Abstract
Several studies investigated the effect of aging on rat and human lacrimal gland physiology. However, in most of these studies, only two age groups were investigated. Furthermore, those studies did not correlate the age-related histological changes that occur in the lacrimal gland to the functional changes (nerve activity and protein secretion) that might occur with aging. Thus, the purpose of the present study was to investigate the effect of aging on lacrimal gland structure, innervation and function using BALB/c mice at different ages. Exorbital lacrimal glands were removed from 3, 8, 12, 24, and 32-month-old, male BALB/c mice, fixed, embedded and processed for histology and immunohistochemistry. Sections were stained with hematoxylin and eosin to determine morphological changes and lymphocytic infiltration; giemsa to identify mast cells; and Kinyoun’s carbol fucsin solution to indicate lipofuscin-like inclusions. Parasympathetic and sympathetic nerves were identified by immunofluorescence techniques. To measure acetylcholine release and protein secretion, lacrimal gland pieces were incubated in Krebs Ringer buffer containing 5 mm KCl (control), 75 mm KCl (depolarizing buffer which activates nerves), carbachol (a cholinergic agonist, 10−4 m), or phenylephrine (an α1-adrenergic agonist, 10−4 m) for 20 min. The media were collected and analysed for acetylcholine and peroxidase using a spectrofluorometric assay. KCl-, carbachol- and phenylephrine-stimulated peroxidase secretion decreased in lacrimal glands from 8, 12, and 24-month-old mice when compared to 3-month-old animals. Both the density and distribution of parasympathetic and sympathetic nerves surrounding the acini decreased with increasing age. Acetylcholine release from lacrimal gland nerves decreased in 24-month-old mice compared to 3- and 12-month-old animals. Similarly, progressive morphological changes, including increased numbers of lipofuscin-like inclusions, mast cells and lymphocytic infiltration occurred in an age-dependent manner. We conclude that structural alterations of mouse lacrimal gland, including increased accumulation of lipofuscin-like inclusions, chronic inflammation and functional alterations including decreased acetylcholine release and protein secretion occurred with aging.
Keywords: tear film, lipofuscin, lymphocyte, mast cells, nerves, aging
1. Introduction
The aqueous layer of the tear film consists of proteins, electrolytes, and water, which are mainly secreted by the lacrimal gland. The lacrimal gland is a tubuloalveolar exocrine gland consisting of acinar cells organized in serous acini surrounded by myoepithelial cells, ductal epithelial cells, blood vessels and nerves. Approximately 80% of the gland consists of acinar cells, which synthesize, store, and secrete proteins, water and electrolytes. Lacrimal gland secretion is regulated by autonomic nerves. Under normal conditions, stimulation of sensory nerves from the ocular surface results in a reflex loop activation of parasympathetic and sympathetic nerves that innervate acini, ducts, and blood vessels (Dartt, 1994). Neurotransmitters such as acetylcholine, the neuropeptide vasoactive intestinal peptide (VIP), and norepinephrine released from parasympathetic and sympathetic nerves are well-known stimuli of lacrimal gland secretion and the signaling pathways activated by these stimuli have been characterized (Dartt, 2001).
An unstable tear film results when the lacrimal gland fails to secrete proteins and fluid in sufficient quantity and/or appropriate composition, compromising the homeostasis of the ocular surface. As a result of this unstable tear film, ocular surface diseases such as dry eye syndromes, which affect about 10 million Americans, can occur (Lemp, 1995). They affect both men and women, although are most common in post-menopausal women (Schaumberg et al., 2001). The incidence of dry eye increases with increasing age (Schaumberg et al., 2002; Sullivan et al., 2002) so that nearly 15% of people over age 65 experience dry eye syndrome (Schaumberg et al., 2002; Sullivan et al., 2002).
Age-related changes in the lacrimal gland are associated with alterations in the structural organization and functional response in the lacrimal gland of diverse mammalian species including humans (Damato et al., 1984; Bromberg et al., 1986; Obata et al., 1995; 1990; Sullivan et al., Ueno et al., 1996; Van Haeringen, 1997; Draper et al., 1999). These changes include increased periductal fibrosis and acinar atrophy, and an increase in inflammatory infiltrates, which contain mast cells and lymphocytes (Damato et al., 1984; Draper et al., 1998; Obata et al., 1995; Williams et al., 1994). Lacrimal glands of aging animals also appear to have reduced ability to synthesize and secrete proteins, which may result in an aqueous tear-deficient dry eye (Roen et al., 1985; Bromberg et al., 1986; Draper et al., 1997). Although age-related changes in lacrimal gland structure and function have been described, in most of these studies, only two age groups have been investigated. Furthermore, those studies did not correlate the age-related histological changes that occur in the lacrimal gland with functional changes in nerve activity and protein secretion that occur with aging.
Thus, in the present study, we used BALB/c mice at different ages (3, 8, 12, 24, and 32 months old), which are essentially the entire life span of the animal, to investigate the effects of aging on lacrimal gland physiology. From the same specimens, we performed both histological, immunohistochemical, and biochemical experiments to determine the effect of aging on lacrimal gland morphology, innervation, and protein secretion, respectively.
2. Materials and methods
Materials
Polyclonal and monoclonal antibodies to the following neural markers were purchased: synaptophysin (NeoMarkers, Fremont, CA), vasoactive intestinal peptide (VIP) (DiaSorin, Stillwater, MN), tyrosine hydroxylase (TH) (Calbiochem, San Diego, CA), and dopamine β-hydroxylase (DBH) (Eugene Tech International, Ridgefield Park, NJ). FITC-conjugated donkey anti-rabbit IgG or donkey anti-mouse IgG were purchased from Jackson Laboratories (West Grove, PA). Carbachol, phenylephrine, neostigmine bromide, choline oxidase, acetylcholinesterase, and horseradish peroxidase were from Sigma (St Louis, MO). Amplex Red was from Molecular Probes (Eugene, OR).
Animals
Male BALB/c mice ranging in age from 3 to 32 months were obtained from the colonies of the National Institute of Aging (Bethesda, MD). Before sacrifice, the eyes of all mice were evaluated under a slit-lamp using 2% fluorescein to investigate tear film integrity and corneal clarity. The mice were then anesthetized for 1 min in CO2 and sacrificed by cervical dislocation. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Histology preparation
Exorbital lacrimal glands were removed from euthanized mice and cut into pieces. Random pieces were fixed in 4% formaldehyde in phosphate buffered saline (PBS, containing in mm: 145 NaCl, 7·3 Na2HPO4, and 2·7 KH2PO4, pH 7·2) for 24hr at 4°C. For histopathology experiments, the tissue was rinsed with PBS, dehydrated (graded alcohol to 95%), and then infiltrated and embedded in Leica Historesin (Leica Deerfield, IL). Five tissue sections (6 µm) were placed on a microscope slide (Superfrost/Plus) by collecting every tenth section. Sections were allowed to dry at 60°C. Slides were stained with either hematoxylin and eosin for histological observation and to identify lymphocytes; with 10% giemsa, to identify mast cells; or with Kinyoun’s carbol fucsin solution to identify lipofuscin-like inclusions.
Histopathological evaluation
Histopathological changes were evaluated using the parameters described by Obata et al. (Obata et al., 1995). The following morphological changes were established as study parameters: acinar atrophy, acinar fibrosis, periductal fibrosis, interlobular ductal dilation, interlobular ductal proliferation, lymphocytic infiltration, mast cell infiltration, and lipofuscin-like accumulation. The criteria used to grade each parameter were as follows: acinar atrophy and fibrosis were graded as absent, focal, lobular or diffuse and were given scores of 0, 1, 2, or 3; respectively. ‘Focal’ was defined as changes present only within one lobule, ‘lobular’ as changes in less than 50% of the lobules, and ‘diffuse’ as changes in more than 50% of the lobules. Periductal fibrosis, interlobular ductal dilation and interlobular ductal proliferation changes were classified negative if not present and positive if present. Lymphocytic infiltration was defined as the number of lymphocytic foci present in the lobular and periductal regions. One lymphocytic focus was defined as consisting of 50 or more cells. The following scores were used to grade lymphocytic infiltration: 0 if no foci were present, grade 1 if one to two foci were present, grade 2 if two to four foci were present, and grade 3 if more than four foci were present. Mast cell infiltration was defined as mast cells infiltrated from the interlobular to the intralobular space. Mast cells were graded as 0 if present in blood vessels and connective tissue, 1 if present in interlobular space, 2 if ≤ 20 mast cells infiltrated the intralobular space, and 3 if more than 20 cells infiltrated the intralobular space. Lipofuscin-like accumulation was defined as the number of cells with age-related pigments accumulated per lobule. The presence of lipofuscin was grade 0 if not present, grade 1 if present within only few lobules, grade 2 if present in less than 50% of the lobules, and grade 3 if present in more than 50% of the lobules. The slides were viewed and graded, in a masked fashion, by three individuals. The results were then averaged.
