates were subsequently found in other countries and these rapidly replaced susceptible strains around the world (Cheng et al., 2009; Dharan et al., 2010; Weinstock and Zuccotti, 2009). The mechanism for the emergence and prevalence of this H275Y variant is not fully understood. In contrast to the resistant isolates identified before 2007, which had compromised growth and infection abilities, this naturally occurring resistant H1N1 strain appeared to cause illness similar to that associated with the wild type virus (Dharan et al., 2009; Hauge et al., 2009). The H275Y variant was found to replicate and transmit similarly to wild type virus in cells and in a competitive mixture model, respectively (Hurt et al., 2010). Nevertheless, it is still not clear how this resistant variant managed to replace the wild type virus and become the predominant population in humans in a relatively short period of time in the apparent absence of drug selection pressure. Resistance to oseltamivir has been detected in some clinical isolates of the pandemic H1N1 virus from patients both with and without a history of exposure to this drug (Chen et al., 2009; Hurt et al., 2011; Meijer et al., 2011). There is a concern that the resistant variant of the 2009 pandemic H1N1 virus may evolve to replace susceptible strains as the dominant population.
Host immunity gained through vaccination or previous infection plays an important role in determining the extent of prevalence of a strain of influenza virus. Influenza viruses undergo continuous antigenic drift to evade existing host immunity. It is believed that the ability of a new virus to rise to prevalence in a population is jointly determined by viral elements which enable robust replication in human tissues and efficient transmission between humans, together with levels of host immunity in the human population. This study compared the antigenic properties of oseltamivir resistant variant and wild type H1N1 viruses, analyzing the reactivity of antisera obtained from individuals who had received seasonal vaccine and from members of the general population who had no history of influenza vaccination to the viruses. We found that 2008–2009 H1N1 influenza A viruses which are resistant to oseltamivir exhibit different antigenic and immunogenic features than the wild type virus, which may contribute to the altered sensitivity of resistant virus strains to neutralizing antibodies.
2. Materials and methods
2.1 Viruses and antibodies
Clinical isolates of oseltamivir resistant and susceptible H1N1 viruses were kindly provided by Dr. W. Lim of the Centre for Health Protection, Hong Kong SAR. The identity of residue 275 of the NA gene was confirmed by sequencing analysis for all viruses used (see Supplementary Table 1 for GenBank accession numbers). Recombinant viruses carrying HA and NA from an oseltamivir resistant seasonal H1N1 influenza virus (A/HK/62768/2008), with the other 6 segments derived from A/Puerto Rico/8/1934 (PR8) virus, were constructed using the pHW2000 system, as described previously (Wang et al., 2009). Briefly, the HA and NA of A/HK/62768/2008 were cloned into the pHW2000 vector (Hoffmann et al., 2000). A Y275H substitution was introduced into the NA segment using the QuikChange II site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions. Recombinant viruses carrying either wild type NA (RG62768 NA275Y) or mutated NA (RG62768 NA275H) plus HA from A/HK/62768/2008 and the remaining six segments from PR8 were rescued. All gene segments from both recombinant viruses were sequenced to confirm the absence of undesired mutations.
Immunized antisera were obtained from individuals (aged from 20 to 50 years old) who received 2008–2009 seasonal trivalent influenza vaccine, which is composed of the A/Brisbane/59/2007 (H1N1), A/Brisbane/10/2007 (H3N2) and B/Florida/4/2006-like virus strains. H1-reactive antisera (176) were identified by screening sera from 1192 individuals from the general population who had not had the influenza vaccine for reactivity with A/Brisbane/59/2007. Murine monoclonal antibodies to A/Brisbane/59/2007 and A/California/7/2009 2009 pandemic H1N1 virus were raised using procedures similar to those previously described (Wu et al., 2008). Briefly, inactivated H1N1 viruses (adjusted to a titer of 5 × 107 TCID50/ml before inactivation) were emulsified with an equal volume of Freund’s complete adjuvant (Sigma, St. Louis, USA) and injected subcutaneously into six-week-old female BALB/c mice at two weekly intervals. A final dose of 100 μl of inactivated virus was injected intravenously 3 days prior to the procedure by which spleen cells from the immunized mice were fused with mouse myeloma cells (SP2/0-Ag14). Hybridomas were screened for the secretion of H1-specific MAbs by HI assay. MAbs were prepared by injecting hybridoma cultures into the peritoneal cavities of pristine-primed BALB/c mice; the ascitic fluid was collected after 9 to 12 days and stored at −20°C. MAbs were purified using ammonium sulfate precipitation, followed by DE-52 ion exchange chromatography. Immunoglobulin (Ig) concentrations were determined by spectrophotometry using a Perkin Elmer MBA 2000 spectrometer.
2.2. Hemagglutination inhibition and microneutralization assays
HI tests and microneutralization assays were performed in accordance with the World Health Organization guidelines (World Health Organization, 2002). Turkey red blood cells (RBC) were used for the standard HI assays. All polyclonal antisera were treated with sialic acid receptor destroying enzyme (RDE, Denka Seiken Co., Tokyo, Japan) prior to testing. HI test serial dilutions were started at 1:10 dilution for antisera and 1:100 dilution for monoclonal antibodies, as previously described (Wu et al., 2008). To determine the effect of neuraminidase activity on the HI assay, viruses were treated with zanamivir (RELENZA, GlaxoSmithKline, UK) prior to the addition of antisera. Briefly, 8 HA units of virus were mixed with 5nM zanamivir at a 1:1(v/v) ratio and incubated at room temperature for 1 hour, then used for HI testing.
2.3. In vitro growth kinetics
Growth kinetics were studied using procedures previously described (Wu et al., 2008). Briefly, 90% confluent MDCK or A549 cells (ATCC, Manassas, USA) were infected with H1N1 viruses at a multiplicity of infection (MOI) of 0.1 for 1 hour at 37°C. Infected cells were incubated in serum free medium in a 5% CO2 atmosphere at 37°C. At 4, 8, 12, 24, and 36 hours post infection (pi), the supernatants of the infected cells were harvested and plaque assays were conducted in MDCK cells to determine virus titers.
2.4. Enzymatic analysis
KM and Vmax values of neuraminidases were estimated using purified virus particles, as previously described (Potier et al., 1979). Briefly, the 2008–2009 H1N1 viruses were cultured in MDCK cells and supernatants were cleared by centrifugation at 5000 rpm for 15 min in JLA16.250 rotor (Beckman-Coulter) and the viruses were pelleted at 40,000 rpm for 1 h at 4 °C in a 45Ti rotor (Beckman-Coulter) and re-suspended in Tris-HCl buffer for enzymatic analysis. NA enzymatic activity was measured using fluorogenic substrate (MUNANA; Sigma). The fluorescence of released 4-methylumbelliferone was measured at excitation and emission wavelengths of 330nm and 450nm, respectively. The enzyme kinetics data were fitted to the Michaelis-Menten equation by using nonlinear regression (Prism; GraphPad) to determine the Michaelis constant (KM) and maximum velocity (Vmax) of substrate conversion.
2.5. Phylogenetic and sequence analyses
HA and NA genes from oseltamivir resistant and susceptible strains of 2008–2009 H1N1 virus were sequenced using standard procedures. Sequence assembly, alignment, and residue analysis of HA and NA genes were performed as previously described (Chen et al., 2006). The phylogenetic trees of nucleotide sequences were analyzed with PAUP* software, using the neighbor-joining method with 1000 bootstraps.
2.6. Preparation of antisera from infected mice
Antibody responses in mice were tested by infecting 6- to 8-week-old female BALB/c mice with H1N1 viruses. Oseltamivir resistant and susceptible strains of 2008–2009 H1N1 and 2009 pandemic H1N1 viruses and recombinant 275H and 275Y versions of one of the 2008–2009 H1N1 viruses were used in the experiment. All procedures were conducted in a Biosafety Level 3 facility at the Department of Microbiology, The University of Hong Kong. Mice were lightly anaesthetized by inhalation of 4% isoflurane (Halocarbon Laboratory, River Edge, NJ, USA) and inoculated intranasally with 25 μl PBS containing 104.5 TCID50 of H1N1 virus. Blood was collected at 21 days post infection and sera were treated with RDE prior to HI testing.
3. Results
3.1 Antigenic analysis of 275Y and wild type 275H isolates with antisera and H1 specific monoclonal antibodies
It has been reported previously that the oseltamivir resistant H1N1 viruses containing H275Y mutant NA replicate similarly to wild type viruses in cell culture using canine MDCK and MDCK-SIAT1 cells (Hurt et al., 2010). We confirmed that there is no statistically significant difference in growth kinetics between oseltamivir resistant and susceptible strains in the human cell line, A549 (Supplementary Figure 1).
To investigate if oseltamivir resistant H1N1 viruses may have other distinct properties, we examined whether the 275Y strain might be equipped with an enhanced ability to escape host immunity, making it more likely to be shed and transmitted between humans. We first conducted an antigenic analysis using a panel of antisera collected from 20 individuals who had received seasonal trivalent influenza vaccine containing the H1N1 vaccine strain A/Brisbane/59/2007, which is an oseltamivir susceptible strain. The level of antibodies to A/Brisbane/59/2007 in these immunized sera were confirmed to be increased at least four fold, compared to unimmunized sera, by HI assay (data not shown). As demonstrated in the reverse cumulative distribution hemagglutination inhibition titer curves, the antisera reacted less strongly with the resistant 275Y strains (N=21) as compared to the susceptible 275H strains (N=10) (Figure 1A, Supplementary Table 1A). To further test if antibodies present in the general population react differently to the resistant variant, we examined 176 antisera reactive to the A/Brisbane/59/2007 H1N1 virus which had been identified in 2009 from among 1192 individuals who did not have an influenza vaccination history. Consistent with the result obtained with sera from vaccinated individuals, the resistant strains analyzed exhibited lower reactivity towards these 176 sera than the oseltamivir susceptible strains (Figure 1B). A similar result was observed when a panel of H1 specific monoclonal antibodies was used in the HI test, with resistant virus strains exhibiting little or no reactivity with some of the H1 monoclonal antibodies (Table 1). To confirm whether the above HI assay observations correlate with the ability of antibodies to inhibit virus attachment during infection, the neutralization activity of antisera obtained from vaccinated people and H1 specific monoclonal antibodies against two resistant and two susceptible H1N1 virus strains was compared in a cell-based neutralization assay. As shown in Table 2, the susceptible strains are inhibited at higher antisera or monoclonal antibody dilutions than the resistant strains. In summary, we found that antibodies against H1N1 virus exhibit less reactivity towards the 275Y resistant variant of H1N1 virus than to wild type virus.
Figure 1.
Comparison of hemagglutination-inhibition activity of antisera against NA 275H and 275Y H1N1 clinical isolates. (A) HI titers were estimated for 20 antisera obtained from individuals immunized with seasonal trivalent vaccine containing A/Brisbane/59/2007-like H1N1 virus. Each of the antisera were tested against 10 susceptible (NA-275H) and 21resistant (NA-275Y) strains of A/Brisbane/59/2007-like H1N1 virus, and the average HI titer for each virus group (susceptible and resistant) calculated for each antisera. The proportion of the antisera samples which inhibited hemagglutination at each progressive antisera dilution based on the average HI titer calculated above was then plotted for the susceptible and resistant virus groups. Error bars represent standard deviation between different virus isolates. (B) HI titers associated with 176 H1 positive antisera identified from 1192 individuals, who had no history of receiving influenza vaccine, were estimated using the A/HK/04633/2008 (NA-275H) and A/HK/17599/2009 (NA-275Y) H1N1 viruses, and plotted as in (A). Statistical significance was determined using the t-test for sera from vaccinated individuals (A) or McNemar test for sera from non-vaccinated individuals (B). Asterisk indicates that the difference in HI reactivity between oseltamivir susceptible and resistant strains is statistically significant (* p<0.05, ** p<0.01).
Table 1.
Hemagglutination inhibition test of H1N1 virus with H1-reactive monoclonal antibodiesa
| Seasonal H1N1 viruses | NAb (275) | Monoclonal antibodiesc |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1D1 | 2E2 | 3A8 | 4C10 | 5H4 | 9D5 | 9E10 | 10G11 | 11A1 | 11E1 | 15G1 | 16C1 | ||
| HK/01236/2008 | H | 1600 | 100 | 400 | 1600 | 400 | 200 | 400 | 200 | 200 | 100 | 800 | < d |
| HK/02181/2008 | 12800 | 100 | 1600 | 12800 | 12800 | 6400 | 200 | 6400 | 6400 | 3200 | 3200 | 3200 | |
| HK/04427/2008 | 12800 | 100 | 400 | 12800 | 800 | 800 | < | 200 | 800 | 200 | 800 | 1600 | |
| HK/01045/2008 | 12800 | 100 | 200 | 12800 | 1600 | 3200 | 100 | 200 | 800 | 100 | 800 | 1600 | |
| HK/03807/2008 | 12800 | 200 | 400 | 12800 | 12800 | 3200 | 200 | 3200 | 3200 | 3200 | 3200 | 1600 | |
| HK/04633/2008 | 12800 | 100 | 800 | 12800 | 12800 | 6400 | 200 | 1600 | 1600 | 1600 | 1600 | 1600 | |
| HK/03138/2008 | 12800 | 200 | 1600 | 12800 | 12800 | 6400 | 400 | 6400 | 3200 | 3200 | 3200 | 640 | |
| HK/02022/2008 | 12800 | 200 | 6400 | 12800 | 12800 | 200 | < | 800 | 1600 | < | < | 3200 | |
|
| |||||||||||||
| HK/17566/2009 | Y | 12800 | < | < | 12800 | < | 3200 | < | < | 6400 | 400 | 800 | 320 |
| HK/16418/2009 | 12800 | 100 | < | 1600 | < | 6400 | 200 | < | 3200 | < | 1600 | 640 | |
| HK/15273/2009 | 12800 | < | 200 | < | < | 6400 | < | < | 1600 | < | < | 320 | |
| HK/15411/2009 | 12800 | < | < | 1600 | < | 3200 | 200 | < | 12800 | < | 1600 | 640 | |
| HK/17270/2009 | 1600 | < | < | < | < | < | < | < | 800 | < | < | 160 | |
| HK/16176/2009 | 12800 | < | < | 200 | < | 100 | < | < | 3200 | < | 200 | 320 | |
| HK/62768/2008 | 12800 | < | < | 12800 | < | 3200 | < | < | < | < | 100 | 320 | |
| HK/65446/2008 | 12800 | < | < | 3200 | < | 1600 | < | < | 1600 | < | < | 160 | |
| HK/63799/2008 | 12800 | < | < | 12800 | < | 400 | < | < | 400 | < | < | 160 | |
H1-reactive monoclonal antibodies were raised against the H1N1 virus strain A/Brisbane/59/2007.