Measurement of acetylcholine release and peroxidase secretion
Lacrimal glands were removed and cut into lobules (~2 mm diameter). The tissue was placed in cell strainers and incubated at 37°C in Krebs-Ringer bicarbonate buffer (KRB, containing in mm: 120 NaCl, 5 KCl, 1 CaCl2, 1·2 MgCl2, 1·2 KH2PO4, and 25 NaHCO3) supplemented with 10 mm Hepes and 5·5 mm glucose, pH 7·4. The cell strainers containing lobules were transferred into fresh KRB solution at 20 min intervals for a total of 60 min. The lobules were then incubated for 20 min in a total volume of 0·8 ml in normal KRB (referred to as spontaneous secretion) followed by depolarizing KRB (evoked secretion) solution in which the concentration of KCl was increased to 75 mm and that of NaCl decreased to 50 mm to maintain isotonicity. Both normal and depolarizing KRB solutions contained neostigmine bromide (0·1 mm), an inhibitor of acetylcholine esterase, in order to allow the accumulation of released acetylcholine in the media. For peroxidase secretion, lacrimal gland lobules were further incubated for 20 min in 0·8 ml of normal KRB containing either carbachol (a cholinergic agonist, 10−4 m) or phenylephrine (an α1-adrenergic agonist, 10−4 m). Following incubation, the media were collected and centrifuged to remove debris. The lobules were then homogenized in 10 mm Tris–-HCl, pH 7·5. The amounts of acetylcholine and peroxidase in the media and tissue homogenate were determined using a spectrofluorometric assay, as previously described (Zoukhri and Kublin, 2001).
Immunohistochemistry
Lacrimal glands were fixed in 4% buffered formaldehyde, cryopreserved overnight in 30% sucrose in PBS at 4°C, and frozen in optimal cutting embedding compound (Miles, Elkhart, IN). Cryostat sections were thawed for 1 hr at room temperature, washed in PBS, and blocked in PBS containing 1% goat serum, 1% bovine albumin serum, and 0·2% Triton X-100 for 1 hr at room temperature. Antibodies against synaptophysin and VIP were diluted 1:1000 and 1:800, respectively, in PBS containing 0·2% Triton X-100. Antibodies against TH and DBH were diluted 1:400 in PBS containing 0·2% Triton X-100. Sections were incubated with the respective primary antibody for either 24 hr (synaptophysin, and VIP), or 36 hr at 4°C (TH and DBH). The secondary antibodies, donkey anti-rabbit IgG or donkey anti-mouse IgG conjugated to FITC were diluted 1:200 in PBS and applied for 1 hr at room temperature. Sections were viewed and photographed using a Nikon UFX II microscope equipped for epi-illumination using the 488 nm wavelength to excite FITC. Sections were also excited at 568 nm to detect lipofuscin-like inclusions (Utsunomiya et al., 2001). The negative controls consisted of omission of the primary antibodies.
Data presentation and statistical analysis
Data are expressed as mean±s.e.m. The data were statistically analysed using Spearman Rank correlation logistic regression (Preisser and Koch, 1997; Zhao et al., 2001) and Student’s t-test for paired and unpaired values. Values of P<0·05 were considered to be significant.
3. Results
Effect of age on the ocular surface
To determine whether aging caused changes in tear film integrity and in corneal clarity, the ocular surface was examined by slit-lamp. Our observations were categorized on the basis of pathological manifestations such as conjunctivitis, superficial punctate keratopathy (SPK), corneal opacity, corneal ulcer and corneal vascularization. Pathological manifestations were not observed in the corneas and conjunctivas of 3 (n = 10) and 8-month-old (n = 4) animals. Conjunctivitis (mucus discharge) was detected in two 12-month-old (n = 10) mice. SPK and corneal ulcers were found in three 24-month-old (n = 8) mice. Corneal ulcers and corneal vascularization were found in two to three of the 32-month-old (n = 8) animals. Tenacious strings of mucus were found on the conjunctiva in three 32-month-old (n = 8) mice. An increase in the incidence of SPK, corneal ulcers and corneal vascularization was observed in mice 24 and 32 months of age compared to mice which were 3, 8, and 12 months old.
These results suggest that tear film and corneal abnormalities appeared more frequently as the animals aged.
Effect of age on lacrimal gland histology
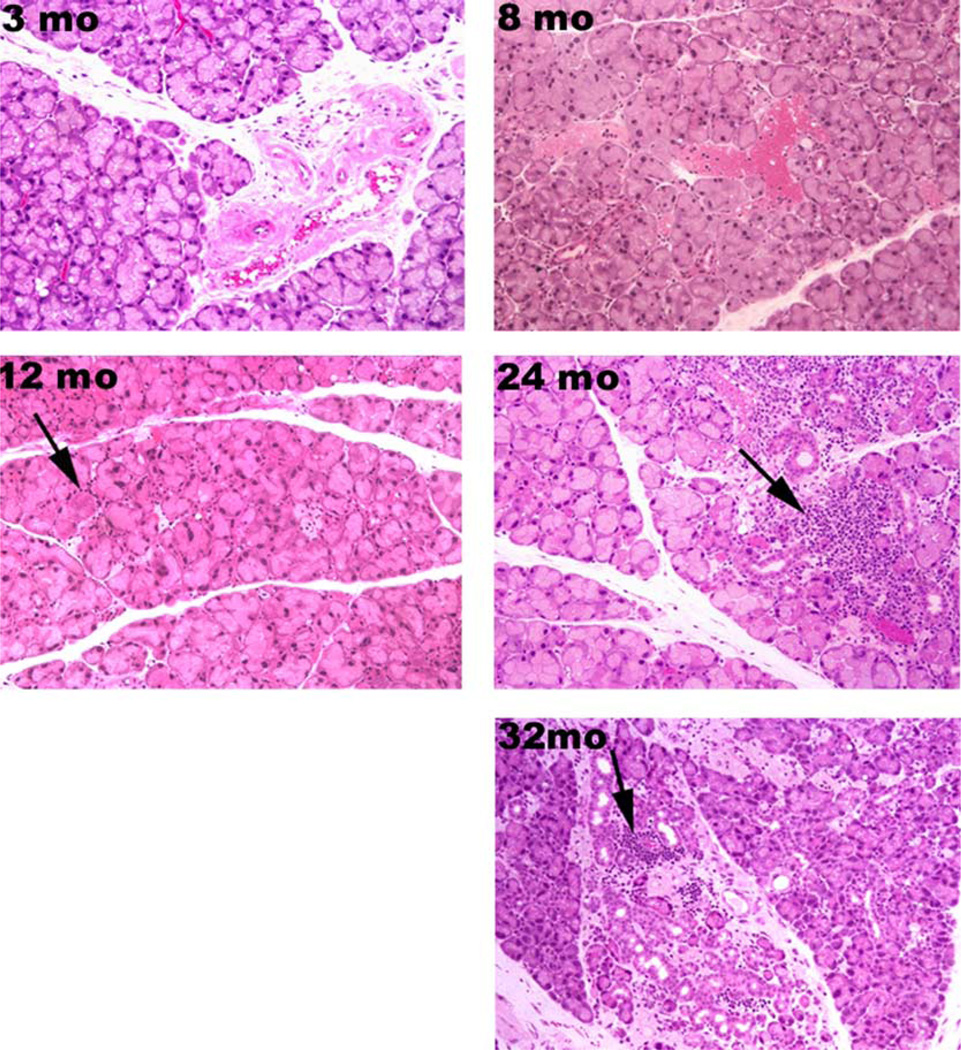
Aging is associated with an array of changes in lacrimal gland structure. To determine the effect of aging on lacrimal gland histology, sections prepared from 3, 8, 12, 24, and 32-month-old animals were stained with hematoxylin and eosin. The slides were viewed, in a masked fashion, by three investigators and scored for acinar atrophy, acinar fibrosis, periductal fibrosis, interlobular ductal dilation, and interlobular ductal proliferation as described in the methods section. As summarized in Table 1, periductal fibrosis, interlobular dilation and interlobular ductal proliferation were not observed in glands from 3, 8, or 12-month-old mice, but they were observed frequently in glands from 24 and 32-month-old animals. Similarly, acinar atrophy and acinar fibrosis were more frequently observed in glands from 12–32-month old animals (Table 1). There was a strong correlation between aging and acinar atrophy and fibrosis.