NA-275H (oseltamivir susceptible), NA-275Y (oseltamivir resistant).
HI titer was determined using an antibody dilution range of 1:100 to 1:12800.
< denotes titer under 100.
Table 2.
Neutralization of H1N1 clinical isolates with flu vaccine immunized human sera and H1-reactive monoclonal antibodies.
| Seasonal H1N1 virusesb |
|||||
|---|---|---|---|---|---|
| Sera/Antibodies | NA-275H
|
NA-275Y
|
|||
| HK/04633/2008 | HK/03138/2008 | HK/17566/2009 | HK/16418/2009 | ||
| Immunized human seraa | HS1 | 320 | 640 | 80 | 80 |
| HS2 | 1280 | 1280 | 40 | < | |
| HS3 | 160 | 160 | 80 | 40 | |
| HS4 | 640 | 640 | 160 | 80 | |
| HS5 | < | < | < | < | |
| HS6 | 160 | 640 | < | 40 | |
| HS7 | 80 | 80 | < | < | |
| HS8 | 160 | 640 | < | < | |
| HS9 | 640 | 640 | 40 | 80 | |
| HS10 | 80 | 80 | 40 | 40 | |
| HS11 | 320 | 320 | < | 40 | |
| HS12 | 80 | 80 | 40 | 40 | |
| HS13 | 80 | 80 | 160 | < | |
| HS14 | < | 40 | 40 | 40 | |
| HS15 | 320 | 320 | 160 | 320 | |
| HS16 | 40 | 40 | 160 | 160 | |
| HS17 | 640 | 1280 | 1280 | 1280 | |
| HS18 | 80 | 80 | 80 | 80 | |
| HS19 | 80 | 320 | 40 | 80 | |
| HS20 | 640 | 1280 | 320 | 160 | |
| H1 monoclonal antibodies | 1D1 | 12800 | 12800 | 12800 | 12800 |
| 2E2 | 200 | 400 | 100 | < | |
| 3A8 | 200 | 800 | < | < | |
| 4C10 | 12800 | 12800 | 6400 | 1600 | |
| 5H4 | 400 | 12800 | < | < | |
| 9D5 | 6400 | 6400 | 400 | 3200 | |
| 9E10 | 200 | 100 | < | < | |
| 10G11 | 800 | 3200 | < | < | |
| 11A1 | 3200 | 3200 | 800 | 1600 | |
| 11E1 | 1600 | 6400 | < | < | |
| 15G1 | 800 | 800 | 100 | 200 | |
| 16C1 | 6400 | 6400 | < | 6400 | |
Note. Numbers represent highest dilution of antiserum (dilution range 1:10 to 1:1280) or monoclonal antibody (dilution range 1:100 to 1:12800) which still exhibits neutralization activity.
HS indicates human sera from immunized individuals.
< denotes titer under 40 for human sera, or under 100 for monoclonal antibodies.
3.2. Phylogenetic and sequence analyses of oseltamivir resistant and susceptible strains
To examine whether oseltamivir resistant and susceptible strains are genetically similar, sequences of HA and NA genes from isolates tested in this study were analyzed. The HA1 and NA phylogenetic trees showed that both oseltamivir resistant and susceptible strains, including the oseltamivir sensitive A/Brisbane/59/2007 vaccine strain, belong to clade 2B (Figure 2). This result excludes the possibility that differences in reactivity to antibodies observed among oseltamivir resistant isolates may be caused by genetically distinct variants. Sequence comparison of NA genes from 275H and 275Y H1N1 strains found that there is a D354G substitution in all resistant strains (Supplementary Table 1). Variations in other NA residues were not common. A substitution at D151 (D151E/N/A) of the NA gene has been found to affect the receptor binding characteristics of human H3N2 viruses, resulting in altered agglutination of red blood cells (Lin et al., 2010). Only one oseltamivir susceptible isolate, A/Hong Kong/02181/2008, was found to contain a D151E substitution. In the HA gene, all resistant strains contain an A189T substitution that is not seen in any of the susceptible strains (Supplementary Table 2). Variations at HA residues 141, 185 and 186 were also found among some resistant variants, but not in susceptible strains. These results indicate that although oseltamivir resistant and susceptible strains are phylogenetically similar, variations occur in both the NA and HA genes of these viruses. The impact of NA D354G, HA A189T and other variations detected in the HA gene on viral fitness and antigenic properties remains to be investigated.
Figure 2.
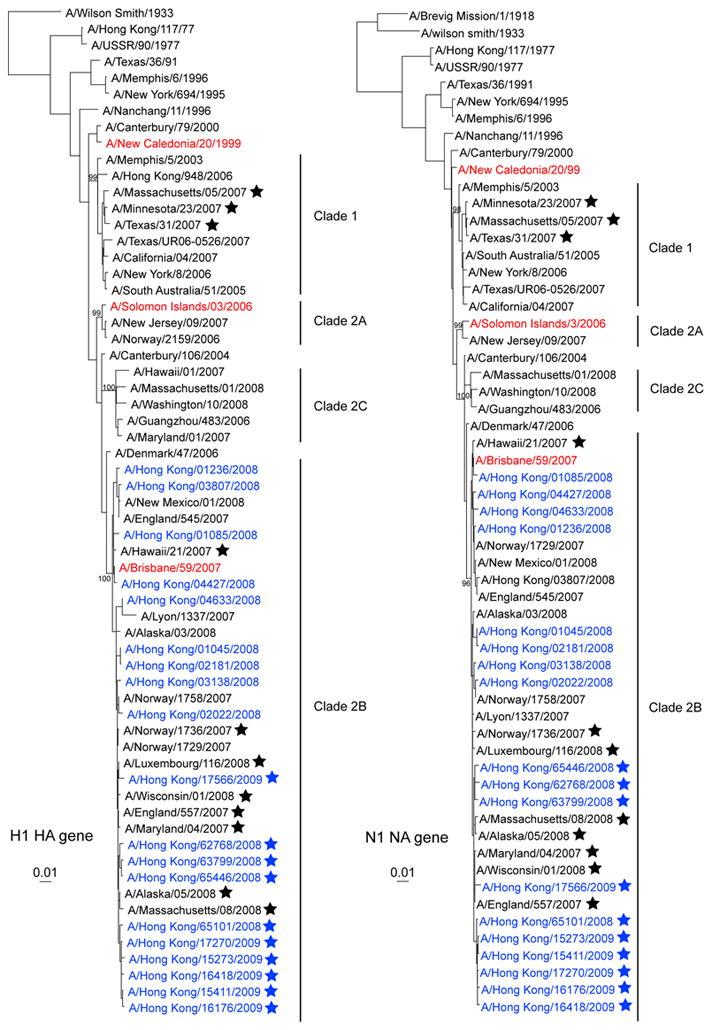
Phylogenetic trees for the HA (A) and NA (B) genes of H1N1 viruses isolated in Hong Kong during the 2008–2009 influenza seasons. The nucleotide sequences were analyzed with PAUP* software, using the neighbor-joining method with 1000 bootstraps. Sequences of H1N1 viruses characterized in this study are colored blue. Red-hued sequences represent H1N1 influenza vaccine strains recommended by the World Health Organization. Star (*) indicates NA H275Y variant of the H1N1 virus. Genetic lineages of Clade 1, 2A, 2B and 2C H1N1 viruses were based on previous reports (Collins et al., 2009; Hauge et al., 2009).