Table 1.
Effect of age on lacrimal gland morphology
| Age (months) | |||||
|---|---|---|---|---|---|
| 3 | 8 | 12 | 24 | 32 | |
| Periductal fibrosis | 0/7 | 0/4 | 0/6 | 3/6 | 5/6 |
| Interlobular ductal dilation | 0/7 | 0/4 | 0/6 | 1/6 | 1/6 |
| Interlobular ductal proliferation | 0/7 | 0/4 | 0/6 | 3/6 | 2/6 |
| Acinar atrophy* | 0 | 0 | 0·5 ± 0·2 | 1·3 ± 0·3 | 2·3 ± 0·3 |
| Acinar fibrosis* | 0 | 0 | 0 | 1±0·4 | 1·7±0·6 |
Lacrimal gland sections prepared from 3 (n = 7), 8 (n = 4), 12 (n = 6), 24 (n = 6), and 32 (n = 6)-month-old mice were stained with hematoxylin and eosin. Sections were examined by three investigators and scored. The criteria used to grade each parameter were classified as follows: periductal fibrosis, interlobular ductal dilation and interlobular ductal proliferation changes were classified negative if not present and positive if present. Acinar atrophy and fibrosis were graded as absent, focal, lobular or diffuse and were given scores of 0, 1, 2, or 3; respectively. ‘Focal’ was defined as changes present only within one lobule, ‘lobular’ as changes in less than 50% of the lobules, and ‘diffuse’ as changes in more than 50% of the lobules.
Data are means±SEM of the individual scores. Data were analysed using the Spearman’s Rank Correlation test and the P values are 0·0001 and 0·0006 for acinar atrophy and fibrosis, respectively.
These results suggest that structural changes occurred in lacrimal gland with aging and were first observed at 12 month of age and progressed as the animals aged.
Effect of age on lacrimal gland protein secretion
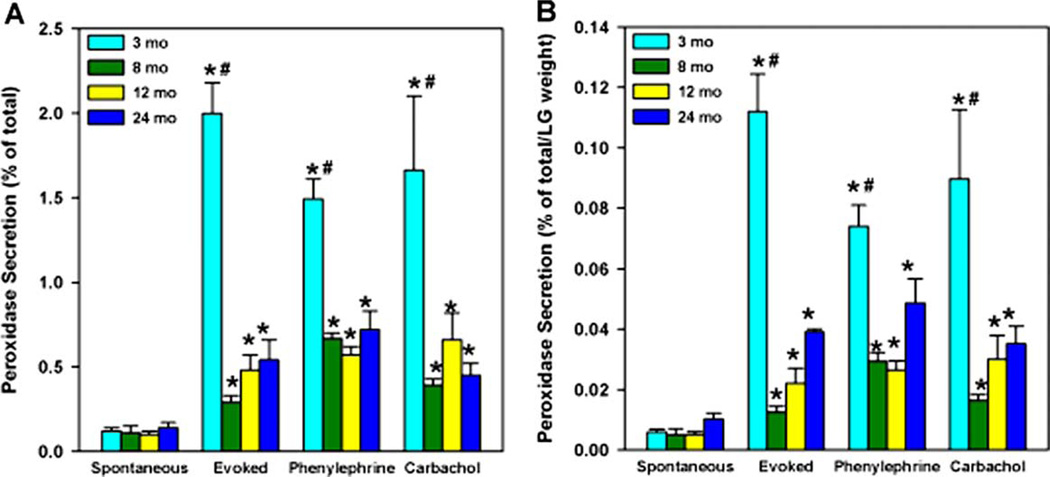
A previous study has shown that peroxidase (a protein secreted by the lacrimal gland) secretion in response to a depolarizing (high) KCl solution can be used as an indirect index to monitor lacrimal gland function and nerve activity (Zoukhri and Kublin, 2001). The high KCl solution depolarizes the nerve endings to release their neurotransmitters that in turn stimulate protein secretion from the acinar cells. We term this evoked release. This can be compared to secretion induced by exogenous agonists. We determined whether the peroxidase secretion was altered with age by using a spectrofluorometric assay (Zoukhri and Kublin, 2001). As shown in Fig. 1(A), spontaneous secretion, that is secretion in the presence of normal (5 mm) KCl, was the same at 3, 8, 12, and 24 months. Compared to spontaneous secretion, evoked secretion (75 mm KCl) was increased 16·7-fold in 3-month-old animals. In 8, 12 and 24-month-old animals evoked secretion was increased by only 2·6, 4·8, and 3·9-fold, respectively. A significant reduction in the amount of peroxidase secreted in response to 75 mm KCl (evoked secretion) was obtained in lacrimal glands of 8, 12 and 24-month-old animals compared with secretion from glands of 3-month-old animals. Carbachol- (a cholinergic agonist) and phenylephrine- (an α1-adrenergic agonist) induced 13·8 and 12·4-fold increases of peroxidase secretion in lobules from 3-month-old animals when compared to spontaneous secretion (Fig. 1). Carbachol and phenylephrine induced peroxidase secretion was significantly reduced in lobules from 8, 12 and 24-month-old animals compared to 3-month-old animals (Fig. 1(A)).
Fig. 1.
Effect of age on KCl-, phenylephrine-, and carbachol-induced peroxidase secretion. Lacrimal glands of 3, 8, 12, and 24-month-old male BALB/c mice were removed and lobules were incubated for 20 min in buffer containing 5 mm KCl (spontaneous secretion), 20 min in 75 mm KCl (evoked secretion), 20 min in the presence of the adrenergic agonist, phenylephrine (10−4 m) or the muscarinic agonist, carbachol (10−4 m) and peroxidase secretion was measured. (1A). Peroxidase secretion is expressed as a percentage of the total. (1B). Peroxidase secretion was normalized to the weight of the lacrimal gland. Data are expressed as means±s.e.m. from 3–10 independent experiments. *Indicates significant difference from spontaneously secreted peroxidase. # Indicates significant difference from 8, 12 and 24-month.
To test whether or not the decreased secretory response from these glands was due to a decrease in total lacrimal gland peroxidase, we measured tissue peroxidase content. As shown in Table 2, compared to 3- and 12-month-old animals, total tissue peroxidase decreased in lacrimal glands from 24-month-old animals. However, lacrimal gland weight also decreased in these animals. Hence, when the amount of total peroxidase was normalized to the lacrimal gland weight, there was no effect of aging on tissue peroxidase (Table 2), suggesting that the decreased secretory response of lacrimal glands from middle and old aged animals was not due to a decrease in total lacrimal gland peroxidase. Similarly, when stimulated peroxidase secretion was normalized to the lacrimal gland weight, the amounts secreted by 12- and 24-month-old lacrimal glands was still significantly lower than that secreted by 3-month-old glands (Fig. 1(B)).
Table 2.
Effect of age on lacrimal gland weight and the amount of lacrimal gland peroxidase
| Age (months) | |||
|---|---|---|---|
| 3 | 12 | 24 | |
| Peroxidase | 72·12 ± 5·98 | 69·09 ± 5·59 | 55·24 ± 7·51* |
| LGW | 17·60 ± 0·75 | 20·70 ± 0·88* | 14·10 ± 0·98* |
| Peroxidase/LGW | 4·24 ± 0·46 | 3·46 ± 0·38 | 4·07 ± 0·88 |
The amount of peroxidase was determined using a spectrofluorometric assay and is expressed in arbitrary units. LGW, lacrimal gland weight in g. Data are expressed as means±S.E.M. (n = 10).
Denotes significant difference from 3-month-old animals.
These results suggest that a reduction in neurally- as well as agonist-stimulated lacrimal gland protein secretion occurred with aging and was first detected in 8-month-old animals.