The swine origin pandemic H1N1 virus has replaced previous seasonal H1N1 virus since 2009 and sporadic oseltamivir resistant variants have also been detected in many countries (Chen et al., 2009; Hurt et al., 2011; Meijer et al., 2011; Ujike et al., 2011). To test if resistant and susceptible strains of the pandemic 2009 virus may also exhibit differences, we compared the antigenic reactivity of a panel of monoclonal antibodies specific for the 2009 H1N1 virus towards three resistant and 10 susceptible strains of the 2009 pandemic H1N1 virus, collected during the 2009 and 2010 seasons. In contrast to the result obtained with 2008–2009 seasonal H1N1 viruses, no apparent difference was observed in the reactivity of the panel of monoclonal antibodies with resistant and susceptible strains of the pandemic 2009 virus (Table 3).
Table 3.
Hemagglutination inhibition assay of the 2009 pandemic H1N1 virus with monoclonal antibodiesa.
| 2009 Pandemic H1N1 virusesd | NA (275)c | Monoclonal antibodiesb |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1E12 | 2B3 | 2F2 | 3G3 | 4A7 | 4A9 | 4G12 | 6B8 | 6B9 | 8H9 | 10B4 | 14D2 | 14H2 | 15H9 | 18A9 | J19B8 | ||
| California/04/2009 | H | < | 12800 | 12800 | 12800 | 6400 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | < | 1600 | 12800 | 12800 | 12800 |
| HK/415742/2009 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/419301/2009 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/420518/2009 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/433397/2009 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | |
| HK/51542/2010 | 12800 | 12800 | 12800 | 12800 | 6400 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/45687/2010 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/40835/2010 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
| HK/00783/2010 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | |
| HK/02645/2010 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | |
|
| |||||||||||||||||
| HK/423432/2009 | Y | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 |
| HK/23369/2009 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 12800 | 3200 | 12800 | 12800 | 12800 | |
| HK/FFD/2009 | 12800 | 12800 | 12800 | 800 | 6400 | 12800 | 3200 | 12800 | 12800 | 12800 | 12800 | 12800 | 1600 | 12800 | 12800 | 12800 | |
Note.
HI titer was estimated using monoclonal antibody dilutions ranging from 1:100 to 1:12800.
Monoclonal antibodies used in this study were raised against the 2009 pandemic H1N1 virus strains A/California/04/2009 and A/California/07/2009.
Ten strains of the 2009 pandemic H1N1 virus characterized between 2009 and 2010 which contain NA-275H and three strains which contain NA-275Y were examined in this study.
3.3. H275Y variants exhibit differential neuraminidase activity
Previous studies showed that H275Y substitution in the NA gene caused changes in the enzymatic properties of the neuraminidase proteins of H1N1 and H5N1 viruses (Collins et al., 2009; Collins et al., 2008; Rameix-Welti et al., 2008). We analyzed the Km and Vmax values of some of the isolates used in this study. The three resistant strains analyzed exhibited higher Km values than susceptible viruses (Table 4), indicating a reduced affinity for substrate. To investigate if the differences in antibody reactivity observed in this study may be due to specific properties associated with variations in the neuraminidase proteins of the tested viruses, we used the neuraminidase inhibitor, zanamivir, to inhibit the neuraminidase activity of both resistant and susceptible strains of H1N1 virus prior to their use in the HI test. Zanamivir binds to a different site on the NA protein than oseltamivir and its action is unaffected by the H275Y substitution in the NA protein of H1N1 virus (Collins et al., 2009; Hurt et al., 2009a; McKimm-Breschkin, 2000). To verify the effect of neuraminidase activity on the hemagglutination inhibition assay, the percentage of sera samples (N=20) with HI titer >1:40 was estimated in the presence or absence of zanamivir. In contrast to the results obtained when zanamivir was not used, no significant difference in antisera reactivity towards resistant and susceptible strains of H1N1 virus was observed when neuraminidase activity was inhibited (Figure 3). To further characterize the effect of the H275Y substitution on reactivity between antibodies and resistant strains, we constructed a pair of recombinant viruses with HA and NA either directly derived from an oseltamivir resistant seasonal H1N1 virus, A/Hong Kong/62768/2008, or with the same HA but the NA of the resistant strain reverse-mutated from 275Y to 275H. The recombinant viruses were rescued in the context of PR8 virus internal genes and the genetic stability of recombinant viruses was verified by sequencing analysis following virus passage in MDCK cells. Using this pair of viruses that differed only at the 275 residue, we repeated the HI assay. Analysis with 20 immunized sera in the HI assay showed that the 275Y virus exhibits statistically lower reactivity compared to the 275H virus (Figure 4). These results suggest that changes in the NA molecule associated with the H275Y substitution may contribute to the lower antibody reactivity of the 275Y variant.
Table 4.
Enzymatic properties of the neuraminidases of 275H and 275Y H1N1 virusesa
| Virus strainb |
NA (275)
|
Km (μM)
|
Vmax (OD)
|
|||
|---|---|---|---|---|---|---|
| HK/02022/2008 | — | H | — | 16.99 | — | 0.421 |
| HK/03138/2008 | 40.66 | 0.394 | ||||
| HK/04633/2008 | 20.68 | 0.472 | ||||
| HK/16418/2009 | Y | 68.19 | 0.243 | |||
| HK/17270/2009 | 61.96 | 1.843 | ||||
| HK/17566/2009 | 59.05 | 0.719 |
Note.
Enzymatic properties of neuraminidases, indicated by Km and Vmax values, were estimated as described in the materials and methods.
Three H1N1 strains of each NA genotype (275H or 275Y) were analyzed in this study.
Figure 3.
Testing of the effect of neuraminidase inhibitor treatment on the reactivity of antibodies with oseltamivir susceptible and resistant H1N1 viruses. Hemagglutination inhibition testing was conducted with 20 immunized human sera, with or without pretreatment of viruses with 5uM of the neuraminidase inhibitor, zanamivir. Percentage of sera samples with HI titer > 1:40 represents average from 3 independent experiments. Error bars represent standard deviations between different isolates. Statistical significance was determined using the McNemar test. Asterisk (*) indicates the difference is statistically significant (p value <0.01).
Figure 4.
Comparison of hemagglutination-inhibition activity of antisera against NA 275Y and 275H H1N1 recombinant viruses. HA and NA derived from A/Hong Kong/62768/2008, together with six internal genes from the PR8 strain, were used to construct recombinant viruses. One version contains wild type HA and NA (275Y) genes while the other contains wild type HA and NA with residue 275Y mutated to 275H. Recombinant viruses were tested against 20 sera obtained from seasonal flu vaccine immunized individuals, as described in Figure 1A. The experiment was repeated three times and data statistically analyzed using the McNemar test. Error bars represent standard deviation.