Effect of age on lacrimal gland innervation
Because lacrimal gland secretion is under neural control, any impairment of either parasympathetic or sympathetic nerves associated with age could account for reduced secretion. A previous study on age-related changes in lacrimal gland innervation showed that the number and intensity acetylcholinesterase (AChE), VIP, neuropeptide Y (NPY), calcitonin gene-related peptide (CGRP), tyrosine hydroxylase, substance P and the phosphoprotein B-50 immunoreactive nerves were reduced in aged rats (24-month-old) (Williams et al., 1994).
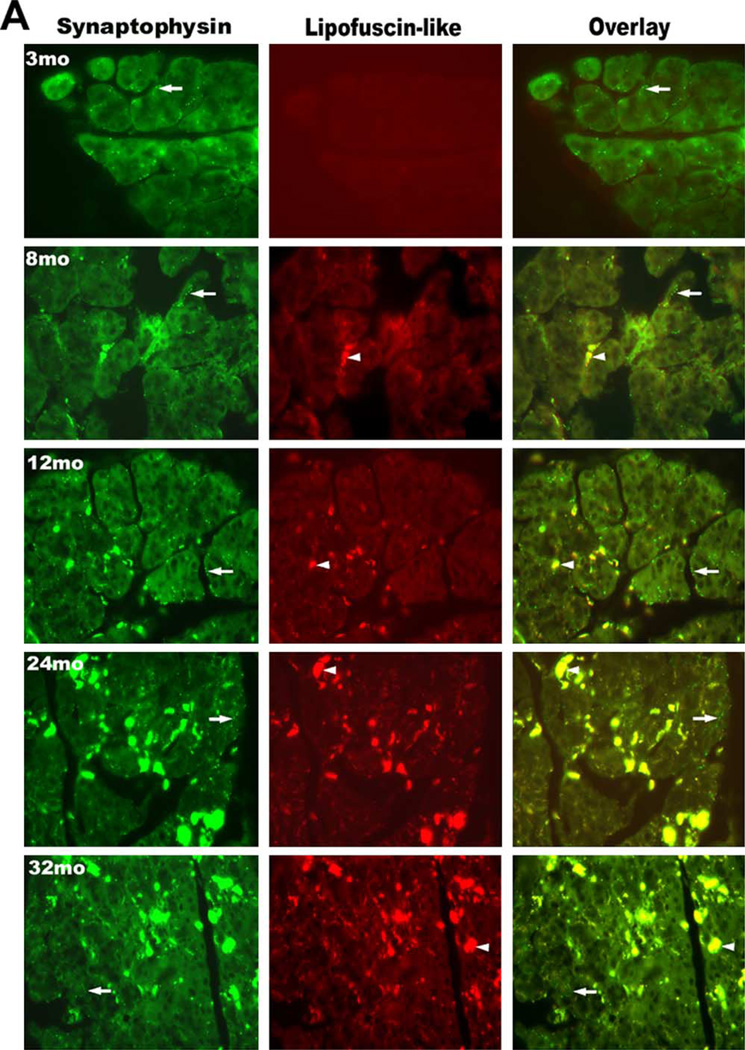
The results in Fig. 1 indicated that the secretory response of lacrimal glands decreased with increasing age starting at 8 months. Therefore, we examined whether the innervation was altered with age, which could account for the diminished secretory response observed in older animals. Since lipofuscin autofluoresces at the same wavelength as FITC, the fluorophore used to indicate the location of nerves, separate micrographs illustrating the localization of lipofuscin were included to differentiate nerves from lipofuscin-like inclusions. The density and pattern of nerve distribution were evaluated by immunofluorescence techniques using an antibody against synaptophysin, a synaptic protein present in all types of nerves. As shown in Fig. 2(A), synaptophysin immunoreactivity appeared as a varicose network surrounding the acinar and ductal cells in lacrimal glands of 3-month-old mice. Both the innervation pattern and nerve density in 8–12-month-old mice were similar to those of 3-month-old animals (Fig. 2(A)). As the animals aged (24 and 32 months) a substantial decrease in nerve density was seen throughout the lacrimal gland (Fig. 2(A)). Omission of the primary antibody resulted in a loss of nerve staining but the lipofuscin autofluorescence was still visible in tissue from old animals (Fig. 2(B)).
Fig. 2.
Immunolocalization of nerves containing synaptophysin in the lacrimal gland. Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and processed for immunohistochemistry with anti-synaptophysin antibody. (2A). Immunofluorescence at 488 nm (green) represents synaptophysin immunoreactivity and autofluorescence of lipofuscin-like inclusions. Fluorescence at 568 nm (red) indicates lipofuscin-like inclusions, which also fluoresce at this wavelength. Overlay of fluorescence observed at 488 and 568 nm distinguishes between the autofluorescence of lipofuscin-like inclusions and the fluorescence of synaptophysin-containing nerves, as synaptophysin-like immunoreactivity is green and whereas lipofuscin-like inclusions are yellow. Arrows indicate areas of nerves. Arrowheads indicate areas of lipofuscin-like inclusions. (2B). Omission of the primary antibody led to a loss of synaptophysin immunoreactivity whereas lipofuscin autofluorescence was still visible in older tissue. Similar results were observed in at least three other experiments. Magnification, ×400.
To evaluate changes in the distribution pattern and density of parasympathetic nerves with age, we used an antibody against VIP, a neuropeptide present in parasympathetic nerve endings. As shown in Fig. 3, a dense network of VIP-containing nerves surrounded most acini from glands of 3-month-old animals. A similar pattern of VIP-containing nerves was observed in glands of 8 and 12-month-old animals (Fig. 3). Similar to synaptophysin, a substantial decrease in the density of VIP-containing nerves was observed in glands from 24 and 32-month-old mice (Fig. 3).
Fig. 3.
Immunolocalization of nerves containing vasoactive intestinal peptide (VIP) in the lacrimal gland. Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and processed for immunohistochemistry with anti-VIP antibody. Immunofluorescence at 488 nm (green) represents VIP-immunoreactivity and autofluorescence of lipofuscin-like inclusions. Fluorescence at 568 nm (red) indicates lipofuscin-like inclusions, which also fluoresce at this wavelength. Overlay of fluorescence observed at 488 and 568 nm distinguishes between the autofluorescence of lipofuscin-like inclusions and the fluorescence of VIP-containing nerves as VIP-like immunoreactivity is green and whereas lipofuscin-like inclusions are yellow. Arrows indicate areas of nerves. Arrowheads indicate areas of lipofuscin-like inclusions. Similar results were observed in at least three other experiments. Magnification, ×400.
We then investigated whether the sympathetic innervation in the gland changed with age using antibodies against tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH), two enzymes located in sympathetic nerve endings. In lacrimal glands of 3-month-old animals, TH-containing nerves were observed around only a few acini and blood vessels (Fig. 4). At 8 months, TH-containing nerves were observed around some acini and blood vessels. A punctuate pattern of TH-containing nerves was seen in glands from 24 and 32-month-old animals. However, compared to glands from 3 and 8-month-old animals, a considerable reduction in the density of TH-containing nerves was observed in glands from 12, 24, and 32-month-old mice (Fig. 4). Similar results were obtained when DBH was used as marker for sympathetic nerve endings (data not shown).
Fig. 4.
Immunolocalization of nerves containing tyrosine hydroxylase (TH) in the lacrimal gland. Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and processed for immunohistochemistry with anti-TH antibody. Immunofluorescence at 488 nm (green) represents TH-immunoreactivity and autofluorescence of lipofuscin-like inclusions. Fluorescence at 568 nm (red) indicates lipofuscin-like inclusions, which also fluoresce at this wavelength. Overlay of fluorescence observed at 488 and 568 nm distinguishes between the autofluorescence of lipofuscin-like inclusions and the fluorescence of TH-containing nerves, as TH-like immunoreactivity is green and whereas lipofuscin-like inclusions are yellow. Arrows indicate areas of nerves. Arrowheads indicate areas of lipofuscin-like inclusions. Similar results were observed in at least three other experiments. Magnification, ×400.
These results show that both the parasympathetic and sympathetic innervation of lacrimal gland acini decreased as the animal aged with a decrease occurring at 24 months. Thus a decrease in nerve density did not appear to account for the decrease in secretion detected at 8 and 12 months of age, but could contribute to the decrease seen at 24 and 32 months of age.
Effect of age on acetylcholine release
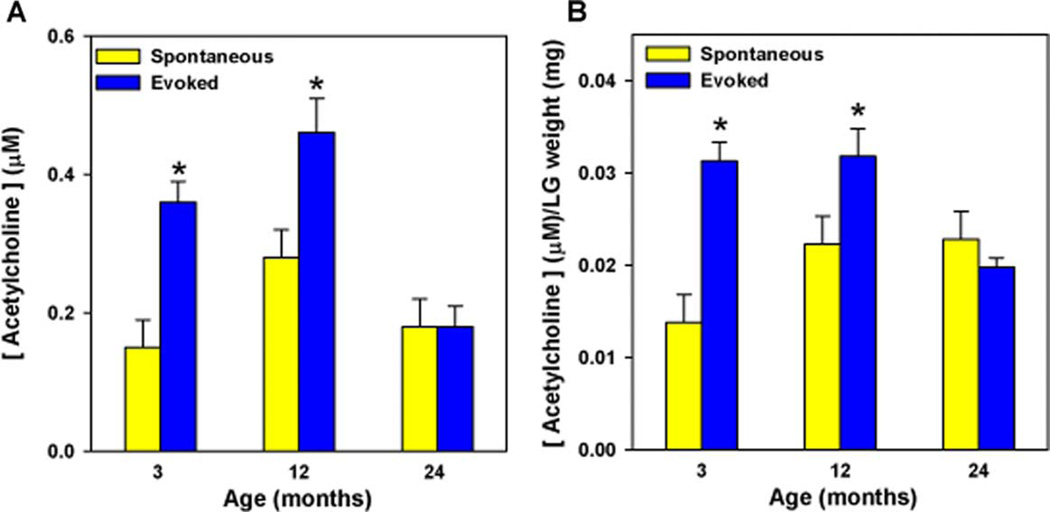
As we demonstrated that a reduction in neurally- as well as agonist-stimulated lacrimal gland protein secretion occurred before a decrease in innervation, we next investigated whether a change in the activity of parasympathetic nerves occurred with age. Lacrimal gland lobules were incubated in buffer containing 75 mm KCl, which will depolarize nerve endings and stimulate them to release their neurotransmitters. As shown in Fig. 5, high KCl (evoked release) induced a significant release of acetylcholine from lacrimal glands of 3- and 12-month old mice compared to 5 mm KCl (spontaneous release). In contrast, nerves of lacrimal glands from 24-month-old mice were not able to release acetylcholine in response to the depolarizing solution (Fig. 5(A)). Compared to spontaneous release, the evoked release increased 3- and 1·7-fold, respectively, in 3 and 12-month-old animals, but was not increased at all in 24-month-old animals. Similar to stimulated peroxidase secretion, when the amount of released acetylcholine was normalized to the weight of the lacrimal gland, evoked acetylcholine release was still impaired in 24-month-old animals (Fig. 5(B)).
Fig. 5.
Effect of age on KCl-evoked acetylcholine release. Lacrimal glands of 3, 12, and 24-month-old male BALB/c mice were removed and lobules were incubated for 20 min in buffer containing 5 mm KCl (spontaneous release) or 75 mm KCl (evoked release). (5A). Acetylcholine release is expressed as micromoles of acetylcholine. (5B). The concentration of released acetylcholine was normalized to the weight of the lacrimal gland. Data are expressed as means±s.e.m. from three independent experiments. *Indicates significant difference from spontaneously released acetylcholine.
These results show that a decrease of parasympathetic nerve activity in the lacrimal gland did not account for the decreased peroxidase secretion that occurred at 12 months of age, but could account for the decrease measured at 24 months.
Effect of age on the accumulation of lipofuscin-like inclusions in the lacrimal gland
Lipofuscin-like inclusions belong to the family of age-related pigment often referred to as fluorescent age pigment (FAP) (Donato and Sohal, 1981). This fluorescent pigment consists of aggregated polymers derived from oxidation products of lipids and proteins (Sohal et al., 1989) and accumulates progressively over time in the lysosomes of post-mitotic cells (Terman and Brunk, 1998). To determine if lipofuscin-like inclusions accumulate progressively with age in the lacrimal gland, and could account for the loss of secretion, tissue sections were stained with Kinyoun’s carbol fucsin solution. As shown in Fig. 6, no lipofuscin accumulation was observed in 3-month-old animals. Lipofuscin-like inclusions appeared in the cytoplasm of acinar cells in glands taken from animals 8–12 months of age. As the animals aged (24 and 32 months old), large cytoplasmic granules of lipofuscin-like inclussions were present in many acinar cells compared to glands from younger animals. When the data presented in Fig. 6 were analysed using Spearman’s Rank Correlation test, there was a strong correlation (P<0·0001) between aging and accumulation of lipofuscin-like inclusions in the lacrimal gland (Fig. 7).
Fig. 6.
Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and stained with Kinyoun’s carbol fuchsin (Kinyoun’s Carbol). Arrows indicate areas of lipofuscin-like inclusions. Similar results were observed in at least three other experiments. Magnification, ×200.
Fig. 7.
Lacrimal gland sections prepared from 3 (n = 7), 8 (n = 4), 12 (n = 6), 24 (n = 6), and 32 (n = 6)-month-old mice were stained with hematoxylin and eosin (for lymphocytic infiltration), giemsa (for mast cell infiltration), or Kinyoun’s carbol fucsin (for lipofuscin-like accumulation). Sections were examined by three investigators and scored using the following criteria: Lymphocytic infiltration was defined as the number of lymphocytic foci present in the lobular and periductal regions and graded as 0 if no foci were present, grade 1 if one to two foci were present, grade 2 if two to four foci were present, and grade 3 if more than four foci were present. Mast cells were graded as 0 if present in blood vessels and connective tissue, 1 if present in interlobular space, 2 if ≤20 mast cells infiltrated the intralobular space, and 3 if more than 20 cells infiltrated the intralobular space. The presence of lipofuscin was grade 0 if not present, grade 1 if present within only few lobules, grade 2 if present in less than 50% of the lobules, and grade 3 if present in more than 50% of the lobules. Data are means±s.e.m. of the individual scores. Data were analysed using the Spearman Rank Correlation test and the P values are shown in the plot.
Lipofuscin is known to auto-fluoresce at both 455 nm (wavelength used to excite FITC) as well as at 568 nm (Utsunomiya et al., 2001). The presence of lipofuscin-like inclusions associated with age was also evaluated by immunofluorescence microcopy in the same tissue sections used to indicate the location of nerves. As shown in Figs. 2–4, accumulation of lipofuscin inclusions was observed throughout the acinar cell cytoplasm either in a packed or diffuse pattern. Accumulation of lipofuscin was not observed in either acinar or ductal cells from the lacrimal glands of 3-month-old animals. Lipofuscin granules were diffusely distributed in a few acinar cells in 8-month-old animals. Increased amounts of lipofuscin granules appeared to preferentially accumulate in the acinar cells as the animal aged from 12 to 32 months.
These results show that accumulation of lipofuscin-like inclusions in the lacrimal gland occurred at 8 months of age and progressed as the animals aged. This process could have contributed to the decrease in secretion that was detected in 8-month-old animals.
Effect of age on the accumulation of inflammatory cells (mast cells and lymphocytes) in the lacrimal gland
Chronic inflammation has been observed in aged lacrimal gland of diverse mammalian species including humans (Damato et al., 1984; Draper et al., 1999). To determine whether the number and distribution of mast cells and lymphocytes changed with increasing age in mice and could account for the decrease in secretion that occurred in 8-month-old animals, tissue sections of lacrimal glands were stained with giemsa, for mast cells or with hematoxylin and eosin for lymphocytes. In the lacrimal glands of 3-month-old mice, mast cells were confined to the interlobular connective tissue and located around blood vessels (Fig. 8). Mast cell infiltration of the interlobular space (grade 1) was observed in glands from 8–12-month-old animals (Fig. 8). Appearance of degranulating mast cells was also observed in 12-month-old mice (Fig. 8, insert). Increased number of mast cells and infiltration of the intralobular space (grade 2–3) were observed in glands from 24- and 32-month-old mice (Fig. 8). When the data presented in Fig. 8 were analysed using the Spearman’s Rank Correlation test, there was a strong correlation (P<0·0001) between aging and infiltration of the lacrimal gland by mast cells (Fig. 7).