3.4. Oseltamivir resistant seasonal H1N1 virus is less immunogenic in infected mice than a susceptible strain
To further examine if the altered antigenic properties of the oseltamivir-resistant seasonal H1N1 virus may result in it being less immunogenic in vivo, we inoculated mice with equivalent doses of either a oseltamivir resistant clinical isolate of the seasonal H1N1 virus (275Y, A/Hong Kong/62768/2008) or a susceptible strain (275H, A/Brisbane/59/2007) through the nasal route. Serum samples were collected at three weeks post infection and the levels of antibodies to inoculated virus were tested. Four of the seven mice infected with NA 275Y virus showed 4-fold lower antibody titers in the HI assay than the median level for mice infected with the NA 275H isolate (Table 5). In contrast to the results using clinical isolates, when mice were infected with reverse genetically modified H1N1 viruses where the only difference between oseltamivir resistant and susceptible strains was the H275Y substitution in the NA gene, there was no apparent difference in antibody levels between the 275Y and 275H strains, suggesting that H275Y alone is not sufficient to alter the antibody response in mice (Table 5). Notably, antibody titers from mice inoculated with oseltamivir resistant (A/Hong Kong/423432/2009) are higher than those elicited by susceptible (A/California/07/2009) strains of the 2009 pandemic H1N1 virus (Table 5), further suggesting that the oseltamivir resistant 2009 pandemic H1N1 virus does not possess similar antigenic properties to the 2008–2009 H1N1 NA-H275Y virus. Further analyses of the cross reactivity of antisera found an 8-fold decrease in median HI titer when oseltamivir-resistant isolates were tested against mouse antisera raised against the H1N1 oseltamivir-susceptible strain, A/Brisbane/59/2007 (Table 6). However, no such difference was observed with oseltamivir resistant 2009 pandemic H1N1 isolates tested against antisera raised against the oseltamivir susceptible strain, A/California/07/2009. These results support the notion that the oseltamivir resistant variant of the 2008–2009 H1N1 virus, but not that of the 2009 pandemic H1N1 virus, is less reactive to antibodies induced by the susceptible strain, and may also be less immunogenic, inducing a comparatively weaker antibody response in vivo.
Table 5.
Hemagglutination inhibition test of H1N1 virus strains with homologous mouse antiseraa
| Inoculated strains | Mouse antisera
|
Mouse control serab |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | median | 1 | 2 | |
| Brisbane/59/2007 | 160c | 80 | 160 | 160 | 160 | 160 | 160 | 160 | <d | < |
| HK/62768/2008 | 40 | 80 | 80 | 80 | 40 | 40 | 40 | 40 | < | < |
| California/7/2009 | 320 | 160 | 320 | 320 | 640 | 320 | 320 | 320 | < | < |
| HK/423432/2009 | 640 | 1280 | 1280 | 1280 | 640 | 1280 | 640 | 1280 | < | < |
| RG 62768 NA-275H | 320 | 160 | 80 | 160 | 160 | 80 | 160 | 160 | < | < |
| RG 62768 NA-275Y | 160 | 320 | 160 | 160 | 160 | 80 | - | 160 | < | < |
Note
Groups of six or seven mice were inoculated with H1N1 virus through the nasal route and antisera were collected at three weeks post infection. One of the mice inoculated with strain RG 62768 NA-275Y died during the expreiment
Mouse control sera were made by inoculated mice with PBS
Numbers represent highest dilution of antiserum (dilution range 1:10 to 1:1280)
denotes HI titer under 10.
Table 6.
Hemagglutination inhibition test of oseltamivir resistant strain reactivity to mouse antisera raised against vaccine strains.
| Serum sample number | Inoculated straina |
Resistant viruses
|
|||
|---|---|---|---|---|---|
| Brisbane/59/2007 | HK/62768/2008 | HK/65101/2008 | HK/15273/2009 | ||
| Seasonal H1N1 | 1 | 160b | 80 | 80 | 40 |
| 2 | 80 | 10 | <c | < | |
| 3 | 160 | 40 | 20 | 20 | |
| 4 | 160 | 20 | 40 | 20 | |
| 5 | 160 | 80 | 40 | 80 | |
| 6 | 160 | 10 | 10 | < | |
| 7 | 160 | 10 | 20 | 10 | |
| Median | 160 | 20 | 20 | 20 | |
|
| |||||
| California/07/2009 | HK/FFD/2009 | HK/23369/2009 | HK/423432/2009 | ||
|
| |||||
| Pandemic H1N1 | 1 | 320 | 320 | 320 | 640 |
| 2 | 160 | 160 | 160 | 640 | |
| 3 | 320 | 320 | 160 | 640 | |
| 4 | 320 | 320 | 320 | 640 | |
| 5 | 640 | 320 | 640 | 640 | |
| 6 | 320 | 320 | 320 | 640 | |
| 7 | 320 | 320 | 320 | 640 | |
| Median | 320 | 320 | 320 | 640 | |
Note
Both Brisbane/59/2007 and California/07/2009 are vaccine strains
Numbers represent highest dilution of antiserum (dilution range 1:10 to 1:1280)
< denotes HI titer under 10.
4. Discussion
Influenza A virus replication fitness is partially achieved by continuous adjustment of the functional balance between affinity of attachment to receptors by hemagglutinin (HA) and activity of cleavage from cellular receptors by neuraminidase during the virus infection cycle (Wagner et al., 2002). Both HA and NA are primary targets for the host immune response. Mutations at sites or residues associated with functions of the HA or NA proteins of influenza A viruses will lead to an alteration in the balance between the attachment and cleavage processes during virus infection and a re-balance between HA and NA functions will be necessary to regain fitness. The rapidly-gained prevalence of a naturally occurring NA 275Y mutant of H1N1 virus raised concern that a similar trend may occur for other influenza viruses, in particular the 2009 H1N1 virus (Moscona, 2009). It is therefore important to understand the mechanisms underlying the emergence and prevalence of H1N1 influenza viruses carrying oseltamivir resistance mutations.
The rapid rise to prevalence of the naturally occurring H275Y resistant of the H1N1 virus since its emergence in late 2007 suggested a possible adaptation mechanism involving restoration of fitness and/or gain of an epidemic advantage. Sequence and structural analyses found that the H275Y resistant strains are predominantly clade 2B lineage H1N1 viruses which carry an additional NA substitution, D344N (Collins et al., 2009; Rameix-Welti et al., 2008). Consistent with this observation, the D344N substitution is present in all of the oseltamivir resistant isolates included in this study (Supplementary Table 1). Another study found that permissive mutations at V234M and R222Q, outside of the active enzyme site, significantly increase the amount of NA protein that reaches the cell surface, while the activity per enzyme molecule is either unaffected or slightly decreased (Bloom et al., 2010). Since both the oseltamivir resistant and susceptible H1N1 viruses examined contained these two mutations (Supplementary Table 1), it seems unlikely that these substitutions are associated with the altered antigenic and immunogenic properties of the H1N1 viruses described in this study. It is notable that all oseltamivir resistant strains of seasonal H1N1 virus examined in this study also contain a D354G substitution in the NA gene that is not seen in any of the susceptible isolates (Supplementary Table 1). The D354G substitution is also observed in some other clade 2B H1N1 viruses, however, there is no known structural basis to support this mutation having a major impact on the enzymatic characteristics of NA (Collins et al., 2009; Rameix-Welti et al., 2008; Russell et al., 2006). Examination of the HA gene also revealed exclusive association of the A189T substitution with oseltamivir resistant (H275Y) 2008–2009 H1N1 viruses, as well as variations at positions 141, 185 and 186 in some resistant variants. It remains to be investigated whether these H275Y-coupled mutations in the NA protein provide the capacity to re-balance HA and NA functions, restoring the H275Y mutation-associated defects in protein folding, substrate interaction, and transport of NA, and possibly reinstating fitness of the virus.