Fig. 8.
Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and stained with Giemsa. Arrows indicate mast cell infiltration while the arrowhead indicates a degranulating mast cell that is shown at a higher magnification in the insert. Similar results were observed in at least three other experiments. Magnification, ×400.
Based on hematoxylin and eosin staining, a progressive increase in lymphocytic infiltration, including periductal and focal infiltration (grade 2–3) was observed in lacrimal glands from older animals (24–32 months) but not in animals at 3, 8 or 12 months of age (Fig. 9). When the data presented in Fig. 7 were analysed using the Spearman’s Rank Correlation test, there was a strong correlation (P<0·0001) between aging and infiltration of the lacrimal gland by lymphocytes with a dramatic increase in lymphocytes occurring at 24 months of age (Fig. 7).
Fig. 9.
Lacrimal glands of 3, 8, 12, 24, and 32-month-old male BALB/c mice were removed and stained with Hematoxylin-eosin (H and E). Arrows indicate areas of lymphocytic infiltration. Similar results were observed in at least three other experiments. Magnification, ×200.
Although the number of lymphocytic foci increased with age, the presence and/or infiltration of neutrophils was not observed. Neutrophils were observed predominantly in the blood vessels and occasionally in the intralobular spaces (data not shown). This observation suggests that the progression of the inflammatory process in the lacrimal gland with aging is not neutrophil-dependent. Identification of other granulocytes such as macrophages or basophils was not determined.
These results show that a progressive infiltration of the gland by mast cells and lymphocytes occurred in an age-dependent manner. These changes are characterized by an early infiltration of mast cells beginning at 8 months followed by a later infiltration of lymphocytes beginning at 24 months (Fig. 7). Thus infiltration of mast cells, but not of lymphocytes, could account for the decrease in secretion that occurred at 8 months. Lymphocytic accumulation could account for the decrease in secretion occurring at 24 months of age.
4. Discussion
This study characterized age-related changes in mouse lacrimal gland associated with its structure, innervation, and secretory response. Our studies showed that neural, cholinergic agonist, and α1-adrenergic agonist stimulated secretion decreased at 8 months of age and continued at this depressed rate for 12–32 months. An increase in lipofuscin-like inclusion accumulation and in mast cell infiltration occured at 8 months of age and appeared to precede lymphocytic infiltration and signs of structural changes. Lipofuscin-like inclusions and mast cell infiltration could account for the decrease in secretion at 8 months of age. A decrease in the density of innervation, a decrease in parasympathetic nerve activity, and an increase in lymphocytic accumulation could account for the loss of secretion at 24 and 32 months of age. This finding suggests that both activation of nerves and receptor mediated signaling processes were impaired.
One of the most prominent changes that occurred in the mouse lacrimal gland with increasing age was the progressive accumulation of lipofuscin-like inclusions in the cytoplasm of acinar cells. Lipofuscin is an accepted biomarker of aging and is composed of a heterogeneous group of complex autofluorescent aggregates derived from oxidative products of lipids, proteins and metal ions (Donato and Sohal, 1981; Terman and Brunk, 1998). It accumulates progressively in lysosomes of post-mitotic tissue because it is not totally eliminated either by degradation or exocytosis (Ivy et al., 1990; Sitte et al., 2000; Spiteller, 2001; Brunk and Terman, 2002). It has been demonstrated that lipofuscin accumulation in cells is accelerated under conditions of increased free radicals and oxidative stress. However, this association is not absolutely proven (Porta, 2002). Lipofuscin accumulation also decreased the efficiency of cellular proteolytic systems (Cuervo and Dice, 2000; Spiteller, 2001).
Increased levels of lipofuscin are associated with progressive injury to the cellular metabolism in neural and cardiac tissues (Reis et al., 1977; Nakamura et al., 1998). It may also diminish autophagocytotic capacity by acting as a sink for newly produced lysosomal enzymes and therefore interfering with the recycling of cellular components (Terman and Brunk, 1999, 1998). Lipofuscin granules have been observed in salivary glands (parotid, submandibular, and minor salivary glands) from older rats, mice and humans (Meisel et al., 1988; Lantini et al., 1990). Reduced secretory activity of acinar cells of the rat parotid gland during aging was also associated with the accumulation of lipofuscin and degenerative secretory granules of acid phosphatase (Kim, 1984).
Interestingly, we found that the reduction in the density of nerves surrounding acinar cells correlated with the presence of lipofuscin-like inclusions in the same tissue sections form older mice. Accumulation of lipofuscin-like inclusions and a decrease over time in the density of the sympathetic nerve network has been observed in various tissues of aged rats (Jaatinen et al., 1989). The presence of lipofuscin-like inclusions in the cytoplasm of lacrimal gland acinar cells in middle-aged animals and its progressive accumulation in the cytoplasmic space suggests that the accumulation of lipofuscin might be related to the reduced level of cellular secretory activity, causing premature senescence of the gland. To date, the effect of age on the accumulation of lipofuscin-like inclusions as a result of an increased oxidative stress in the lacrimal gland has not been investigated in depth. Therefore, the contributions of lipofuscin accumulation to cumulative oxidative damage to the acinar cells or to the loss of secretory responses to nerve-stimulation need to be addressed in further investigations.
Chronic inflammation and fibrosis are often found in the lacrimal and salivary glands of elderly patients without autoimmune diseases (Damato et al., 1984; Obata et al., 1995). It is then possible that the impairment of peroxidase secretion is mediated by an inhibitory effect of proinflammatory cytokines released from either mast cells or lymphocytes. Previous studies indicated that a reduction in the density of nerves surrounding acinar cells, combined with an increase in the number of mast cells associated with neural and vascular tissues also occurs in the lacrimal glands of aged rats (Williams et al., 1994). Our data shows that an increase in the score for mast cells was observed as early as 8 months (middle age) before any manifestation of acinar atrophy or lymphocytic infiltration. As the animals aged, a progressive increase in the number of mast cells and mast cell degranulation was observed throughout the interlobular and intralobular space. Most mast cells were observed in close proximity to the acini and to the periductal lymphocytic foci as the animals aged. Structural interactions between mast cells and a variety of other cell types have been observed and their close proximity to nerve endings in tissues in which mast cells are especially active (Purcell and Atterwill, 1995; Dines and Powell, 1997) suggest that mast cells can modulate events of tissue repair or destruction in their microenvironment through either autocrine and/or paracrine mechanisms. In addition to histamine release, these cells are capable of synthesizing, and responding to trophic factors such as nerve growth factor, and can also produce and respond to proinflammatory cytokines such as TNFα and IL-4 (Burd et al., 1989; Gordon and Galli, 1990, 1991). It is possible that in the lacrimal gland, mast cell degranulation may lead to the recruitment of lymphocytes at the site of inflammation leading to the destruction of acinar- and ductal-cells or nerve degeneration progressively with age.
Several studies reported that the mechanisms involved in inflammation-induced inhibition of neurotransmitter release in the lacrimal gland are mediated by the production of proinflammatory cytokines (IL-1α, IL-1β and TNFα), autoantibodies and metalloproteases (Lambert, 1998; Fox and Stern, 2002; Zoukhri et al., 2002). Furthermore, elevated levels of IL-1β, as occurs with aging or inflammation also inhibits neurotransmitter release (Collins et al., 1992; Lynch, 1998). Zoukhri et al. (Zoukhri and Kublin, 2001) demonstrated that concomitant with inflammation of lacrimal glands in MRL/lpr mice, a murine model of human Sjögren’s syndrome, there is increased production of IL-1β that inhibits both neural as well as adrenergic stimulation of lacrimal gland secretion. This study suggested that proinflammatory cytokines contribute to a dysfunction in the nerve endings that control lacrimal gland secretion by interfering with neurotransmitter release (Zoukhri and Kublin, 2001). Therefore, an impairment of peroxidase secretion mediated by an inhibitory effect of proinflammatory cytokines released from either mast cells or lymphocytes could occur with age.
Age-related changes in the lacrimal gland may cause a dysfunction in intracellular signaling pathways and result in a decrease in protein secretion. Differences in protein and peroxidase secretion in response to sympathomimetic stimulation have been reported in the glands of aged compared to young male rats (Bromberg et al., 1986). Cholinergic stimulation also resulted in significantly less protein secretion by the lacrimal tissue of aged male rats (Draper et al., 1997). In rat parotid gland, α1-adrenergic-stimulated secretion is reduced in part due to an alteration in the coupling of the ligand to α1-adrenergic receptors (Ishikawa et al., 1989; Roka et al., 2000) with age. Loss of muscarinic receptor responsiveness is caused by increased sensitivity to oxidative stress in senescence (Joseph et al., 1996). Decreases in membrane fluidity due to oxidative damage are associated with age-related changes in membrane receptor number and binding affinity (Miyamoto et al., 1992; Spiteller, 2002; Choi and Yu, 1995; Gao et al., 1998). Thus, age-related peroxidation of membrane phospholipids increases membrane rigidity and may compromise many membrane-associated functions in the lacrimal gland. It is possible that an increasing oxidative stress in the gland associated with age could lead to an inability of the acini to synthesize or to secrete secretory proteins in response to nerve-stimulation. Another possibility is that a reduction or an inability of the acini to synthesize and to secrete serous proteins because of lipofuscin accumulation induced apoptosis in acini as animals aged. It is not yet possible to discriminate between these alternatives.
Since it is known that striking differences exist in the morphology, biochemistry, immunology and genetics between adult males and females lacrimal gland tissues from either rodents or human, it would be interesting to determine whether age-dependent changes associated with the structure, innervation, and secretory response observed in males also occur in female mice. However, we could not explore this possibility because the NIA rodent colony did not have female BALB/c mice to conduct this study. However, this possibility will be pursued in further studies.
In summary, our data demonstrate that the mouse lacrimal gland undergoes both morphological and functional changes with age. These findings show that, a progressive accumulation of lipofuscin-like inclusions, as well as inflammatory infiltrate containing mast cells followed by lymphocytes, and extensive structural alterations occurred as the animals aged. In accordance with our findings, the presence of lipofuscin-like inclusion in acinar cells as well as the infiltration of mast cells occurred at early stage in the middle age (8-month-old) and before the presence of acinar atrophy. Infiltration of lymphocytes progressively increased in advanced age (24–32-month-old) concomitant with structural changes. The decrease in peroxidase secretion before an overall degeneration of the gland implies that lacrimal gland protein secretion is impaired at an early stage during the aging process. Furthermore, an alteration in parasympathetic and sympathetic innervation of the lacrimal gland occurred in older animals (24–32-month-old), as did a decrease in acetylcholine release.
This evidence supports the hypothesis that with increasing age, a combination of factors including increased lipofuscin-like inclusions (an index of oxidative stress), and increased inflammatory cell infiltration, may result in an early decrease of peroxidase secretion with an immunerelated damage to the lacrimal gland nerves and/or the impairment of neurotransmitter release. This leads to decreased fluid production from acinar and ductal cells. Thus the age-related reduction in tear secretion could be due to the destruction of acinar cells associated with oxidative stress and subsequent chronic inflammatory processes. The inflammatory reactions could then exacerbate the loss of neural responsiveness, leading to a spiral of destructive interaction among nerve terminals, acinar and ductal cells, and the immune system. This evidence also suggests that the influence of aging in lacrimal gland characterized by morphological and functional alterations might be correlated with the incidence of dry eye syndrome in elderly population.
Acknowledgements
The authors thank Mrs Patricia Pearson, and Ms Marita Sandstrom for their excellent technical assistance. The grant support was given by NIH EY12383, EY06177 and Fight For Sight, Research Division of Prevent Blindness America, Grant-In Aid.
References
- Bromberg BB, Cripps MM, Welch MH. Sympathomimetic protein secretion by young and aged lacrimal gland. Curr. Eye Res. 1986;5:217–223. doi: 10.3109/02713688609020046. [DOI] [PubMed] [Google Scholar]
- Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- Burd PR, Rogers HW, Gordon JR, Martin CA, Jayaraman S, Wilson SD, Dvorak AM, Galli SJ, Dorf ME. Interleukin 3-dependent and -independent mast cells stimulated with IgE and antigen express multiple cytokines. J. Exp. Med. 1989;170:245–257. doi: 10.1084/jem.170.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Yu BP. Brain synaptosomal aging: free radicals and membrane fluidity. Free Radic. Biol. Med. 1995;18:133–139. doi: 10.1016/0891-5849(94)00106-t. [DOI] [PubMed] [Google Scholar]
- Collins SM, Hurst SM, Main C, Stanley E, Khan I, Blennerhassett P, Swain M. Effect of inflammation of enteric nerves. Cytokine-induced changes in neurotransmitter content and release. Ann. N Y Acad. Sci. 1992;664:415–424. doi: 10.1111/j.1749-6632.1992.tb39780.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Dice JF. When lysosomes get old. Exp. Gerontol. 2000;35:119–131. doi: 10.1016/s0531-5565(00)00075-9. [DOI] [PubMed] [Google Scholar]
- Damato BE, Allan D, Murray SB, Lee WR. Senile atrophy of the human lacrimal gland: the contribution of chronic inflammatory disease. Br. J. Opthalmol. 1984;68:674–680. doi: 10.1136/bjo.68.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartt DA. Regulation of tear secretion. Adv. Exp. Med. Biol. 1994;350:1–9. doi: 10.1007/978-1-4615-2417-5_1. [DOI] [PubMed] [Google Scholar]
- Dartt DA. Regulation of lacrimal gland secretion by neurotransmitters and the EGF family of growth factors. Exp. Eye Res. 2001;73:741–752. doi: 10.1006/exer.2001.1076. [DOI] [PubMed] [Google Scholar]
- Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. J. Neuropathol. Exp. Neurol. 1997;56:627–640. [PubMed] [Google Scholar]
- Donato HJ, Sohal RS. Handbook of Biochemistry in Ageing. Boca Raton: CRC Press; 1981. [Google Scholar]
- Draper CEJS, Pallot DJ, Adeghate EA. The effect of age on protein and peroxidase secretion and morphological changes in the isolated rat lacrimal gland. J. Physiol. 1997;1997:155. [see also page 501] [Google Scholar]
- Draper CE, Adeghate E, Lawrence PA, Pallot DJ, Garner A, Singh J. Age-related changes in morphology and secretory responses of male rat lacrimal gland. J. Auton. Nerv. Syst. 1998;69:173–183. doi: 10.1016/s0165-1838(98)00026-5. [DOI] [PubMed] [Google Scholar]
- Draper CE, Adeghate EAJS, Pallot DJ. Evidence to suggest morphological and physiological alteration of lacrimal gland acini with aging. Exp. Eye Res. 1999;68:265–276. doi: 10.1006/exer.1998.0605. [DOI] [PubMed] [Google Scholar]
- Fox RI, Stern M. Sjogren’s syndrome: mechanisms of pathogenesis involve interaction of immune and neurosecretory systems. Scand. J. Rheumatol. Suppl. 2002;116:3–13. [PubMed] [Google Scholar]
- Gao E, Snyder DL, Roberts J, Friedman E, Cai G, Pelleg A, Horwitz J. Age-related decline in beta adrenergic and adenosine A1 receptor function in the heart are attenuated by dietary restriction. J. Pharmacol. Exp. Ther. 1998;285:186–192. [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J. Exp. Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Gee MV, Baum BJ, Roth GS. Decreased signal transduction in rat parotid cell aggregates during aging is not due to loss of alpha 1-adrenergic receptors. Exp. Gerontol. 1989;24:25–36. doi: 10.1016/0531-5565(89)90032-6. [DOI] [PubMed] [Google Scholar]