For an emerging influenza virus strain to become prevalent, it requires fitness in receptor binding (via HA) and cleavage (via NA), replication efficiency and transmissibility. It is also necessary for a prevalent strain to adapt to or evade host immune surveillance (Palese and Wang, 2011). We found that antisera obtained from vaccine immunized or naturally infected people, and monoclonal antibodies raised against HA of vaccine strains reacted less strongly with H275Y resistant strains of seasonal 2008–2009 H1N1 viruses. Our data also showed that the oseltamivir resistant strain is less immunogenic in mice. While permissive mutations can restore the optimal functioning of neuraminidases carrying the 275Y mutation, the distinct antigenic and immunogenic properties possessed by the 2008–2009 H1N1 oseltamivir resistant variant may provide a further advantage, allowing the virus to evade the immune system and tolerate neutralizing antibodies. Our data using recombinant viruses varying only by the H275Y substitution suggests that molecular changes caused by H275Y substitution in the NA of the 2008–2009 oseltamivir resistant H1N1 virus can cause reduced reactivity to antibodies raised against the susceptible H1N1 strain in the in vitro HI assay (Figure 4 and Table 6). However, in contrast to the 275Y clinical isolate, which was less immunogenic than the tested 275H isolate, recombinant viruses carrying either NA-275Y or altered from NA-275Y to NA-275H by site mutagenesis show no difference in antibody induction in vivo (Table 5). It appears the H275Y substitution alone is insufficient to account for the altered immunogenic properties of 2008–2009 oseltamivir resistant H1N1 viruses. Further study is required to understand the composite roles of substitutions in both HA and NA in conferring replication fitness and the distinct immunogenic properties associated with the oseltamivir resistant H1N1 variant that enabled it to rise to, and sustain, worldwide prevalence between 2007 and 2009.
While resistant variants of the 2009 H1N1 virus carrying the H275Y substitution have been detected (Chen et al., 2009; Hurt et al., 2011; Meijer et al., 2011; Ujike et al., 2011), current circulating strains remain mostly susceptible to oseltamivir. Assessment of the fitness of resistant strains in animal models suggests that the H275Y variant of pandemic 2009 H1N1 virus may not be less transmissible or virulent than wild type (Hamelin et al., 2010; Kiso et al., 2010; Seibert et al., 2010). Our analysis showed that, different to the 2008–2009 seasonal H1N1 viruses, both susceptible and resistant strains of the 2009 pandemic virus are similar in their sensitivity to neutralizing antibodies and their immunogenicity in infected mice (Table 4 and Table 5). The 2009 pandemic and the preceding 2008–2009 seasonal H1N1 viruses have traversed different evolutionary paths and exhibit distinct antigenic properties. It is likely that the adaptive changes that allow H275Y variation in the NA of the pandemic H1N1 virus may be different to those which occur in the 2008–2009 H1N1 viruses. As the 2009 pandemic H1N1 virus continues to circulate in humans, and neuraminidase inhibitors are used increasingly for treatment of influenza virus infection (Sugaya, 2011), it is necessary to monitor the antigenic and immunogenic properties which may allow drug resistant strains of circulating influenza viruses to gain an advantage and rise to prevalence.
Supplementary Material
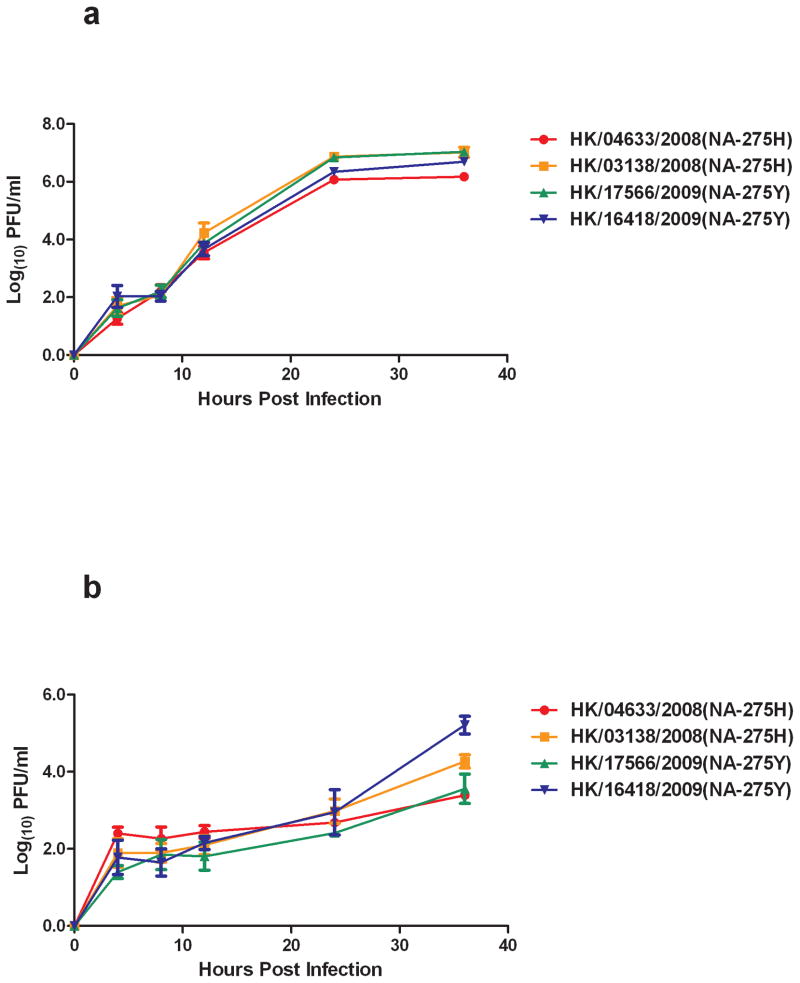
Supplementary Figure 1. Comparison of the growth kinetics of clinical isolates of oseltamivir resistant and susceptible strains of the A/Brisbane/59/2007-like H1N1 virus in MDCK cells (A) and A549 cells (B). A/HK/04633/2008 and A/HK/03138/2008 are oseltamivir susceptible, while A/HK/17566/2009 and A/HK/16418/2009 are oseltamivir resistant. Techniques for virus infection and estimation of virus titer (plaque forming units, PFU) are described in the materials and methods. Results were obtained from three repeated experiments and error bars indicate standard deviation.