- Ivy GO, Kanai S, Ohta M, Smith G, Sato Y, Kobayashi M, Kitani K. Lipofuscin-like substances accumulate rapidly in brain, retina and internal organs with cysteine protease inhibition. In: Porta EA, editor. Lipofuscin and Ceroid Pigments. New York: Plenum Press; 1990. [DOI] [PubMed] [Google Scholar]
- Jaatinen P, Koistinaho J, Hervonen A. Age-related morphometric and histochemical features of rat sympathetic neurons. Adv. Exp. Med. Biol. 1989;266:61–72. doi: 10.1007/978-1-4899-5339-1_5. [DOI] [PubMed] [Google Scholar]
- Joseph J, Villalobos-Molina R, Denisova N, Erat S, Jimenez N, Strain J. Increased sensitivity to oxidative stress and loss of muscarinic receptors responsiveness in senescence. Ann. N Y Acad. Sci. 1996;786:112–119. doi: 10.1111/j.1749-6632.1996.tb39056.x. [DOI] [PubMed] [Google Scholar]
- Kim SK. Changes in the secretory acinar cells of the rat parotid gland during aging. Anat. Rec. 1984;209:345–354. doi: 10.1002/ar.1092090313. [DOI] [PubMed] [Google Scholar]
- Lambert RW. Do cytokines have a role in the regulation of lacrimal gland acinar cell ion transport and protein secretion? Adv. Exp. Med. Biol. 1998;438:499–503. doi: 10.1007/978-1-4615-5359-5_69. [DOI] [PubMed] [Google Scholar]
- Lantini MS, Proto E, Puxeddu P, Riva A, Testa Riva F. Fine structure of excretory ducts of human salivary glands. J. Submicrosc. Cytol. Pathol. 1990;22:465–475. [PubMed] [Google Scholar]
- Lemp MA. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232. [PubMed] [Google Scholar]
- Lynch MA. Age-related impairment in long-term potentiation in hippocampus: a role for the cytokine, interleukin-1 beta? Prog. Neurobiol. 1998;56:571–589. doi: 10.1016/s0301-0082(98)00054-9. [DOI] [PubMed] [Google Scholar]
- Meisel DL, Skobe Z, Prostak KS, Shklar G. A light and electron microscope study of aging parotid and submandibular salivary glands of Swiss-Webster mice. Exp. Gerontol. 1988;23:197–210. doi: 10.1016/0531-5565(88)90007-1. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Villalobos-Molina R, Kowatch MA, Roth GS. Altered coupling of a1-adrenergic receptor-G protein in rat parotid during aging. Am. Physiol. Soc. 1992;31:C1181–C1188. doi: 10.1152/ajpcell.1992.262.5.C1181. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Lane MA, Roth GS, Ingram DK. A strategy for identifying biomarkers of aging: further evaluation of hematology and blood chemistry data from a calorie restriction study in rhesus monkeys. Exp. Gerontol. 1998;33:421–443. doi: 10.1016/s0531-5565(97)00134-4. [DOI] [PubMed] [Google Scholar]
- Obata H, Yamamoto S, Horiuchi H, Machinami R. Histologic study of human lacrimal gland statistical analysis with special reference to aging. Opthalmology. 1995;102:678–686. doi: 10.1016/s0161-6420(95)30971-2. [DOI] [PubMed] [Google Scholar]
- Porta EA. Dietary factors in lipofuscinogenesis and ceroidogenesis. Arch. Gerontol. Geriatr. 2002;34:319–327. doi: 10.1016/s0167-4943(02)00009-2. [DOI] [PubMed] [Google Scholar]
- Preisser JS, Koch GG. Categorical data analysis in public health. Annu. Rev. Public Health. 1997;18:51–82. doi: 10.1146/annurev.publhealth.18.1.51. [DOI] [PubMed] [Google Scholar]
- Purcell WM, Atterwill CK. Mast cells in neuroimmune function: neurotoxicological and neuropharmacological perspectives. Neurochem. Res. 1995;20:521–532. doi: 10.1007/BF01694534. [DOI] [PubMed] [Google Scholar]
- Reis DJ, Ross RA, Joh TH. Changes in the activity and amounts of enzymes synthesizing catecholamines and acetylcholine in brain, adrenal medulla, and sympathetic ganglia of aged rat and mouse. Brain Res. 1977;136:465–474. doi: 10.1016/0006-8993(77)90071-3. [DOI] [PubMed] [Google Scholar]
- Roen JL, Stasior OG, Jakobiec FA. Aging changes in the human lacrimal gland: role of ducts. CLAO J. 1985;11:237–242. [PubMed] [Google Scholar]
- Roka F, Freissmuth M, Nanoff C. G protein-dependent signalling and ageing. Exp. Gerontol. 2000;35:133–143. doi: 10.1016/s0531-5565(99)00092-3. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Buring JE, Sullivan DA, Dana MR. Hormone replacement therapy and dry eye syndrome. JAMA. 2001;286:2114–2119. doi: 10.1001/jama.286.17.2114. [DOI] [PubMed] [Google Scholar]
- Schaumberg DA, Sullivan DA, Dana MR. Epidemiology of dry eye syndrome. Adv. Exp. Med. Biol. 2002;506:989–998. doi: 10.1007/978-1-4615-0717-8_140. [DOI] [PubMed] [Google Scholar]
- Sitte N, Huber M, Grune T, Ladhoff A, Doecke WD, von Zglinicki T, Davies KJ. Proteasome inhibition by lipofuscin/ceroid during postmitotic aging of fibroblasts. FASEB J. 2000;14:1490–1498. doi: 10.1096/fj.14.11.1490. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Marzabadi MR, Gallaris D, Brunk UT. Effect of ambient oxygen concentration on lipofuscin accumulation in cultured rat heart myocytes—a novel in vitro model of lipofusciogenesis. Free Radic. Biol. Med. 1989;6:23–30. doi: 10.1016/0891-5849(89)90155-x. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp. Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Spiteller G. Are changes of the cell membrane structure causally involved in the aging process? Ann. N Y Acad. Sci. 2002;959:30–44. doi: 10.1111/j.1749-6632.2002.tb02080.x. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Hann LE, Yee L, Allansmith MR. Age- and gender-related influence on the lacrimal gland and tears. Acta Ophthalmol. (Copenh) 1990;68:188–194. doi: 10.1111/j.1755-3768.1990.tb01902.x. [DOI] [PubMed] [Google Scholar]
- Sullivan DA, Yamagami H, Liu M, Steagall RJ, Schirra F, Suzuki T, Krenzer KL, Cermak JM, Sullivan RM, Richards SM, Schaumberg DA, Dana MR, Sullivan BD. Sex steroids, the meibomian gland and evaporative dry eye. Adv. Exp. Med. Biol. 2002;506:389–399. doi: 10.1007/978-1-4615-0717-8_56. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Ceroid/lipofuscin formation in cultured human fibroblasts: the role of oxidative stress and lysosomal proteolysis. Mech. Ageing Dev. 1998;104:277–291. doi: 10.1016/s0047-6374(98)00073-6. [DOI] [PubMed] [Google Scholar]
- Terman A, Brunk UT. Lipofuscin: mechanisms of formation and increase with age. Apmis. 1999;106:265–276. doi: 10.1111/j.1699-0463.1998.tb01346.x. [DOI] [PubMed] [Google Scholar]
- Ueno H, Ariji E, Izumi M, Uetani M, Hayashi K, Nakamura T. MR imaging of the lacrimal gland. Age-related and gender-dependent changes in size and structure. Acta Radiol. 1996;37:714–719. doi: 10.1177/02841851960373P259. [DOI] [PubMed] [Google Scholar]
- Utsunomiya I, Ren J, Taguchi K, Ariga T, Tadashi T, Ihara Y, Miyatake T. Immunohistochemical detection of verotoxin receptors in nervous system. Brain Res. Protocols. 2001;8:99–103. doi: 10.1016/s1385-299x(01)00091-5. [DOI] [PubMed] [Google Scholar]
- Van Haeringen NJ. Aging and the lacrimal system. Br. J. Ophthalmol. 1997;81:824–826. doi: 10.1136/bjo.81.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RM, Singh J, Sharkey KA. Innervation and mast cells of the rat exorbital lacrimal gland: the effect of age. J. Auton. Nerv. Syst. 1994;47:95–108. doi: 10.1016/0165-1838(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Y, Schaffner DW. Comparison of logistic regression and linear regression in modeling percentage data. Appl. Environ. Microbiol. 2001;67:2129–2135. doi: 10.1128/AEM.67.5.2129-2135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Kublin CL. Impaired neurotransmitter release from lacrimal and salivary gland nerves of a murine model of Sjögren’s syndrome. Invest. Ophthalmol Vis. Sci. 2001;42:925–932. [PMC free article] [PubMed] [Google Scholar]
- Zoukhri D, Hodges RR, Byon D, Kublin CL. Role of proinflammatory cytokines in the impaired lacrimation associated with autoimmune xerophthalmia. Invest. Ophthalmol. Vis. Sci. 2002;43:1429–1436. [PMC free article] [PubMed] [Google Scholar]