Acknowledgments
The authors would like to thank Dr. W. Lim (Center for Health Protection, Hong Kong SAR, China) for providing clinical isolates of H1N1 viruses for this study. This study was partially supported by the Research Grants Council of the Hong Kong SAR (7500/06M and 7620/10M), the Research Fund for the Control of Infectious Diseases of the Health, Welfare and Food Bureau of the Hong Kong SAR, the Areas of Excellence Scheme of the University Grants Committee (Grant AoE/M-12/06), the National Institutes of Health (NIAID contract HHSN2662007 00005C), the Providence Foundation Limited in memory of the late Dr. Lui Hac Minh and the Li Ka Shing Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bloom JD, Gong LI, Baltimore D. Permissive secondary mutations enable the evolution of influenza oseltamivir resistance. Science. 2010;328:1272–1275. doi: 10.1126/science.1187816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier NM, Lowen AC, Palese P. Oseltamivir-resistant influenza A viruses are transmitted efficiently among guinea pigs by direct contact but not by aerosol. J Virol. 2008;82:10052–10058. doi: 10.1128/JVI.01226-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright RA, Medina MJ, Xu X, Perez-Oronoz G, Wallis TR, Davis XM, Povinelli L, Cox NJ, Klimov AI. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet. 2005;366:1175–1181. doi: 10.1016/S0140-6736(05)67338-2. [DOI] [PubMed] [Google Scholar]
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA. 2006;295:891–894. doi: 10.1001/jama.295.8.joc60020. [DOI] [PubMed] [Google Scholar]
- Carr J, Ives J, Kelly L, Lambkin R, Oxford J, Mendel D, Tai L, Roberts N. Influenza virus carrying neuraminidase with reduced sensitivity to oseltamivir carboxylate has altered properties in vitro and is compromised for infectivity and replicative ability in vivo. Antiviral Res. 2002;54:79–88. doi: 10.1016/s0166-3542(01)00215-7. [DOI] [PubMed] [Google Scholar]
- Chen H, Cheung CL, Tai H, Zhao P, Chan JF, Cheng VC, Chan KH, Yuen KY. Oseltamivir-resistant influenza A pandemic (H1N1) 2009 virus, Hong Kong, China. Emerg Infect Dis. 2009;15:1970–1972. doi: 10.3201/eid1512.091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Smith GJ, Li KS, Wang J, Fan XH, Rayner JM, Vijaykrishna D, Zhang JX, Zhang LJ, Guo CT, Cheung CL, Xu KM, Duan L, Huang K, Qin K, Leung YH, Wu WL, Lu HR, Chen Y, Xia NS, Naipospos TS, Yuen KY, Hassan SS, Bahri S, Nguyen TD, Webster RG, Peiris JS, Guan Y. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc Natl Acad Sci U S A. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PK, Leung TW, Ho EC, Leung PC, Ng AY, Lai MY, Lim WW. Oseltamivir- and amantadine-resistant influenza viruses A (H1N1) Emerg Infect Dis. 2009;15:966–968. doi: 10.3201/eid1506.081357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Martin SR, Daniels RS, Gregory V, Skehel JJ, Gamblin SJ, Hay AJ. Structural basis for oseltamivir resistance of influenza viruses. Vaccine. 2009;27:6317–6323. doi: 10.1016/j.vaccine.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Collins PJ, Haire LF, Lin YP, Liu J, Russell RJ, Walker PA, Skehel JJ, Martin SR, Hay AJ, Gamblin SJ. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature. 2008;453:1258–1261. doi: 10.1038/nature06956. [DOI] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Klimov AI, Fiore AE, Bresee JS, Fry AM. Antiviral treatment of patients with oseltamivir-resistant and oseltamivir-susceptible seasonal Influenza A (H1N1) infection during the 2007–2008 influenza season in the United States. Clin Infect Dis. 2010;50:621–622. doi: 10.1086/650178. [DOI] [PubMed] [Google Scholar]
- Dharan NJ, Gubareva LV, Meyer JJ, Okomo-Adhiambo M, McClinton RC, Marshall SA, St George K, Epperson S, Brammer L, Klimov AI, Bresee JS, Fry AM. Infections with oseltamivir-resistant influenza A(H1N1) virus in the United States. JAMA. 2009;301:1034–1041. doi: 10.1001/jama.2009.294. [DOI] [PubMed] [Google Scholar]
- Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, Okomo-Adhiambo M, Gubareva L, Barnes J, Smith CB, Emery SL, Hillman MJ, Rivailler P, Smagala J, de Graaf M, Burke DF, Fouchier RA, Pappas C, Alpuche-Aranda CM, Lopez-Gatell H, Olivera H, Lopez I, Myers CA, Faix D, Blair PJ, Yu C, Keene KM, Dotson PD, Jr, Boxrud D, Sambol AR, Abid SH, St George K, Bannerman T, Moore AL, Stringer DJ, Blevins P, Demmler-Harrison GJ, Ginsberg M, Kriner P, Waterman S, Smole S, Guevara HF, Belongia EA, Clark PA, Beatrice ST, Donis R, Katz J, Finelli L, Bridges CB, Shaw M, Jernigan DB, Uyeki TM, Smith DJ, Klimov AI, Cox NJ. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin ME, Baz M, Abed Y, Couture C, Joubert P, Beaulieu E, Bellerose N, Plante M, Mallett C, Schumer G, Kobinger GP, Boivin G. Oseltamivir-resistant pandemic A/H1N1 virus is as virulent as its wild-type counterpart in mice and ferrets. PLoS Pathog. 2010;6:e1001015. doi: 10.1371/journal.ppat.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauge SH, Dudman S, Borgen K, Lackenby A, Hungnes O. Oseltamivir-resistant influenza viruses A (H1N1), Norway, 2007–08. Emerg Infect Dis. 2009;15:155–162. doi: 10.3201/eid1502.081031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlocher ML, Carr J, Ives J, Elias S, Truscon R, Roberts N, Monto AS. Influenza virus carrying an R292K mutation in the neuraminidase gene is not transmitted in ferrets. Antiviral Res. 2002;54:99–111. doi: 10.1016/s0166-3542(01)00214-5. [DOI] [PubMed] [Google Scholar]
- Herlocher ML, Truscon R, Elias S, Yen HL, Roberts NA, Ohmit SE, Monto AS. Influenza viruses resistant to the antiviral drug oseltamivir: transmission studies in ferrets. J Infect Dis. 2004;190:1627–1630. doi: 10.1086/424572. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Deng YM, Ernest J, Caldwell N, Leang L, Iannello P, Komadina N, Shaw R, Smith D, Dwyer DE, Tramontana AR, Lin RT, Freeman K, Kelso A, Barr IG. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill. 2011;16 [PubMed] [Google Scholar]
- Hurt AC, Holien JK, Parker M, Kelso A, Barr IG. Zanamivir-resistant influenza viruses with a novel neuraminidase mutation. J Virol. 2009a;83:10366–10373. doi: 10.1128/JVI.01200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt AC, Holien JK, Parker MW, Barr IG. Oseltamivir resistance and the H274Y neuraminidase mutation in seasonal, pandemic and highly pathogenic influenza viruses. Drugs. 2009b;69:2523–2531. doi: 10.2165/11531450-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hurt AC, Nor’e SS, McCaw JM, Fryer HR, Mosse J, McLean AR, Barr IG. Assessing the viral fitness of oseltamivir-resistant influenza viruses in ferrets, using a competitive-mixtures model. J Virol. 2010;84:9427–9438. doi: 10.1128/JVI.00373-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives JA, Carr JA, Mendel DB, Tai CY, Lambkin R, Kelly L, Oxford JS, Hayden FG, Roberts NA. The H274Y mutation in the influenza A/H1N1 neuraminidase active site following oseltamivir phosphate treatment leave virus severely compromised both in vitro and in vivo. Antiviral Res. 2002;55:307–317. doi: 10.1016/s0166-3542(02)00053-0. [DOI] [PubMed] [Google Scholar]
- Kiso M, Mitamura K, Sakai-Tagawa Y, Shiraishi K, Kawakami C, Kimura K, Hayden FG, Sugaya N, Kawaoka Y. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364:759–765. doi: 10.1016/S0140-6736(04)16934-1. [DOI] [PubMed] [Google Scholar]
- Kiso M, Shinya K, Shimojima M, Takano R, Takahashi K, Katsura H, Kakugawa S, Le MT, Yamashita M, Furuta Y, Ozawa M, Kawaoka Y. Characterization of oseltamivir-resistant 2009 H1N1 pandemic influenza A viruses. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno S, Kida H, Mizuguchi M, Hirotsu N, Ishida T, Kadota J, Shimada J. Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011;55:2803–2812. doi: 10.1128/AAC.01718-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Gregory V, Collins P, Kloess J, Wharton S, Cattle N, Lackenby A, Daniels R, Hay A. Neuraminidase receptor binding variants of human influenza A(H3N2) viruses resulting from substitution of aspartic acid 151 in the catalytic site: a role in virus attachment? J Virol. 2010;84:6769–6781. doi: 10.1128/JVI.00458-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J, Trivedi T, Hampson A, Hay A, Klimov A, Tashiro M, Hayden F, Zambon M. Neuraminidase sequence analysis and susceptibilities of influenza virus clinical isolates to zanamivir and oseltamivir. Antimicrob Agents Chemother. 2003;47:2264–2272. doi: 10.1128/AAC.47.7.2264-2272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin JL. Resistance of influenza viruses to neuraminidase inhibitors--a review. Antiviral Res. 2000;47:1–17. doi: 10.1016/s0166-3542(00)00103-0. [DOI] [PubMed] [Google Scholar]
- Meijer A, Jonges M, Abbink F, Ang W, van Beek J, Beersma M, Bloembergen P, Boucher C, Claas E, Donker G, van Gageldonk-Lafeber R, Isken L, de Jong A, Kroes A, Leenders S, van der Lubben M, Mascini E, Niesters B, Oosterheert JJ, Osterhaus A, Riesmeijer R, Riezebos-Brilman A, Schutten M, Sebens F, Stelma F, Swaan C, Timen A, Van’t Veen A, van der Vries E, Te Wierik M, Koopmans M. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res. 2011 doi: 10.1016/j.antiviral.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Monto AS, McKimm-Breschkin JL, Macken C, Hampson AW, Hay A, Klimov A, Tashiro M, Webster RG, Aymard M, Hayden FG, Zambon M. Detection of influenza viruses resistant to neuraminidase inhibitors in global surveillance during the first 3 years of their use. Antimicrob Agents Chemother. 2006;50:2395–2402. doi: 10.1128/AAC.01339-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med. 2009;360:953–956. doi: 10.1056/NEJMp0900648. [DOI] [PubMed] [Google Scholar]
- Oxford J. Oseltamivir in the management of influenza. Expert Opin Pharmacother. 2005;6:2493–2500. doi: 10.1517/14656566.6.14.2493. [DOI] [PubMed] [Google Scholar]
- Palese P, Wang TT. Why do influenza virus subtypes die out? A hypothesis. MBio. 2011;2 doi: 10.1128/mBio.00150-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Rameix-Welti MA, Enouf V, Cuvelier F, Jeannin P, van der Werf S. Enzymatic properties of the neuraminidase of seasonal H1N1 influenza viruses provide insights for the emergence of natural resistance to oseltamivir. PLoS Pathog. 2008;4:e1000103. doi: 10.1371/journal.ppat.1000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D. Responding to pandemic (H1N1) 2009 influenza: the role of oseltamivir. J Antimicrob Chemother. 2010;65(Suppl 2):ii35–ii40. doi: 10.1093/jac/dkq014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RJ, Haire LF, Stevens DJ, Collins PJ, Lin YP, Blackburn GM, Hay AJ, Gamblin SJ, Skehel JJ. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature. 2006;443:45–49. doi: 10.1038/nature05114. [DOI] [PubMed] [Google Scholar]
- Seibert CW, Kaminski M, Philipp J, Rubbenstroth D, Albrecht RA, Schwalm F, Stertz S, Medina RA, Kochs G, Garcia-Sastre A, Staeheli P, Palese P. Oseltamivir-resistant variants of the 2009 pandemic H1N1 influenza A virus are not attenuated in the guinea pig and ferret transmission models. J Virol. 2010;84:11219–11226. doi: 10.1128/JVI.01424-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, Bright RA, Butler EN, Wallis TR, Klimov AI, Gubareva LV. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008;52:3284–3292. doi: 10.1128/AAC.00555-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya N. Widespread use of neuraminidase inhibitors in Japan. J Infect Chemother. 2011;17:595–601. doi: 10.1007/s10156-011-0288-0. [DOI] [PubMed] [Google Scholar]
- Ujike M, Ejima M, Anraku A, Shimabukuro K, Obuchi M, Kishida N, Hong X, Takashita E, Fujisaki S, Yamashita K, Horikawa H, Kato Y, Oguchi A, Fujita N, Tashiro M, Odagiri T. Monitoring and characterization of oseltamivir-resistant pandemic (H1N1) 2009 virus, Japan, 2009–2010. Emerg Infect Dis. 2011;17:470–479. doi: 10.3201/eid1703.101188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Matrosovich M, Klenk HD. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev Med Virol. 2002;12:159–166. doi: 10.1002/rmv.352. [DOI] [PubMed] [Google Scholar]
- Wang P, Song W, Mok BW, Zhao P, Qin K, Lai A, Smith GJ, Zhang J, Lin T, Guan Y, Chen H. Nuclear factor 90 negatively regulates influenza virus replication by interacting with viral nucleoprotein. J Virol. 2009;83:7850–7861. doi: 10.1128/JVI.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock DM, Zuccotti G. The evolution of influenza resistance and treatment. JAMA. 2009;301:1066–1069. doi: 10.1001/jama.2009.324. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization; 2002. [Google Scholar]
- Wu WL, Chen Y, Wang P, Song W, Lau SY, Rayner JM, Smith GJ, Webster RG, Peiris JS, Lin T, Xia N, Guan Y, Chen H. Antigenic profile of avian H5N1 viruses in Asia from 2002 to 2007. J Virol. 2008;82:1798–1807. doi: 10.1128/JVI.02256-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Herlocher LM, Hoffmann E, Matrosovich MN, Monto AS, Webster RG, Govorkova EA. Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility. Antimicrob Agents Chemother. 2005;49:4075–4084. doi: 10.1128/AAC.49.10.4075-4084.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen HL, Hoffmann E, Taylor G, Scholtissek C, Monto AS, Webster RG, Govorkova EA. Importance of neuraminidase active-site residues to the neuraminidase inhibitor resistance of influenza viruses. J Virol. 2006;80:8787–8795. doi: 10.1128/JVI.00477-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon M, Hayden FG. Position statement: global neuraminidase inhibitor susceptibility network. Antiviral Res. 2001;49:147–156. doi: 10.1016/s0166-3542(01)00124-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Comparison of the growth kinetics of clinical isolates of oseltamivir resistant and susceptible strains of the A/Brisbane/59/2007-like H1N1 virus in MDCK cells (A) and A549 cells (B). A/HK/04633/2008 and A/HK/03138/2008 are oseltamivir susceptible, while A/HK/17566/2009 and A/HK/16418/2009 are oseltamivir resistant. Techniques for virus infection and estimation of virus titer (plaque forming units, PFU) are described in the materials and methods. Results were obtained from three repeated experiments and error bars indicate standard deviation.